Abstract
Aims
Low-density lipoprotein cholesterol (LDL-C) predicts heart disease onset and may be reduced by intermittent fasting. Some studies, though, reported that fasting increased LDL-C; however, no study evaluated LDL-C as the primary endpoint. This randomized controlled trial evaluated the effect of low-frequency intermittent fasting on LDL-C and other biomarkers.
Methods and results
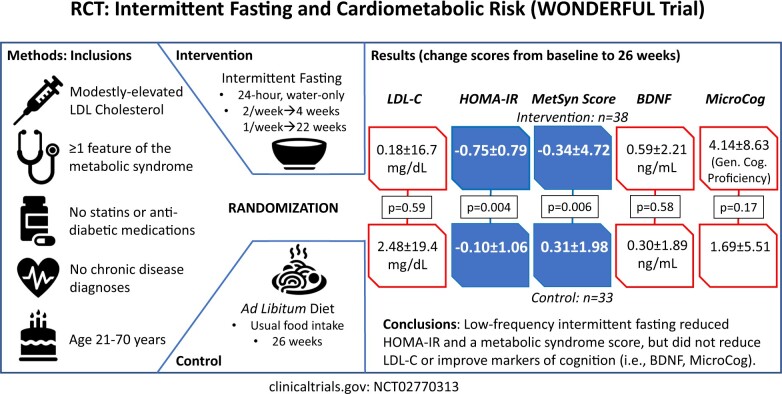
Adults aged 21–70 years were enrolled who were not taking a statin, had modestly elevated LDL-C, had ≥1 metabolic syndrome feature or type 2 diabetes, and were not taking anti-diabetic medication (N = 103). Water-only 24-h fasting was performed twice weekly for 4 weeks and then once weekly for 22 weeks; controls ate ad libitum. The primary outcome was 26-week LDL-C change score. Secondary outcomes (requiring P ≤ 0.01) were 26-week changes in homeostatic model assessment of insulin resistance (HOMA-IR), Metabolic Syndrome Score (MSS), brain-derived neurotrophic factor (BDNF), and MicroCog general cognitive proficiency index (GCPi). Intermittent fasting (n = 50) and control (n = 53) subjects were, respectively, aged 49.3 ± 12.0 and 47.0 ± 9.8 years, predominantly female (66.0% and 67.9%), and overweight (103 ± 24 and 100 ± 21 kg) and had modest LDL-C elevation (124 ± 19 and 128 ± 20 mg/dL). Drop-outs (n = 12 fasting, n = 20 control) provided an evaluable sample of n = 71 (n = 38 fasting, n = 33 control). Intermittent fasting did not change LDL-C (0.2 ± 16.7 mg/dL) vs. control (2.5 ± 19.4 mg/dL; P = 0.59), but it improved HOMA-IR (−0.75 ± 0.79 vs. −0.10 ± 1.06; P = 0.004) and MSS (−0.34 ± 4.72 vs. 0.31 ± 1.98, P = 0.006). BDNF (P = 0.58), GCPi (P = 0.17), and weight (−1.7 ± 4.7 kg vs. 0.2 ± 3.5 kg, P = 0.06) were unchanged.
Conclusions
A low-frequency intermittent fasting regimen did not reduce LDL-C or improve cognitive function but significantly reduced both HOMA-IR and MSS.
Trial registration
clinicaltrials.gov, NCT02770313.
Keywords: Therapeutic fasting, Cholesterol, Cognitive function, Metabolic syndrome, Pre-diabetes, Insulin resistance
Graphical Abstract
Graphical Abstract.
Introduction
Fasting was speculated to have a host of benefits as early as 1915 when Otto Folin recommended short periods of starvation as an effective means to reduce weight in obese individuals.1 In 1946, Anton Carlson coined the term ‘intermittent fasting’ when investigating its effect on longevity in rats.2 Carlson also noted that many religious individuals historically attributed their long lifespan to periodically abstaining from food.2 Most fasting studies in humans have evaluated high frequency fasting as a weight loss intervention.3–8 Unfortunately, weight change due to intense intermittent fasting regimens is generally no more effective than caloric restriction (CR), and drop-outs from those regimens are high.5–8
While weight loss—due to CR, intermittent fasting, or other energy restriction methods—has its health benefits, fasting may impact health independent of weight loss.9 This may include a lower risk of coronary artery disease,10 diabetes,11 and cognitive dysfunction [e.g. measured by brain-derived neurotrophic factor (BDNF)].4 As a risk factor for coronary disease, low-density lipoprotein cholesterol (LDL-C) is of particular interest as it plays an integral part in the development of atherosclerotic plaque by causing endothelial damage.12 A 2019 meta-analysis found that intermittent fasting may improve lipid and lipoprotein parameters.13 For LDL-C, though, the relationship is complex and may depend on nutrient intake.14 Furthermore, some trials found that LDL-C was higher in the intermittent fasting group compared to CR,5 or to control.15,16 However, no randomized trial of intermittent fasting has assessed LDL-C as the primary outcome.
This study evaluated the effect of a low-frequency intermittent fasting regimen on the 26-week LDL-C change score compared to ad libitum control. Secondary objectives were to evaluate the influence of intermittent fasting on change scores of secondary endpoints (pre-specified to require P ≤ 0.01 to achieve statistical significance): the homeostatic model assessment of insulin resistance (HOMA-IR), the Metabolic Syndrome Score (MSS), the MicroCog general cognitive proficiency index (GCPi), and BDNF.
Methods
Design
This randomized controlled interventional trial evaluated the impact of intermittent fasting in people with modestly elevated LDL-C and mild metabolic derangement who were at risk for poor cardiovascular, metabolic, and cognitive outcomes. Subjects were randomized 1:1 by permuted block design to water-only intermittent fasting or ad libitum control using sequentially numbered envelopes that were separately numbered for two separate strata. The randomization was stratified by history of routine periodic fasting (∼181–720 total hours of deliberate fasting >15 h during the previous 2 years, allowing for 3–15 days of fasting history per year since many in the local general population engage in periodic fasting for ≈24 h 12 times per year [10,11] or allowing for other very low-frequency fasting) and no history of fasting (≤180 h in the previous 2 years). No CR arm was evaluated in the trial because rapid weight loss was neither the goal nor an expected outcome from the study’s low-frequency intermittent fasting regimen. The Intermountain Healthcare Institutional Review Board approved the study protocol and subjects provided written consent prior to the initiation of any study procedures. The study was registered as NCT02770313 at clinicaltrials.gov on 12 May 2016, and subject enrolment began on 30 November 2016. The final subject’s 26-week study visit occurred on 19 February 2020.
Study population
The study recruited 103 patients from Intermountain hospitals and clinics and from local community volunteers. Knowledge of the study was disseminated via advertising in medical facilities (in clinics and at health fairs) and news stories in traditional and social media. Most potential subjects were previous patients whose electronic records suggested the potential to meet inclusion and exclusion criteria; these patients were contacted by the Intermountain Heart Institute medical director and invited to contact study investigators.
The study protocol included men or non-pregnant women aged 21–70 years of age who met the following criteria: (i) they were not taking statin medication and had no indication for statin therapy, (ii) they had modestly elevated LDL-C (for ages 21–39 years: 90–189 mg/dL; for ages 40–70 years: 90–159 mg/dL, or any qualifying age subject with LDL-C of 90 mg/dL or greater who had tried statins and stopped due to intolerance or had one or more contraindications to statins), and (iii) they met one of the following conditions: they had pre-diabetes (fasting glucose >100 mg/dL, haemoglobin A1c >5.6%, or a clinical diagnosis) and were not taking anti-diabetic medications, diagnosed type 2 diabetes that was diet-controlled (i.e. they were not taking anti-diabetic medications), or had ≥1 of the other components of the metabolic syndrome: elevated blood pressure (systolic ≥130 mmHg, diastolic ≥85 mmHg, or use of an antihypertensive medication), high waist circumference (≥40 inches/102 cm for males or ≥35 inches/88 cm for females) or high BMI (>25 kg/m2), high triglycerides (≥150 mg/dL or use of a non-statin triglyceride-lowering medication like a fibrate or niacin), or low HDL-C (<40 mg/dL for males or <50 mg/dL for females). Qualifying laboratory tests had to be measured within 6 months of screening.
Potential subjects were excluded if they had a history of fasting for a total of >720 h (>45 episodes of 16 h, or >30 days of 24-h fasting) during the previous 2 years (except that participation in Ramadan, Baha’i fasting, or similar practices was allowed if the 6-month study period did not overlap the religious fasting period; however, no enrolled subject followed these practices). They were also excluded if they had a prior diagnosis of a chronic disease (i.e. symptomatic coronary artery disease, coronary revascularization, myocardial infarction, unstable angina, stroke, transient ischaemic attack, peripheral vascular thromboembolism, chronic kidney disease stage III or higher, chronic obstructive pulmonary disease, immunodeficiency, solid organ transplant, eating disorder, type I diabetes, dementia, traumatic brain injury, and cancer of any type within the last 5 years except for non-melanoma skin cancer), were participating in another clinical trial, or (for women) were pregnant and/or lactating.
Study outcomes
The primary outcome of the study was change in LDL-C from baseline to 26 weeks. Secondary endpoints requiring a pre-specified Bonferroni-corrected P ≤ 0.01 for significance were 26-week change in HOMA-IR, MSS,17,18 BDNF,4,19,20 and the MicroCog (Pearson Assessments, San Antonio, TX, USA) GCPi score. MicroCog is a computerized cognitive screening attention/mental control, reasoning/calculation, memory, spatial processing, and simple reaction time. Performance scores are aggregated into index scores, including the GCPi. The GCPi is a global measure of cognitive performance that includes the speed and accuracy index scores with greater weighting given to the accuracy of responses. Scaled scores from the GCPi were used for analysis. Exploratory analyses evaluated the change in LDL-C, HOMA-IR, and MSS from baseline to 4 weeks and baseline to 13 weeks. Other exploratory endpoints included changes in other components of the lipid panel [i.e. total cholesterol, high-density lipoprotein cholesterol (HDL-C), triglycerides, very low-density lipoprotein cholesterol, non-HDL-C] and related factors (apolipoprotein B), fasting glucose, fasting insulin, systolic blood pressure (SBP), diastolic blood pressure (DBP), high-sensitivity C-reactive protein (hsCRP), the MicroCog general cognitive functioning index (that gives equal weighting to speed and accuracy of responses), weight, and waist circumference. All outcome measurements were performed after a 12-h (range: 8–16 h) overnight fast.
Procedures
The study covered a 26-week period (6 months). Following recruitment and completion of informed consent, subjects participated in preliminary screening using the lipid panel, pregnancy test (for women of child-bearing age only), and fasting glucose. Those qualifying at screening had a baseline visit within 1–7 days, at which time randomization occurred, and follow-up visits at 4, 13, and 26 weeks after randomization. Baseline and follow-up testing included measurements of height, weight, waist circumference, medical history, dietary history, vital signs, lipid panel, fasting glucose, fasting insulin, hsCRP, BDNF, HOMA-IR, MSS, and GCPi. The GCPi was measured only at baseline and 26 weeks due to the required testing time.
Following baseline screening and randomization, subjects participated in their designated trial groups. Subjects allocated to the fasting arm underwent a fasting regimen of twice-per-week 24-h fasting (defined as a 21–27-h water-only fast) on non-consecutive days during the first 4 weeks of the study, then once-per-week 24-h fasting during the rest of the study (22 weeks). A fasting diary was kept to record deviations from 24-h water-only fasting and was reviewed with research personnel at the three follow-up study visits to evaluate adherence. Between fasting days, eating was ad libitum. Subjects randomized to the control group ate ad libitum at all times during the 26 weeks of the trial and were only contacted to schedule and conduct the three follow-up visits. No detailed dietary intake information was collected because the study was intended to be as similar to the real world as possible, dietary monitoring could trigger all subjects to alter their eating behaviour, and the randomization to fasting or control arms should have evenly distributed different eating patterns between the two study groups. Major changes in diet were evaluated at each follow-up visit using general questions about dietary status (Supplementary material online, Appendix S1).
Statistical considerations
Summary statistics (means with standard deviations or counts with percentages) were used to describe the baseline characteristics of the study population. Correlations between change scores were examined to determine which changes were potentially related and these analyses utilized the Pearson correlation.
Analyses of primary, secondary, and exploratory outcomes were performed using Student’s T-test under the intent-to-treat approach to compare 26-week change scores between intermittent fasting and control groups for changes in LDL-C and the other outcome measures. As the primary endpoint, the change score for LDL-C was required to reach P ≤ 0.05 to declare statistical significance. Not directly related to the primary endpoint, multiple pre-specified secondary outcomes of changes in HOMA-IR, MSS, BDNF, and GCPi were evaluated to examine unrelated metabolic and cognitive mechanisms of potential benefit, and to do so a conservative Bonferroni-corrected P-value of P ≤ 0.010 was pre-defined as necessary to achieve significance. Exploratory 26-week outcomes and the 13-week and 4-week changes in LDL-C were considered hypothesis-generating. Analyses were conducted using SPSS v26.0 (IBM SPSS, Armonk, NY, USA) by a trained statistician who was blinded to which group was fasting and control using a randomly assigned alphabetic code.
As an exploratory analysis, a mixed model approach was used to evaluate the repeated measurement of the primary and secondary outcomes (except GCPi since it was not measured at weeks 4 or 13), and weight. These analyses evaluated the value of each parameter such as LDL-C at baseline, week 4, week 13, and week 26 in the same linear model as the dependent variables, with trial randomization assignment utilized as the independent variable. For LDL-C, one control subject was excluded from repeated measures analysis of LDL-C because their week 4 LDL-C was 97 mg/dL higher than baseline (69% higher) and the week 13 value was equal to the baseline value.
This trial was powered to detect a 20 mg/dL reduction in LDL-C for the intermittent fasting group based on a prior single-arm trial21 and compared to a 5 mg/dL decrease in the control arm (allowing for some regression to the mean), assuming a common standard deviation of 24.7 mg/dL. With α = 0.05 and β = 0.20 in a two-sided test of hypothesis, a sample size of 102 was anticipated to provide 80% power to detect the expected difference between intermittent fasting and control arms, allowing for a 15% dropout rate.
Results
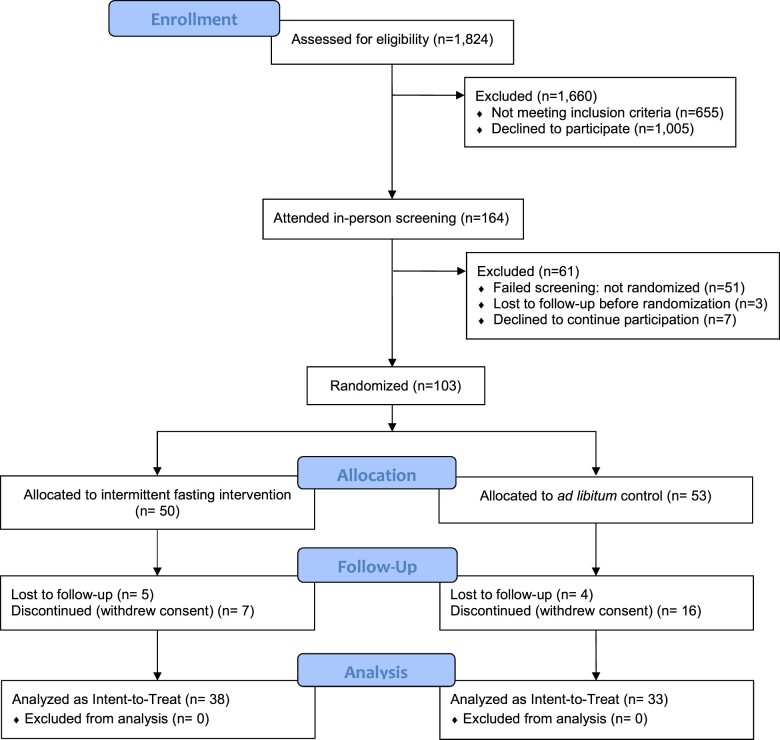
Of 164 individuals who were screened for the trial (Figure 1), N = 103 were randomized to the fasting (n = 50) or control (n = 53) groups. Baseline characteristics are shown in Table 1. Of those in the fasting group, 5 were lost to follow-up and 7 withdrew from the trial. The control group had 4 that were lost to follow-up and 16 withdrew from the trial giving a final sample of n = 71 (n = 38 fasting, n = 33 control). The main reasons for the 23 withdrawals were that: (i) 8 subjects in the control group indicated a discomfort with their assigned randomization group and (ii) 7 subjects (in both arms) had conflicts with attending study follow-up visits due to their employment schedule (5 subjects), a family issue (1 subject), and a personal unwillingness to attend (1 subject). While withdrawals were higher in the control group, baseline differences between fasting and control arms of those who completed the trial were similar (Supplementary material online, Table S1).
Figure 1.
CONSORT diagram. Of the 1824 potential subjects, 21.6% became aware of the study due to advertising, among whom 68.0% (n = 70) were randomized. The other 78.4% of the potential subjects were patients identified through historical electronic health record data who were sent an invitation letter, among whom 32.0% (n = 33) were randomized.
Table 1.
Baseline characteristics of subjects randomized to the intermittent fasting intervention or to the ad libitum control group
| Intermittent fasting, n = 50 | Ad libitum control, n = 53 | |
|---|---|---|
| Demographics | ||
| Age, years | 49.3 ± 12.0 | 47.0 ± 9.8 |
| Sex, male | 17 (34.0%) | 17 (32.1%) |
| Race (non-white) | 3 (6.0%) | 1 (1.9%) |
| Ethnicity | ||
| Hispanic/Latino | 3 (6.0%) | 5 (9.4%) |
| Not Hispanic/Latino | 47 (94.0%) | 47 (88.7%) |
| Unknown | 0 (0%) | 1 (1.9%) |
| Alcohol (drinks) | ||
| None | 26 (52.0%) | 21 (39.6%) |
| <1 per week | 2 (4.0%) | 6 (11.3%) |
| 1–7 per week | 1 (2.0%) | 5 (9.4%) |
| Declined to answer | 21 (42.0%) | 21 (39.6%) |
| Lipid profile | ||
| Total cholesterol (mg/dL) | 196.1 ± 25.4 | 201.8 ± 27.8 |
| LDL-C (mg/dL) | 123.9 ± 19.2 | 127.5 ± 19.5 |
| HDL-C (mg/dL) | 46.1 ± 10.2 | 47.0 ± 14.2 |
| Triglycerides (mg/dL) | 131.7 ± 76.6 | 137.8 ± 63.3 |
| Non-HDL-C (mg/dL) | 149.3 ± 24.9 | 154.9 ± 25.1 |
| Apolipoprotein B (mg/dL) | 81.6 ± 50.5 | 75.9 ± 45.0 |
| Comorbiditiesa | ||
| Autoimmune disease history | 5 (10.0%) | 9 (17.0%) |
| Depression history | 6 (12.0%) | 9 (17.0%) |
| Diabetes (type 2) | 0 (0%) | 2 (3.8%) |
| Pre-diabetes | 6 (12.0%) | 7 (13.2%) |
| Liver disease history | 0 (0%) | 1 (1.9%) |
| Neurological disease history | 1 (2.0%) | 1 (1.9%) |
| Parkinson’s disease | 2 (4.0%) | 0 (0%) |
| Physical examination and vital signs | ||
| Systolic blood pressure (mmHg) | 127.5 ± 12.2 | 127.7 ± 13.8 |
| Diastolic blood pressure (mmHg) | 82.1 ± 8.0 | 81.2 ± 10.4 |
| Heart rate (bpm) | 69.8 ± 9.1 | 70.6 ± 10.9 |
| Weight (kg) | 103.0 ± 24.2 | 99.7 ± 20.7 |
| Height (cm) | 171.5 ± 10.2 | 171.5 ± 9.9 |
| Body mass index (kg/m2) | 35.1 ± 8.2 | 34.0 ± 7.3 |
| Waist circumference (cm) | 107 ± 20.2 | 106 ± 17.1 |
| Temperature (°C) | 36.7 ± 0.2 | 36.7 ± 0.2 |
| Other measurements | ||
| Fasting glucose (mg/dL) | 91.7 ± 14.4 | 90.2 ± 9.5 |
| BDNF (ng/mL) | 2.63 ± 1.66 | 2.66 ± 1.83 |
| HOMA-IR | 2.3 ± 1.2 | 2.8 ± 1.9 |
| Fasting insulin (mIU/L) | 11.5 ± 11.5 | 11.9 ± 7.0 |
| Metabolic syndrome score | 0.641 ± 3.54 | 0.379 ± 3.80 |
| MicroCog GCPi score | 96.3 ± 13.0 | 100.3 ± 11.7 |
| MicroCog GCFi score | 99.7 ± 11.7 | 101.8 ± 10.4 |
| eGFR (mL/min/1.73 m2) | 97.6 ± 9.0 | 98.9 ± 7.1 |
| Troponin I (ng/mL) | 0.011 ± 0.006 | 0.010 ± 0.000 |
| hsCRP (mg/L) | 4.1 ± 5.2 (median: 2.3) | 4.8 ± 5.2 (median: 2.8) |
BDNF, brain-derived neurotrophic factor; eGFR, estimated glomerular filtration rate; GCFi, general cognitive functioning index; GCPi, general cognitive proficiency index; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; hsCRP, high-sensitivity C-reaction protein; LDL-C, low-density lipoprotein cholesterol.
See the list of chronic disease exclusions in the Methods for other comorbidities not listed here.
Adherence to the fasting regimen was 95 ± 12% in the 38 fasting subjects who completed the 26-week visit. This included 22 with 100% adherence, 6 with 97%, 3 with 93%, 3 with 90%, 2 with 80%, 1 with 70%, and 1 with 37% adherence. Thus, 95% of subjects in the fasting arm had adherence ≥80%. The adverse events reported at study visits were all mild (Supplementary material online, Table S2), with the majority occurring in the intermittent fasting group. Most were reported at the end of the 4-week twice weekly fasting segment by subjects in the fasting arm. No major changes in diet that investigators considered of concern for the trial were noted in either study arm across the duration of the study.
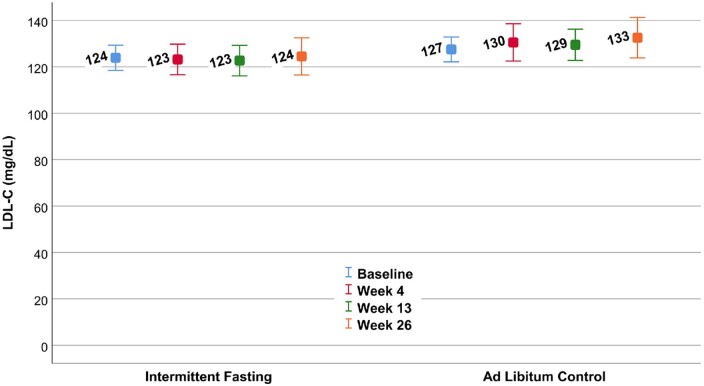
The change score of LDL-C at 26 weeks (Table 2) was not different compared to the control group (P = 0.59), exhibiting a 0.15% increase in the fasting group and 2.0% increase in the control arm. The mean LDL-C levels at each of the four study visits are shown in Figure 2. Results were similar for the change in LDL-C at 4 weeks (−0.22 ± 15.8 mg/dL vs. 5.64 ± 23.4 mg/dL, P = 0.17) and 13 weeks (−1.38 ± 17.7 mg/dL vs. 0.68 ± 16.6 mg/dL, P = 0.59). Exclusion of one subject with an outlying LDL-C at 4 weeks (an LDL-C increase of 97.0 mg/dL) reduced the change in the control group at 4 weeks to 3.41 ± 18.6 mg/dL (P = 0.33). Analysis of LDL-C at all four timepoints using a repeated measures mixed model confirmed no difference in LDL-C between trial arms (P = 0.11).
Table 2.
Change scores for the primary and pre-specified secondary trial outcomes
| Intermittent fasting | Ad libitum control | P-value | |
|---|---|---|---|
| Primary endpoint | |||
| LDL-C (mg/dL) | 0.18 ± 16.7 | 2.48 ± 19.4 | 0.59 |
| Secondary endpoints | |||
| HOMA-IR | −0.75 ± 0.79 | −0.10 ± 1.06 | 0.004a |
| MSS | −0.34 ± 4.72 | 0.31 ± 1.98 | 0.006a |
| BDNF (ng/mL) | 0.59 ± 2.21 | 0.30 ± 1.89 | 0.58 |
| MicroCog GCPi Score | 4.14 ± 8.63 | 1.69 ± 5.51 | 0.17 |
BDNF, brain-derived neurotrophic factor; GCPi, general cognitive proficiency index; HOMA-IR, homeostatic model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol; MSS, metabolic syndrome score.
Significant at P ≤ 0.01 when corrected for the 4 secondary endpoints and the primary endpoint, as designated a priori at the start of the study.
Figure 2.
Mean low-density lipoprotein cholesterol in the intermittent fasting and ad libitum control arms of the trial at each of the four study visits. No differences between randomization groups were found in the change scores (P = 0.59 at 26 weeks) or differences in means across the four timepoints (P = 0.11).
The 26-week change in LDL-C was correlated with 26-week MSS change (r = −0.257, P = 0.031), but not with 26-week changes in HOMA-IR (r = −0.127, P = 0.29), BDNF (r = 0.102, P = 0.41), or GCPi (r = 0.108, P = 0.38). HOMA-IR change was correlated with MSS change (r = 0.440, P < 0.001) and BDNF change (r = −0.301, P = 0.013). Changes in GCPi and BDNF were not correlated (r = 0.039, P = 0.76).
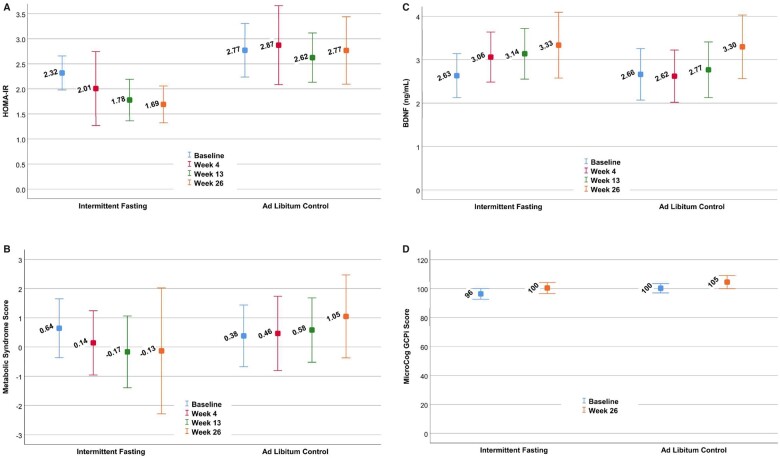
HOMA-IR improved by 32.5% in the fasting group vs. 3.7% in the control group, achieving the pre-specified multiple comparison-corrected P-value ≤0.01 with P = 0.004 (Table 2). The mean HOMA-IR at each study timepoint is provided in Figure 3A. The change in HOMA-IR was primarily due to improvement in insulin levels (P = 0.001, with a 24.0% decrease in the fasting group vs. a 2.8% decrease in the control arm) and to a lesser extent to lower glucose concentrations (P = 0.039, with a 7.9% decrease for fasting subjects vs. 2.4% in control subjects) (see Table 3). At 13 weeks, the change in HOMA-IR was −0.63 ± 1.00 vs. −0.16 ± 1.10 for fasting vs. control groups, respectively (P = 0.045); at 4 weeks, the change in HOMA-IR was −0.34 ± 2.37 vs. −0.19 ± 1.56 for fasting vs. control arms (P = 0.23). Changes in insulin sensitivity categories are provided in Supplementary material online, Table S3.
Figure 3.
Mean results for the intermittent fasting and ad libitum control arms at each of the four study visits for: (A) homeostatic model assessment of insulin resistance, (B) metabolic syndrome score, (C) brain-derived neurotrophic factor, and (D) the MicroCog GCPi Score. Differences were found between randomization groups for the change scores of homeostatic model assessment of insulin resistance (P = 0.004 at 26 weeks) and metabolic syndrome score (P = 0.006 at 26 weeks) and for the differences in means across the four timepoints for homeostatic model assessment of insulin resistance (P = 0.007). Values are means and whiskers are 95% confidence intervals of the means. Data are drawn from all subjects at each time point, thus simple subtraction of means will not necessarily provide the change score values in Table 2. GCPi was only measured at baseline and week 26 (see Methods).
Table 3.
Change scores for exploratory outcomes, including cardiovascular risk factors and factors related to the primary and secondary trial outcomes
| Intermittent fasting | Ad libitum control | P-value | |
|---|---|---|---|
| Changes in the lipid profile | |||
| Total cholesterol (mg/dL) | 1.21 ± 19.6 | 3.91 ± 19.6 | 0.57 |
| HDL-C (mg/dL) | 2.37 ± 6.20 | −0.39 ± 5.17 | 0.05 |
| Triglycerides (mg/dL) | −6.8 ± 51.9 | 12.4 ± 46.6 | 0.11 |
| Non-HDL-C (mg/dL) | 0.47 ± 16.6 | 4.30 ± 18.6 | 0.36 |
| Apolipoprotein B (mg/dL) | 0.21 ± 41.4 | −5.73 ± 34.4 | 0.29 |
| Changes in the metabolism | |||
| Fasting glucose (mg/dL) | −7.21 ± 9.30 | −2.12 ± 11.1 | 0.04 |
| Fasting insulin (mIU/L) | −2.76 ± 2.61 | −0.33 ± 3.51 | 0.001a |
| Changes in blood pressure and inflammation | |||
| Systolic blood pressure (mmHg) | −3.3 ± 12.2 | 0.0 ± 16.4 | 0.33 |
| Diastolic blood pressure (mmHg) | −1.7 ± 9.1 | 4.9 ± 11.8 | 0.01 |
| hsCRP (mg/L) | 1.02 ± 2.73 | −0.25 ± 5.66 | 0.23 |
| Changes in other MicroCog factors | |||
| GCFi score | 1.86 ± 7.09 | 2.97 ± 5.49 | 0.48 |
| Changes in anthropometrics | |||
| Weight (kg) | −1.70 ± 4.69 | 0.20 ± 3.45 | 0.06 |
| Waist circumference (cm) | −2.88 ± 11.47 | −0.35 ± 4.41 | 0.40 |
GCFi, general cognitive functioning index; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reaction protein.
Significant at P < 0.0028 when corrected for 18 comparisons (the primary outcome, 4 secondary outcomes, and the 13 outcomes in the table).
MSS was also improved (Figure 3B), with MSS declining in the intermittent fasting group but increasing in controls (P = 0.006; see Table 2). The significant improvement in MSS was due to the aggregation of mild to moderate declines in DBP, triglycerides, and glucose, and a moderate increase in HDL-C (Table 3), while changes in SBP and waist circumference were not statistically different although both changed qualitatively in the correct direction. At 13 weeks, MSS change averaged −0.71 ± 1.72 vs. 0.06 ± 1.71 for fasting vs. control groups, respectively (P = 0.056); at 4 weeks, the MSS change was −0.90 ± 3.03 vs. −0.05 ± 2.28 for fasting vs. control arms (P = 0.22). Because the MSS ranged from −8.37 to 11.86 at baseline and from −6.48 to 32.39 at 26 weeks, with no absolute zero, no percentage changes in MSS were calculated. For the cognitive biomarkers (Table 2), no differences were found for changes in BDNF (Figure 3C) or GCPi (Figure 3D). Repeated measures mixed modelling showed that HOMA-IR (P = 0.007) was significant when all timepoints were taken into account, but variation in MSS across all four visits produced a weaker outcome (P = 0.32), while BDNF (P = 0.80) remained not significant (GCPi was not measured at 4 or 13 weeks: see Methods).
Weight was minimally affected (Table 3) by the low-frequency intermittent fasting regimen (fasting: −1.65% vs. baseline; control: +0.20% vs. baseline) and no change was found in waist circumference (see Table 3). Changes in other cholesterol parameters, blood pressures, and cardiovascular risk factors are shown in Table 3. In repeated measures mixed modelling, weight was not different for fasting vs. controls (P = 0.84).
With a higher than anticipated drop-out rate, post hoc power calculations were conducted. Based on the expected changes in LDL-C for intermittent fasting and control arms prior to trial inception (see Methods), the final evaluable sample size of 71 subjects provided 71% power to detect an LDL-C difference between the study arms. However, using the observed LDL-C changes (i.e. fasting: 0.18 ± 16.7 mg/dL; control: 2.48 ± 19.4 mg/dL), the actual sample size provided <10% power to detect the targeted difference and, to have 80% power, it would have required a sample size of 1936 subjects (968 per arm).
Discussion
Summary
In a randomized controlled trial of low-frequency intermittent fasting, change in LDL-C over 26 weeks was not different compared to a parallel ad libitum diet control group. Cognitive performance measured by the MicroCog, a clinical screening exam, and BDNF, a circulating biomarker of cognitive function, were also not different. In contrast, and despite conservative multiple comparisons correction, insulin resistance measured by HOMA-IR and metabolic syndrome quantified by the MSS were significantly improved by intermittent fasting. These improvements were in the context of minimal, non-significant changes in body weight and waist circumference. The decline in HOMA-IR in the intermittent fasting arm was associated with substantially reduced insulin levels and moderately decreased glucose.
Context of these findings
While numerous studies previously evaluated the relationship between intermittent fasting and LDL-C,5–7,13–16 those results are complicated to interpret. Generally, they reported that intermittent fasting was associated with declines in LDL-C.13 Larger trials (i.e. >60 subjects), however, showed over 12 months that LDL-C changes were similar in intermittent fasting and parallel CR arms,6,7 with no differences between fasting and control groups.5,6 In one trial, LDL-C was reduced by CR but ended significantly higher with intermittent fasting compared to CR.5 Achieving clarity from these results is challenging, perhaps because intermittent fasting indirectly reduced LDL-C in some studies by reducing weight and it was the weight loss that decreased LDL-C since weight changes alter cardiovascular risks.22–25
The challenges of interpretation are also partly due to a lack of consistency in what was considered to be fasting. Many fasts were no longer than 16 h—only minimally longer than a usual overnight fast—and some regimens allowed consumption of a small meal during the fast, making them very-low-calorie diets rather than true fasting. In addition, the frequencies of the fasts were inconsistent—twice per week, every other day, or every day for a portion of the day—and most studies were of relatively short length, with some as short as 2 days, many up to 12 weeks, and only a few out to 12 months. Very little information is available on lower-frequency fasting, which may be more sustainable for periods of years or decades but may not change weight.
Evidence exists from trials and epidemiologic studies that intermittent fasting has direct, weight-independent effects on health risks.9–11,26 Such direct effects likely involve activation of physiological survival responses during each fasting episode regardless of the fasting frequency. From an evolutionary perspective, the human body may have historically responded with survival benefits related to stress, nutrient deprivation, risks due to infection, or other mechanisms that were triggered by each fasting episode. In such a context, it is reasonable to expect that a longer duration of fasting would produce a larger endogenous survival benefit. Even low-frequency fasting of meaningful duration (e.g. 24 h) could alert the body to a threat to survival at each instance and, thereby, induce a meaningful survival benefit. In contrast, most intermittent fasting regimens today are weight loss diets that generate an energy deficit through high frequency, short duration food deprivation, with the duration of the energy intake-free period being approximately 16 h or less.5–7,15
If a weight loss-independent survival response exists, it would not be surprising that LDL-C did not decline in the present study. Weight loss was minimal in this study and the survival hazard of LDL-C, as currently understood, is indirect through a decade-long accumulation of atherosclerosis that eventually results in coronary artery disease.27 For people who develop coronary disease, some but not all will subsequently experience life-threatening acute events such as acute myocardial infarction, incident heart failure, or cardiac arrhythmias. LDL-C, however, does not predict the occurrence of such major adverse cardiovascular events once coronary disease has developed.28
Other explanations for this trial’s negative result for LDL-C changes may exist. With a higher-than-expected drop-out rate (24.0%) in the intermittent fasting arm, the statistical power of the trial may be an issue. However, post hoc power calculations estimated that the study needed >1900 subjects to detect statistical significance based on the observed LDL-C changes. Hence, an overestimate of the treatment effect presents a much larger issue. Furthermore, drop-outs were greater in the control arm (37.7%), perhaps due to the increasing popularity of fasting. This is in contrast to studies of more intensive intermittent fasting regimens in which the fasting arm had more drop-outs than the control and/or CR arm.5–7 Furthermore, the 24.0% drop-outs in the fasting arm were less than in studies of more intensive regimens: 38.2% in the fasting arm,5 31.3% in the fasting arm,7 38.9% overall,15 and 48.8% overall8 [the exception: an 8.2% drop-out rate in the fasting arm in a trial in which participants received a monetary incentives if they completed study milestones6].
Metabolic improvements
In contrast to risk factors for coronary disease onset, diabetes remains a risk factor for mortality once coronary disease is diagnosed.29 Generally, life expectancy is more seriously blunted by diabetes and metabolic syndrome than by elevated cholesterol.30,31 Furthermore, hypoglycaemia and uncontrolled hyperglycaemia constitute serious short-term threats to survival regardless of the presence of chronic diseases such as coronary disease.31 Indeed, the metabolome is a central biological system deeply involved in chronic cardiovascular diseases.
Insulin resistance, the primary cause of diabetes, arises from or in concert with a variety of factors, including higher weight.32 Intermittent fasting causes the body to extract fatty acids from adipose tissue to convert them to ketones for energy once glucose, glycogen, and resources for gluconeogenesis are consumed.33 Fasting may also trigger the body to dispose of stored energy through mitochondrial uncoupling.34 Other mechanisms are also under investigation, but these various mechanisms should reduce insulin resistance if, as the literature indicates,5,7,9,35 intermittent fasting has a beneficial effect on the metabolism. In the present trial, subjects in the intermittent fasting arm experienced improved insulin sensitivity associated with modest declines in glucose and substantial reductions in insulin after 26 weeks. The effect on insulin is of note clinically, as relatively less insulin was required to stimulate the needed glucose utilization.
The MSS, a weighted composite of all metabolic syndrome factors, was used previously in studies evaluating nutrition and exercise effects on the metabolic syndrome.18 MSS was significantly reduced by intermittent fasting in the present study. While no individual component of MSS changed significantly, the aggregated effect of mild-to-moderate improvements in DBP, HDL-C, glucose, and triglycerides in the intermittent fasting arm resulted in a significant decline in the summary MSS metric. This occurred despite no significant changes in SBP or waist circumference compared to controls. Finally, the Bonferroni multiple comparisons correction assumes that all studied outcomes are unrelated, but changes in HOMA-IR and MSS were modestly correlated (r = 0.440), suggesting that the use of P ≤ 0.01 herein as a multiple comparisons correction was conservative, but given the lack of change in LDL-C in the trial, cautious interpretation and additional validation are indicated.
Strengths and limitations
This trial evaluated a low-frequency intermittent fasting regimen that produced minimal weight loss, which is inconsistent with the weight loss reported by other trials of intermittent fasting. However, other health effects (e.g. reduced HOMA-IR and MSS) due to repeated fasting were found in this trial that may directly influence human health. Drop-outs were >15%, especially in controls; thus, statistical power and the LDL-C change were lower than expected, so caution should be used in the interpretation of study results. Assessment of cognitive function using a biomarker (BDNF) and a clinical test (MicroCog) did not show substantial improvements by intermittent fasting. The lack of significance of changes in BDNF and MicroCog measures and the use of the low-frequency intermittent fasting regimen, however, do not rule out small improvements in cognitive function due to fasting or small declines in control subjects, which should be investigated further. It may be that longer-duration studies are needed to observe cognitive changes, or that other devices should be used to assess cognitive function. Finally, the lack of detailed information on dietary intake did not allow the investigation of nutritional differences at baseline or changes in nutrition over the course of the study; thus, it is not possible to evaluate the implications of changes that were observed relative to nutritional status.
Conclusions
Low-frequency 24-h water-only intermittent fasting did not reduce LDL-C or improve markers of cognitive function but did reduce both HOMA-IR and MSS. As a genetically encoded response with few major side effects and a very low financial cost, intermittent fasting has the potential to be used by most people worldwide as a clinical therapeutic to treat cardiometabolic disease. More importantly, low-frequency intermittent fasting may be an optimal preventive lifestyle that can produce life-long impact on many cardiometabolic risk factors and help inhibit the development of chronic disease. Low-frequency intermittent fasting regimens should be further investigated for health and survival benefits. In future trials of intermittent fasting, markers of insulin sensitivity and glucose metabolism may be better endpoints to study than LDL-C.
Lead author biography

Ciera Bartholomew received a bachelor’s degree in Exercise and Wellness and a master’s degree in Exercise Sciences from Brigham Young University. She is pursuing a PhD at the University of Alabama at Birmingham under the tutelage of Dr. Courtney Peterson. During her master’s degree programme, Ms. Bartholomew developed an interest in intermittent fasting and its effects on health outcomes. As a PhD student, she is working on projects funded by RO1 and US Department of Defense grants. These projects are exploring the effects of meal timing on 24-h glucose control and blood pressure in people with pre-diabetes and diabetes.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Data Availability
The data underlying this article cannot be shared publicly due to United States privacy laws. The data will be shared contractually on reasonable request to the corresponding author.
Funding
This research was funded by the Intermountain Research and Medical Foundation grant number 759 (PI: BDH) and a supplemental grant from the Intermountain Research and Medical Foundation that was provided through the philanthropy of the Dell Loy Hansen Heart Foundation. The funding source had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Conflict of interest: B.D.H. is PI of other intermittent fasting grants from the Intermountain Research and Medical Foundation and a member of the scientific advisory board of Sylfph. The authors have no other conflicts of interest to report.
Supplementary Material
Contributor Information
Ciera L Bartholomew, Department of Exercise Sciences, Brigham Young University, Provo, UT, USA.
Joseph B Muhlestein, Intermountain Medical Center Heart Institute, 5121 S. Cottonwood St., Salt Lake City, UT 84107, USA; Cardiology Division, Department of Internal Medicine, University of Utah, Salt Lake City, UT, USA.
Heidi T May, Intermountain Medical Center Heart Institute, 5121 S. Cottonwood St., Salt Lake City, UT 84107, USA.
Viet T Le, Intermountain Medical Center Heart Institute, 5121 S. Cottonwood St., Salt Lake City, UT 84107, USA; Rocky Mountain University of Health Professions, Provo, UT, USA.
Oxana Galenko, Intermountain Medical Center Heart Institute, 5121 S. Cottonwood St., Salt Lake City, UT 84107, USA.
Kelly Davis Garrett, Neuropsychology, Intermountain Medical Center, Salt Lake City, UT, USA; Center for Aging, University of Utah, Salt Lake City, UT, USA.
Cherie Brunker, Geriatric Medicine, Department of Internal Medicine, Intermountain Medical Center; Division of Geriatrics, Department of Internal Medicine, University of Utah, Salt Lake City, UT, USA.
Ramona O Hopkins, Pulmonary Division, Department of Internal Medicine, Intermountain Medical Center, Salt Lake City, UT, USA; Psychology Department and Neuroscience Center, Brigham Young University, Provo, UT, USA.
John F Carlquist, Intermountain Medical Center Heart Institute, 5121 S. Cottonwood St., Salt Lake City, UT 84107, USA; Cardiology Division, Department of Internal Medicine, University of Utah, Salt Lake City, UT, USA.
Kirk U Knowlton, Intermountain Medical Center Heart Institute, 5121 S. Cottonwood St., Salt Lake City, UT 84107, USA; Division of Cardiovascular Medicine, Department of Medicine, University of California San Diego, La Jolla, CA, USA.
Jeffrey L Anderson, Intermountain Medical Center Heart Institute, 5121 S. Cottonwood St., Salt Lake City, UT 84107, USA; Cardiology Division, Department of Internal Medicine, University of Utah, Salt Lake City, UT, USA.
Bruce W Bailey, Department of Exercise Sciences, Brigham Young University, Provo, UT, USA.
Benjamin D Horne, Intermountain Medical Center Heart Institute, 5121 S. Cottonwood St., Salt Lake City, UT 84107, USA; Division of Cardiovascular Medicine, Department of Medicine, Stanford University, Stanford, CA, USA.
References
- 1. Folin O, Denis W. On starvation and obesity with special regerence to acidosis. J Biol Chem 1915;21:183–192. [Google Scholar]
- 2. Carlson AJ, Hoelzel F. Apparent prolongation of the life span of rats by intermittent fasting. J Nutr 1946;31:363–375. [DOI] [PubMed] [Google Scholar]
- 3. Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr 2005;81:69–73. [DOI] [PubMed] [Google Scholar]
- 4. Johnson JB, Summer W, Cutler RG, Martin B, Hyun D-H, Dixit VD, Pearson M, Nassar M, Telljohann R, Tellejohan R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med 2007;42:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, Gabel K, Freels S, Rigdon J, Rood J, Ravussin E, Varady KA. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med 2017;177:930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schübel R, Nattenmüller J, Sookthai D, Nonnenmacher T, Graf ME, Riedl L, Schlett CL, von Stackelberg O, Johnson T, Nabers D, Kirsten R, Kratz M, Kauczor HU, Ulrich CM, Kaaks R, Kühn T. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. Am J Clin Nutr 2018;108:933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carter S, Clifton PM, Keogh JB. Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes. A randomized noninferiority trial. JAMA Netw Open 2018;1:e180756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gray KL, Clifton PM, Keogh JB. The effect of intermittent energy restriction on weight loss and diabetes risk markers in women with a history of gestational diabetes: a 12-month randomized control trial. Am J Clin Nutr 2021;doi:10.1093/ajcn/nqab058. [DOI] [PubMed] [Google Scholar]
- 9. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 2018;27:1212–1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horne BD, May HT, Anderson JL, Kfoury AG, Bailey BM, McClure BS, Renlund DG, Lappé DL, Carlquist JF, Fisher PW, Pearson RR, Bair TL, Adams TD, Muhlestein JB. Usefulness of routine periodic fasting to lower risk of coronary artery disease in patients undergoing coronary angiography. Am J Cardiol 2008;102:814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horne BD, Muhlestein JB, May HT, Carlquist JF, Lappé DL, Bair TL, Anderson JL., for the Intermountain Heart Collaborative Study Group. Relation of routine, periodic fasting to risk of diabetes mellitus, and coronary artery disease in patients undergoing coronary angiography. Am J Cardiol 2012;109:1558–1562. [DOI] [PubMed] [Google Scholar]
- 12. Huff T, Jialal I. Physiology, cholesterol. Treasure Island, FL: StatPearls Publishing, 2019. [PubMed] [Google Scholar]
- 13. Mirmiran P, Bahadoran Z, Gaeini Z, Moslehi N, Azizi F. Effects of Ramadan intermittent fasting on lipid and lipoprotein parameters: an updated meta-analysis. Nutr Metab Cardiovasc Dis 2019;29:906–915. [DOI] [PubMed] [Google Scholar]
- 14. Klempel MC, Kroeger CM, Varady KA. Alternate day fasting increases ldl particle size independently of dietary fat content in obese humans. Eur J Clin Nutr 2013;67:783–785. [DOI] [PubMed] [Google Scholar]
- 15. Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 2019;11:1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horne BD, Muhlestein JB, Lappé DL, May HT, Carlquist JF, Galenko O, Brunisholz KD, Anderson JL. Randomized cross-over trial of short-term water-only fasting: metabolic and cardiovascular consequences. Nutr Metab Cardiovasc Dis 2013;23:1050–1057. [DOI] [PubMed] [Google Scholar]
- 17. Wijndaele K, Beunen G, Duvigneaud N, Matton L, Duquet W, Thomis M, Lefevre J, Philippaerts R. A continuous metabolic syndrome risk score: utility for epidemiological analyses. Diabetes Care 2006;29:2329. [DOI] [PubMed] [Google Scholar]
- 18. Johnson JL, Slentz CA, Houmard JA, Samsa GP, Duscha BD, Aiken LB, McCartney JS, Tanner CJ, Kraus WE. Exercise training amount and intensity effects on metabolic syndrome (from Studies of a Targeted Risk Reduction Intervention through Defined Exercise). Am J Cardiol 2007;100:1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Amidfar M, de Oliveira J, Kucharska E, Budni J, Kim YK. The role of CREB and BDNF in meurobiology and treatment of Alzheimer’s disease. Life Sci 2020;257:118020. [DOI] [PubMed] [Google Scholar]
- 20. Walsh EI, Smith L, Northey J, Rattray B, Cherbuin N. Towards an understanding of the physical activity-BDNF-cognition triumvirate: a review of associations and dosage. Ageing Res Rev 2020;60:101044. [DOI] [PubMed] [Google Scholar]
- 21. Horne BD, Muhlestein JB, Anderson JL. Letter by Horne et al regarding article, “Prognostic value of fasting versus nonfasting low-density lipoprotein cholesterol levels on long-term mortality: Insight from the National Health and Nutrition Survey III (NHANES-III)”. Circulation 2015;131:e472. [DOI] [PubMed] [Google Scholar]
- 22. Wilson PWF, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk. The Framingham experience. Arch Intern Med 2002;162:1867–1872. [DOI] [PubMed] [Google Scholar]
- 23. Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr 1992;56:320–328. [DOI] [PubMed] [Google Scholar]
- 24. Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994;17:30–36. [DOI] [PubMed] [Google Scholar]
- 25. Moore LL, Visioni AJ, Qureshi MM, Bradlee ML, Ellison RC, D'Agostino RB. Weight loss in overweight adults and the long-term risk of hypertension. Arch Intern Med 2005;165:1298–1303. [DOI] [PubMed] [Google Scholar]
- 26. Bartholomew CL, Muhlestein JB, Anderson JL, May HT, Knowlton KU, Bair TL, Le VT, Bailey BW, Horne BD. Association of periodic fasting lifestyles with survival and incident major adverse cardiovascular events in patients undergoing cardiac catheterization. Eur J Prev Cardiol 2021. doi:10.1093/eurjpc/zwaa050. [DOI] [PubMed] [Google Scholar]
- 27. Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, Daemen MJ, Demer LL, Hegele RA, Nicholls SJ, Nordestgaard BG, Watts GF, Bruckert E, Fazio S, Ference BA, Graham I, Horton JD, Landmesser U, Laufs U, Masana L, Pasterkamp G, Raal FJ, Ray KK, Schunkert H, Taskinen MR, van de Sluis B, Wiklund O, Tokgozoglu L, Catapano AL, Ginsberg HN. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2020;41:2313–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, Brenner H, Schöttker B, Lääperi M, Kauhanen D, Koistinen KM, Jylhä A, Huynh K, Mellett NA, Tonkin AM, Sullivan DR, Simes J, Nestel P, Koenig W, Rothenbacher D, Nygård O, Laaksonen R. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur Heart J 2020;41:371–380. [DOI] [PubMed] [Google Scholar]
- 29. Muhlestein JB, Anderson JL, Horne BD, Lavasani F, Allen Maycock CA, Bair TL, Pearson RR, Carlquist JF. Effect of fasting glucose levels on mortality in non-diabetic and diabetic patients with coronary artery disease undergoing percutaneous coronary intervention. Am Heart J 2003;146:351–358. [DOI] [PubMed] [Google Scholar]
- 30. Pencina MJ, D'Agostino RB, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease. The Framingham Heart Study. Circulation 2009;119:3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med 2007;167:1145–1151. [DOI] [PubMed] [Google Scholar]
- 32. Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867. [DOI] [PubMed] [Google Scholar]
- 33. Ruderman NB, Aoki TT, Cahill GF. Jr. Gluconeogenesis and its disorders in man. In Hanson RW, Mehlman MA, eds. Gluconeogenesis: Its Regulation in Mammalian Species. New York: Wiley; 1976. pp 515–530. [Google Scholar]
- 34. Walton CM, Jacobsen SM, Dallon BW, Saito ER, Bennett SLH, Davidson LE, Thomson DM, Hyldahl RD, Bikman BT. Ketones elicit distinct alterations in adipose mitochondrial bioenergetics. Int J Molec Sci 2020;21:6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res 2014;164:302–311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to United States privacy laws. The data will be shared contractually on reasonable request to the corresponding author.