Abstract
A simple method for the nontoxic, specific, and efficient secretion of active single-chain Fv antibodies (scFvs) into the supernatants of Escherichia coli cultures is reported. The method is based on the well-characterized hemolysin transport system (Hly) of E. coli that specifically secretes the target protein from the bacterial cytoplasm into the extracellular medium without a periplasmic intermediate. The culture media that accumulate these Hly-secreted scFv's can be used in a variety of immunoassays without purification. In addition, these culture supernatants are stable over long periods of time and can be handled basically as immune sera.
The current methodology for the selection and production of antibody fragments in Escherichia coli involves the generation of large repertories of human or murine immunoglobulin (Ig) V gene segments, either from naive or immunized individuals, and its cloning in filamentous phage (or phagemid) vectors that allow both the phage display and the production of the reconstructed antibody Fv fragments (17, 19, 25, 27). After a selection (biopanning) of Fv clones capable of binding a given antigen, the recombinant Fv antibodies are produced individually in E. coli and tested for their antigen-binding properties (16, 22). The standard Ig fragments produced in E. coli are the so-called single-chain Fv (scFv) in which the variable domains from the heavy (VH) and light (VL) chains are linked in a single polypeptide. The standard protocol for production of scFv's require their translocation to the periplasmic space using an N-terminal signal peptide (SP) that is recognized by the general secretion pathway of E. coli, encoded by the sec genes, and which is responsible for the export of most cellular proteins targeted to the extracytoplasmic compartments (12, 31). Next, the scFv polypeptides are purified, using chromatographic techniques, from periplasmic protein extracts obtained from those cells (30).
Besides being time-consuming, the major problem associated with the production of scFv in E. coli is the toxicity caused by their periplasmic export and accumulation, which eventually leads to the lysis of the bacterial cell (25, 30). The export of scFvs gives rise to a number of toxic effects, such as the jamming of the Sec pathway, the titration of periplasmic-folding catalysts, the induction of periplasmic proteases, and an enhanced outer membrane permeability (3, 6, 7, 20, 32). All of these events have important biotechnological consequences, such as low production yields and the formation of scFv aggregates. Thus, an ideal method for scFv production should allow their secretion to the extracellular space without a periplasmic intermediate and by a Sec-independent pathway.
The hemolysin transport system (Hly) is a type I secretory apparatus that forms a protein channel between the inner and outer membranes of E. coli through which the hemolysin toxin (HlyA) is secreted (5). The protein machinery of Hly is independent of the cellular sec genes and consists in two inner membrane components, HlyB and HlyD, and the outer membrane protein TolC. The HlyB-HlyD complex recognizes the last ∼60 amino acids of the C terminus of HlyA as the secretion signal and, therefore, there is no N-terminal SP involved. The HlyA secretion is a posttranslational process that is thought to occur without a periplasmic intermediate by the direct passage of the HlyA polypeptide from the cytoplasm to the extracellular medium (5, 34). A conformational change, energized by the hydrolysis of ATP in HlyB, allows the translocation of HlyA from the cytoplasm through the hydrophilic pore formed in the outer membrane by TolC oligomers (23, 24, 34). Importantly, the Hly system has been proved competent for the secretion of heterologous hybrid proteins, including single Ig domains, containing the C domain of HlyA fused at their C terminus (5, 21). These features prompted us to envision the E. coli Hly system as an attractive candidate for the secretion of scFv's into the extracellular medium.
MATERIALS AND METHODS
Bacterial strains, growth, and induction conditions.
All of the bacterial strains used here were derivatives of E. coli K-12 and are listed in Table 1. Bacteria harboring the plasmids indicated in each case were grown at 30°C in Luria-Bertani (LB) medium-agar plates (26) containing 2% (wt/vol) glucose (for repressing the lac promoter) and the antibiotics appropriate for plasmid selection. For induction of scFv and HlyA derivatives, single colonies were inoculated in liquid LB medium containing 2% (wt/vol) glucose and grown at 30 or 37°C until reaching an optical density at 600 nm (OD600) of ∼0.5. At this point bacteria were harvested by centrifugation, resuspended at the same density in LB medium containing 0.25 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and further incubated (at 30 or 37°C) for 4 to 16 h, as indicated. Expression of scFv's in the periplasm of E. coli was induced at 30°C, unless noted otherwise. Secretion of HlyA derivatives was carried out at either 30 or 37°C, as indicated. Antibiotics were added to the culture media at the following concentrations: ampicillin, 100 μg/ml; chloramphenicol, 40 μg/ml.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| E. coli HB2151 | Δlac-pro ara Nalr thi F′ (proAB lacIq lacZΔM15) | 9 |
| E. coli XL1-Blue | endA1 hsdR17 supE44 thi-1 recA1 gyrA96 relA1 lac F′(proAB lacIqlacZΔM15 Tn10) | 8 |
| Plasmids | ||
| pCANTAB-5E | Apr; vector for periplasmic production of scFv's with E-tag epitope at C terminus | Pharmacia |
| pCANTAB-5Ehis | Apr; pCANTAB-5E with His6 tag incorporated before E-tag | This work |
| p6AC3g3 | Apr; scFv 6AC3 cloned between SfiI-NotI sites of pCANTAB-5Ehis | This work |
| pB4g3 | Apr; scFv B4 cloned between SfiI-NotI sites of pCANTAB-5Ehis | This work |
| pVDL9.3 | Cmr; production of HlyB and HlyD transporters | 35 |
| pLG612-1B | Apr; 23-kDa C-terminal domain of HlyA | 35 |
| pLG612-1SD | Apr; 23-kDa C-terminal domain of HlyA with Shine-Dalgarno sequence and NcoI site | 35 |
| pEHlyA | Apr; same as pLG612-1B with E-tag epitope incorporated at the 23-kDa C domain of HlyA | This work |
| pEHlyA1SD | Apr; same as pLG612-1SD with E-tag epitope incorporated at the 23-kDa C domain of HlyA | This work |
| pEHlyA2-SD | Apr; same as pEHlyA1SD with polylinker for cloning of scFv's in frame with E-tagged ′hlyA | This work |
| p6AC3HLYA | Apr; scFv 6AC3 cloned between NcoI and XmaI sites in pEHlyA2-SD | This work |
Apr, ampicillin resistant; Cmr, chloramphenicol resistant.
DNA constructs, oligonucleotides, and plasmids.
DNA manipulations and PCR were made using standard methods (2). Oligonucleotides were synthesized by Isogen Bioscience BV. The plasmids used in this study are listed in Table 1. The scFv 6AC3 was assembled in a VH-linker-VL DNA fragment in a homology-driven reaction using Taq DNA polymerase and according to published protocols (25). The DNA sequence of 6AC3 VH was amplified from plasmid pINHC6A (10) with the degenerated oligonucleotides VH1BACK (5′-AAG TSM ARC TGC AGS AGT CWG G-3′) and VH1FOR-2 (5′-TGA GGA GAC GGT GAC CGT GGT CCC TTG GCC CC-3′). Similarly, the 6AC3 VL segment was amplified from plasmid pINLC6A (10) with oligonucleotides VKBACK-2 (5′-GAC ATT GAG CTC ACC CAG TCT C-3′) and MJK4FONX (5′-CCG TTT TAT TTC CAA CTT TGT CCC-3′). The DNA encoding the (Gly4Ser)3 linker was amplified from the sequence of a preexisting scFv named B4 (4) using oligonucleotides LINKBACK (5′-GGG ACC ACG GTC ACC GTC TCC TCA-3′) and LINKFOR5′-2 (5′-GAG ACT GGG TGA GCT CAA TGT C-3′). To construct pEHlyA2-SD, the plasmid pLG612-1SD (35) was linearized with XmaI and religated in the presence of the E-tag XmaI linker (obtained by hybridizing the oligonucleotides 5′-CC GGG GGT GCG CCG GTG CCG TAT CCG GAT CCG CTG GAA CCG G-3′ and 5′-CC GGC CGG TTC CAG CGG ATC CGG ATA CGG CAC CGG CGC ACC C-3′). The plasmid obtained (pEHlyA1-SD) was then digested with NcoI and SalI and religated in the presence of the SfiI linker (generated by hybridizing the oligonucleotides 5′-C ATG GCT AGC ACG GCC TCG GGG GCC GCG-3′ and 5′-T CGA CGC GGC CCC CGA GGC CGT GCT AGC-3′) to finally produce pEHlyA2-SD. The plasmid pEHlyA was constructed by religation of XmaI-linearized pLG612-1B (35) in the presence of E-tag XmaI linker (see above). Plasmid p6AC3HlyA was obtained by cloning into pEHlyA2-SD, a ∼0.7-kb NcoI-XmaI insert, encoding the SP-less scFv 6AC3, amplified from p6AC3g3 with oligonucleotides NcoI-VHBACK (5′-GCC CAG CCG GCC ATG GCC CAG GT-3′) and JK4-XmaI (5′-TGC GGC CCC GGG TTT TAT TTC CAA CTT-3′). To construct p6AC3g3 (Apr), the ∼0.7-kb DNA encoding 6AC3 scFv was cloned, after SfiI and NotI digestion, into the phagemid pCANTAB5-Ehis. This vector is a variant of pCANTAB5-E (Apr; Pharmacia), in which inserts become added with a His6 tag because of the presence at the former NotI site of the artificial linker Not-His6 which results from hybridizing the oligonucleotides Not-His6-A (5′-G GCC GCA CAT CAT CAT CAC CAT CAC GTG GG-3′) and Not-His6-B (5′-GGC CCC CAC GTG ATG GTG ATG ATG ATG TGC-3′). Control construct pB4g3 (Apr) was obtained by placing the 0.7-kb SfiI-NotI insert from pHEN1-B4 (4) into the same sites of pCANTAB5-Ehis.
ELISAs.
Enzyme-linked immunosorbent assays (ELISAs) were performed at room temperature (RT) as follows: purified transmissible gastroenteritis virus (TGEV; 5 μg/ml) (10) or ovalbumin (10 μg/ml; Sigma) as a negative control was adsorbed for 2 h to ELISA plates (Maxisorb; Nunc) in 50 mM NaHCO3 (pH 9.0). The excess antigen was washed out, and the plates were blocked for 2 h in B buffer (3% [wt/vol] skim milk and 1% [wt/vol] bovine serum albumin [BSA] in phosphate-buffered saline [PBS]). Primary antibodies (scFvs or monoclonal antibodies [MAbs]), prepared in INC buffer (PBS, 1% [wt/vol] BSA, 1% [wt/vol] skim milk), were added to the wells at the concentrations indicated in each case and then incubated for 1 h. Unbound antibodies were removed by four 3-min washings of the wells with PBS. For detection of the bound E-tagged scFvs, the anti-E-tag MAb-horseradish peroxidase (HRP) conjugate (Pharmacia; 1 μg/ml) was added for 1 h in INC buffer. Bound MAb 6AC3 was revealed with a goat anti-mouse IgG-HRP conjugate in INC buffer (0.03 U/ml; Boehringer Mannheim). In every case, the ELISAs were developed using o-phenylenediamine (Sigma) as a substrate of the peroxidase. The reaction was allowed to proceed for 10 min and stopped with 0.6 N HCl, and the OD492 of the plates was determined (Benchmark Microplate Reader; Bio-Rad).
Protein techniques.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by standard methods through 4% stacking and 10 or 12% separating gels (2). Silver-staining of polyacrylamide gels was performed as described by Ansorge (1). For immunoblotting, the proteins separated in the gels were transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore) using a semidry electrophoresis transfer apparatus (Bio-Rad, Cupertino, Calif.). Membranes were blocked for 16 h at 4°C using B buffer (see above) containing 0.1% (vol/vol) Tween 20. For immunodetection of the E-tagged proteins, the blots were incubated for 1 h at RT with anti-E-tag MAb-HRP conjugate (1 μg/ml; Pharmacia) in the same buffer. After three washings in PBS containing 0.1% (vol/vol) Tween 20, the bound antibody-HRP conjugate was detected by a chemiluminescence reaction. To develop chemiluminescence, we prepared a 1.25 mM luminol (Sigma)–42 μM luciferin (Boehringer Mannheim) mixture in 100 mM Tris-HCl (pH 8.0). The membrane (rinsed in PBS) was soaked in this mixture, and H2O2 was added at 0.0075% (vol/vol). After a 1-min incubation, the membrane was exposed to an X-ray film (usually from 5 s to 1 min).
For purification of scFv's from the periplasm, mid-log cultures (500 ml) of E. coli HB2151 cells harboring p6AC3g3 (or plasmid pB4g3 encoding a control scFv) were induced for 16 h at 30°C by the addition of 0.2 mM IPTG. Periplasmic extracts from these cells were obtained (19), and the His6-tagged scFv's were allowed to bind to the cobalt-containing Talon resin (Clontech). The resin was later collected in a chromatography column and washed extensively with DI buffer (40 mM Tris-HCl, 0.5 M NaCl, 5 mM MgCl2; pH 8.0). The bound His-tagged scFv's were eluted by adding buffer DI plus 100 mM imidazole (pH 8.0). The fractions containing the scFv's (as determined by immunoblottings using anti-E-tag MAb) were pooled and dialyzed against buffer DII (40 mM Tris-HCl, 0.15 M NaCl, 1 mM EDTA, 5% glycerol; pH 8.0). The concentrations of 6AC3 and B4 scFv's purified were determined by silver staining of SDS-polyacrylamide gels and immunoblots using the anti-E-tag MAb. The concentrations of the secreted EHlyA and scFv-HlyA polypeptides present in the E. coli culture supernatants were determined by measuring the intensity of the corresponding protein bands in silver-stained gels or, alternatively, in immunoblots revealed with anti-E-tag MAb. In either case, samples were compared to serial dilutions of either molecular weight markers (Bio-Rad) or an E-tagged scFv purified from the periplasm.
Virus neutralization assays.
The TGEV strain PUR46-MAD, grown and titrated in swine testis (ST) cells, was used in these experiments (10). ST cells were grown at 37°C and 5% CO2 in Dulbecco modified Eagle medium (DMEM) supplemented with 5% (vol/vol) fetal calf serum (FCS) and 4 mM l-glutamine. For assaying the neutralizing activity of antibodies, 50 μl of a TGEV preparation of known titer, in DMEM containing 2% (vol/vol) FCS, was combined with 50 μl of the purified scFv, MAb, or the culture supernatants at the concentrations indicated, adjusted to PBS 1×. After 30 min of incubation at 37°C, the mixtures were added to confluent monolayers of ST cells grown on 24-well tissue culture dishes (Nalgene; Nunc). After adsorption of TGEV to ST cells (45 min, 37°C), the medium was aspirated and replaced by overlay medium (DMEM, 4 mM l-glutamine, 2% [vol/vol] FCS, 1% [vol/vol] DEAE-dextrane, 0.1% [wt/vol] agarose, 0.1% gentamicin) at 1 ml per well. Plates were further incubated for 48 h at 37°C, and the cells were fixed afterward for 15 min at RT by adding 10% (vol/vol) formaldehyde solution in 1× PBS (1 ml per well). The liquid overlay was then poured from the plates, and the cells were stained for 30 min by adding 0.1% (wt/vol) crystal violet in 20% (vol/vol) methanol (1 ml per well). The staining solution was discarded, and the plates were rinsed extensively with deionized water to visualize the TGEV plaques.
RESULTS AND DISCUSSION
A hemolysin-based vector for the nontoxic secretion of scFv's.
An expression vector for the secretion of scFv's by the Hly transport system was constructed (pEHlyA2-SD; Fig. 1). In this vector, the DNA fragments encoding scFv's can be cloned in frame with the 3′ end of hlyA. The production of HlyB and HlyD components of the Hly machinery is induced with IPTG from the compatible plasmid pVDL9.3 (35). The restriction sites included in pEHlyA2-SD allow the cloning of scFv genes obtained either from current phage display vectors or from scFv assembly PCRs (25). The vector pEHlyA2-SD placed the scFv-hlyA gene fusions under the control of the lac promoter of E. coli and provides a ribosome-binding sequence (SD) and an ATG start codon for their translation. The hybrid scFv-HlyA proteins produced using pEHlyA2-SD lacked the N-terminal SP since it could interfere with the Hly-dependent secretion (15). Furthermore, an epitope tag (E-tag) is included along with the ∼23-kDa C-terminal domain of HlyA for the detection of scFv-HlyA hybrids. This epitope tag is introduced in some phagemid vectors (see below) so that the scFv's produced by these two different secretion systems, which are Sec or Hly dependent, can be detected using the same secondary monoclonal antibody (MAb anti-E-tag–HRP conjugate). Therefore, this expression system requires an E. coli wild-type strain (TolC+) cotransformed with plasmids pVDL9.3 (containing hylB and hylD) and a derivative of pEHlyA2-SD in which an scFv gene is cloned. Induction of these cells with IPTG is predicted to elicit the secretion of an E-tagged scFv-HlyA hybrid protein into the culture medium.
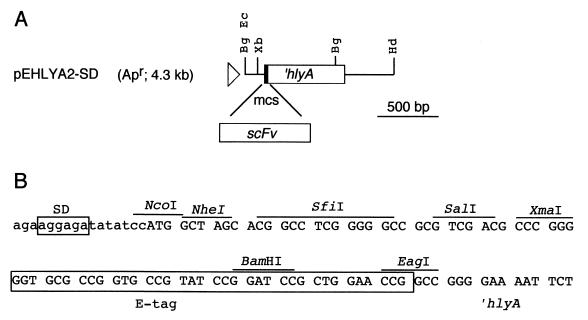
FIG. 1.
Vector for the secretion of scFv using the hemolysin transport system. (A) Schematic representation of the DNA region of plasmid pEHlyA2-SD in which the scFv-encoding genes can be cloned in a multiple cloning site (mcs) in frame with the ∼0.6-kb 3′ end of hlyA. The position of the lac promoter (triangle) and that of relevant restriction sites outside the multiple cloning site are labeled (Bg, BglII; Ec, EcoRI; Hd, HindIII; Xb, XbaI). (B) The DNA sequence of the multiple cloning site of pEHlyA2-SD is shown. The sites of the restriction enzymes that cut pEHlyA2-SD only once, the ribosome-binding sequence (SD), and the DNA encoding the E-tag epitope are marked. The reading frame used to translate the 3′ end of hlyA is indicated from the start codon ATG at the NcoI site.
To demonstrate the functionality of this system, the secretion of a model scFv was studied. To this end, an scFv was assembled from the VH and VL domains of the MAb 6AC3, which neutralizes the infection of the coronavirus responsible for the transmissible gastroenteritis in pigs (TGEV) by recognizing a conserved epitope of the Spike viral protein (13, 14, 33). The gene fusion encoding the scFv 6AC3, in a VH-linker-VL configuration (25), was cloned in the phagemid pCANTAB-5Ehis, giving rise to plasmid p6AC3g3 (Table 1). After induction with IPTG of E. coli HB2151 cells harboring p6AC3g3, a ∼30-kDa scFv 6AC3 protein with the E-tag epitope fused at its C terminus is accumulated in the periplasm (not shown). For the secretion of this scFv by the Hly transporter, the DNA encoding the scFv 6AC3 devoid of the N-terminal SP was cloned into pEHlyA2-SD to obtain p6AC3HlyA. As a positive control for Hly secretion, the plasmid pEHlyA was constructed, which encodes an E-tagged version of the ∼23-kDa C-terminal domain of HlyA named EHlyA.
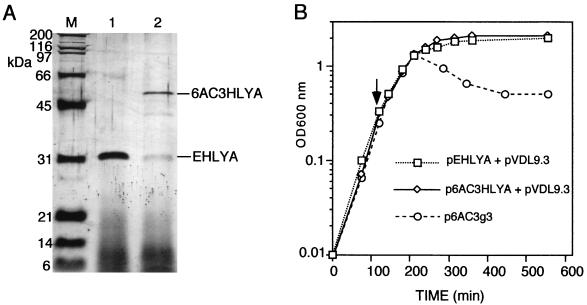
Induction of E. coli HB2151(TolC+) cells harboring pVDL9.3 and p6AC3HlyA or cells harboring pVDL9.3 and pEHlyA resulted in the specific accumulation of the 6AC3HlyA (∼55 kDa) or EHlyA (∼26 kDa), respectively, as the sole extracellular proteins (Fig. 2A). The level of secreted 6AC3HlyA protein, after 5 h of induction at 37°C of standard E. coli cultures in shake flasks, was estimated to be in the range of ∼1 to 2 mg/liter, whereas the concentration of secreted EHlyA was ∼5 to 10 mg/liter (see Materials and Methods). Some proteolytic fragments derived from 6AC3HlyA could also be detected both intra- and extracellularly, although at concentrations much lower than those of the secreted full-length hybrid (see Fig. 2A). This low-level proteolysis was independent of the outer membrane protease OmpT (18) and could be diminished by inducing the cells at 30°C (not shown). Importantly, secretion of 6AC3HlyA or EHlyA did not lead to any significant cell lysis or alteration in the growth of the producing E. coli cultures (Fig. 2B). In contrast, when the scFv 6AC3 was accumulated into the periplasm of E. coli HB2151 cells harboring p6AC3g3, an apparent growth arrest and lysis of the cells could be observed (Fig. 2B).
FIG. 2.
Nontoxic secretion of 6AC3HlyA hybrid to the extracellular medium of E. coli cultures. (A) Denaturing SDS-polyacrylamide gel (12%) stained with silver (1) showing the proteins secreted to the extracellular medium after 5 h of induction with 0.25 mM IPTG of E. coli HB2151(pVDL9.3) cells grown at 37°C and harboring pEHlyA (lane 1) or p6AC3HlyA (lane 2). Protein samples were prepared by adding to 50-μl portions of the culture supernatants an identical volume of 2× SDS-PAGE denaturing sample buffer (2). The mixtures were boiled for 5 min, and 10 μl of each sample was loaded per lane. For the molecular mass standards (M; Bio-Rad), 0.1 μg of each protein was loaded. (B) Growth curves in LB medium at 37°C of E. coli HB2151 cells harboring the indicated plasmids. The cultures were induced by 0.25 mM IPTG when the OD600 was ∼0.4 (arrow).
Activity of the secreted scFv-HlyA hybrids.
Next, the activity of scFv secreted by Hly-transporter was compared to that of the scFv exported into the periplasm. For these experiments the samples containing 6AC3HlyA were supernatants from induced E. coli (p6AC3HlyA and pVDL9.3) cultures without further purification besides the removal of E. coli cells by centrifugation. On the contrary, the scFv 6AC3 was purified by metal affinity chromatography from the periplasmic fraction of E. coli (p6AC3g3) cells. The supernatant of in vitro tissue culture of hybridoma 6AC3, containing MAb 6AC3, was utilized as a positive control. Negative controls for these experiments were the supernatants of E. coli (pEHlyA and pVDL9.3) cultures, containing EHlyA, and one unrelated E-tagged scFv purified from the periplasm of E. coli (pB4g3) cells. The concentrations of the secreted 6AC3HlyA and EHlyA proteins in these supernatants and that of the purified scFv's were determined as described in Materials and Methods.
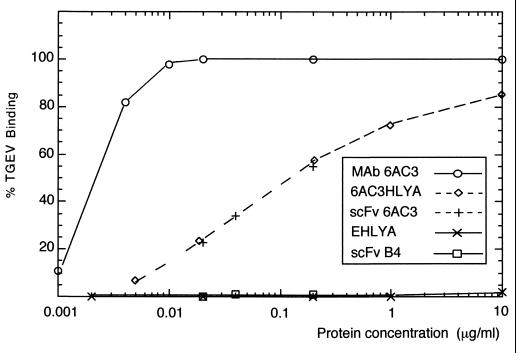
First, the binding of the different scFv's to TGEV was tested in vitro using ELISA (see Materials and Methods). As shown in Fig. 3, the binding curves for TGEV of the scFv 6AC3, purified from the periplasm, and of the 6AC3HlyA, secreted into the extracellular medium, were identical, clearly demonstrating that these two molecules have the same binding activity. The 6AC3 MAb bound TGEV with a ∼50-fold-higher apparent affinity than the E. coli recombinant antibodies (Fig. 3). This value is within the range expected from the transformation from monovalent to bivalent miniantibodies (28, 29) and probably reflects the substantial decrease in avidity of the 6AC3 MAb after becoming a monovalent scFv fragment. No TGEV-binding was detected using the EHlyA protein and an unrelated E-tagged scFv (B4).
FIG. 3.
Binding activity of the secreted scFv-HlyA hybrid in ELISA. Relative binding to TGEV as a function of the concentration of antibodies (MAb 6AC3, 6AC3HlyA, and the scFv 6AC3). The EHlyA protein and the scFv B4 were used as negative controls in the ELISAs. Maximal binding was considered when the OD492 reached 2. The values shown are the average of at least two independent experiments in which binding to TGEV was determined in triplicate. No detectable signals (OD492 of ≤0.02) were observed in parallel ELISAs using ovalbumin as a specificity control antigen.
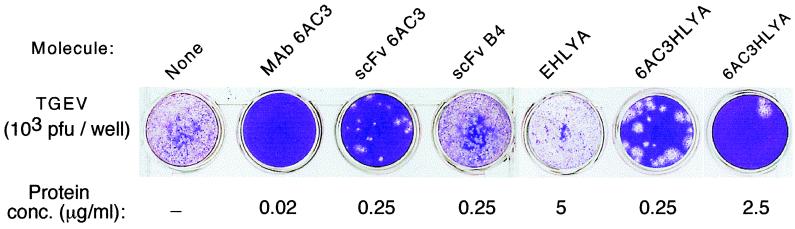
The same protein samples were also used to test whether or not the secreted 6AC3HlyA maintained the ability to neutralize TGEV infection over swine epithelial cells (ST cells), grown in vitro. To this end, monolayers of ST cells, grown in tissue culture plates, were challenged with various numbers of TGEV infective virions. The TGEVs were preincubated for 1 h at 37°C with the 6AC3 derivatives and control antibodies prior to their adsorption to ST cells. After 48 h of further incubation, the ST-cell monolayers were stained to visualize the plaques formed by TGEV replication. As shown in Fig. 4, a distinct and specific neutralization of TGEV became apparent when the virus was incubated with the scFv 6AC3 and 6AC3HlyA samples. Furthermore, a good reciprocity was obtained between the concentration of 6AC3HlyA and the extent of TGEV neutralization, ranging from >95 to >99.5% for concentrations of the recombinant antibodies ranging from 0.25 to 2.5 μg/ml, respectively. As expected from the in vitro binding data, the degree of TGEV neutralization elicited by the bivalent MAb 6AC3 was higher than that obtained with the monovalent scFv molecules.
FIG. 4.
Neutralization of TGEV infection by the secreted scFvHlyA. A defined number of TGEV (103 PFU) were incubated for 30 min at 37°C with the antibodies and proteins indicated and then added to monolayers of ST cells grown in vitro. After 48 h of further growth, the plaques of TGEV replication were visualized by fixing and staining the ST cells monolayers as described in Materials and Methods. The final concentration in the assay, and the antibodies and protein molecules used were as follows: MAb 6AC3 (0.02 μg/ml), purified scFv 6AC3 (0.25 μg/ml), the purified control B4 scFv (0.25 μg/ml), the nonpurified supernatant from E. coli HB2151 (pEHlyA; pVDL9.3) containing EHlyA (5.0 μg/ml), the nonpurified supernatant from E. coli HB2151 (p6AC3HLYA; pVDL9.3) containing 6AC3-HlyA (0.25 and 2.5 μg/ml), or buffer (None).
Conclusion.
Taken together, the experiments reported here demonstrate that fully active scFv's can be produced and secreted by E. coli cells with the Hly transport system and that this process is apparently without toxic effects on the producing cells. Furthermore, yields are similar to those obtained using periplasmic expression systems. The scFv-HlyA hybrids accumulate in the extracellular medium as the major polypeptide (Fig. 2) and, therefore, the culture supernatants obtained can directly be used in different immune assays, such as ELISA (Fig. 3), virus neutralization (Fig. 4), and Western blottings (data not shown). These supernatants can be stored for several weeks at 4°C and for up to a year at −80°C without apparent loss of their activity. Some minor proteolytic fragments (∼5 to 10% of the total protein secreted) other than the whole-length scFv-HlyA hybrid were also observed in the culture supernatants (Fig. 2A). However, they seem not to interfere with any of the assays made. Other scFv-HlyA hybrids unrelated to 6AC3 are now routinely produced in our laboratory, with yields and performances similar to that of 6AC3 (data not shown). This is largely due to the effective formation of disulfide bonds within the secreted scFv's (L. A. Fernández and V. de Lorenzo, unpublished data). In conclusion, the simplicity of the production method reported here can greatly increase the utility of recombinant scFv antibodies and accelerate the characterization of scFv's obtained from high-throughput biopannings (16, 22) and affinity-maturation procedures (11).
ACKNOWLEDGMENTS
The excellent technical assistance of Sofía Fraile is greatly appreciated.
I.S. is the holder of a Postdoctoral fellowship from the Spanish Ministry of Education. This work is partially supported by FAIR contract CT96-1339 and by the Spanish CICYT grants BIO98-0808 and BIO98-0756.
REFERENCES
- 1.Ansorge W. Fast and sensitive detection of protein and DNA bands by treatment with potassium permanganate. J Biochem Biophys Methods. 1985;11:13–20. doi: 10.1016/0165-022x(85)90037-5. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 3.Baneyx F. Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol. 1999;10:411–421. doi: 10.1016/s0958-1669(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 4.Beckman C, Haase B, Timmis K N, Tesar M. Multifunctional g3p-peptide tag for current phage display systems. J Immunol Methods. 1998;212:131–138. doi: 10.1016/s0022-1759(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 5.Blight M A, Holland I B. Heterologous protein secretion and the versatile Escherichia coli haemolysis translocator. Trends Biotechnol. 1994;12:450–455. doi: 10.1016/0167-7799(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 6.Bothmann H, Pluckthun A. The periplasmic E. coli peptidyl-prolyl isomerase FkpA (I): increased functional expression of antibody fragments with and without cis-prolines. J Biol Chem, 2000;275:17100–17105. doi: 10.1074/jbc.M910233199. [DOI] [PubMed] [Google Scholar]
- 7.Bothmann H, Pluckthun A. Selection for periplasmic factor improving phage display and functional periplasmic expression. Nat Biotechnol. 1998;16:376–380. doi: 10.1038/nbt0498-376. [DOI] [PubMed] [Google Scholar]
- 8.Bullock W O, Fernández J M, Short J M. XL1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with beta-galactosidase selection. BioTechniques. 1987;4:376–378. [Google Scholar]
- 9.Carter P, Bedouelle H, Winter G. Improved oligonucleotide site-directed mutagenesis using M13 vectors. Nucleic Acids Res. 1985;13:4431–4443. doi: 10.1093/nar/13.12.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castilla J, Sola I, Enjuanes L. Interference of coronavirus infection by expression of immunoglobulin G (IgG) or IgA virus-neutralizing antibodies. J Virol. 1997;71:5251–5258. doi: 10.1128/jvi.71.7.5251-5258.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhury P S, Pastan I. Improving antibody affinity by mimicking somatic hypermutation in vitro. Nat Biotechnol. 1999;17:568–572. doi: 10.1038/9872. [DOI] [PubMed] [Google Scholar]
- 12.Economou A. Following the leader: bacterial protein export through the Sec pathway. Trends Microbiol. 1999;7:315–319. doi: 10.1016/s0966-842x(99)01555-3. [DOI] [PubMed] [Google Scholar]
- 13.Enjuanes L, Van der Zeijst B A M. Molecular basis of transmissible gastroenteritis coronavirus epidemiology. In: Siddell S G, editor. The coronaviridae. New York, N.Y: Plenum Press; 1995. pp. 337–376. [Google Scholar]
- 14.Gebauer F, Posthumus W P A, Correa I, Suñé C, Smerdou C, Sánchez C M, Lenstra J A, Meloen R H, Enjuanes L. Residues involved in the antigenic sites of transmissible gastroenteritis coronavirus S glycoprotein. Virology. 1991;183:225–238. doi: 10.1016/0042-6822(91)90135-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentschev I, Hess J, Goebel W. Change in the cellular localization of alkaline phosphatase by alteration of its carboxy-terminal sequence. Mol Gen Genet. 1990;222:211–216. doi: 10.1007/BF00633820. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths A D, Ducan A R. Strategies for selection of antibodies by phage display. Curr Opin Biotechnol. 1998;9:102–108. doi: 10.1016/s0958-1669(98)80092-x. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths A D, Williams S C, Hartley O, Tomlinson I M, Waterhouse P, Crosby W L, Kontermann R E, Jones P T, Low N M, Allison T J, Prospero T D, Hoogenboom H R, Nissim A, Cox J P L, Harrison J L, Zaccolo M, Gherardi E, Winter G. Isolation of high affinity human antibodies directly from large synthetic repertoires. EMBO J. 1994;13:3245–3260. doi: 10.1002/j.1460-2075.1994.tb06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grodberg J, Dunn J J. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J Bacteriol. 1988;170:1245–1253. doi: 10.1128/jb.170.3.1245-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison J L, Williams S, Winter G, Nissim A. Screening of phage antibody libraries. Methods Enzymol. 1996;267:83–109. doi: 10.1016/s0076-6879(96)67007-4. [DOI] [PubMed] [Google Scholar]
- 20.Hayhurst A, Harris W J. Escherichia coli Skp chaperone coexpression improves solubility and phage display of single chain antibody fragments. Prot Expr Purif. 1999;15:336–343. doi: 10.1006/prep.1999.1035. [DOI] [PubMed] [Google Scholar]
- 21.Holland I B, Kenny B, Steipe B, Plückthun A. Secretion of heterologous proteins in Escherichia coli. Methods Enzymol. 1990;182:132–143. doi: 10.1016/0076-6879(90)82013-r. [DOI] [PubMed] [Google Scholar]
- 22.Hoogenboom H R. Designing and optimizing library selection strategies for generating high-affinity antibodies. Trends Biotechnol. 1997;15:62–70. doi: 10.1016/S0167-7799(97)84205-9. [DOI] [PubMed] [Google Scholar]
- 23.Koronakis E, Hughes C, Milisav I, Koronakis V. Protein exporter function and in vitro ATPase activity are correlated in ABC-domain mutants of HlyB. Mol Microbiol. 1995;16:87–96. doi: 10.1111/j.1365-2958.1995.tb02394.x. [DOI] [PubMed] [Google Scholar]
- 24.Koronakis V, Li J, Koronakis E, Stauffer K. Structure of TolC, the outer membrane component of the bacterial type I efflux system, derived from two-dimensional crystals. Mol Microbiol. 1997;23:617–626. doi: 10.1046/j.1365-2958.1997.d01-1880.x. [DOI] [PubMed] [Google Scholar]
- 25.McCafferty J, Johnson K S. Construction and screening of antibody display libraries. In: Kay B K, Winter J, McCafferty J, editors. Phage display of peptides and proteins. San Diego, Calif: Academic Press, Inc.; 1996. pp. 79–111. [Google Scholar]
- 26.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 27.Nissim A, Hoogenboom H R, Tomlinson I M, Flynn G, Midgley C, Lane D, Winter G. Antibody fragments from a “single pot” phage display library as immunochemical reagents. EMBO J. 1994;13:692–698. doi: 10.1002/j.1460-2075.1994.tb06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pack P, Müller K, Zahn R, Plückthun A. Tetravalent miniantibodies with high avidity assembling in Escherichia coli. J Mol Biol. 1995;246:28–34. doi: 10.1006/jmbi.1994.0062. [DOI] [PubMed] [Google Scholar]
- 29.Pack P, Plückthun A. Miniantibodies: use of amphipathic helices to produce functional, flexibly linked dimeric Fv fragments with high avidity in Escherichia coli. Biochemistry. 1992;31:1579–1584. doi: 10.1021/bi00121a001. [DOI] [PubMed] [Google Scholar]
- 30.Plückthun A, Krebber C, Krebber U, Horn U, Knüpfer U, Wenderoth R, Nieba L, Proba K, Riesenberg D. Producing antibodies in Escherichia coli: from PCR to fermentation. In: McCafferty J, Hoogenboom H R, editors. Antibody engineering: a practical approach. Oxford, England: IRL Press; 1996. pp. 203–252. [Google Scholar]
- 31.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raivio T L, Silhavy T. The ςE and Cpx regulatory pathways: overlapping but distinct envelope stress responses. Curr Opin Microbiol. 1999;2:159–165. doi: 10.1016/S1369-5274(99)80028-9. [DOI] [PubMed] [Google Scholar]
- 33.Sola I, Castilla J, Pintado B, Sanchez-Morgado J M, Whitelaw B, Clark J, Enjuanes L. Transgenic mice secreting coronavirus neutralizing antibodies into the milk. J Virol. 1998;72:3762–3772. doi: 10.1128/jvi.72.5.3762-3772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thanabalu T, Koronakis E, Hughes C, Koronakis V. Substrate-induced assembly of a contiguous channel for protein export from E. coli: reversible bridging of a inner-membrane translocase to an outer membrane exit pore. EMBO J. 1998;17:6487–6496. doi: 10.1093/emboj/17.22.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tzschaschel B D, Guzman C A, Timmis K N, de Lorenzo V. An Escherichia coli hemolysin transport system-based vector for the export of polypeptides: export of Shiga-like toxin IIeB subunit by Salmonella typhimurium aroA. Nat Biotechnol. 1996;14:765–769. doi: 10.1038/nbt0696-765. [DOI] [PubMed] [Google Scholar]