ABSTRACT
Pet bite-related infections are commonly caused by the pet’s oral flora transmitted to the animal handlers through the bite wounds. In this study, we isolated a streptococcus, HKU75T, in pure culture from the purulent discharge collected from a guinea pig bite wound in a previously healthy young patient. HKU75T was alpha-hemolytic on sheep blood agar and agglutinated with Lancefield group D and group G antisera. API 20 STREP showed that the most likely identity for HKU75T was S. suis I with 85.4% confidence while Vitek 2 showed that HKU75T was unidentifiable. MALDI-TOF MS identified HKU75T as Streptococcus suis (score of 1.86 only). 16S rRNA gene sequencing showed that HKU75T was most closely related to S. parasuis (98.3% nucleotide identity), whereas partial groEL and rpoB gene sequencing showed that it was most closely related to S. suis (81.8% and 89.8% nucleotide identity respectively). Whole genome sequencing and intergenomic distance determined by ANI revealed that there was <85% identity between the genome of HKU75T and those of all other known Streptococcus species. Genome classification using concatenated sequences of 92 bacterial core genes showed that HKU75T belonged to the Suis group. groEL gene sequences identical to that of HKU75T could be directly amplified from the oral cavities of the two guinea pigs owned by the patient. HKU75T is a novel Streptococcus species, which we propose to be named S. oriscaviae. The oral cavity of guinea pigs is presumably a reservoir of S. oriscaviae. Some of the reported S. suis strains isolated from clinical specimens may be S. oriscaviae.
IMPORTANCE We reported the discovery of a novel Streptococcus species, propose to be named Streptococcus oriscaviae, from the pus collected from a guinea pig bite wound in a healthy young patient. The bacterium was initially misidentified as S. suis/S. parasuis by biochemical tests, mass spectrometry. and housekeeping genes sequencing. Its novelty was confirmed by whole genome sequencing. Comparative genomic studies showed that S. oriscaviae belongs to the Suis group. S. oriscaviae sequences were detected in the oral cavities of the two guinea pigs owned by the patient, suggesting that the oral cavity of guinea pigs could be a reservoir of S. oriscaviae. Some of the reported S. suis strains may be S. oriscaviae. Further studies are warranted to refine our knowledge on this novel Streptococcus species.
KEYWORDS: Streptococcus oriscaviae, novel species, guinea pigs, bite wound, infection
INTRODUCTION
There is an increasing number of pet-related infections worldwide (1). A significant proportion of these infections are due to the owners being accidentally bitten by their pets, leading to dog-bite infections, cat-bite infections, etc. caused by the oral flora of the pets being transmitted to the animal handlers through the bite wounds (1). In fact, some bacterial species are characteristically found in bite wounds inflicted by a specific group of animals. For instance, Pasteurella canis and Capnocytophaga canimorsus are frequently isolated in dog-bite wound infections (2, 3), as these are part of the normal flora of the canine oral cavity. In addition to dogs and cats, guinea pigs are becoming an increasingly popular choice of pet (4). Previous studies have shown that a diverse population of Streptococcus species, such as Streptococcus parasanguinis, Streptococcus mitis, and Streptococcus suis, as well as some unidentified hemolytic isolates, inhabited the oral cavities of guinea pigs (4, 5). These streptococci are potentially pathogenic for the animal host, and can cause zoonotic infections in humans.
The genus Streptococcus currently comprises more than 112 species, some of which are important human pathogens that cause significant morbidity and mortality worldwide. Traditionally, streptococci have been classified into alpha-hemolytic, beta-hemolytic, and non-hemolytic depending on the type of hemolysis that the bacterium generated on blood agar. The beta-hemolytic streptococci were further subclassified by Lancefield grouping, although some alpha-hemolytic and nonhemolytic streptococci also reacted with certain Lancefield antisera. As a result of the widespread use of PCR and DNA sequencing throughout the last 2 decades, genotypic methods like amplification and sequencing of universal gene targets represent an alternative method for classification and identification of Streptococcus. Among the various universal gene targets that have been studied, the 16S rRNA gene has been the most widely used (6, 7). However, some studies have shown that the 16S rRNA gene failed to provide sufficient resolution and to delineate Streptococcus species into proper taxonomic groupings under some circumstances (8–11).
Recently, a Gram-positive coccus, strain HKU75T, was isolated from the purulent discharge of a wound induced by a guinea pig bite. Although the bacterium was identified as S. suis by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) with a score of 1.86, the history of the patient was not compatible with a case of S. suis infection, which is often associated with a contact history of pigs. Moreover, the phenotypic characteristics of the bacterium also did not fit into patterns of any known species. A comparison based on the complete 16S rRNA gene sequences (1,557 bp) showed that there was 98.3% base identity between the 16S rRNA gene of HKU75T and that of the most closely related species, S. parasuis SUT-286T, and whole genome sequencing confirmed that it is a previously undescribed bacterium. In this study, we describe the phenotypic and genotypic characterization of this novel bacterium. In addition, we also investigated the presence of it in the oral cavity of the corresponding guinea pigs. On the basis of these studies, we propose a new species, Streptococcus oriscaviae sp. nov., to describe this bacterium.
RESULTS
Patient.
A 24-year-old Chinese man, with good past health, was admitted because of a guinea pig bite, resulting in a 2 mm bite mark at the dorsum of the left hand between the heads of the second and third metacarpals. There was a hematoma and purulent discharge a few hours after bitten by a guinea pig. Two guinea pigs (GP1 and GP2, Fig. S1 in the supplemental material) were purchased from a local pet store as pets a few years ago. They were fed with grass or vegetables purchased from the pet store and were apparently healthy all along. In the evening on the day of admission, the patient was bitten by one of the two guinea pigs (GP1) while he was trying to stop the two guinea pigs from fighting. The swelling increased in the next few hours. There was no numbness or any symptoms suggestive of compartment syndrome. Examination showed that the patient was afebrile and vital signs were normal. Neurological examination did not reveal any sensory or motor deficit. The complete blood count and liver and renal function tests were within normal limits. Radiographic examination did not reveal any fracture. The pus from the wound was sent for bacterial culture. Intravenous amoxicillin-clavulanate 1.2 g q8h was commenced. After 24 h of incubation, a Gram-positive aerobic nonsporulating coccus (the strain tentatively named HKU75T) was isolated from the purulent discharge. The swelling gradually subsided and the patient was discharged on day 3. The patient was continued with oral amoxicillin-clavulanate 1 g q12 h for four more days. The patient as well as the two guinea pigs remained asymptomatic at the time of writing, 10 months after discharge.
Phenotypic characterizations.
HKU75T grew on sheep blood agar as alpha-hemolytic, and gray colonies of 0.5–1 mm in diameter after 24 h of incubation at 37°C in aerobic environment (Fig. S2). Growth enhancement was observed under 5% CO2 conditions. The strain did not grow on bile esculin agar, or in 6.5% NaCl. Serogrouping results showed that HKU75T reacted with antisera of both Lancefield groups D and G. It was resistant to optochin, but was sensitive to bacitracin and was non-motile at both 25°C and 37°C. MALDI-TOF MS identified HKU75T as S. suis, with a score of 1.86. The biochemical profile of strain HKU75T is shown in Table 1; it was Voges-Proskauer test negative. It produced leucine, alanine, and tyrosine arylamidase, but did not produce catalase or urease as determined by the Vitek 2 system. It hydrolyzed esculin (API 20) and arginine (Vitek 2 and API 20), and utilized lactose (Vitek 2 and API 20), mannitol (Vitek 2 and API 20), salicin (Vitek 2), sucrose (Vitek 2), trehalose (Vitek 2 and API 20), inulin (API20), mannose (Vitek 2), maltose (Vitek 2), starch (API20), glycogen (API20), amygdalin (Vitek 2), and galactose (Vitek 2). The Vitek 2 system identified HKU75T as “unidentified organism.” The API system showed that its identity was most likely S. suis I with 85.4% confidence. It was sensitive to penicillin (MIC ≤0.016 μg/mL), ceftriaxone, cefepime, levofloxacin, clindamycin, erythromycin, ofloxacin, tetracycline, and vancomycin.
TABLE 1.
Biochemical profile of S. oriscaviae HKU75T and S. suis S735T by Vitek 2 and API 20 STREPa
| Biochemical reaction or enzyme | Result by testing method |
|||
|---|---|---|---|---|
| Vitek 2 | API 20 STREP | |||
| S. suis | S. oriscaviae | S. suis | S. oriscaviae | |
| Resistance to bacitracin | − | − | ||
| Resistance to optochin | + | + | ||
| Growth in 6.5% NaCl | − | − | ||
| Esculin hydrolysis | + | + | ||
| Hippurate hydrolysis | − | − | ||
| Arginine hydrolysis | + | + | + | + |
| Urease | − | − | ||
| Voges-Proskauer test | − | − | ||
| Resistance to novobiocin | − | − | ||
| Resistance to polymyxin B | + | + | ||
| Utilization of: | ||||
| Lactose | + | + | + | + |
| Mannitol | − | + | − | + |
| Raffinose | + | − | + | − |
| Salicin | + | + | ||
| Sorbitol | + (v) | − | − | − |
| Sucrose | + | + | ||
| Trehalose | + | + | + | + |
| Arabinose | − | − | ||
| Pullulan | − | − | ||
| Inulin | + | + | ||
| Ribose | − | − | − | − |
| Xylose | − | − | ||
| D-mannose | + | + | ||
| Maltose | + | + | ||
| Starch | + | + | ||
| Glycogen | + | + | ||
| Methyl-β-D-glucopyranoside | + | − | ||
| Cyclodextrin | − | − | ||
| D-amygdalin | − | + | ||
| D-galactose | + | + | ||
| N-acetyl-D-glucosamine | + | − | ||
| Pyrrolidonylarylamidase | − | − | − | − |
| α-galactosidase | + | − | + | − |
| β-glucuronidase | − (v) | − | + | − |
| β-galactosidase | − | − | − | − |
| Leucine arylamidase | + | + | ||
| Leucine aminopeptidase | + | + | ||
| Alkaline phosphatase | − | − | ||
| Alanine-phenylalanine-proline arylamidase | + | − | ||
| Phosphatidylinositol phospholipase C | − | − | ||
| α-glucosidase | + | − | ||
| L-aspartate arylamidase | − | − | ||
| β-galactopyranosidase | − | − | ||
| α-mannosidase | − | − | ||
| Phosphatase | − | − | ||
| L-proline arylamidase | − | − | ||
| β-glucuronidase | + | − | ||
| Alanine arylamidase | + | + | ||
| Tyrosine arylamidase | + | + | ||
| L-lactate alkalinization | − | − | ||
| O/129 resistance (comp. vibrio) | − | − | ||
| Arginine dihydrolase 2 | + | − | ||
−, negative; +, positive; (v), variable from previously reported strains (51). All data were obtained by the same methodology using the same culture conditions.
Molecular characterizations.
PCR (PCR) of the 16S rRNA, partial groEL, and partial rpoB genes of HKU75T yielded DNA products with lengths of approximately 1,500, 600, and 700 bp, respectively. Pairwise alignment showed that the complete 16S rRNA sequence of HKU75T possessed a 98.3% nucleotide identity to Streptococcus parasuis SUT-286T, a 97.8% nucleotide identity to Streptococcus porcorum DSM 28302T, a 96.7% nucleotide identity to Streptococcus gordonii ATCC 10558T, and 96.3% nucleotide identity to S. suis S735T; the partial groEL sequence of HKU75T possessed an 81.8% nucleotide identity to S. suis S735T, an 81.7% nucleotide identity to S. parasuis SUT-286T, an 81.4% nucleotide identity to Streptococcus dentisani 7747T, and a 78.2% nucleotide identity to Streptococcus rubneri DSM 26920T; the partial rpoB sequence of HKU75T possessed an 89.8% nucleotide identity to S. suis S735T, an 89.5% nucleotide identity to S. parasuis SUT-286T, an 88.8% nucleotide identity to Streptococcus gallinaceus CIP 107087T, and an 87.6% nucleotide identity to Streptococcus merionis NCTC 13778T (Fig. 1). These results suggested that phylogenetic analyses using sequences of single gene loci, 16S rRNA, groEL, and rpoB, failed to determine the taxonomic position of HKU75T within the genus Streptococcus.
FIG 1.
Phylogenetic trees showing the relationship of S. oriscaviae HKU75T to its closely related Streptococcus species. The tree was inferred from the sequence data of (a) the 16S rRNA gene, (b) the partial groEL gene, and (c) the partial rpoB gene by the maximum-likelihood method using Kimura’s two parameter correction (16S rRNA) and general time reversible (groEL and rpoB) models, with Lactococcus lactis ATCC 19435T as the outgroup. The scale bar indicates the estimated number of substitutions per base. Numbers at nodes indicate levels of bootstrap support calculated from 1,000 pseudoreplicates (values lower than 70 are not shown). Names and nucleotide accession numbers are given as cited in GenBank/JGI/PATRIC. The accession numbers for L. lactis ATCC 19435T are NR_040955.1 (16S rRNA), FMTF01000003 (groEL), and FMTF01000007 (rpoB).
Screening of HKU75T in guinea pigs.
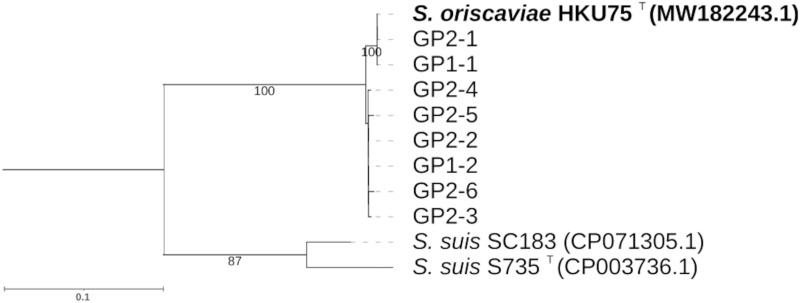
Direct cultures of the two oral swabs of GP1 and GP2 failed to isolate HKU75T. PCR targeting the partial groEL gene fragment of HKU75T yielded DNA products with lengths of approximately 600 bp in DNA samples extracted from the two oral swabs of the guinea pigs. Sequencing and phylogenetic analysis of the clones from each sample showed that GP1 contained two sequence types (GP1-1 and GP1-2) while GP2 contained six sequence types (GP2-1, GP2-2, GP2-3, GP2-4, GP2-5, and GP2-6). There were five (0.85%) nucleotide differences between GP1-1 and GP1-2, and five to seven (0.85–1.19%) nucleotide differences among the six sequence types of GP2. GP1-1 was identical to GP2-1, and both types showed 100% nucleotide identity to the groEL gene sequence of HKU75T; the next closest match was S. suis SC183, which only shared 85.20% of the nucleotide identity (Fig. 2).
FIG 2.
Phylogenetic analysis of groEL sequences detected in the oral swabs of guinea pigs GP-1 and GP-2. The tree was constructed by the maximum-likelihood method using the Tamura 3-parameter model with L. lactis ATCC 19435T as the outgroup. A total of 588 nucleotide positions was included in the analysis. Bootstrap values were calculated as percentages from 1,000 pseudoreplicates (values lower than 70 are not shown). The scale bar indicates the estimated number of substitutions per 100 bases. Names and GenBank nucleotide accession numbers are given as cited in GenBank. The accession number for the groEL gene sequence of L. lactis ATCC 19435T is FMTF01000003.
Comparative genomic characterizations.
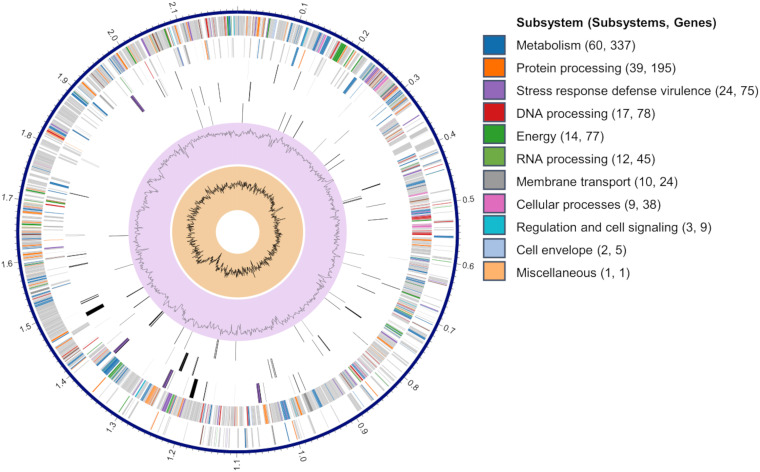
The de novo assembly conducted using both Illumina and Nanopore reads generated one contig, giving a total genome size of 2,197,335 bp (N50 = 2,197,335 bp, 348× coverage) with an average G+C content of 44.1% (Table 2). The contig was submitted to NCBI Prokaryotic Genome Annotation Pipeline (PGAP) for annotation, resulting in 2,119 protein-coding sequences (CDSs), 4 rRNA operons, and 57 tRNA-encoding genes (Table 2). The result of subsystem analysis is summarized in Fig. 3 and Table 3. An in-silico genome-to-genome comparison showed that HKU75T was closest to Streptococcus porcorum DSM 28302T (average nucleotide identity [ANI] value of 84.7%), followed by Streptococcus ferus DSM 20646T (ANI value of 84.2%) and Streptococcus porci DSM 23759T (ANI value of 83.9%), with ANI values <95%, a threshold value for species boundary (12, 13) (Table 2; Table S1). This supports that HKU75T should be proposed as a novel Streptococcus species, tentatively named Streptococcus oriscaviae HKU75T. Further comparative genomic analyses with closest Streptococcus species revealed that HKU75T were typical of members of Streptococcus (Table 2).
TABLE 2.
Comparative genomic analysis between S. oriscaviae HKU75T and the next 19 ANI closest Streptococcus genomes
| Species | Genome size (bp) | G+C content (%) | No. of proteins | No. of rRNA (5S, 16S, 23S) | No. of tRNA | ANI (%) | G+C content difference (%) | GenBank accession no. |
|---|---|---|---|---|---|---|---|---|
| Streptococcus oriscaviae HKU75T | 2,197,335 | 44.1 | 2,119a | 12a | 57a | GCA_018137985.1 | ||
| Streptococcus porcorum DSM 28302T | 1,899,330 | 38.2 | 1,846 | 3 | 41 | 84.7 | 5.8 | 2928214140 b |
| Streptococcus ferus DSM 20646T | 1,872,314 | 42.8 | 1,819 | 15 | 64 | 84.2 | 1.2 | GCA_900475025.1 |
| Streptococcus porci DSM 23759T | 2,289,031 | 40.8 | 2,297 | 6 | 32 | 83.9 | 3.3 | GCA_000423765.1 |
| Streptococcus orisratti DSM 15617T | 2,415,121 | 38.5 | 2,350 | 3 | 26 | 83.9 | 5.5 | GCA_000380105.1 |
| Streptococcus pseudoporcinus NCTC13786T | 2,156,061 | 37.1 | 2,004 | 18 | 67 | 83.6 | 6.9 | GCA_900637075.1 |
| Streptococcus dysgalactiae subsp. equisimilis NCTC13762T | 2,285,205 | 39.5 | 2,324 | 18 | 69 | 83.5 | 4.5 | GCA_900459095.1 |
| Streptococcus agalactiae NCTC8181T | 2,448,053 | 35.7 | 2,555 | 21 | 80 | 83.3 | 8.3 | GCA_900458965.1 |
| Streptococcus plurextorum DSM 22810T | 2,100,658 | 41.1 | 2,091 | 6 | 31 | 83.3 | 3.0 | GCA_000423745.1 |
| Streptococcus parauberis NCFD 2020T | 2,164,480 | 35.5 | 2,129 | 15 | 58 | 82.9 | 8.6 | GCA_000187935.2 |
| Streptococcus urinalis NCTC13766T | 2,144,000 | 34.3 | 2,212 | 18 | 70 | 82.8 | 9.8 | GCA_900636885.1 |
| Streptococcus canis NCTC12191T | 2,084,744 | 39.9 | 1,974 | 18 | 67 | 82.7 | 4.2 | GCA_900636575.1 |
| Streptococcus uberis NCTC3858T | 1,975,601 | 36.6 | 1,919 | 18 | 69 | 82.6 | 7.5 | GCA_900475595.1 |
| Streptococcus gallolyticus subsp. pasteurianus WUSP067T | 2,149,841 | 37.3 | 2,054 | 18 | 70 | 82.4 | 6.7 | GCA_004843545.1 |
| Streptococcus equi subsp. equi NCTC9682T | 2,253,416 | 41.3 | 2,303 | 18 | 65 | 82.1 | 2.8 | GCA_900637675.1 |
| Streptococcus pyogenes DSM 20565T | 1,914,862 | 38.5 | 1,899 | 18 | 67 | 82.0 | 5.6 | GCA_002055535.1 |
| Streptococcus suis S735T | 1,980,887 | 41.4 | 1,858 | 12 | 56 | 81.9 | 2.7 | GCA_000294495.1 |
| Streptococcus iniae QMA0248T | 2,116,570 | 36.8 | 2,006 | 15 | 58 | 81.9 | 7.2 | GCA_002220115.1 |
| Streptococcus dysgalactiae FDAARGOS 1157T | 2,151,179 | 39.3 | 2,142 | 18 | 67 | 81.8 | 4.7 | GCA_016724885.1 |
| Streptococcus salivarius NCTC8618T | 2,206,150 | 40.1 | 2,001 | 18 | 68 | 81.7 | 3.9 | GCA_900636435.1 |
FIG 3.
A circular genomic map of S. oriscaviae HKU75T, showing a circular graphical display of the distribution of S. oriscaviae genome annotations. From outer to inner rings: contigs, CDS on the forward strand, CDS on the reverse strand, RNA genes, CDS with homology to known antimicrobial resistance genes, CDS with homology to know virulence factors, G+C content, and GC skew. The colors of the CDSs on the forward and reverse strands indicate the subsystems that these genes belong to.
TABLE 3.
Distributions of predicted coding sequence function and potential virulence genes in the annotated genome of S. oriscaviae HKU75T
| Genome features | ||
|---|---|---|
| Subsystema | No. of subsystems | No. of genes |
| Class | ||
| Metabolism | 60 | 337 |
| Protein processing | 39 | 195 |
| Stress response, defense, virulence | 24 | 75 |
| DNA processing | 17 | 78 |
| Energy | 14 | 77 |
| RNA processing | 12 | 45 |
| Membrane transport | 10 | 24 |
| Cellular processes | 9 | 38 |
| Regulation and cell signaling | 3 | 9 |
| Cell envelope | 2 | 5 |
| Miscellaneous | 1 | 1 |
| Virulence factorsb | Gene | Locus tag |
| Class | ||
| Adherence | Alpha-glucosyltransferase (gftA)c | INT76_01865 |
| Collagen binding protein (cpbA) | INT76_08030 | |
| Fibronectin-binding proteins (pavA) | INT76_02425 | |
| Laminin-binding protein (lmb) | INT76_05165 | |
| Sortase A (srtA) | INT76_02095 | |
| Streptococcal lipoprotein rotamase A (slrA) | INT76_10040 | |
| Streptococcal plasmin receptor/GAPDH (plr/gapA) | INT76_05895 | |
| Enzyme | Hyaluronidase (hysA) | INT76_10670 |
| Streptococcal enolase (eno) | INT76_02460 | |
| Protease | C5a peptidase (scpA/scpB) | INT76_05155 |
| Serine protease (htrA/degP) | INT76_07235 | |
| Trigger factor (tig/ropA) | INT76_05170 | |
| Zinc metalloproteinase (zmpC) | INT76_08495 |
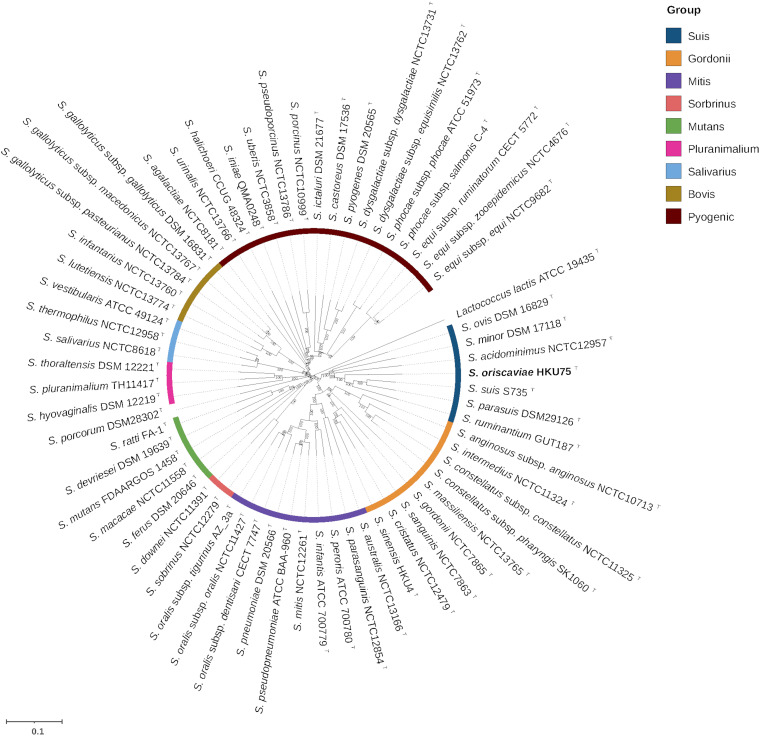
To elucidate the phylogenetic position of S. oriscaviae HKU75T among the genus Streptococcus, a multigene-based phylogenomic analysis was performed. The tree based on the concatenated nucleotide sequences of 92 bacterial core genes showed that S. oriscaviae clustered with members of the Suis group, but not S. porcorum (undefined taxonomic group) (Fig. 4). The Suis group currently includes some medically important pathogens such as S. acidominimus and S. ovis, forming a distinct and well supported phylogenetic clade (Fig. 4). The tree was also able to recover members of the remaining 8 taxonomic groups, including Bovis, Gordonii, Mitis, Mutans, Pluranimalium, Pyogenic, Salivarius, and Sobrinus (Fig. 4), as described in previous studies (14–18).
FIG 4.
Phylogenetic tree constructed by using concatenated nucleotide sequences of 92 core genes of S. oriscaviae HKU75T and its closely related Streptococcus species. The tree was constructed by the neighbor-joining method using MEGA and Lactococcus lactis as the root. The gene names and accession numbers are given as cited in the Database of Clusters of Orthologous Genes (COGs) listed in supplemental Table S3. The Streptococcus genomes were classified into 9 taxonomic groups, namely, Bovis, Gordonii, Mitis, Mutans, Pluranimalium, Pyogenic, Salivarius, Sobrinus, and Suis. The color represents different taxonomic groups. The scale bar corresponds to the mean number of nucleotide substitutions per site on the respective branch. Bootstrap values were calculated as percentages from 1,000 pseudoreplicates (values lower than 70 are not shown).
Chemotaxonomic characteristics of S. oriscaviae HKU75T.
Peptidoglycan type of HKU75T was A3α l-lysine-l-alanine, with rhamnose, ribose, and glucose as major cell-wall constituents. Menaquinones and ubiquinones were not detected. The major cellular fatty acids are C16:0 (35.98%) and summed feature 5 (comprising C18:2 ω6,9c/C18:0 ante) (11.38%).
Potential virulence factors of S. oriscaviae HKU75T.
Streptococci is commonly found in the oral cavities of pet animals, representing one of the most common genera isolated from animal bite wounds (1). Similar to other streptococci, S. oriscaviae HKU75T may possess virulence factors that enable it to colonize the oral cavity of guinea pigs and to establish bite wound infection. The complete genome of S. oriscaviae HKU75T identified in this study contained homologs of several virulence genes found in streptococci (Table 3). Examples of these genes include glucosyltransferases (gtfA), fibronectin-binding protein (pavA), collagen-binding protein (cpbA), laminin-binding protein (lmb) and enolase (eno); these genes are known to be involved in adhesion, colonization, internalization, or invasion (19–24).
TAXONOMY
Description of Streptococcus oriscaviae sp. nov.
Streptococcus oriscaviae (o.ris.ca'vi.ae. L. neut. n. os (gen. oris), mouth; N.L. fem. n. cavia, a guinea pig (genus Cavia); N.L. gen. n. oriscaviae, of the mouth of a cavia).
Aerobic. Gram-stain positive. Non-motile. Non-spore-forming. Negative for catalase and urease. Grows best on Columbia agar with 5% defibrinated sheep blood agar. Grows as alpha-hemolytic and gray colonies of 0.5–1 mm in diameter after a 24 h of incubation at 37°C in an aerobic environment. Growth occurs at 37°C but not at 10°C or 42°C. Capable of growing on brain heart infusion agar, nutrient agar, Trypticase soy agar, and chocolate agar. Using the API 20 STREP system, it can assimilate d-mannitol, esculin, d-lactose, d-trehalose, inulin, starch, l-leucine-ß-naphthylamide, l-arginine, and glycogen, but not l-arabinose, d-ribose, d-sorbitol, d-raffinose, pyroglutamic acid-ß-naphthylamide, 6-bromo-2-naphthyl-αD-galactopyranoside, naphthol ASBI-glucuronic acid, 2-naphthyl-ßD-galactopyranoside, or 2-naphthyl phosphate. The peptidoglycan type of HKU75T is A3α l-lysine-l-alanine. Menaquinones and ubiquinones are absent.
The type strain, HKU75T (= CCUG 75141T = JCM 34455T), was isolated from the guinea pig bite wound of a patient in Hong Kong. The G+C content of the DNA of the type strain HKU75T was 44.1%. The GenBank accession numbers of the whole genome, 16S rRNA, groEL, and rpoB genes for the strain HKU75T are CP073084, ON000582, MW182243, and MW182242, respectively.
DISCUSSION
In this study, we report the isolation of HKU75T in pure culture from the pus collected from a guinea pig bite wound in a healthy young patient. HKU75T is an alpha-hemolytic streptococcus that agglutinates with Lancefield group D and group G antisera. When we first tried to identify HKU75T to the species level, MALDI-TOF MS, the platform we currently used for rapid identification of bacterial isolates in our clinical microbiology laboratory (25–27), showed that the bacterium was most compatible with S. suis, but with a top match score of only 1.86. Therefore, more phenotypic tests were performed using two commercially available kits. One of the kits (API 20 STREP) showed that the most likely identity for HKU75T was S. suis I with 85.4% confidence while the other (Vitek 2) showed that HKU75T was unidentifiable (Table 1). In view of the ambiguous phenotypic profile and inconclusive MALDI-TOF MS results, 16S rRNA and partial groEL and rpoB gene sequencing were performed. Although the complete 16S rRNA gene of HKU75T was most closely related to that of S. parasuis (98.3% sequence identity), partial groEL and rpoB gene sequence comparison showed that it was more closely related to S. suis (81.8% and 89.8% sequence identity respectively) than S. parasuis (81.7% and 89.5% sequence identity respectively) (Fig. 2). Accurate identification of the bacterium was not only of biological interest but also important clinically because if it is a strain of S. suis, the patient could have a significant risk of meningitis and hearing loss (28–31). Finally, whole genome sequencing was performed and intergenomic distance determined by ANI revealed that there was less than 85% identity between the genome of HKU75T and those of all other known species in the Streptococcus genus, confirming that HKU75T is a novel Streptococcus species, which we propose to be named S. oriscaviae.
The oral cavity of guinea pigs is presumably a reservoir of S. oriscaviae. Although guinea pigs are common household pets of children, unlike dog bite and cat bite wound infections, guinea pig bite wound infections were uncommonly reported. In the literature, guinea pig bite wound infections caused by Haemophilus influenzae and Pasteurella species have been described (32, 33). In the present study, when S. oriscaviae was first isolated from the pus collected from the patient’s wound, we suspected that the bacterium could have originated from the oral cavities of the guinea pigs owned by the patient, as the wound was inflicted by a bite by the pet. Since S. oriscaviae was highly susceptible to most antibiotics, no selective medium could be successfully generated (data not shown). Therefore, we tried to directly amplify the groEL gene of the bacterium from DNA extracts obtained from the oral cavities of the two guinea pigs at the patient’s home using specific PCR primers (Fig. S1). Results showed that groEL gene sequences (GP1-1 and GP2-1) identical to that of the HKU75T isolate could be amplified from the oral cavities of both guinea pigs (Fig. 2), indicating that guinea pigs are likely a reservoir of S. oriscaviae. It is also notable that groEL gene sequences (GP1-2 and GP2-2 to GP2-6) that differed by 5–7 bases from HKU75T were also present (Fig. 2), suggesting that multiple strains of S. oriscaviae or other very closely related streptococci may be present in the oral cavities of the guinea pigs.
S. oriscaviae is a member of the Suis group in the Streptococcus genus. With the advancement of sequencing technologies and accumulation of more and more complete bacterial genomes, streptococci can now be classified using the core gene sequences of their genomes (14–18). In general, the conclusions drawn from phenotypic classification match quite well with those obtained from genomic classification. For example, the beta-hemolytic streptococci are clustered and form a Pyogenic group; and members of the unique S. milleri group, namely, S. anginosus, S. intermedius and S. constellatus, are also closely related to each other phylogenetically. Previously, we have also used its genome sequence to characterize S. sinensis, which we discovered and found to be a cause of infective endocarditis (34), and observed that what we suspected about its phylogenetic position from its phenotypic characteristics could be confirmed by genome classification (35–37). As for S. oriscaviae in the present study, biochemically the best match was S. suis. When MALDI-TOF MS was used, it was also most closely related to S. suis. 16S rRNA and groEL/rpoB gene sequence analysis showed that it was most closely related to S. parasuis and S. suis respectively (Fig. 1). Genome classification using concatenated sequences of 92 bacterial core genes also showed that it was most closely related to the S. suis/S. parasuis/S. ruminatium cluster (Fig. 4), confirming that it is a member of the Suis group. It is of note that both S. parasuis and S. ruminatium were previously different serotypes of S. suis but were recently reclassified using data from molecular and genetic tests. S. parasuis was formerly S. suis serotype 20, 22, and 26 but was reclassified as S. parasuis in 2015, whereas S. ruminatium was formerly S. suis serotype 33 but was reclassified as S. ruminatium in 2017 (38, 39). We speculate that some of the reported S. suis isolated from clinical specimens may in fact be S. parasuis, S. ruminatium, or S. oriscaviae. Further studies on these isolates will reveal the relative clinical importance of these Streptococcus species.
MATERIALS AND METHODS
Patient and strains.
Clinical specimens were collected and handled according to standard protocols as described previously (40), and were cultured on sheep blood agar at 37°C with 5% CO2 to obtain the case isolate HKU75T. Clinical data were collected by retrieving and analyzing the hospital record of the patient. The type strain of S. suis S735T was originally isolated from cases of bacteremia/meningitis in piglets; and it was obtained from the Biological Resource Center of Institut Pasteur, France. The quality control strain, Streptococcus pneumoniae ATCC 49619, was obtained for the susceptibility test from the American Type Culture Collection, USA. The study was approved by the Institute Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (reference number: UW 16–365).
Microbiological methods and phenotypic characterizations.
Bacterial cultures and phenotypic identification were performed according to standard protocols (40). In addition, the Vitek 2 System (bioMérieux, Marcy l’Etoile, France) and the API system (20 STREP) (bioMérieux, France) were used to identify the bacterial isolate in this study. Lancefield serogrouping was performed using Streptex (Murex Biotech Ltd., Dartford, United Kingdom) according to the manufacturer’s instructions. MALDI-TOF MS was performed by the direct transfer method, as described previously, with modifications (41). This was conducted using the Microflex LT system with MALDI Biotyper 3.0 and Reference Library V.3.1.2.0 (Bruker Daltonik). Antibiotic susceptibility testing was performed using the Etest method for penicillin and the Kirby Bauer disk diffusion method for the other antibiotics; the results were interpreted according to the Clinical and Laboratory Standards Institute for alpha-hemolytic streptococci.
16S rRNA, partial groEL, and partial rpoB gene sequencing; sequence identity analyses and phylogenetic analyses.
Extractions of bacterial DNA, PCR, and sequencing of the 16S rRNA, groEL, and rpoB genes for the case isolate HKU75T were carried out following the methods outlined in a previous publication, with slight modifications (42, 43). The primer pairs LPW40131 (5′-CTAAGGCCCCACAAGACCTC-3′) and LPW40132 (5′-CAGAGTGCTTAGCCGGACAA-3′), LPW15046 (5′-GAHGTNGTiGAAGGiATGCA-3′) and LPW15047 (5′-ATTTGRCGiAYWGGYTCTTC-3′), and LPW38616 (5′-TCGTCAACCATGTGGTGA-3′) and LPW38617 (5′-GGGCCTGAAGAAATCACC-3′) were used for the 16S rRNA, groEL and rpoB genes, respectively, for the respective PCR and DNA sequencing. The DNA sequences obtained, together with those of other closely related species accessioned in the DDBJ/ENA/GenBank/JGI/PATRIC databases (Table S2), were then compared by pairwise alignment, using MEGA 11 (version 11.0.11) (44). These sequences were also analyzed via multiple sequence alignment, using ClustalW (45). Tests for substitution models and the phylogenetic tree construction were performed using the maximum likelihood method, using MEGA 11 (version 11.0.11) (44). Phylogenetic analyses included 1,287, 666, and 628 nucleotide positions of the 16S rRNA, partial groEL, and partial rpoB sequences, respectively.
Sample collection from guinea pigs and identification of HKU75T.
Two oral swabs were prospectively collected from each of the two guinea pigs (GP1 and GP2) (Fig. S1). Before sample collection, the guinea pigs were not allowed to eat or drink for 30 min. Briefly, the swabs (Oxoid) were inserted into the mouths of the guinea pigs and slowly twirled, and the swab was then rubbed across the tooth surface/mucosa to collect the samples. The swabs were immediately stored in Amies agar gel and transported to a laboratory at ambient temperature. One swab was cultured on sheep blood agar at 37°C with 5% CO2 for 48 h. The genus identities of all suspected Streptococcus-like isolates were confirmed by MALDI-TOF MS, as described above. Another oral swab was subjected to direct DNA extraction using the alkaline lysis method, as described previously (42). The HKU75T in the DNA extracts, as well as in all Streptococcus species isolated from the swab samples, was detected using PCR targeting its partial 588-bp fragment of the groEL gene. The obtained sequences were compared with the groEL gene sequence of HKU75T.
As double or multiple nucleotide peaks were observed in the sequencing results, the corresponding purified PCR product was cloned into the pCR-II-TOPO vector (Invitrogen), according to manufacturer’s instructions. Eight and 11 clones were selected for GP-1 and GP-2 respectively. Both strands of each clone were sequenced using the primers 5′-GTAAAACGACGGCCAG-3′ and 5′-CAGGAAACAGCTATGAC-3′. The sequences of the clones were compared with the HKU75T genome and other groEL gene sequences of Streptococcus species available in the GenBank database. Phylogenetic analysis was performed as described above, using the 588 nucleotide positions of the partial groEL sequences.
Chemotaxonomic characterizations.
Analysis of peptidoglycan, cell-wall sugars, quinones, and fatty acid were carried out by DSMZ Services, Leibniz-Institut DSMZ-Deutsche Sammlung von Mikroongranismen und Zellkulturen GmbH, Braunschweig, Germany.
Whole genome sequencing and hybrid genome assembly.
The complete genome of the case isolate HKU75T was sequenced using Illumina and Oxford Nanopore technologies. Genomic DNA was extracted from an overnight culture (37°C) grown on blood agar using a genomic DNA purification kit (Qiagen, Hilden, Germany), as described previously (42). The Illumina DNA library was prepared using a Nextera XT DNA Sample Prep Kit (Illumina, San Diego, CA, USA) and was sequenced on a NovaSeq 6000 instrument (run type: PE151 bp). The ONT long-read library was prepared with SQK-RAD004 rapid sequencing kit (Oxford Nanopore Technologies, Oxford, UK) according to the manufacturer’s instructions, and sequenced on a MinION sequencer. Illumina reads and Oxford Nanopore MinION reads were assembled by Unicycler/0.4.8 to obtain the whole genome of HKU75T.
Genome sequence analyses.
Intergenomic distances (i.e., ANI values) between the proposed novel species (i.e., the case isolate HKU75T) and the type and reference strains of the corresponding closest species were calculated using the web service available at http://enve-omics.ce.gatech.edu/ani/ (46). In addition to the case isolate HKU75T, which was sequenced to completion as part of this study, the remaining 104 complete genome sequences of 104 Streptococcus species were downloaded from NCBI, JGI, and PATRIC databases (Table S2). The G+C content of HKU75T was determined based on the genome sequence. Predictions of protein coding regions and automatic functional annotations were performed using the PGAP (47). Virulence genes were identified by using the Virulence Factor Database (VFDB) (48). Comparative genomic analysis between HKU75T and the next 19 ANI closest Streptococcus genomes was performed using the Type Strain Genome Server (49).
Phylogenomic characterization.
To determine the phylogenetic position of S. oriscaviae HKU75T among the current 9 taxonomic groups within the genus Streptococcus (14), a multigene-based phylogenomic treeing approach based on concatenated nucleotide sequences of 92 bacterial core genes was used (Table S3). This approach has been shown to be useful for phylogenetic delineation of bacterial species in previous studies (14–17). The alignment of concatenated 92 core genes of 62 Streptococcus genomes and one Lactococcus genome was first generated using an up-to-date bacterial core gene (UBCG) pipeline (https://www.ezbiocloud.net/tools/ubcg) with the default parameters as described by Na et al. (Table S4) (50). The Neighbor-joining tree was constructed using MEGA 11 (version 11.0.11) (44).
Data availability.
The whole genome sequence of HKU75T has been deposited at GenBank under the accession CP073084. The version described in this article is CP073084. The 16S rRNA, rpoB, and groEL gene sequences of HKU75T have been deposited at GenBank under the accession numbers ON000582, MW182242, and MW182243 respectively.
ACKNOWLEDGMENTS
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
We declare no conflicts of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Susanna K. P. Lau, Email: skplau@hku.hk.
Patrick C. Y. Woo, Email: pcywoo@hku.hk.
Jasna Kovac, The Pennsylvania State University.
REFERENCES
- 1.Abrahamian FM, Goldstein EJ. 2011. Microbiology of animal bite wound infections. Clin Microbiol Rev 24:231–246. doi: 10.1128/CMR.00041-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers EM, Ward SL, Myers JP. 2012. Life-threatening respiratory pasteurellosis associated with palliative pet care. Clin Infect Dis 54:e55-7–e57. doi: 10.1093/cid/cir975. [DOI] [PubMed] [Google Scholar]
- 3.Markewitz RDH, Graf T. 2020. Capnocytophaga canimorsus Infection. N Engl J Med 383:1167. doi: 10.1056/NEJMicm1916407. [DOI] [PubMed] [Google Scholar]
- 4.Król J, Nowakiewicz A, Błaszków A, Brodala M, Domagała A, Prassol AN, Sławska D, Wojtynia J. 2022. Genetic diversity of oral streptococci in the guinea pig as assessed by sequence analysis of the 16S rRNA and groEL genes. Folia Microbiol (Praha) 67:311–318. doi: 10.1007/s12223-021-00936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minarikova A, Hauptman K, Knotek Z, Jekl V. 2016. Microbial flora of odontogenic abscesses in pet guinea pigs. Vet Rec 179:331. doi: 10.1136/vr.103551. [DOI] [PubMed] [Google Scholar]
- 6.Kawamura Y, Hou XG, Sultana F, Miura H, Ezaki T. 1995. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int J Syst Bacteriol 45:406–408. [7537076]. doi: 10.1099/00207713-45-2-406. [DOI] [PubMed] [Google Scholar]
- 7.Facklam R. 2002. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev 15:613–630. doi: 10.1128/CMR.15.4.613-630.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tapp J, Thollesson M, Herrmann B. 2003. Phylogenetic relationships and genotyping of the genus Streptococcus by sequence determination of the RNase P RNA gene, rnpB. Int J Syst Evol Microbiol 53:1861–1871. doi: 10.1099/ijs.0.02639-0. [DOI] [PubMed] [Google Scholar]
- 9.Glazunova OO, Raoult D, Roux V. 2010. Partial recN gene sequencing: a new tool for identification and phylogeny within the genus Streptococcus. Int J Syst Evol Microbiol 60:2140–2148. doi: 10.1099/ijs.0.018176-0. [DOI] [PubMed] [Google Scholar]
- 10.Park HK, Yoon JW, Shin JW, Kim JY, Kim W. 2010. rpoA is a useful gene for identification and classification of Streptococcus pneumoniae from the closely related viridans group streptococci. FEMS Microbiol Lett 305:58–64. doi: 10.1111/j.1574-6968.2010.01913.x. [DOI] [PubMed] [Google Scholar]
- 11.Zbinden A, Kohler N, Bloemberg GV. 2011. recA-based PCR assay for accurate differentiation of Streptococcus pneumoniae from other viridans streptococci. J Clin Microbiol 49:523–527. doi: 10.1128/JCM.01450-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain C, Rodriguez-R LM, Phillippy AM, Konstantinidis KT, Aluru S. 2018. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun 9:5114. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richter M, Rosselló-Móra R. 2009. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci USA 106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan Y, An H, Fu T, Zhao S, Zhang C, Xiao G, Zhang J, Zhao X, Hu G. 2018. Characterization of Streptococcus pluranimalium from a cattle with mastitis by whole genome sequencing and functional validation. BMC Microbiol 18:182. doi: 10.1186/s12866-018-1327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel S, Gupta RS. 2018. Robust demarcation of fourteen different species groups within the genus Streptococcus based on genome-based phylogenies and molecular signatures. Infect Genet Evol 66:130–151. doi: 10.1016/j.meegid.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Gao XY, Zhi XY, Li HW, Klenk HP, Li WJ. 2014. Comparative genomics of the bacterial genus streptococcus illuminates evolutionary implications of species groups. PLoS One 9:e101229. doi: 10.1371/journal.pone.0101229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Póntigo F, Moraga M, Flores SV. 2015. Molecular phylogeny and a taxonomic proposal for the genus Streptococcus. Genet Mol Res 14:10905–10918. doi: 10.4238/2015.September.21.1. [DOI] [PubMed] [Google Scholar]
- 18.Barajas HR, Romero MF, Martinez-Sanchez S, Alcaraz LD. 2019. Global genomic similarity and core genome sequence diversity of the Streptococcus genus as a toolkit to identify closely related bacterial species in complex environments. PeerJ 6:e6233. doi: 10.7717/peerj.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu RR, Yang WD, Niu KX, Wang B, Wang WM. 2018. An update on the evolution of glucosyltransferase (GTF) genes in Streptococcus. Front Microbiol 9:2979–2979. doi: 10.3389/fmicb.2018.02979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarz-Linek U, Höök M, Potts JR. 2006. Fibronectin-binding proteins of Gram-positive cocci. Microbes Infect 8:2291–2298. doi: 10.1016/j.micinf.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Natanson S, Sela S, Moses AE, Musser JM, Caparon MG, Hanski E. 1995. Distribution of fibronectin-binding proteins among group A streptococci of different M types. J Infect Dis 171:871–878. [7706813]. doi: 10.1093/infdis/171.4.871. [DOI] [PubMed] [Google Scholar]
- 22.Arora S, Gordon J, Hook M. 2021. Collagen binding proteins of Gram-positive pathogens. Front Microbiol 12:628798. doi: 10.3389/fmicb.2021.628798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linke C, Caradoc-Davies TT, Young PG, Proft T, Baker EN. 2009. The laminin-binding protein Lbp from Streptococcus pyogenes is a zinc receptor. J Bacteriol 191:5814–5814. doi: 10.1128/JB.00485-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu H, Lei S, Jia L, Xia X, Sun Y, Jiang H, Zhu R, Li S, Qu G, Gu J, Sun C, Feng X, Han W, Langford PR, Lei L. 2021. Streptococcus suis serotype 2 enolase interaction with host brain microvascular endothelial cells and RPSA-induced apoptosis lead to loss of BBB integrity. Vet Res 52:30. doi: 10.1186/s13567-020-00887-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang BSF, Lau SKP, Teng JLL, Chan TM, Chan WS, Wong TY, Tong YT, Fan RYY, Yuen KY, Woo PCY. 2013. Matrix-assisted laser desorption ionisation-time of flight mass spectrometry for rapid identification of Laribacter hongkongensis. J Clin Pathol 66:1081–1083. doi: 10.1136/jclinpath-2013-201651. [DOI] [PubMed] [Google Scholar]
- 26.Lau SKP, Tang BSF, Teng JLL, Chan TM, Curreem SOT, Fan RYY, Ng RHY, Chan JFW, Yuen KY, Woo PCY. 2014. Matrix-assisted laser desorption ionisation time-of-flight mass spectrometry for identification of clinically significant bacteria that are difficult to identify in clinical laboratories. J Clin Pathol 67:361–366. doi: 10.1136/jclinpath-2013-201818. [DOI] [PubMed] [Google Scholar]
- 27.Teng JLL, Tang Y, Wong SSY, Fong JYH, Zhao Z, Wong CP, Chen JHK, Ngan AHY, Wu AKL, Fung KSC, Que TL, Lau SKP, Woo PCY. 2018. MALDI-TOF MS for identification of Tsukamurella species: Tsukamurella tyrosinosolvens as the predominant species associated with ocular infections. Emerging Microbes & Infections 7:1–11. doi: 10.1038/s41426-018-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arends JP, Zanen HC. 1988. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis 10:131–137. [3353625]. doi: 10.1093/clinids/10.1.131. [DOI] [PubMed] [Google Scholar]
- 29.Ip M, Fung KSC, Chi F, Cheuk ESC, Chau SSL, Wong BWH, Lui SL, Hui M, Lai RWM, Chan PKS. 2007. Streptococcus suis in Hong Kong. Diagn Microbiol Infect Dis 57:15–20. doi: 10.1016/j.diagmicrobio.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 30.van Samkar A, Brouwer MC, Schultsz C, van der Ende A, van de Beek D. 2015. Streptococcus suis meningitis in the Netherlands. J Infect 71:602–604. doi: 10.1016/j.jinf.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Mai NTH, Hoa NT, Nga TVT, Linh LD, Chau TTH, Sinh DX, Phu NH, Chuong L, Diep TS, Campbell J, Nghia HDT, Minh TN, Chau NVV, De Jong MD, Chinh NT, Hien TT, Farrar J, Schultsz C. 2008. Streptococcus suis meningitis in adults in Vietnam. Clin Infect Dis 46:659–667. [DOI] [PubMed] [Google Scholar]
- 32.Rolle U, Handrick W. 2000. Haemophilus influenzae cellulitis after bite injuries in children. J Pediatr Surg 35:1408–1409. doi: 10.1053/jpsu.2000.9355. [DOI] [PubMed] [Google Scholar]
- 33.Lion C, Conroy MC, Dupuy ML, Escande F. 1995. Pasteurella “SP” group infection after a guineapig bite. Lancet 346:901–902. doi: 10.1016/S0140-6736(95)92741-7. [DOI] [PubMed] [Google Scholar]
- 34.Woo PCY, Tam DMW, Leung KW, Lau SKP, Teng JLL, Wong MKM, Yuen KY. 2002. Streptococcus sinensis sp. nov., a novel species isolated from a patient with infective endocarditis. J Clin Microbiol 40:805–810. doi: 10.1128/JCM.40.3.805-810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teng JL, Huang Y, Tse H, Chen JH, Tang Y, Lau SK, Woo PC. 2014. Phylogenomic and MALDI-TOF MS analysis of Streptococcus sinensis HKU4T reveals a distinct phylogenetic clade in the genus Streptococcus. Genome Biol Evol 6:2930–2943. doi: 10.1093/gbe/evu232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woo PC, Teng JL, Leung KW, Lau SK, Tse H, Wong BH, Yuen KY. 2004. Streptococcus sinensis may react with Lancefield group F antiserum. J Med Microbiol 53:1083–1088. doi: 10.1099/jmm.0.45745-0. [DOI] [PubMed] [Google Scholar]
- 37.Woo PC, Teng JL, Tsang SN, Tse CW, Lau SK, Yuen KY. 2008. The oral cavity as a natural reservoir for Streptococcus sinensis. Clin Microbiol Infect 14:1075–1079. doi: 10.1111/j.1469-0691.2008.02083.x. [DOI] [PubMed] [Google Scholar]
- 38.Nomoto R, Maruyama F, Ishida S, Tohya M, Sekizaki T, Osawa R. 2015. Reappraisal of the taxonomy of Streptococcus suis serotypes 20, 22 and 26: Streptococcus parasuis sp. nov. Int J Syst Evol Microbiol 65:438–443. doi: 10.1099/ijs.0.067116-0. [DOI] [PubMed] [Google Scholar]
- 39.Tohya M, Arai S, Tomida J, Watanabe T, Kawamura Y, Katsumi M, Ushimizu M, Ishida-Kuroki K, Yoshizumi M, Uzawa Y, Iguchi S, Yoshida A, Kikuchi K, Sekizaki T. 2017. Defining the taxonomic status of Streptococcus suis serotype 33: the proposal for Streptococcus ruminantium sp. nov. Int J Syst Evol Microbiol 67:3660–3665. doi: 10.1099/ijsem.0.002204. [DOI] [PubMed] [Google Scholar]
- 40.Jorgensen JH, Pfaller MA. 2015. Manual of clinical microbiology 11th ed. John Wiley & Sons, Inc., New York, NY. [Google Scholar]
- 41.Chen JH, She KK, Wong OY, Teng JL, Yam WC, Lau SK, Woo PC, Cheng VC, Yuen KY. 2015. Use of MALDI Biotyper plus ClinProTools mass spectra analysis for correct identification of Streptococcus pneumoniae and Streptococcus mitis/oralis. J Clin Pathol 68:652–656. doi: 10.1136/jclinpath-2014-202818. [DOI] [PubMed] [Google Scholar]
- 42.Teng JLL, Fong JYH, Fok KMN, Lee HH, Chiu TH, Tang Y, Ngan AHY, Wong SSY, Que TL, Lau SKP, Woo PCY. 2020. Tsukamurella asaccharolytica sp. nov., Tsukamurella conjunctivitidis sp. nov. and Tsukamurella sputi sp. nov., isolated from patients with bacteraemia, conjunctivitis and respiratory infection in Hong Kong. Int J Syst Evol Microbiol 70:995–1006. doi: 10.1099/ijsem.0.003861. [DOI] [PubMed] [Google Scholar]
- 43.Lau SKP, Curreem SOT, Lin CCN, Fung AMY, Yuen K-Y, Woo PCY. 2013. Streptococcus hongkongensis sp. nov., isolated from a patient with an infected puncture wound and from a marine flatfish. Int J Syst Evol Microbiol 63:2570–2576. doi: 10.1099/ijs.0.045120-0. [DOI] [PubMed] [Google Scholar]
- 44.Tamura K, Stecher G, Kumar S. 2021. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol 38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4673. [7984417]. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez-R LM, Konstantinidis KT. 2016. The enveomics collection: a toolbox for specialized analyses of microbial genomes and metagenomes. PeerJ Preprints 4:e1900v1. doi: 10.7287/PEERJ.PREPRINTS.1900V1. [DOI] [Google Scholar]
- 47.NCBI Resource Coordinators. 2016. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 44:D7–D19. doi: 10.1093/nar/gkv1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu B, Zheng D, Zhou S, Chen L, Yang J. 2022. VFDB 2022: a general classification scheme for bacterial virulence factors. Nucleic Acids Res 50:D912–D917. doi: 10.1093/nar/gkab1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meier-Kolthoff JP, Göker M. 2019. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat Commun 10:2182. doi: 10.1038/s41467-019-10210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Na SI, Kim YO, Yoon SH, Ha S, Baek I, Chun J. 2018. UBCG: up-to-date bacterial core gene set and pipeline for phylogenomic tree reconstruction. J Microbiol 56:280–285. doi: 10.1007/s12275-018-8014-6. [DOI] [PubMed] [Google Scholar]
- 51.Kilpper-Balz R, Schleifer KH. 1987. Streptococcus suis sp. nov., nom. rev. Int J Systematic Bacteriology 37:160–162. doi: 10.1099/00207713-37-2-160. [DOI] [Google Scholar]
- 52.Nordberg H, Cantor M, Dusheyko S, Hua S, Poliakov A, Shabalov I, Smirnova T, Grigoriev IV, Dubchak I. 2014. The genome portal of the Department of Energy Joint Genome Institute: 2014 updates. Nucleic Acids Res 42:D26–D31. doi: 10.1093/nar/gkt1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis JJ, Wattam AR, Aziz RK, Brettin T, Butler R, Butler RM, Chlenski P, Conrad N, Dickerman A, Dietrich EM, Gabbard JL, Gerdes S, Guard A, Kenyon RW, MacHi D, Mao C, Murphy-Olson D, Nguyen M, Nordberg EK, Olsen GJ, Olson RD, Overbeek JC, Overbeek R, Parrello B, Pusch GD, Shukla M, Thomas C, Vanoeffelen M, Vonstein V, Warren AS, Xia F, Xie D, Yoo H, Stevens R. 2020. The PATRIC Bioinformatics Resource Center: expanding data and analysis capabilities. Nucleic Acids Res 48:D606–D612. doi: 10.1093/nar/gkz943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.00014-22-s001.pdf, PDF file, 0.4 MB (385.1KB, pdf)
Data Availability Statement
The whole genome sequence of HKU75T has been deposited at GenBank under the accession CP073084. The version described in this article is CP073084. The 16S rRNA, rpoB, and groEL gene sequences of HKU75T have been deposited at GenBank under the accession numbers ON000582, MW182242, and MW182243 respectively.