Abstract
Background:
Major depressive disorder (MDD) is a highly heterogenous disease, both in terms of clinical profiles and pathobiological alterations. Recently, immunometabolic dysregulations were shown to be correlated with atypical, energy-related symptoms but less so with the Melancholic or Anxious distress symptom dimensions of depression in The Netherlands Study of Depression and Anxiety (NESDA) study. In this study, we aimed to replicate these immunometabolic associations and to characterize the metabolomic correlates of each of the three MDD dimensions.
Methods:
Using three clinical rating scales, Melancholic, and Anxious distress, and Immunometabolic (IMD) dimensions were characterized in 158 patients who participated in the Predictors of Remission to Individual and Combined Treatments (PReDICT) study and from whom plasma and serum samples were available. The NESDA-defined inflammatory index, a composite measure of interleukin-6 and C-reactive protein, was measured from pre-treatment plasma samples and a metabolomic profile was defined using serum samples analyzed on three metabolomics platforms targeting fatty acids and complex lipids, amino acids, acylcarnitines, and gut microbiome-derived metabolites among other metabolites of central metabolism.
Results:
The IMD clinical dimension and the inflammatory index were positively correlated (r=0.19, p=.019) after controlling for age, sex, and body mass index, whereas the Melancholic and Anxious distress dimensions were not, replicating the previous NESDA findings. The three symptom dimensions had distinct metabolomic signatures using both univariate and set enrichment statistics. IMD severity correlated mainly with gut-derived metabolites and a few acylcarnitines and long chain saturated free fatty acids. Melancholia severity was significantly correlated with several phosphatidylcholines, primarily the ether-linked variety, lysophosphatidylcholines, as well as several amino acids. Anxious distress severity correlated with several medium and long chain free fatty acids, both saturated and polyunsaturated ones, sphingomyelins, as well as several amino acids and bile acids.
Conclusion:
The IMD dimension of depression appears reliably associated with markers of inflammation. Metabolomics provides powerful tools to inform about depression heterogeneity and molecular mechanisms related to clinical dimensions in MDD, which include a link to gut microbiome and lipids implicated in membrane structure and function.
INTRODUCTION
Major depressive disorder (MDD) is an important public health problem, with a lifetime incidence of 16.6% (Kessler et al., 2005). Although MDD has been defined by a consistent set of clinical criteria since the introduction of the DSM-III in 1980, the problem of clinical heterogeneity across individuals has severely hampered progress in achieving a more precise, biologically-based classification system for the syndrome (Fried and Nesse 2015, Park, Kim et al. 2017, Zimmerman, Martin et al. 2019). This clinical heterogeneity likely arises from substantial biological variability which, if better understood, could yield more personalized treatments for patients presenting with the MDD syndrome (Chen, Eaton et al. 2000, Goldberg 2011, Milaneschi, Lamers et al. 2016).
Blood-based metabolomic analyses, which measure the presence of small molecules (e.g., amino acids, lipids, organic acids) in the systemic circulation, body fluids and tissues, is a powerful emerging tool for parsing heterogeneous complex disorders. Metabolomics measures the inputs, intermediate products, and outputs of biological pathways, thereby informing the systems and components (such as enzymes) that are disrupted in disease states. Metabolomics is particularly valuable in heterogeneous syndromes such as MDD, where the specific biological systems disrupted may vary between individuals and lead to disparate symptom presentations. Previous work by our group and others have identified correlations between serum or plasma metabolomic profiles and clinically defined features of MDD including response to treatment (Kaddurah-Daouk, Bogdanov et al. 2013, Bhattacharyya, Ahmed et al. 2019, Ahmed, MahmoudianDehkordi et al. 2020, MahmoudianDehkordi, Ahmed et al. 2021). Prior work has focused primarily on metabolomic analyses conducted on a single analytic platform (Brydges et al. 2021), usually utilizing a method such as nuclear magnetic resonance, gas chromatography–mass spectrometry, liquid chromatography–mass spectrometry, or capillary electrophoresis–mass spectrometry (Shulaev 2006). Analysis of samples across multiple platforms that assess a broad range of molecules of differing polarity and molecular weight ranges involved in chemical reactions, including gut-derived metabolites, offers the promise of a more thorough biological characterization of distinct symptom dimensions that comprise MDD.
The importance of inflammation on MDD symptoms has recently gained increased attention. Epidemiologic data indicate MDD patients are at increased risk of inflammation-related comorbidities, including obesity and metabolic syndrome, cardiovascular disease, and diabetes mellitus (Otte, Gold et al. 2016). These elevated risks are not fully explained by lifestyle factors, such as smoking and a sedentary lifestyle (Penninx 2017). Many MDD patients demonstrate increased circulating levels of pro-inflammatory proteins, including interleukin (IL)-6, and C-reactive protein (CRP) (Dowlati, Herrmann et al. 2010, Kiecolt-Glaser, Derry et al. 2015, Kohler-Forsberg, Buttenschon et al. 2017, Haroon, Daguanno et al. 2018). Clinical trials suggest that the presence of high inflammation in MDD impedes response to monoamine modulating antidepressants and such patients may benefit from more specific anti-inflammatory treatments (Raison, Rutherford et al. 2013, Liao, Xie et al. 2019).
Two historically important clinical dimensions in MDD are melancholic features and anxiety. The clinical features of Melancholia, characterized by psychomotor changes, guilt, and the neurovegetative symptoms of early insomnia (difficulty falling asleep for the first time in the night) plus appetite or weight loss, have long been recognized as an important clinical subtype of depression (Kendler 2020). Anxious distress is characterized by prominent worry or dread, feeling tense, and restlessness (Zimmerman, Martin et al. 2019). Multiple studies have identified poorer treatment outcomes among patients with anxiety (Fava, Rush et al. 2006, Howland, Rush et al. 2009, Gaspersz, Lamers et al. 2017) and mixed results in patients with melancholia (McGrath et al., 2008; Gili et al., 2012; Imai et al., 2021), suggesting that more efficacious treatments will require a deeper understanding of the biological characteristics of these MDD dimensions.
Recently, in the Netherlands Study of Depression and Anxiety (NESDA) cohort of depressed adults, an immunometabolic dimension (IMD) was identified, consisting of the atypical and energy related symptoms of increased appetite, weight gain, hypersomnia, low energy, and leaden paralysis (Lamers, Milaneschi et al. 2018, Alshehri, Boone et al. 2019). Severity of IMD symptoms in this cohort positively associated with a measure of inflammation derived from serum CRP and IL-6 concentrations (Lamers, Milaneschi et al. 2018, Lamers, Milaneschi et al. 2019, Lamers, Milaneschi et al. 2020, Milaneschi, Lamers et al. 2020). Importantly, the association of inflammation with the IMD dimension was specific, with no significant association found with the Melancholia or Anxious distress dimensions (Lamers, Milaneschi et al. 2020). It should be noted that the crux of IMD is both atypical energy-related symptoms and biological inflammatory metabolic dysregulation, as opposed to just the symptomology. Although others have also found associations between related symptom profiles and inflammation (Simmons, Burrows et al. 2020), there have been no attempts to replicate the IMD specific findings of the NESDA analyses. The metabolomic profile of IMD has not previously been examined; characterizing this profile may yield insights into the pathobiology of the IMD dimension and identify potential novel targets for intervention.
In the current study, we assessed both inflammatory and metabolomic markers as they relate to the three MDD dimensions of IMD, Melancholia, and Anxious distress. Using samples from a randomized controlled trial of treatment-naïve adults with MDD (Dunlop, Binder et al. 2012), we aimed to: 1) replicate the positive association between immune-inflammatory dysregulation and IMD severity and the non-association between immune-inflammatory dysregulation and severity of Melancholia and Anxious distress symptoms reported by the NESDA group (Lamers, Milaneschi et al. 2020); and 2) conduct an exploratory analysis to characterize the metabolomic signature of IMD, Melancholia, and Anxious distress dimensions of depression.
Methods
Study Design
The Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT) study was conducted from 2007–2013 through the Mood and Anxiety Disorders Program of Emory University. The study was approved by the Institutional Review Board at Emory University and all patients provided written informed consent before participating. The PReDICT study protocol (Dunlop, Binder et al. 2012) and clinical results (Dunlop, Kelley et al. 2017) have been published previously.
Briefly, PReDICT randomly assigned treatment-naïve adult outpatients with non-psychotic MDD to one of three treatments: duloxetine (30–60 mg/day), escitalopram (10–20 mg/day), or cognitive behavior therapy (CBT, 16 one-hour individual sessions) for 12 weeks. Eligibility required a score ≥ 18 at screening and ≥ 15 at baseline on the 17-item Hamilton Depression Rating Scale (HAM-D) (Hamilton 1960). Participants were excluded if they had any medically significant or unstable medication condition that could impact study participation, safety, or data interpretation, any current eating disorder, or any current substance abuse or dependence. A medically significant condition was one that could have physiologically contributed to depressive symptoms (e.g., history of stroke, poorly controlled diabetes mellitus), or interfered with ability to respond to the study treatments (e.g. gastric bypass surgery, significant liver disease). Examples of common medical conditions that did not exclude patients from the study include well-controlled diabetes mellitus, hypertension, hypercholesterolemia, and irritable bowel syndrome.
Symptom Assessments
The analyses conducted herein utilized only baseline (i.e., pre-treatment) measures, which were collected on the day of a patient’s randomization to one of the study treatments. Patients were assessed by trained interviewers using the HAM-D 24-item with atypical symptoms, the Hamilton Anxiety Rating Scale (HAMA) (Hamilton 1959), and the Quick Inventory of Depressive Symptomatology Self-Report (QIDS-SR) (Rush, Trivedi et al. 2003). The NESDA study derived the three dimensions of MDD evaluated here based on items from the Inventory of Depressive Symptoms Self-Report (IDS-SR) (Rush et al., 1996) and Beck Anxiety Inventory (BAI) (Beck et al., 1988). The IDS-SR, QIDS-SR, and BAI items are all scored from 0–3. In order to replicate the NESDA analyses, to construct the atypical/energy related (A/ER) and Melancholia dimensions in PReDICT we used the same items from the QIDS-SR where possible and substituted similar items from the HAMD as described below.
For the IMD symptom dimension, the NESDA study summed the five IDS item scores for hypersomnia, increased appetite, increased weight, low energy, and leaden paralysis (Lamers, Milaneschi et al. 2020). The first four of these symptoms are present in the QIDS-SR used in PReDICT (items 4, 7, 9, and 14, respectively). For the IDS-SR leaden paralysis item, we substituted item E (fatiguability) of the HAM-D. This last item was recoded such that scores of 3 and 4 were replaced with values of 2 and 3, respectively, so that each item was on the same scale and to maintain the overall clinical IMD symptom score range of 0–15. A higher score indicated more severe IMD symptomology.
The Melancholia score was defined in NESDA by summing the 8 IDS-SR items for early morning insomnia, decreased appetite, decreased weight, view of myself, psychomotor slowing, and psychomotor agitation, mood worse in the morning, and distinct quality of mood (Lamers, Milaneschi et al. 2020). The first six of these items are present on the QIDS-SR (items 3, 6, 8, 11, 15, and 16, respectively). There were no substitutable items from the PReDICT scales for the last two symptoms on the NESDA Melancholia dimension. Thus, the current analysis used a Melancholia dimension score range of 0–18, with a higher score being indicative of more severe symptomology.
The Anxious distress dimension was defined by NESDA using three items from the IDS-SR (feeling tense, restless, concentration/worry) and two items from the BAI (fear of awful events and feeling like losing control). The PReDICT scales did not contain items that could be substituted for the BAI items, so we opted to define the Anxious distress dimension in PReDICT using the Psychic Anxiety subscale (items 1–6 and 14, all scored 0–4) of the HAMA (Maier, Buller et al. 1988). We (Brydges et al. 2021) have previously demonstrated the Psychic Anxiety subscale has utility in biological assessments of anxiety. The score range was 0–28, and a higher score indicated more severe symptomology.
Blood Sampling
The decision to collect blood samples for metabolomics was made part-way through the enrollment period of the PReDICT study, resulting in 158 subjects with blood samples available for metabolic analyses. On the day of randomization (without consideration of diet or fasting status) 10cc of whole blood was collected in an EDTA tube (for plasma for inflammatory markers) and 10cc in a red-top silicone-coated tube (for serum for metabolomics) from an antecubital vein between 8 am and 4 pm. EDTA tubes were immediately centrifuged at 1400g at 4°C for 10 mins; for serum the same centrifugation parameters were applied after the blood had clotted for 20 minutes. The resulting plasma and serum were then pipetted into 1 ml vials and frozen at −80°C until thawed for analysis.
Inflammatory Markers
Following Lamers et al. (2020), an inflammation index was calculated from plasma levels of CRP and IL-6. Plasma high sensitivity (hs)-CRP concentrations were assayed using the Ultra WR CRP immunoturbidimetric assay from Sekisui Diagnostics (Exton, PA) implemented on the AU480 chemistry analyzer (Beckman Coulter, Brea, CA; detection limit 0.01 mg/L) (Le, Innis-Whitehouse et al. 2000, Mehta, Raison et al. 2013, Raison, Rutherford et al. 2013, Felger, Li et al. 2016, Bekhbat, Chu et al. 2018, Goldsmith, Bekhbat et al. 2020). Concentrations of plasma IL-6 were assessed using high-sensitivity Quantikine enzyme-linked immunosorbent assay (ELISA) kits from R&D systems (Minneapolis, MN; detection limit 0.04 pg/ml) (Raison, Borisov et al. 2009, Raison, Borisov et al. 2010, Musselman, Royster et al. 2013, Smith, Conneely et al. 2014). Assays were performed in duplicate according to the manufacturer’s instructions. Intra- and inter-assay coefficients of variation (CVs) were reliably <10%. To calculate the inflammation index, the CRP and IL-6 measures were each log transformed, standardized, and then averaged, following the procedure described by Lamers et al. (2020).
Metabolic Profiling
We examined metabolomics associations with MDD using three platforms that provide complementary insights to the metabolism. Metabolomics data focused on primary and polar metabolites using gas chromatography – time of flight mass spectrometry (Fiehn 2016). Briefly, 30 μl of plasma was extracted at −20°C with 1 mL degassed isopropanol/acetonitrile/water (3/3/2). Extracts were dried down, cleaned from triacylglycerides using acetonitrile/water (1/1), and derivatized with methoxyamine and trimethylsilylation. Samples (0.5 μL) were injected at 250°C to a 30 m rtx5-SilMS column, ramped from 50–300°C at 15°C/min, and analyzed by −70 eV electron ionization at 17 spectra/s. Raw data were deconvoluted and processed using ChromaTOF vs. 4.1 and uploaded to the UC Davis BinBase database (Fiehn, Wohlgemuth et al. 2008) for data curation and compound identification (Lai, Tsugawa et al. 2018). Result data were normalized by systematic error removal using random forests (SERRF) software to correct for drift or batch effects (Fan, Kind et al. 2019).
Bile acids were quantified using targeted metabolomics protocols and profiling protocols established in previous studies (Qiu, Cai et al. 2009, Xie, Wang et al. 2015), and ultra-performance liquid chromatography triple quadrupole mass spectrometry (UPLC-TQMS) (Waters XEVO TQ-S, Milford, USA).
A third metabolite platform with a targeted metabolomics approach used the AbsoluteIDQ® p180 Kit (BIOCRATES Life Science AG, Innsbruck, Austria), with an ultra-performance liquid chromatography (UPLC)/MS/MS system [Acquity UPLC (Waters), TQ-S triple quadrupole MS/MS (Waters)]. This procedure provides measurements of up to 186 endogenous metabolites in quantitative mode (amino acids and biogenic amines) and semi-quantitative mode (acylcarnitines, sphingomyelins, phosphatidylcholines and lysophosphatidylcholines across multiple classes). The AbsoluteIDQ® p180 kit has been fully validated according to European Medicine Agency Guidelines on bioanalytical method validation. Additionally, the kit plates include an automated technical validation to assure the validity of the run and provide verification of the actual performance of the applied quantitative procedure including instrumental analysis. The technical validation of each analyzed kit plate was performed using MetIDQ® software based on results obtained and defined acceptance criteria for blank, zero samples, calibration standards and curves, low/medium/high-level quality control (QC) samples, and measured signal intensity of internal standards over the plate. De-identified samples were analyzed following the manufacturer’s protocol, with metabolomics labs blinded to the clinical data. The raw metabolomic profiles included 182 metabolite measurements of serum samples. Each assay plate included a set of duplicates obtained by combining approximately 10 μl from the first 76 samples in the study (QC pool duplicates: SPQC) to allow for appropriate inter-plate abundance scaling based specifically on this cohort of samples (n = 24 across all plates). Metabolites with 1) >40% of measurements below the lower limit of detection (LOD) and 2) >30% of SPQC coefficient of variation were excluded from the analysis (n = 160 metabolites passed QC filters). To adjust for the batch effects, a correction factor for each metabolite in a specific plate was obtained by dividing the metabolite’s QC global average by QC average within the plate. Missing values were imputed using each metabolite’s LOD/2 value followed by log2 transformation to obtain a normal distribution of metabolite levels.
Statistical Analyses
In order to investigate associations between the metabolome, inflammation, and scores on the A/ER, Melancholia and Anxious distress dimensions, partial Spearman rank correlations were conducted between baseline intensity of each compound, the inflammatory index, and the three symptom dimensions after accounting for age, sex, and body mass index (BMI; height and weight were obtained from laboratory measures). The resulting correlation coefficients and p values were also entered into the ChemRICH tool (Barupal and Fiehn 2017) to test for statistically significant enrichment in sets of structurally similar chemicals using the Kolmogorov-Smirnov statistics. All reported p values are uncorrected for multiple comparisons, given the exploratory nature of these analyses. Additionally, due to the complex relationship between BMI, metabolic alterations and specific atypical energy-related depressive symptoms, all analyses were repeated without accounting for BMI. It is known indeed that risk variants for metabolic pathway alterations (e.g., leptin receptor, melanocortin 4 receptor) and depression (e.g. NEGR1 [Neuronal Growth Regulator 1]) have a major role in BMI genetic architecture, determining substantial genetic covariance between BMI, metabolic alterations and atypical symptoms (Milaneschi, Lamers et al. 2017, Badini, Coleman et al. 2020, Kappelmann, Arloth et al. 2021, Milaneschi, Lamers et al. 2021). Note that the MDD dimensions are not mutually exclusive “subtypes” of MDD; analyses of all three dimensions use all 158 patients’ scores on each dimension.
We also used Bayes factors (BFs) to investigate the non-associations of inflammation and Melancholia and Anxious distress symptoms (Wagenmakers, 2007). BFs are advantageous over traditional p values because they allow for evidence in support of one hypothesis over another to be quantified (including evidence supporting the null hypothesis). Conversely, a p value is limited in that a non-significant p value cannot distinguish between a true null effect and an effect that is underpowered. Following Jeffreys’ (1961) guidelines, a BF of at least 3 was used as a cut-off for sufficient evidence in favor of the alternative hypothesis (i.e., an association between inflammation and a symptom dimension), and less than 1/3 was considered as sufficient evidence in favor of the null hypothesis. That is, one hypothesis had to be at least three times more likely, given the data, to be considered sufficient evidence in favor of that hypothesis. BFs between 1/3 and 3 were considered as weak evidence, which is indicative of an underpowered effect, and a BF of 1 implies that the null and alternative hypotheses are equally likely, given the data. A scaled beta (1/3, 1/3) distribution was used as the prior distribution.
RESULTS
Patient characteristics
Of the 344 patients randomized in PReDICT, 158 had all measures available for analysis at baseline. The demographic and clinical characteristics of these subjects are presented in Table 1. IL-6 and CRP were significantly correlated (r = 0.54, p < 0.001).
Table 1.
Subject Demographic and Clinical Characteristics
| Variable | M (SD) (N = 158) |
|---|---|
|
| |
| Age (years) | 39.09 (12.00) |
| Sex (N, % female) | 102 (64.56%) |
| Body mass index | 28.65 (6.47) |
| Race | |
| White | 62 (39.24%) |
| Native American | 44 (27.85%) |
| Black | 32 (20.25%) |
| Asian | 2 (1.27%) |
| Multiracial | 10 (6.33%) |
| Unknown/Not Reported | 8 (5.06%) |
| Hispanic ethnicity (%) | 57 (36.08%) |
| Immunometabolic dimension score (baseline; max score = 15) | 5.25 (2.57) |
| Melancholia dimension score (baseline; max score = 18) | 5.73 (2.68) |
| Anxious distress dimension score (baseline; max score = 28) | 10.62 (2.75) |
Associations between Depression Symptoms Dimensions and Inflammation
Table 2 shows the correlations and Bayes Factor (BF) values between the three symptom dimension scores and the inflammation index. IMD symptom dimension scores significantly and positively correlated with inflammation marker levels, but Melancholia and Anxious distress scores did not. Additionally, the BF values for Melancholia and Anxious distress indicated moderate evidence in favor of the null hypothesis (i.e., no association), replicating the results from the NESDA study (Lamers et al 2020). The fact that the correlations between depression dimensions were also quite low (IMD-Anxious distress r = .18; IMD-melancholia r = .09; Anxious distress-melancholia r =.31), highlights the importance of separating these dimensions and implying that these dimensions may be different disorders.
Table 2.
Correlations and Bayes Factors between Depression Symptom Dimensions and Inflammation Index
| Age and sex accounted for | Age, sex, and BMI accounted for | |||||
|---|---|---|---|---|---|---|
| Symptom Dimension | r | p | BF | r | p | BF |
| IMD | 0.22 | .005 | 7.35 | .19 | .019 | 2.11 |
| Melancholia | −0.09 | .280 | 0.20 | 0.03 | .734 | 0.24 |
| Anxious Distress | 0.06 | .444 | 0.23 | 0.10 | .224 | 0.31 |
Note. BF refers to Bayes Factor in favor of the alternative hypothesis over the null hypothesis. A BF < 1/3 indicates moderate evidence in favor of the null hypothesis; a BF > 3 indicates moderate evidence in favor of the alternative hypothesis. All p values are uncorrected for multiple comparisons. IMD: Immunometabolic depression.
Biochemical Profile of Immunometabolic Depression
As shown in Figure 1, IMD dimension scores were significantly positively correlated with indoxyl sulfate and indole-3-lactate (tryptophan metabolites generated from specific gut bacteria), and propane-1,3-diol (another microbial product). A short chain free fatty acid, butyric acid (also a microbial product) and the TCA cycle metabolite fumaric acid were positively correlated with IMD scores. Among the acylcarnitines, the short chain C4:1 and C3:1 and the medium chain C10 showed significant inverse correlations with IMD. Several long chain saturated free fatty acids, C16, C17, C18 were inversely correlated to IMD.
Figure 1.
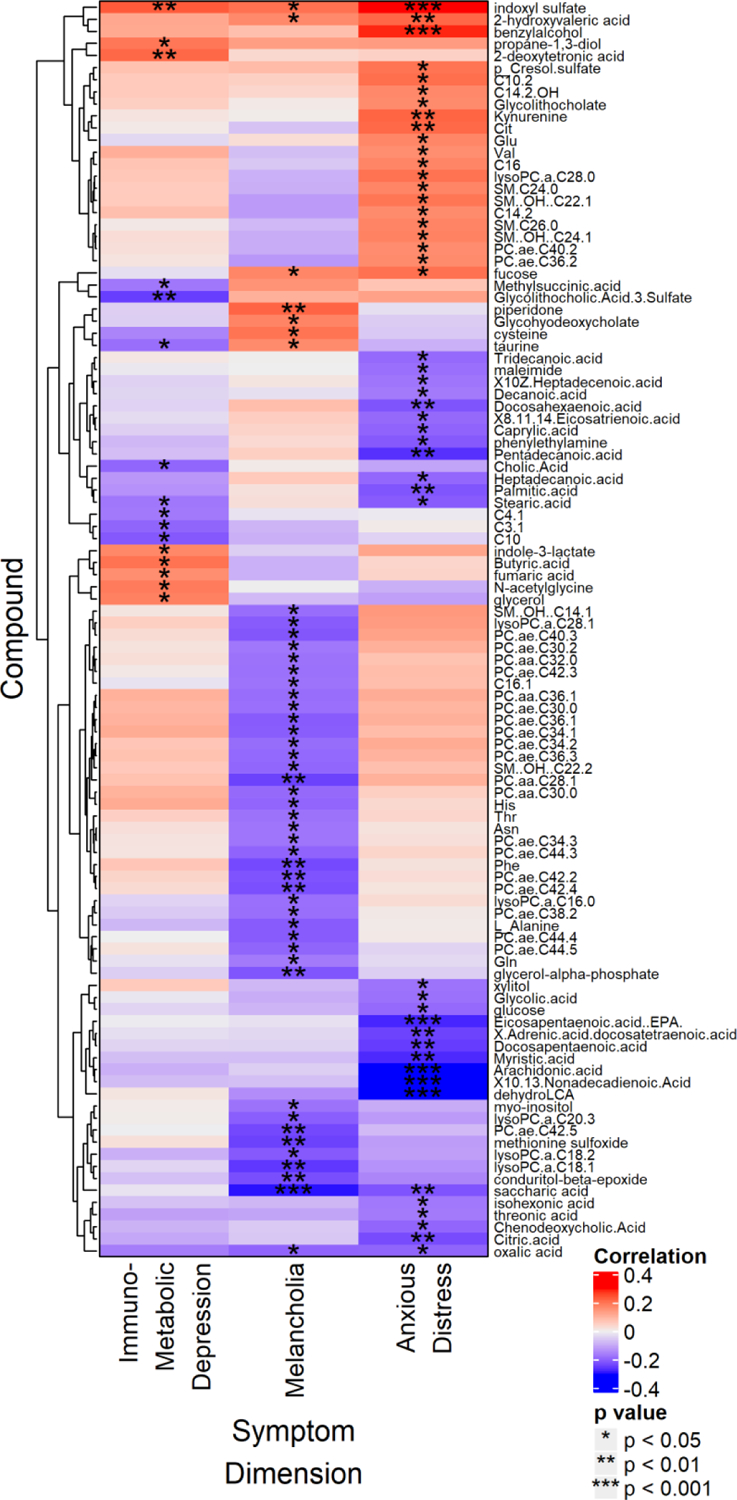
Heat map displaying partial Spearman rank correlations between compound intensity and Immunometabolic, Anxious distress, and Melancholia dimension scores at baseline, after controlling for age, sex, and BMI. Only identified metabolites that correlated significantly with at least one of the three variables are shown. All p values are uncorrected for multiple comparisons.
Given the significant correlation between IMD symptom dimension and the inflammatory index, we also compared the metabolomic profiles of the inflammatory index with that of IMD, but few metabolites showed significant correlations with both measures. One compound that was positively correlated with both IMD and the inflammatory index was 2-deoxytetronic acid, a gamma-aminobutyric acid (GABA) breakdown metabolite. One bile acid produced by the gut bacteria, glycolithocholic acid-3-sulfate, was inversely correlated with both measures.
Biochemical profile of the Melancholia Dimension
The symptom dimension of Melancholia showed significant inverse correlations to several long chain, saturated and unsaturated fatty acyl containing phosphatidylcholines (PCs), and lysoPCs, with or without adjusting for BMI (Figure 1 and supplemental Figures). Among the significantly inversely correlated PCs were a few diacyl ester-linked PCs, including PCaaC28:1, -C30:0, -C32:0, -C36:1, -C38:3. However, the majority of the PCs that showed inverse correlation to melancholia were presumably the ether-linked PCs such as PCaeC30:0, -C30:2, -C34:1, -C34:2, -C34:3, -C36:1, -C36:2, -C36:3, -C38:2, -C40:2, -C40:3, -C42:2, -C42:3, -C42:4, -C42:5, -C44:3, -C44:4, -C44:5, -C44:6. Among the ester lysoPCs, -C16:0, -C18:1,-C18:2, -C20:3, -C28:1 were inversely correlated to severity of Melancholia.
Besides the lipids, several amino acids, including asparagine, glutamine, histidine, phenylalanine, threonine, and alanine, were significantly negatively correlated to the Melancholia dimension score. Saccharic acid, a derivative of glucose, was also significantly inversely correlated with Melancholia severity. In contrast, the gut microbiome-derived tryptophan metabolite indoxyl sulfate was significantly positively correlated to severity of Melancholia, as was 2-hydroxyvaleric acid, a highly hydrophobic, short chain hydroxyl fatty acid.
Biochemical profile of the Anxious Distress Dimension
The Anxious distress dimension was significantly negatively correlated with several medium and long chain free fatty acids (FFAs), both saturated and unsaturated, including polyunsaturated fatty acids (Figure 1). Among the saturated FFAs were the medium chain C8:0 and C10:0, and the long chain C13:0, C14:0, C15:0, C16:0, C17:0, and C18:0 with both odd-chain FAs and even-chain FAs showing significant correlations to the symptom severity . Among the polyunsaturated fatty acids there were the omega-3 FFAs like Docosahexaenoic acid (DHA), Docosapentaenoic acid (DPA), Eicosapentaenoic acid (EPA), and several omega-6 FFAs as well, e.g., arachidonic acid (AA), C19:2(cis_10,13), C20:3(cis_8,11,14) and C22:4(cis_7,10,13,16). However, no significant associations of this dimension were detected with the ratios of omega3/omega-6 FFAs. In contrast, several amino acid levels, including citrulline, glutamate, valine, and kynurenine, were all significantly positively correlated with this dimension. Gut-microbiome-derived secondary bile acids like glycolithocholic acid and its sulfate also showed positive correlations with severity of anxiety distress, whereas the primary bile acid, CDCA showed opposite relationship. Other gut-microbiome related metabolites that were significantly positively correlated to anxiety severity were indoxyl sulfate, p-cresol sulfate, benzyl alcohol, and 2-hydroxyvaleric acid. Finally, several medium and long chain acylcarnitines (C10:2, C14:2, C14:2OH, C16) as well as a number of hydroxy-sphingolipids were positively correlated with Anxious distress scores.
Comparison across MDD dimensions and inflammation
Indoxyl sulfate was the only metabolite that showed a significant positive correlation to all three symptom dimensions, with or without controlling for BMI (Figure 1). Other metabolites significantly correlated in the same direction with both Melancholia and Anxious distress were: 1) 2-hydroxyvaleric acid (positive correlations), 2) fucose, a monosaccharide (positive correlations), 3) oxalic acid (negative correlations), and 4) saccharic acid, a hydrophobic glucuronic acid derivative (negative correlations). The only compound significantly correlated with both IMD and Melancholia was taurine, though the correlations were in opposite directions: Melancholia (positive) and IMD (negative).
This low level of overlap in biochemistry across these clinical dimensions was further supported by the ChemRICH enrichment analyses (Figure 2). The heatmap shows that no chemical clusters were common to any of the three symptom dimensions with the exception of sphingolipids for Melancholia and Anxious distress, but in that case the associations were opposite directions.
Figure 2.

ChemRICH enrichment results for Immunometabolic, Melancholia, and Anxious distress dimensions after controlling for age, sex, and BMI. The color of each cell indicates the direction of the correlations in the chemical cluster (blue = all compounds negatively correlate with phenotype; red = all compounds positively correlate with phenotype; pink/purple = correlations in both directions).
Note. PC aa: diacyl ester-linked phosphatidylcholines. PC ae: ether-linked phosphatidylcholines. FA: fatty acids.
Discussion
The results of the analyses reported here identify two important findings for understanding the heterogeneity of MDD. First, we replicated the NESDA study findings of a specific association of the inflammatory index (defined by plasma CRP and IL-6 concentrations) with the clinical dimension of IMD, and the lack of association of the inflammatory index with the Melancholia and Anxious distress dimensions (Lamers et al., 2020). This replication provides further support for the pathogenic impact of elevated states of inflammation for certain MDD patients, particularly those who report very low energy despite increases in appetite and sleep as part of their major depressive episode. Second, our exploratory analyses identified specific, minimally overlapping metabolomic associations of the three MDD clinical dimensions. These biochemical differences are likely to contribute to the biological processes that underlie the variability in clinical presentations of MDD. Taken together, this and others’ work (Ahmed, MahmoudianDehkordi et al 2020, Liu, Yieh et al 2016, Alshehri, Mook-Kanamori et al 2021, Kunugi, Hori et al 2015) demonstrate the utility of using biochemistry for dissecting the heterogeneity of MDD.
Although MDD is recognized as a heterogeneous condition, efforts to identify replicable biological characteristics of clinical sub-phenotypes has eluded the field. Most often, publications have used split-sample or cross-fold validation techniques to validate their biological associations; out-of-sample replications are rare. This study’s use of the PReDICT sample from the USA to replicate the Netherlands’ NESDA study report of a two-component index of inflammation specifically characterizing the IMD dimension (with no association with the dimensions of Melancholia or Anxious distress) represents an important replication. Although the effect size of the association in the PReDICT sample was not large, the fact that it was detected despite the presence of several potentially confounding variables, including uncontrolled diet, timing of blood draw, and differing ethnic compositions of the studies, argues that the association is robust (Choi et al. 2013). The association of inflammation with IMD symptoms is consistent with prior work evaluating inflammation non-MDD samples, including increased sleepiness (Imeri & Opp 2009) and fatigue (Dantzer et al. 2008). Converging evidence in large cohorts (Mileneschi et al. 2020) showed that markers of inflammation map more consistently to IMD atypical energy-related symptoms, and that this phenotypic link may be traced back at the level of shared genetic liability (Badini et al. 2020; Milaneschi et al. 2017). More recently, a large-scale study leveraging genetic data and Mendelian randomization analyses showed that the IL-6 pathway could be causally linked to the development of the depressive symptoms of fatigue and sleep problems (Milaneschi, Kappelmann et al. 2021). It is estimated that about 15% of the variability in CRP concentrations across individuals is related to adiposity (Aronson et al. 2004), so the identified association between weight gain and inflammation in our sample may reflect a bidirectional process, given the well-established link between obesity and chronic low-grade inflammation (Gregor & Hotamisligil 2011), including in depressed adults (Capuron, Lasselin, & Castanon 2017). Adiposity may hold different roles in the (unknown) underlying pathways linking inflammation to IMD-related symptoms, by acting as a confounder with a causal role in the development of depression and inflammation, as a mediator (e.g., physical inactivity secondary to depression leading to increased adiposity that, in turn, determines heightened inflammation), or as a collider emerging from the same antecedent causal pathways determining depressive symptoms and inflammation (common genetic liability, alterations in systems involved in homeostatic adjustments and brain circuitries integrating homeostatic and mood regulatory responses). Alterations in energy utility and storage could also shift the energy homeostasis and adiposity. Finally, one report found an association between inflammation and the symptom of leaden paralysis in bipolar adults (Gan et al. 2019). Given that inflammation is not likely to be part of the pathophysiology of all cases of MDD, further characterization of these inflammation-specific symptom-profiles will inform selection of patients for future anti-inflammatory therapeutic approaches.
Using metabolomics to further explore the biological underpinnings of the IMD dimension, we identified positive associations of IMD with several gut metabolites (i.e., high metabolite levels were associated with more severe symptomology), including indole-derivates of tryptophan metabolism. Tryptophan is an essential amino acid that is metabolized into a broad variety of molecules in the gastrointestinal tract via the indole, serotonin, and kynurenine metabolic pathways, which have been associated with a range of health and neuropsychiatric pathologies including inflammatory diseases, metabolic syndrome, depression, and anxiety disorders (Agus, Planchais et al. 2018, Gao, Xu et al. 2018). Interestingly, certain bacterial derivatives of tryptophan, including indole (Bansal et al. 2010; Beaumont et al. 2018), indole-3-propionic acid (Jennis et al. 2018), and indole-3-lactate (Ehrlich et al. 2020), have demonstrated anti-inflammatory properties and enhancement of intestinal epithelial cell barrier function in the gastrointestinal tract in preclinical models. It may be that individual differences in the generation of specific microbiota-host co-metabolites (i.e., the bacterial metabolites subsequently modified by host enzymes) contribute to pathogenicity (Beaumont et al. 2018). Differences in sulfation of indole during phase II hepatic metabolism may yield higher concentrations of pathogenic indoxyl sulfate in certain individuals (Hubbard et al. 2015), which has previously been found to be associated with depression (Philippe, Szabo de Edelenyi et al. 2021). Additionally, indole-3-lactic acid and other gut microbiome-linked metabolites such as propane-1,3-diol, which is produced from glycerol by Clostridium diolis bacteria and Enterobacteriaceae, were also positively associated with IMD scores.
Also of interest was the positive correlation between IMD severity and 2-deoxytetronic acid, a metabolite formed through a GABA degradation pathway. 2-deoxytetronic acid is a marker of succinic semialdehyde dehydrogenase deficiency (Shinka, Inoue et al. 2002) that is associated with a rare autosomal recessive disorder and with Alzheimer’s disease (Mousavi, Jonsson et al. 2014). Given that inflammation is considered to be a potential pathophysiologic contributor to Alzheimer’s disease, insights into the metabolomic abnormalities that occur with IMD may inform efforts to understand the biological basis of the neuropsychiatric symptoms, such as aspects of depression, that often precede the clinical manifestation of Alzheimer’s dementia. The ChemRICH analysis (Figure 2) also identified a specific negative correlation of several acylcarnitines, including the short chain C4:1 and C3:1 and the medium chain C10 with IMD scores. Acylcarnitines play a central role in regulating mitochondrial energy homeostasis, specifically in the β-oxidation of fatty acids (Fritz & McEwen 1959). Earlier work in MDD identified abnormal acylcarnitine profiles that correlate with symptom severity and antidepressant treatment response (Ahmed et al. 2020; MahmoudianDehkordi et al. 2021) and recent genomic based data supports a potential causal role for acylcarnitines in depression (Milaneschi, Arnold et al. 2021). Our results advance this work by more specifically associating short-chain acylcarnitine disruptions with the IMD dimension of MDD.
Lipids are involved in crucial brain functions, including cell membrane structure, vesicle trafficking, energy metabolism, and neuroendocrine function (MahmoudianDehkordi et al. 2021). Blood lipid profile changes have been implicated in the pathophysiology of depression, schizophrenia, and Alzheimer’s disease. Associations between altered lipid profiles and the presence of depression and anxiety have been previously reported in several studies (Ahmed et al. 2020; MahmoudianDehkordi et al. 2021). The findings in the current study clearly indicate the association of aberrated lipid metabolism with psychiatric disorders.
It is striking that in our study lipid associations (shown in Figure 2) were quite unique for dimensions evaluated. Anxious distress had a cluster of free fatty acids, melancholia had a strong cluster of phospholipids with ether linkages and no lipid links were noted for IMD dimension. Patients with greater Melancholia dimension severity were characterized by significantly low baseline levels of PCs such as several diacyl PCs and lysoPCs; almost 80% of the PCs significantly associated with severity of Melancholia were ether-linked. FFAs function as the building blocks of conjugated lipids and FFA derivatives function in cell signaling. Phosphatidylcholines PCs, are a major component of cell membranes with distinctive chemical feature for the ether PC which have ether bond at the sn-1 position of the glycerol backbone where fatty alcohol is attached as opposed to the more common diacyl moiety containing phospholipids. The emerging role of these lipids in neurological diseases like Alzheimer’s disease and autism is currently garnering much interest. Ether-linked PCs act as potent antioxidants in cellular membranes by preventing oxidative degradation of polyunsaturated fatty acid containing phospholipids (Liu, Yieh et al 2016, Dorninger, Forss-Petter et al 2020, Dean and Lodhi 2018). A major class of ether-linked phospholipids are plasmalogens that are ubiquitous in mammalian cell membranes and plasma lipoproteins. They are key components of “lipid rafts,” the glycolipoprotein micro-domains in the plasma membrane, comprised of glycosphingolipids, cholesterol, and receptors, and thought to regulate receptor trafficking (Di Paolo and Kim 2011, Dean and Lodhi 2018, Lefevre-Arbogast, Hejblum et al. 2021) and contribute to neurotransmission and synaptic plasticity (Sebastiao, Colino-Oliveira et al. 2013). The enriched heterotrimeric G protein Gs-alpha, which activates adenylyl cyclase to produce cyclic adenosine monophosphate, is less efficient when located within lipid rafts than when outside of lipid rafts in the plasma membrane (Allen et al. 2009). Emerging data suggest that MDD patients may have enriched concentrations of Gs-alpha within lipid rafts (Donati et al. 2008) and that antidepressants may act in part by enabling G protein exodus from lipid rafts (Singh et al. 2018).
There are several potential mechanisms that could result in our finding of reduced levels of ether-linked PCs among the patients scoring higher on the Melancholia dimension. Phospholipases A2 (PLA2s) are enzymes that cleave fatty acid in position two of phospholipids, hydrolyzing the bond between the second fatty acid “tail” and the glycerol molecule, releasing lysoPCs and FFAs (Figure 3). The lower level of these end-products of PLA2 may suggest lower activity of this enzyme, but the lower overall PC concentrations cannot be explained by altered PLA2 activity. Another possibility is increased activity of lecithin cholesterol acyltransferase (LCAT). LCAT uses PCs to esterify free cholesterol on high density lipoproteins (HDL), thereby releasing lysoPCs, but does not do so selectively for ether-lipids (as observed in our data), making this explanation unlikely. Thus, the combination of reduced levels of both PC and lysoPC, along with low or unchanged masses of FFAs, appear more likely to be explained by defects in PC biosynthesis, as occurs in the de novo synthesis of phospholipids via the Kennedy pathway and the biosynthesis of plasmalogen precursor. This explanation better fits the observed low levels of both PC and lysoPC since PC serves as the precursor of lysoPC production. This hypothesis of a defect in PC biosynthesis will need to be tested with measurement of liver function including hepatic peroxisomal activity or HDL levels. Whatever the explanation, plasmalogens are thought to play a significant role in Alzheimer’s disease (Su, Wang et al. 2019) and other genetic neurological and metabolic diseases, though whether their role is causal or a consequence of the underlying pathophysiology is yet to be ascertained (Paul, Lancaster et al. 2019).
Figure 3.

Pathway showing phosphatidylcholine metabolism by phospholipase A2 to produce lysophosphatidylcholine and a free fatty acid, particularly Arachidonic Acid which goes on to produce the inflammation-mediating eicosanoids.
In contrast to the findings for the Melancholia dimension, higher levels of PCs with both the diacyl-ester linked and the ether-linked fatty acyl chains were associated with greater scores on the Anxious distress dimension. Although this association did not reach statistical significance, overall they were positively correlated to more severe symptoms of anxiety. Significantly low levels of the serum free fatty acid levels, both saturated and unsaturated, were also be observed in patients with higher Anxious distress dimension scores. This may suggest a possible disruption in the activity of PLA2 function. In particular, PLA2 has been shown to release arachidonic acid that, upon downstream modification by cyclooxygenases or lipoxygenases, is modified into active compounds called eicosanoids (Figure 3). Eicosanoids include prostaglandins and leukotrienes, which can function as both anti-inflammatory and pro-inflammatory mediators by regulating the immune response (Dennis 1994). We found a highly significant inverse correlation between Anxious distress scores and levels of arachidonic acid, suggesting a possible role of inflammatory mediators for this dimension. However, we did not detect significant correlations between the inflammatory index and the Anxious distress dimension score.
Additionally, several other polyunsaturated free fatty acids were also significantly inversely correlated to severity of Anxious distress, such as EPA, DHA, and docosapentaenoic acid. DHA, followed by its precursor EPA, are the most common omega-3 fatty acids in human body (Schmitz and Ecker 2008, Parekh, Smeeth et al. 2017). DHA alone comprises 15%–20% of human brain lipid and lower concentrations in this group has been shown to have strong association to depression due to dysregulation of inflammatory responses, decreased antioxidant capacity and disrupted neurotransmission (Parekh, Smeeth et al. 2017). Several previous reports have indicated that patients with MDD have a lower level of the PUFAs such as EPA and DHA in their peripheral tissues (plasma, serum and red blood cells) than control subjects, and furthermore, the severity of depressive symptoms is correlated with lower omega-3 PUFAs (Edwards, Peet et al. 1998). Here we show that among the MDD patients, specifically the Anxious distress dimension, there were significant inverse correlations of EPA and DHA with increased severity of the symptoms. PUFAs are important components of phospholipids and cholesterol esters of the neuronal cell membrane, especially of dendritic and synaptic membranes and constitute principal regulating factors of neurogenesis, cell survival and neurotransmission. Higher circulating n-3 PUFAs are known to be associated with a lower risk for metabolic syndrome. This syndrome refers to a cluster of three or more components of cardiometabolic risk factors, including central obesity, hypertension, dyslipidemia and impaired glucose tolerance. Quality of dietary fat is known to play significant role in the development of metabolic syndrome. Both omega-3 and omega-6 PUFAs are inversely associated with more severe Anxious distress in our study which may suggest presence of metabolic syndrome in these patients, partly secondary to poor dietary sources. However genetic or hormonal factors may also play a role. Additionally, the PUFAs being low may result from increased lipid peroxidation from reactive oxygen species due to increase in oxidative stress.
Besides the phospholipids and the FFAs, several medium and long chain acylcarnitines such as C10:2, C14:2OH, C16 and C14:2 were positively correlated with Anxious distress dimension scores, likely indicating disrupted beta-oxidation in the mitochondrial energy metabolism in these patients. We have previously reported a decrease in several medium and long chain acylcarnitines after selective serotonin reuptake inhibitor (SSRI) treatment within the clinical subtype of anxiety in a separate MDD cohort (Ahmed, MahmoudianDehkordi et al. 2020, MahmoudianDehkordi, Ahmed et al. 2021), which suggests that the SSRI Citalopram/Escitalopram treatment may restore beta-oxidation related mitochondrial dysfunction in clinically anxious MDD patients.
Other molecules of interest in the Anxious distress dimension were the significant positive correlations of several gut-bacteria derived metabolites such as indoxyl sulfate, p-cresol sulfate, 2-hydroxyvaleric acid, benzyl-alcohol and the toxic bile acid glycolithocholate with increased symptom severity, suggesting possible gut-dysbiosis in these patients.
Among the amino acids, kynurenine and citrulline showed positive correlations to severity of the Anxious distress dimension. Our previous work has shown that kynurenine, a tryptophan metabolite, was strongly associated with depressive symptoms in the Mayo-PGRN cohort of 290 MDD outpatients (Liu, Ray et al. 2018). Inflammatory cytokines can increase expression of the enzyme indoleamine 2,3-dioxygenase, thus activating the kynurenine branch of tryptophan metabolism resulting in depressive-like behavior in mouse model (O’Connor, Andre et al. 2009).
Citrulline is an intermediate of the urea cycle and previous studies have observed significantly low levels of arginine and citrulline in MDD patients compared to healthy controls (Hess, Baker et al. 2017). We have shown increase in these urea cycle intermediates in MDD patients in response to exposure to the SSRI escitalopram/citalopram (MahmoudianDehkordi, Ahmed et al. 2021) which may be indicative of alterations in urea cycle, removal of nitrogen waste and nitric acid production in these patients.
There are some limitations to these analyses. Although the sample size included over 150 treatment naïve MDD adults, we did not have sufficient power to meaningfully apply corrections for multiple comparisons. We could not control for diet and the samples were collected without regard to fasting status, which may have particularly impacted some of the lipids reported. Another limitation is that the Biocrates p180 kit coupled with the mass-spectrometer platform used cannot discern lipid molecules with very small mass differences (i.e., less than 0.05 Da) and therefore it is possible that the SM(OH)s might be long-chain SMs instead (Li, Alam et al. 2021). Similarly, the platform also has limited resolution to differentiate between long chain lysophosphatidylcholines and phosphatidylcholines. Finally, due to differences in rating scales employed between studies, we could not exactly replicate the symptom structures for each dimension as defined by NESDA. Although our IMD dimension differed on only a single scale item, and the Melancholia dimension by two scale items; the Anxious distress dimension had to be defined using a different rating scale.
In conclusion, this study presented exploratory findings that a multifaceted disruption in the delicate balance between the gut microbiota, dietary lipids and host lipid metabolism triad relationship may be a cause of distinct symptom dimensions of MDD. Given the exploratory nature of the metabolomic associations with symptom dimensions identified in this work, additional studies should be conducted to establish their replicability.
Supplementary Material
Major depressive disorder (MDD) can be theorized as a series of symptom dimensions
We examined metabolic associations of three MDD symptom dimensions
Metabolic associations were largely independent across symptoms dimensions
Acknowledgements
We acknowledge the assistance of Ms. Lisa Howerton (Duke). This work was funded by grant support to Dr. Rima Kaddurah-Daouk (PI) through NIH grants R01MH108348, R01AG046171 and U01AG061359. Dr. Boadie Dunlop has support from NIH grants P50-MH077083 (PI Mayberg), R01-MH080880 (PI Craighead), UL1-RR025008 (PI Stevens), M01-RR0039 (PI Stevens) and the Fuqua Family Foundations. The PReDICT study was supported by NIH Grants numbered: P50 MH077083 and RO1 MH080880. Dr. Penninx has received research funding (unrelated to the work reported here) from Jansen Research and Boehringer Ingelheim. Drs. Gabi Kastenmüller and Matthias Arnold have support from NIH grants U01AG061359 (PI Kaddurah-Daouk), 1RF1AG059093 (PI Kaddurah-Daouk), 1RF1AG058942 (PI Kaddurah-Daouk), and 1U19AG063744 (PI Kaddurah-Daouk). Dr. Bhattacharyya had support for this work from the NIH grant R01MH108348 (PI Kaddurah-Daouk and Dunlop).
Disclosures
Dr. Dunlop has received research support from Acadia, Compass, Aptinyx, NIMH, Sage, and Takeda, and has served as a consultant to Greenwich Biosciences, Myriad Neuroscience, Otsuka, Sage, and Sophren Therapeutics.
Dr. Rush has received consulting fees from Compass Inc., Curbstone Consultant LLC, Emmes Corp., Holmusk, Johnson and Johnson (Janssen), Liva-Nova, Neurocrine Biosciences Inc., Otsuka-US, Sunovion; speaking fees from Liva-Nova, Johnson and Johnson (Janssen); and royalties from Guilford Press and the University of Texas Southwestern Medical Center, Dallas, TX (for the Inventory of Depressive Symptoms and its derivatives). He is also named co-inventor on two patents: U.S. Patent No. 7,795,033: Methods to Predict the Outcome of Treatment with Antidepressant Medication, Inventors: McMahon FJ, Laje G, Manji H, Rush AJ, Paddock S, Wilson AS; and U.S. Patent No. 7,906,283: Methods to Identify Patients at Risk of Developing Adverse Events During Treatment with Antidepressant Medication, Inventors: McMahon FJ, Laje G, Manji H, Rush AJ, and Paddock S.
Dr. Kaddurah-Daouk in an inventor on a series of patents on use of metabolomics for the diagnosis and treatment of CNS diseases and holds equity in Metabolon Inc. and Chymia LLC. Dr. Milaneschi reported no biomedical financial interests or potential conflicts of interest. Dr. Mayberg has received consulting and intellectual property licensing fees from Abbott Labs. Dr. Penninx reports no conflicts of interest with regard to this study. Drs. Kastenmüller and Arnold are inventors on a series of patents on the use of metabolomics for the diagnosis and treatment of CNS diseases. Dr. Bhattacharyya is a co-inventor in a patent on the use of metabolomics in major depression.
Dr. Craighead receives research support from the NIH and private foundations, including the Fuqua family foundations, William I. H. and Lula E. Pitts Foundation, and the Mary and John Brock Foundation. He receives book royalties from John Wiley & Sons. He is on the Board of Directors of Hugarheill ehf, an Icelandic company dedicated to prevention of depression. He serves on the Scientific Advisory Boards of Anxiety and Depression Association of America, the George West Mental Health Foundation, and AIM for Mental Health.
Dr. Kristal is the inventor on general metabolomics-related IP that has been licensed to Metabolon via Weill Medical College of Cornell University and for which he received royalty payments via Weill Medical College of Cornell University and currently has an equity interest in the company. Metabolon offers biochemical profiling services and is developing molecular diagnostic assays detecting and monitoring disease. Metabolon has no rights or proprietary access to the research results presented and/or new IP generated under these grants/studies. Dr. Kristal’s interests were reviewed by the Brigham and Women’s Hospital and Partners Healthcare in accordance with their institutional policy. Accordingly, upon review, the institution determined that Dr. Kristal’s financial interest in Metabolon does not create a significant financial conflict of interest (FCOI) with this research. The addition of this statement where appropriate was explicitly requested and approved by Dr. Kristal.
Footnotes
All other authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agus A, Planchais J and Sokol H (2018). “Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease.” Cell Host Microbe 23(6): 716–724. [DOI] [PubMed] [Google Scholar]
- Ahmed AT, MahmoudianDehkordi S, Bhattacharyya S, Arnold M, Liu D, Neavin D, Moseley MA, Thompson JW, Williams LSJ, Louie G, Skime MK, Wang L, Riva-Posse P, McDonald WM, Bobo WV, Craighead WE, Krishnan R, Weinshilboum RM, Dunlop BW, Millington DS, Rush AJ, Frye MA, Kaddurah-Daouk R and Mood C Disorders Precision Medicine (2020). “Acylcarnitine metabolomic profiles inform clinically-defined major depressive phenotypes.” J Affect Disord 264: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JA, Yu JZ, Dave RH, Bhatnagar J, Roth BL and Rasenick MM (2009). “Caveolin-1 and lipid microdomains regulate Gs trafficking and attenuate Gs/adenylyl cyclase signaling.” Mol Pharmacol 76:1082–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri T, Boone S, de Mutsert R, Penninx B, Rosendaal F, le Cessie S, Milaneschi Y and Mook-Kanamori D (2019). “The association between overall and abdominal adiposity and depressive mood: A cross-sectional analysis in 6459 participants.” Psychoneuroendocrinology 110: 104429. [DOI] [PubMed] [Google Scholar]
- Alshehri T, Mook-Kanamori DO, Willems van Dijk K, Dinga R, Penninx BWJH, Rosendaal FR, le Cessie S, Milaneschi Y Metabolomics dissection of depression heterogeneity and related cardiometabolic risk. Psychol Med. 2021. Jun 3:1–10. doi: 10.1017/S0033291721001471. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson D, Bartha P, Zinder O, Kerner A, Markiewicz W, Avizohar O, Brook GJ and Levy Y (2004). “ Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome.” Int J Obes 28:674–679. [DOI] [PubMed] [Google Scholar]
- Badini I, Coleman JRI, Hagenaars SP, Hotopf M, Breen G, Lewis CM and Fabbri C (2020). “Depression with atypical neurovegetative symptoms shares genetic predisposition with immuno-metabolic traits and alcohol consumption.” Psychol Med: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal T, Alaniz RC, Wood TK and Jayaraman A (2010). “ The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation.” Proc Natl Acad Sci 107(1): 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barupal DK and Fiehn O (2017). “Chemical Similarity Enrichment Analysis (ChemRICH) as alternative to biochemical pathway mapping for metabolomic datasets.” Sci Rep 7(1): 14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont M, Neyrinck AM, Olivares M, Rodriguez J, de Rocca Serra A, Roumain M, Bindels LB, Cani PD, Evenepoel P, Muccioli GG and Demoulin JB (2018). “The gut microbiota metabolite indole alleviates liver inflammation in mice.” The FASEB Journal 32(12): 6681–6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Chu K, Le NA, Woolwine BJ, Haroon E, Miller AH and Felger JC (2018). “Glucose and lipid-related biomarkers and the antidepressant response to infliximab in patients with treatment-resistant depression.” Psychoneuroendocrinology 98: 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Ahmed AT, Arnold M, Liu D, Luo C, Zhu H, Mahmoudiandehkordi S, Neavin D, Louie G, Dunlop BW, Frye MA, Wang L, Weinshilboum RM, Krishnan RR, Rush AJ and Kaddurah-Daouk R (2019). “Metabolomic signature of exposure and response to citalopram/escitalopram in depressed outpatients.” Transl Psychiatry 9(1): 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges CR, Fiehn O, Mayberg HS, Schreiber H, Dehkordi SM, Bhattacharyya S, Cha J, Choi KS, Craighead WE, Krishnan RR, Rush AJ, Dunlop BW and Kaddurah-Daouk R (2021). “Indoxyl sulfate, a gut microbiome-derived uremic toxin, is associated with psychic anxiety and its functional magnetic resonance imaging-based neurologic signature.” Sci Rep, 11(1): 21011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Lasselin J and Castanon N (2017). “Role of adiposity-driven inflammation in depressive morbidity.” Neuropsychopharmacol 42(1): 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Eaton WW, Gallo JJ and Nestadt G (2000). “Understanding the heterogeneity of depression through the triad of symptoms, course and risk factors: a longitudinal, population-based study.” J Affect Disord 59(1): 1–11. [DOI] [PubMed] [Google Scholar]
- Choi J, Joseph L and Pilote L (2013). “Obesity and C-reactive protein in various populations: a systematic review and meta-analysis.” Obes Rev, 14(3): 232–244. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW and Kelley KW (2008). “ From inflammation to sickness and depression: when the immune system subjugates the brain.” Nat Rev Neurosci 9(1): 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JM and Lodhi IJ (2018). “Structural and functional roles of ether lipids.” Protein Cell 9(2): 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EA (1994). “Diversity of group types, regulation, and function of phospholipase A2.” J Biol Chem 269(18): 13057–13060. [PubMed] [Google Scholar]
- Di Paolo G and Kim TW (2011). “Linking lipids to Alzheimer’s disease: cholesterol and beyond.” Nat Rev Neurosci 12(5): 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati RJ, Dwivedi Y, Roberts RC, R Conley R, Pandey GN and Rasenick MM (2008). “Post-mortem brain tissue of depressed suicides reveals increased Gs localization in lipid raft domains where it is less likely to activate adenylyl cyclase.” J Neurosci 28:3042–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK and Lanctot KL (2010). “A meta-analysis of cytokines in major depression.” Biol Psychiatry 67(5): 446–457. [DOI] [PubMed] [Google Scholar]
- Dorninger F, Forss-Petter S, Wimmer I, Berger J Plasmalogens, platelet-activating factor and beyond - Ether lipids in signaling and neurodegeneration. Neurobiol Dis. 2020. Nov;145:105061. doi: 10.1016/j.nbd.2020.105061. Epub 2020 Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Binder EB, Cubells JF, Goodman MM, Kelley ME, Kinkead B, Kutner M, Nemeroff CB, Newport DJ, Owens MJ, Pace TW, Ritchie JC, Rivera VA, Westen D, Craighead WE and Mayberg HS (2012). “Predictors of remission in depression to individual and combined treatments (PReDICT): study protocol for a randomized controlled trial.” Trials 13: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Kelley ME, Aponte-Rivera V, Mletzko-Crowe T, Kinkead B, Ritchie JC, Nemeroff CB, Craighead WE, Mayberg HS and Team PR (2017). “Effects of Patient Preferences on Outcomes in the Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT) Study.” Am J Psychiatry 174(6): 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R, Peet M, Shay J and Horrobin D (1998). “Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients.” J Affect Disord 48(23): 149–155. [DOI] [PubMed] [Google Scholar]
- Ehrlich AM, Pacheco AR, Henrick BM, Taft D, Xu G, Huda MN, Mishchuk D, Goodson ML, Slupsky C, Barile D and Lebrilla CB (2020). “Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells.” BMC microbiol, 20(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Kind T, Cajka T, Hazen SL, Tang WHW, Kaddurah-Daouk R, Irvin MR, Arnett DK, Barupal DK and Fiehn O (2019). “Systematic Error Removal Using Random Forest for Normalizing Large-Scale Untargeted Lipidomics Data.” Anal Chem 91(5): 3590–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Carmin CN, Balasubramani GK, Wisniewski SR, Trivedi MH, Biggs MM and Shores-Wilson K (2006). “What clinical and symptom features and comorbid disorders characterize outpatients with anxious major depressive disorder: a replication and extension.” Can J Psychiatry 51(13): 823–835. [DOI] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X and Miller AH (2016). “Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression.” Mol Psychiatry 21(10): 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehn O (2016). “Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling.” Curr Protoc Mol Biol 114: 30 34 31–30 34 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehn O, Wohlgemuth G, Scholz M, Kind T, Lee DY, Lu Y, Moon S and Nikolau B (2008). “Quality control for plant metabolomics: reporting MSI-compliant studies.” Plant J 53(4): 691–704. [DOI] [PubMed] [Google Scholar]
- Fried EI and Nesse RM (2015). “Depression is not a consistent syndrome: An investigation of unique symptom patterns in the STAR*D study.” J Affect Disord 172: 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz IB and McEwen B (1959). “Effects of carnitine on fatty-acid oxidation by muscle.” Science 129(3345):334–335. [DOI] [PubMed] [Google Scholar]
- Gan Z, Wu X, Liao Y, He Z, Yang Z and Zhang Q (2019). “ The association between low-grade inflammation and the clinical features of bipolar disorder in Han Chinese population.” Psychoneuroendocrinol 101:286–294. [DOI] [PubMed] [Google Scholar]
- Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T and Yin Y (2018). “Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism.” Front Cell Infect Microbiol 8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspersz R, Lamers F, Kent JM, Beekman AT, Smit JH, van Hemert AM, Schoevers RA and Penninx BW (2017). “Longitudinal Predictive Validity of the DSM-5 Anxious Distress Specifier for Clinical Outcomes in a Large Cohort of Patients With Major Depressive Disorder.” J Clin Psychiatry 78(2): 207–213. [DOI] [PubMed] [Google Scholar]
- Gili M, Roca M, Armengol S, Asensio D, Garcia-Campayo J, Parker G Clinical patterns and treatment outcome in patients with melancholic, atypical and non-melancholic depressions. PLoS One. 2012;7(10):e48200. doi: 10.1371/journal.pone.0048200. Epub 2012 Oct 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D (2011). “The heterogeneity of “major depression”.” World Psychiatry 10(3): 226–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Bekhbat M, Le NA, Chen X, Woolwine BJ, Li Z, Haroon E and Felger JC (2020). “Protein and gene markers of metabolic dysfunction and inflammation together associate with functional connectivity in reward and motor circuits in depression.” Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor MF and Hotamisligil GS (2011). “Inflammatory mechanisms in obesity.” Annu Rev Immunol 29:415–445. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1959). “The assessment of anxiety states by rating.” Br J Med Psychol 32(1): 50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). “A rating scale for depression.” J Neurol Neurosurg Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Daguanno AW, Woolwine BJ, Goldsmith DR, Baer WM, Wommack EC, Felger JC and Miller AH (2018). “Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder.” Psychoneuroendocrinology 95: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess S, Baker G, Gyenes G, Tsuyuki R, Newman S and Le Melledo JM (2017). “Decreased serum L-arginine and L-citrulline levels in major depression.” Psychopharmacology (Berl) 234(21): 3241–3247. [DOI] [PubMed] [Google Scholar]
- Howland RH, Rush AJ, Wisniewski SR, Trivedi MH, Warden D, Fava M, Davis LL, Balasubramani GK, McGrath PJ and Berman SR (2009). “Concurrent anxiety and substance use disorders among outpatients with major depression: clinical features and effect on treatment outcome.” Drug Alcohol Depend 99(1–3): 248–260. [DOI] [PubMed] [Google Scholar]
- Hubbard TD, Murray IA and Perdew GH (2015). “Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation.” Drug Metabol Disposition 43(10): 1522–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imeri I and Opp MR (2009). “How (and why) the immune system makes us sleep.” Nat Rev Neurosci 10(3): 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H, Noma H, Furukawa TA Melancholic features (DSM-IV) predict but do not moderate response to antidepressants in major depression: an individual participant data meta-analysis of 1219 patients. Eur Arch Psychiatry Clin Neurosci. 2021. Apr;271(3):521–526. doi: 10.1007/s00406-020-01173-4. Epub 2020 Jul 26. [DOI] [PubMed] [Google Scholar]
- Jennis M, Cavanaugh CR, Leo GC, Mabus JR, Lenhard J and Hornby PJ (2018). “ Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo.” Neurogastroenterol Motil 30(2): e13178. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, Bogdanov MB, Wikoff WR, Zhu H, Boyle SH, Churchill E, Wang Z, Rush AJ, Krishnan RR, Pickering E, Delnomdedieu M and Fiehn O (2013). “Pharmacometabolomic mapping of early biochemical changes induced by sertraline and placebo.” Transl Psychiatry 3: e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappelmann N, Arloth J, Georgakis MK, Czamara D, Rost N, Ligthart S, Khandaker GM and Binder EB (2021). “Dissecting the Association Between Inflammation, Metabolic Dysregulation, and Specific Depressive Symptoms: A Genetic Correlation and 2-Sample Mendelian Randomization Study.” JAMA Psychiatry 78(2): 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005. Jun;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kendler KS The Origin of Our Modern Concept of Depression-The History of Melancholia From 1780-1880: A Review. JAMA Psychiatry. 2020. Aug 1;77(8):863–868. doi: 10.1001/jamapsychiatry.2019.4709. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Derry HM and Fagundes CP (2015). “Inflammation: depression fans the flames and feasts on the heat.” Am J Psychiatry 172(11): 1075–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler-Forsberg O, Buttenschon HN, Tansey KE, Maier W, Hauser J, Dernovsek MZ, Henigsberg N, Souery D, Farmer A, Rietschel M, McGuffin P, Aitchison KJ, Uher R and Mors O (2017). “Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression.” Brain Behav Immun 62: 344–350. [DOI] [PubMed] [Google Scholar]
- Kunugi H, Hori H, Ogawa S Biochemical markers subtyping major depressive disorder. Psychiatry Clin Neurosci. 2015. Oct;69(10):597–608. doi: 10.1111/pcn.12299. Epub 2015 May 13. [DOI] [PubMed] [Google Scholar]
- Lai Z, Tsugawa H, Wohlgemuth G, Mehta S, Mueller M, Zheng Y, Ogiwara A, Meissen J, Showalter M, Takeuchi K, Kind T, Beal P, Arita M and Fiehn O (2018). “Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics.” Nat Methods 15(1): 53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers F, Milaneschi Y, de Jonge P, Giltay EJ and Penninx B (2018). “Metabolic and inflammatory markers: associations with individual depressive symptoms.” Psychol Med 48(7): 1102–1110. [DOI] [PubMed] [Google Scholar]
- Lamers F, Milaneschi Y, Smit JH, Schoevers RA, Wittenberg G and Penninx B (2019). “Longitudinal Association Between Depression and Inflammatory Markers: Results From the Netherlands Study of Depression and Anxiety.” Biol Psychiatry 85(10): 829–837. [DOI] [PubMed] [Google Scholar]
- Lamers F, Milaneschi Y, Vinkers CH, Schoevers RA, Giltay EJ and Penninx B (2020). “Depression profilers and immuno-metabolic dysregulation: Longitudinal results from the NESDA study.” Brain Behav Immun 88: 174–183. [DOI] [PubMed] [Google Scholar]
- Le NA, Innis-Whitehouse W, Li X, Bakker-Arkema R, Black D and Brown WV (2000). “Lipid and apolipoprotein levels and distribution in patients with hypertriglyceridemia: effect of triglyceride reductions with atorvastatin.” Metabolism 49(2): 167–177. [DOI] [PubMed] [Google Scholar]
- Lefevre-Arbogast S, Hejblum BP, Helmer C, Klose C, Manach C, Low DY, Urpi-Sarda M, Andres-Lacueva C, Gonzalez-Dominguez R, Aigner L, Altendorfer B, Lucassen PJ, Ruigrok SR, De Lucia C, Du Preez A, Proust-Lima C, Thuret S, Korosi A and Samieri C (2021). “Early signature in the blood lipidome associated with subsequent cognitive decline in the elderly: A case-control analysis nested within the Three-City cohort study.” EBioMedicine 64: 103216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Xie B, Zhang H, He Q, Guo L, Subramaniapillai M, Fan B, Lu C and McLntyer RS (2019). “Efficacy of omega-3 PUFAs in depression: A meta-analysis.” Transl Psychiatry 9(1): 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Ray B, Neavin DR, Zhang J, Athreya AP, Biernacka JM, Bobo WV, Hall-Flavin DK, Skime MK, Zhu H, Jenkins GD, Batzler A, Kalari KR, Boakye-Agyeman F, Matson WR, Bhasin SS, Mushiroda T, Nakamura Y, Kubo M, Iyer RK, Wang L, Frye MA, Kaddurah-Daouk R and Weinshilboum RM (2018). “Beta-defensin 1, aryl hydrocarbon receptor and plasma kynurenine in major depressive disorder: metabolomics-informed genomics.” Transl Psychiatry 8(1): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yieh L, Yang T, Drinkenburg W, Peeters P, Steckler T, Narayan VA, Wittenberg G, Ye J Metabolomic biosignature differentiates melancholic depressive patients from healthy controls. BMC Genomics. 2016. Aug 23;17(1):669. doi: 10.1186/s12864-016-2953-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Alam AB, Yu F, Kucharska-Newton A, Windham BG, Alonso A Sphingolipids and physical function in the Atherosclerosis Risk in Communities (ARIC) study. Sci Rep. 2021. Jan 13;11(1):1169. doi: 10.1038/s41598-020-80929-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MahmoudianDehkordi S, Ahmed AT, Bhattacharyya S, Han X, Baillie RA, Arnold M, Skime MK, John-Williams LS, Moseley MA, Thompson JW, Louie G, Riva-Posse P, Craighead WE, McDonald W, Krishnan R, Rush AJ, Frye MA, Dunlop BW, Weinshilboum RM, Kaddurah-Daouk R and Mood C Disorders Precision Medicine (2021). “Alterations in acylcarnitines, amines, and lipids inform about the mechanism of action of citalopram/escitalopram in major depression.” Transl Psychiatry 11(1): 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W, Buller R, Philipp M and Heuser I (1988). “The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders.” J Affect Disord 14(1): 61–68. [DOI] [PubMed] [Google Scholar]
- McGrath PJ, Khan AY, Trivedi MH, Stewart JW, Morris DW, Wisniewski SR, Miyahara S, Nierenberg AA, Fava M, Rush AJ Response to a selective serotonin reuptake inhibitor (citalopram) in major depressive disorder with melancholic features: a STAR*D report. J Clin Psychiatry. 2008. Dec;69(12):1847–55. doi: 10.4088/jcp.v69n1201. Epub 2008 Nov 18. [DOI] [PubMed] [Google Scholar]
- Mehta D, Raison CL, Woolwine BJ, Haroon E, Binder EB, Miller AH and Felger JC (2013). “Transcriptional signatures related to glucose and lipid metabolism predict treatment response to the tumor necrosis factor antagonist infliximab in patients with treatment-resistant depression.” Brain Behav Immun 31: 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaneschi Y, Arnold M, Kastenmüller G, Mahmoudian Dehkordi S, Krishnan RR, Dunlop BW, Rush AJ, Penninx BWJH, Kaddurah-Daouk R, for the Mood Disorders Precision Medicine Consortium (MDPMC) (2021). “Genomics-based identification of a potential causal role for acylcarnitine metabolism in depression.” medRxiv 2021.10.18.21265157; doi: 10.1101/2021.10.18.21265157 [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Lamers F, Berk M and Penninx B (2020). “Depression Heterogeneity and Its Biological Underpinnings: Toward Immunometabolic Depression.” Biol Psychiatry 88(5): 369–380. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Lamers F and Penninx B (2021). “Dissecting Depression Biological and Clinical Heterogeneity-The Importance of Symptom Assessment Resolution.” JAMA Psychiatry 78(3): 341. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Lamers F, Peyrot WJ, Abdellaoui A, Willemsen G, Hottenga JJ, Jansen R, Mbarek H, Dehghan A, Lu C, C. i. w. group, Boomsma DI and Penninx BW (2016). “Polygenic dissection of major depression clinical heterogeneity.” Mol Psychiatry 21(4): 516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaneschi Y, Lamers F, Peyrot WJ, Baune BT, Breen G, Dehghan A, Forstner AJ, Grabe HJ, Homuth G, Kan C, Lewis C, Mullins N, Nauck M, Pistis G, Preisig M, Rivera M, Rietschel M, Streit F, Strohmaier J, Teumer A, Van der Auwera S, Wray NR, Boomsma DI, Penninx B, Group CIW and the C Major Depressive Disorder Working Group of the Psychiatric Genomics (2017). “Genetic Association of Major Depression With Atypical Features and Obesity-Related Immunometabolic Dysregulations.” JAMA Psychiatry 74(12): 1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaneschi Y, Kappelmann N, Ye Z, Lamers F, Moser S, Jones PB, Burgess S, Penninx BWJH and Khandaker GM (2021). “ Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts.” Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi M, Jonsson P, Antti H, Adolfsson R, Nordin A, Bergdahl J, Eriksson K, Moritz T, Nilsson LG and Nyberg L (2014). “Serum metabolomic biomarkers of dementia.” Dement Geriatr Cogn Dis Extra 4(2): 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CP, Reichel M, Mühle C, Rhein C, Gulbins E and Kornhuber J (2015). “Brain membrane lipids in major depression and anxiety disorders.” Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 1851(8): 1052–1065. [DOI] [PubMed] [Google Scholar]
- Musselman D, Royster EB, Wang M, Long Q, Trimble LM, Mann TK, Graciaa DS, McNutt MD, Auyeung NS, Oliver L, Lawson DH and Miller AH (2013). “The impact of escitalopram on IL-2-induced neuroendocrine, immune, and behavioral changes in patients with malignant melanoma: preliminary findings.” Neuropsychopharmacology 38(10): 1921–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW and Dantzer R (2009). “Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin.” J Neurosci 29(13): 4200–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC and Schatzberg AF (2016). “Major depressive disorder.” Nat Rev Dis Primers 2: 16065. [DOI] [PubMed] [Google Scholar]
- Parekh A, Smeeth D, Milner Y and Thure S (2017). “The Role of Lipid Biomarkers in Major Depression.” Healthcare (Basel) 5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SC, Kim JM, Jun TY, Lee MS, Kim JB, Yim HW and Park YC (2017). “How many different symptom combinations fulfil the diagnostic criteria for major depressive disorder? Results from the CRESCEND study.” Nord J Psychiatry 71(3): 217–222. [DOI] [PubMed] [Google Scholar]
- Paul S, Lancaster GI and Meikle PJ (2019). “Plasmalogens: A potential therapeutic target for neurodegenerative and cardiometabolic disease.” Prog Lipid Res 74: 186–195. [DOI] [PubMed] [Google Scholar]
- Penninx BW (2017). “Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms.” Neurosci Biobehav Rev 74(Pt B): 277–286. [DOI] [PubMed] [Google Scholar]
- Philippe C, Szabo de Edelenyi F, Naudon L, Druesne-Pecollo N, Hercberg S, Kesse-Guyot E, Latino-Martel P, Galan P and Rabot S (2021). “Relation between Mood and the Host-Microbiome Co-Metabolite 3-Indoxylsulfate: Results from the Observational Prospective NutriNet-Sante Study.” Microorganisms 9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Cai G, Su M, Chen T, Zheng X, Xu Y, Ni Y, Zhao A, Xu LX, Cai S and Jia W (2009). “Serum metabolite profiling of human colorectal cancer using GC-TOFMS and UPLC-QTOFMS.” J Proteome Res 8(10): 4844–4850. [DOI] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, Vogt GJ, Massung B and Miller AH (2009). “Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression.” Biol Psychiatry 65(4): 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G and Miller AH (2010). “Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior.” Mol Psychiatry 15(5): 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E and Miller AH (2013). “A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers.” JAMA Psychiatry 70(1): 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH and Keller MB (2003). “The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression.” Biol Psychiatry 54(5): 573–583. [DOI] [PubMed] [Google Scholar]
- Schmitz G and Ecker J (2008). “The opposing effects of n-3 and n-6 fatty acids.” Prog Lipid Res 47(2): 147–155. [DOI] [PubMed] [Google Scholar]
- Sebastiao AM, Colino-Oliveira M, Assaife-Lopes N, Dias RB and Ribeiro JA (2013). “Lipid rafts, synaptic transmission and plasticity: impact in age-related neurodegenerative diseases.” Neuropharmacology 64: 97–107. [DOI] [PubMed] [Google Scholar]
- Shinka T, Inoue Y, Ohse M, Ito A, Ohfu M, Hirose S and Kuhara T (2002). “Rapid and sensitive detection of urinary 4-hydroxybutyric acid and its related compounds by gas chromatography-mass spectrometry in a patient with succinic semialdehyde dehydrogenase deficiency.” J Chromatogr B Analyt Technol Biomed Life Sci 776(1): 57–63. [DOI] [PubMed] [Google Scholar]
- Shulaev V (2006). “Metabolomics technology and bioinformatics.” Brief Bioinform 7(2): 128–139. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Burrows K, Avery JA, Kerr KL, Taylor A, Bodurka J, Potter W, Teague TK and Drevets WC (2020). “Appetite changes reveal depression subgroups with distinct endocrine, metabolic, and immune states.” Mol Psychiatry 25(7): 1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Wray N, Schappi J and Rasenick MM (2018). “Disruption of lipid-raft localized Gαs/tubulin complexes by antidepressants: a common feature of HDAC6 inhibitors, SSRI and Tricyclic compounds.” Neuropsychopharmacol 43:1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Smith AK, Conneely KN, Pace TW, Mister D, Felger JC, Kilaru V, Akel MJ, Vertino PM, Miller AH and Torres MA (2014). “Epigenetic changes associated with inflammation in breast cancer patients treated with chemotherapy.” Brain Behav Immun 38: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su XQ, Wang J and Sinclair AJ (2019). “Plasmalogens and Alzheimer’s disease: a review.” Lipids Health Dis 18(1): 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Wang Y, Wang X, Zhao A, Chen T, Ni Y, Wong L, Zhang H, Zhang J, Liu C, Liu P and Jia W (2015). “Profiling of serum bile acids in a healthy Chinese population using UPLC-MS/MS.” J Proteome Res 14(2): 850–859. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Martin J, McGonigal P, Harris L, Kerr S, Balling C, Kiefer R, Stanton K and Dalrymple K (2019). “Validity of the DSM-5 anxious distress specifier for major depressive disorder.” Depress Anxiety 36(1): 31–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


