Parkinson’s disease (PD) is a heterogeneous multifactorial disorder and, during the last years, new scientific evidence has supported this concept. The principal hallmarks of PD are the loss of dopaminergic neurons in the substantia nigra pars compacta and the aggregation of misfolded alpha-synuclein (α-syn). In particular, α-syn is receiving great attention for its key role in PD neuropathology. Both genetic mutations and post-translational modifications (i.e., α-syn phosphorylation) can induce protein misfolding. An abnormal accumulation of this misfolded α-syn crushes both the ubiquitin-proteosome and autophagy systems, which are involved in protein clearance. As a result, α-syn aggregation leads to neuronal dysfunction and neurodegeneration. Along with this pathological condition, dysregulated mitochondrial activity, reactive oxygen species production, oxidative stress, and blood-brain barrier alteration, are typical features of neurological disorders such as PD. In addition, neuroinflammatory processes are critical for PD pathogenesis and strictly interconnected to α-syn pathology. According to this, neuroinflammation could be considered as a potential early drug target.
The primary hallmark of PD is α-syn, one of the main components of Lewy bodies. Cardinale et al. (2021) have shown how mapping the journey of α-syn and its region-dependent effects is one of the stumbling blocks that researchers have encountered over the years to explain if neurodegeneration is closely intertwined with α-syn misfolded forms.
The spreading of misfolded α-syn through the brain depends on disease progression (Braak et al., 2003). α-syn is highly expressed in the substantia nigra pars compacta and other regions affected by PD (Calabresi et al., 2014). In the normal brain of a rhesus monkey, α-syn is largely distributed in the whole brain; in particular, the substantia nigra, the hippocampus, and the neocortex express high α-syn levels and they are also notably vulnerable during the progression of idiopathic PD (Yang et al., 2021).
The topic concerning the role of the different species of α-syn also seems to be rather controversial. The toxicity of α-syn is associated with its oligomeric and protofibrillar forms (Bras et al., 2020). On the other hand, there is controversial evidence of the physiological α-syn monomer role in the presynaptic vesicle formation mediated by soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE) complex and neurotransmission (Calo et al., 2021).
The molecular mechanisms underlying how α-syn oligomers drive the death of neurons remain unclear. Yoo et al. (2021) recently proposed an interesting and original question: can the physiological function of monomers and the toxicity of oligomers influence synaptic transmission in a correlative way? As other studies report, both monomers and oligomers to combine inhibit SNARE-mediated vesicle fusion. Yoo et al. (2021) have shown that the low concentration of oligomers, together with abundant monomers at the presynaptic terminals, can inhibit vesicle fusion efficiently. This ambitious work sets new prospects no longer directed to the singular study of a specific form of pathological α-syn. Therefore, it seems necessary not to exclude any factor to investigate what may occur during their interactions, in order to understand the pathology generated by α-syn oligomers and to develop improved therapeutic strategies.
Regarding the protofibrillar form of α-syn, in the last ten years, several experimental studies have increasingly enhanced understanding of the role of this form in synaptic transmission and plasticity.
A recent study by Tozzi et al. (2021) identified two early time points, at 6 weeks and 12 weeks after protofibril α-syn intrastriatal injection in adult male Wistar rats, to clarify how α-syn induces alterations in distinct forms of corticostriatal plasticity. The authors found that the spontaneous firing rate was absent in the cells recorded at 6 weeks post-injection, long-term potentiation was blocked, together with a reduction of dopamine release in striatum. On the contrary, at 12 weeks post-injection, dopaminergic neurons showed an increased firing rate, an impaired long-term depression, and a decrease of dopamine release compared to sham-operated rats, when a marked death of TH+ cells arose. From this latest study, it can be inferred that the specific mechanism of neurotoxicity induced by α-syn at different time points remains an open field of exploration.
Neuroinflammation is strictly associated with α-syn pathology and neuronal degeneration in PD. As mentioned previously, we described the mechanism by which α-syn alters neuronal functions in PD and how this causes inflammation signs (Cardinale et al., 2021).
Which came first, the chicken or the egg? We could extend this ancient paradox to PD. Which came first, neuroinflammation or dopaminergic cell death? Over many years, the long debate on neuroinflammation and neurodegeneration has generated many studies to understand which of the two came first. Nowadays, it is generally accepted that the innate immune system is activated when plasticity alteration and degeneration of dopaminergic terminals in the striatum occur, many years before the death of Substantia Nigra pars compacta neurons (Harry, 2021). Indeed, the onset of PD symptoms appears when neurodegeneration has already been in progress for several years and has already caused the death of 50% of dopamine neurons and the loss of 70–80% of dopaminergic fibers (Parnetti et al., 2019).
By means of microglia cells activity, the immune system keeps brain health under control. Therefore, when the central nervous system could be in danger, immune cells activate pro-inflammatory processes. In physiological conditions, the activation of microglial cells is the dynamic adopting of the anti-inflammatory phenotype if the central nervous system environment is safe. In a PD context, where many biological processes are modified (i.e., altered ATP production and release of neurotransmitters, synaptic loss, reactive oxygen species production, and oxidative stress), the brain could be under chronic activation of pro-inflammatory mechanisms, producing detrimental effects, that ultimately lead to neuronal death (Picca et al., 2021). Thus, neuroinflammation could be considered the trigger of dopaminergic cell loss and strictly related to α-syn pathology. Indeed, as we discussed in our previous work (Cardinale et al., 2021), α-syn oligomers and fibrils cause the activation of glial cells. Several works have highlighted this process, particularly in protein-based animal models, by which the early activation of both the innate and adaptive immune systems was demonstrated. Moreover, the early activation of microglial cells was also demonstrated in patients with prodromal PD signs (rapid-eye-movement sleep disorders) by means of positron emission tomography analysis (Ferreira and Romero-Ramos, 2018). Therefore, the immune system could be an early target to ameliorate PD prognosis (Krashia et al., 2019).
Misfolded α-syn and inflammatory response act alongside one another providing the PD phenotype. This detrimental relationship provides fertile ground for cellular death (Figure 1). As is well known, currently, available treatments are only used to reduce symptoms. Furthermore, in this context, there is the urgency and the challenge to find an early therapeutic target and/or biomarkers in order to assist in diagnosis and prognostication and to develop new drugs, capable of delaying or stopping the disease. α-syn and pro-inflammatory agents could be still considered powerful candidates for this challenge looking for new personalized therapies.
Figure 1.
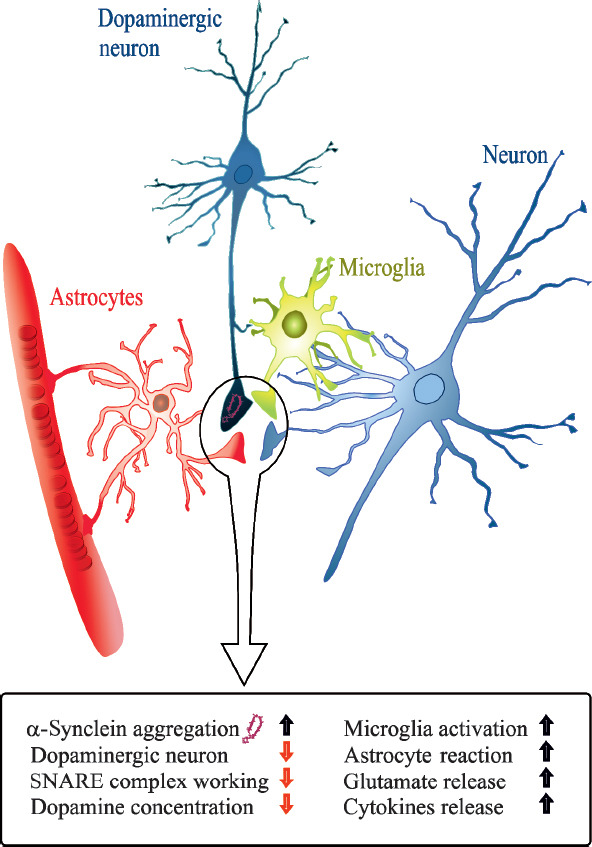
The inflammatory mechanism induced by alpha-synuclein accumulation.
Formation of toxic α-synuclein in Parkinson’s disease (PD), specifically at the presynaptic terminal, leads to changes of proteins involved in synaptic transmission (i.e., SNARE complex alteration and increased glutamate release), determining synaptic dysfunction and contributing to degeneration of dopaminergic neurons. Moreover, α-synuclein aggregation could be considered as a causal link between neurodegeneration and neuroinflammation in PD, inducing astrocyte and microglia reaction, consequent release of cytokines and activation of adaptive immune system. SNARE: Soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor.
This work was supported by a grant from NYU Grossman School of Medicine and The Marlene and Paolo Fresco Institute for Parkinson’s and Movement Disorders, which was made possible with support from Marlene and Paolo Fresco (to AC).
We thank Antonio de Iure for figure illustration. We thank Barbara Picconi for her constant support.
Footnotes
C-Editors: Zhao M, Liu WJ, Wang Lu; T-Editor: Jia Y
References
- 1.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 2.Bras IC, Xylaki M, Outeiro TF. Mechanisms of alpha-synuclein toxicity:An update and outlook. Prog Brain Res. 2020;252:91–129. doi: 10.1016/bs.pbr.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. Direct and indirect pathways of basal ganglia:a critical reappraisal. Nat Neurosci. 2014;17:1022–1030. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- 4.Calo L, Hidari E, Wegrzynowicz M, Dalley JW, Schneider BL, Podgajna M, Anichtchik O, Carlson E, Klenerman D, Spillantini MG. CSPalpha reduces aggregates and rescues striatal dopamine release in alpha-synuclein transgenic mice. Brain. 2021;144:1661–1669. doi: 10.1093/brain/awab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardinale A, Calabrese V, de Iure A, Picconi B. Alpha-synuclein as a prominent actor in the inflammatory synaptopathy of Parkinson's disease. Int J Mol Sci. 2021;22:6517. doi: 10.3390/ijms22126517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira SA, Romero-Ramos M. Microglia response during Parkinson's disease:Alpha-synuclein intervention. Front Cell Neurosci. 2018;12:247. doi: 10.3389/fncel.2018.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harry GJ. Microglia in neurodegenerative events-an initiator or a significant other? Int J Mol Sci. 2021;22:5818. doi: 10.3390/ijms22115818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krashia P, Cordella A, Nobili A, La Barbera L, Federici M, Leuti A, Campanelli F, Natale G, Marino G, Calabrese V, Vedele F, Ghiglieri V, Picconi B, Di Lazzaro G, Schirinzi T, Sancesario G, Casadei N, Riess O, Bernardini S, Pisani A, et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson's disease. Nat Commun. 2019;10:3945. doi: 10.1038/s41467-019-11928-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parnetti L, Gaetani L, Eusebi P, Paciotti S, Hansson O, El-Agnaf O, Mollenhauer B, Blennow K, Calabresi P. CSF and blood biomarkers for Parkinson's disease. Lancet Neurol. 2019;18:573–586. doi: 10.1016/S1474-4422(19)30024-9. [DOI] [PubMed] [Google Scholar]
- 10.Picca A, Guerra F, Calvani R, Romano R, Coelho-Junior HJ, Bucci C, Marzetti E. Mitochondrial dysfunction, protein misfolding and neuroinflammation in Parkinson's disease:Roads to biomarker discovery. Biomolecules. 2021;11:1508. doi: 10.3390/biom11101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tozzi A, Sciaccaluga M, Loffredo V, Megaro A, Ledonne A, Cardinale A, Federici M, Bellingacci L, Paciotti S, Ferrari E, La Rocca A, Martini A, Mercuri NB, Gardoni F, Picconi B, Ghiglieri V, De Leonibus E, Calabresi P. Dopamine-dependent early synaptic and motor dysfunctions induced by alpha-synuclein in the nigrostriatal circuit. Brain. 2021;144:3477–3491. doi: 10.1093/brain/awab242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang JL, Gao JH, Du TF, Yi HK, Ma KL. Distribution of the alpha-synuclein in the brain and the primary organs of the rhesus monkey. Appl Biochem Biotechnol. 2021;193:3187–3201. doi: 10.1007/s12010-021-03586-w. [DOI] [PubMed] [Google Scholar]
- 13.Yoo G, Yeou S, Son JB, Shin YK, Lee NK. Cooperative inhibition of SNARE-mediated vesicle fusion by alpha-synuclein monomers and oligomers. Sci Rep. 2021;11:10955. doi: 10.1038/s41598-021-90503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


