ABSTRACT
Serological assays for SARS-CoV-2 antibodies must be validated for performance with a large panel of clinical specimens. Most existing assays utilize a single antigen target and may be subject to reduced diagnostic specificity. This study evaluated a multiplex assay that detects antibodies to three SARS-CoV-2 targets. Human serum specimens (n = 323) with known previous SARS-CoV-2 exposure status were tested on a commercially available multiplex bead assay (MBA) measuring IgG to SARS-CoV-2 spike protein receptor-binding domain (RBD), nucleocapsid protein (NP), and RBD/NP fusion antigens. Assay performance was evaluated against reverse transcriptase PCR (RT-PCR) results and also compared with test results for two single-target commercial assays. The MBA had a diagnostic sensitivity of 89.8% and a specificity of 100%, with serum collection at >28 days following COVID-19 symptom onset showing the highest seropositivity rates (sensitivity: 94.7%). The MBA performed comparably to single-target assays with the ability to detect IgG against specific antigen targets, with 19 (5.9%) discrepant specimens compared to the NP IgG assay and 12 (3.7%) compared to the S1 RBD IgG assay (kappa coefficients 0.92 and 0.88 compared to NP IgG and S1 RBD IgG assays, respectively. These findings highlight inherent advantages of using a SARS-CoV-2 serological test with multiple antigen targets; specifically, the ability to detect IgG against RBD and NP antigens simultaneously. In particular, the 100.0% diagnostic specificity exhibited by the MBA in this study is important for its implementation in populations with low SARS-CoV-2 seroprevalence or where background antibody reactivity to SARS-CoV-2 antigens has been detected.
IMPORTANCE Reporting of SARS-CoV-2 infections through nucleic acid or antigen based diagnostic tests severely underestimates the true burden of exposure in a population. Serological data assaying for antibodies against SARS-CoV-2 antigens offers an alternative source of data to estimate population exposure, but most current immunoassays only include a single target for antibody detection. This report outlines a direct comparison of a multiplex bead assay to two other commercial single-target assays in their ability to detect IgG against SARS-CoV-2 antigens. Against a well-defined panel of 323 serum specimens, diagnostic sensitivity and specificity were very high for the multiplex assay, with strong agreement in IgG detection for single targets compared to the single-target assays. Collection of more data for individual- and population-level seroprofiles allows further investigation into more accurate exposure estimates and research into the determinants of infection and convalescent responses.
KEYWORDS: COVID-19, SARS-CoV-2, antibody, multiplex bead assay
INTRODUCTION
Development and implementation of high-quality serological assays for SARS-CoV-2 antibody detection is an important component in the ongoing COVID-19 pandemic response (1). Detection of antibodies can serve a critical public health role in identifying individuals with prior SARS-CoV-2 infection and estimating seroprevalence in populations, especially for patients who have had subclinical infection (2, 3). As of October 2021, more than 600 commercial SARS-CoV-2 serological assays were currently on the global market or in development (4); however, the majority of these rely on a single antigen target for antibody capture and detection. The spike glycoprotein (often the receptor-binding domain [RBD]) or the nucleocapsid protein (NP) are commonly utilized antigens in antibody detection assays. While single-target antibody detection assays can exhibit diagnostic sensitivity and specificity approaching 100%, they may have suboptimal performance in low-seroprevalence populations or, perhaps, be subject to cross-reactivity from antibodies to other human coronaviruses (5–7) or to other pathogens in the areas of endemicity, including malaria and dengue (8–10). Serology assays which utilize multiple targets represent a viable strategy for achieving robust seroprevalence estimates, because their result algorithms offer inherent redundancy based on a consensus call (11). Multiplex assays may also serve a role in differentiating the antibody responses induced by vaccination or from natural infection (12). Although several commercial multiplex bead assays (MBAs) have been recently released for SARS-CoV-2 antibody detection (13, 14), additional studies with this type of assay platform are needed, ideally utilizing larger clinical specimen cohorts.
In this study, we evaluated the FlexImmArray SARS-CoV-2 Human IgG Antibody Test developed by Tetracore, Inc. (Rockville, MD). This commercially available serological MBA allows the simultaneous detection of IgG antibodies against the receptor-binding domain epitope of the spike glycoprotein S1 subunit, IgG against nucleocapsid protein, and IgG against a fusion protein of the two (RBD/NP). Notably, this assay generates an overall call of “seropositive” for SARS-CoV-2 IgG only if all three targets are individually positive.
Here, we evaluated the sensitivity and specificity of this MBA, and also compared its performance to two commercially available single-antigen SARS-CoV-2 serology assays, using human serum specimens provided by the Centers for Disease Control and Prevention (CDC) COVID-19 Laboratory Task Force.
RESULTS
For the 323 serum specimens utilized in this study, patient demographics and COVID-19 RT-PCR testing data are listed in Table 1. Univariate and adjusted modeling was performed for potential associations between patient demographics and SARS-CoV-2 assay seropositivity. After adjusting for covariates, the only significant association was seen with reduced odds of seropositivity for participants in the “Other” race category. The number of days between COVID-19 symptom onset and subsequent serum collection ranged from 6 to 83 days; a histogram showing the distribution of days between COVID-19 symptom onset and serum collection is shown in Fig. S1 in the supplemental material.
TABLE 1.
Characteristics of the study population and associations with SARS-CoV-2 multiplex seropositivitya
| Characteristic | No. with data available, n (%) | Association with SARS-CoV-2 multiplex assay seropositivity |
|
|---|---|---|---|
| Univariate analysis result (95% CI) | Multivariate analysis result (95% CI) | ||
| Sex | 322 | ||
| Male | 203 (63) | Referent (1.0) | Referent (1.0) |
| Female | 119 (37.0) | 4.82 (2.86–8.11) | 0.89 (0.41–1.92) |
| Age (mean = 46 yrs, range = 19–88 yrs) | 312 | 1.00 (0.99–1.02) | 1.01 (0.98–1.03) |
| Race | 280 | ||
| African American | 46 (16.4) | 0.07 (0.03–0.16) | Invalid (0.001–999.9) |
| American Indian | 2 (0.7) | 0.34 (0.02–5.53) | 0.22 (0.01–4.10) |
| Asian | 5 (1.8) | 1.36 (0.15–12.43) | Invalid (0.001–999.9) |
| Caucasian | 209 (74.6) | Referent (1.0) | Referent (1.0) |
| Other | 18 (6.4) | 0.10 (0.03–0.31) | 0.08 (0.02–0.32) |
| Ethnicity | 213 | ||
| Hispanic/Latino | 111 (52.1) | 0.47 (0.24–0.89) | 0.85 (0.35–2.07) |
| Non-Hispanic/Latino | 102 (47.9) | Referent (1.0) | Referent (1.0) |
| COVID-19 symptoms | 298 | ||
| Symptomatic | 174 (58.4) | 2.20 (1.37–3.54) | 1.20 (0.49–2.94) |
| Asymptomatic | 124 (41.6) | Referent (1.0) | Referent (1.0) |
| Days between symptom onset and serum collection (mean = 28, median = 22, range = 6–83) | 135 | See analysis in Table 3, Table S1, Fig. S1 | |
95% CI, 95% confidence interval.
We performed the MBA on all serum specimens on three consecutive days and found a high degree of agreement for the qualitative results obtained: 311 (or 96.3%) specimens concordantly tested either seropositive or seronegative across all 3 days. The 12 specimens with variation had a high degree of “equivocal” calls for one or more antigen targets, indicating a quantitative result near the assay seropositivity threshold.
Corresponding patient results from previous SARS-CoV-2 RT-PCR testing were used to calculate overall diagnostic sensitivity and specificity (Table 2). Eleven specimens (3.4%) were excluded from this analysis because they did not have corresponding data for SARS-CoV-2 RT-PCR diagnostic testing. The MBA results yielded a diagnostic sensitivity of 89.8% and a specificity of 100.0%. Because an overall seropositive call for the MBA requires IgG positivity to all three antigen targets (recommended by the manufacturer), we also performed additional exploratory analysis using data from each antigen target individually (RBD, NP, or the RBD/NP fusion antigen) (Table 2). Sensitivity estimates were comparable among the three targets, ranging from a low of 90.8% for the RBD/NP fusion to a high of 92.9% for NP antigen. Specificity was lowest for the NP antigen (94.8%), followed by the RBD (95.7%), and was highest for RBD/NP fusion antigen (100.0%). Positivity to a single antigen target and previous RT-PCR positivity were also investigated. Among all specimens with corresponding RT-PCR results (n = 312), 12 (3.8%) had positive calls for only one or two targets and were therefore called SARS-CoV-2 IgG seronegative. Of these 12, 1 was positive only to NP, 9 were positive to both RBD and NP, and 2 were positive to NP and the RBD/NP fusion.
TABLE 2.
RT-PCR-identified diagnostic sensitivity and specificity of the multiplex bead assay kit by overall call and individual antigen targetsa
| Target | Sensitivity (%) |
Specificity (%) |
||||
|---|---|---|---|---|---|---|
| Valueb | LL | UL | Valueb | LL | UL | |
| Overall | 89.80 | 84.68 | 93.65 | 100.00 | 96.87 | 100.00 |
| RBD | 91.84 | 87.08 | 95.26 | 95.69 | 90.23 | 98.59 |
| NP | 92.86 | 88.31 | 96.04 | 94.83 | 89.08 | 98.08 |
| RBD/NP fusion | 90.82 | 85.87 | 94.47 | 100.00 | 96.87 | 100.00 |
RT-PCR, reverse transcriptase PCR; n = 312 specimens with full data available for analysis.
Values with 95% confidence intervals (LL, lower limit; UL, upper limit) were calculated for the assays’ overall call and for each individual antigen (RBD, receptor binding domain of spike protein; NP, nucleocapsid protein).
Outcomes of univariate and multivariate logistic regression between patient covariates and the overall MBA test result are summarized in the final column of Table 1. Positive associations were found between assay results and female sex, as well as with previous reporting of COVID-19 symptoms, using univariate logistic regression. However, these associations were not found to be statistically significant after adjusting for other variables with multivariate analysis.
The assay’s diagnostic sensitivity was examined for impact by the time between an individuals’ onset of symptoms and when a serum specimen was collected. Sensitivity gradually increased with increasing time since infection and was the highest for serum specimens collected more than 28 days following symptom onset, 94.7% (95% confidence interval [CI]: 82.3 to 99.4%), versus 87.5% (47.4 to 99.7%) for specimens collected less than 15 days post-symptom onset and 86.9% (77.8 to 93.3%) for those collected between 15 and 28 days post-symptom onset (Table 3). Analyzing time to serum collection as a continuous variable yielded steady increases in seropositivity to individual targets (Fig. S2). Only minor and nonsignificant variation was noted between time to serum collection and a seropositive call for individual antigen targets between 18 and 60 days after COVID-19 symptom onset.
TABLE 3.
Diagnostic sensitivity of overall multiplex assay call stratified by the time between COVID-19 symptom onset and serum collection (n = 135)
| Collection time (days) | N of samples | Sensitivity (%) |
||
|---|---|---|---|---|
| Valuea | LL | UL | ||
| 0−14 | 10 | 87.50 | 47.35 | 99.68 |
| 15−28 | 87 | 86.90 | 77.78 | 93.28 |
| >28 | 38 | 94.74 | 82.25 | 99.36 |
Values shown with 95% confidence intervals (LL, lower limit; UL, upper limit).
The MBA was compared against two commercially available SARS-CoV-2 serological assays which each detect antibodies to a single antigen target: NP for the Abbott SARS-CoV-2 IgG assay, and S1 for the Ortho VITROS Anti-SARS-CoV-2 Total assay. Compared to the Abbott assay, the positive percent agreement (PPA) of the multiplex NP antigen result was 95.26%, the negative percent agreement (NPA) was 98.50%, and the overall agreement was 96.59% (kappa coefficient: 0.88). A power function trendline and R2 value (0.61) for the quantitative results are shown in Fig. 1A. Of the 323 specimens, 19 (5.9%) had discrepant results between the two assays. Most of these (n = 17) had positive results for the MBA and negative results for the Abbott assay. Compared to the Ortho assay, the PPA of the multiplex RBD antigen result was 97.78%, the NPA was 95.10%, and the overall agreement was 96.59% (kappa coefficient 0.92). Figure 1B shows the power function trendline and R2 value (0.67) for comparison of quantitative results. Discrepant results were obtained for 12/323 (3.7%) of specimen calls. Of the 31 discrepant results from both assay comparisons, 6 corresponded to the same specimen, for a total of 25 discrepant specimens.
FIG 1.
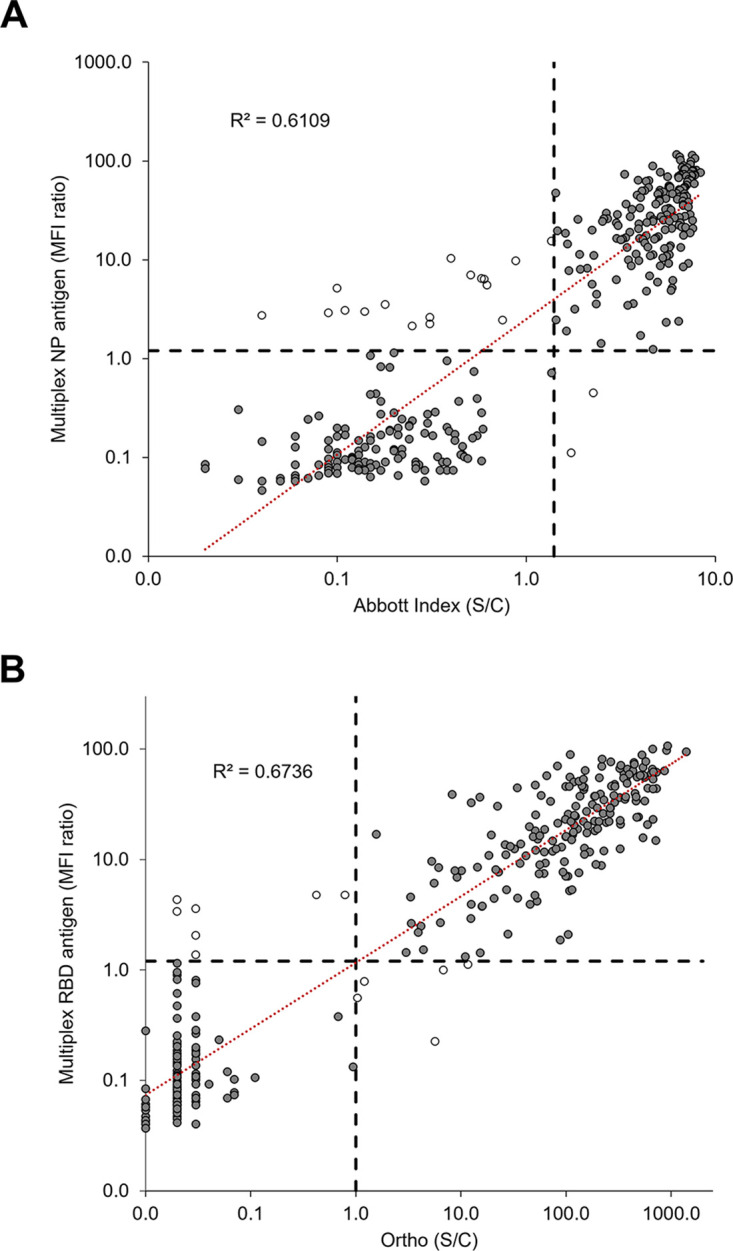
Comparison of multiplex bead assay results to results from two single antigen-based SARS-CoV-2 antibody assays. (A) The nucleocapsid protein (NP) antigen results from the multiplex assay were compared to those from the Abbott SARS-CoV-2 IgG assay. (B) Receptor-binding domain (RBD) antigen results from the multiplex assay were compared to those from the Ortho VITROS Anti-SARS-CoV-2 Total assay. Black dashed lines represent assay cutoffs: median fluorescence intensity (MFI) ratio of 1.2 for the multiplex assay, index of stored calibrator luminescent signal divided by a stored calibrator luminescent signal (S/C) of 1.4 for Abbott, and specimen luminescent signal divided by the luminescent signal at cutoff (S/C) of 1.0 for Ortho. Power function trendlines (red dashed lines) are shown with R2 values. Unfilled data points represent specimens with discrepant results between the two assays.
Based on RT-PCR determination of SARS-CoV-2 exposure, for these 25 discrepant specimens, most (18; 72%) were falsely negative for SARS-CoV-2 IgG (type 2 error), 1 specimen (4.0%) was falsely positive by the Abbott assay (type 1 error), and 6 (33.3%) were falsely positive on the Tetracore MBA for only one or two antigens; however, the overall assay call was negative and therefore correct (Table S2 in the supplemental material). Of the 12 specimens described above with only one or two positive antigen targets on the MBA, six were true negatives for SARS-CoV-2 infection and also tested negative on the Abbott and Ortho assays. The multiplex results for these included two specimens positive for both RBD and NP, and one specimen positive for NP only. The other six specimens were true positives for previous SARS-CoV-2 infection. Two tested positive on both the Abbott and Ortho assays (and were positive for both the NP and RBD/NP fusion targets on the multiplex assay). The other four yielded false-negative results from the Abbott assay (and were positive for both the RBD and NP targets on the MBA).
DISCUSSION
In response to the COVID-19 pandemic, numerous SARS-CoV-2 serological assays have been developed in a relatively short time frame (1, 4). Most commercially available assays detect antibodies to a single target antigen; while many single-target assays exhibit high sensitivity and specificity, these important metrics of assay performance can be negatively impacted when testing is performed in areas of low SARS-CoV-2 seroprevalence and waning IgG responses (11, 15) and can also be subject to cross-reactivity with antibodies against other pathogens (5–10). Assays with more than one target antigen can help address these issues and may offer additional advantages, such as differentiation of antibodies produced during natural SARS-CoV-2 infection from those that are vaccine-induced (16). It is not currently recommended to use antibody test results to assess immunity to SARS-CoV-2 following COVID-19 vaccination, in part because some antibody tests will not detect the antibodies generated by COVID-19 vaccines (17). If multiplex serological assays are employed for vaccination surveillance studies, careful consideration should be given to the vaccine(s) available to a particular region or population and which specific viral elements they include. Additionally, there is currently no level of IgG antibodies known to have a protective association against SARS-CoV-2 infection, so serosurveillance could be utilized to estimate exposure to either natural infection or vaccination but not, presently, to estimate individual or population protection against the virus.
Here, we evaluated a commercially available multiplex bead assay kit (FlexImmArray SARS-CoV-2 Human IgG Antibody Test, Tetracore, Inc.) which measures IgG antibodies against RBD, NP, and RBD/NP fusion proteins. Against this serum panel of 312 specimens with RT-PCR results, the MBA demonstrated high diagnostic sensitivity (89.8%) and specificity (100.0%). While the overall call of “IgG seropositive” for this assay relies on detection of IgG against all three target antigens, we also performed exploratory analysis of the sensitivity and specificity using each individual antigen target. Relying on a single target did not negatively impact diagnostic sensitivity, but it did lead to modest reduction of diagnostic specificity if considering the RBD or NP targets alone. This observed impact on assay specificity highlights the inherent advantage of using an assay with multiple target antigens (11, 18). A crude odds ratio estimate noted a significant, positive association between the multiplex result for IgG seropositivity and previously reported COVID-19 symptoms, and this relationship has been reported before (19, 20). This characterization of asymptomatic SARS-CoV-2 infections suggests association with shorter-lived antibody responses and reduced viral loads, which may have contributed to the observations in this current study (21, 22).
Testing on three consecutive days demonstrated the high reproducibility of the MBA (96.3% of specimens had full qualitative agreement). Specimens with different results across the 3 days were noted to have a high number of “equivocal” calls for one or more antigens. It was initially attempted to resolve these following the assay kit instructions for use (IFU), which advise re-testing specimens with any equivocal results in duplicate to allow for a tiebreaker. However, there were three specimens where the output was consistently “equivocal” for two antigens and seropositive for the third from all technical replicates. Because there was no possibility of collecting or testing serum specimens from later in convalescence, consultation with the assay manufacturer recommended calling these repeating “equivocal” sera negative. Additional studies from our group have demonstrated the high inter-laboratory reproducibility of this assay, its scalability for large numbers of specimens, and its accurate performance with dried blood spot specimens (manuscript in preparation). The inclusion of positive and negative assay controls and clearly defined cutoffs in this kit represent important quality indicators for serological testing of clinical specimens (23–25).
This study characterized the impact of time between onset of COVID-19 symptoms and serum collection for serology testing on the MBA’s diagnostic sensitivity. Sensitivity increased as the time to serum collection increased, with the highest value (94.7%) observed for specimens collected >28 days after the patient’s initial reporting of symptoms. This increasing trend was clear, although sample size varied substantially between the three categories of time to serum collection and likely accounted for the similarity in upper limits of variation. Time to collection was also analyzed as a continuous variable for each individual antigen target, and it was similarly found that the probability of a seropositive call increased steadily over time, plateauing at around 60 days post-symptom reporting. We observed no substantial variations in these trends among the three antigens. While the full duration of detectable SARS-CoV-2 IgG antibodies after infection remains unclear, multiple studies have demonstrated an initial rise of IgG and IgM antibodies within the first few weeks and reported detectable antibodies months later (26–28). We speculate that specimens with a false-negative serological result may have been collected at an earlier time following COVID-19 symptom onset; the average time between symptom onset and serum collection for this group was 19.6 days, ranging from 14 to 47 days. Of the seven specimens with false-positive results (or partial false positivity on the MBA), six had been collected in September 2019 and one had been collected in May 2020, but the latter patient had a negative SARS-CoV-2 RT-PCR result and was IgG-positive for the NP only by MBA. It is possible this individual had a viral load below the detection limit of RT-PCR or, more likely, a positive NP IgG signal due to previous exposure to other human coronaviruses and IgG cross-binding to similar epitopes, as has been observed previously (6, 10). The dynamics of antibody development and persistence have been shown to impact the performance of SARS-CoV-2 serological assays, with optimal sensitivity reported for specimens collected at least 15 days after the onset of symptoms (29). This is reflected in current CDC guidelines, which recommend collecting serum following a 3-week period after the onset of illness for accurate seropositivity assessment (2, 17, 30). Determining these time frames is especially difficult for people with asymptomatic SARS-CoV-2 infections, who may develop antibody responses outside a 3-week period or develop no apparent response (31, 32).
Overall good agreement was noted in comparison with the Abbott and Ortho assays, though there were low proportions of discrepant results between Tetracore and Abbott (5.9%) and between Tetracore and Ortho (3.7%) which corresponded to 25 total specimens. Reviewing SARS-CoV-2 infection data showed that discrepant specimens were most associated with a false-negative call by either the Abbot or Ortho assays. This discrepant group had a modest time post-symptom onset (19.6 days on average), which may indicate a lower concentration of IgG antibodies in the specimen; this time period falls before the CDC-recommended 3-week window for serum collection (17, 29, 30). This is supported by other findings: shorter times from symptom onset to serum collection were associated with reduced diagnostic sensitivity, and survival analysis showed a period of 19.6 days to be associated with a probability of MBA seropositivity of only about 0.3. However, discrepancies between these results could also be related to several technical or biological factors, including differences in assay technologies, different operators, or degree of epitope similarity for the target antigens in each assay (which is not readily available for proprietary assay kits). Utilizing clinical specimens from heterogeneous human populations also introduces an inherent range of immune responses, with subtle changes expected based on genetics, immune experience, and the specific pathogen. Few studies have directly compared the results of multiple SARS-CoV-2 serological assays, but assay sensitivity values ranging from 63.0% to 94.8% have been reported and vary by assay platform, targeted antibody isotype(s), and study population (2, 33).
This study is limited in that all specimens were obtained from commercial sources within the United States, so our findings are not necessarily generalizable to patient populations in other global regions. Additionally, all RT-PCR specimens were from 2020, so infection with other SARS-CoV-2 variants which have arisen after 2020 may elicit different IgG-binding capacities for this assay (34, 35). The sample set of persons without SARS-CoV-2 infection exposure partially relied on negative RT-PCR results from a single time point, which may not preclude prior exposure in early 2020. Finally, serum was collected at a single time point for each subject; since the probability of seropositivity increases with time following SARS-CoV-2 infection, inclusion of specimens from earlier time points may have increased the number of false-negative assay results. Including serial specimens from the same individuals would better confirm their true SARS-CoV-2 IgG status, especially for specimens with assay signal values near the seropositivity thresholds.
The MBA developed by Tetracore, Inc. was found to be a highly sensitive and specific method for detecting antibodies to three SARS-CoV-2 targets. The use of multiple antigen targets showed a diagnostic specificity of 100% in this sample set, which is an especially key factor in populations with low disease prevalence or where background seropositivity has been observed.
MATERIALS AND METHODS
Study specimens.
Residual human serum specimens (n = 323) were provided by the CDC COVID-19 Laboratory Task Force and sourced in 2020 via BioIVT, iSpecimen, StemExpress, and Emory University (36), and included 196 (60.7%) with a previous positive RT-PCR SARS-CoV-2 result. Specimens were considered negative for SARS-CoV-2 exposure if they were collected prior to September 2019 (prior to the COVID-19 pandemic), or if they were collected after January 2020 but the patient had a negative SARS-CoV-2 RT-PCR test result from a nasopharyngeal specimen. Upon receipt at the CDC in Atlanta, GA, each specimen was thawed and aliquoted into multiple vials for storage at −80°C and later distribution. Specimens were heat-treated for 10 min at 56°C to inactivate any pathogens, and then assayed in a blinded fashion.
Human serum specimens were provided for this study by the CDC. This activity was reviewed by the CDC and conducted in a manner consistent with applicable federal law and CDC policy (e.g., 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.). This study was approved by the CDC Institutional Review Board (project ID 0900f3eb81c1b13d).
Multiplex bead assay performance.
All specimens were tested with the Tetracore FlexImmArray SARS-CoV-2 IgG kit (Tetracore, Inc., Rockville, MD) following the manufacturer’s instructions for use (37). In summary, this assay utilizes magnetic microspheres coupled with unique recombinant proteins for SARS-CoV-2 (RBD, NP, and RBD/NP fusion). The assay kit also includes four different internal controls for monitoring each step of assay performance. External positive-control, negative control, and calibrator reagents are also provided in the kit and were run in duplicate wells on every assay plate. Serum specimens tested in singlet were diluted 1:400 in assay kit sample dilution buffer, immediately mixed with the antigen-coated microspheres in a 96-well plate, and incubated for 20 min by gentle shaking at room temperature protected from light. Plates were washed four times with assay wash buffer, and serum IgG antibodies were detected by incubation with phycoerythrin (PE)-conjugated anti-human IgG for 20 min under gentle shaking protected from light. Plates were washed four times and the microspheres were resuspended in wash buffer and analyzed using a Luminex MAGPIX instrument (Luminex Corporation, Austin, TX) with a target of 50 beads per region.
Data analysis.
Specimen testing results were calculated following the Tetracore IFU and using the mean of the calibrator median fluorescence intensity (MFI) for each antigen target. The MFI for each test specimen was divided by the mean calibrator MFI to determine an MFI ratio. The algorithm used to calculate a qualitative estimate of human IgG against the three SARS-CoV-2 antigens is shown in Table S1 in the supplemental material. For calculating assay sensitivity and specificity, SARS-CoV-2 RT-PCR results were considered the gold-standard assay designation, in accordance with the time frames in 2019 and 2020 described above.
Specimens had been previously tested by the CDC Laboratory Task Force using two commercially available SARS-CoV-2 serology assays: the Abbott SARS-CoV-2 IgG assay (Abbott Diagnostics, Abbott Park, IL) and the Ortho VITROS Anti-SARS-CoV-2 Total assay (Ortho Clinical Diagnostics, Rochester, NY). Both assays, authorized by the FDA under an emergency use authorization, utilize a single antigen target: the Abbott SARS-CoV-2 IgG assay measures IgG against NP, while the Ortho VITROS Anti-SARS-CoV-2 Total assay measures total Ig against the S1 subunit of the spike protein. Results from the Abbott assay were provided as indices of the specimen luminescent signal divided by a stored calibrator luminescent signal (S/C), while results from the Ortho assay were provided as the specimen luminescent signal divided by the luminescent signal at cutoff (also abbreviated S/C) (38, 39). Since neither of these assays represent a “gold standard” immunoassay, positive percent agreement and negative percent agreement were calculated to compare the results from these assays to those of the MBA.
Associations between population demographics, individual antigen results, and overall MBA results were investigated in SAS v9.4 (SAS Institute, Cary, NC) by the “proc logistic” procedure with an alpha significance level of 0.05. Survival analysis and hazard ratios for time to seropositivity for individual targets were generated by “proc phreg” by the Cox proportional hazards model with an alpha significance level of 0.05.
Supplementary Material
ACKNOWLEDGMENTS
We thank the CDC Division of Laboratory Systems for facilitating the transfer of specimens used in this study. We appreciate the support provided by Cecilia Nelson and Peter McElroy in conducting this work.
This work was supported with funding by the Coronavirus Aid, Relief, and Economic Security (CARES) Act of 2020, which provided the CDC with funding to support operational readiness for COVID-19 preparedness and response, as well as to develop tools and strategies to monitor, respond to, and prevent COVID-19 through expansion of testing, contact tracing, and enhanced disease surveillance.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the CDC. Tetracore, Inc. provided FlexImmArray kits but had no role in data analysis for this project; use of these products, trade names, and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services or the CDC.
The authors report no conflicts of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Eric Rogier, Email: erogier@cdc.gov.
Kileen L. Shier, Quest Diagnostics Nichols Institute
Steven Drews, Canadian Blood Services.
Dale Schwab, Quest Diagnostics (United States).
REFERENCES
- 1.Galipeau Y, Greig M, Liu G, Driedger M, Langlois M-A. 2020. Humoral responses and serological assays in SARS-CoV-2 infections. Front Immunol 11:610688. doi: 10.3389/fimmu.2020.610688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitman JD, Hiatt J, Mowery CT, Shy BR, Yu R, Yamamoto TN, Rathore U, Goldgof GM, Whitty C, Woo JM, Gallman AE, Miller TE, Levine AG, Nguyen DN, Bapat SP, Balcerek J, Bylsma SA, Lyons AM, Li S, Wong AW-Y, Gillis-Buck EM, Steinhart ZB, Lee Y, Apathy R, Lipke MJ, Smith JA, Zheng T, Boothby IC, Isaza E, Chan J, Acenas DD, Lee J, Macrae TA, Kyaw TS, Wu D, Ng DL, Gu W, York VA, Eskandarian HA, Callaway PC, Warrier L, Moreno ME, Levan J, Torres L, Farrington LA, Loudermilk RP, Koshal K, Zorn KC, Garcia-Beltran WF, Yang D, et al. 2020. Evaluation of SARS-CoV-2 serology assays reveals a range of test performance. Nat Biotechnol 38:1174–1183. doi: 10.1038/s41587-020-0659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang YW, Schmitz JE, Persing DH, Stratton CW. 2020. Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol 58:e00512-20. doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO/FIND. SARS-CoV-2 diagnostic pipeline. https://www.finddx.org/covid-19/pipeline. Accessed April 30, 2021.
- 5.Lv H, Wu NC, Tsang OT, Yuan M, Perera R, Leung WS, So RTY, Chan JMC, Yip GK, Chik TSH, Wang Y, Choi CYC, Lin Y, Ng WW, Zhao J, Poon LLM, Peiris JSM, Wilson IA, Mok CKP. 2020. Cross-reactive antibody response between SARS-CoV-2 and SARS-CoV infections. Cell Rep 31:107725. doi: 10.1016/j.celrep.2020.107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson EM, Goodwin EC, Verma A, Arevalo CP, Bolton MJ, Weirick ME, Gouma S, McAllister CM, Christensen SR, Weaver J, Hicks P, Manzoni TB, Oniyide O, Ramage H, Mathew D, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, Alanio C, D'Andrea K, Kuthuru O, Dougherty J, Pattekar A, Kim J, Han N, Apostolidis SA, Huang AC, Vella LA, Kuri-Cervantes L, Pampena MB, Betts MR, Wherry EJ, Meyer NJ, Cherry S, Bates P, Rader DJ, Hensley SE, UPenn COVID Processing Unit. 2021. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell 184:1858.e10–1864.e10. doi: 10.1016/j.cell.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sagara I, Woodford J, Dicko A, Zeguime A, Doucoure M, Kwan J, Zaidi I, Doritchamou J, Snow-Smith M, Alani N, Renn J, Kosik I, Holly J, Yewdell J, Esposito D, Sadtler K, Duffy P. 2021. SARS-CoV-2 seroassay optimization and performance in a population with high background reactivity in Mali. medRxiv. doi: 10.1101/2021.03.08.21252784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lustig Y, Keler S, Kolodny R, Ben-Tal N, Atias-Varon D, Shlush E, Gerlic M, Munitz A, Doolman R, Asraf K, Shlush LI, Vivante A. 2020. Potential antigenic cross-reactivity between SARS-CoV-2 and dengue viruses. Clin Infect Dis 73:e2444–e2449. doi: 10.1093/cid/ciaa1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masyeni S, Santoso MS, Widyaningsih PD, Asmara DW, Nainu F, Harapan H, Sasmono RT. 2021. Serological cross-reaction and coinfection of dengue and COVID-19 in Asia: experience from Indonesia. Int J Infect Dis 102:152–154. doi: 10.1016/j.ijid.2020.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinhardt LC, Ige F, Iriemenam NC, Greby SM, Hamada Y, Uwandu M, Aniedobe M, Stafford KA, Abimiku A, Mba N, Agala N, Okunoye O, Mpamugo A, Swaminathan M, Onokevbagbe E, Olaleye T, Odoh I, Marston BJ, Okoye M, Abubakar I, Rangaka MX, Rogier E, Audu R. 2021. Cross-reactivity of two SARS-CoV-2 serological assays in a setting where malaria is endemic. J Clin Microbiol 59:e0051421. doi: 10.1128/JCM.00514-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fotis C, Meimetis N, Tsolakos N, Politou M, Akinosoglou K, Pliaka V, Minia A, Terpos E, Trougakos IP, Mentis A, Marangos M, Panayiotakopoulos G, Dimopoulos MA, Gogos C, Spyridonidis A, Alexopoulos LG. 2021. Accurate SARS-CoV-2 seroprevalence surveys require robust multi-antigen assays. Sci Rep 11:6614–6614. doi: 10.1038/s41598-021-86035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suhandynata RT, Bevins NJ, Tran JT, Huang D, Hoffman MA, Lund K, Kelner MJ, McLawhon RW, Gonias SL, Nemazee D, Fitzgerald RL. 2021. SARS-CoV-2 serology status detected by commercialized platforms distinguishes previous infection and vaccination adaptive immune responses. J Appl Lab Med 5:1109–1122. doi: 10.1093/jalm/jfab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Assis RR, Jain A, Nakajima R, Jasinskas A, Felgner J, Obiero JM, Norris PJ, Stone M, Simmons G, Bagri A, Irsch J, Schreiber M, Buser A, Holbro A, Battegay M, Hosimer P, Noesen C, Adenaiye O, Tai S, Hong F, Milton DK, Davies DH, Contestable P, Corash LM, Busch MP, Felgner PL, Khan S. 2021. Analysis of SARS-CoV-2 antibodies in COVID-19 convalescent blood using a coronavirus antigen microarray. Nat Commun 12:6–6. doi: 10.1038/s41467-020-20095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pisanic N, Randad PR, Kruczynski K, Manabe YC, Thomas DL, Pekosz A, Klein SL, Betenbaugh MJ, Clarke WA, Laeyendecker O, Caturegli PP, Larman HB, Detrick B, Fairley JK, Sherman AC, Rouphael N, Edupuganti S, Granger DA, Granger SW, Collins MH, Heaney CD. 2020. COVID-19 serology at population scale: SARS-CoV-2-specific antibody responses in saliva. J Clin Microbiol 59:e02204-20. doi: 10.1128/JCM.02204-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National SARS-CoV-2 Serology Assay Evaluation Group. 2020. Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis 20:1390–1400. doi: 10.1016/S1473-3099(20)30634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradley T, Grundberg E, Selvarangan R, LeMaster C, Fraley E, Banerjee D, Belden B, Louiselle D, Nolte N, Biswell R, Pastinen T, Myers A, Schuster J. 2021. Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med 384:1959–1961. doi: 10.1056/NEJMc2102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CDC. 2021. Interim guidelines for COVID-19 antibody testing in clinical and public health settings. Available from https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html. Accessed October 12, 2021.
- 18.Marien J, Ceulemans A, Michiels J, Heyndrickx L, Kerkhof K, Foque N, Widdowson MA, Mortgat L, Duysburgh E, Desombere I, Jansens H, Van Esbroeck M, Arien KK. 2021. Evaluating SARS-CoV-2 spike and nucleocapsid proteins as targets for antibody detection in severe and mild COVID-19 cases using a Luminex bead-based assay. J Virol Methods 288:114025. doi: 10.1016/j.jviromet.2020.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchi S, Viviani S, Remarque EJ, Ruello A, Bombardieri E, Bollati V, Milani GP, Manenti A, Lapini G, Rebuffat A, Montomoli E, Trombetta CM. 2021. Characterization of antibody response in asymptomatic and symptomatic SARS-CoV-2 infection. PLoS One 16:e0253977. doi: 10.1371/journal.pone.0253977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dufloo J, Grzelak L, Staropoli I, Madec Y, Tondeur L, Anna F, Pelleau S, Wiedemann A, Planchais C, Buchrieser J, Robinot R, Ungeheuer MN, Mouquet H, Charneau P, White M, Levy Y, Hoen B, Fontanet A, Schwartz O, Bruel T. 2021. Asymptomatic and symptomatic SARS-CoV-2 infections elicit polyfunctional antibodies. Cell Rep Med 2:100275. doi: 10.1016/j.xcrm.2021.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sui Z, Dai X, Lu Q, Zhang Y, Huang M, Li S, Peng T, Xie J, Zhang Y, Wu C, Xia J, Dong L, Yang J, Huang W, Liu S, Wang Z, Li K, Yang Q, Zhou X, Wu Y, Liu W, Fang X, Peng K. 2021. Viral dynamics and antibody responses in people with asymptomatic SARS-CoV-2 infection. Signal Transduct Target Ther 6:181. doi: 10.1038/s41392-021-00596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Yang M, Peng Y, Liang Y, Wei J, Xing L, Guo L, Li X, Li J, Wang J, Li M, Xu Z, Zhang M, Wang F, Shi Y, Yuan J, Liu Y. 2022. Longitudinal analysis of antibody dynamics in COVID-19 convalescents reveals neutralizing responses up to 16 months after infection. Nat Microbiol 7:423–433. doi: 10.1038/s41564-021-01051-2. [DOI] [PubMed] [Google Scholar]
- 23.Theel ES, Couturier MR, Filkins L, Palavecino E, Mitchell S, Campbell S, Pentella M, Butler-Wu S, Jerke K, Dharmarha V, McNult P, Schuetz AN. 2020. Application, verification, and implementation of SARS-CoV-2 serologic assays with emergency use authorization. J Clin Microbiol 59:e02148-20. doi: 10.1128/JCM.02148-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martins TB. 2002. Development of internal controls for the Luminex instrument as part of a multiplex seven-analyte viral respiratory antibody profile. Clin Vaccine Immunol 9:41–45. doi: 10.1128/CDLI.9.1.41-45.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons JH. 2008. Development, application, and quality control of serology assays used for diagnostic monitoring of laboratory nonhuman primates. Ilar J 49:157–169. doi: 10.1093/ilar.49.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, Nakao C, Rayaprolu V, Rawlings SA, Peters B, Krammer F, Simon V, Saphire EO, Smith DM, Weiskopf D, Sette A, Crotty S. 2021. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duysburgh E, Mortgat L, Barbezange C, Dierick K, Fischer N, Heyndrickx L, Hutse V, Thomas I, Van Gucht S, Vuylsteke B, Ariën KK, Desombere I. 2021. Persistence of IgG response to SARS-CoV-2. Lancet Infect Dis 21:163–164. doi: 10.1016/S1473-3099(20)30943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shrotri M, Harris RJ, Rodger A, Planche T, Sanderson F, Mahungu T, McGregor A, Heath PT, Brown CS, Dunning J, Hopkins S, Ladhani S, Chand M, The LondonCOVID Group. 2021. Persistence of SARS-CoV-2 N-antibody response in healthcare workers, London, UK. Emerg Infect Dis 27:1155–1158. doi: 10.3201/eid2704.204554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, Adriano A, Beese S, Dretzke J, Ferrante di Ruffano L, Harris IM, Price MJ, Dittrich S, Emperador D, Hooft L, Leeflang MM, Van den Bruel A, Cochrane COVID-19 Diagnostic Test Accuracy Group. 2020. Antibody tests for identification of current and past infection with SARS‐CoV‐2. Cochrane Database Syst Rev 6:CD013652. doi: 10.1002/14651858.CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakano Y, Kurano M, Morita Y, Shimura T, Yokoyama R, Qian C, Xia F, He F, Kishi Y, Okada J, Yoshikawa N, Nagura Y, Okazaki H, Moriya K, Seto Y, Kodama T, Yatomi Y. 2021. Time course of the sensitivity and specificity of anti-SARS-CoV-2 IgM and IgG antibodies for symptomatic COVID-19 in Japan. Sci Rep 11:2776. doi: 10.1038/s41598-021-82428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milani GP, Dioni L, Favero C, Cantone L, Macchi C, Delbue S, Bonzini M, Montomoli E, Bollati V, UNICORN Consortium. 2020. Serological follow-up of SARS-CoV-2 asymptomatic subjects. Sci Rep 10:20048. doi: 10.1038/s41598-020-77125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choe PG, Kang CK, Suh HJ, Jung J, Kang E, Lee SY, Song KH, Kim HB, Kim NJ, Park WB, Kim ES, Oh MD. 2020. Antibody responses to SARS-CoV-2 at 8 weeks postinfection in asymptomatic patients. Emerg Infect Dis 26:2484–2487. doi: 10.3201/eid2610.202211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tešija Kuna A, Hanžek M, Vukasović I, Nikolac Gabaj N, Vidranski V, Ćelap I, Miler M, Stančin N, Šimac B, Živković M, Žarak M, Kmet M, Jovanović M, Tadinac S, Šupraha Goreta S, Periša J, Šamija I, Štefanović M. 2021. Comparison of diagnostic accuracy for eight SARS-CoV-2 serological assays. Biochem Med (Zagreb) 31:e010708. doi: 10.11613/BM.2021.010708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dowell AC, Butler MS, Jinks E, Tut G, Lancaster T, Sylla P, Begum J, Bruton R, Pearce H, Verma K, Logan N, Tyson G, Spalkova E, Margielewska-Davies S, Taylor GS, Syrimi E, Baawuah F, Beckmann J, Okike IO, Ahmad S, Garstang J, Brent AJ, Brent B, Ireland G, Aiano F, Amin-Chowdhury Z, Jones S, Borrow R, Linley E, Wright J, Azad R, Waiblinger D, Davis C, Thomson EC, Palmarini M, Willett BJ, Barclay WS, Poh J, Amirthalingam G, Brown KE, Ramsay ME, Zuo J, Moss P, Ladhani S. 2022. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat Immunol 23:40–49. doi: 10.1038/s41590-021-01089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez E, Haycroft ER, Adair A, Mordant FL, O’Neill MT, Pymm P, Redmond SJ, Lee WS, Gherardin NA, Wheatley AK, Juno JA, Selva KJ, Davis SK, Grimley SL, Harty L, Purcell DFJ, Subbarao K, Godfrey DI, Kent SJ, Tham W-H, Chung AW. 2021. Simultaneous evaluation of antibodies that inhibit SARS-CoV-2 variants via multiplex assay. JCI Insight 6:150012. doi: 10.1172/jci.insight.150012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kainulainen MH, Bergeron E, Chatterjee P, Chapman AP, Lee J, Chida A, Tang X, Wharton RE, Mercer KB, Petway M, Jenks HM, Flietstra TD, Schuh AJ, Satheshkumar PS, Chaitram JM, Owen SM, McMullan LK, Flint M, Finn MG, Goldstein JM, Montgomery JM, Spiropoulou CF. 2021. High-throughput quantitation of SARS-CoV-2 antibodies in a single-dilution homogeneous assay. Sci Rep 11:12330. doi: 10.1038/s41598-021-91300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tetracore, Inc. 2020. Instructions for use: Tetracore FlexImmArray SARS-CoV-2 Human IgG Antibody Test. Available from https://tetracore.com/wp-content/uploads/2021/04/FlexImmArraySARS-CoV-2IgGkitIFU_ver05122020_web.pdf. Tetracore, Inc., Rockville, MD. [Google Scholar]
- 38.Ortho Clinical Diagnostics. 2020. Instructions for Use: VITROS Immunodiagnostic Products Anti-SARS-CoV-2 Total Reagent Pack. Available from https://www.fda.gov/media/136967/download. Accessed May 10, 2021. Ortho Clinical Diagnostics, Rochester, NY. [Google Scholar]
- 39.Abbott Diagnostics. 2020. Instructions for Use: SARS-CoV-2 IgG. Abbott Diagnostics, Abbot Park, IL. https://www.fda.gov/media/137910/download. Accessed May 10, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.01054-22-s0001.pdf, PDF file, 0.2 MB (226.9KB, pdf)


