Abstract
Selective adhesion to only certain epithelia is particularly common among the bacterial members of the indigenous microflora of mammals. We have found that the stratified squamous epithelium of the nonsecreting area of horse stomach is colonized by gram-positive rods. The microscopic features of a dense layer of these bacteria on the epithelium were found to be similar to those reported in mice, rats, and swine. Adhering microorganisms were isolated and identified as Lactobacillus salivarius, L. crispatus, L. reuteri, and L. agilis by DNA-DNA hybridization and 16S rRNA gene sequencing techniques. These lactobacilli associated with the horse, except for L. reuteri, were found to adhere to horse epithelial cells in vitro but not to those of rats. A symbiotic relationship of these lactobacilli with the horse is suggested.
In a series of studies on the relationships between intestinal microflora and host animals, we have demonstrated that lactobacilli indigenous to and dominant in the upper digestive tract control the population levels of other bacterial species in the stomach and the upper small intestine (13, 25). We have also shown that indigenous lactobacilli can attach host specifically to keratinized epithelial cells of the rat stomach in vitro (20).
In our recent study examining microbial colonization of the intestinal tract in newborn foals (15), we noticed a dense layer of gram-positive rods on the stratified squamous epithelium in the nonsecreting area of the stomach of the horse. This is the first report on the isolation and identification of indigenous Lactobacillus species adhering to the horse stomach.
MATERIALS AND METHODS
Preparation of specimens for isolation of bacteria and histology.
Samples of the nonsecreting area of the stomach, obtained from a healthy 7-year-old female thoroughbred immediately after euthanasia, were washed three times with vigorous agitation in buffered saline (BS; 0.8% NaCl, 0.121% K2HPO4, 0.034% KH2PO4; pH 7.2). A schematic drawing of the sampling locations of the horse stomach is shown in Fig. 1. After washing the specimen, some parts of the tissue were fixed with formalin and then embedded in paraffin, processed for histology, and stained with hematoxylin and eosin and the Gram stain. Epithelial cells were recovered from the remaining portion of fresh tissue by scraping and then homogenized in a Teflon grinder. The homogenates were plated at appropriate dilutions on MRS agar (Difco Laboratories, Detroit, Mich.). The plates were incubated anaerobically (AnaeroPack; Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan) at 37°C for 72 to 96 h. Before homogenization, a portion of epithelial cells were placed on slides, stained with Giemsa stain, and examined for the detection of adherent bacteria by microscope.
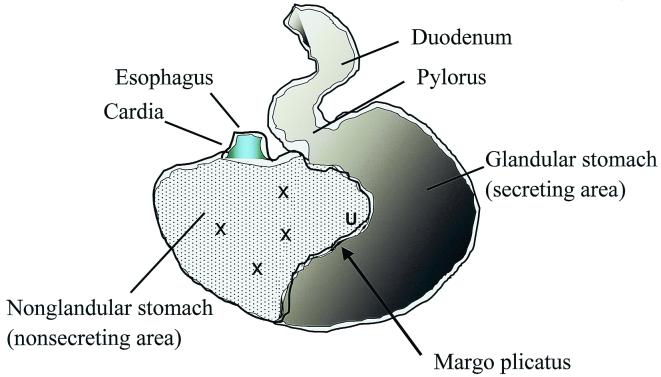
FIG. 1.
Schematic drawing of the nonsecreting area of the horse stomach. The mucosal surface of this area is lined by a keratinized squamous epithelium. X, sampling location; U, ulcer.
DNA preparation.
Bacteria were grown in MRS broth overnight at 37°C. Chromosomal DNA to be used as the template for RAPD [random(ly) amplified polymorphic DNA] PCR and 16S rRNA gene amplification was prepared from bacterial strains by the method of Zhu et al. (27). Intact DNA suitable for use in DNA-DNA hybridization was isolated by the method of Marmur (11) with slight modification, in that the bacterial cells were treated with 100 μg of the peptidoglycan N-acetylmuramoylhydrolase (N-acetylmuramidase SG; Seikagaku Corp., Tokyo, Japan) per ml at 50°C for 30 min after lysozyme treatment by the original method.
Determination of G+C content.
The guanine-plus-cytosine (G+C) content was determined by hydrolyzing the DNA enzymatically and then quantifying the nucleosides by high-performance liquid chromatography by the method of Ezaki et al. (4).
RAPD fingerprinting.
PCR-based RAPD fingerprinting was carried out by the method of Akopyanz et al. (1) using two primers (GAGGACAAAG and GGCGTCGGTT). The PCR products were electrophoresed in 2% agarose gels and photographed under UV light.
DNA-DNA hybridization.
Fluorometric DNA-DNA hybridization in microdilution wells was carried out by the method of Ezaki et al. (5), using 16 type strains of Lactobacillus species as standards. These were L. acidophilus YIT 0070 (ATCC 4356T), L. amylovorus YIT 0211 (JCM 1126T), L. brevis YIT 0076 (ATCC 14869T), L. buchneri YIT 0077 (ATCC 4005T), L. casei YIT 0180 (ATCC 334T), L. coryniformis subsp. coryniformis YIT 0237 (JCM 1164T), L. crispatus YIT 0212 (JCM 1185T), L. fermentum YIT 0081 (ATCC 14931T), L. gasseri YIT 0192 (DSM 20243T), L. graminis YIT 0260 (NRIC 1775T), L. johnsonii YIT 0219 (JCM 2012T), L. plantarum YIT 0102 (ATCC 14917T), L. reuteri YIT 0197 (JCM 1112T), L. rhamnosus YIT 0105 (ATCC 7469T), L. salivarius subsp. salicinius YIT 0089 (ATCC 11742T), and L. salivarius subsp. salivarius YIT 0104 (ATCC 11741T).
16S rRNA gene sequencing.
In vitro PCR amplification of 16S rRNA genes and direct sequencing of the amplified DNA fragments were performed. Details of the procedures, except for the sequence of primer 8F (5′-AGAGTTTGATCMTGGCTCAG), have been described previously (12). Primers 8F and 15R were used for PCR, and primers 8F, 520F, 930F, 1100F, 15R, 520R, 800R, and 1100R were used for 16S rRNA gene sequencing.
In vitro test for adhesion.
The in vitro test for adhesion was performed by the method described previously (20) with slight modifications. A 16-h culture of each of the test strains in MRS broth was centrifuged, and the cells were resuspended in BS at a cell density corresponding to an optical density of 1 unit at 660 nm (OD660; ca. 3 × 108 microorganisms/ml). To 3 ml of this bacterial cell suspension, a piece (ca. 1 by 1 cm) of bacterium-free tissue from the nonsecreting area of the stomach wall was added. The mixture was shaken at 60 rpm for 30 min at 37°C. At the end of this period, the piece of stomach tissue was washed three times with BS, and epithelial cells were scraped off with a surgical knife. The cells were placed on slides, stained with Giemsa stain, and examined microscopically. Bacterium-free stomach was obtained from a newborn foal euthanized because of bone fracture at delivery. Bacterium-free rat stomachs were obtained from germfree Fischer 344 rats.
Coaggregation assay.
The coaggregation test was performed according to Vandevoorde et al. (24) with slight modifications. Overnight cultures in MRS broth were harvested by centrifugation and washed twice with phosphate-buffered saline. They were resuspended in the same buffer at an OD600 of 0.6. Mixtures (1:1; total, 1 ml) of the cell suspension of both strains (28 pairs of eight strains) were shaken for 30 min at 150 rpm and left to stand at room temperature for 1 h before measuring the OD. The coaggregation was expressed according to the equation of Handley et al. (10).
RESULTS
Histological observation.
In preparations from the nonsecreting portion of the stomach, dense layers of short rods could be seen on the keratinized stratified squamous epithelium (Fig. 2a). Examination of the epithelial cells obtained in scrapings indicated that the number of bacteria naturally attached per cell was more than 100 (Fig. 2b). Most frequently seen were short rods with rounded ends. These bacteria were gram positive. Although there were no signs of gastrointestinal problems in this horse, we found a small ulcer in the stratified squamous epithelial mucosa adjacent to the margo plicatus. Interestingly, no layers of gram-positive rods were observed in this area, whereas sporadic microcolonies of gram-positive cocci surrounded by neutrophilic inflammatory cell infiltrates were observed (Fig. 3).
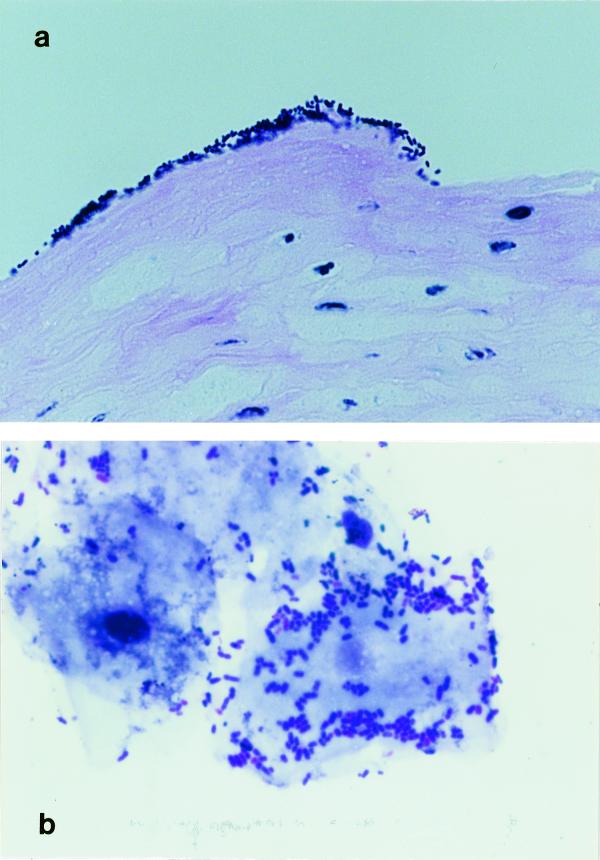
FIG. 2.
Dense layer of bacteria on the keratinized stratified squamous epithelium of the nonsecreting portion of the stomach (a; hematoxylin and eosin stained; ×780) and adherent bacteria on epithelial cells obtained in scrapings (b; Giemsa stained; ×1,040).
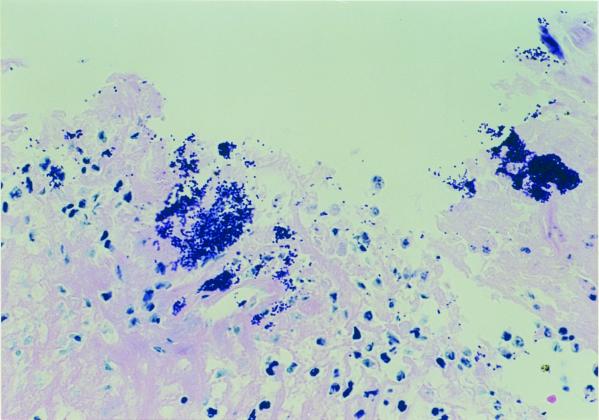
FIG. 3.
Hematoxylin-and-eosin-stained tissue section of an ulcer in the stratified squamous epithelial mucosa adjacent to the margo plicatus, showing invasion of gram-positive cocci surrounded by neutrophilic inflammatory cell infiltrates (magnification, ×520).
Identification of bacteria by DNA-DNA hybridization.
Among the colonies of gram-positive rods isolated, eight different strains distinguished by RAPD DNA fingerprinting, designated as H-2, H-3, H-5, H-8, H-9, H-10, H-12, and H-14 (Table 1), were selected. The DNA-DNA relatedness of these strains to 16 standard type strains was then examined. Three heterofermentative strains, H-3, H-12, and H-14, were identified (>70% reassociation) as L. reuteri. Strains H-8 and H-9 were identified as L. salivarius and L. crispatus, respectively. DNA from strain H-5 did not hybridize to the DNA from any of the 16 type strains. Two strains, H-2 and H-10, were excluded from the DNA-DNA hybridization test because samples of chromosomal DNA could not be isolated from these strains due to their resistance to lysis by lysozyme, N-acetylmuramidase, and sodium dodecyl sulfate.
TABLE 1.
Identification of Lactobacillus species isolated from horse stomach and adhesion of these bacteria to keratinized epithelial cells of the stomach in vitro
| Strain | Fermentationa | % GC content | Identification by:
|
In vitro adhesionb
|
||
|---|---|---|---|---|---|---|
| DNA-DNA hybridization | 16S rRNA sequencing | Horse | Rat | |||
| H-2 | Homo | 35 | Not done | L. salivarius | +++ | − |
| H-3 | Hetero | 43 | L. reuteri | Not done | − | − |
| H-5 | Homo | 40 | Not identified | L. agilis | + | − |
| H-8 | Homo | 37 | L. crispatus | Not done | + | − |
| H-9 | Homo | 33 | L. salivarius | Not done | + | − |
| H-10 | Homo | 35 | Not done | L. salivarius | +++ | − |
| H-12 | Hetero | 43 | L. reuteri | Not done | − | − |
| H-14 | Hetero | 42 | L. reuteri | Not done | − | − |
Fermentation types were determined as homogeneous (Homo) or heterogeneous (Hetero) on the basis of gas production in 2% glucose broth (MRS broth).
The number of bacteria attached per epithelial cell was obtained by direct microscope count, examining 10 to 20 epithelial cells. Score: +++, >100; ++, 10 to 100; +, 1 to 10; −, 0. Details are as described in Materials and Methods.
Identification by 16S rRNA gene sequencing.
The amplified fragments (ca. 1,500 bp) encoding the 16S rRNA gene sequences of the strains H-2, H-5, and H-10 were sequenced. When compared with other rRNA gene sequences available in the DNA Data Bank of Japan (http://www.ddbj.nig.ac.jp/), the sequence of strain H-5 was found to be identical (100% similarity) to the 16S rRNA gene sequence of the type strain of Lactobacillus agilis (accession no. M58803). Although strains H-2 and H-10 showed different RAPD profiles (data not shown), their 16S rRNA gene sequences were identical. These strains were tentatively identified as L. salivarius because the sequence of the 16S rRNA gene of these strains was found to share more than 99.6% similarity with the corresponding gene sequences of the type strains of L. salivarius subsp. salivarius (accession no. AF089108) and L. salivarius subsp. salicinius (accession no. M59054).
In vitro test for adhesion.
The finding that epithelial cells from the stomach of a newborn foal that had died immediately after birth did not have bacteria attached to them allowed us to use them to examine the cell adherence properties of bacterial isolates. Cells from germfree rats were also used to examine the host specificity of adhesion. L. salivarius H-2 and H-10 adhered well to the horse epithelial cells (Fig. 4a), and L. agilis H-5, L. crispatus H-8, and L. salivarius H-9 showed moderate adhesion (Table 1). Although these strains adhered to the host cells, none of these strains isolated from the horse adhered to the keratinized epithelial cells of germ-free rat stomach (Table 1). Three strains of L. reuteri did not adhere to either the horse epithelial cells (Fig. 4b) or the cells of the rat stomach (Table 1).
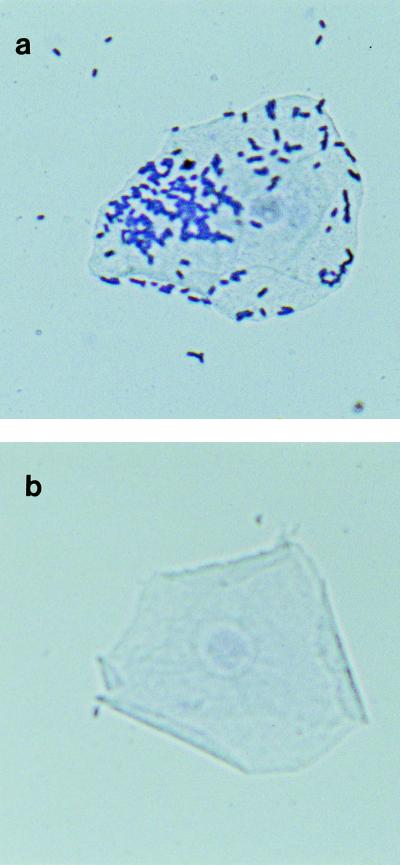
FIG. 4.
Photomicrographs of Giemsa-stained epithelial cells, showing an adhering strain of Lactobacillus, L. salivarius H-2 (a), and a nonadhering strain, L. reuteri H-14 (b), attached to the keratinized epithelial cells of the horse stomach in vitro. Magnification, ×1,040.
Coaggregation test.
Because strains of L. reuteri did not adhere to the horse epithelial cells in vitro, interbacterial adherence (coaggregation) of the isolated strains was tested. None of 28 combinations involving 2 strong adhering strains (Table 1) were shown to be coaggregative under the experimental conditions used.
DISCUSSION
It is well known that indigenous microorganisms of many types associate closely with the mucosal epithelia in the gastrointestinal tract of the host (for a review, see reference 17). One of the types of surface cells involved in this interaction is keratinized stratified squamous epithelial cells found in parts of the stomach and esophagus of some monogastric animals such as rats, mice, and pigs and in the crops of chickens (2, 3, 6–8, 16–19, 21–23). We found a layer of lactobacilli lining the nonsecreting area of horse stomach. Although the isolation and identification of the bacteria reported here is a case report of specimens from a 7-year-old female thoroughbred horse, such bacterial layers were observed in specimens from four out of four other horses (data not shown). These specimens were from two 1-year-old foals euthanized because of dysstasia and fracture of ribs, respectively; a 21-year-old female that died due to uterus rupture; and a 23-year-old female sacrificed. These data, together with the results of our previous study on the development of the intestinal microflora in newborn foals (15) indicate that the lactobacillus flora become established soon after birth and adhere to the stomach epithelium throughout the life of the horse.
The mechanisms by which indigenous intestinal bacteria adhere to the gastrointestinal epithelial cells of host animals are not fully understood. As we and others have reported previously (6, 20, 21), the Lactobacillus strains adhering to the gastric epithelium of the horse also appear to be highly host specific; these strains adhered aggressively to cells from horse epithelium in vitro but not to cells from rats (Table 1). In the present study, some strains isolated from the horse stomach did not show the ability to adhere to horse epithelial cells in vitro (Table 1). We then examined interbacterial adherence (coaggregation) of all strains isolated because the bacterial coaggregation has been demonstrated as highly specific partnerships between some strains of indigenous bacteria in the oral cavity and gastrointestinal tracts (24, 26). Although Vandevoorde et al. reported that the coaggregation reactions were prevalent among chicken lactobacilli, no coaggregation of the horse Lactobacillus strains (28 combinations of 8 strains listed in Table 1) was detected. At present, the reason not all strains isolated from the epithelial sample did not adhere to the epithelial cells in vitro is unclear. It is reported that not all gastrointestinal strains of lactobacilli are able to adhere to the mucosal surfaces of the host, as some are inhabitants of the gastrointestinal lumen (7, 23). Although the significance of the adhering lactobacilli in the stomach is unclear, the ability to adhere to host cells seems to be an important factor in determining whether a particular bacterial strain colonizes the intestinal tract of a specific host. Desquamation of the stratified squamous epithelium of the stomach releases cells with attached lactobacilli into the lumen as an inoculum. Therefore, as suggested by Fuller et al. (7), these attached bacteria could prove to be an important mechanism of regulating the microflora of not only the stomach but also of the whole gastrointestinal tract by supplying a continuous inoculum of specific lactobacilli.
Gastric ulcer is one of the most common diseases in horses (14). We found a small ulcer, associated with which only gram-positive cocci were observed (Fig. 3). We did not isolate and identify these cocci because the focus of this study was to examine the layer of bacteria on the stratified squamous epithelial mucosa and to identify these members of the indigenous microflora of the horse stomach. Loss of the superficial mucosa with layers of indigenous lactobacilli may have allowed invasion by these gram-positive cocci. The ability of indigenous lactobacilli to inhibit the growth of potentially pathogenic bacteria is well documented and has been attributed to a number of possible mechanisms, including competition for adhesion sites (17). Further studies of the role of bacteria in the pathogenesis of gastric ulcers in the horse are needed.
In conclusion, the selectivity of bacterial adhesion to surfaces is proposed as a critical ecological determinant affecting bacterial colonization in environments with open surfaces exposed to bathing fluids (9).
ACKNOWLEDGMENTS
We thank Shin Iwata and Shouichi Kado for technical assistance with the histological studies.
REFERENCES
- 1.Akopyanz N, Bukanov N O, Westblom T U, Kresovich S, Berg D E. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992;20:5137–5142. doi: 10.1093/nar/20.19.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brockett M, Tannock G W. Dietary components influence tissue-associated lactobacilli in the mouse stomach. Can J Microbiol. 1981;27:452–455. doi: 10.1139/m81-068. [DOI] [PubMed] [Google Scholar]
- 3.Brownlee A, Moss W. The influence of diet on lactobacilli in the stomach of the rat. J Pathol Bacteriol. 1961;82:513–516. doi: 10.1002/path.1700820227. [DOI] [PubMed] [Google Scholar]
- 4.Ezaki T, Saidi S M, Liu S L, Hashimoto Y, Yamamoto H, Yabuuchi E. Rapid procedure to determine the DNA base composition from small amounts of gram-positive bacteria. FEMS Microbiol Lett. 1990;55:127–130. doi: 10.1016/0378-1097(90)90180-x. [DOI] [PubMed] [Google Scholar]
- 5.Ezaki T, Hashimoto Y, Takeuchi N, Yamamoto H, Liu S L, Miura H, Matsui K, Yabuuchi E. Simple genetic method to identify viridans group streptococci by colorimetric dot hybridization and fluorometric hybridization in microdilution wells. J Clin Microbiol. 1988;26:1708–1813. doi: 10.1128/jcm.26.9.1708-1713.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuller R. Ecological studies on the Lactobacillus flora associated with the crop epithelium of the fowl. J Appl Bact. 1973;36:131–139. [Google Scholar]
- 7.Fuller R, Barrow P A, Brooker B E. Bacteria associated with the gastric epithelium of neonatal pigs. Appl Environ Microbiol. 1978;35:582–591. doi: 10.1128/aem.35.3.582-591.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuller R, Brooker B E. Lactobacilli which attach to the crop epithelium of the fowl. Am J Clin Nutr. 1974;27:1305–1312. doi: 10.1093/ajcn/27.11.1305. [DOI] [PubMed] [Google Scholar]
- 9.Gibbons R J, van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971;3:567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Handley P S, Harty D W, Wyatt J E, Brown C R, Doran J P, Gibbs A C. A comparison of the adhesion, coaggregation and cell-surface hydrophobicity properties of fibrillar and fimbriate strains of Streptococcus salivarius. J Gen Microbiol. 1987;133:3207–3217. doi: 10.1099/00221287-133-11-3207. [DOI] [PubMed] [Google Scholar]
- 11.Marmur J. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. Mol Biol. 1961;3:208–218. [Google Scholar]
- 12.Miyake T, Watanabe K, Watanabe T, Oyaizu H. Phylogenetic analysis of the genus Bifidobacterium and related genera based on 16S rDNA sequences. Microbiol Immunol. 1998;42:661–667. doi: 10.1111/j.1348-0421.1998.tb02337.x. [DOI] [PubMed] [Google Scholar]
- 13.Morotomi M, Watanabe T, Suegara N, Kawai Y, Mutai M. Distribution of indigenous bacteria in the digestive tract of conventional and gnotobiotic rats. Infect Immun. 1975;11:962–968. doi: 10.1128/iai.11.5.962-968.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray M J. Gastric ulceration in horses: 91 cases (1987–1990) J Am Vet Med Assoc. 1992;201:117–120. [PubMed] [Google Scholar]
- 15.Sakaitani Y, Yuki N, Nakajima F, Nakanishi S, Tanaka H, Tanaka R, Morotomi M. Colonization of intestinal microflora in newborn foals. J Intestinal Microbiol. 1999;13:9–14. . (In Japanese with English summary.) [Google Scholar]
- 16.Savage D C. Microbial interference between indigenous yeast and lactobacilli in the rodent stomach. J Bacteriol. 1969;98:1278–1283. doi: 10.1128/jb.98.3.1278-1283.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savage D C. Introduction to mechanisms of association of indigenous microbes. Am J Clin Nutr. 1979;32:113–118. doi: 10.1093/ajcn/32.1.113. [DOI] [PubMed] [Google Scholar]
- 18.Savage D C, Dubos R, Schaedler R W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968;127:67–80. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savage D C, Blumershine R V. Surface-surface associations in microbial communities populating epithelial habitats in the murine gastrointestinal ecosystem: scanning electron microscopy. Infect Immun. 1974;10:240–250. doi: 10.1128/iai.10.1.240-250.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suegara N, Morotomi M, Watanabe T, Kawai Y, Mutai M. Behavior of microflora in the rat stomach: adhesion of lactobacilli to the keratinized epithelial cells of the rat stomach in vitro. Infect Immun. 1975;12:173–179. doi: 10.1128/iai.12.1.173-179.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tannock G W. Demonstration of mucosa-associated microbial populations in the colons of mice. Appl Environ Microbiol. 1987;53:1965–1968. doi: 10.1128/aem.53.8.1965-1968.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tannock G W, Szylit O, Duval Y, Raibaud P. Colonization of tissue surfaces in the gastrointestinal tract of gnotobiotic animals by Lactobacillus strains. Can J Microbiol. 1982;28:1196–1198. doi: 10.1139/m82-177. [DOI] [PubMed] [Google Scholar]
- 23.Tannock G W, Smith J M. The microflora of the pig stomach and its possible relationship to ulceration of the pars oesophagea. J Comp Pathol. 1970;80:359–367. doi: 10.1016/0021-9975(70)90066-6. [DOI] [PubMed] [Google Scholar]
- 24.Vandevoorde L, Christiaens H, Verstraete W. Prevalence of coaggregation reactions among chicken lactobacilli. J Appl Bacteriol. 1992;72:214–219. doi: 10.1111/j.1365-2672.1992.tb01826.x. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe T, Morotomi M, Suegara N, Kawai Y, Mutai M. Distribution of indigenous lactobacilli in the digestive tract of conventional and gnotobiotic rats. Microbiol Immunol. 1977;21:183–191. doi: 10.1111/j.1348-0421.1977.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 26.Weiss E I, Shenitzki B, Leibusor R. Microbial coaggregation in the oral cavity. Adv Exp Med Biol. 1996;408:233–240. doi: 10.1007/978-1-4613-0415-9_28. [DOI] [PubMed] [Google Scholar]
- 27.Zhu H, Qu F, Zhu L H. Isolation of genomic DNAs from plants, fungi and bacteria using benzyl chloride. Nucleic Acids Res. 1993;21:5279–5280. doi: 10.1093/nar/21.22.5279. [DOI] [PMC free article] [PubMed] [Google Scholar]