Abstract
Stroke is the second leading cause of death and a major cause of disability worldwide, and biological sex is an important determining factor in stroke incidence and pathology. From childhood through adulthood, men have a higher incidence of stroke compared with women. Abundant research has confirmed the beneficial effects of estrogen in experimental ischemic stroke but genetic factors such as the X-chromosome complement can also play an important role in determining sex differences in stroke. Autophagy is a self-degrading cellular process orchestrated by multiple core proteins, which leads to the engulfment of cytoplasmic material and degradation of cargo after autophagy vesicles fuse with lysosomes or endosomes. The levels and the activity of components of these signaling pathways and of autophagy-related proteins can be altered during ischemic insults. Ischemic stroke activates autophagy, however, whether inhibiting autophagy after stroke is beneficial in the brain is still under a debate. Autophagy is a potential mechanism that may contribute to differences in stroke progression between the sexes. Furthermore, the effects of manipulating autophagy may also differ between the sexes. Mechanisms that regulate autophagy in a sex-dependent manner in ischemic stroke remain unexplored. In this review, we summarize clinical and pre-clinical evidence for sex differences in stroke. We briefly introduce the autophagy process and summarize the effects of gonadal hormones in autophagy in the brain and discuss X-linked genes that could potentially regulate brain autophagy. Finally, we review pre-clinical studies that address the mechanisms that could mediate sex differences in brain autophagy after stroke.
Keywords: autophagy, brain, estrogen, gonadal hormones, neurodegeneration, neuron, stroke, X-chromosome
Introduction
Growing evidence supports the idea that the onset, progression, prevention, and treatment of diseases are influenced by sex and gender (Mauvais-Jarvis et al., 2020). Considering these factors, gender medicine, as a part of precision medicine, aims to design therapies that include the use of a specific drug, in a specific dose, for each individual (Shang et al., 2021).
Biologic and epidemiologic differences importantly contribute to sex differences in stroke incidence, prevalence, severity, and case fatality (Zhang et al., 2021b). Identifying risk factors that lead to sex differences in stroke is challenging, as these factors may be dependent on other variables, such as age. Exploring different biological factors that affect the incidence, severity, and recovery from ischemic stroke may help elucidate the mechanisms that determine sex differences in ischemic stroke. One of the most studied factors is gonadal hormones, but genetic factors including the sex chromosome complement (XX vs. XY) may also play an important role in mediating sex differences in stroke.
Ischemic stroke triggers multiple pathological events, including oxidative stress, adenosine triphosphate (ATP) deprivation, mitochondrial dysfunction, cellular ion overload, and cytotoxic edema, all of which contribute to neuronal death. Ischemic stroke also activates autophagy, which influences ischemic stroke outcomes (Wang et al., 2021). Autophagy is a self-degrading cellular process that is tightly regulated by multiple signaling pathways regulated by internal and external stimuli. Macroautophagy, the best-studied autophagy mechanism, is orchestrated by numerous core proteins to engulf cytoplasmic material and degrade cargo after autophagy vesicles eventually fuse with lysosomes (Klionsky et al., 2021; Figure 1). The levels and the activity of these signaling pathways and of autophagy-related proteins are altered during ischemic insults (Wang et al., 2021). Gonadal hormones regulate several signaling pathways associated with autophagy and several autophagy-related genes (ATG) are located on the X-chromosome (Congdon, 2018; Azcoitia et al., 2019), suggesting that autophagy may be regulated by sex-related factors.
Figure 1.
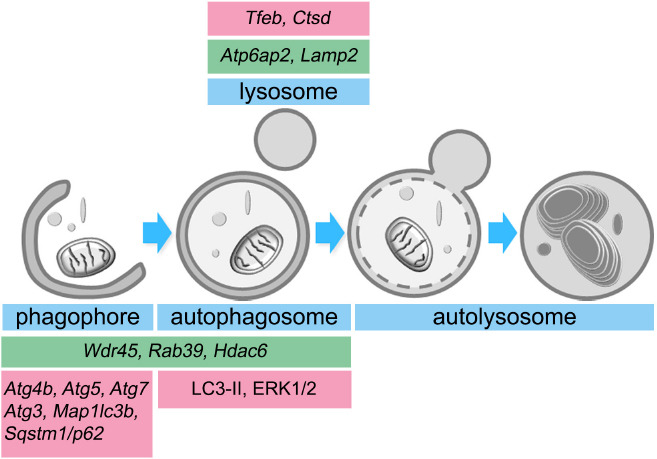
Estrogen and X-linked genes contribute to autophagy in the brain.
Estradiol (pink boxes) upregulates the expression of several autophagy-related genes (ATGs) and induces the activation of different proteins that are involved in phagophore formation, autophagosome maturation, and lysosomal activity. Similarly, several genes located in the X-chromosome (green boxes) encode proteins that participate in autophagy progression. Atg: Autophagy-related gene; Atp6ap2: ATPase H+ transporting accessory protein 2; Ctsd: cathepsin D; ERK1/2: extracellular signal-regulated kinases 1/2; Hdac6: histone deacetylase 6; Lamp2: lysosomal associated membrane protein-2; Map1lc3b: microtubule-associated protein 1A/1B-light chain 3 B; Rab39: Ras-related protein Rab-39; Sqstm1/p62: Sequestosome 1; Tfeb: transcription factor EB; Wdr45: WD40 repeat protein interacting with phosphoinositides 4.
Recent reviews have comprehensively summarized clinical and pre-clinical studies on the role of brain autophagy in stroke (Ajoolabady et al., 2021; Wang et al., 2021). However, none have specifically addressed sex-biased autophagy as a contributor to differences in stroke outcome between the sexes. Most preclinical studies in the stroke field still do not include both sexes in their in vitro and in vivo analyses. Identifying the cellular mechanisms that govern these differences is required to reach the ultimate goal of providing precision medicine for patients. In this review, we highlight the evidence for sex differences in stroke in human and in animal models, and how these differences can be explained by gonadal hormones and the sex chromosome complement. We discuss the contribution of gonadal hormones and the X-chromosome as biological factors in brain autophagy. We also discuss the limited studies that investigate sex differences in autophagy in preclinical stroke models.
Search Strategy
Publications included in this narrative review were retrieved from the PubMed database by using the following sets of search terms: (1) “sex differences”[title/abstract], brain[title/abstract], stroke[title/abstract], and (2) stroke[title], brain[title/abstract], autophagy[title]. Retrieval time: up to November 2021.
Sex Differences in Stroke
Stroke is a leading cause of mortality in the United States, and sex is a determining factor in the incidence and pathology of stroke (Bushnell et al., 2018). Age-specific incidence rates are substantially lower in females than males in younger and middle-aged groups (Bushnell et al., 2018). However, age reverses the “female protected” phenotype. Incidence rates in females are similar or even higher than those in males in the oldest age groups (Howard et al., 2019; Madsen et al., 2020). Currently, more women (4.1 million) live with stroke-related disability than men (3.1 million), and women are more likely to die than men (6.2% vs. 4.3%) after a stroke (Benjamin et al., 2017).
In pre-clinical studies, young female mice and rats subjected to transient ischemia induced by middle cerebral artery occlusion (MCAO) have reduced brain injury compared to young males (Acaz-Fonseca et al., 2020; Patrizz et al., 2021). Similarly, in spontaneously hypertensive stroke-prone rats (a model relevant to human lacunar stroke, a common type of ischemic stroke), young male rats had their first stroke 3 weeks after a high-salt diet while females were event-free for 6 weeks. Further, females had significantly longer survival time, and brain damage progressed at a slower rate (detectable with magnetic resonance imaging) in females compared with males (Ballerio et al., 2007). This protection was lost after ovariectomy in MCAO models and restored with estrogen supplementation (Acaz-Fonseca et al., 2020; Patrizz et al., 2021). As observed in humans, age plays a critical role in the response to experimental stroke. Manwani et al. (2013) found that infarct size was affected by aging in mice subjected to experimental MCAO. Mice were subjected to focal transient cerebral ischemia for 60 minutes followed by reperfusion. Young (5–6 months old) males had larger infarcts compared to young females; however, in middle-aged (14–15 months old) male mice, the infarct volume was smaller compared with young males, but middle-aged females had larger infarcts than young females. The infarct volume did not differ between sexes in aged (20–22 months old) mice (Manwani et al., 2013). Clinical evidence also supports the hypothesis that aging worsens stroke outcomes disproportionally in women compared to men.
After menopause, the decline in gonadal hormones is associated with an increased risk of stroke, suggesting that women lose the protection that estradiol confers, which may explain the shift in the stroke incidence in women with aging. Differences in life expectancy between sexes and other factors also contribute to the reversal of the trend (Bushnell et al., 2018).
Considerable research has confirmed the beneficial effects of estrogen in stroke in preclinical models (Sohrabji et al., 2019). Importantly, the timing in the initiation of hormone therapy appears to be critical for determining its beneficial effects. Oral administration of estradiol within 6 years after menopause mitigated the increase of carotid-artery intima-media thickness seen with aging, a measure of atherosclerotic burden. However, the beneficial effect of estradiol was absent when the hormone was administered more than 10 years after menopause (Hodis et al., 2016). A similar effect was observed in experimental models. Chronic estrogen replacement therapy initiated in late middle-aged (16 months old) mice reduced infarct volume, led to improved neurological outcomes, and decreased neuroinflammation in both sexes, but more significantly in aged females (Liu et al., 2012). Female mice treated with estradiol for 3 months prior to an induced stroke had smaller infarcts compared to animals treated acutely for 2 weeks prior to stroke (Liu et al., 2012) mirroring clinical findings that earlier treatment increases benefit. Sexually mature rats subjected to MCAO and supplemented with estradiol showed reduced infarct size compared with stroke rats treated with placebo (Selvamani and Sohrabji, 2010). However, estradiol supplementation increased the infarct size in reproductively senescent rats compared with control ovariectomized mature rats (Selvamani and Sohrabji, 2010). Thus, early administration of female hormones in post-menopausal women may reduce stroke severity; the timing of initiation of hormone therapy matters.
In contrast to the beneficial effects of estrogen, clinical and preclinical studies have demonstrated that the male gonadal hormone testosterone contributes to enhanced vulnerability to cerebral ischemia (Uchida et al., 2009). Therefore, gonadal hormones play a critical role in ischemic vulnerability. However, not all sex differences in stroke are associated with sex hormones. Evidence demonstrates a sex bias in stroke incidence in pre-pubertal children, where the levels of gonadal hormones are equivalent between sexes; boys have a higher incidence of stroke and poorer outcomes (Dunbar et al., 2020). Indeed, using a mouse model in which the effects of sex chromosome complement can be analyzed independently of gonadal sex (the four-core-genotype (FCG) model) found that both sex chromosomes and gonadal hormones contribute to sex differences (Cambiasso et al., 2017; Vousden et al., 2018).
The FCG mouse model has been used to evaluate the role of the chromosomal sex (XX or XY) in the response to stroke. In the FCG model, the gene Sry, the mammalian testes determining-gene, is deleted from the Y-chromosome and inserted on autosomal chromosome 3 (A+Sry). This leads to a male that inherits Y-Sry, but still has a copy of the Sry gene on chromosome 3. Thus, mice that inherit chromosome 3 with a copy of the Sry gene (represented as A+Sry) will develop the secondary sex characteristics of male mice, such as testis development and typical male-like behavior (Qi et al., 2021). Genetic crosses between a wild-type female, XX(A), and the phenotypically normal male, XY-Sry(A+Sry), will produce four distinct genotypes: phenotypically female with Y-chromosome, XY-Sry(A); wild-type female, XX(A); phenotypically normal male, XY-Sry(A+Sry); and a phenotypically male with two X-chromosomes, XX(A+Sry)M (Figure 2).
Figure 2.
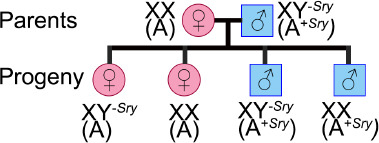
The Four Core Genotype mouse model.
Crossing a wild-type mouse female, XX(A), with a mouse male, XY-Sry(A+Sry), in which the gene that determines testes (Sry) was deleted from the Y-chromosome and inserted on an autosomal chromosome (A), produces litters with four genotypes, characterized by the number of X-chromosomes and the presence of the Sry gene. Thus, we have wild-types females, XX(A), phenotypically females with a Y-chromosome without Sry, XY-Sry(A), phenotypically normal males, XY-Sry(A+Sry), and phenotypically males with two X-chromosomes with an autosomal chromosome with Sry, XX(A+Sry).
Studies with this model helped determine that ischemic sensitivity is driven by the gonads in young mice. Young gonadal male mice, XX(A+Sry) and XY-Sry(A+Sry), that had testis (as they inherited the Sry), had higher larger infarct volumes after MCAO, compared with animals with ovaries and estrogen, XX(A) and XY-Sry(A). However, the protection was lost with gonadectomy, meaning all infarcts were equalized (Manwani et al., 2015). Thus, ischemic sensitivity is primarily driven by gonadal hormones in young mice. However, the X-chromosome complement plays a critical role in ischemic sensitivity in aging mice. 18–20-month-old mice with an XX-chromosome complement, XX(A) and XX(A+Sry), regardless of gonads, had larger strokes than animals with only one X-chromosome, XY-Sry(A+Sry) and XY-Sry(A) (McCullough et al., 2016). Therefore, gonadal hormones drive the “female protected” phenotype seen in young mice, but in older reproductively senescent animals, the X-complement (XX) leads to ischemic sensitivity. Sex-biased differences in ischemic sensitivity driven by the X-chromosome may be present during the lifespan, but these sex differences may be held in check by estrogen. We subsequently found that two genes that escape from X-inactivation (Kdm5c and Kdm6a), which regulate inflammatory interferon regulatory factors, were upregulated in the brain from mice with two X-chromosomes (XX(A) and XX(A+Sry)), compared with brains from XY (XY-Sry(A) and XY-Sry(A+Sry)) mice (Qi et al., 2021). This indicates that the X-chromosome contributes to sex differences in stroke sensitivity of aged mice. Thus, with aging, detrimental genes on the second X-chromosome are revealed.
Autophagy Overview
Autophagy is a self-degrading mechanism that mediates the degradation of intracellular material, such as protein aggregates, damaged organelles, and microorganisms. Basically, autophagy (1) recycles molecules to rebuild new ones, (2) recovers energy by breaking intramolecular bonds, (3) allows cells to adapt to rapid changes in the cellular environment, and (4) protects cells by eliminating pathogens and toxic aggregates (Klionsky et al., 2021).
Three types of autophagy are classified by the manner in which the cargo is delivered into the lysosomes: macroautophagy, microautophagy, and chaperone-mediated autophagy. Chaperone-mediated autophagy specifically degrades proteins containing a KFERQ-like motif. Microautophagy non-specifically degrades cytoplasmic material by lysosome-mediated phagocytosis. Macroautophagy is the most well-studied autophagy type and is characterized by using double-membrane structures (phagophores) isolated from different intracellular lipid membrane sources (endoplasmic reticulum, Golgi apparatus, mitochondria, and plasma membrane) (Pavel and Rubinsztein, 2017). During macroautophagy (hereafter called autophagy), a phagophore elongates while engulfing cytoplasmic material, and intermediate filaments and microfilaments mediate phagophore closure to form a double-membrane vesicle (autophagosome) that later fuses with endosomes (amphisome) and/or lysosomes (autolysosome). The hydrolytic content of lysosomes/endosomes releases into the double-membrane vesicle and degrades the cargo (Loeffler, 2019; Figure 1).
Many neurodegenerative disorders exhibit autophagy impairment, which may interfere with the degradation of protein aggregates, toxic compounds, and damaged organelles. Differentiated neurons are especially vulnerable to protein aggregate accumulation as they cannot dilute harmful cellular material by cell division, and neurons importantly depend on autophagy for clearing these substrates (Kulkarni et al., 2018). Adequate autophagy regulation helps to remove damaged mitochondria that cause cellular stress and provides energy in energy-depleting conditions. Thus, autophagy is beneficial for many aspects of brain function. However, autophagy also has deleterious effects on the brain. Overactivation of autophagy can induce apoptosis. For example, in the ischemic stroke field, several studies have shown that inhibiting autophagy reduces brain damage (Liu et al., 2016; Zhang et al., 2019), and the activation of autophagy-related signaling pathways induces oxidative stress and apoptosis (Li and McCullough, 2010). Therefore autophagy can play a dual role in the ischemic stroke brain. Identifying the mechanisms that regulate the activation and progression of autophagy is critical to the development of therapeutic approaches to mitigate brain damage after stroke.
Summary of autophagy core proteins
Over 30 ATG’s participate in the machinery of autophagy. The essential autophagy core proteins can be classified into four functional groups: (1) UNC-51-like kinase 1 (ULK1), ATG13, and ATG17 form the initiation complex; (2) phosphatidylinositol 3-kinase (PI3K), Beclin1, ATG14, and AMBRA1 form the autophagy activating class III PI3K complex to form the phagophore; and (3) the ATG12 conjugation system (ATG12, ATG5, ATG16L1, and ATG10) in conjunction with (4) the microtubule-associated protein 1A/1B-light chain 3 (LC3) conjugation system (LC3, ATG3, ATG7, and ATG4B) lipidate and conjugate LC3 to phosphatidylethanolamine (LC3-II) to the membrane of the autophagosome and hence promote autophagosome maturation (Ajoolabady et al., 2021; Table 1). LC3-II in combination with Sequestosome 1 (SQSTM1 or p62) is commonly used as autophagy markers. LC3-II is found in the outer and inner membrane of autophagy vesicles autophagosomes, and p62 is a marker of autophagy cargo. Thus, increased levels of LC3-II (suggesting a higher number of autophagosomes) and reduced levels of p62 (suggesting enhanced autophagy cargo degradation) are generally interpreted as increased autophagy flux. Autophagy flux is the progression of the entire process of autophagy since autophagosome formation progresses until fusion with lysosomes and cargo degradation. However, it is important to consider that inner-membrane LC3-II is degraded after autophagosome-lysosome fusion (autolysosome). Thus, in conditions that interrupt fusion and/or lysosomal activity, we may expect enhanced levels of both markers LC3-II and p62. Otherwise, autophagy studies should not be limited to the use of only these two markers. The use of multiple autophagy markers and several techniques is strongly recommended to avoid ambiguous conclusions (Klionsky et al., 2021).
Table 1.
Different protein complexes in each autophagy stage contribute to the progression of autophagy
| Autophagy stage | Protein complex/system | Core autophagy proteins |
|---|---|---|
| Initiation complex | ULK1 complex | ULK1, ATG13, ATG17, ATG101, FIP200 |
| Phagophore formation | Class III PI3K complex | PIK3R4, PIK3C3, Beclin1, ATG14, AMBRA1 |
| Autophagosome formation and maturation | ATG12 conjugation system | ATG12, ATG5, ATG16L1, ATG10 |
| LC3 conjugation system | LC3, ATG4B, ATG3, ATG7 |
AMBRA1: Activating molecule in Beclin 1-regulated autophagy; ATG: autophagyrelated gene; FIP200: focal adhesion kinase family interacting protein of 200 kDa; LC3: microtubule-associated protein light chain 3; PI3K: phosphoinositide 3-kinase; PIK3C3: phosphatidylinositol 3-kinase catalytic subunit type 3; PIK3R4: phosphoinositide 3-kinase regulatory subunit 4; ULK1: Unc-51 like autophagy activating kinase 1.
Summary of autophagy-related signaling pathways
Multiple signaling pathways that respond to extracellular and intracellular stimuli are coordinated to regulate autophagy. There have been several excellent recent reviews on the mechanisms that govern brain autophagy during the ischemic stroke (Ajoolabady et al., 2021; Wang et al., 2021). Here, we briefly introduce the pathways that might have specific relevance for understanding potential sex differences in autophagy in stroke.
mTOR is a serine/threonine kinase that inhibits autophagy initiation by phosphorylating ULK1. mTOR plays a central role not only in autophagy but also in cell growth, protein synthesis, cell cycle, and apoptosis (Kim and Guan, 2015). The activity of mTOR is regulated by the PI3K family. PI3Ks are classified into three classes (class I, II, and III). While class I PI3Ks are negative regulators of autophagy by activating mTOR via phosphorylation, class III PI3Ks promote autophagy progression by producing the phospholipid phosphatidylinositol 3-phosphate (Yu et al., 2015).
5′-AMP-activated protein kinase (AMPK) is a serine/threonine kinase that is activated when the ATP/AMP ratio decreases, which occurs after ischemic stroke. AMPK inhibits mTOR and consequently activates autophagy (Zhang and Miao, 2018). Indeed, pharmacological and genetic studies have demonstrated that activating AMPK exacerbates stroke outcome, and that AMPK inhibition can provide neuroprotection in stroke models (Li and McCullough, 2010).
Nuclear factor-kappa B (NF-κB) is a transcription factor that is involved in autophagy by regulating the expression of mTOR in ischemic mice (Li et al., 2013). Thus, the upregulation of NF-κB leads to autophagy repression.
B-cell lymphoma-2 (Bcl-2) binds to and inhibits Beclin-1, which is required for autophagy initiation. When Bcl-2 is phosphorylated, Beclin-1 is released from the complex Beclin-1/Bcl-2 and joins other autophagy-related proteins to form the phagophore assembly site. Thus, a phagophore expands, which engulfs cytoplasmic material until its extremes fuse to form an autophagosome (Menon and Dhamija, 2018).
In the sections below, we summarize the evidence for sex-dependent regulation of these pathways.
Sex-Biased Regulation of Autophagy in the Brain
Role of sex steroid hormones in brain autophagy
Autophagy is importantly regulated by sex steroid hormones, such androgens, estrogens, and progesterone via their receptors, androgen receptor (AR), estrogen receptors α and β (ERα, ERβ), and progesterone receptors, respectively. These receptors localize to the gonads, but they are also found in the human and rodent brains. Androgens, estrogens, and progesterone influence different stages of the autophagy machinery via their receptors by the regulation of the expression of multiple ATG’s. AR, ERα, and ERβ act on phagophore induction and phagophore expansion by mediating the expression of Atg3, Atg4b, Atg5, Atg7, Lc3b, and Sqstm1/p62 (Congdon, 2018). AR is also important for regulating autophagosome maturation as ERα contributes to elevating LC3-II levels (Li et al., 2015). In lysosome biogenesis, AR can upregulate the expression of the gene encoding the transcription factor EB, Tfeb, which controls the expression of lysosomal genes (Blessing et al., 2017). Furthermore, ERα is suggested to induce the lysosomal proteinase cathepsin D (Augereau et al., 1994; Figure 1). Thus, steroid hormones contribute not only to the formation of autophagy vesicles but also to maintaining lysosomal integrity for efficient cargo degradation during autophagy.
Sex differences exist in the expression of these sex steroid hormone receptors, which is also brain region-specific. The expression of Ar is higher in the hypothalamus of men than in women (Fernandez-Guasti et al., 2000). Female rats express higher levels of ERα, ERβ, and G protein-coupled estrogen receptor than males in the trigeminal ganglia (Warfvinge et al., 2020). Furthermore, the expression of these receptors varies with the cellular context in a sex-dependent manner. For example, while Erα and G protein-coupled estrogen receptor genes are upregulated, Ar is downregulated in male rats after stroke. However, female rats did not show significant differences in the expression of these sex steroid hormone receptors after MCAO, compared with sham rats (Acaz-Fonseca et al., 2020). Thus, these differences in the expression of gonadal hormone receptors in the brain between sexes may determine sex differences in the regulation of autophagy.
Estradiol activates autophagy in the brain
Estradiol acts on multiple signaling pathways associated with brain autophagy (Azcoitia et al., 2019; Xiang et al., 2019). Estradiol activates the PI3K/AKT signaling pathway in the brain. PI3K activity induces the phosphorylation and activation of AKT, which phosphorylates and inhibits the serine/threonine kinase glycogen synthase kinase 3β (GSK3β) (Saraceno et al., 2018). Pharmacological inhibition of GSK3β increases levels of LC3-II and decreases levels of p62 (Wang et al., 2019). Alternatively, class III PI3K/AKT activation by estradiol can also inhibit mTORC1 (Perez-Alvarez et al., 2018), a master nutrient sensor that inhibits autophagy. Estradiol can activate AMPK (Guo et al., 2017a), an energy sensor that is activated with ATP depletion and acts as the antithesis of mTORC1 by activating autophagy. Furthermore, estradiol inhibits the activity of the transcription factor NF-κB (Cook et al., 2018; Yun et al., 2018). Enhanced activity of the transcription factor NF-κB is associated with reduced autophagy during neural progenitor cell differentiation (FitzPatrick et al., 2018) and in the cortex of a barrel cortex ischemic stroke mouse model (Li et al., 2013). This data suggests that estradiol activates autophagy via inhibiting NF-κB. However, the activation of NF-κB exacerbates autophagy in a traumatic brain injury rat model (Liu et al., 2020). The signaling pathways linked to brain autophagy positively regulated by estradiol are summarized in Figure 3.
Figure 3.
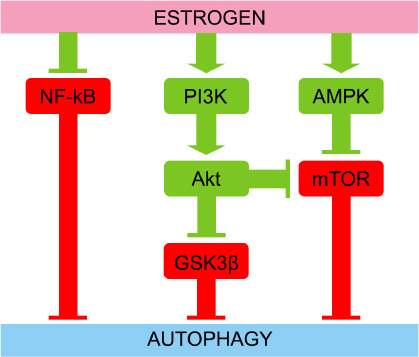
Estrogen induces autophagy through several signaling pathways.
Estrogen inhibits NF-κB, GSK3β, and mTOR that are direct autophagy inhibitors as occurs for NF-κB, or by mediating different kinases. Estrogen activates the PI3K/Akt signaling pathway that ultimately phosphorylates and inhibits GSK3β and mTOR. Estrogen also activates AMPK that phosphorylates and inhibits mTOR. Thus, inhibiting NF-κB, GSK3β, and mTOR estrogen promotes autophagy. Red symbolizes negative effect on autophagy, while green symbolizes positive effect on autophagy. Akt: Protein kinase B; AMPK: AMP-activated protein kinase; GSK3β: glycogen synthase kinase-3β; mTOR: mammalian target of rapamycin; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K: phosphoinositide 3-kinases.
Estradiol inhibits autophagy in the brain
In ischemic stroke mouse models, estradiol supplementation may also have an inhibitory effect on autophagy in the brain by downregulating hypoxia inducible factor 1α (HIF-1α), which is a transcription factor that induces autophagy (Hsieh et al., 2015). Estradiol can also inhibit autophagy by increasing levels of p62 and by reducing levels of LC3-II, ATG5 (a component of the ATG12 conjugation system), and Beclin1 (involved in phagophore formation), in the hippocampus after stroke (Li et al., 2017). Thus, in which conditions estradiol can act as an autophagy stimulator or inhibitor in stroke models need to be identified for the proper application of therapeutic strategies.
Effects of other gonadal hormones in autophagy in the brain
Progesterone first appeared as a potential neuroprotective steroid by upregulating autophagy in an amyotrophic lateral sclerosis mouse model (Kim et al., 2013). In cultured astrocytes, progesterone activates autophagy and prevents the accumulation of neurotoxic aggregates (Kim et al., 2012). In ischemic brains of male rats and in ex vivo microglia derived from adult male rats treated with the toxin lipopolysaccharide, progesterone administration enhanced autophagy and reduced inflammasome activation (Espinosa-Garcia et al., 2020). These studies highlight the beneficial effects of progesterone in the brain.
Few studies have investigated the effects of testosterone on autophagy in the brain, and existing data from rat cardiac tissue indicate that testosterone supplementation improved heart function by upregulating the expression of Bcl-2, which binds to Beclin1 and interrupts autophagy initiation (Fu et al., 2017). However, testosterone can be aromatized into estradiol by aromatase (Shay et al., 2018). Thus, the inhibitory effects of testosterone in autophagy could turn out to autophagy stimulation induced by aromatization-derived estradiol. Androstenedione is another androgen that can upregulate autophagy via reactive oxidative species in osteosarcoma (Liu et al., 2014). However, there is not much data about the role of androstenedione in autophagy in the brain.
In conclusion, estrogen is a master regulator of autophagy in the brain by altering the expression of multiple ATG’s and by modulating different signaling pathways. Overall, estrogen is an activator of autophagy; however, under certain circumstances, estrogen can inhibit autophagy (Hsieh et al., 2015; Li et al., 2017). Additional research is necessary to determine the sex-biased effects of the different gonadal hormones in autophagy in ischemic stroke. Potential sex-specific signaling pathways seen in both health and disease need to be elucidated.
Role of the X-chromosome in autophagy in the brain
Several genes involved in the machinery and signaling pathways that regulate autophagy in the brain localize to the X-chromosome. So far, no ATG’s linked to the Y-chromosome have been found, suggesting that the sex differences in autophagy mediated by the sex chromosomes are likely due to the X-chromosome. Known genes associated with autophagy in the brain and localize to the X-chromosome are listed (Table 2). Abnormalities in these genes have deleterious consequences for brain function. Given that males only have one copy of the X-chromosome, alterations in X-linked genes are associated with worse symptoms in males compared to females. Generally, in neuropathology associated with X-linked genes, heterozygous females have less severe phenotypes, or no clinical phenotype, compared with hemizygous males.
Table 2.
Several autophagy-related genes localize in the X-chromosome
| Gene symbol | Gene name | Function in autophagy | Effects caused by protein deficiency in the brain | References |
|---|---|---|---|---|
| Wdr45/Wipi4 | WD repeat domain 45 | WDR45 participates in the elongation, closure of isolation membrane, and autophagosome formation. | Lethal in males. Females have seizures and develop neurodegeneration in infancy and childhood. | Zhao et al., 2015; Carvill et al., 2018; Chowdhury et al., 2018; Stanga et al., 2019 |
| Rab39b | Ras-related protein rab-39B | RAB39B regulates vesicle trafficking, and contributes to autophagosome formation and maturation. | Reduction of the growth cone, synapse formation, and excitatory synaptic transmission. | Tang, 2021 |
| Hdac6 | Histone deacetylase 6 | HDAC6 is a microtubule-associated deacetylase that acts on different cytoplasmic proteins. Recruits autophagic components to aggresomes for degradation (i.e. damaged mitochondria) | Hyperphosphorylation of mTOR and consequently reduced autophagy and neuronal death. However, it is not relevant for neurodegenerative disorders, such as Huntington’s disease. | Lee et al., 2010; Bobrowska et al., 2011; Zhu et al., 2016 |
| Atp6ap2 | ATPase H+ transporting accessory protein 2 | ATP6AP2 is a lysosomal transmembrane domain protein that maintains lysosomal pH via the V-ATPase complex. | Inefficient endosome acidification, and impaired endocytosis, vesicular trafficking, and autophagy. | Korvatska et al., 2013 |
| Lamp2 | Lysosomal associated membrane protein 2 | LAMP2 is a lysosomal membrane protein that confers lysosomal integrity and contributes to the fusion between autophagosomes and lysosomes. | Deficient clearance of cellular components, leading to cognitive dysfunction and accumulation of lipofuscin, polyglucose aggregates, and intranuclear inclusions in their neurons. | Nishino et al., 2000; Spinazzi et al., 2008; Maron et al., 2009; Rothaug et al., 2015 |
ATP6AP2: ATPase H+ transporting accessory protein-2; HDAC6: histone deacetylase 6; LAMP2: lysosomal associated membrane protein-2; RAB39: Ras-related GTPbinding protein rab-39B; WDR45/WIPI4: WD repeat domain-45 or WD repeat domain phosphoinositide-interacting protein-4.
The X-chromosome plays an important role in autophagy in the brain as it has genes that encode proteins that regulate: the formation and maturation of autophagosomes (Wdr45, Rab39b); vesicle trafficking (Rab39b); autophagy cargo recruitment (Hdac6); and the maintenance of lysosomal integrity (Atp6ap2 and Lamp2) (Figure 1). Deficiency in any of these genes negatively affects the progression of autophagy in the brain and leads to neurodegeneration and brain dysfunction.
No sex differences have been reported yet in the expression of the X-linked genes associated with autophagy in the brain, except for Wdr45, as WDR45 deficiency is lethal only for males (Carvill et al., 2018). This is likely due to the X-chromosome inactivation (XCI) that occurs in females to compensate for double X-chromosome dosage (Marks et al., 2015). Thus, males and females may have equivalent expression levels of X-linked genes. However, the XCI mechanism becomes less effective with aging, and some X-linked genes may escape from XCI (skewing) (Shvetsova et al., 2019). Reduced XCI also occurs more frequently in the brain compared to other tissues (Nguyen and Disteche, 2006), which could lead to altered expression of key autophagy genes located on the X-chromosome during reproductive senescence. Changes in gene expression could alter the autophagic response to ischemia. Thus, identifying escapee genes associated with autophagy in the brain could lead to novel treatment strategies.
In conclusion, differential expression of X-linked genes associated with autophagy may occur during aging and contribute to sex differences in brain autophagy. Future research may focus on identifying these autophagy-related X-linked genes that escape from XCI.
Autophagy in Ischemic Stroke
The clinical research in autophagy in stroke faces a challenging methodological situation: currently, there is a lack of methods that can measure the dynamic cellular process of autophagy in the most relevant human tissue, the brain, which is often inaccessible. Thus, blood samples are often used as a surrogate to determine changes that occur with stroke in the brain. Autophagy-associated circular RNAs, which are a noncoding RNA isoform with the potential to regulate neurological diseases, are differentially expressed in the plasma of large-artery ischemic stroke patients. Levels of these are positively correlated with neurological deficits and infarct volumes (Li et al., 2021). Gene microarrays analyses of blood samples from ischemic stroke patients revealed that several autophagy-associated genes (AKT, ATG4B, HDAC6, and LAMP1) are upregulated in stroke patients compared with controls (Guo et al., 2017b). Patients with a variant in the promoter of the autophagy-inducer lncRNA MALAT1 have a higher risk of ischemic stroke (Wang et al., 2020c). Thus, clinical data suggest that autophagy is altered in stroke patients.
There is a general assumption that autophagy is beneficial under basal conditions, and that upregulating autophagy improves brain function in neurodegenerative disorders and in the aged brains, which shows reduced autophagy (Loeffler, 2019). For example, rapamycin, an inhibitor of mTOR that plays a central role in energy homeostasis and autophagy inhibition, confers protection to the brain after ischemic stroke in clinical and pre-clinical studies (reviewed in (Hadley et al., 2019). More specifically, restoring autophagy by enhancing NAD+:NADH levels or upregulating Beclin1 improved mitochondrial function and reduced stroke development in stroke-prone spontaneously hypertensive rats (Forte et al., 2020). However, enhanced autophagy can be deleterious under stressful conditions such as stroke, which may lead to consider autophagy inhibition as a strategy to reduce infarct volume. Recent reviews have highlighted the evidence on the role of autophagy in ischemic stroke (Ajoolabady et al., 2021; Wang et al., 2021). During an ischemic insult, a plethora of intertwined pathways is coordinated to activate autophagy (Wang et al., 2020a). For example, there is a positive correlation between LC3-II levels (enhanced number of autophagosomes) and cortical infarct volume after stroke in MCAO rat models (Acaz-Fonseca et al., 2020). However, as we discuss below, the activation of autophagy by ischemia may occur in a sex-dependent manner. Autophagy can lead to cytoprotective (adaptive autophagy) or detrimental (maladaptive autophagy) consequences depending on the cellular context (Wang et al., 2020a; Ajoolabady et al., 2021). Thus, inhibiting autophagy may not have similar effects in males and females.
Manipulating autophagy may be a feasible approach to prevent or mitigate brain damage after ischemic stroke. However, before the development or implementation of therapeutic strategies for stroke via inhibition or promotion of autophagy, it is critical to first elucidate the effects of manipulating autophagy in both sexes.
Sex Differences in Autophagy in Stroke
Autophagy is importantly regulated by sex hormones, and several ATG’s locate on the X-chromosome. Thus, it is reasonable to think that sex differences exist in autophagy and that these differences contribute to sex differences in stroke outcomes.
Sex differences in autophagy in stroke animal models
Acaz-Fonseca et al. (2020) found that autophagy is upregulated by an ischemic stroke in a sex-dependent manner by examining the levels of two ATG’s, uncoupling protein 2 (Ucp-2) and Hif-1α. HIF-1α is a transcription factor that induces autophagy (Lu et al., 2018), and UCP-2 is a mitochondrial protein involved in the regulation of autophagy (Mao et al., 2021). Authors found that these genes were upregulated in males after stroke compared with females, which showed no changes in these markers. The upregulation of Ucp-2 and Hif-1α expression was associated with enhanced levels of LC3-II after stroke only in male rats. Consistent with this work, we found that autophagy was enhanced in the brain of mouse males after stroke, compared with sham males; both Beclin1 and LC3-II were upregulated, and p62 was reduced. However, changes in autophagy in females after stroke were less evident; LC3-II and ATG7, which contribute to LC3 conjugation and autophagosome maturation, were upregulated, but Beclin1 was downregulated and p62 levels remained unaltered. This suggests that stroke-induced autophagy may be muted in females (Patrizz et al., 2021).
A study using a neonatal hypoxia-ischemia (HI) rat model compared the levels of Beclin1 between sham and HI in each sex and found no differences. This suggests that the early steps of autophagy were not stimulated by HI. However, they found that HI males had reduced levels of LC3-II than sham males, suggesting a reduction of autophagosomes/autolysomes after HI. Contrarily, LC3-II levels were increased (increased autophagy vesicles) in HI females vs. sham females (Weis et al., 2014). Considering that LC3-II is degraded when autophagosomes fuse with lysosomes, we may hypothesize that the autophagy flux is stimulated in males after HI, but blocked in females. However, analyses of additional markers, such as p62, would help for a better interpretation of this data. Another study found postnatal day 8 male rats eliminate damaged mitochondria (likely by mitochondrial autophagy, known as mitophagy) more efficiently than female rat pups after HI (Demarest et al., 2016). HI males showed reduced mitochondrial pool and mitochondrial size compared with HI females. Thus, the studies above suggest that the autophagy process is upregulated in males compared to females after ischemia.
Enhanced levels of LC3-II correlate with increased cortical infarct volume after stroke in MCAO rat models (Acaz-Fonseca et al., 2020), and that inhibition of autophagy may reduce brain injury (Tower et al., 2020). Given that stroke-induced autophagy appears to be more relevant in male mice than in females, the inhibition of autophagy could lead to neuroprotection to a greater extent in males. In a focal model of stroke we found that inhibiting autophagy with 3-methyladenine (3-MA), which blocks the signaling pathway that induces phagophore formation and autophagosome nucleation (class III PI3K signaling pathway), reduced infarct volume by 6-fold in young (8–12 weeks old) male mice subjected to MCAO. In contrast, 3-MA exacerbated ischemic damage in MCAO female mice (Patrizz et al., 2021). To determine if this was secondary to gonadal hormones, female mice were ovariectomized and treated with 3-MA or vehicle after stroke. Ovariectomized females treated with 3-MA had reduced infarct volume compared with ovariectomized females treated with vehicle, suggesting that gonadal hormones mediate the deleterious effects of autophagy in stroke, independent of sex. 3-MA also had beneficial effects in young (3-months old) ovariectomized females by preventing the increase of pro-inflammatory cytokines induced by stroke (Li et al., 2017). However, despite these studies demonstrate the inhibitory effect of 3-MA on autophagy, others found that 3-MA may stimulate autophagy in ischemic stroke samples (Zhang et al., 2021a). Thus, it is necessary to specifically target autophagy components to inhibit autophagy. In a cardiac arrest and global ischemia using 16–18-day-old postnatal rats (with equivalent estradiol levels between sexes), intracisternal administration of siRNA against ATG7 led to an improvement in motor function in female rat pups but had no benefit in male pups (Au et al., 2015). Moreover, the treatment led to a reduction of degeneration of cerebellar Purkinje neurons in females, but not in males. This seems to contradict evidence from 3-MA studies. Note that using alternative genetic tools may lead to different outcomes compared with using non-specific approaches to inhibit autophagy. In addition, ATG7-independent autophagy has been demonstrated in different cell lines, such as MEF and HEK293T cells (Nishida et al., 2009; Ra et al., 2016). Whether there are differences in ATG7-dependent autophagy pathways between sexes has not been elucidated.
Sex differences in autophagy in cultured brain cells
At the cellular level, cultured neurons from embryonic male rat pups exhibit enhanced autophagy compared with female neurons. In in vitro assays, male neurons showed higher levels of LC3-II (Du et al., 2009; Patrizz et al., 2021) and ATG7 (Patrizz et al., 2021), and reduced levels of the autophagy cargo p62 (Patrizz et al., 2021), compared with female neurons, suggesting that basal autophagy is enhanced in male vs. female neurons. After oxygen and glucose deprivation (OGD), male neurons showed an increase in LC3-II levels, and a more dramatic reduction in p62 levels, compared with female neurons, which supports that the activation of autophagy is greater in male neurons than in female cells after OGD (Du et al., 2009). Thus, ATG7 may act as a limiting factor in both basal autophagy and autophagy activation in female neurons.
It is evident that neurons play a major role in brain function, but they only represent approximately 50% of all cells in the human brain. The remaining cells are primarily glial cells (Wang et al., 2020b), which also regulate neuronal autophagy (Kulkarni et al., 2018). It is reasonable to think that sex-biased mechanisms could govern autophagy in the brain via non-neuronal cell types, such as microglia, astrocytes, and endothelial cells. However, no studies have explored sex differences in autophagy in these brain cell types. Sex hormones affect the fate of brain cells in ischemia models. For example, estradiol prevents microglia activation after MCAO (Perez-Alvarez et al., 2012; Zhang et al., 2018) and in cultured microglia exposed to hypoxia (Slowik et al., 2018). In co-cultures of astrocytes and neurons, activation of ERβ in astrocytes by estradiol leads to neuronal protection after OGD (Ma et al., 2016). Furthermore, estradiol plays a beneficial role in the brain by preventing BBB disruption and leakage during and after ischemic injury (Sohrabji, 2015), and prevents tight junction impairment in cultured brain-derived endothelial cells after OGD (Na et al., 2015). However, whether the effects of estradiol in glia and endothelial cells are mediated by autophagy needs to be examined. It is imperative to determine if sex hormones and X-linked autophagy genes regulate autophagy in specific cell types in the brain.
Conclusions
The incidence, prevalence, and outcomes after ischemic stroke are strongly influenced by sex. Age, gonadal hormone exposure, and the sex chromosome complement are important factors in the sex differences seen in stroke. Elderly women have poorer functional outcomes, and more women live with a disability after stroke compared to men. This is in part due to the older age of women when they have their first stroke. Pre-clinical studies have found that both gonadal hormones and the X-chromosome complement play important roles in the sex-biased outcomes seen after stroke, but their contribution differs throughout the lifespan. Autophagy may be a mechanism that contributes to these differences. In the brain, autophagy is importantly regulated by estrogen. However, despite our knowledge of multiple X-linked autophagy genes, there is no evidence yet that these genes contribute to sex differences in brain autophagy after ischemic stroke.
In males, ischemic stroke activates autophagy. Given that autophagy inhibition reduces volume infarction after stroke in males, we could hypothesize that maladaptive autophagy is more importantly upregulated in male mice than adaptive autophagy. However, while females show reduced brain damage after ischemic stroke compared with males, autophagy stimulation after stroke in female brains is less evident than in males. Thus, inhibiting autophagy benefited males, but not females, an effect that is mediated by gonadal hormones. This could be explained if stroke induces maladaptive autophagy in males. However, in females, the stroke did not stimulate autophagy and the brain tissue from females may retain only basal autophagy levels. Using autophagy inhibitors as 3-MA in females may then block adaptive autophagy, which is beneficial and neuroprotective. Thus, blocking basal autophagy in females may expose brain cells to energy deficits making them more vulnerable to ischemia (Figure 4).
Figure 4.
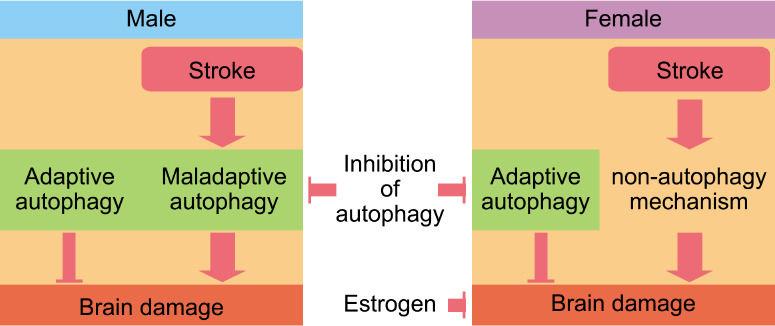
Stroke may upregulate autophagy more in males than in females, which may have alternative mechanisms to respond to the high cellular stress during and after an ischemic stroke.
Inhibition of autophagy appears to be more beneficial for males than for females, likely due to the excessive autophagy triggered by a stroke in males, which leads to cell death and an exacerbation of brain damage. However, during and after stroke, females may maintain basal autophagy that is beneficial for brain function, and hence its inhibition is detrimental for females. In addition, when females are young, they are protected from ischemia due to the neuroprotective effects of estrogen. This is lost after menopause and likely contributes to the vulnerability seen in stroke in older females.
Research in sex differences in autophagy in stroke is still in its infancy, as the mechanisms and signaling pathways that regulate the different steps of autophagy in a sex-dependent manner have not been identified. Furthermore, published studies have important limitations in using multiple autophagy markers for an accurate interpretation of data. Future studies on this topic should follow appropriate guidelines for monitoring autophagy (Klionsky et al., 2021). Using existing tools such as the FCG mouse model, transgenic mice with ATG deletions, and cell and animal models with autophagy-flux reporters will help to identify the mechanisms that regulate autophagy after stroke in a sex-dependent manner, but both sexes need to be evaluated. This knowledge could contribute to the development of therapeutic targets for both men and women.
Footnotes
Funding: This work was supported by the American Heart Association (856061) to JFMM and by the NINDS (R01 5R01NS108779 and 5R01NS094543) to LDM.
Conflicts of interest: The authors declare no conflicts of interest.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Acaz-Fonseca E, Castello-Ruiz M, Burguete MC, Aliena-Valero A, Salom JB, Torregrosa G, Garcia-Segura LM. Insight into the molecular sex dimorphism of ischaemic stroke in rat cerebral cortex:Focus on neuroglobin, sex steroids and autophagy. Eur J Neurosci. 2020;52:2756–2770. doi: 10.1111/ejn.14731. [DOI] [PubMed] [Google Scholar]
- 2.Ajoolabady A, Wang S, Kroemer G, Penninger JM, Uversky VN, Pratico D, Henninger N, Reiter RJ, Bruno A, Joshipura K, Aslkhodapasandhokmabad H, Klionsky DJ, Ren J. Targeting autophagy in ischemic stroke:from molecular mechanisms to clinical therapeutics. Pharmacol Ther. 2021;225:107848. doi: 10.1016/j.pharmthera.2021.107848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Au AK, Chen Y, Du L, Smith CM, Manole MD, Baltagi SA, Chu CT, Aneja RK, Bayir H, Kochanek PM, Clark RS. Ischemia-induced autophagy contributes to neurodegeneration in cerebellar Purkinje cells in the developing rat brain and in primary cortical neurons in vitro. Biochim Biophys Acta. 2015;1852:1902–1911. doi: 10.1016/j.bbadis.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augereau P, Miralles F, Cavailles V, Gaudelet C, Parker M, Rochefort H. Characterization of the proximal estrogen-responsive element of human cathepsin D gene. Mol Endocrinol. 1994;8:693–703. doi: 10.1210/mend.8.6.7935485. [DOI] [PubMed] [Google Scholar]
- 5.Azcoitia I, Barreto GE, Garcia-Segura LM. Molecular mechanisms and cellular events involved in the neuroprotective actions of estradiol. Analysis of sex differences. Front Neuroendocrinol. 2019;55:100787. doi: 10.1016/j.yfrne.2019.100787. [DOI] [PubMed] [Google Scholar]
- 6.Ballerio R, Gianazza E, Mussoni L, Miller I, Gelosa P, Guerrini U, Eberini I, Gemeiner M, Belcredito S, Tremoli E, Sironi L. Gender differences in endothelial function and inflammatory markers along the occurrence of pathological events in stroke-prone rats. Exp Mol Pathol. 2007;82:33–41. doi: 10.1016/j.yexmp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, et al. Heart Disease and Stroke Statistics-2017 Update:a report from the American Heart Association. Circulation. 2017:135, e146–603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blessing AM, Rajapakshe K, Reddy Bollu L, Shi Y, White MA, Pham AH, Lin C, Jonsson P, Cortes CJ, Cheung E, La Spada AR, Bast RC, Jr, Merchant FA, Coarfa C, Frigo DE. Transcriptional regulation of core autophagy and lysosomal genes by the androgen receptor promotes prostate cancer progression. Autophagy. 2017;13:506–521. doi: 10.1080/15548627.2016.1268300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bobrowska A, Paganetti P, Matthias P, Bates GP. Hdac6 knock-out increases tubulin acetylation but does not modify disease progression in the R6/2 mouse model of Huntington's disease. PLoS One. 2011;6:e20696. doi: 10.1371/journal.pone.0020696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bushnell CD, Chaturvedi S, Gage KR, Herson PS, Hurn PD, Jimenez MC, Kittner SJ, Madsen TE, McCullough LD, McDermott M, Reeves MJ, Rundek T. Sex differences in stroke:challenges and opportunities. J Cereb Blood Flow Metab. 2018;38:2179–2191. doi: 10.1177/0271678X18793324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cambiasso MJ, Cisternas CD, Ruiz-Palmero I, Scerbo MJ, Arevalo MA, Azcoitia I, Garcia-Segura LM. Interaction of sex chromosome complement, gonadal hormones and neuronal steroid synthesis on the sexual differentiation of mammalian neurons. J Neurogenet. 2017;31:300–306. doi: 10.1080/01677063.2017.1390572. [DOI] [PubMed] [Google Scholar]
- 12.Carvill GL, Liu A, Mandelstam S, Schneider A, Lacroix A, Zemel M, McMahon JM, Bello-Espinosa L, Mackay M, Wallace G, Waak M, Zhang J, Yang X, Malone S, Zhang YH, Mefford HC, Scheffer IE. Severe infantile onset developmental and epileptic encephalopathy caused by mutations in autophagy gene WDR45. Epilepsia. 2018;59:e5–13. doi: 10.1111/epi.13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhury S, Otomo C, Leitner A, Ohashi K, Aebersold R, Lander GC, Otomo T. Insights into autophagosome biogenesis from structural and biochemical analyses of the ATG2A-WIPI4 complex. Proc Natl Acad Sci U S A. 2018;115:E9792–9801. doi: 10.1073/pnas.1811874115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Congdon EE. Sex differences in autophagy contribute to female vulnerability in Alzheimer's disease. Front Neurosci. 2018;12:372. doi: 10.3389/fnins.2018.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook S, Hung V, Duncan KA. Crosstalk between estrogen withdrawal and NFkappaB signaling following penetrating brain injury. Neuroimmunomodulation. 2018;25:193–200. doi: 10.1159/000493506. [DOI] [PubMed] [Google Scholar]
- 16.Demarest TG, Waite EL, Kristian T, Puche AC, Waddell J, McKenna MC, Fiskum G. Sex-dependent mitophagy and neuronal death following rat neonatal hypoxia-ischemia. Neuroscience. 2016;335:103–113. doi: 10.1016/j.neuroscience.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du L, Hickey RW, Bayir H, Watkins SC, Tyurin VA, Guo F, Kochanek PM, Jenkins LW, Ren J, Gibson G, Chu CT, Kagan VE, Clark RS. Starving neurons show sex difference in autophagy. J Biol Chem. 2009;284:2383–2396. doi: 10.1074/jbc.M804396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunbar M, Mineyko A, Hill M, Hodge J, Floer A, Kirton A. Population based birth prevalence of disease-specific perinatal stroke. Pediatrics. 2020;146:e2020013201. doi: 10.1542/peds.2020-013201. [DOI] [PubMed] [Google Scholar]
- 19.Espinosa-Garcia C, Atif F, Yousuf S, Sayeed I, Neigh GN, Stein DG. Progesterone attenuates stress-induced NLRP3 inflammasome activation and enhances autophagy following ischemic brain injury. Int J Mol Sci. 2020;21:3740. doi: 10.3390/ijms21113740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez-Guasti A, Kruijver FP, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol. 2000;425:422–435. doi: 10.1002/1096-9861(20000925)425:3<422::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 21.FitzPatrick LM, Hawkins KE, Delhove J, Fernandez E, Soldati C, Bullen LF, Nohturfft A, Waddington SN, Medina DL, Bolanos JP, McKay TR. NF-kappaB activity initiates human ESC-derived neural progenitor cell differentiation by inducing a metabolic maturation program. Stem Cell Reports. 2018;10:1766–1781. doi: 10.1016/j.stemcr.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forte M, Bianchi F, Cotugno M, Marchitti S, De Falco E, Raffa S, Stanzione R, Di Nonno F, Chimenti I, Palmerio S, Pagano F, Petrozza V, Micaloni A, Madonna M, Relucenti M, Torrisi MR, Frati G, Volpe M, Rubattu S, Sciarretta S. Pharmacological restoration of autophagy reduces hypertension-related stroke occurrence. Autophagy. 2020;16:1468–1481. doi: 10.1080/15548627.2019.1687215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu L, Liu Y, Wang J, Sun Y, Zhang L, Wu T, Li Y, Wang B, Huang S, Bu H, Sun H. Cardioprotection by low-dose of estrogen and testosterone at the physiological ratio on ovariectomized rats during ischemia/reperfusion injury. J Cardiovasc Pharmacol. 2017;70:87–93. doi: 10.1097/FJC.0000000000000497. [DOI] [PubMed] [Google Scholar]
- 24.Guo JM, Shu H, Wang L, Xu JJ, Niu XC, Zhang L. SIRT1-dependent AMPK pathway in the protection of estrogen against ischemic brain injury. CNS Neurosci Ther. 2017a;23:360–369. doi: 10.1111/cns.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Y, Ma Y, Zhang Y, Zhou L, Huang S, Wen Y, Zou F, Cheng J. Autophagy-related gene microarray and bioinformatics analysis for ischemic stroke detection. Biochem Biophys Res Commun. 2017b;489:48–55. doi: 10.1016/j.bbrc.2017.05.099. [DOI] [PubMed] [Google Scholar]
- 26.Hadley G, Beard DJ, Couch Y, Neuhaus AA, Adriaanse BA, DeLuca GC, Sutherland BA, Buchan AM. Rapamycin in ischemic stroke:Old drug, new tricks? J Cereb Blood Flow Metab. 2019;39:20–35. doi: 10.1177/0271678X18807309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N, Stanczyk FZ, Selzer RH, Azen SP, Group ER. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374:1221–1231. doi: 10.1056/NEJMoa1505241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard VJ, Madsen TE, Kleindorfer DO, Judd SE, Rhodes JD, Soliman EZ, Kissela BM, Safford MM, Moy CS, McClure LA, Howard G, Cushman M. Sex and race differences in the association of incident ischemic stroke with risk factors. JAMA Neurol. 2019;76:179–186. doi: 10.1001/jamaneurol.2018.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh DJ, Kuo WW, Lai YP, Shibu MA, Shen CY, Pai P, Yeh YL, Lin JY, Viswanadha VP, Huang CY. 17beta-Estradiol and/or estrogen receptor beta attenuate the autophagic and apoptotic effects induced by prolonged hypoxia through HIF-1alpha-mediated BNIP3 and IGFBP-3 signaling blockage. Cell Physiol Biochem. 2015;36:274–284. doi: 10.1159/000374070. [DOI] [PubMed] [Google Scholar]
- 30.Kim HN, Lee SJ, Koh JY. The neurosteroids, allopregnanolone and progesterone, induce autophagy in cultured astrocytes. Neurochem Int. 2012;60:125–133. doi: 10.1016/j.neuint.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Kim TY, Cho KS, Kim HN, Koh JY. Autophagy activation and neuroprotection by progesterone in the G93A-SOD1 transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2013;59:80–85. doi: 10.1016/j.nbd.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Kim YC, Guan KL. mTOR:a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S, Abeliovich H, Abildgaard MH, Abudu YP, Acevedo-Arozena A, Adamopoulos IE, Adeli K, Adolph TE, Adornetto A, Aflaki E, Agam G, Agarwal A, Aggarwal BB, Agnello M, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1) Autophagy. 2021;17:1–382. doi: 10.1080/15548627.2020.1797280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korvatska O, Strand NS, Berndt JD, Strovas T, Chen DH, Leverenz JB, Kiianitsa K, Mata IF, Karakoc E, Greenup JL, Bonkowski E, Chuang J, Moon RT, Eichler EE, Nickerson DA, Zabetian CP, Kraemer BC, Bird TD, Raskind WH. Altered splicing of ATP6AP2 causes X-linked parkinsonism with spasticity (XPDS) Hum Mol Genet. 2013;22:3259–3268. doi: 10.1093/hmg/ddt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kulkarni A, Chen J, Maday S. Neuronal autophagy and intercellular regulation of homeostasis in the brain. Curr Opin Neurobiol. 2018;51:29–36. doi: 10.1016/j.conb.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol. 2010;189:671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, McCullough LD. Effects of AMP-activated protein kinase in cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:480–492. doi: 10.1038/jcbfm.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Chen J, Sun S, Zhao J, Dong X, Wang J. Effects of estradiol on autophagy and Nrf-2/ARE signals after cerebral ischemia. Cell Physiol Biochem. 2017;41:2027–2036. doi: 10.1159/000475433. [DOI] [PubMed] [Google Scholar]
- 39.Li S, Hu W, Deng F, Chen S, Zhu P, Wang M, Chen X, Wang Y, Hu X, Zhao B, Zhong W, Ma G, Li Y. Identification of circular RNA hsa_circ_0001599 as a novel biomarker for large-artery atherosclerotic stroke. DNA Cell Biol. 2021;40:457–468. doi: 10.1089/dna.2020.5662. [DOI] [PubMed] [Google Scholar]
- 40.Li WL, Yu SP, Chen D, Yu SS, Jiang YJ, Genetta T, Wei L. The regulatory role of NF-kappaB in autophagy-like cell death after focal cerebral ischemia in mice. Neuroscience. 2013;244:16–30. doi: 10.1016/j.neuroscience.2013.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li XZ, Sui CY, Chen Q, Chen XP, Zhang H, Zhou XP. Upregulation of cell surface estrogen receptor alpha is associated with the mitogen-activated protein kinase/extracellular signal-regulated kinase activity and promotes autophagy maturation. Int J Clin Exp Pathol. 2015;8:8832–8841. [PMC free article] [PubMed] [Google Scholar]
- 42.Liu F, Benashski SE, Xu Y, Siegel M, McCullough LD. Effects of chronic and acute oestrogen replacement therapy in aged animals after experimental stroke. J Neuroendocrinol. 2012;24:319–330. doi: 10.1111/j.1365-2826.2011.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Zhu Z, Wang L, Du J, Zhang B, Feng X, Zhang G. Functional suppression of Ripk1 blocks the NF-kappaB signaling pathway and induces neuron autophagy after traumatic brain injury. Mol Cell Biochem. 2020;472:105–114. doi: 10.1007/s11010-020-03789-5. [DOI] [PubMed] [Google Scholar]
- 44.Liu W, Shang G, Yang S, Huang J, Xue X, Lin Y, Zheng Y, Wang X, Wang L, Lin R, Tao J, Chen L. Electroacupuncture protects against ischemic stroke by reducing autophagosome formation and inhibiting autophagy through the mTORC1-ULK1 complex-Beclin1 pathway. Int J Mol Med. 2016;37:309–318. doi: 10.3892/ijmm.2015.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Zhao L, Ju Y, Li W, Zhang M, Jiao Y, Zhang J, Wang S, Wang Y, Zhao M, Zhang B, Zhao Y. A novel androstenedione derivative induces ROS-mediated autophagy and attenuates drug resistance in osteosarcoma by inhibiting macrophage migration inhibitory factor (MIF) Cell Death Dis. 2014;5:e1361. doi: 10.1038/cddis.2014.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loeffler DA. Influence of normal aging on brain autophagy:a complex scenario. Front Aging Neurosci. 2019;11:49. doi: 10.3389/fnagi.2019.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu N, Li X, Tan R, An J, Cai Z, Hu X, Wang F, Wang H, Lu C, Lu H. HIF-1alpha/Beclin1-mediated autophagy is involved in neuroprotection induced by hypoxic preconditioning. J Mol Neurosci. 2018;66:238–250. doi: 10.1007/s12031-018-1162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma Y, Guo H, Zhang L, Tao L, Yin A, Liu Z, Li Y, Dong H, Xiong L, Hou W. Estrogen replacement therapy-induced neuroprotection against brain ischemia-reperfusion injury involves the activation of astrocytes via estrogen receptor beta. Sci Rep. 2016;6:21467. doi: 10.1038/srep21467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madsen TE, Khoury JC, Leppert M, Alwell K, Moomaw CJ, Sucharew H, Woo D, Ferioli S, Martini S, Adeoye O, Khatri P, Flaherty M, De Los Rios La Rosa F, Mackey J, Mistry E, Demel SL, Coleman E, Jasne A, Slavin SJ, Walsh K, et al. Temporal trends in stroke incidence over time by sex and age in the GCNKSS. Stroke. 2020;51:1070–1076. doi: 10.1161/STROKEAHA.120.028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol. 2013;249:120–131. doi: 10.1016/j.expneurol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manwani B, Bentivegna K, Benashski SE, Venna VR, Xu Y, Arnold AP, McCullough LD. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J Cereb Blood Flow Metab. 2015;35:221–229. doi: 10.1038/jcbfm.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mao JY, Su LX, Li DK, Zhang HM, Wang XT, Liu DW. The effects of UCP2 on autophagy through the AMPK signaling pathway in septic cardiomyopathy and the underlying mechanism. Ann Transl Med. 2021;9:259. doi: 10.21037/atm-20-4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marks H, Kerstens HH, Barakat TS, Splinter E, Dirks RA, van Mierlo G, Joshi O, Wang SY, Babak T, Albers CA, Kalkan T, Smith A, Jouneau A, de Laat W, Gribnau J, Stunnenberg HG. Dynamics of gene silencing during X inactivation using allele-specific RNA-seq. Genome Biol. 2015;16:149. doi: 10.1186/s13059-015-0698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maron BJ, Roberts WC, Arad M, Haas TS, Spirito P, Wright GB, Almquist AK, Baffa JM, Saul JP, Ho CY, Seidman J, Seidman CE. Clinical outcome and phenotypic expression in LAMP2 cardiomyopathy. JAMA. 2009;301:1253–1259. doi: 10.1001/jama.2009.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, De Vries GJ, Epperson CN, Govindan R, Klein SL, Lonardo A, Maki PM, McCullough LD, Regitz-Zagrosek V, Regensteiner JG, Rubin JB, Sandberg K, Suzuki A. Sex and gender:modifiers of health, disease, and medicine. Lancet. 2020;396:565–582. doi: 10.1016/S0140-6736(20)31561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCullough LD, Mirza MA, Xu Y, Bentivegna K, Steffens EB, Ritzel R, Liu F. Stroke sensitivity in the aged:sex chromosome complement vs. gonadal hormones. Aging (Albany NY) 2016;8:1432–1441. doi: 10.18632/aging.100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menon MB, Dhamija S. Beclin 1 phosphorylation - at the center of autophagy regulation. Front Cell Dev Biol. 2018;6:137. doi: 10.3389/fcell.2018.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Na W, Lee JY, Kim WS, Yune TY, Ju BG. 17beta-estradiol ameliorates tight junction disruption via repression of MMP transcription. Mol Endocrinol. 2015;29:1347–1361. doi: 10.1210/ME.2015-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen DK, Disteche CM. Dosage compensation of the active X chromosome in mammals. Nat Genet. 2006;38:47–53. doi: 10.1038/ng1705. [DOI] [PubMed] [Google Scholar]
- 60.Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, Shimizu S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 61.Nishino I, Fu J, Tanji K, Yamada T, Shimojo S, Koori T, Mora M, Riggs JE, Oh SJ, Koga Y, Sue CM, Yamamoto A, Murakami N, Shanske S, Byrne E, Bonilla E, Nonaka I, DiMauro S, Hirano M. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- 62.Patrizz AN, Moruno-Manchon JF, O'Keefe LM, Doran SJ, Patel AR, Venna VR, Tsvetkov AS, Li J, McCullough LD. Sex-specific differences in autophagic responses to experimental ischemic stroke. Cells. 2021;10:1825. doi: 10.3390/cells10071825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pavel M, Rubinsztein DC. Mammalian autophagy and the plasma membrane. FEBS J. 2017;284:672–679. doi: 10.1111/febs.13931. [DOI] [PubMed] [Google Scholar]
- 64.Perez-Alvarez MJ, Villa Gonzalez M, Benito-Cuesta I, Wandosell FG. Role of mTORC1 controlling proteostasis after brain ischemia. Front Neurosci. 2018;12:60. doi: 10.3389/fnins.2018.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perez-Alvarez MJ, Maza Mdel C, Anton M, Ordonez L, Wandosell F. Post-ischemic estradiol treatment reduced glial response and triggers distinct cortical and hippocampal signaling in a rat model of cerebral ischemia. J Neuroinflammation. 2012;9:157. doi: 10.1186/1742-2094-9-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qi S, Al Mamun A, Ngwa C, Romana S, Ritzel R, Arnold AP, McCullough LD, Liu F. X chromosome escapee genes are involved in ischemic sexual dimorphism through epigenetic modification of inflammatory signals. J Neuroinflammation. 2021;18:70. doi: 10.1186/s12974-021-02120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ra EA, Lee TA, Won Kim S, Park A, Choi HJ, Jang I, Kang S, Hee Cheon J, Cho JW, Eun Lee J, Lee S, Park B. TRIM31 promotes Atg5/Atg7-independent autophagy in intestinal cells. Nat Commun. 2016;7:11726. doi: 10.1038/ncomms11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rothaug M, Stroobants S, Schweizer M, Peters J, Zunke F, Allerding M, D'Hooge R, Saftig P, Blanz J. LAMP-2 deficiency leads to hippocampal dysfunction but normal clearance of neuronal substrates of chaperone-mediated autophagy in a mouse model for Danon disease. Acta Neuropathol Commun. 2015;3:6. doi: 10.1186/s40478-014-0182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saraceno GE, Bellini MJ, Garcia-Segura LM, Capani F. Estradiol activates PI3K/Akt/GSK3 pathway under chronic neurodegenerative conditions triggered by perinatal asphyxia. Front Pharmacol. 2018;9:335. doi: 10.3389/fphar.2018.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Selvamani A, Sohrabji F. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging. 2010;31:1618–1628. doi: 10.1016/j.neurobiolaging.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shang D, Wang L, Klionsky DJ, Cheng H, Zhou R. Sex differences in autophagy-mediated diseases:toward precision medicine. Autophagy. 2021;17:1065–1076. doi: 10.1080/15548627.2020.1752511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shay DA, Vieira-Potter VJ, Rosenfeld CS. Sexually dimorphic effects of aromatase on neurobehavioral responses. Front Mol Neurosci. 2018;11:374. doi: 10.3389/fnmol.2018.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shvetsova E, Sofronova A, Monajemi R, Gagalova K, Draisma HHM, White SJ, Santen GWE, Chuva de Sousa Lopes SM, Heijmans BT, van Meurs J, Jansen R, Franke L, Kielbasa SM, den Dunnen JT, t Hoen PAC, consortium B, Go NLc. Skewed X-inactivation is common in the general female population. Eur J Hum Genet. 2019;27:455–465. doi: 10.1038/s41431-018-0291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Slowik A, Lammerding L, Zendedel A, Habib P, Beyer C. Impact of steroid hormones E2 and P on the NLRP3/ASC/Casp1 axis in primary mouse astroglia and BV-2 cells after in vitro hypoxia. J Steroid Biochem Mol Biol. 2018;183:18–26. doi: 10.1016/j.jsbmb.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 75.Sohrabji F. Estrogen-IGF-1 interactions in neuroprotection:ischemic stroke as a case study. Front Neuroendocrinol. 2015;36:1–14. doi: 10.1016/j.yfrne.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sohrabji F, Okoreeh A, Panta A. Sex hormones and stroke:beyond estrogens. Horm Behav. 2019;111:87–95. doi: 10.1016/j.yhbeh.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spinazzi M, Fanin M, Melacini P, Nascimbeni AC, Angelini C. Cardioembolic stroke in Danon disease. Clin Genet. 2008;73:388–390. doi: 10.1111/j.1399-0004.2008.00971.x. [DOI] [PubMed] [Google Scholar]
- 78.Stanga D, Zhao Q, Milev MP, Saint-Dic D, Jimenez-Mallebrera C, Sacher M. TRAPPC11 functions in autophagy by recruiting ATG2B-WIPI4/WDR45 to preautophagosomal membranes. Traffic. 2019;20:325–345. doi: 10.1111/tra.12640. [DOI] [PubMed] [Google Scholar]
- 79.Tang BL. RAB39B's role in membrane traffic, autophagy, and associated neuropathology. J Cell Physiol. 2021;236:1579–1592. doi: 10.1002/jcp.29962. [DOI] [PubMed] [Google Scholar]
- 80.Tower J, Pomatto LCD, Davies KJA. Sex differences in the response to oxidative and proteolytic stress. Redox Biol. 2020;31:101488. doi: 10.1016/j.redox.2020.101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Uchida M, Palmateer JM, Herson PS, DeVries AC, Cheng J, Hurn PD. Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. J Cereb Blood Flow Metab. 2009;29:1454–1462. doi: 10.1038/jcbfm.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vousden DA, Corre C, Spring S, Qiu LR, Metcalf A, Cox E, Lerch JP, Palmert MR. Impact of X/Y genes and sex hormones on mouse neuroanatomy. Neuroimage. 2018;173:551–563. doi: 10.1016/j.neuroimage.2018.02.051. [DOI] [PubMed] [Google Scholar]
- 83.Wang M, Lee H, Elkin K, Bardhi R, Guan L, Chandra A, Geng X, Ding Y. Detrimental and beneficial effect of autophagy and a potential therapeutic target after ischemic stroke. Evid Based Complement Alternat Med. 2020a;2020:8372647. doi: 10.1155/2020/8372647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang X, Fang Y, Huang Q, Xu P, Lenahan C, Lu J, Zheng J, Dong X, Shao A, Zhang J. An updated review of autophagy in ischemic stroke:From mechanisms to therapies. Exp Neurol. 2021;340:113684. doi: 10.1016/j.expneurol.2021.113684. [DOI] [PubMed] [Google Scholar]
- 85.Wang X, Allen M, Li S, Quicksall ZS, Patel TA, Carnwath TP, Reddy JS, Carrasquillo MM, Lincoln SJ, Nguyen TT, Malphrus KG, Dickson DW, Crook JE, Asmann YW, Ertekin-Taner N. Deciphering cellular transcriptional alterations in Alzheimer's disease brains. Mol Neurodegener. 2020b;15:38. doi: 10.1186/s13024-020-00392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, Meng C, Zhang J, Wu J, Zhao J. Inhibition of GSK-3beta alleviates cerebral ischemia/reperfusion injury in rats by suppressing NLRP3 inflammasome activation through autophagy. Int Immunopharmacol. 2019;68:234–241. doi: 10.1016/j.intimp.2018.12.042. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y, Gu XX, Huang HT, Liu CH, Wei YS. A genetic variant in the promoter of lncRNA MALAT1 is related to susceptibility of ischemic stroke. Lipids Health Dis. 2020c;19:57. doi: 10.1186/s12944-020-01236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Warfvinge K, Krause DN, Maddahi A, Edvinsson JCA, Edvinsson L, Haanes KA. Estrogen receptors alpha, beta and GPER in the CNS and trigeminal system -molecular and functional aspects. J Headache Pain. 2020;21:131. doi: 10.1186/s10194-020-01197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weis SN, Toniazzo AP, Ander BP, Zhan X, Careaga M, Ashwood P, Wyse AT, Netto CA, Sharp FR. Autophagy in the brain of neonates following hypoxia-ischemia shows sex- and region-specific effects. Neuroscience. 2014;256:201–209. doi: 10.1016/j.neuroscience.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 90.Xiang J, Liu X, Ren J, Chen K, Wang HL, Miao YY, Qi MM. How does estrogen work on autophagy? Autophagy. 2019;15:197–211. doi: 10.1080/15548627.2018.1520549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu X, Long YC, Shen HM. Differential regulatory functions of three classes of phosphatidylinositol and phosphoinositide 3-kinases in autophagy. Autophagy. 2015;11:1711–1728. doi: 10.1080/15548627.2015.1043076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yun J, Yeo IJ, Hwang CJ, Choi DY, Im HS, Kim JY, Choi WR, Jung MH, Han SB, Hong JT. Estrogen deficiency exacerbates Abeta-induced memory impairment through enhancement of neuroinflammation, amyloidogenesis and NF-kB activation in ovariectomized mice. Brain Behav Immun. 2018;73:282–293. doi: 10.1016/j.bbi.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 93.Zhang A, Song Y, Zhang Z, Jiang S, Chang S, Cai Z, Liu F, Zhang X, Ni G. Effects of autophagy inhibitor 3-Methyladenine on ischemic stroke:a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021a;100:e23873. doi: 10.1097/MD.0000000000023873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang DM, Zhang T, Wang MM, Wang XX, Qin YY, Wu J, Han R, Sheng R, Wang Y, Chen Z, Han F, Ding Y, Li M, Qin ZH. TIGAR alleviates ischemia/reperfusion-induced autophagy and ischemic brain injury. Free Radic Biol Med. 2019;137:13–23. doi: 10.1016/j.freeradbiomed.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 95.Zhang H, Lin S, McElroy CL, Wang B, Jin D, Uteshev VV, Jin K. Circulating pro-inflammatory exosomes worsen stroke outcomes in aging. Circ Res. 2021b;129:e121–140. doi: 10.1161/CIRCRESAHA.121.318897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Y, Miao JM. Ginkgolide K promotes astrocyte proliferation and migration after oxygen-glucose deprivation via inducing protective autophagy through the AMPK/mTOR/ULK1 signaling pathway. Eur J Pharmacol. 2018;832:96–103. doi: 10.1016/j.ejphar.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Z, Qin P, Deng Y, Ma Z, Guo H, Guo H, Hou Y, Wang S, Zou W, Sun Y, Ma Y, Hou W. The novel estrogenic receptor GPR30 alleviates ischemic injury by inhibiting TLR4-mediated microglial inflammation. J Neuroinflammation. 2018;15:206. doi: 10.1186/s12974-018-1246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao YG, Sun L, Miao G, Ji C, Zhao H, Sun H, Miao L, Yoshii SR, Mizushima N, Wang X, Zhang H. The autophagy gene Wdr45/Wipi4 regulates learning and memory function and axonal homeostasis. Autophagy. 2015;11:881–890. doi: 10.1080/15548627.2015.1047127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu T, Zhao D, Song Z, Yuan Z, Li C, Wang Y, Zhou X, Yin X, Hassan MF, Yang L. HDAC6 alleviates prion peptide-mediated neuronal death via modulating PI3K-Akt-mTOR pathway. Neurobiol Aging. 2016;37:91–102. doi: 10.1016/j.neurobiolaging.2015.09.021. [DOI] [PubMed] [Google Scholar]


