Keywords: bioinformatics, complement cascade, mass spectrometry, neuroinflammation, proteomics, secondary injury, subacute phase, tandem mass tag, transcription factor, traumatic brain injury
Abstract
Proteomics is a powerful tool that can be used to elucidate the underlying mechanisms of diseases and identify new biomarkers. Therefore, it may also be helpful for understanding the detailed pathological mechanism of traumatic brain injury (TBI). In this study, we performed Tandem Mass Tag-based quantitative analysis of cortical proteome profiles in a mouse model of TBI. Our results showed that there were 302 differentially expressed proteins in TBI mice compared with normal mice 7 days after injury. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway analyses showed that these differentially expressed proteins were predominantly involved in inflammatory responses, including complement and coagulation cascades, as well as chemokine signaling pathways. Subsequent transcription factor analysis revealed that the inflammation-related transcription factors NF-κB1, RelA, IRF1, STAT1, and Spi1 play pivotal roles in the secondary injury that occurs after TBI, which further corroborates the functional enrichment for inflammatory factors. Our results suggest that inflammation-related proteins and inflammatory responses are promising targets for the treatment of TBI.
Introduction
Traumatic brain injury (TBI) is a leading cause of mortality and disability across all age groups and imposes a tremendous burden on families and society (Maas et al., 2017). Increasing evidence shows that TBI is a chronic disease process (Masel and DeWitt, 2010; Gilmore et al., 2020) that is associated with neurodegenerative diseases (Xu et al., 2021) and mental health disorders (Stein et al., 2019). However, to date, no effective treatments are available to promote functional recovery (Ghiam et al., 2021). Therefore, gaining a better understanding of the pathophysiology of TBI is critical to breaking the bottleneck for the treatment of patients with TBI (Dixon, 2017; Thapa et al., 2021).
Omics-based technologies, including genomics, transcriptomics, proteomics, and metabolomics (Samia Hassan, 2019), have revolutionized biomedical research and provided a more holistic perspective of specific biological functions. The aim of omics-based approaches is to collectively identify, characterize, and quantify all phenotype-associated biomolecular changes (Vailati-Riboni et al., 2017; Abu Hamdeh et al., 2021). Proteins are the ultimate executors of physiological processes (Tang et al., 2019) and key indicators of disease states (Williams et al., 2019). Therefore, interrogating changes in protein dynamics, which is essentially the scope of proteomics, provides crucial information for disease-specific diagnosis and treatment (Krzyszczyk et al., 2018). Proteomics, a discipline that has arisen in the post-genomic era, comprises proteome analysis and large-scale systematic analysis of protein expression (Pandey and Mann, 2000; Hristova and Chan, 2019). An individual’s genome is unique and constant, whereas the proteome is diverse and dynamic, which makes proteomics a powerful tool for elucidating the underlying mechanisms of diseases and identifying novel biomarkers.
Due to the complexity of the brain and the heterogeneity of TBI-related pathogenesis (Jassam et al., 2017; Yang et al., 2021), the detailed pathological mechanisms underlying TBI are not completely known (Qin et al., 2021). The TBI field could benefit from the use of proteomics analyses, given the diversity of primary insults and secondary injury-associated processes (Sarkis et al., 2017). Proteomics studies of TBI have involved a variety of species, animal models, tissue samples, time points, and approaches (Xu et al., 2016; Ojo et al., 2018; Pham et al., 2021; Wang et al., 2021b; Zhang et al., 2021). Moreover, technical development in proteomics, such as the tandem mass tag (TMT) labeling approach, can provide novel methods for TBI proteomic investigation (Thompson et al., 2003; Zhang et al., 2014).
The subacute phase, which is often defined as 7 days post-injury, is a turning point in the pathophysiology of TBI (Kaindl et al., 2007; Guglielmetti et al., 2017; Robinson et al., 2017). In the present work, we took advantage of TMT-based proteomics and a mouse model of controlled cortical impact (CCI) to identify critical proteins and biological processes involved in the subacute phase of TBI.
Methods
Animals
Twenty specific pathogen-free male C57BL/6 mice (2 months old) weighing 24–26 g were supplied by Beijing Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China; license No. SCXK (Jing) 2016-0006). All mice were maintained in groups of five mice per cage in a barrier housing facility with a 12/12-hour light/dark cycle. Laboratory rodent chow and tap water were provided ad libitum. All animals in this study were acclimated for at least 2 weeks before any procedures were performed. The mice were randomized into either the CCI (n = 10) or the control group (anesthesia only, n = 10). The study was approved by the Animal Welfare Ethics Committee of Beijing Neurosurgical Institute, Capital Medical University on March 24, 2020 (approval No. 201904011). All experiments were designed and reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (Percie du Sert et al., 2020). During the surgical procedure, a small animal anesthesia machine (RWD Life Science Co., Shenzhen, China) was used for induction or maintenance of general anesthesia via inhalation anesthesia with isoflurane (RWD Life Science Co.) at concentrations of 2% or 1.5%, respectively. The flow chart was shown in Figure 1.
Figure 1.
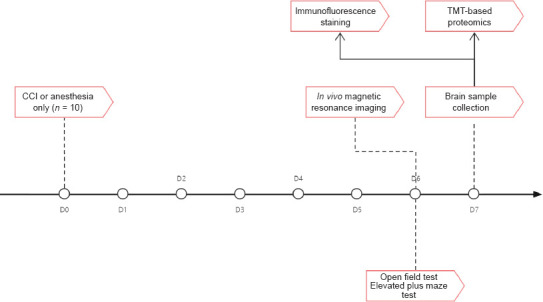
Study flow chart.
CCI: Controlled cortical impact; TMT: tandem mass tag.
CCI model
TBI was induced in mice utilizing a CCI model, as previously described (Smith et al., 1995). To make the mice more comfortable and reduce the risks associated with anesthesia, a temperature maintenance device (RWD Life Science Co.) was used when the mice were under anesthesia. Following loss of consciousness, the head was secured in a stereotaxic instrument (RWD Life Science Co.) that held the head firmly without damaging the skull. After depilation, iodophors were used to sterilize the surgical site. The following procedures were performed using aseptic techniques. A midline scalp incision was performed and the muscles were retracted to expose the calvarium. Then, a 5 mm-diameter craniotomy was performed over the parietal cortex, 2 mm to the right of the midline, between lambda and bregma (Wu et al., 2021), using a dental drill, while keeping the dura undamaged. The PCI3000 PinPoint Precision Cortical Impactor (Hatteras Instruments, Cary, NC, USA) with a 3 mm-diameter impactor tip at a velocity of 3 m/s, depth of 1.5 mm, and dwelling time of 20 ms was used to induce moderate TBI. Finally, the skull defect was repaired with bone wax, and the scalp incision was closed with stitches.
In vivo magnetic resonance imaging
At 6 days post-injury, in vivo magnetic resonance imaging (MRI) was used to confirm the radiological abnormalities characteristic of the subacute stage of TBI. The mice were scanned using a 7.0 Tesla small-bore animal scanner (Bruker Biospec, Ettlingen, Germany). Mice were anesthetized with 2% isoflurane in ambient air, then fastened to an adapter to immobilize the heads. During the imaging, anesthesia was maintained using isoflurane via a nasal catheter, and respiration was monitored using the monitoring system that the scanner is equipped with (Bruker Biospec). T2-weighted structural images were obtained using axial, 2D, T2-TurboRARE sequences. The imaging parameters were as follows: repetition time = 2795 ms, echo time = 35 ms, echo spacing = 11.667 ms, rare factor = 8, field of view = 22 mm × 22 mm, and slice thickness = 0.5 mm.
MRI analysis
MRI is a sensitive and noninvasive tool that can be used to detect lesions in the brain. Brain lesions were defined as focal regions of abnormally hypo- or hyperintense signals in the white or gray matter. Brain lesion volume was measured on the T2-weighted image (T2WI) using a semiautomatic segmentation method. ITK-SNAP (version 3.8.0) (Yushkevich et al., 2006) was used to trace and calculate regions of interest on 25 consecutive axial MRI T2-weighted structural images. First, the structure of interest was defined. Next, the multiple structural images were transformed into a speed image with the voxels inside the lesion and non-lesion having positive and negative speed values, respectively. Then, seeds were placed inside the structure of interest. Last, seeds were expanding to fill the structure of interest and the segmentation was completed. The ITK-SNAP automatically calculates the volume after the segmentation of the structure. All of the MRI images were acquired and analyzed by a researcher who was blinded to the experimental injury conditions.
Behavioral assessment
Small rodents, including mice, have an innate aversion to unfamiliar open fields and intense light (Choleris et al., 2001). Thus, the elevated plus maze (EPM) and open field test (OFT) were used to evaluate anxiety-like behavior in the mice. All behavioral assessments were performed at 6 days post-injury. One hour before the experiment, the mice were transferred into the behavioral testing room for acclimation. The apparatuses were cleaned with 70% ethanol before each test. The tests were performed as described previously (Chen et al., 2021). An overhead night vision camera was used to record the activities of the mice. A blinded investigator carried out all behavioral assessments and data analyses.
Elevated plus maze
The EPM includes a central platform (10 cm × 10 cm), a pair of open arms (50 cm × 10 cm), and a pair of closed arms (50 cm × 10 cm × 10 cm). The apparatus was elevated 50 cm above the ground. The mouse was gently placed in the center of the maze, facing a closed arm. The free and uninterrupted activity of the mouse was recorded for 5 minutes. The time each mouse spent in the open arms and the number of times it entered the open arms were quantified using the SMART video tracking system (SMART v3.0, Panlab, Spain).
Open field test
An opaque plastic container (24 cm × 24 cm × 50 cm) and a lid with a hole in the center to accommodate the night vision camera were used for the OFT. For each trial, a mouse was gently grasped and placed in the middle of the bottom of the container. The mouse was allowed to move freely and uninterruptedly for 5 minutes and was monitored by the camera. The total distance traveled and the time spent in the inner area (8 cm × 8 cm) of the open field were measured using the SMART video tracking system (SMART v3.0).
Sample collection
Brain samples were collected at 7 days post-injury. For TMT-based proteomics, mice were anesthetized and then perfused with cold physiological saline via the left ventricle. The mouse was decapitated after confirmed death, and the entire brain was isolated. Approximately 2 mm of tissue representing the ipsilateral cortices around the injury site was dissected from the CCI mouse brains, and the corresponding part of the control mouse brains were dissected, as described previously (Wang et al., 2021b). The brain samples were placed in cryogenic storage tubes and flash-frozen using liquid nitrogen, after which they were stored at –80°C until further processing.
For immunofluorescence imaging, mice were euthanized and then perfused with cold physiological saline and 4% paraformaldehyde. Next, the cortices were extracted and fixed in 4% paraformaldehyde overnight at 4°C. The next day, the samples were immersed in 30% sucrose in phosphate-buffered saline (PBS) until they sank, at which point they were cut into 20 µm-thick coronal sections.
Protein extraction, digestion, and TMT labeling
The cortices were homogenized in SDT lysis buffer (2% sodium dodecyl sulfate, 100 mM dithiothreitol, 100 mM Tris-HCl, pH 7.6) twice at 6.0 m/s for 30 seconds each time using a FastPrep-24 Classic homogenizer (MP Biomedicals, Irvine, CA, USA). After sonication, the homogenate was incubated in boiling water for 15 minutes and centrifuged at 14,000 × g for 40 minutes. The amount of protein in the filtered supernatant was quantified using a bicinchoninic acid protein assay kit (Beyotime, Shanghai, China). Then, 20 μg of protein from each sample was individually mixed with 6× loading buffer and boiled for 5 minutes. The boiled proteins were separated on a 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and subjected to Coomassie Blue R-250 staining for visualization. Protein digestion was performed using the filter-aided sample preparation method, as previously described (Wiśniewski et al., 2009; Wang et al., 2021a). The samples were labeled as TBI1-126, TBI2-127, TBI3-128, CON1-129, CON2-130, and CON3-131, with three biological replicates per group, using a TMT 6 plex Isobaric Mass Tag Labeling kit (Thermo Fisher Scientific, Waltham, MA, USA). Afterward, the TMT-labeled peptides were fractionated by reverse-phase chromatography using a 1260 Infinity II High Performance Liquid Chromatography apparatus (Agilent Technologies, Santa Clara, CA, USA), as described earlier (Wang et al., 2021a).
Mass spectrometry analysis
All fractions were injected for nano-liquid chromatography-tandem mass spectrometry analysis. The mixture of peptides was loaded onto a C18 reverse-phase analytical column (Thermo Fisher Scientific) in buffer A (0.1% formic acid) and diluted with a linear gradient of buffer B (0.1% formic acid and 80% acetonitrile) at a flow velocity of 300 nL/min.
A Q Exactive Plus mass spectrometer was used, coupled to an Easy nLC system (Thermo Fisher Scientific) and manipulated in positive ion mode. Mass spectrometry (MS) data were acquired via a data-dependent top 10 method that dynamically chose the most abundant precursor ions from the survey scan for higher-energy collisional dissociation fragmentation. The parameters of the survey scans were as follows: resolution = 70,000, AGC target = 3e6, maximum injection time = 50 ms. The parameters of the MS2 scans were as follows: resolution = 17,500, AGC target = 3e6, maximum injection time = 45 ms, isolation width = 2 m/z. Only ions with a minimum intensity of 2e3 and a charge state between 2 and 6 were chosen for fragmentation.
Raw data were processed using the MASCOT engine 2.6 (Matrix Science, London, UK) embedded in the Proteome Discoverer 2.2 software program (Thermo Fisher Scientific). Protein sequences for Mus musculus were collected from the UniProt database. The principal parameters were set as follows: max missed cleavages = 2; precursor mass tolerance = ± 10 ppm; fragment mass tolerance = 0.05 Da; and peptide false discovery rate ≤ 0.01. Differentially expressed proteins (DEPs) were defined as those exhibiting a fold change > 1.2 for upregulation and < 0.83 for downregulation at P < 0.05, in accordance with previous studies (Casey et al., 2017; Song et al., 2019).
Bioinformatics analysis
Gene Ontology (GO) was used to annotate the functions of the DEPs in three categories, namely, biological process, cellular component, and molecular function (Ashburner et al., 2000). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was applied to identify signal transduction pathways and biochemical metabolic pathways related to the DEPs (Kanehisa and Goto, 2000). The open-access platform Metascape (https://metascape.org) was used to perform the GO and KEGG analyses. The Benjamini-Hochberg procedure was performed to compute false discovery rates to correct for multiple comparisons. A false discovery rate of less than 0.05 was considered significant enrichment. The DEP volcano plot and heatmap were graphed using R software (https://www.r-project.org/). The volcano plot was generated based on log2(fold change) at the x-axis and -log10(p-value) at the y-axis.
The Animal Transcription Factor DataBase (AnimalTFDB, http://bioinfo.life.hust.edu.cn/AnimalTFDB/#!/) was used to annotate transcription factors (TFs) (Hu et al., 2019). Differentially expressed genes (DEGs) and TFs after TBI were imported into the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING; https://string-db.org) database to identify protein-protein interactions (PPIs). The open-source software Cytoscape (https://cytoscape.org) was used to visualize the PPI network (Shannon et al., 2003). The cytoHubba plugin in Cytoscape was used to predict and discover hub genes and hub TFs with the highest node degrees via the Degree method, which is a local-based topological analysis method provided by cytoHubba (Chin et al., 2014). TF regulatory networks were visualized using Cytoscape software. For all TMT-based proteomics analyses, the investigator was blinded to the raw data.
Immunofluorescence staining
Immunofluorescence staining was performed on the sections taken from CCI and control mice as described previously (Henry et al., 2020). Brain tissue located 0.5 mm to –2.5 mm from the bregma based on stereotaxic coordinates was dissected out and sectioned. After washing three times in PBS, the sections were blocked in goat serum (Zhongshan Goldenbridge Biotechnology, Beijing, China) for 1 hour at room temperature, and then incubated overnight at 4°C in the primary antibodies, including rabbit anti-C3 (1:300, Proteintech, Rosemont, IL, USA, Cat# 21337-1-AP, RRID: AB_2878843) and rabbit anti-Iba1 (official symbol: allograft inflammatory factor 1, Aif1; 1:500, Wako Chemicals, Richmond, VA, USA, Cat# 019-19741, RRID: AB_839504). After 24 hours, the sections were washed with PBS three times and incubated in Cy3-conjugated goat anti-rabbit IgG (1:300, Abcam, Cambridge, UK, Cat# ab97080, RRID: AB_10679808) at room temperature for 1 hour to visualize the target antigens. Finally, the sections were washed with PBS three times, counterstained with 4,6-diamidino-2-phenylindole (Thermo Fisher Scientific), and mounted with coverslips. The sections were then imaged using a ZEISS/LSM 880 confocal laser scanning microscope (Carl Zeiss AG, Oberkochen, Germany). ImageJ software (National Institutes of Health, Bethesda, MD USA) (Schneider et al., 2012) was used to quantify the immunofluorescence signal intensities by a blinded investigator.
Statistical analysis
The statistics program GraphPad Prism 8.0.2 (GraphPad Software, San Diego, CA, USA, www.graphpad.com) was used for statistical analyses and graphical representations. The variance in lesion volumes on MRI was measured using relative standard deviation. For the behavioral assessment and immunofluorescence imaging, differences were analyzed by unpaired t-test to compare the control and CCI groups. Differences in the average expression levels of a given protein between two groups were assessed by independent two-sample t-test. Data are presented as mean ± standard error of the mean (SEM) and coefficient of variation. For all comparisons, the statistical significance was set as a P-value of less than 0.05.
Results
Brain lesions on T2-weighted MRI after CCI
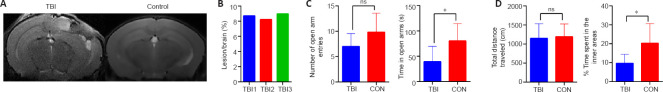
Brain abnormalities were investigated by T2WI at 6 days post-injury. As illustrated in Figure 2A, abnormal hypo- or hyperintensity was detected at the injury site on T2WI. Compared with controls, CCI caused brain edema and structural abnormalities in the ipsilateral hemisphere, including the cortex, hippocampus, corpus callosum, and lateral ventricles. No evidence of pronounced midline shift was found on T2WI in CCI mice. There was minimal variability in the lesion volumes of the three CCI mice used for proteomic analysis (8.637 ± 0.219 mm3, coefficient of variation = 4.381%; Figure 2B), indicating consistency among the biological replicates and the reliability of the proteomics data.
Figure 2.
Neuroimaging and neurobehavioral alterations at 6 days post-TBI.
(A) Representative T2-weighted imaging of TBI (left) and control (right) mice at 6 days post-TBI. Compared with the control mice, the TBI mice exhibited brain edema and structural abnormalities in the ipsilateral hemisphere. (B) Quantification of lesion volume on T2-weighted imaging in TBI mice used for proteomic analysis. (C) Behavioral alteration was evaluated by the elevated plus maze test 6 days after injury for a 5-minute period. (D) Behavioral alteration was evaluated by the open field test 6 days after injury for a 5-minute period. Data are expressed as mean ± SEM (n = 5–9 mice/group). *P < 0.05 (unpaired t-test). CON: Control; ns: not significant; TBI: traumatic brain injury.
Behavioral changes after CCI
The anxiety levels of mice are inversely proportional to the time spent in the open arms of the EPM or the inner area during the OFT (Shavit-Stein et al., 2021). CCI animals spent significantly less time in the open arms of the EPM compared with the control group (39.31± 10.13 seconds vs. 80.15 ± 15.32 seconds, P = 0.0397; Figure 2C). The difference between the two groups in terms of the number of times the mice entered the open arms fell just short of statistical significance (7.000 ± 0.8498 vs. 9.800 ± 1.685, P = 0.1213). In the OFT, CCI mice spent significantly less time in the inner area compared with control mice (9.68 ± 1.68% vs. 20.26 ± 4.65%, P = 0.0277; Figure 2D). However, the total traveled distance in the OFT did not differ between the groups (1148 ± 135.9 cm vs. 1191 ± 150.3 cm, P = 0.8391). Altogether, these results indicated that TBI in the subacute stage increased anxiety-like behavior, as demonstrated by a decreased risk-taking behavior in an unfamiliar environment.
Quantitative proteomic analysis of cortical proteome profiles after CCI
To gain a detailed understanding of the molecular changes that occur during the subacute stage of TBI, the proteomic profiles in six mouse cortex samples from the CCI and control groups were analyzed using TMT 6-plex tagging and LC-MS/MS. Principal component analysis was performed to estimate variations between and within groups. The principal component analysis plot demonstrated a clear distinction between groups and consistency within each group (Figure 3A).
Figure 3.
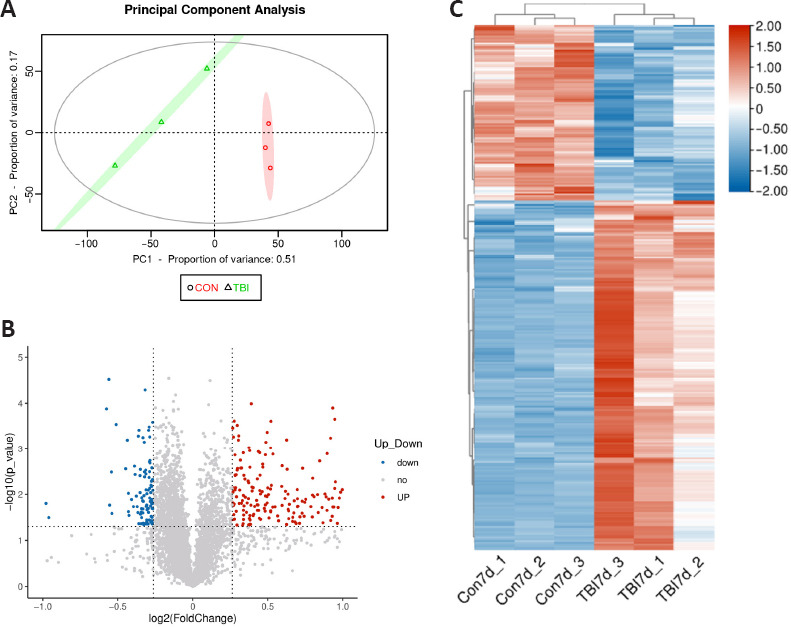
Analysis of cortical DEPs in TBI and control mice.
(A) Principal component analysis plot generated from the proteomics analysis results for the TBI (triangles) and control (circles) mice. (B) Volcano plot of DEPs between the TBI and control mice. Blue, gray, and red dots indicate downregulated, unchanged, or upregulated proteins following controlled cortical impact, respectively. (C) Heatmap of DEPs between the TBI and control mice. The color scale indicates significant differences in the levels of protein expression. CON: Control; DEP: differentially expressed protein; TBI: traumatic brain injury.
We identified and quantified a total of 5738 proteins in cortex tissues 7 days post-TBI via large-scale proteomics analysis (Additional Table 1 (8.7MB, pdf) ). Twenty of these proteins were upregulated, and 100 were downregulated. A volcano plot of the DEPs is shown in Figure 3B. A heatmap of the DEPs showed distinct protein profiles for the CCI and control samples (Figure 3C). Hierarchical clustering analysis demonstrated a trend toward increased or decreased protein expression following subacute TBI. These results demonstrate that protein expression profiles are significantly altered in the subacute phase (7 days) following CCI. Additionally, the majority of the DEPs appeared consistently within all members of a single group, which indicated good reproducibility.
GO functional classification of DEPs after CCI
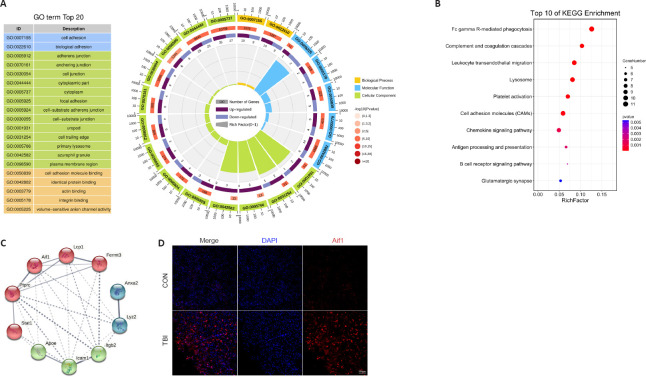
The enrichment analysis was performed to determine the function of the DEPs according to the three aspects mentioned above. The 20 most significantly enriched GO terms, consisting of two biological processes, five molecular functions, and thirteen cellular components, are shown in Figure 4A. Cell adhesion and biological adhesion were the most overrepresented biological processes. The significantly enriched molecular functions comprised cell adhesion molecule binding, identical protein binding, actin-binding, integrin binding, and volume-sensitive anion channel activity. As for the cellular component annotation, the significantly enriched terms included adherens junction, anchoring junction, cell junction, focal adhesion, cell-substrate adherens junction, cell-substrate junction, and more. GO enrichment analysis of the DEPs illustrated that these proteins are related to the immune system and inflammation-related processes.
Figure 4.
Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and protein-protein interaction (PPI) analysis of cortical DEPs between the TBI and control mice.
(A) GO functional enrichment analysis of DEPs at 7 days post-TBI. (B) KEGG pathway analysis of DEPs at 7 days post-TBI. (C) PPI network analysis of DEPs at 7 days post-TBI. (D) Representative immunofluorescence images of Aif1 staining (red, stained with Cy3) in the ipsilateral cortex from the TBI and control mice. Aif1 expression was significantly higher in the TBI mice compared with the control mice. The nuclei were stained with DAPI (blue). Scale bar: 100 μm. CON: Control; DAPI: 4,6-diamidino-2-phenylindole; DEP: differentially expressed protein; TBI: traumatic brain injury.
KEGG pathway analysis of DEPs after CCI
A pathway represents the sequence of chemical reactions in the cell that causes a particular biological effect (Schmidt et al., 2014). KEGG pathway analysis demonstrated that the significantly enriched pathways in the CCI group were mainly involved in inflammation (Figure 4B). Among the identified KEGG pathways, Fc gamma R-mediated phagocytosis exhibited the highest statistical significance and the largest number of genes, including ASAP2, FCGR2, FCGR1, RAC2, and LYN. Additionally, inflammation-related pathways such as complement and coagulation cascades, leukocyte transendothelial migration, antigen processing and presentation, and the B cell receptor signaling pathway were significantly enriched and share proteins with altered expression levels such as RAC2, FCGR2, and LYN. Other noticeably enriched pathways included lysosome, platelet activation, cell adhesion molecules, chemokine signaling pathway, and glutamatergic synapse. Taken together, KEGG pathway analysis of the DEPs revealed that these proteins are mainly involved in inflammatory signaling pathways, which was consistent with the GO annotation results.
PPI network construction and identification of hub genes based on DEPs after CCI
PPI networks are interaction networks that represent the high connectivity and complexity of hub proteins. PPI network analysis of the DEPs showed direct and indirect interconnections among 302 DEPs. Based on the generated PPI network, the ten most highly connected hub genes, which are the significant nodes in PPI networks and represent DEGs with a large number of associations with other DEGs (Chen et al., 2020a), were identified (Figure 4C). Among these hub genes, Lyz2 exhibited the most significant difference between the CCI and control groups. GO and KEGG analyses demonstrated that the hub genes, such as Itgb2, Icam1, Fermt3, Lcp1, Aif1, and Ptprc, were primarily associated with inflammatory processes and complement systems. Immunofluorescence analysis further confirmed the significant upregulation of Aif1 in the CCI group compared with the control group (Figure 4D).
TF regulatory network analysis
TFs control the initiation of gene transcription, regulate gene expression, and play an essential role in almost all biological processes (Lambert et al., 2018; Mitsis et al., 2020). Therefore, TBI-related alterations in TF expression were investigated to gain better insight into the pathophysiologic mechanism of TBI. The nine most connected hub TFs were identified from the PPI networks: NF-κB1, RelA, STAT1, IRF1, CEBPA, Spi1, E2F1, SP1, and NFYA. Next, genes upregulated and downregulated by the hub TFs were predicted and used to construct a TF regulatory network that included 46 edges, nine TFs, and 27 target genes (Figure 5). Among the identified hub TFs, NF-κB1, RelA, STAT1, IRF1, and Spi1 played pivotal roles in the inflammatory response.
Figure 5.
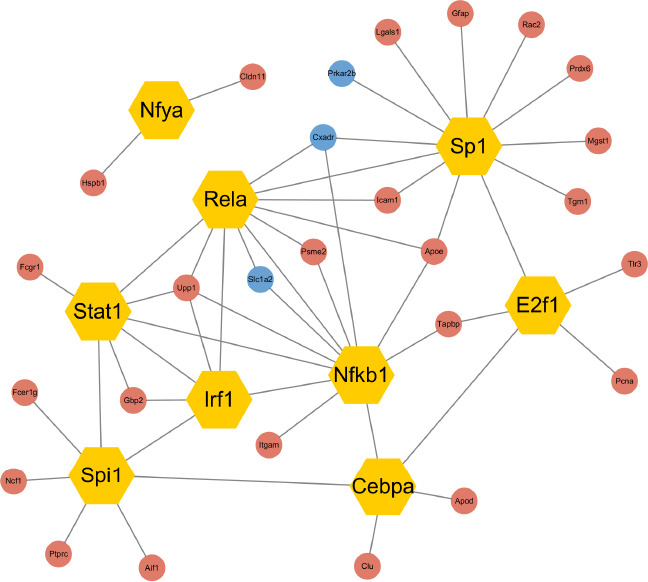
Regulatory network analysis of transcription factors that were differentially expressed in TBI and control mice.
Regulatory transcription factor interactions with corresponding target genes are illustrated. TFs are shown as hexagonal nodes. Up- or downregulated target genes are shown as red or blue circular nodes, respectively. CON: Control; TBI: traumatic brain injury.
Complement C3 is upregulated following TBI, as detected by immunofluorescence
The functional analyses described above suggested that the complement cascade may exert a detrimental effect in the context of TBI. To confirm this finding, the expression of the hub protein complement C3, which is a central molecule in the complement cascade and the inflammatory response (Ricklin et al., 2016), was investigated by immunofluorescence. There was a highly statistically significant increase in total C3 protein immunopositivity in cortices from mice 7 days post-TBI compared with control mice (P < 0.001; Figure 6), which verified the bioinformatics-based functional analysis.
Figure 6.
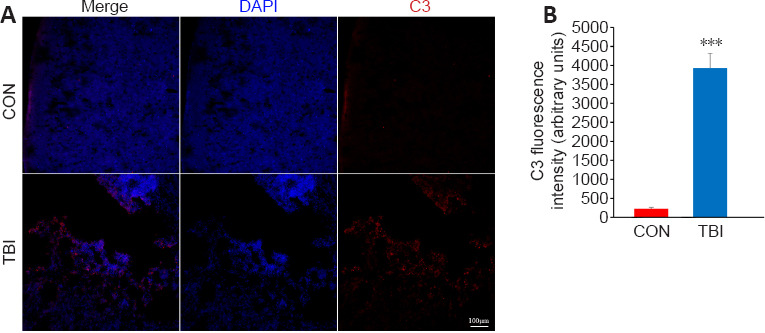
Validation of the differential expression of the identified hub protein C3 in the cortex between TBI and control mice by immunofluorescence staining.
(A) Representative immunofluorescence images of C3 staining (red, stained by Cy3) in the ipsilateral cortex from the TBI and control mice. C3 immunopositivity was significantly increased in the TBI mice compared with the control mice. The nuclei were stained with DAPI (blue). Scale bar: 100 μm. (B) Quantification of C3 immunofluorescence intensity. Data are expressed as mean ± SEM (n = 5 mice/group). ***P < 0.001 (unpaired t-test). CON: Control; DAPI: 4, 6-diamidino-2-phenylindole; TBI: traumatic brain injury; TF: transcription factor.
Discussion
Despite the progress that has been made in the management of TBI, its physiopathology is not completely understood, especially at the protein level (Sempere et al., 2019). To provide new insights into the nature and progression of TBI, in this study we performed global proteomic profiling in the well-established murine CCI model of TBI using TMT-based quantitative proteomics. The results showed that TBI evoked dramatic changes in the protein expression profile that were predominantly associated with inflammatory responses, including complement and coagulation cascades. Furthermore, we identified several inflammation-related TFs that may account for TBI-induced dysfunctional biologic processes. Although proteomic alteration following CCI was previously investigated, the present study differed from published studies in terms of the model species (Pumiglia et al., 2021), biosamples (Thelin et al., 2018), time points (Zhou et al., 2020), and approaches (Kobeissy et al., 2016). Use of the TMT-labeling approach has increased progressively because of its high throughput and excellent precision (Rauniyar and Yates, 2014; Zhang et al., 2014). TMT-based proteomics has been used to analyze protein profiles following blast-induced or weight drop-induced TBI (Song et al., 2019; Wang et al., 2021b). In addition, TMT-labeling proteomics has been used in a rat CCI model of TBI (Zhou et al., 2020). Our study adds to this body of research by focusing on TMT-based analysis of secondary injury in a mouse model of subacute CCI.
The rapid development of omics technologies, such as transcriptomics and proteomics, has enabled high-throughput analysis of TBI-induced molecular biological alterations to be performed (Abu Hamdeh et al., 2021). Transcriptomic studies of TBI have revealed distinct changes in gene expression that are associated with multiple biological processes and provide potential targets for treatment (Samal et al., 2015; Lipponen et al., 2016; Chakraborty et al., 2021). Nevertheless, transcriptomics is limited in that it provides information about the mechanisms of diseases at the DNA and RNA levels, but not specifically at the protein level (Denny and Kalesh, 2020). As proteins serve as the primary functional effector molecules in cells, proteomics can offer a more comprehensive understanding of molecular and cellular events in disease development. Several proteins involved in underlying pathophysiological processes have been identified using the proteomics technique following experimental or clinical TBI (Xu et al., 2016; Liang et al., 2019; Wang et al., 2021b; Zhang et al., 2021). However, these studies mainly focused on acute proteome alterations following TBI. Our study aimed to reveal the cortex proteomic profile in mice in the subacute stage of TBI. Because TBI is a progressive disease, and its pathophysiology varies in different stages, understanding the multitemporal pathophysiological cascade could expand the therapeutic window for TBI and help provide stage-specific therapy.
The physiopathology of TBI comprises irreversible primary injuries and secondary injuries, both of which serve as therapeutic targets (Thapa et al., 2021). Hence, understanding secondary brain injuries has become a principal concern in TBI. Neuroinflammation is considered a significant and manageable aspect of secondary injuries following TBI (Corrigan et al., 2016). TBI-induced inflammation may have detrimental or beneficial effects (Simon et al., 2017). On one hand, neuroinflammation contributes to clearances of necrotic debris, neural regeneration, repair of neuronal circuits, and reconstruction of the central nervous system barrier (Jassam et al., 2017; Clark et al., 2019; Bodnar et al., 2021). On the other hand, neuroinflammation plays a significant role in neuronal death, neuronal circuit destruction, central nervous system barriers disruption, post-traumatic epilepsy, and progressive neurodegeneration (Chen et al., 2018a; Shi et al., 2019; Bodnar, 2021; Pham et al., 2021). In addition, following TBI-induced neuroinflammation, inflammatory mediators cause systemic inflammatory responses in other organs, such as the lungs, kidneys, and intestines (Sabet et al., 2021). In addition, inflammation can alter coagulation following TBI (Maegele et al., 2020). The goal of immunomodulatory therapies for TBI is to constrain the acute inflammatory response and discontinue chronic progressive neuroinflammation (Simon et al., 2017). There is a great need to identify the underlying mechanisms of TBI-induced inflammation, as well as corresponding biomarkers and therapeutic targets.
The complement system is pivotal for multiple biological progress and plays a crucial role in the neuroinflammatory response (Dinet et al., 2019; Ziabska et al., 2021). Following TBI, dysfunctional complement cascades cause secondary damage, epileptogenesis, synaptic degeneration, and cognitive deficit (Chen et al., 2020b; Alawieh et al., 2021; Mallah et al., 2021). Moreover, the complement system interferes with the coagulation cascade and homeostasis, which are closely related to TBI patient outcomes (Ricklin et al., 2010; Fletcher-Sandersjöö et al., 2020). However, the complement system also promotes neuroprotection and regeneration after TBI (Hammad et al., 2018), which suggests that the complement system is a double-edged sword in TBI pathophysiology (Ziabska et al., 2021). A better understanding of the complement system could open up new avenues for stage-specific management of TBI.
As the pivotal regulators of gene expression, TFs play crucial roles in complex biological processes (Lee and Young, 2013; Zhang et al., 2020). TBI could disrupt transcription factor activity, which in turn may exacerbate brain injury by mediating secondary injuries, including inflammation (Kumar et al., 2020). In this study we found that pro-inflammatory TFs, including NF-κB1, RelA, STAT1, IRF1, and Spi1, exert a notable influence on secondary injury after TBI. NF-κB1 and RelA are two of the five members of the NF-κB transcription factor family, which is a critical mediator of various biological processes such as inflammation and cell survival (Liu et al., 2017; Kumar et al., 2020). The upregulation of cortical NF-κB expression was detected in experimental and clinical TBI (Yang et al., 1995; Hang et al., 2006). Lian et al. (2012) revealed that upregulated NF-κB in astrocytes following TBI was associated with impaired inflammatory responses and worsened damage, including blood-brain barrier dysfunction and increased lesion volumes. Furthermore, Mettang et al. (2018) showed that NF-κB inhibition in neurons was associated with poorer outcomes in TBI mice. These studies indicate that NF-κB activation in neurons following TBI is beneficial, and NF-κB activation in glial cells is detrimental. In addition, activation of the HMGB1/NF-κB signaling pathway can block TBI-induced neuroinflammation (Chen et al., 2018b). Taken together, these findings suggest that NF-κB could be a potential target for TBI treatment. In addition, our earlier transcriptomic study revealed that the STAT and IRF families of TFs play critical roles in the cellular and molecular events that occur following TBI, which is consistent with the findings from the current proteomics study (Yang et al., 2021). Developing precise stage-specific therapy for TBI will require a better knowledge of the relevant TFs (Kumar et al., 2020).
There were some limitations to our study. Firstly, the sample size was small, because of both financial and ethical considerations. Secondly, given that this was a cross-sectional study that assessed one specific point in time (7 days post-TBI), we were unable to explore the possibility of a temporal relationship between TBI and neuroinflammation or complement. Additional studies involving more time points are needed to clarify the role of neuroinflammation and complement in TBI. Finally, the TMT proteomics analysis carried out as part of this study was limited to the ipsilateral cortex. Hence, future studies should validate these alterations and investigate potential changes in different brain regions. We used male mice, in accordance with a previous proteomic study (Nebie et al., 2021). Female laboratory rodents have an estrous cycle that lasts for four or five days, which is regarded as a quicker version of the human reproductive cycle (Becker et al., 2017). If female mice are at different stages in their estrous cycle, their responses during behavioral assessments will vary significantly (Estrela et al., 2021). Gender bias has long been present in animal studies, and studies that include both male and female animals are advocated (Beery and Zucker, 2011). Thus, in future studies we will consider sex as a critical variable.
In summary, TBI is a biologically complex progressive disease, and its pathophysiology is far from clear. Proteomics is an ideal tool for illustrating the mechanism of TBI at the molecular and cellular levels. In this study, we used TMT-labeled proteomics and bioinformatics to identify the hub genes, pathways, and TFs in the subacute phase of TBI. The results indicated that inflammation-related biological processes, including the complement cascade, may increase anxiety after TBI. Having a comprehensive understanding of the neuroinflammatory responses that occur in TBI would advance the management of this condition.
Additional files:
Additional Table 1 (8.7MB, pdf) : The identified proteins in 7 days post-TBI cortex tissues using a large-scale proteomics investigation.
The identified proteins in 7 days post-TBI cortex tissues using a large-scale proteomics investigation
Additional file 1: Open peer review report 1 (78.8KB, pdf) .
Acknowledgments:
We would like to thank Dr. Zhong-Fang Shi from Beijing Neurosurgical Institute for her generous technical assistance
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81771327, and a grant for the Platform Construction of Basic Research and Clinical Translation of Nervous System Injury, China, No. PXM2020_026280_000002 (both to BYL).
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open peer reviewer: Renata Ciccarelli, University of Chieti-Pescara, Italy.
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
References
- 1.Abu Hamdeh S, Tenovuo O, Peul W, Marklund N. “Omics”in traumatic brain injury:novel approaches to a complex disease. Acta Neurochir (Wien) 2021;163:2581–2594. doi: 10.1007/s00701-021-04928-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alawieh A, Chalhoub RM, Mallah K, Langley EF, York M, Broome H, Couch C, Adkins D, Tomlinson S. Complement drives synaptic degeneration and progressive cognitive decline in the chronic phase after traumatic brain injury. J Neurosci. 2021;41:1830–1843. doi: 10.1523/JNEUROSCI.1734-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology:tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker JB, McClellan ML, Reed BG. Sex differences, gender and addiction. J Neurosci Res. 2017;95:136–147. doi: 10.1002/jnr.23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodnar CN, Watson JB, Higgins EK, Quan N, Bachstetter AD. Inflammatory regulation of CNS barriers after traumatic brain injury:a tale directed by interleukin-1. Front Immunol. 2021;12:688254. doi: 10.3389/fimmu.2021.688254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey TM, Khan JM, Bringans SD, Koudelka T, Takle PS, Downs RA, Livk A, Syme RA, Tan KC, Lipscombe RJ. Analysis of reproducibility of proteome coverage and quantitation using Isobaric Mass Tags (iTRAQ and TMT) J Proteome Res. 2017;16:384–392. doi: 10.1021/acs.jproteome.5b01154. [DOI] [PubMed] [Google Scholar]
- 8.Chakraborty N, Hammamieh R, Gautam A, Miller SA, Condlin ML, Jett M, Scrimgeour AG. TBI weight-drop model with variable impact heights differentially perturbs hippocampus-cerebellum specific transcriptomic profile. Exp Neurol. 2021;335:113516. doi: 10.1016/j.expneurol.2020.113516. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Chen Y, Olivero A, Chen X. Identification and validation of potential biomarkers and their functions in acute kidney injury. Front Genet. 2020a;11:411. doi: 10.3389/fgene.2020.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018a;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Liu K, Wang Y, Liu N, Yao M, Hu J, Wang G, Sun Y, Pan J. Phosphodiesterase-2 inhibitor reverses post-traumatic stress induced fear memory deficits and behavioral changes via cAMP/cGMP pathway. Eur J Pharmacol. 2021;891:173768. doi: 10.1016/j.ejphar.2020.173768. [DOI] [PubMed] [Google Scholar]
- 12.Chen M, Edwards SR, Reutens DC. Complement in the development of post-traumatic epilepsy:prospects for drug repurposing. J Neurotrauma. 2020b;37:692–705. doi: 10.1089/neu.2019.6942. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Chen C, Fan S, Wu S, Yang F, Fang Z, Fu H, Li Y. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-κB pathway following experimental traumatic brain injury. J Neuroinflammation. 2018b;15:116. doi: 10.1186/s12974-018-1151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba:identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(Suppl 4):S11. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test:effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- 16.Clark DPQ, Perreau VM, Shultz SR, Brady RD, Lei E, Dixit S, Taylor JM, Beart PM, Boon WC. Inflammation in traumatic brain injury:roles for toxic A1 astrocytes and microglial-astrocytic crosstalk. Neurochem Res. 2019;44:1410–1424. doi: 10.1007/s11064-019-02721-8. [DOI] [PubMed] [Google Scholar]
- 17.Corrigan F, Mander KA, Leonard AV, Vink R. Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation. J Neuroinflammation. 2016;13:264. doi: 10.1186/s12974-016-0738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denny PW, Kalesh K. How can proteomics overhaul our understanding of Leishmania biology? Expert Rev Proteomics. 2020;17:789–792. doi: 10.1080/14789450.2020.1885375. [DOI] [PubMed] [Google Scholar]
- 19.Dinet V, Petry KG, Badaut J. Brain-immune interactions and neuroinflammation after traumatic brain injury. Front Neurosci. 2019;13:1178. doi: 10.3389/fnins.2019.01178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon KJ. Pathophysiology of traumatic brain injury. Phys Med Rehabil Clin N Am. 2017;28:215–225. doi: 10.1016/j.pmr.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Estrela FN, Guimarães ATB, Araújo A, Silva FG, Luz TMD, Silva AM, Pereira PS, Malafaia G. Toxicity of polystyrene nanoplastics and zinc oxide to mice. Chemosphere. 2021;271:129476. doi: 10.1016/j.chemosphere.2020.129476. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher-Sandersjöö A, Maegele M, Bellander BM. Does complement-mediated hemostatic disturbance occur in traumatic brain injury?A literature review and observational study protocol. Int J Mol Sci. 2020;21:1596. doi: 10.3390/ijms21051596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghiam MK, Patel SD, Hoffer A, Selman WR, Hoffer BJ, Hoffer ME. Drug repurposing in the treatment of traumatic brain injury. Front Neurosci. 2021;15:635483. doi: 10.3389/fnins.2021.635483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilmore CS, Lim KO, Garvin MK, Wang JK, Ledolter J, Fenske AL, Gentz CL, Nellis J, Armstrong MT, Kardon RH. Association of optical coherence tomography with longitudinal neurodegeneration in veterans with chronic mild traumatic brain injury. JAMA Netw Open. 2020;3:e2030824. doi: 10.1001/jamanetworkopen.2020.30824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guglielmetti C, Chou A, Krukowski K, Najac C, Feng X, Riparip LK, Rosi S, Chaumeil MM. In vivo metabolic imaging of traumatic brain injury. Sci Rep. 2017;7:17525. doi: 10.1038/s41598-017-17758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammad A, Westacott L, Zaben M. The role of the complement system in traumatic brain injury:a review. J Neuroinflammation. 2018;15:24. doi: 10.1186/s12974-018-1066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hang CH, Chen G, Shi JX, Zhang X, Li JS. Cortical expression of nuclear factor kappaB after human brain contusion. Brain Res. 2006;1109:14–21. doi: 10.1016/j.brainres.2006.06.045. [DOI] [PubMed] [Google Scholar]
- 28.Henry RJ, Ritzel RM, Barrett JP, Doran SJ, Jiao Y, Leach JB, Szeto GL, Wu J, Stoica BA, Faden AI, Loane DJ. Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits. J Neurosci. 2020;40:2960–2974. doi: 10.1523/JNEUROSCI.2402-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hristova VA, Chan DW. Cancer biomarker discovery and translation:proteomics and beyond. Expert Rev Proteomics. 2019;16:93–103. doi: 10.1080/14789450.2019.1559062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu H, Miao YR, Jia LH, Yu QY, Zhang Q, Guo AY. AnimalTFDB 3.0:a comprehensive resource for annotation and prediction of animal transcription factors. Nucleic Acids Res. 2019;47:D33–D38. doi: 10.1093/nar/gky822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of traumatic brain injury:time for a paradigm shift. Neuron. 2017;95:1246–1265. doi: 10.1016/j.neuron.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaindl AM, Zabel C, Stefovska V, Lehnert R, Sifringer M, Klose J, Ikonomidou C. Subacute proteome changes following traumatic injury of the developing brain:Implications for a dysregulation of neuronal migration and neurite arborization. Proteomics Clin Appl. 2007;1:640–649. doi: 10.1002/prca.200600696. [DOI] [PubMed] [Google Scholar]
- 33.Kanehisa M, Goto S. KEGG:kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobeissy FH, Guingab-Cagmat JD, Zhang Z, Moghieb A, Glushakova OY, Mondello S, BouttéAM, Anagli J, Rubenstein R, Bahmad H, Wagner AK, Hayes RL, Wang KK. Neuroproteomics and systems biology approach to identify temporal biomarker changes post experimental traumatic brain injury in rats. Front Neurol. 2016;7:198. doi: 10.3389/fneur.2016.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krzyszczyk P, Acevedo A, Davidoff EJ, Timmins LM, Marrero-Berrios I, Patel M, White C, Lowe C, Sherba JJ, Hartmanshenn C, O'Neill KM, Balter ML, Fritz ZR, Androulakis IP, Schloss RS, Yarmush ML. The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci) 2018;6:79–100. doi: 10.1142/S2339547818300020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar S, Fritz Z, Sulakhiya K, Theis T, Berthiaume F. Transcriptional factors and protein biomarkers as target therapeutics in traumatic spinal cord and brain injury. Curr Neuropharmacol. 2020;18:1092–1105. doi: 10.2174/1570159X18666200522203542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert SA, Jolma A, Campitelli LF, Das PK, Yin Y, Albu M, Chen X, Taipale J, Hughes TR, Weirauch MT. The human transcription factors. Cell. 2018;172:650–665. doi: 10.1016/j.cell.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Lee TI, Young RA. Transcriptional regulation and its misregulation in disease. Cell. 2013;152:1237–1251. doi: 10.1016/j.cell.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lian H, Shim DJ, Gaddam SS, Rodriguez-Rivera J, Bitner BR, Pautler RG, Robertson CS, Zheng H. IκBαdeficiency in brain leads to elevated basal neuroinflammation and attenuated response following traumatic brain injury:implications for functional recovery. Mol Neurodegener. 2012;7:47. doi: 10.1186/1750-1326-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang Y, Tong F, Zhang L, Zhu L, Li W, Huang W, Zhao S, He G, Zhou Y. iTRAQ-based proteomic analysis discovers potential biomarkers of diffuse axonal injury in rats. Brain Res Bull. 2019;153:289–304. doi: 10.1016/j.brainresbull.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Lipponen A, Paananen J, Puhakka N, Pitkänen A. Analysis of post-traumatic brain injury gene expression signature reveals Tubulins, Nf|ne2l2, Nfkb, Cd44, and S100a4 as treatment targets. Sci Rep. 2016;6:31570. doi: 10.1038/srep31570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Büki A, Chesnut RM, Citerio G, Coburn M, Cooper DJ, Crowder AT, Czeiter E, Czosnyka M, Diaz-Arrastia R, Dreier JP, Duhaime AC, Ercole A, et al. Traumatic brain injury:integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 44.Maegele M, Aversa J, Marsee MK, McCauley R, Chitta SH, Vyakaranam S, Walsh M. Changes in coagulation following brain injury. Semin Thromb Hemost. 2020;46:155–166. doi: 10.1055/s-0040-1702178. [DOI] [PubMed] [Google Scholar]
- 45.Mallah K, Couch C, Alshareef M, Borucki D, Yang X, Alawieh A, Tomlinson S. Complement mediates neuroinflammation and cognitive decline at extended chronic time points after traumatic brain injury. Acta Neuropathol Commun. 2021;9:72. doi: 10.1186/s40478-021-01179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masel BE, DeWitt DS. Traumatic brain injury:a disease process, not an event. J Neurotrauma. 2010;27:1529–1540. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- 47.Mettang M, Reichel SN, Lattke M, Palmer A, Abaei A, Rasche V, Huber-Lang M, Baumann B, Wirth T. IKK2/NF-κB signaling protects neurons after traumatic brain injury. FASEB J. 2018;32:1916–1932. doi: 10.1096/fj.201700826R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitsis T, Efthimiadou A, Bacopoulou F, Vlachakis D, Chrousos GP, Eliopoulos E. Transcription factors and evolution:an integral part of gene expression (Review) World Acad Sci J. 2020;2:3–8. [Google Scholar]
- 49.Nebie O, Carvalho K, Barro L, Delila L, Faivre E, Renn TY, Chou ML, Wu YW, Nyam-Erdene A, Chou SY, Buée L, Hu CJ, Peng CW, Devos D, Blum D, Burnouf T. Human platelet lysate biotherapy for traumatic brain injury:preclinical assessment. Brain. 2021;144:3142–3158. doi: 10.1093/brain/awab205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ojo JO, Crynen G, Reed JM, Ajoy R, Vallabhaneni P, Algamal M, Leary P, Rafi NG, Mouzon B, Mullan M, Crawford F. Unbiased proteomic approach identifies unique and coincidental plasma biomarkers in repetitive mTBI and AD pathogenesis. Front Aging Neurosci. 2018;10:405. doi: 10.3389/fnagi.2018.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pandey A, Mann M. Proteomics to study genes and genomes. Nature. 2000;405:837–846. doi: 10.1038/35015709. [DOI] [PubMed] [Google Scholar]
- 52.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, et al. The ARRIVE guidelines 2.0:Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pham L, Wright DK, O'Brien WT, Bain J, Huang C, Sun M, Casillas-Espinosa PM, Shah AD, Schittenhelm RB, Sobey CG, Brady RD, O'Brien TJ, Mychasiuk R, Shultz SR, McDonald SJ. Behavioral, axonal, and proteomic alterations following repeated mild traumatic brain injury:Novel insights using a clinically relevant rat model. Neurobiol Dis. 2021;148:105151. doi: 10.1016/j.nbd.2020.105151. [DOI] [PubMed] [Google Scholar]
- 54.Pumiglia L, Williams AM, Kemp MT, Wakam GK, Alam HB, Biesterveld BE. Brain proteomic changes by histone deacetylase inhibition after traumatic brain injury. Trauma Surg Acute Care Open. 2021;6:e000682. doi: 10.1136/tsaco-2021-000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qin D, Wang J, Le A, Wang TJ, Chen X, Wang J. Traumatic Brain injury:ultrastructural features in neuronal ferroptosis, glial cell activation and polarization, and blood-brain barrier breakdown. Cells. 2021:10. doi: 10.3390/cells10051009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rauniyar N, Yates JR., 3rd Isobaric labeling-based relative quantification in shotgun proteomics. J Proteome Res. 2014;13:5293–5309. doi: 10.1021/pr500880b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement:a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ricklin D, Reis ES, Mastellos DC, Gros P, Lambris JD. Complement component C3 - The “Swiss Army Knife”of innate immunity and host defense. Immunol Rev. 2016;274:33–58. doi: 10.1111/imr.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson S, Berglass JB, Denson JL, Berkner J, Anstine CV, Winer JL, Maxwell JR, Qiu J, Yang Y, Sillerud LO, Meehan WP, 3rd, Mannix R, Jantzie LL. Microstructural and microglial changes after repetitive mild traumatic brain injury in mice. J Neurosci Res. 2017;95:1025–1035. doi: 10.1002/jnr.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sabet N, Soltani Z, Khaksari M. Multipotential and systemic effects of traumatic brain injury. J Neuroimmunol. 2021;357:577619. doi: 10.1016/j.jneuroim.2021.577619. [DOI] [PubMed] [Google Scholar]
- 61.Samal BB, Waites CK, Almeida-Suhett C, Li Z, Marini AM, Samal NR, Elkahloun A, Braga MF, Eiden LE. Acute response of the hippocampal transcriptome following mild traumatic brain injury after controlled cortical impact in the rat. J Mol Neurosci. 2015;57:282–303. doi: 10.1007/s12031-015-0626-2. [DOI] [PubMed] [Google Scholar]
- 62.Samia Hassan R. Risk-Benefit Evaluation in Clinical Research Practice. In:Ethics in Research Practice and Innovation. In: Antonio S, Ana F, Elena U, editors. Hershey, PA, USA: IGI Global; 2019. pp. 148–170. [Google Scholar]
- 63.Sarkis GA, Mangaonkar MD, Moghieb A, Lelling B, Guertin M, Yadikar H, Yang Z, Kobeissy F, Wang KK. The application of proteomics to traumatic brain and spinal cord injuries. Curr Neurol Neurosci Rep. 2017;17:23. doi: 10.1007/s11910-017-0736-z. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt A, Forne I, Imhof A. Bioinformatic analysis of proteomics data. BMC Syst Biol. 2014;8(Suppl 2):S3. doi: 10.1186/1752-0509-8-S2-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ:25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sempere L, Rodríguez-Rodríguez A, Boyero L, Egea-Guerrero JJ. Experimental models in traumatic brain injury:from animal models to in vitro assays. Med Intensiva (Engl Ed) 2019;43:362–372. doi: 10.1016/j.medin.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 67.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape:a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shavit-Stein E, Gerasimov A, Aharoni S, Gofrit SG, Pikus E, Pick CG, Maggio N. Unexpected role of stress as a possible resilience mechanism upon mild traumatic brain injury (mTBI) in mice. Mol Cell Neurosci. 2021;111:103586. doi: 10.1016/j.mcn.2020.103586. [DOI] [PubMed] [Google Scholar]
- 69.Shi K, Zhang J, Dong JF, Shi FD. Dissemination of brain inflammation in traumatic brain injury. Cell Mol Immunol. 2019;16:523–530. doi: 10.1038/s41423-019-0213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simon DW, McGeachy MJ, Bayır H, Clark RS, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13:171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. A model of parasagittal controlled cortical impact in the mouse:cognitive and histopathologic effects. J Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- 72.Song H, Chen M, Chen C, Cui J, Johnson CE, Cheng J, Wang X, Swerdlow RH, DePalma RG, Xia W, Gu Z. Proteomic analysis and biochemical correlates of mitochondrial dysfunction after low-intensity primary blast exposure. J Neurotrauma. 2019;36:1591–1605. doi: 10.1089/neu.2018.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stein MB, Jain S, Giacino JT, Levin H, Dikmen S, Nelson LD, Vassar MJ, Okonkwo DO, Diaz-Arrastia R, Robertson CS, Mukherjee P, McCrea M, Mac Donald CL, Yue JK, Yuh E, Sun X, Campbell-Sills L, Temkin N, Manley GT, Adeoye O, et al. Risk of posttraumatic stress disorder and major depression in civilian patients after mild traumatic brain injury:a TRACK-TBI study. JAMA Psychiatry. 2019;76:249–258. doi: 10.1001/jamapsychiatry.2018.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang M, Huang H, Li S, Zhou M, Liu Z, Huang R, Liao W, Xie P, Zhou J. Hippocampal proteomic changes of susceptibility and resilience to depression or anxiety in a rat model of chronic mild stress. Transl Psychiatry. 2019;9:260. doi: 10.1038/s41398-019-0605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thapa K, Khan H, Singh TG, Kaur A. Traumatic brain injury:mechanistic insight on pathophysiology and potential therapeutic targets. J Mol Neurosci. 2021;71:1725–1742. doi: 10.1007/s12031-021-01841-7. [DOI] [PubMed] [Google Scholar]
- 76.Thelin EP, Just D, Frostell A, Häggmark-Månberg A, Risling M, Svensson M, Nilsson P, Bellander BM. Protein profiling in serum after traumatic brain injury in rats reveals potential injury markers. Behav Brain Res. 2018;340:71–80. doi: 10.1016/j.bbr.2016.08.058. [DOI] [PubMed] [Google Scholar]
- 77.Thompson A, Schäfer J, Kuhn K, Kienle S, Schwarz J, Schmidt G, Neumann T, Johnstone R, Mohammed AK, Hamon C. Tandem mass tags:a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal Chem. 2003;75:1895–1904. doi: 10.1021/ac0262560. [DOI] [PubMed] [Google Scholar]
- 78.Vailati-Riboni M, Palombo V, Loor JJ. What are omics sciences?In:periparturient diseases of dairy cows:a systems biology approach. In: Ametaj BN, editor. Cham: Springer International Publishing; 2017. pp. 1–7. [Google Scholar]
- 79.Wang H, Li B, Yan K, Wu Y, Wen Y, Liu Y, Fan P, Ma Q. Protein and Signaling pathway responses to rhIL-6 intervention before lobaplatin treatment in osteosarcoma cells. Front Oncol. 2021a;11:602712. doi: 10.3389/fonc.2021.602712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang H, Chen J, Gao C, Chen W, Chen G, Zhang M, Luo C, Wang T, Chen X, Tao L. TMT-based proteomics analysis to screen potential biomarkers of acute-phase TBI in rats. Life Sci. 2021b;264:11. doi: 10.1016/j.lfs.2020.118631. [DOI] [PubMed] [Google Scholar]
- 81.Williams SA, Kivimaki M, Langenberg C, Hingorani AD, Casas JP, Bouchard C, Jonasson C, Sarzynski MA, Shipley MJ, Alexander L, Ash J, Bauer T, Chadwick J, Datta G, DeLisle RK, Hagar Y, Hinterberg M, Ostroff R, Weiss S, Ganz P, et al. Plasma protein patterns as comprehensive indicators of health. Nat Med. 2019;25:1851–1857. doi: 10.1038/s41591-019-0665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 83.Wu H, Zheng J, Xu S, Fang Y, Wu Y, Zeng J, Shao A, Shi L, Lu J, Mei S, Wang X, Guo X, Wang Y, Zhao Z, Zhang J. Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. J Neuroinflammation. 2021;18:2. doi: 10.1186/s12974-020-02041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu B, Tian R, Wang X, Zhan S, Wang R, Guo Y, Ge W. Protein profile changes in the frontotemporal lobes in human severe traumatic brain injury. Brain Res. 2016;1642:344–352. doi: 10.1016/j.brainres.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 85.Xu XJ, Yang MS, Zhang B, Niu F, Dong JQ, Liu BY. Glucose metabolism:A link between traumatic brain injury and Alzheimer's disease. Chin J Traumatol. 2021;24:5–10. doi: 10.1016/j.cjtee.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang K, Mu XS, Hayes RL. Increased cortical nuclear factor-kappa B (NF-kappa B) DNA binding activity after traumatic brain injury in rats. Neurosci Lett. 1995;197:101–104. doi: 10.1016/0304-3940(95)11919-n. [DOI] [PubMed] [Google Scholar]
- 87.Yang MS, Xu XJ, Zhang B, Niu F, Liu BY. Comparative transcriptomic analysis of rat versus mouse cerebral cortex after traumatic brain injury. Neural Regen Res. 2021;16:1235–1243. doi: 10.4103/1673-5374.301028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures:significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Q, Liu W, Zhang HM, Xie GY, Miao YR, Xia M, Guo AY. hTFtarget:a comprehensive database for regulations of human transcription factors and their targets. Genomics Proteomics Bioinformatics. 2020;18:120–128. doi: 10.1016/j.gpb.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Z, Wu S, Stenoien DL, Paša-Tolić L. High-throughput proteomics. Annu Rev Anal Chem (Palo Alto Calif) 2014;7:427–454. doi: 10.1146/annurev-anchem-071213-020216. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Z, Yu J, Wang P, Lin L, Liu R, Zeng R, Ma H, Zhao Y. iTRAQ-based proteomic profiling reveals protein alterations after traumatic brain injury and supports thyroxine as a potential treatment. Mol Brain. 2021;14:25. doi: 10.1186/s13041-021-00739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou D, Liu J, Hang Y, Li T, Li P, Guo S, Liu T, Xia Z, Wang Y. TMT-based proteomics analysis reveals the protective effects of Xuefu Zhuyu decoction in a rat model of traumatic brain injury. J Ethnopharmacol. 2020;258:112826. doi: 10.1016/j.jep.2020.112826. [DOI] [PubMed] [Google Scholar]
- 93.Ziabska K, Ziemka-Nalecz M, Pawelec P, Sypecka J, Zalewska T. Aberrant complement system activation in neurological disorders. Int J Mol Sci. 2021;22:4675. doi: 10.3390/ijms22094675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The identified proteins in 7 days post-TBI cortex tissues using a large-scale proteomics investigation