Abstract
Alzheimer’s disease (AD) is a degenerative neurological disease that primarily affects the elderly. Drug therapy is the main strategy for AD treatment, but current treatments suffer from poor efficacy and a number of side effects. Non-drug therapy is attracting more attention and may be a better strategy for treatment of AD. Hypoxia is one of the important factors that contribute to the pathogenesis of AD. Multiple cellular processes synergistically promote hypoxia, including aging, hypertension, diabetes, hypoxia/obstructive sleep apnea, obesity, and traumatic brain injury. Increasing evidence has shown that hypoxia may affect multiple pathological aspects of AD, such as amyloid-beta metabolism, tau phosphorylation, autophagy, neuroinflammation, oxidative stress, endoplasmic reticulum stress, and mitochondrial and synaptic dysfunction. Treatments targeting hypoxia may delay or mitigate the progression of AD. Numerous studies have shown that oxygen therapy could improve the risk factors and clinical symptoms of AD. Increasing evidence also suggests that oxygen therapy may improve many pathological aspects of AD including amyloid-beta metabolism, tau phosphorylation, neuroinflammation, neuronal apoptosis, oxidative stress, neurotrophic factors, mitochondrial function, cerebral blood volume, and protein synthesis. In this review, we summarized the effects of oxygen therapy on AD pathogenesis and the mechanisms underlying these alterations. We expect that this review can benefit future clinical applications and therapy strategies on oxygen therapy for AD.
Keywords: Alzheimer’s disease, amyloid-beta metabolism, clinical symptoms, hypoxia, neuroinflammation, neuronal apoptosis, oxygen therapy, pathogenesis, risk factor, tau phosphorylation
Introduction
Alzheimer’s disease (AD) is the most common senile dementia, and causes severe disability in the elderly (McKhann et al., 2011). The pathological features include amyloid-beta (Aβ) deposition, abnormal tau phosphorylation, and neuronal loss in the brain (Ou et al., 2021; Roda et al., 2022). At present, the primary treatment for AD is cholinesterase inhibitors (Haake et al., 2020). However, due to limitations in efficacy and side effects, non-drug treatment of AD has recently received more attention.
Oxygen is necessary for life (Reinhard et al., 2016), and different organs consume different amounts of oxygen. Oxygen consumption in the brain accounts for more than 20% of total human oxygen consumption. Hypoxia immediately causes impairment of brain function and can lead to rapid cell death.
Hypoxia in the brain can be caused by environmental and pathological conditions. Pathological conditions include aging, cerebrovascular diseases, hypertension, type 2 diabetes, traumatic brain injury (TBI), and sleep-disordered breathing, all of which are major risk factors for AD (Reitz and Mayeux, 2014). Studies have shown that hypoxia may affect many pathological aspects of AD including Aβ metabolism (Liu et al., 2016), tau phosphorylation (Zhang et al., 2018), autophagy (Karabiyik et al., 2021), neuroinflammation, oxidative stress, endoplasmic reticulum (ER) stress, and mitochondrial and synaptic dysfunction, which may collectively result in neurodegeneration in the brain (Zhang and Le, 2010; Zhang et al., 2019).
Therefore, treatments targeting hypoxia may delay or mitigate progression of AD. Oxygen inhalation (referred to as oxygen therapy) is a medical procedure to relieve hypoxia by increasing the partial pressure of oxygen in the inhaled gas, thus improving oxygen saturation in the blood (Horner and O’Driscoll, 2018). Two other types of oxygen therapy are normobaric oxygen therapy (NBOT) and hyperbaric oxygen therapy (HBOT) (Zhang and Barralet, 2017). Normobaric oxygen therapy is characterized by administration of high concentrations of oxygen through a facemask or nasal cannula at normal atmospheric pressure. In contrast, HBOT is a treatment in which the patient breathes 100% oxygen while exposed to environmental pressure > 1 atmosphere (Deng et al., 2018).
Oxygen therapy has been used medically for more than 200 years (Kelly, 2014). Oxygen therapy is widely used for treatment of central nervous system disease (Baratz-Goldstein et al., 2017; Biggs et al., 2021). Some clinical studies have indicated that HBOT may provide benefits in the ultra-acute stage of brain ischemia. Moreover, HBOT has been used to treat brain and spinal cord injuries (Shytle et al., 2019), carbon monoxide poisoning (Megas et al., 2021), and decompression illness involving the central nervous system (Kohshi et al., 2021).
Alzheimer’s disease is common, and the nervous system pathologies associated with AD are difficult to treat. However, some studies have suggested that oxygen therapy may significantly improve AD symptoms and pathologies (Zhang et al., 2019; Chen et al., 2020). Studies have shown that oxygen therapy may affect many pathological aspects of AD including Aβ metabolism (Gao et al., 2011; Shapira et al., 2018; Choi et al., 2019), tau phosphorylation (Shapira et al., 2018), neuroinflammation (Shapira et al., 2018), neuronal apoptosis (Tian et al., 2012), oxidative stress (Tian et al., 2012), neurotrophic factors (Choi et al., 2019), mitochondrial function (Wang et al., 2017a), cerebral blood volume (Shapira et al., 2021), and protein synthesis (Wang et al., 2016). Therefore, in this review, we summarized the effects of oxygen therapy on AD pathogenesis and discussed the underlying mechanisms.
Retrieval Strategy
Literature cited in this narrative review was published from 1997 to 2021 and was searched on PubMed and Google Scholar. We searched using the following search strategies: Alzheimer’s disease (MeSH Terms) AND (oxygen therapy (MeSH Terms) OR hyperbaric oxygen therapy (MeSH Terms) OR normobaric oxygen therapy (MeSH Terms) OR normobaric oxygen (MeSH Terms)). The reviewer (CY) selected these articles by reviewing their titles and abstracts. Eligible studies included those that evaluated oxygen therapy, hyperbaric oxygen therapy, or normobaric oxygen therapy conducted on any species. Review articles were excluded.
Hypoxia Is a Risk Factor for Alzheimer’s Disease
The pathogenesis of AD is related to genetic and nongenetic factors. Genetic factors include dominant inherited mutations in amyloid precursor protein (APP), presenilin 1, and presenilin 2 (PSEN2), and risk genes including apolipoprotein E and others (Chouraki and Seshadri, 2014; Karch et al., 2014). There are several major nongenetic factors such as aging, cerebrovascular diseases, hypertension, type 2 diabetes, TBI, and sleep-disordered breathing (Reitz and Mayeux, 2014). Aging is the key risk factor and a fundamental driver for development of AD (Riedel et al., 2016). The age-specific prevalence of AD nearly doubles every 5 years after age 65, resulting in a prevalence greater than 25% in individuals over age 90 (Jonsson et al., 2012). The ability to delay the age at onset (AAO) of AD through preventive or therapeutic approaches would have significant benefits, but no therapies have achieved this important goal (Guerreiro and Bras, 2015).
Obstructive sleep apnea syndrome and chronic obstructive pulmonary disease
Obstructive sleep apnea syndrome (OSAS) (Liguori et al., 2017) and chronic obstructive pulmonary disease (COPD) have been reported to be closely associated with AD (Daulatzai, 2013; Zhang et al., 2019). Obstructive sleep apnea syndrome is a chronic condition characterized by repetitive repression of breathing in the upper airway during sleep, leading to intermittent hypoxia and recurrent awakening. Some epidemiological studies have suggested a pathophysiological link between OSAS and AD. Treatment of OSAS has been shown to delay mild cognitive impairment (MCI) and improve cognition in patients with AD. In addition, OSAS may accelerate amyloid deposition and increase CSF T-tau and P-tau levels over time in individuals with normal cognition and those with MCI (Bubu et al., 2019). Moreover, CSF Aβ40 and Aβ42 levels were lower in patients with OSAs than those in controls, but higher in OSAS patients compared to those in patients with AD. However, patients with AD had lower CSF Aβ42 levels, but similar CSF Aβ40 levels, than those in controls (Liguori et al., 2019).
The brain may also be particularly susceptible to the systemic effects of COPD. Significantly increased levels of serum Aβ40, Aβ42, and total Aβ were found in patients with COPD compared with those in healthy controls. Serum Aβ levels were significantly higher in COPD patients with worse pulmonary function (Bu et al., 2015b). Moreover, pulmonary dysfunction or behavioral comorbidities can lead to hypoxia, oxidative stress, and neurological damage. Damaged brain cells eventually trigger cognitive deficits in patients with COPD (Karamanli et al., 2015).
Traumatic brain injury
Traumatic brain injury is one of the strongest risk factors for development of AD (Alali et al., 2019). Studies have shown that TBI might facilitate development of AD by delaying glial activation, intercorrelating synaptic dysfunction, and inducing endoplasmic reticulum stress, oxidative stress, and neuroinflammation, resulting in APP and tau cleavage (Mei et al., 2017; Lou et al., 2018; Ramos-Cejudo et al., 2018; Zyśk et al., 2019; Wu et al., 2020; Li et al., 2021). Prevention and treatment of secondary brain injury is the major clinical concern in management of TBI. Cerebral ischemia and hypoxia are the main causes of secondary brain injury.
Vascular risk factors
Vascular risk factors of AD include hypertension, type 2 diabetes, and cardiovascular and cerebrovascular diseases. All vascular risk factors synergistically promote diverse pathological mechanisms including cerebral hypoperfusion and glucose hypometabolism (Daulatzai, 2017). Animal models of induced and spontaneous hypertension found that pathological changes in AD (beta-amyloid plaques and neurofibrillary tangles) occurred within weeks of hypertensive injury. Human imaging and autopsy studies have shown that hypertension is associated with increased pathological changes in AD (Lennon et al., 2021). In addition, a number of hypotheses have been presented to explain the relationship between AD and type 2 diabetes. For example, hyperglycemia leads to glutamate-induced excitatory toxicity of nerve cells. This may contribute to Aβ accumulation, tau phosphorylation, and oxidative stress in addition to insulin resistance in the brain (Diniz Pereira et al., 2021). Cardiovascular diseases are also closely related to AD. The Framingham study showed that lower heart rate index was associated with a nearly three-fold increased risk of AD (Jefferson et al., 2015). The risk of AD was 1.8 times higher in patients with heart failure than in those without heart failure (Qiu et al., 2006). Moreover, cerebrovascular diseases are associated with increased Aβ production through modulation of β and γ-secretase, and with impaired Aβ clearance, which is mainly driven by vascular-mediated systems (Saito and Ihara, 2016).
Hypoxia can be caused by respiratory dysfunction, cardiovascular dysfunction, or environmental conditions (Peers et al., 2007; Snyder et al., 2021; Figure 1). Hypoxia may affect the functions of organs, especially the central nervous system (Burtscher et al., 2021; Dunn and Isaacs, 2021; Xu et al., 2021). Stroke or TBI can result in acute hypoxia, whereas OSAS and COPD can cause chronic hypoxia. Hypoxia has been shown to be associated with familial and sporadic AD. Increasing evidence has shown that hypoxia may affect many pathological aspects of AD including Aβ metabolism (Liu et al., 2016), tau phosphorylation (Zhang et al., 2018), autophagy, neuroinflammation, oxidative stress, ER stress, and mitochondrial and synaptic dysfunction, which may collectively result in neurodegeneration in the brain (Zhang and Le, 2010; Zhang et al., 2019).
Figure 1.
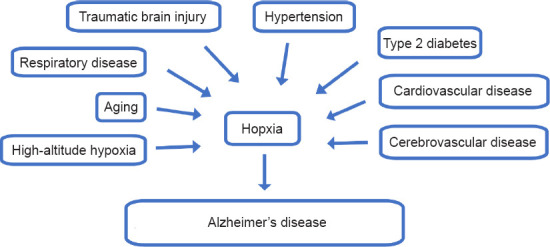
Hypoxia is an important risk factor for Alzheimer’s disease.
Oxygen Therapy for Alzheimer’s Disease
As hypoxia is involved in AD pathogenesis, preventive measures and treatments targeting hypoxia may delay or mitigate the progression of neurodegenerative diseases. Oxygen therapy can significantly improve the oxygen content in the body to reverse acute or chronic hypoxia (Hamilton et al., 2003; Cheng et al., 2021; Guan et al., 2021). Numerous studies have shown that oxygen therapy can improve the risk factors of AD (Hachmo et al., 2020; Deng et al., 2018) and the clinical symptoms of AD (Chen et al., 2020).
Effects of oxygen therapy on major risk factors for AD
Anti-aging effect of oxygen therapy
Oxygen is a modulator of aging. Hyperbaric oxygen therapy may exert significant anti-aging effects (Hachmo et al., 2020). Two key hallmarks of the aging process include telomere length shortening and cellular senescence. A study showed that HBOT (2 ATA, 100% O2, 90 min/d, 60 days) increased the telomere lengths in helper T cells NK cells, cytotoxic T cells, and B cells, and significantly reduced the number of senescent helper T cells and cytotoxic T cells in a healthy aging adult population (Hachmo et al., 2020). Another study also showed that 14 days of HBOT (2ATA, 100% O2, 80 min/d) notably restored insulin sensitivity, hippocampal function, and cognition in aging and obese aging rats (Shwe et al., 2021). Given the results of these two studies, oxygen therapy may improve symptoms of aging and may delay the onset and development of AD.
Oxygen therapy for respiratory diseases
Constant positive airway pressure (CPAP) is the most effective therapy for moderate to severe OSA (Marin et al., 2005; Siachpazidou et al., 2020). A study showed that CPAP therapy (after one night, 3 and 6 weeks, 13.3 months, and 1 year of treatment) significantly improved cognitive function in individuals with OSAS (Siachpazidou et al., 2020). Oxygen therapy is also critical for treatment of COPD. Patients with COPD who received continuous oxygen therapy (24 h/d) at home via nasal prongs adequate to maintain oxygen saturation between 88% and 95% experienced improved severe resting hypoxemia and cognitive function (Karamanli et al., 2015). Therefore, we propose that oxygen therapy may delay or prevent the onset and development of AD by improving oxygenation in lung disease.
Oxygen therapy for TBI
Oxygen therapy has been reported to exert therapeutic effects on brain injuries. A study showed that 1 day or 3 days of HBOT (2.2 ATA, 100% O2, 60 min/d, twice a day) or NBOT (80% FIO2, 4 h/d, twice a day) improved neurological function and reduced levels of neuron specific enolase (NSE), tau protein, and interleukin-6 (IL-6). Neuron specific enolase is localized in the cytoplasm of neurons and acts as a specific index to determine the severity of neuronal damage. Tau protein is an indicator of neuronal axonal injury. Interleukin-6 is an inflammatory cytokine that plays an important role in the acute inflammatory response (Li et al., 2020). Early intervention with NBOT and HBOT reduced brain edema and the number of apoptotic cells in the hippocampus in a rat model of TB (Li et al., 2020). In clinical studies, HBOT also reduced clinical symptoms and improved cognitive function in patients with TBI (Baratz-Goldstein et al., 2017; Biggs et al., 2021), and NBO was shown to promote improved brain metabolism (Deng et al., 2018) in patients who had suffered strokes.
Oxygen therapy also has been shown to mitigate vascular risk factors. Studies have shown that HBOT significantly improved peripheral insulin resistance, and reduced blood glucose and hemoglobin (Xu et al., 2017; Irawan et al., 2018; Baitule et al., 2021). In addition, HBOT also improved left ventricular function, especially in the apical segments, and was associated with better cardiac performance (Leitman et al., 2020). Oxygen therapy has also shown positive therapeutic effects in cerebrovascular diseases. Studies have shown that oxygen therapy can reduce hyperglycolysis through modulation of the adenosine monophosphate-activated protein kinase signaling pathway and alleviate oxidative injury in ischemic stroke (Cheng et al., 2021). Of note, HBOT treatment does not significantly increase blood pressure (Heyboer Rd et al., 2017).
Oxygen therapy improves clinical symptoms of AD
It is reasonable to speculate that oxygen therapy may improve the symptoms of AD. In our previous clinical study, we recruited 42 patients with AD, 11 patients with amnestic MCI, and 30 control patients. The patients with AD or MCI were treated with HBOT (99.9% O2, 0.4 to 0.7 MPa) for 40 minutes, once per day, for 20 days. Cognitive function and daily living activities were evaluated before HBOT and at 1-, 3-, and 6-month follow-ups. We found that HBOT ameliorated cognitive impairment in patients with AD patients or amnestic MCI (Chen et al., 2020). Another study also showed that HBOT (60 daily HBOT sessions within 3 months) increased cerebral blood flow and improved cognitive performance in older adult patients with significant memory loss at baseline (Shapira et al., 2021). Furthermore, HBOT also promoted good cognitive function healthy individuals (Yu et al., 2015).
18Fluorodeoxyglucose brain positron emission tomography imaging showed that the metabolic rate of focal or diffuse FDG decreased with the development of AD in the medial parietal lobe, posterior temporal lobe, and posterior cingulate cortex (Jansen et al., 2015). Hyperbaric oxygen therapy ameliorated AD-related reductions in brain glucose metabolism in some patients with AD or MCI (Harch and Fogarty, 2018; Chen et al., 2020). Resting-state functional magnetic resonance imaging has been widely used to study AD. Regional homogeneity (ReHo) reflects the synchronization of local neuronal activity in a brain region (Zang et al., 2004). Previous studies have shown that ReHo is a valuable index to reflect the neuronal abnormalities involved in diseases characterized by cognitive impairments, such as AD (He et al., 2007). Reduced memory and cognitive abilities are indicated by lower ReHo in patients with MCI and AD (Zhang et al., 2012). After 5 consecutive days of HBOT (100% O2, 2ATA, 80 min/d), the ReHo value was significantly increased in healthy young adults (Yu et al., 2015).
Underlying Mechanisms of Oxygen Therapy on Alzheimer’s Disease
Clinical studies have shown that oxygen therapy significantly improved cognitive function and brain metabolism, and positively regulated the activity of local neurons in brain regions. Furthermore, laboratory experiments have shown that oxygen therapy improved cognitive function in a mouse model of AD through modulation of Aβ metabolism, tau phosphorylation, neuroinflammation, neuronal apoptosis, oxidative stress, neurotrophic factors, mitochondrial function, cerebral blood volume, and protein synthesis. A better understanding of the mechanisms of oxygen therapy in AD pathogenesis will provide valuable information for prevention and treatment of AD.
Oxygen therapy affects Aβ metabolism
Aβ plays an important role in the pathology of AD and is considered to be an early trigger for onset of AD (Kukar et al., 2005). Aβ is produced by sequential cleavages of APP protein by β- and γ-secretase, and is mainly present as Aβ40 and Aβ42 (Chow et al., 2010; Murphy and LeVine, 2010). Studies have shown that hypoxia may play a role in Aβ metabolism (Li et al., 2009; Liu et al., 2016). Hypoxia can cause increased β- and γ-cleavage of APP (Li et al., 2009). Hypoxia can also increase mitochondria-derived reactive oxygen species (ROS) production and enhance amyloidogenic APP processing (Leuner et al., 2012). Furthermore, hypoxia-induced activation of autophagy may lead to the aggregation of C99, which can be further cleaved by γ -secretase to form Aβ (Li et al., 2009). In addition, hypoxia has been shown to mediate upregulation of β-site APP cleaving enzyme 1 (BACE1) expression (Guglielmotto et al., 2009). Neprilysin (NEP) (Zhang et al., 2019) and insulin-degrading enzymes (IDE) (Kurochkin et al., 2018) are the major enzymes responsible for Aβ degradation. The receptor responsible for the clearance of Aβ across the blood-brain barrier (BBB) is low-density lipoprotein receptor related protein-1 (LRP-1) (Cai et al., 2018). The main receptor for Aβ influx from the blood to the brain is a receptor for advanced glycation end products. Hypoxia results in decreased expression of NEP, IDE, and LRP-1, and increased expression of receptors for advanced glycation end products (Zhang et al., 2019).
Studies have shown that oxygen therapy may modulate Aβ metabolism (Gao et al., 2011; Shapira et al., 2018; Choi et al., 2019). A study found that 4 weeks or 8 weeks of NBOT (O2 40%, 8 h/d) improved spatial learning and memory deficits, and decreased Aβ deposition and neuritic plaque formation in the cortices and hippocampi of mice. Furthermore, increased APP C-terminal fragments, β-secretase generated C99, and α-secretase generated C83 fragments levels, and decreased Aβ production in the brains of NBOT treated transgenic mice indicated that NBOT inhibited γ-secretase cleavage of APP proteins (Gao et al., 2011). Beta-site APP cleaving enzyme 1 (BACE1) is a rate-limiting enzyme for hydrolysis of APP to Aβ, and plays a pivotal role in the process of conversion of APP to Aβ (Hampel et al., 2021). Inhibition of BACE1 to reduce production of Aβ can prevent onset of AD. Another study showed that HBOT (O2 100%, 2 ATA, 14 days) downregulated BACE1, Presenilin 1, a component of the γ-secretase complex and a disintegrin, and downregulated metalloproteinase 10 (ADAM10), an α-secretase enzyme, which resulted in reduced Aβ deposition, and relieved memory deficits in triple-transgenic (3×Tg) mice (Shapira et al., 2018). Furthermore, 28 days of HBOT (O2 100%, 2 ATA) suppressed Aβ accumulation in the brains of APP/PS1 mice (Choi et al., 2019).
HBOT has been reported to promote degradation and clearance of Aβ. Insulin-degrading enzymes are responsible for proteolytic degradation of intracellular and extracellular Aβ (Kurochkin et al., 2018). The main receptor for the clearance of Aβ across the blood-brain barrier (BBB) is LRP-1 (Cai et al., 2018). A study showed that HBOT (O2 100%, 2 ATA, 60 minutes per day, 5 days a week for 4 weeks) significantly increased IDE and LRP1 levels in 5XFAD mice (Shapira et al., 2021; Table 1).
Table 1.
Hypoxia, oxygen therapy and Aβ metabolism
| Aβ metabolism | hypoxia | Oxygen therapy | |
|---|---|---|---|
| Production of Aβ | APH-1α | ↑ | – |
| BACE1 | ↑ | ↓ | |
| PEN2 | ↑ | – | |
| NCSTN | ↑ | – | |
| Degradation and clearance of Aβ | IDE | ↓ | ↑ |
| NEP | ↓ | – | |
| RAGE | ↑ | – | |
| LRP-1 | ↓ | ↑ | |
APH-1α: Anterior pharynx-defective 1α; Aβ: amyloid-β; BACE1: β-site APP cleaving enzyme 1; IDE: insulin-degrading enzyme; LRp-1: low-density lipoprotein receptor related protein-1; NCSTN: nicastrin; NEP: Neprilysin; PEN2: presenilin enhancer 2; RAGE: receptor for advanced glycation end products.
Oxygen therapy influences tau hyperphosphorylation
Abnormal tau phosphorylation is a typical pathological feature of AD (Cárdenas-Aguayo Mdel et al., 2014) and results from an imbalance of regulation between protein kinase and protein phosphorylase activities (Iqbal et al., 2016). Basic and clinical studies have reported that hypoxia could enhance tau phosphorylation (Gao et al., 2013; Zhang et al., 2014; Bu et al., 2015a). Furthermore, a study that used 3×Tg mice showed that HBOT (O2 100%, 2 ATA, 14 days) reduced tau phosphorylation at Ser202/Thr205 in the hippocampus and subiculum, but did not affect total tau levels. Glycogen synthase kinase 3b (GSK3b) is a kinase associated with tau phosphorylation in AD. Hyperbaric oxygen therapy downregulated total GSK3b (tGSK3b) and upregulated the ratio between pGSK3b and tGSK3b (pGSK3b/tGSK3b), which was associated with reduced tau phosphorylation (Shapira et al., 2018).
Anti-neuroinflammatory effect of oxygen therapy
Neuroinflammation is defined as brain activation of the innate immune system, and plays a significant role in the pathophysiology of AD (Leng and Edison, 2021). Previous studies have shown that hypoxia can increase neuroinflammation, which can worsen nerve damage (Smith et al., 2013; Wang et al., 2014). A study indicated that 14 days of HBOT (O2 100%, 2 ATA) reduced astrogliosis and the number of microglia near plaques, and induced microglia sprouting in 3×Tg mice (Shapira et al., 2018). Furthermore, HBOT also increased microglial expression of scavenger receptor class A, which has been shown to mediate Aβ clearance (Yuan et al., 2020), and arginase 1, which contributes to Aβ plaque reduction during sustained neuroinflammation. Activated microglia can be divided into M1-like and M2-like phenotypes (Jin et al., 2020). M1-like phenotype microglia are pro-inflammatory, play a dominant role at the site of neuroinflammation, and are related to acute inflammation and release of pro-inflammatory cytokines. M2-like phenotype microglia appear later in the inflammatory response and are associated with tissue repair and resolution of inflammation (DeRidder et al., 2021). Hyperbaric oxygen therapy was shown to reduce production of M1-like phenotype microglia-associated proinflammatory cytokines, such as IL-1β and tumor necrosis factor-α, and increase production of M2-like phenotype microglia-associated anti-inflammatory cytokines, such as IL-4 and IL-10 (Shapira et al., 2018). These findings suggest that HBOT may shift the microglial population from the M1-like to the M2-like phenotype, resulting in neuroprotection function and increased Aβ clearance. Other studies showed that HBOT (O2 100%, 2 ATA, 5 days) reduced astrocyte activation and tumor necrosis factor-α expression in a rat model of AD (were induced by injecting Aβ1–40 into the hippocampus) (Zhao et al., 2017).
Oxygen therapy and neuronal apoptosis
Neuronal damage and apoptosis are hallmarks of AD. Hypoxia can aggravate neuronal damage and apoptosis (Choudhary et al., 2022). In a rat model of AD (induced by injection of Aβ1–40 into the hippocampus), the neurons were loosely distributed and exhibited swelling and vacuolation (Zhao et al., 2017). Treatment with HBOT (O2 100%, 2ATA) for 5 days prevented neuronal damage and loss of dendritic spines in a rat model of AD (Zhao et al., 2017). Furthermore, apoptosis and expression of cleaved caspase 3 were significantly increased remarkably in the AD model group, and this increase was reversed by HBOT, potentially through modulation of p38 mitogen-activated protein kinase (Zhao et al., 2017). Studies have also shown that 20 days of HBOT (O2 100%, 2 ATA) reduced neuronal damage and apoptosis in a rat model of AD (induced by injecting Aβ25–35 into the hippocampus) via modulation of the nuclear factor-κB (NF-κB) pathway or inhibition of mitochondria-mediated apoptosis signaling (Tian et al., 2013; Zhang et al., 2015).
Effect of oxygen therapy on oxidative stress
Oxidative stress plays a significant role in the pathogenesis of AD and progression of AD (Butterfield and Mattson, 2020; Plascencia-Villa and Perry, 2021). Oxidative stress is a disturbance in the balance between the production of ROS and antioxidant defenses. Hypoxia can aggravate oxidative stress (Snyder et al., 2017). Studies showed that HBOT (O2 100%, 2 ATA) reduced oxidative stress in models of AD (Tian et al., 2012, 2013; Choi et al., 2019). Reactive oxygen species levels were increased in the hippocampi of APP/PS1 mice, and HBOT reversed this increase (Choi et al., 2019). In addition, the expression of peroxiredoxin 3 (an antioxidant enzyme) increased after HBOT in APP/PS1 mice (Choi et al., 2019). In addition, previous studies have shown that superoxide dismutase (SOD) and glutathione (GSH) are important endogenous antioxidant enzymes that mitigate the effects of free radicals, and prevent subsequent lipid peroxidation (Braidy et al., 2019). Malondialdehyde (MDA) is a terminal product of lipid peroxidation of biomembranes, and MDA content typically reflects the level of lipid peroxidation and indirectly reflects the extent of oxidative injury (Tsikas, 2017). In the AD group, SOD and GSH activities were significantly decreased, and MDA levels were significantly increased. However, HBOT significantly enhanced the activities of SOD and GSH, as evidenced by decreased MDA levels (Tian et al., 2012, 2013). In addition, nitric oxide (NO) production was significantly increased in the AD group, and was significantly decreased following HBOT (Tian et al., 2012).
Oxygen therapy and neurotrophic factors
Neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3), and neurotrophin 4/5 (NT4/5), play a vital role in changes in neuritic morphology and synapse formation (Dechant and Neumann, 2002). The levels of BDNF and NT3 were reduced in the hippocampi of patients with AD (Kobayashi et al., 2012; Siuda et al., 2017). Decreased expression of BDNF in the forebrain or hippocampus impairs cognitive function, and administration of BDNF has been shown to attenuate memory deficits (de Pins et al., 2019). Therefore, neurotrophic factors are critical modulators of AD pathology. Hypoxia has been shown to decrease BDNF, and induce cognitive dysfunction (Li et al., 2012).
A study showed that HBOT reversed AD pathology through upregulation of neurotrophic factors in vitro and in vivo. HT22 cells treated with Aβ42 showed reduced expression of BDNF, NT3, NT4/5, and Trkb, and oxygen therapy reversed these decreases in expression. The expression of BDNF, NT3, and NT4/5 was also increased in the hippocampus of wild-type mice treated with HBOT (100% O2, 2 ATA, 60 min/d) for more than 7 days. In APP/PS1 mice, the levels of BDNF, NT3, and NT4/5 were reduced in the hippocampus, and HBOT reversed these decreases in expression (Choi et al., 2019). Furthermore, the levels of TrkB (a common receptor for BDNF, NT3, andNT4/5) and its key signaling mediators, p-Akt, p-ERK1/2, and p-CaMKII, were decreased in the hippocampi of APP/PS1 mice, and HBOT reversed these decreases. Furthermore, HBOT was shown to upregulate neurotrophic factors via methyl-CpG binding protein 2 (MeCP2) and cAMP response element binding protein (CREB), which regulate the expression of BDNF (Choi et al., 2019).
Oxygen therapy improves mitochondrial functions
Mitochondrial dysfunction plays a pivotal role in AD (Yoo et al., 2020). Impaired brain mitochondrial function in AD, characterized by reduced membrane potential, increased permeability, and excessive ROS production, is believed to be an underlying cause of neurodegeneration (Onyango et al., 2016). The expression of enzyme complexes in the tricarboxylic acid cycle and respiratory chain complexes is reduced in the brain in AD (Carvalho et al., 2009). Furthermore, hypoxia can aggravate mitochondrial dysfunction (Jain et al., 2015). A supplemental oxygen study demonstrated that 2 months of oxygen treatments (O2 40% for 20 min/d), resulted in significant upregulation of hippocampal mitochondrial proteins that participate in oxidative phosphorylation and energy production. Oxidative phosphorylation-associated proteins are likely to have direct effects on redox homeostasis. Moreover, energy production pathways are critical to neuronal energy homeostasis. Therefore, upregulation of components of energy production pathways would be expected to promote repair of dysfunctional mitochondria (Wang et al., 2017a).
Oxygen therapy enhances cerebral blood flow and protein synthesis
Regulation of cerebral blood flow (CBF) is a critical factor in normal brain function. The mammalian brain has evolved a unique mechanism for CBF control known as neurovascular coupling. This mechanism ensures a rapid increase in CBF and oxygen delivery to activated brain structures (Kisler et al., 2017). Early impairments to neurovascular coupling have been proposed to be key pathogenic factors in the onset and progression of AD (Solis et al., 2020). A study indicated that HBOT (O2 100%, 2 ATA, 60 min/d, 5 days per week for 4 weeks) alleviated the reduction in vessel diameter, and increased blood flow and arteriolar lumen size in 5XFAD mice (Shapira et al., 2021). Another study showed that 2 seconds and 16 seconds of hyperoxia stimulation (O2 100%) substantially enhanced baseline blood volume and saturation of all vascular compartments in the brains of J20-hAPP mice, a model of AD, using 2D-optical imaging spectroscopy. Therefore, enhanced blood volume in oxygen therapy may provide a therapeutic strategy for AD (Shabir et al., 2020).
Disordered protein synthesis may be an early pathological effector of AD (Ding et al., 2006). Our previous study showed that hypoxia altered histone modification of the DNMT3b promoter and downregulated its expression (Liu et al., 2016). A supplemental oxygen study demonstrated that 2 months of oxygen treatments (O2 40% for 20 min/d) significantly upregulated the synthesis of numerous proteins involved in mRNA splicing, transcription regulation, and translation, and upregulated several proteins associated with antioxidant defense. Supplemental oxygen therapy also reversed protein synthesis impairment in AD model mice. Importantly, oxygen therapy may support activation of antioxidant defenses and promote cellular redox homeostasis (Wang et al., 2017b). Proteomic analysis of the lenses of AD mice after oxygen supplement therapy showed 205 differentially expressed proteins relative to lenses from the control group, including proteins that involved in the clearance of Aβ (Wang et al., 2016). Therefore, oxygen therapy may improve cognitive function in AD by regulating protein synthesis.
Finally, although oxygen therapy appears to be beneficial for AD, it has been reported that oxygen therapy may aggravate the pathology of neurodegenerative diseases. Improper oxygen therapy can seriously damage the structure and function of tissues and organs (Sen and Sen, 2021). A study showed that NBOT (exposure of cells to 40% oxygen for 5 days) induced macroautophagy and accumulation of Aβ within lysosomes, which resulted in apoptosis of differentiated SH-SY5Y neuroblastoma cells (Zheng et al., 2011). Therefore, more studies are needed to understand the efficacy and mechanisms of oxygen therapy in AD.
Limitations
The limitations of this review include potential reporting bias, incomplete retrieval of completed research studies, and data extraction errors.
Conclusions and Future Perspectives
With the increasing older adult population, AD has become a serious health problem. Unfortunately, existing treatments only minimally slow the progression of the disease. Therefore, researchers have begun to evaluate non-drug treatments. Oxygen therapy could improve the symptoms of AD through modulation of multiple pathological aspects of AD including Aβ metabolism, tau phosphorylation, neuroinflammation, neuronal apoptosis, oxidative stress, neurotrophic factors, mitochondrial function, cerebral blood volume, mitochondrial function, and protein synthesis. Therefore, oxygen therapy may emerge as an effective therapeutic strategy for AD (Figure 2 and Table 2). However, previous studies mainly focused on the short-term therapeutic effects of oxygen treatment, and the related mechanisms. Therefore, the long-term effects and associated mechanisms, and the safety of oxygen therapy for treatment of AD as a long-term strategy should be further clarified.
Figure 2.
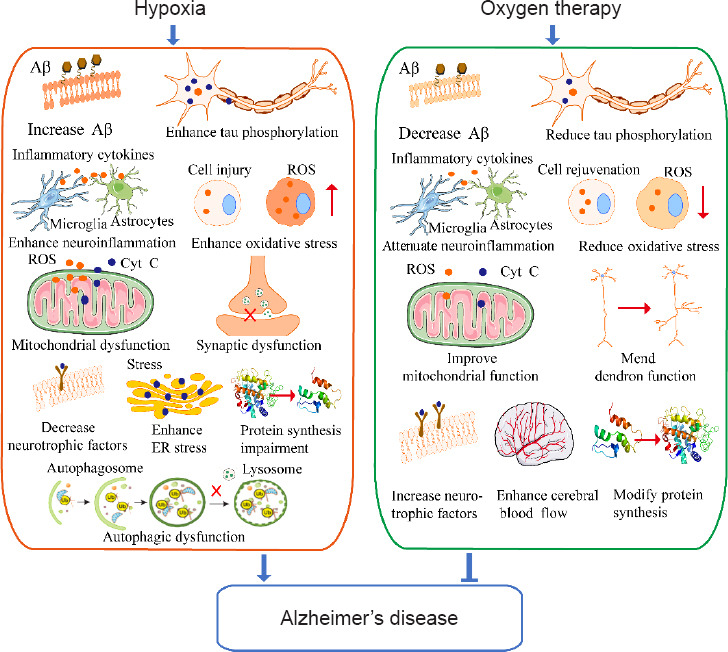
The mechanism underlying hypoxia and oxygen therapy in Alzheimer’s disease.
Aβ: Amyloid-beta; Cyt C: cytochrome C; ER: endoplasmic reticulum; ROS: reactive oxygen species.
Table 2.
Oxygen therapy ameliorates neuropathology in the models of AD
| Study | Transgenic line or treatment agent | Age and gender | Intervention | Result of cognitive function | The underlying mechanism of oxygen therapy |
|---|---|---|---|---|---|
| Gao et al., 2011 | APP/PS1 mice | 10 wk, male | O2 40%, normobaric 8 h/d for 4 wk/8 wk | Oxygen therapy improved the spatial learning and memory deficits | Decrease Aβ deposition and senile plaque formation in the cortex and hippocampus and reduce Aβ production by inhibiting β-secretase cleavage of APP. |
| Shapira et al., 2018 | 3×Tg-AD mice | 17 mon, male | O2 100%, 2ATA 60 min/d for 14 d | Oxygen therapy improved the performance of behavioral tasks | Reduce Aβ burden, ameliorate tau hyperphosphorylation, reduce the presence of hypoxia in the hippocampal formation, reduce astrogliosis and the number of microglia near plaques and promoted microglial sprouting, reduce proinflammatory cytokines, and increase the expression of anti-inflammatory cytokines and phagocytic markers. |
| Choi et al., 2019 | APP/PS1 mice | 7 mon, either gender | O2 100%, 2ATA 60 min/d for 28 d | Oxygen therapy improved cognitive function. | Reduce Aβ accumulation and hippocampal neuritic atrophy, increased hippocampal neurogenesis, increased the expression of BDNF, NT3, and NT4/5 through the upregulation of MeCP2/p-CREB activity in the hippocampus of mice. |
| Shapira et al., 2021 | 5×FAD mice | 6 mon, male | O2 100%, 2ATA 60 min/d, 5 d a wk for 4 wk | Oxygen therapy improved cognitive function | Reduce Aβ burden by reducing the volume of pre-existing plaques and attenuating the formation of new ones, increase arteriolar luminal diameter and elevate cerebral blood flow |
| Zhao et al., 2017 | SD rats-Aβ1–40 | 4 mon, male | O2 100%, 2ATA 60 min/d for 5 d | Oxygen therapy improved the spatial learning and memory deficits | Lower rates of neuronal damage, astrocyte activation, dendritic spine loss, and hippocampal neuron apoptosis via p38 mitogen-activated protein kinase |
| Zhang et al., 2015 | SD rats-Aβ25–35 | 5–6 mon, male | O2 100%, 2ATA 60 min/day, 20 d | Oxygen therapy improved cognitive and memory capacity | Reduce apoptosis via NF-κB pathway activation in hippocampus neurons |
| Tian et al., 2013 | SD rats-Aβ25–35 | 4–5 mon, male | O2 100%, 2ATA 60 min/d for 20 d | Oxygen therapy improved cognitive and memory capacity | Reduce cell toxicity and oxidative stress by blocking mitochondria-mediated apoptosis signaling |
| Tian et al., 2012 | SD rats-Aβ25–35 | 3–4 mon, either gender | O2 100%, 2ATA 60 min/d for 20 d | Oxygen therapy improved cognitive and memory capacity | Reduce cell toxicity and oxidative stress |
| Shabir et al., 2020 | J20-hAPP mice | 6 mon, male | O2 100%, 2 s/16 s | N/A | Enhance the baseline blood volume and saturation of all vascular compartments in the brains of J20-hAPP mice |
| Wang et al., 2017a | 3×Tg-AD mice | 6 mon | O2 40%, 20 min/d for 2 mon | N/A | Alleviate mitochondrial damage |
| Wang et al., 2016 | 3×Tg-AD mice | 6 mon | O2, 40%, 20 min/d for 2 mon | Oxygen therapy improved cognitive function | Alter protein expression in a manner consistent with improved redox regulation |
| Wang et al., 2017b | 3×Tg-AD mice | 6 mon | O2 40%, 20 min/d for 2 mon | N/A | Upregulate the synthesis of numerous proteins involved in mRNA splicing, transcription regulation, and translation in cortex tissues |
APP: Amyloid precursor protein; Aβ: amyloid-beta; BDNF: brain-derived neurotrophic factor; CREB: cAMP response element binding protein; MeCP2: methyl-CpG binding protein 2; N/A: not applicable; NF-κB: nuclear factor κB; NT3: neurotrophin 3; NT4/5: neurotrophin 4/5.
Additional file: Open peer review report 1 (76.7KB, pdf) .
Footnotes
Funding: This work was supported by the Key Research and Development Support Project of Chengdu Science and Technology Bureau, No. 2019-YF05-00655-SN (to WDL) and the Key Project of the Medical Science Department, University of Electronic Science and Technology of China, No. ZYGX2020ZB035 (to WDL).
Conflicts of interest: The authors declare no competing financial interests.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open peer reviewers: Sudip Dhakal, RMIT University, Australia; Valeria Bortolotto, Universita degli Studi del Piemonte Orientale Amedeo Avogadro, Italy.
P-Reviewers: Dhakal S, Bortolotto V; C-Editor: Zhao M; L-Editors: Li CH, Song LP; T-Editor: Jia Y
References
- 1.Alali AS, Temkin N, Vavilala MS, Lele AV, Barber J, Dikmen S, Chesnut RM. Matching early arterial oxygenation to long-term outcome in severe traumatic brain injury:target values. J Neurosurg. 2019;132:537–544. doi: 10.3171/2018.10.JNS18964. [DOI] [PubMed] [Google Scholar]
- 2.Baitule S, Patel AH, Murthy N, Sankar S, Kyrou I, Ali A, Randeva HS, Robbins T. A systematic review to assess the impact of hyperbaric oxygen therapy on glycaemia in people with diabetes mellitus. Medicina (Kaunas) 2021;57:1134. doi: 10.3390/medicina57101134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baratz-Goldstein R, Toussia-Cohen S, Elpaz A, Rubovitch V, Pick CG. Immediate and delayed hyperbaric oxygen therapy as a neuroprotective treatment for traumatic brain injury in mice. Mol Cell Neurosci. 2017;83:74–82. doi: 10.1016/j.mcn.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Biggs AT, Dainer HM, Littlejohn LF. Effect sizes for symptomatic and cognitive improvements in traumatic brain injury following hyperbaric oxygen therapy. J Appl Physiol 1985. 2021;130:1594–1603. doi: 10.1152/japplphysiol.01084.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braidy N, Zarka M, Jugder BE, Welch J, Jayasena T, Chan DKY, Sachdev P, Bridge W. The precursor to glutathione (GSH), γ-glutamylcysteine (GGC), can ameliorate oxidative damage and neuroinflammation induced by Aβ(40) oligomers in human astrocytes. Front Aging Neurosci. 2019;11:177. doi: 10.3389/fnagi.2019.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bu XL, Liu YH, Wang QH, Jiao SS, Zeng F, Yao XQ, Gao D, Chen JC, Wang YJ. Serum amyloid-beta levels are increased in patients with obstructive sleep apnea syndrome. Sci Rep. 2015a;5:13917. doi: 10.1038/srep13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bu XL, Cao GQ, Shen LL, Xiang Y, Jiao SS, Liu YH, Zhu C, Zeng F, Wang QH, Wang YR, He Y, Zhou HD, Wang YJ. Serum amyloid-beta levels are increased in patients with chronic obstructive pulmonary disease. Neurotox Res. 2015b;28:346–351. doi: 10.1007/s12640-015-9552-x. [DOI] [PubMed] [Google Scholar]
- 8.Bubu OM, Pirraglia E, Andrade AG, Sharma RA, Gimenez-Badia S, Umasabor-Bubu OQ, Hogan MM, Shim AM, Mukhtar F, Sharma N, Mbah AK, Seixas AA, Kam K, Zizi F, Borenstein AR, Mortimer JA, Kip KE, Morgan D, Rosenzweig I, Ayappa I, et al. Obstructive sleep apnea and longitudinal Alzheimer's disease biomarker changes. Sleep. 2019;42:zsz048. doi: 10.1093/sleep/zsz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burtscher J, Mallet RT, Burtscher M, Millet GP. Hypoxia and brain aging:Neurodegeneration or neuroprotection? Ageing Res Rev. 2021;68:101343. doi: 10.1016/j.arr.2021.101343. [DOI] [PubMed] [Google Scholar]
- 10.Butterfield DA, Mattson MP. Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer's disease. Neurobiol Dis. 2020;138:104795. doi: 10.1016/j.nbd.2020.104795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Z, Qiao PF, Wan CQ, Cai M, Zhou NK, Li Q. Role of blood-brain barrier in Alzheimer's disease. J Alzheimers Dis. 2018;63:1223–1234. doi: 10.3233/JAD-180098. [DOI] [PubMed] [Google Scholar]
- 12.Cárdenas-Aguayo Mdel C, Gómez-Virgilio L, DeRosa S, Meraz-Ríos MA. The role of tau oligomers in the onset of Alzheimer's disease neuropathology. ACS Chem Neurosci. 2014;5:1178–1191. doi: 10.1021/cn500148z. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho C, Correia SC, Santos RX, Cardoso S, Moreira PI, Clark TA, Zhu X, Smith MA, Perry G. Role of mitochondrial-mediated signaling pathways in Alzheimer disease and hypoxia. J Bioenerg Biomembr. 2009;41:433–440. doi: 10.1007/s10863-009-9247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Zhang F, Zhao L, Cheng C, Zhong R, Dong C, Le W. Hyperbaric oxygen ameliorates cognitive impairment in patients with Alzheimer's disease and amnestic mild cognitive impairment. Alzheimers Dement (N Y) 2020;6:e12030. doi: 10.1002/trc2.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Z, Li FW, Stone CR, Elkin K, Peng CY, Bardhi R, Geng XK, Ding YC. Normobaric oxygen therapy attenuates hyperglycolysis in ischemic stroke. Neural Regen Res. 2021;16:1017–1023. doi: 10.4103/1673-5374.300452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi J, Kwon HJ, Lee JE, Lee Y, Seoh JY, Han PL. Hyperoxygenation revitalizes Alzheimer's disease pathology through the upregulation of neurotrophic factors. Aging Cell. 2019;18:e12888. doi: 10.1111/acel.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudhary R, Kumar M, Katyal A. 12/15-Lipoxygenase debilitates mitochondrial health in intermittent hypobaric hypoxia induced neuronal damage:An in vivo study. Redox Biol. 2022;49:102228. doi: 10.1016/j.redox.2021.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chouraki V, Seshadri S. Genetics of Alzheimer's disease. Adv Genet. 2014;87:245–294. doi: 10.1016/B978-0-12-800149-3.00005-6. [DOI] [PubMed] [Google Scholar]
- 19.Chow VW, Mattson MP, Wong PC, Gleichmann M. An overview of APP processing enzymes and products. Neuromolecular Med. 2010;12:1–12. doi: 10.1007/s12017-009-8104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daulatzai MA. Death by a thousand cuts in Alzheimer's disease:hypoxia--the prodrome. Neurotox Res. 2013;24:216–243. doi: 10.1007/s12640-013-9379-2. [DOI] [PubMed] [Google Scholar]
- 21.Daulatzai MA. Cerebral hypoperfusion and glucose hypometabolism:Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer's disease. J Neurosci Res. 2017;95:943–972. doi: 10.1002/jnr.23777. [DOI] [PubMed] [Google Scholar]
- 22.de Pins B, Cifuentes-Díaz C, Farah AT, López-Molina L, Montalban E, Sancho-Balsells A, López A, Ginés S, Delgado-García JM, Alberch J, Gruart A, Girault JA, Giralt A. Conditional BDNF delivery from astrocytes rescues memory deficits, spine density, and synaptic properties in the 5xFAD mouse model of Alzheimer disease. J Neurosci. 2019;39:2441–2458. doi: 10.1523/JNEUROSCI.2121-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dechant G, Neumann H. Neurotrophins. Adv Exp Med Biol. 2002;513:303–334. doi: 10.1007/978-1-4615-0123-7_11. [DOI] [PubMed] [Google Scholar]
- 24.Deng Z, Chen W, Jin J, Zhao J, Xu H. The neuroprotection effect of oxygen therapy:A systematic review and meta-analysis. Niger J Clin Pract. 2018;21:401–416. doi: 10.4103/njcp.njcp_315_16. [DOI] [PubMed] [Google Scholar]
- 25.DeRidder L, Sharma A, Liaw K, Sharma R, John J, Kannan S, Kannan RM. Dendrimer-tesaglitazar conjugate induces a phenotype shift of microglia and enhances β-amyloid phagocytosis. Nanoscale. 2021;13:939–952. doi: 10.1039/d0nr05958g. [DOI] [PubMed] [Google Scholar]
- 26.Ding Q, Markesbery WR, Cecarini V, Keller JN. Decreased RNA, and increased RNA oxidation, in ribosomes from early Alzheimer's disease. Neurochem Res. 2006;31:705–710. doi: 10.1007/s11064-006-9071-5. [DOI] [PubMed] [Google Scholar]
- 27.Diniz Pereira J, Gomes Fraga V, Morais Santos AL, Carvalho MDG, Caramelli P, Braga Gomes K. Alzheimer's disease and type 2 diabetes mellitus:A systematic review of proteomic studies. J Neurochem. 2021;156:753–776. doi: 10.1111/jnc.15166. [DOI] [PubMed] [Google Scholar]
- 28.Dunn JF, Isaacs AM. The impact of hypoxia on blood-brain, blood-CSF, and CSF-brain barriers. J Appl Physiol 1985. 2021;131:977–985. doi: 10.1152/japplphysiol.00108.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao B, Long Z, Zhao L, He G. Effect of normobaric hyperoxia on behavioral deficits and neuropathology in Alzheimer's disease mouse model. J Alzheimers Dis. 2011;27:317–326. doi: 10.3233/JAD-2011-110308. [DOI] [PubMed] [Google Scholar]
- 30.Gao L, Tian S, Gao H, Xu Y. Hypoxia increases Aβ-induced tau phosphorylation by calpain and promotes behavioral consequences in AD transgenic mice. J Mol Neurosci. 2013;51:138–147. doi: 10.1007/s12031-013-9966-y. [DOI] [PubMed] [Google Scholar]
- 31.Guan Y, Niu H, Liu Z, Dang Y, Shen J, Zayed M, Ma L, Guan J. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci Adv. 2021;7:eabj0153. doi: 10.1126/sciadv.abj0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerreiro R, Bras J. The age factor in Alzheimer's disease. Genome Med. 2015;7:106. doi: 10.1186/s13073-015-0232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guglielmotto M, Aragno M, Autelli R, Giliberto L, Novo E, Colombatto S, Danni O, Parola M, Smith MA, Perry G, Tamagno E, Tabaton M. The up-regulation of BACE1 mediated by hypoxia and ischemic injury:role of oxidative stress and HIF1alpha. J Neurochem. 2009;108:1045–1056. doi: 10.1111/j.1471-4159.2008.05858.x. [DOI] [PubMed] [Google Scholar]
- 34.Haake A, Nguyen K, Friedman L, Chakkamparambil B, Grossberg GT. An update on the utility and safety of cholinesterase inhibitors for the treatment of Alzheimer's disease. Expert Opin Drug Saf. 2020;19:147–157. doi: 10.1080/14740338.2020.1721456. [DOI] [PubMed] [Google Scholar]
- 35.Hachmo Y, Hadanny A, Abu Hamed R, Daniel-Kotovsky M, Catalogna M, Fishlev G, Lang E, Polak N, Doenyas K, Friedman M, Zemel Y, Bechor Y, Efrati S. Hyperbaric oxygen therapy increases telomere length and decreases immunosenescence in isolated blood cells:a prospective trial. Aging (Albany NY) 2020;12:22445–22456. doi: 10.18632/aging.202188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamilton G, Mathur R, Allsop JM, Forton DM, Dhanjal NS, Shaw RJ, Taylor-Robinson SD. Changes in brain intracellular pH and membrane phospholipids on oxygen therapy in hypoxic patients with chronic obstructive pulmonary disease. Metab Brain Dis. 2003;18:95–109. doi: 10.1023/a:1021938926807. [DOI] [PubMed] [Google Scholar]
- 37.Hampel H, Vassar R, De Strooper B, Hardy J, Willem M, Singh N, Zhou J, Yan R, Vanmechelen E, De Vos A, Nisticò R, Corbo M, Imbimbo BP, Streffer J, Voytyuk I, Timmers M, Tahami Monfared AA, Irizarry M, Albala B, Koyama A, et al. The β-secretase BACE1 in Alzheimer's disease. Biol Psychiatry. 2021;89:745–756. doi: 10.1016/j.biopsych.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harch PG, Fogarty EF. Hyperbaric oxygen therapy for Alzheimer's dementia with positron emission tomography imaging:a case report. Med Gas Res. 2018;8:181–184. doi: 10.4103/2045-9912.248271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, Jiang T. Regional coherence changes in the early stages of Alzheimer's disease:a combined structural and resting-state functional MRI study. Neuroimage. 2007;35:488–500. doi: 10.1016/j.neuroimage.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 40.Heyboer Rd M, Wojcik SM, Smith G, Santiago W. Effect of hyperbaric oxygen therapy on blood pressure in patients undergoing treatment. Undersea Hyperb Med. 2017;44:93–99. doi: 10.22462/3.4.2017.2. [DOI] [PubMed] [Google Scholar]
- 41.Horner D, O'Driscoll R. Oxygen therapy for medical patients. BMJ. 2018;363:k4436. doi: 10.1136/bmj.k4436. [DOI] [PubMed] [Google Scholar]
- 42.Iqbal K, Liu F, Gong CX. Tau and neurodegenerative disease:the story so far. Nat Rev Neurol. 2016;12:15–27. doi: 10.1038/nrneurol.2015.225. [DOI] [PubMed] [Google Scholar]
- 43.Irawan H, Semadi IN, Widiana IGR. A pilot study of short-duration hyperbaric oxygen therapy to improve HbA1c, leukocyte, and serum creatinine in patients with diabetic foot ulcer Wagner 3-4. ScientificWorldJournal. 2018;2018:6425857. doi: 10.1155/2018/6425857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain K, Prasad D, Singh SB, Kohli E. Hypobaric hypoxia imbalances mitochondrial dynamics in rat brain hippocampus. Neurol Res Int. 2015;2015:742059. doi: 10.1155/2015/742059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, Visser PJ Amyloid Biomarker Study Group. Aalten P, Aarsland D, Alcolea D, Alexander M, Almdahl IS, Arnold SE, Baldeiras I, Barthel H, van Berckel BN, Bibeau K, Blennow K, Brooks DJ, et al. Prevalence of cerebral amyloid pathology in persons without dementia:a meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jefferson AL, Beiser AS, Himali JJ, Seshadri S, O'Donnell CJ, Manning WJ, Wolf PA, Au R, Benjamin EJ. Low cardiac index is associated with incident dementia and Alzheimer disease:the Framingham Heart Study. Circulation. 2015;131:1333–1339. doi: 10.1161/CIRCULATIONAHA.114.012438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin J, Guo J, Cai H, Zhao C, Wang H, Liu Z, Ge ZM. M2-like microglia polarization attenuates neuropathic pain associated with Alzheimer's disease. J Alzheimers Dis. 2020;76:1255–1265. doi: 10.3233/JAD-200099. [DOI] [PubMed] [Google Scholar]
- 48.Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jönsson EG, et al. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 49.Karabiyik C, Frake RA, Park SJ, Pavel M, Rubinsztein DC. Autophagy in ageing and ageing-related neurodegenerative diseases. Ageing Neur Dis. 2021;2021(1):2. [Google Scholar]
- 50.Karamanli H, Ilik F, Kayhan F, Pazarli AC. Assessment of cognitive impairment in long-term oxygen therapy-dependent COPD patients. Int J Chron Obstruct Pulmon Dis. 2015;10:2087–2094. doi: 10.2147/COPD.S88326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karch CM, Cruchaga C, Goate AM. Alzheimer's disease genetics:from the bench to the clinic. Neuron. 2014;83:11–26. doi: 10.1016/j.neuron.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelly C. Oxygen therapy:time to move on? Ther Adv Respir Dis. 2014;8:191–199. doi: 10.1177/1753465814549011. [DOI] [PubMed] [Google Scholar]
- 53.Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419–434. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobayashi N, Nagata T, Shinagawa S, Nakayama R, Kondo K, Nakayama K, Yamada H. Association between neurotrophin-3 polymorphisms and executive function in Japanese patients with amnestic mild cognitive impairment and mild Alzheimer disease. Dement Geriatr Cogn Disord. 2012;34:190–197. doi: 10.1159/000343075. [DOI] [PubMed] [Google Scholar]
- 55.Kohshi K, Denoble PJ, Tamaki H, Morimatsu Y, Ishitake T, Lemaitre F. Decompression illness in repetitive breath-hold diving:why ischemic lesions involve the brain? Front Physiol. 2021;12:711850. doi: 10.3389/fphys.2021.711850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kukar T, Murphy MP, Eriksen JL, Sagi SA, Weggen S, Smith TE, Ladd T, Khan MA, Kache R, Beard J, Dodson M, Merit S, Ozols VV, Anastasiadis PZ, Das P, Fauq A, Koo EH, Golde TE. Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Abeta42 production. Nat Med. 2005;11:545–550. doi: 10.1038/nm1235. [DOI] [PubMed] [Google Scholar]
- 57.Kurochkin IV, Guarnera E, Berezovsky IN. Insulin-degrading enzyme in the fight against Alzheimer's disease. Trends Pharmacol Sci. 2018;39:49–58. doi: 10.1016/j.tips.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 58.Leitman M, Efrati S, Fuchs S, Hadanny A, Vered Z. The effect of hyperbaric oxygenation therapy on myocardial function. Int J Cardiovasc Imaging. 2020;36:833–840. doi: 10.1007/s10554-020-01773-0. [DOI] [PubMed] [Google Scholar]
- 59.Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease:where do we go from here? Nat Rev Neurol. 2021;17:157–172. doi: 10.1038/s41582-020-00435-y. [DOI] [PubMed] [Google Scholar]
- 60.Lennon MJ, Koncz R, Sachdev PS. Hypertension and Alzheimer's disease:is the picture any clearer? Curr Opin Psychiatry. 2021;34:142–148. doi: 10.1097/YCO.0000000000000684. [DOI] [PubMed] [Google Scholar]
- 61.Leuner K, Schutt T, Kurz C, Eckert SH, Schiller C, Occhipinti A, Mai S, Jendrach M, Eckert GP, Kruse SE, Palmiter RD, Brandt U, Drose S, Wittig I, Willem M, Haass C, Reichert AS, Muller WE. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxid Redox Signal. 2012;16:1421–1433. doi: 10.1089/ars.2011.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li L, Zhang X, Yang D, Luo G, Chen S, Le W. Hypoxia increases Abeta generation by altering beta- and gamma-cleavage of APP. Neurobiol Aging. 2009;30:1091–1098. doi: 10.1016/j.neurobiolaging.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 63.Li L, Liang J, Fu H. An update on the association between traumatic brain injury and Alzheimer's disease:Focus on Tau pathology and synaptic dysfunction. Neurosci Biobehav Rev. 2021;120:372–386. doi: 10.1016/j.neubiorev.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li P, Zhang G, You HY, Zheng R, Gao YQ. Training-dependent cognitive advantage is suppressed at high altitude. Physiol Behav. 2012;106:439–445. doi: 10.1016/j.physbeh.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Li Y, Lv W, Cheng G, Wang S, Liu B, Zhao H, Wang H, Zhang L, Dong C, Zhang J. Effect of early normobaric hyperoxia on blast-induced traumatic brain injury in rats. Neurochem Res. 2020;45:2723–2731. doi: 10.1007/s11064-020-03123-x. [DOI] [PubMed] [Google Scholar]
- 66.Liguori C, Mercuri NB, Izzi F, Romigi A, Cordella A, Sancesario G, Placidi F. Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer's disease biomarkers changes. Sleep. 2017 doi: 10.1093/sleep/zsx011. doi:10.1093/sleep/zsx011. [DOI] [PubMed] [Google Scholar]
- 67.Liguori C, Mercuri NB, Nuccetelli M, Izzi F, Cordella A, Bernardini S, Placidi F. Obstructive sleep apnea may induce orexinergic system and cerebral β-amyloid metabolism dysregulation:is it a further proof for Alzheimer's disease risk? Sleep Med. 2019;56:171–176. doi: 10.1016/j.sleep.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 68.Liu H, Qiu H, Yang J, Ni J, Le W. Chronic hypoxia facilitates Alzheimer's disease through demethylation of gamma-secretase by downregulating DNA methyltransferase 3b. Alzheimers Dement. 2016;12:130–143. doi: 10.1016/j.jalz.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 69.Lou D, Du Y, Huang D, Cai F, Zhang Y, Li T, Zhou W, Gao H, Song W. Traumatic brain injury alters the metabolism and facilitates Alzheimer's disease in a murine model. Mol Neurobiol. 2018;55:4928–4939. doi: 10.1007/s12035-017-0687-z. [DOI] [PubMed] [Google Scholar]
- 70.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure:an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 71.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Jr, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease:recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Megas IF, Beier JP, Grieb G. The history of carbon monoxide intoxication. Medicina (Kaunas) 2021;57:400. doi: 10.3390/medicina57050400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mei Z, Zheng P, Tan X, Wang Y, Situ B. Huperzine A alleviates neuroinflammation, oxidative stress and improves cognitive function after repetitive traumatic brain injury. Metab Brain Dis. 2017;32:1861–1869. doi: 10.1007/s11011-017-0075-4. [DOI] [PubMed] [Google Scholar]
- 74.Murphy MP, LeVine H., 3rd Alzheimer's disease and the amyloid-beta peptide. J Alzheimers Dis. 2010;19:311–23. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Onyango IG, Dennis J, Khan SM. Mitochondrial dysfunction in Alzheimer's disease and the rationale for bioenergetics based therapies. Aging Dis. 2016;7:201–214. doi: 10.14336/AD.2015.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ou GY, Lin WW, Zhao WJ. Neuregulins in neurodegenerative diseases. Front Aging Neurosci. 2021;13:662474. doi: 10.3389/fnagi.2021.662474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peers C, Pearson HA, Boyle JP. Hypoxia and Alzheimer's disease. Essays Biochem. 2007;43:153–164. doi: 10.1042/BSE0430153. [DOI] [PubMed] [Google Scholar]
- 78.Plascencia-Villa G, Perry G. Preventive and therapeutic strategies in Alzheimer's disease:focus on oxidative stress, redox metals, and ferroptosis. Antioxid Redox Signal. 2021;34:591–610. doi: 10.1089/ars.2020.8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease:a population-based cohort study. Arch Intern Med. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 80.Ramos-Cejudo J, Wisniewski T, Marmar C, Zetterberg H, Blennow K, de Leon MJ, Fossati S. Traumatic brain injury and Alzheimer's disease:the cerebrovascular link. EBioMedicine. 2018;28:21–30. doi: 10.1016/j.ebiom.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reinhard CT, Planavsky NJ, Olson SL, Lyons TW, Erwin DH. Earth's oxygen cycle and the evolution of animal life. Proc Natl Acad Sci U S A. 2016;113:8933–8938. doi: 10.1073/pnas.1521544113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reitz C, Mayeux R. Alzheimer disease:epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88:640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex:Triad of risk of Alzheimer's disease. J Steroid Biochem Mol Biol. 2016;160:134–147. doi: 10.1016/j.jsbmb.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roda AR, Serra-Mir G, Montoliu-Gaya L, Tiessler L, Villegas S. Amyloid-beta peptide and tau protein crosstalk in Alzheimer's disease. Neural Regen Res 2022. 2022 Aug;17(s8):1666–1674. doi: 10.4103/1673-5374.332127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saito S, Ihara M. Interaction between cerebrovascular disease and Alzheimer pathology. Curr Opin Psychiatry. 2016;29:168–173. doi: 10.1097/YCO.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 86.Sen S, Sen S. Therapeutic effects of hyperbaric oxygen:integrated review. Med Gas Res. 2021;11:30–33. doi: 10.4103/2045-9912.310057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shabir O, Sharp P, Rebollar MA, Boorman L, Howarth C, Wharton SB, Francis SE, Berwick J. Enhanced cerebral blood volume under normobaric hyperoxia in the J20-hAPP mouse model of Alzheimer's disease. Sci Rep. 2020;10:7518. doi: 10.1038/s41598-020-64334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shapira R, Solomon B, Efrati S, Frenkel D, Ashery U. Hyperbaric oxygen therapy ameliorates pathophysiology of 3xTg-AD mouse model by attenuating neuroinflammation. Neurobiol Aging. 2018;62:105–119. doi: 10.1016/j.neurobiolaging.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 89.Shapira R, Gdalyahu A, Gottfried I, Sasson E, Hadanny A, Efrati S, Blinder P, Ashery U. Hyperbaric oxygen therapy alleviates vascular dysfunction and amyloid burden in an Alzheimer's disease mouse model and in elderly patients. Aging (Albany NY) 2021;13:20935–20961. doi: 10.18632/aging.203485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shwe T, Bo-Htay C, Ongnok B, Chunchai T, Jaiwongkam T, Kerdphoo S, Kumfu S, Pratchayasakul W, Pattarasakulchai T, Chattipakorn N, Chattipakorn SC. Hyperbaric oxygen therapy restores cognitive function and hippocampal pathologies in both aging and aging-obese rats. Mech Ageing Dev. 2021;195:111465. doi: 10.1016/j.mad.2021.111465. [DOI] [PubMed] [Google Scholar]
- 91.Shytle RD, Eve DJ, Kim SH, Spiegel A, Sanberg PR, Borlongan CV. Retrospective case series of traumatic brain injury and post-traumatic stress disorder treated with hyperbaric oxygen therapy. Cell Transplant. 2019;28:885–892. doi: 10.1177/0963689719853232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Siachpazidou DI, Stavrou VT, Astara K, Pastaka C, Gogou E, Hatzoglou C, Economou NT, Gourgoulianis KI. Alzheimer's disease in patients with obstructive sleep apnea syndrome. Tanaffos. 2020;19:176–185. [PMC free article] [PubMed] [Google Scholar]
- 93.Siuda J, Patalong-Ogiewa M, Żmuda W, Targosz-Gajniak M, Niewiadomska E, Matuszek I, Jędrzejowska-Szypułka H, Lewin-Kowalik J, Rudzińska-Bar M. Cognitive impairment and BDNF serum levels. Neurol Neurochir Pol. 2017;51:24–32. doi: 10.1016/j.pjnns.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 94.Smith SM, Friedle SA, Watters JJ. Chronic intermittent hypoxia exerts CNS region-specific effects on rat microglial inflammatory and TLR4 gene expression. PLoS One. 2013;8:e81584. doi: 10.1371/journal.pone.0081584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Snyder B, Shell B, Cunningham JT, Cunningham RL. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol Rep. 2017;5:e13258. doi: 10.14814/phy2.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Snyder B, Simone SM, Giovannetti T, Floyd TF. Cerebral hypoxia:its role in age-related chronic and acute cognitive dysfunction. Anesth Analg. 2021;132:1502–1513. doi: 10.1213/ANE.0000000000005525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Solis E, Jr, Hascup KN, Hascup ER. Alzheimer's disease:the link between amyloid-βand neurovascular dysfunction. J Alzheimers Dis. 2020;76:1179–1198. doi: 10.3233/JAD-200473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tian X, Wang J, Dai J, Yang L, Zhang L, Shen S, Huang P. Hyperbaric oxygen and Ginkgo Biloba extract inhibit Aβ25-35-induced toxicity and oxidative stress in vivo:a potential role in Alzheimer's disease. Int J Neurosci. 2012;122:563–569. doi: 10.3109/00207454.2012.690797. [DOI] [PubMed] [Google Scholar]
- 99.Tian X, Zhang L, Wang J, Dai J, Shen S, Yang L, Huang P. The protective effect of hyperbaric oxygen and Ginkgo biloba extract on Aβ25-35-induced oxidative stress and neuronal apoptosis in rats. Behav Brain Res. 2013;242:1–8. doi: 10.1016/j.bbr.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 100.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples:Analytical and biological challenges. Anal Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 101.Wang CY, Wang ZY, Xie JW, Cai JH, Wang T, Xu Y, Wang X, An L. CD36 upregulation mediated by intranasal LV-NRF2 treatment mitigates hypoxia-induced progression of Alzheimer's-like pathogenesis. Antioxid Redox Signal. 2014;21:2208–2230. doi: 10.1089/ars.2014.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang H, Wang Y, Hong X, Li S, Wang Y. Quantitative proteomics reveals the mechanism of oxygen treatment on lenses of Alzheimer's disease model mice. J Alzheimers Dis. 2016;54:275–286. doi: 10.3233/JAD-160263. [DOI] [PubMed] [Google Scholar]
- 103.Wang H, Hong X, Wang Y. Mitochondrial repair effects of oxygen treatment on Alzheimer's disease model mice revealed by quantitative proteomics. J Alzheimers Dis. 2017a;56:875–883. doi: 10.3233/JAD-161010. [DOI] [PubMed] [Google Scholar]
- 104.Wang H, Hong X, Li S, Wang Y. Oxygen supplementation improves protein milieu supportive of protein synthesis and antioxidant function in the cortex of Alzheimer's disease model mice-a quantitative proteomic study. J Mol Neurosci. 2017b;63:243–253. doi: 10.1007/s12031-017-0975-0. [DOI] [PubMed] [Google Scholar]
- 105.Wu Z, Wang ZH, Liu X, Zhang Z, Gu X, Yu SP, Keene CD, Cheng L, Ye K. Traumatic brain injury triggers APP and Tau cleavage by delta-secretase, mediating Alzheimer's disease pathology. Prog Neurobiol. 2020;185:101730. doi: 10.1016/j.pneurobio.2019.101730. [DOI] [PubMed] [Google Scholar]
- 106.Xu L, Song H, Qiu Q, Jiang T, Ge P, Su Z, Ma W, Zhang R, Huang C, Li S, Lin D, Zhang J. Different expressions of HIF-1alpha and metabolism in brain and major visceral organs of acute hypoxic mice. Int J Mol Sci. 2021;22:6705. doi: 10.3390/ijms22136705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu Q, Wei YT, Fan SB, Wang L, Zhou XP. Repetitive hyperbaric oxygen treatment increases insulin sensitivity in diabetes patients with acute intracerebral hemorrhage. Neuropsychiatr Dis Treat. 2017;13:421–426. doi: 10.2147/NDT.S126288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yoo SM, Park J, Kim SH, Jung YK. Emerging perspectives on mitochondrial dysfunction and inflammation in Alzheimer's disease. BMB Rep. 2020;53:35–46. doi: 10.5483/BMBRep.2020.53.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu R, Wang B, Li S, Wang J, Zhou F, Chu S, He X, Wen X, Ni X, Liu L, Xie Q, Huang R. Cognitive enhancement of healthy young adults with hyperbaric oxygen:A preliminary resting-state fMRI study. Clin Neurophysiol. 2015;126:2058–2067. doi: 10.1016/j.clinph.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 110.Yuan C, Aierken A, Xie Z, Li N, Zhao J, Qing H. The age-related microglial transformation in Alzheimer's disease pathogenesis. Neurobiol Aging. 2020;92:82–91. doi: 10.1016/j.neurobiolaging.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 111.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 112.Zhang CE, Yang X, Li L, Sui X, Tian Q, Wei W, Wang J, Liu G. Hypoxia-induced tau phosphorylation and memory deficit in rats. Neurodegener Dis. 2014;14:107–116. doi: 10.1159/000362239. [DOI] [PubMed] [Google Scholar]
- 113.Zhang F, Zhong R, Qi H, Li S, Cheng C, Liu X, Liu Y, Le W. Impacts of acute hypoxia on Alzheimer's disease-like pathologies in APP(swe)/PS1(dE9) mice and their wild type littermates. Front Neurosci. 2018;12:314. doi: 10.3389/fnins.2018.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang F, Niu L, Li S, Le W. Pathological impacts of chronic hypoxia on Alzheimer's disease. ACS Chem Neurosci. 2019;10:902–909. doi: 10.1021/acschemneuro.8b00442. [DOI] [PubMed] [Google Scholar]
- 115.Zhang H, Barralet JE. Mimicking oxygen delivery and waste removal functions of blood. Adv Drug Deliv Rev. 2017;122:84–104. doi: 10.1016/j.addr.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 116.Zhang LD, Ma L, Zhang L, Dai JG, Chang LG, Huang PL, Tian XQ. Hyperbaric oxygen and Ginkgo biloba extract ameliorate cognitive and memory impairment via nuclear factor kappa-B pathway in rat model of Alzheimer's disease. Chin Med J (Engl) 2015;128:3088–3093. doi: 10.4103/0366-6999.169105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang X, Le W. Pathological role of hypoxia in Alzheimer's disease. Exp Neurol. 2010;223:299–303. doi: 10.1016/j.expneurol.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Z, Liu Y, Jiang T, Zhou B, An N, Dai H, Wang P, Niu Y, Wang L, Zhang X. Altered spontaneous activity in Alzheimer's disease and mild cognitive impairment revealed by regional homogeneity. Neuroimage. 2012;59:1429–1440. doi: 10.1016/j.neuroimage.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 119.Zhao B, Pan Y, Wang Z, Xu H, Song X. Hyperbaric oxygen pretreatment improves cognition and reduces hippocampal damage via p38 mitogen-activated protein kinase in a rat model. Yonsei Med J. 2017;58:131–138. doi: 10.3349/ymj.2017.58.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng L, Terman A, Hallbeck M, Dehvari N, Cowburn RF, Benedikz E, Kågedal K, Cedazo-Minguez A, Marcusson J. Macroautophagy-generated increase of lysosomal amyloid β-protein mediates oxidant-induced apoptosis of cultured neuroblastoma cells. Autophagy. 2011;7:1528–1545. doi: 10.4161/auto.7.12.18051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zyśk M, Clausen F, Aguilar X, Sehlin D, Syvänen S, Erlandsson A. Long-term effects of traumatic brain injury in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2019;72:161–180. doi: 10.3233/JAD-190572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


