ABSTRACT
Avian pathogenic Escherichia coli (APEC) associated with colibacillosis results in high morbidity and mortality, and severe economic losses to the poultry industry. APEC is a zoonotic pathogen and can infect humans through contaminated poultry products. Vaccination and antibiotic treatment are currently used to control APEC infections; however, the limited effect of vaccines and the emergence of antibiotic-resistant strains have necessitated the development of novel therapeutics. Here, we evaluated seven quorum sensing inhibitors (QSI) identified in our previous study, in APEC-infected chickens. QSIs were administered orally (~92 to 120 μg/bird) and chickens were challenged subcutaneously with APEC. Among them, QSI-5 conferred the best protection (100% reduction in mortality, 82% to 93% reduction in lesions [airsacculitis, perihepatitis, lung congestion, pericarditis] severity, and 5.2 to 6.1 logs reduction in APEC load). QSI-5 was further tested in chickens raised on built-up floor litter using an optimized dose (1 mg/L) in drinking water. QSI-5 reduced the mortality (88.4%), lesion severity (72.2%), and APEC load (2.8 logs) in chickens, which was better than the reduction observed with currently used antibiotic sulfadimethoxine (SDM; mortality 35.9%; lesion severity up to 36.9%; and APEC load up to 2.4 logs). QSI-5 was detected in chicken's blood after 0.5 h with no residues in muscle, liver, and kidney. QSI-5 increased the body weight gain with no effect on the feed conversion ratio and cecal microbiota of the chickens. Metabolomic studies revealed reduced levels of 5′-methylthioadenosine in QSI-5-treated chicken serum. In conclusion, QSI-5 displayed promising effects in chickens and thus, represents a novel anti-APEC therapeutic.
IMPORTANCE Avian pathogenic Escherichia coli (APEC), a subgroup of ExPEC, is a zoonotic pathogen with public health importance. Quorum sensing is a mechanism that regulates virulence, biofilm formation, and pathogenesis in bacteria. Here, we identified a novel quorum sensing autoinducer-2 inhibitor, QSI-5, which showed higher anti-APEC efficacy in chickens compared to the currently used antibiotic, sulfadimethoxine at a much lower dose (up to 4,500 times). QSI-5 is readily absorbed with no residues in the tissues. QSI-5 also increased the chicken’s body weight gain and did not impact the cecal microbiota composition. Overall, QSI-5 represents a promising lead compound for developing novel anti-virulence therapies with significant implications for treating APEC infections in chickens as well as other ExPEC associated infections in humans. Further identification of its target(s) and understanding the mechanism of action of QSI-5 in APEC will add to the future novel drug development efforts that can overcome the antimicrobial resistance problem.
KEYWORDS: APEC, quorum sensing, inhibitors, anti-virulence, auto-inducer 2, QSI-5, sulfadimethoxine, 5'-methylthioadenosine, chickens
INTRODUCTION
Avian pathogenic Escherichia coli (APEC), is one of the major poultry pathogens causing significant economic losses to the poultry industry worldwide (1). APEC is an extra-intestinal pathogenic E. coli (ExPEC) and infections are characterized by a wide range of localized and systemic infections such as septicemia, salpingitis, airsacculitis, arthritis, peritonitis, yolk sac infection, swollen head syndrome, and respiratory tract infection (2, 3). APEC can be transmitted to humans through the consumption of contaminated poultry products and fresh produce that is amended with contaminated poultry manure (4). APEC strains share genetic similarity and virulence genes with human ExPECs such as uropathogenic E. coli (UPEC) and neonatal meningitis E. coli (NMEC) and has been reported to cause urinary tract infections and meningitis in rodent models and manifest in the form of foodborne gastrointestinal illnesses that are often accompanied by the ingestion of contaminated foods (4–6). APEC infects a wide range of poultry species of all ages and is common in chickens, turkeys, and ducks in different production systems and can negatively impact the body weight gain and the feed conversion ratio (2, 3).
APEC is prevalent in all ages of chickens, although, a higher prevalence is detected in adult layer chickens (36.7%) (7). At least 30% of the commercial poultry flocks in the United States have been estimated to be affected with colibacillosis at any given point of time (8). APEC infections also result in the reduction of meat production (2% reduction in chicken’s live weight), feed conversion ratio (up to 2.7%), and egg production (up to 15%), increased carcass condemnation at slaughter age (up to 45%) (5), and high morbidity and mortality of chickens (up to 20%) especially in young chickens (53.5% of the total mortality), leading to severe economic losses. Annual losses to the poultry industry in the United States due to APEC infection have previously been estimated at $40 million (9). Thus, APEC poses a significant threat to global poultry production as well as sustainable animal agriculture worldwide (5).
APEC infections in poultry are treated with antibiotics worldwide (quinolones, tetracycline, cephalosporins, aminoglycosides, sulfonamides, and colistin) and/or by vaccination (Poulvac E. coli). However, antibiotics have limited effect due to the emergence of multidrug-resistant (MDR) APEC strains and vaccine failure is associated with infection by heterologous serotypes (1, 10–12). For example, in the United States, Europe, and Australia, approximately 92% of APEC isolates with resistance to three or more classes of antibiotics, including to some of the most commonly used drugs such as tetracycline, streptomycin, and sulfonamides have been isolated (1). Furthermore, APEC isolates with resistance to colistin (possessing mcr-1) and β-lactam antibiotics (possessing extended-spectrum-β-lactamase, ESBL genotype) have been isolated from chickens in China, Egypt, and France (13, 14). Therefore, novel approaches are needed to effectively control APEC infections in poultry. Additionally, APEC is considered as a zoonotic foodborne pathogen and shares several important traits with human ExPEC (8). Thus, the control of APEC infections in poultry also has a public health significance.
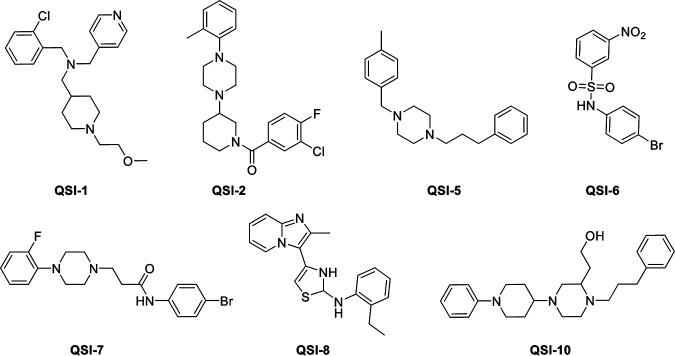
In several bacteria including E. coli, quorum sensing autoinducer-2 (QS AI-2) plays a critical role in virulence and biofilm formation (15). AI-2 is unique because it serves as the universal signal between interspecies QS communication for both Gram negative and Gram positive bacteria (16). In our previous study (17), we identified 10 novel QS AI-2 inhibitors by using Vibrio harveyi AI-2 indicator bacteria, that did not inhibit the APEC’s growth, but impacted the QS- regulated processes and showed efficacy against APEC infections in vitro. Previously, these quorum sensing inhibitors (QSI) were designated C-1 to C-10 (17); however, in the current study they are referred as QSI-1 to QSI-10. Here, we evaluated the efficacy of the seven (QS-1, -2, -5, -6, -7, -8, and -10; Fig. 1) potent AI-2 inhibitors from our previous in vitro studies in APEC-infected chickens. We then selected the best anti-APEC compound (QSI-5) that significantly reduced the mortality, APEC load and pathological lesion severity in infected chickens and (i) optimized its dose for delivery in drinking water; (ii) compared its efficacy with the currently used antibiotic (sulfadimethoxine, SDM) in a field simulated setting; (iii) evaluated its effect on gut microbiota and serum metabolites; (iv) measured its residue in muscle, liver, and kidney; and (v) conducted pharmacokinetic studies.
FIG 1.
Chemical structures of the Quorum-Sensing AI-2 Inhibitors (QSIs) investigated in this study.
RESULTS
The QSI-5 and QSI-10 showed higher anti-APEC efficacy compared with other QSIs in chickens.
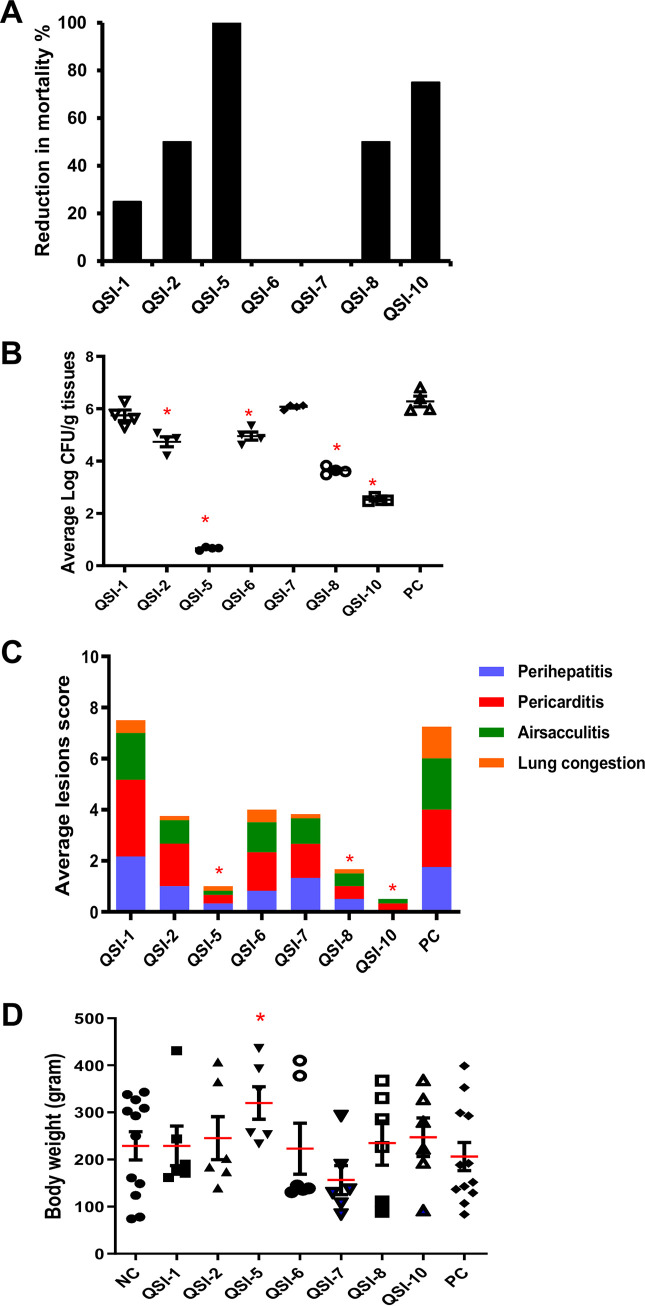
Treatment of 1-day-old chickens once daily with QSIs orally reduced the mortality, APEC load, and lesion severity in the internal organs compared to the positive control (PC; not treated and infected) group. To calculate the mortality reduction in the treated groups compared with the PC group, the mortality in the PC group was normalized to 100%. Our results showed that the treatment of chickens with QSI-5 and QSI-10 resulted in 100% and 75% reduction in mortality, respectively; while QSI-2 and QSI-8 resulted in a 50% reduction in mortality compared with the PC group. Further, treatment of chickens with QSI-1 reduced the mortality by 25%, while QSI-6 and QSI-7 did not reduce mortality compared to the PC group (Fig. 2A). Mortality details in QSIs-treated and control groups are shown in Table S1 and Fig. S1A.
FIG 2.
Effect of QSIs on (A) chicken's mortality, (B) APEC lesion severity in the internal organs (liver, heart, airsacs, and lung), (C) APEC load in the internal organs (liver, lung, heart, and kidney), and (D) body weight gain of QSIs-treated groups compared to the PC group. QSIs were administered once daily for 5 days using oral gavage and chickens were infected with APEC using s/c route. The average lesion score and APEC load was calculated, and the data were presented as an average of all the organs in each chicken and cumulative lesion scores for each group, respectively. *Significant difference between treated chickens (P < 0.05) and the PC group.
The average APEC load reduction in the internal organs (liver, heart, lung, kidney) of QSI-5-treated chickens ranged between 5.2 and 6.1 logs CFU/g of tissues (P < 0.05); while the average reduction in APEC load in QSI-10 and QSI-8-treated chickens ranged between (3.4 to 4.3 logs; P < 0.05) and (2.2 to 3.1 logs CFU/g of tissues), respectively, compared with the PC group. Further, QSI-2 and QSI-6 reduced the APEC load between 1.1 logs and 1.8 logs CFU/g of tissues; whereas QSI-1 and QSI-7 did not reduce the APEC load compared with the PC group (Fig. 2B). Similarly, to calculate the reduction of lesion (perihepatitis, pericarditis, lung congestion, and airsacculitis) severity in internal organs, the lesion severity in the PC group was considered as 100%. The reduction of lesion severity in the QSI-5-treated group ranged between 78% and 93%, while QSI-10 resulted in 85% to 100% reduction of lesion severity compared to the PC group. Further, treatment of the chickens with QSI-8 resulted in 67% to 89% reduction of lesion severity, while QSI-2, QSI-6, QSI-7, and QSI-1 resulted in (26% to 89%), (33% to 67%), (11% to 89%), and (0% to 67%) reduction, respectively, compared with the PC group (Fig. 2C). None of the tested QSIs affected the body weight of treated chickens, except for QSI-5-treated chickens which showed increased average body weight (mean difference = 90.9 g) compared with negative control (NC; not treated and not infected) group (P < 0.05; Fig. 2D). Details of average APEC load and average lesion severity in each organ in QSIs treated and control groups are shown in Table S1.
QSI-5 reduced the level of 5′-Methylthioadenosine, a component of QS AI-2 activated methyl cycle.
Untargeted metabolomic profiling was performed using LC-MS to determine the effect of QSIs (QSI-5, QSI-8, and QSI-10) treatment on chicken serum metabolites compared with the PC group. The QSI-5 treatment significantly reduced the level of 5′-methylthioadenosine (7 folds; P = 0.0009) that is involved in methionine metabolism pathway and spermidine and spermine biosynthesis pathway compared to the PC group (Table 1). Similarly, QSI-8 treatment significantly increased the abundance of all-trans-Carophyll yellow, a member of the triterpenoid family, (51.8 folds; P = 0. 000002); 9,10-DiHODE, a member of the linoleic acids, which are involved in lipid transport, lipid and fatty acid metabolism (3.6 folds; P = 0. 00008); and LysoPE (0:0/20:3[11Z,14Z,17Z]), a phospholipid, which is involved in the glycerophospholipid metabolism and lipid metabolism pathways (5.1 folds; P = 0. 0008), compared with the PC group. Treatment of chickens with QSI-10 increased the level of Tetranor-PGF1alpha, a prostaglandin (9.3 folds; P = 0. 0008) and LysoPE (20:4 (8Z,11Z,14Z,17Z)/0:0) (5.4 folds; P = 0. 0008), which are involved in glycerophospholipid metabolism and lipid metabolism pathways; while reduced the level of Betavulgaroside VIII, a member of the diterpene glycosides (17.6 folds; P = 0. 0002), which is involved in lipid metabolism pathway compared with the PC group (Table 1).
TABLE 1.
The altered metabolites in chicken’s serum after the treatment with QSIs
| QSI | Metabolites | Fold changea | P-value |
|---|---|---|---|
| QSI-5 | 5′-Methylthioadenosine | ↓7.0 | 0.0009 |
| QSI-8 | all-trans-Carophyll yellow | ↑51.8 | 0.000002 |
| LysoPE (0:0/20:3(11Z,14Z,17Z)) | ↑5.1 | 0.0008 | |
| 9,10-DiHODE | ↑3.6 | 0.0008 | |
| QSI-10 | Betavulgaroside VIII | ↓17.6 | 0.0002 |
| Tetranor-PGF1alpha | ↑9.3 | 0.0008 | |
| LysoPE (20:4(8Z,11Z,14Z,17Z)/0:0) | ↑5.4 | 0.0008 |
Fold change was calculated by comparing to the abundance of metabolite in serum of the PC group. The arrows represent whether given metabolites are up (increased) or down (decreased) regulated.
To explain the variance of the metabolite profiles between treated and control groups, PCA was conducted with two principal components for QSI-5 (PC3 = 6.78%, PC5 = 3.5%), and QSI-8, and QSI-10 (PC1 = 30.5%, PC7 = 3.97%). The principal-component analysis (PCA) scores revealed that the metabolite profiles and composition of QSI-5-treated chickens clustered differently than the metabolites profile of PC (P = 0.04) and NC groups (0.0007) (Fig. S2A). Similarly, the metabolite profiles of QSI-8- (P = 0.0002) and QSI-10-treated (P = 0.009) chickens also clustered differently than the metabolite profiles of the PC group. Interestingly, metabolites profiles of QSI-8- and QSI-10-treated chickens clustered together (Fig. S2B).
QSI-5 has no/minimal impact on the gut microbiota, QSI-10 significantly increased Lactobacillus, and QSI-8 significantly increased Butyricicoccus abundance.
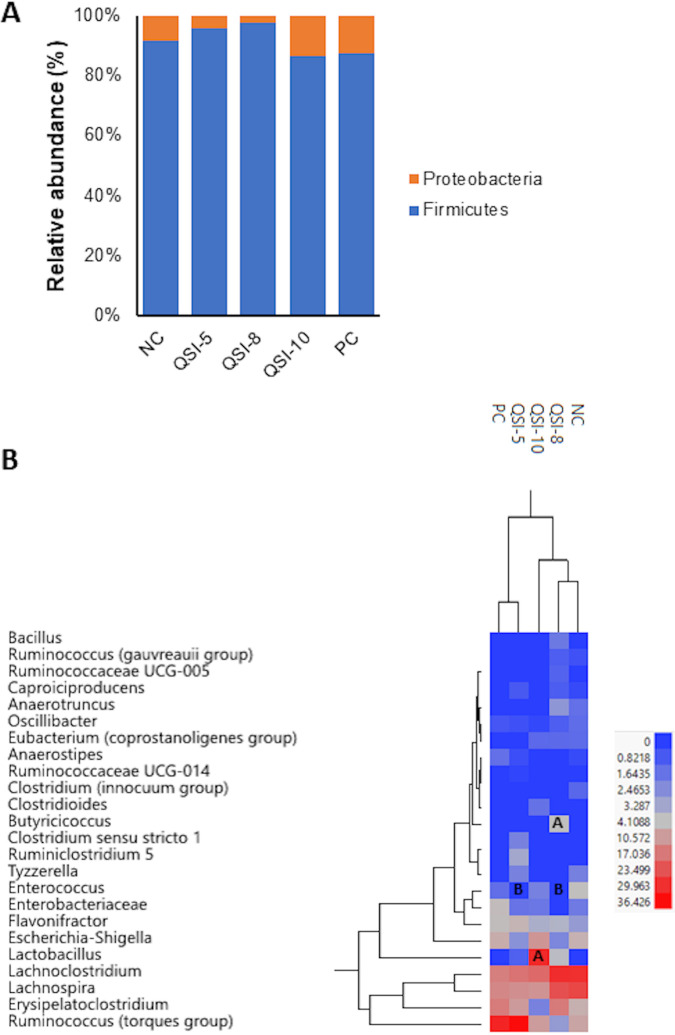
The alpha diversity analysis showed no difference in the phylogenetic diversity (P = 0.3; H = 5.1), richness (P = 0.1; H = 7.4), and evenness (P = 0.22; H = 5.7) of the gut microbiota between treated (QSI-5, QSI-8, and QSI-10) groups compared with both NC and PC (P <0.05) (Fig. S3A). Further, there was no spatial separation observed in the cecal microbiota of the QSIs-treated groups compared to the PC and NC groups when the principal coordinates analysis (PCoA) was performed using the unweighted uniFrac data (P <0.05) (Fig. S3B). Firmicutes was the most abundant bacterial phylum present in all chicken groups (87.3% to 95.7%) followed by Proteobacteria (4.3% to 13.7%). Interestingly, the treatment of chickens with QSI-5, QSI-8 and QSI-10 did not cause significant alterations in the gut microbiota compared to the NC or PC group at the phylum level (P > 0.05). Only QSI-5 and QSI-8 increased the Firmicutes (91.4% to 95%); whereas QSI-10 increased the Proteobacteria abundance (8.6% to 13.7%) compared with the NC group; however, this increase was not significant (Fig. 3A). Treatment of chickens with QSI-5 increased the abundance of Ruminococcus (torques group) (9% to 6%), Flavonifractor (2.9% to 6%), Lactobacillus (0% to 1.1%), Clostridium sensu stricto 1 (0% to 2.1%), Ruminiclostridium 5 (0%to 3.3%), and Erysipelatoclostridium (7.2% to 10.8%); while reduced Enterococcus (4% to 0%; P <0.05) compared with the NC group. Treatment of chickens with QSI-8 increased the abundance of Butyricicoccus (0% to 4.3%; P > 0.05), Bacillus (0% to 1.8%), Lactobacillus (0% to 4%), Erysipelatoclostridium (7.2% to 16.6%); while reduced Enterococcus (4.3% to 0%; P > 0.05) compared with the NC group. The high abundance of all the aforementioned genera explained the high abundance of phylum Firmicutes in the cecum of the QSI-5- and QSI-8-treated groups (Fig. 3B). Notably, treatment of chickens with QSI-10 significantly increased Lactobacillus (0% to 31%) abundance compared with the NC and PC groups (P <0.05) which explained the high abundance of phylum Firmicutes, while the increased abundance of Escherichia-Shigella (6.7% to 11.9%) explained the high abundance of phylum Proteobacteria (Fig. 3B).
FIG 3.
Relative abundance of gut microbial community at (A) phylum and (B) genus levels in QSI-treated chickens compared with the NC and PC groups. *Significant difference between treated chickens (P < 0.05) and the control groups. The heat maps generated using JMP PRO 13 software (SAS Institute). The letters A and B on the heat map indicate whether the OTUs were significantly increased or decreased, respectively, compared with the PC group (P < 0.05).
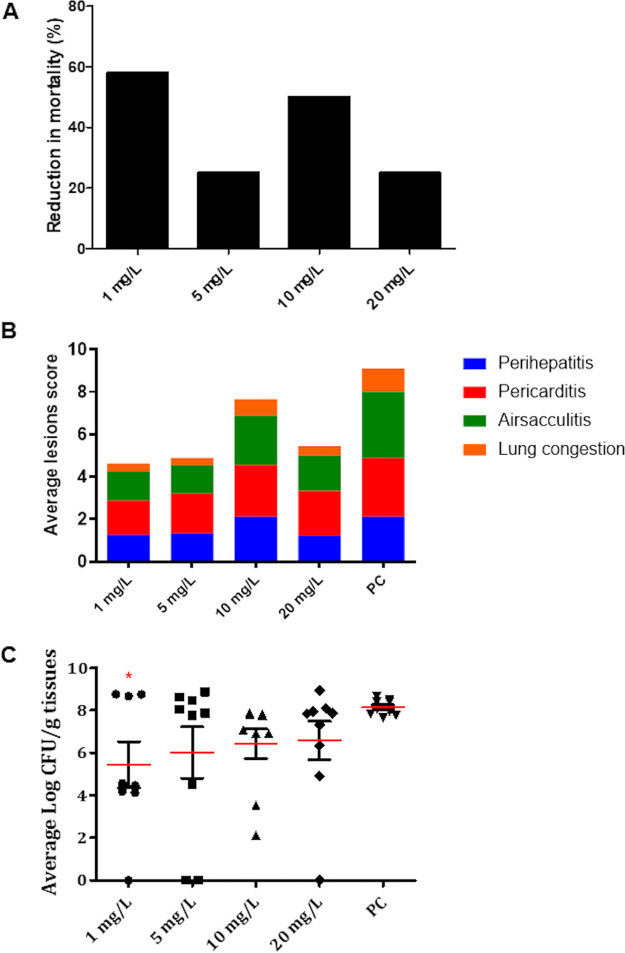
The optimal therapeutic dose of QSI-5 for treatment of APEC-infected chickens is determined to be 1 mg/L.
We optimized the dose of QSI-5 for drinking water delivery in chickens, a common industry practice, given that QSI-5 showed the best anti-APEC activity in chickens when administered by oral gavage in the pilot experiment above. Our results showed that treatment of chickens with 1 mg/L of QSI-5 reduced the mortality by 58%; while treatment with 5 mg/L, 10 mg/L, and 20 mg/L reduced the mortality by 25%, 50%, and 25%, respectively, compared with the PC group. The mortality observed in the PC group (60%) was normalized to 100% (Fig. 4A). Mortality details in QSI-treated and control groups are shown in Table S2 and Fig. S1B. Additionally, the reduction in pathological lesions (perihepatitis, pericarditis, airsacculitis, and lung congestion) in the 1 mg/L treated group ranged between 40.8% to 70%, which was higher than the reduction observed in the 5 mg/L (32% to 66.3%), 10 mg/L (0% to 30%), and 20 mg/L (24% to 60%) groups compared with the PC group (Fig. 4B). Similarly, APEC load in the internal organs (liver, heart, lung, kidney) of the 1 mg/L treated group was significantly (P < 0.05) reduced by approximately 2.3 to 3.1 logs CFU/g of tissues compared to the PC group; while the reduction ranged between (1.7 to 2.4), (1 to 2.6), and (1.3 to 1.9) logs CFU/g of tissues in 5 mg/L, 10 mg/L, and 20 mg/L treated groups, respectively, compared with PC group (Fig. 4C). Details about the average APEC load reduction and average lesion severity reduction in each organ in QSI-5-treated chickens at 1 mg/L, 5 mg/L, 10 mg/L, and 20 mg/L are shown in Table S2. The nature of this unexpected dose-response relationship is not clear but could conceivably be influenced by competing alternative mechanisms of action at higher concentrations.
FIG 4.
Effect of QSI-5 at 1 mg/L, 5 mg/L, 10 mg/L, and 20 mg/L on (A) chicken's mortality, (B) APEC lesion severity in the internal organs (liver, heart, airsacs, and lung), and (C) APEC load in the internal organs (liver, lung, heart, and kidney). QSI-5 was administered continuously in drinking water for 7 days and chickens were infected with APEC using s/c route. The average lesion score and APEC load was calculated, and the data were presented as an average of all the organs in each chicken and cumulative lesions score for each group, respectively. *Significant difference between treated and the PC group (P < 0.05) groups.
QSI-5 possessed higher anti-APEC efficacy than antibiotic SDM in chickens.
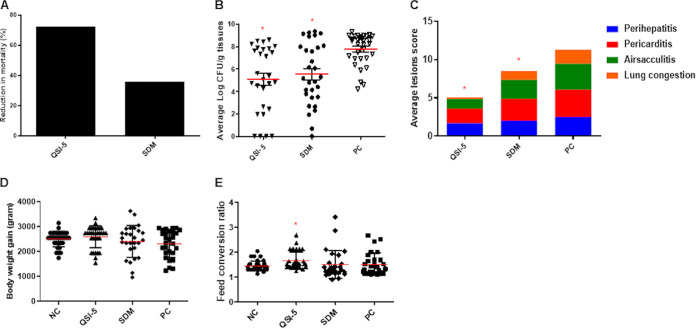
We used the optimized dose of QSI-5 (1 mg/L) and therapeutic dose of SDM (0.05%/495.3 mg/L) to compare the efficacy of QSI-5 with SDM in commercial broiler chickens raised on built-up floor litter. Chickens treated with QSI-5 showed 72.2% reduction in mortality compared with the PC group; whereas a 35.9% reduction was observed in the SDM-treated group (Fig. 5A). Mortality details in the QSIs-treated and control groups are shown in Table S3 and Fig. S1C. In the QSI-5-treated group, APEC load in internal organs was reduced by 2.3 to 2.8 logs CFU/g of tissues compared with the PC group, depending on the organ; while the reduction was 1.9 to 2.4 logs CFU/g of tissue in SDM-treated group (Fig. 5B). Notably, on day 42, no APEC was recovered from any organs. Additionally, the reduction in APEC lesion severity in the QSI-5-treated group was 33.3% to 88.4%, which were higher than those observed in the SDM-treated group (from 19.2% to 36.9% reduction), respectively (Fig. 5C). Details about the average APEC load reduction and average lesion severity reduction in each organ of QSI-5- and SDM-treated groups are shown in Table S3. Further, body weight gain (BWG) and feed conversion ratio (FCR) were increased in the QSI-5-treated group (BWG: 2,659.1 g; FCR: 1.48) compared with the NC (BWG: 2,501.2 g; FCR: 1.46) and SDM-treated (BWG: 2,408.4 g; FCR: 1. 46) groups (Fig. 5D and E); however, this increase was not significant. Details about weekly BWG and FCR are shown in Table S4 and S5.
FIG 5.
Comparing the efficacy of QSI-5 and sulfadimethoxine on (A) chicken’s mortality, (B) APEC lesion severity in the internal organs (liver, heart, airsacs, and lung), (C) APEC load in the internal organs (liver, lung, heart, and kidney), (D) body weight gain, and (E) feed conversion ratio. QSI-5 and sulfadimethoxine were administered continuously in drinking water for 7 days and chickens were infected with APEC using s/c route. The average lesion score and APEC load was calculated, and the data were presented as an average of all the organs in each chicken and cumulative lesions score for each group, respectively. *Significant difference between treated and control chicken (P < 0.05) groups.
QSI-5 was rapidly absorbed into chickens’ blood and no residues were detected in the chicken tissues.
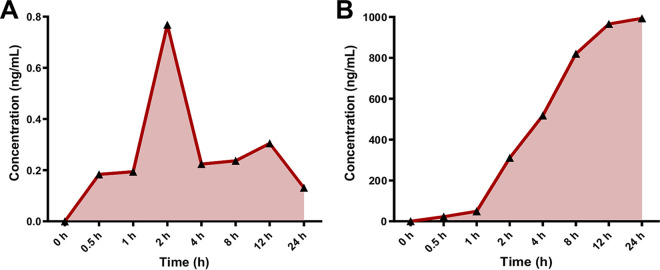
The pharmacokinetic (PK) profile of QSI-5 in plasma and its residue in different tissues was analyzed using liquid chromatography-mass spectrometry (LC-MS). The PK profile was measured (five chickens/group) at each time point (0 h, 0.5 h, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h) after administration of QSI-5 and SDM. Our results showed that QSI-5 was rapidly absorbed (0.5-h posttreatment [HPT]), reached the peak concentration at 2 HPT with a short half-life, and was excreted by 24 HPT (Fig. 6A), while the absorption of SDM was slower (2 HPT) and peak concentration was reached at 24 HPT (Fig. 6B). Additionally, the maximum concentration of QSI-5 in plasma (Cmax) values (0.76 ng/ML) was observed at 2 HPT, whereas the Cmax of SDM (933.6 ng/ML) was observed at 24 HPT.
FIG 6.
Pharmacokinetic profile of (A) QSI-5 and (B) sulfadimethoxine in chicken’s plasma. Data were analyzed using LC-MS. The PK profile was measured in five chickens per group at different time points (0 h, 0.5 h, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h) after administration of the drugs.
Further, the safety of QSI-5 was demonstrated by measuring the level of drug residue in the muscle, liver, and kidney. Tissue samples (five chickens/group) were collected at 2-day post last treatment (DPLT), 5 DPLT, and 35 DPLT (slaughter age). Interestingly, no QSI-5 residue (0 ppm) in the muscle, liver, and kidney of treated chickens was detected at all time points. On the contrary, SDM residue was 0.17 ppm, 0.15 ppm, and 0.99 ppm at 2 DPLT and 0.0 ppm, 0.04 ppm, and 0.09 pp at 5 DPLT in muscle, liver and kidney, respectively. At 35 DPLT, no detectable residues of SDM were observed in any tissues (Table 2).
TABLE 2.
Drug accumulation in the kidney, liver, and muscle in QSI-5- and sulfadimethoxine-treated groupsa
| Treatment group | Time point | Muscle (ppm± SD) | Liver (ppm± SD) | Kidney (ppm± SD) |
|---|---|---|---|---|
| QSI-5 | 2 DPLT | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 5 DPLT | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| 35 DPLT (slaughter age) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Sulfadimethoxine | 2 DPLT | 0.17 ± 0.17 | 0.15 ± 0.04 | 0.99 ± 0.15 |
| 5 DPLT | 0.02 ± 0.02 | 0.04 ± 0.02 | 0.09 ± 0.05 | |
| 35 DPLT (slaughter age) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
DPLT, day post last treatment.
DISCUSSION
Previously, we identified novel quorum sensing inhibitors (QSI) that did not affect the growth of APEC, but impacted the QS- regulated processes and showed promising efficacy against APEC infections in vitro (17). Using QSIs is an important approach to attenuate APEC pathogenicity and reduce the probability of development of resistant APEC strains. Interestingly, out of the seven QSIs tested in our study, two QSIs (QSI-5 and QSI-10) showed promising anti-APEC efficacy in chickens by (i) reducing the mortality up to 100% (Fig. 2A); (ii) reducing APEC load in the internal organs up to 6 logs (Fig. 2B); (iii) minimizing the pathological lesion severity in the internal organs up to 100% compared with the PC group (Fig. 2C); and (iv) maintaining body weight gain consistent with the NC group (Fig. 2D). Both QSI-5 and QSI-10 belong to the same chemical class with phenyl piperazinyl functional groups (17). Previously, amoxicillin and surfactin combination has been reported to reduce the mortality and bacterial loads in the liver of 1-day-old broiler chickens infected subcutaneously (s/c) with APEC and vaccinated with Marek’s disease vaccine (18), while oxytetracycline, trimethoprim-sulfadimethoxine, and enrofloxacin have been reported to reduce mortality and pathological lesions severity in 1-day-old chickens infected with APEC via airsac and vaccinated using infectious bronchitis vaccine (19). Similarly, ciprofloxacin has been reported to reduce mortality and APEC load in the liver of 1-day-old broilers infected orally with APEC O157 (20). Our study suggests that QSIs can be promising lead compounds to control the mortality and carcass condemnation due to APEC infection in poultry flocks without affecting the production performance. The challenge model used in this study was the s/c route using 107 CFU/bird of APEC, which is considered as an acute infection model resulting in rapid progression of disease and high mortality (21). However, in the field conditions, chickens are exposed to much lower APEC (102 to 103) doses via oral or aerosol routes and the APEC infection develops slowly (21, 22). Therefore, these QSIs (QSI-5 and QSI-10) might be more effective in controlling APEC infection in chickens, if they are applied in field simulated settings using the oral (natural) route of infection, or when they are modified to improve oral bioavailability in chickens.
Oral administration of antimicrobials has been shown to affect the gut microbial community and immune responses (23, 24). Gut microbiota protects the host from pathogenic bacteria colonization and serve as source of amino acids, vitamins, enzymes, and short chain fatty acids to the host (25, 26). The misuse of antibiotics may affect the abundance of microbial species, microbial diversity, leading to increased susceptibility to pathogenic bacteria (27). This alteration might result in inflammation of the gut mucosal epithelium and subsequently mucosal colonization by pathogens (28). In this study, QSIs did not cause an alteration in the diversity of the gut microbial community. Treatment of chickens with QSI-5 and QSI-8 increased the Firmicutes (91.4% to 95%); whereas QSI-10 increased the Proteobacteria (8.6%-13.7%) compared with the NC group (Fig. 3A). Similar results were previously obtained in the gut microbiota of chickens after administration of antibiotics, small molecules, peptides, and probiotics (29–35). Previous reports have shown that increased Firmicutes abundance in the gut positively correlated with feed efficiency and chicken’s performance (36, 37). Therefore, we suggest that increase in the chicken’s body weight in QSI-5-, QSI-8-, and QSI-10-treated groups (Fig. 2D) might be due to the high abundance of Firmicutes population in the cecum. Notably, QSI-8 and QSI-10 significantly increased the abundance of Butyricicoccus (>4 folds) and Lactobacillus (>30 folds) genera in the gut (P < 0.05), respectively (Fig. 3B). Lactobacillus plays a role in enhancing innate and adaptive immunity, attenuating the inflammatory processes, and inhibiting pathogens growth (38, 39). Further, QSI-8 increased butyrate-producing bacteria, Butyricicoccus which plays a role in cell permeability and intestinal barrier functions (40). Previously, B. pullicaecorum has been reported to reduce Salmonella, Campylobacter, and Clostridium perfringens infections in chickens (41, 42). In a similar study, small molecules treatment increased the abundance of Butyricicoccus in the gut of chickens infected with Salmonella (30). We suggest that the anti-APEC activity of QSI-8 and QSI-10 may be enhanced by their growth-promoting effect on Butyricicoccus and Lactobacillus, respectively.
Interestingly, QSI-5 reduced levels of 5-methylthioadenosine (MTA) up to 7 folds (Table 1). The Methylthioadenosine/S-adenosylhomocysteine nucleosidase (MTA/SAH or MTAN) is involved in the AI-2 methylation cycle. MTAN is involved in 5′-methylthioadenosine recycling to S-adenosylmethionine (43) and inhibition of MTA/SAH resulted in suppression of AI-2 and subsequently reduced biofilm formation and bacterial virulence (44). The MTA/SAH or MTAN nucleosidase inhibitors were reported to be effective against Borrelia burgdorferi (45), Helicobacter pylori (43), Mycobacterium tuberculosis (46), and E. coli (47). Therefore, we suggest that the anti-quorum sensing efficacy of QSI-5 might be attributed to its inhibitory effect on the 5′-methylthioadenosine; however, additional studies are necessary to confirm this hypothesis and define the mechanisms of action of QSI-5. Additionally, the treatment of chickens with QSI-8 significantly increased the abundance of 9,10-DiHODE, a member of the class of linoleic acids, by 3.6 folds (Table 1). Linoleic acids are involved in lipid transport, lipid metabolism, and fatty acid metabolism. Several strains of gut bacteria have the ability to metabolize linoleic acids including Lactobacilli, Lactococcus, Propionibacteria, Bifidobacteria, Faecalibacteria, Eubacteria, Anaerostipes, Roseburia, Clostridium, and Butyrivibrio (48). Previously, it has been reported that Gamma-linolenic acid has anti-inflammatory effects on broiler chickens’ gut (49). Further, Lactobacillus strains producing conjugated linolenic acid have efficacy against enterohemorrhagic E. coli (50). The deficiency of linoleic acid in the diet of young chickens leads to reduced growth rate, enlarged liver, and reduced resistance to respiratory infections, while the deficiency of linoleic acid in laying hens results in reduced egg production, laying of small sized eggs, and reduced fertility and hatchability (51). We suggest that the increased level of linoleic acids in chicken’s serum might be due to the high abundance of butyrate-producing bacteria such as Butyricicoccus and Lactobacillus in chicken’s gut that is caused by QSI-8 (Fig. 3B).
In our study, QSI-5 possessed higher anti-APEC efficacy compared with SDM when administered at 1 mg/L in drinking water in a field simulated condition. QSI-5 demonstrated a 72.2% reduction in mortality, up to 2.8 logs reduction in APEC load, and up to 88.4% reduction in lesion severity (Fig. 5). The QSI-5 showed better efficacy even at a dose lower than SDM, an antibiotic used to treat APEC infections in poultry (19, 52). Further, QSI-5 was absorbed quickly into the blood circulation (at 0.5 HPT) and reached the peak concentration after 2 HPT. QSI-5 also showed good aqueous solubility, based on the observations during dosing in drinking water. These properties indicate that QSI-5 might have high bioavailability compared with SDM (53). No accumulated QSI-5 residues were detected in the edible tissues of chickens (Table 2), suggesting safety of the treated chickens for human consumption (54). Notably, the dose of QSI-5 (1 mg/L) is very low (up to 4,500 times) compared with the doses of antibiotics that are commonly used in the poultry industry to treat APEC such as SDM (495 mg/L), chlortetracycline (4.5 g/L), ampicillin (1.65 g/L), and sulfaquinoxaline (200 mg/ L) (55). This has a significant importance in terms of treatment costs and accumulation of drug residues in chickens tissues which is crucial for the safety of food for human consumption (56). Interestingly, increasing the dose of QSI-5 did not result in better anti-APEC efficacy in chickens. Furthermore, resistance to QSI-5 is less likely to occur as it does not affect the growth of APEC. Therefore, QSI-5 can be developed as an alternative to the current treatments for APEC infections in poultry in the field. Though, previously we have shown that these QSIs including QSI-5 are effective against multiple APEC serotypes in vitro (17), further studies are needed to demonstrate the effect of QSI-5 in chickens infected with other APEC serotypes that are implicated in colibacillosis.
In summary, our studies showed that QSI-5 is a promising novel anti-APEC therapeutic. QSI-5 showed the best anti-APEC efficacy among other tested QSIs in chickens with an optimal dose of 1 mg/L. Further, QSI-5 possessed higher anti-APEC efficacy compared with SDM in infected chickens with no impact the BWG, FCR, and cecal microbiota of the treated chickens. Further, there were no detectable residues in muscle, liver, and kidney. Our future studies will focus on testing QSI-5 in APEC-infected chickens using the natural route of infection in a field simulated conditions, improving the QSI-5 efficacy using medicinal chemistry, and elucidating the mechanisms of action of QSI-5. Furthermore, APEC shares genetic similarity to human ExPECs; therefore, our findings will have implications for developing novel antibacterials against human ExPEC infections.
MATERIALS AND METHODS
Ethics statement.
All the experimental procedures were carried out in accordance with approved Ohio State University Institutional Animal Care and Use Committee (IACUC) guidelines under protocol number 2010A00000149. The experiments were conducted according to approved husbandry practices.
Bacterial inoculums and culture conditions.
APEC O78 (GenBank accession no. CP004009) (Tim Johnson, University of Minnesota, Saint Paul, MN, USA) (57). Rifampicin resistant APEC O78 (RifR) was isolated on Luria-Bertani (LB) agar (Sigma-Aldrich, Inc. MO, USA)containing 50 μg/mL of rifampicin (EMD Millipore, USA) (58). For the preparation of bacterial inoculums, APEC O78 (RifR) was grown overnight in LB media containing 50 μg/mL of rifampicin at 37°C with shaking at 200 rpm. The bacteria were then diluted 1:100 in fresh LB broth and was incubated with shaking at 200 rpm at 37°C for 3 h. Logarithmic phase grown culture of RifR APEC O78 (OD600 ~ 0.5) was washed twice with PBS and adjusted to the required concentration (OD600 = 0.1).
Efficacy of QSIs in APEC-infected chickens.
The chicken experiment was carried out using 1-day-old broiler chickens (Mayer Hatchery, OH, USA). Feed and water were given ad libitum. Chickens (n = 6/group) were administered with the QSIs (QSI-1, QSI-2, QSI-5 − 8, QSI-10, ChemBridge, San Diego, CA); previously designated as C1, C2, C5, C6, C7, C8, C10 (17) using oral gavage. These compounds were selected based on their high efficacy in in vitro studies and the doses correspond to 30X of the initial in vitro screening concentration (17). The chemical structures of the QSIs used in this study are shown in Fig. 1. The QSIs were suspended in dimethyl sulfoxide (DMSO; used as a vehicle) and administered once daily for 5 successive days (starting from day 4 to day 8). The first dose was administered 1 day before the challenge, followed by the second dose on the challenge day (2 h before challenge), followed by three additional doses on subsequent days. Feeders were removed 1 h prior to the treatment and replaced 1 h after the treatment. The dose used for each QSI is shown in Table 3. Chickens were challenged subcutaneously (s/c) on day 5 with RifR APEC O78 (1 × 107 CFU/bird) in PBS using syringe (27 gauge, 0.5 in.). The challenge dose, which reduced mortality by 50%, was chosen based on preliminary experiments conducted with different infection routes (subcutaneous s/c, intra-tracheal and intra-airsacs) and different doses (106, 107, and 108 CFU/chicken). Chicken group infected s/c with 107 CFU/bird possessed clear APEC colonization in liver, heart, lung, and kidney. Therefore, we selected this challenge dose and route for evaluation of QSIs in chickens (34). The positive (PC; infected with APEC O78 and DMSO treated) and negative (NC; non-infected and non-treated) control groups were included.
TABLE 3.
Treatment groups and the dose of each AI-2 inhibitor
| Groups | SM dose (μg/bird) | APEC O78 |
|---|---|---|
| QSI-1 | 116.4 | Yes |
| QSI-2 | 128 | Yes |
| QSI-5 | 92.6 | Yes |
| QSI-6 | 107.2 | Yes |
| QSI-7 | 121.89 | Yes |
| QSI-8 | 100.32 | Yes |
| QSI-10 | 122.28 | Yes |
| Positive control (PC) | DMSO | Yes |
| Negative control NC) | None | No |
Chickens were monitored for clinical signs for 8 DPI. Any chickens moribund during this period were humanely euthanized and necropsied to determine bacterial load in the internal organs (liver, heart, lung, and kidney). Internal organs were aseptically collected, weighed and suspended in 1× PBS, the amount of PBS added was adjusted based on the organ size to make a suspension, the organ size and amount of PBS added were included in the final calculation of the CFU. The samples were homogenized, serially diluted 10-fold, plated on MacConkey agar (Remel, CA, USA) containing 50 μg/mL rifampicin, and incubated at 37°C for 24 h to determine the CFU/g. Pathological lesions severity in internal organs (pericarditis, perihepatitis, airsacculitis, and lung congestion) was scored as described previously (21, 59). At 8 DPI, the remaining chickens were humanely euthanized and necropsied, lesions were scored, and the APEC load was quantified in internal organs as described above.
Quantification of serum metabolites.
In order to determine the effect of the QSI treatment on the metabolites present in chicken’s serum, untargeted metabolomic profiling was carried out using LC-MS as described before (34, 60). Blood was collected from the treated chicken groups that showed efficacy against APEC (QSI-5, QSI-8, and QSI-10-treated groups) and control groups (PC and NC), serum was separated and stored at −80°C for LC-MS analysis. Serum protein was precipitated by mixing cold methanol at −20°C for 30 min. The protein was then removed by centrifugation at 13,000 × g for 30 min. Five μL of the supernatant was transferred to glass vials for LC-MS analysis (2 to 3 runs for each sample). For quality control, equal portions of serum samples from QSIs-treated and control groups were pooled and run for LC-MS analysis every eight sample runs. Samples were run on a Thermo Orbitrap LTQ XL in positive mode analysis with high-performance liquid chromatography (HPLC) separation in a Poroshell 120 SB-C18 (2 × 100 mm in diameter, 2.7 μm particle sizes) columns using Thermo Fisher Scientific RLCS Ultimate 3000 LC system. The data analysis was performed in two separate groups (NC, QSI-5, PC) and (NC, QSI-8, QSI-10, PC). The metabolites were analyzed at the Campus Chemical Instrumentation Center, Mass Spectrometry and Proteomics Facility (CCIC, MS&PF), The Ohio State University (https://www.ccic.osu.edu/MSP). Progenesis QI (http://www.nonlinear.com/progenesis/qi/) and XCMS Online (https://xcmsonline.scripps.edu/) were used to identify the metabolites, retention time correction, feature detection, alignment, annotation, statistical analysis, and data visualization. The samples were aligned with a score of ≥ 88% and database matching was performed using the Human Metabolome Database, selecting for adducts M+H, M+Na, M+K, and M + 2H and <10 ppm mass error. Human metabolome database was used since no chicken metabolome database is available.
Effect of the QSIs on the gut microbiota of chickens.
To determine the effect of the QSIs on the gut microbiota, metagenomic analysis targeting 16S rRNA was conducted as described previously (32). Genomic DNA extracted only from the cecum of the treated groups that possessed high efficacy against APEC (QSI-5, QSI-8, and QSI-10) and control groups (PC and NC) were analyzed. Quantitative Insights Into Microbial Ecology (QIIME 2) bioinformatics platform (61) was used for metagenomic analysis. DADA2 was used to create the feature table and make additional sequence filtering (62). The SILVA classifier was used for the taxonomy analysis, and the align-to-tree-mafft-fast tree pipeline was used for phylogenetic diversity analysis. The core-metrics-phylogenetic pipeline was used to analyze the alpha (Shannon’s diversity) and the beta diversity (Bray-Curtis distance).
Optimization of the therapeutic dose of QSI-5 in drinking water.
QSI-5 showed the best activity against APEC when administered to chickens orally in the pilot study; therefore, the dose of QSI-5 was optimized for delivery in drinking water. One-day-old broiler chickens (n = 10/group) (Case Farms Ohio Hatchery, Strasburg, OH, USA) were used in this experiment. QSI-5 was synthesized in-house in the Fuchs laboratory (College of Pharmacy, OSU) via reductive amination reaction of commercially available 3-phenylpropionaldehyde with 1-(4-methylbenzyl) piperazine in the presence of acetic acid and sodium triacetoxyborohydride (Fig. S4). QSI-5 was administered daily in drinking water containing 0.05% DMSO at doses of 1 mg/L, 5 mg/L, 10 mg/L, and 20 mg/L for seven consecutive days starting from day 4 to day 10 of age. The amount of drinking water given daily was calculated based on the age of chickens (http://www.poultryhub.org/nutrition/nutrient-requirements/water-consumption-rates-for-chickens/). Chickens were infected with Rifr APEC O78 (5 × 106 CFU/chicken) on day 5 as described above. The PC (0.05% DMSO treated and infected) and NC (non-treated and non-infected) control groups were included. The clinical signs and the daily mortality were recorded for 8 DPI. Dead chickens were necropsied, and APEC load was quantified in the internal organs as mentioned above. At 8 DPI, the remaining chickens were necropsied, lesions were scored, and the APEC load was quantified in internal organs as described above.
Comparative efficacy of QSI-5 and SDM in field simulated conditions.
To compare the efficacy of QSI-5 with an antibiotic SDM currently used in the field, chickens (n = 70) were raised on built-up floor litter in a field-simulated conditions. One-day-old broiler chickens (Case Farms Ohio Hatchery, OH, USA) were used for conducting the experiment. The optimized dose of QSI-5 (1 mg/L) and the therapeutic dose of SDM (495.323 mg/L) (0.05%) were given in drinking water daily for 7 days (starting from day 5 to day 11 of age). The volume of drinking water needed daily was determined as described above. On day 6, the chickens were infected s/c with Rifr APEC O78 (5 × 106 CFU/chicken, s/c). PC (0.05% DMSO treated and infected) and NC (non-infected and non-treated) control groups were included. The mortality was recorded daily until the end of the experiment (42 days of age: slaughter age). At 8 DPI, half of the chickens from each group were necropsied, lesions were scored, and the APEC load was quantified in the internal organs as described above. The other half of the chickens were raised until day 42 to determine the impact of treatment on the body weight gain and feed intake. To calculate the FCR, body weight was measured once every week and feed intake was recorded every day. On day 42, the remaining chickens were euthanized, body weight was measured and 10 chickens from each group were randomly selected and necropsied, lesions and APEC load were assessed in the internal organs.
PK profile of QSI-5 and SDM.
The amount of QSI-5 and SDM in chicken plasma was measured using LC-MS. The optimized dose of QSI-5 (1 mg/L) and the therapeutic dose of SDM (495.323 mg/L) (0.05%) were administered orally as a single dose. Blood was collected individually from five chickens per group at different time points (0 h, 0.5 h, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h) after administration of QSI-5 and SDM in vacutainer EDTA (10.8 mg) tubes (Becton, Dickinson, NJ, USA), blood was placed on ice for 1 h to clot, plasma was separated by centrifuging at 2,000 × g for 10 min at 4°C and stored at −80°C °C for LC-MS analysis. Plasma protein was precipitated by adding cold methanol and 10 μL (1 μg/mL) of an internal standard (IS) heavy-labeled phenylalanine (dissolved in 0.1% formic acid) was added to each 100 μL aliquot. The mixture was then incubated at −20°C for 30 min and centrifuged at 1300 × g for 25 min at 2°C. Sixty μL of the supernatant was pipetted into LC vials. For standard calibration, solutions of 0.0, 0.001, 0.005, 0.01, 0.05, 0.1, 0.5, 1, and 5 μg/mL of QSI-5 and SDM were prepared and 20 μL of each standard solution was added to 80 μL of cold methanol and IS as mentioned above. Calibration standard curves for QSI-5 and SDM are shown in Fig. S5A and S5B. All samples were run by injecting 5 μL on an Agilent Poroshell 120 SB-C18 (2 × 100 mm in diameter, 2.7 μm particle sizes). The LC system (Thermo Fisher Scientific UltiMate 3000 HPLC) was used with solvent A (10 mM ammonium formate and 0.1% formic acid) and solvent B (methanol with a flow rate of 200 μL/min). The gradient was set to 2% B at 2 min, increased from 2% to 20% at 5 min, 40% at 7.5 min, and reached 90% after 9 min. The gradient was sustained at 90% B for 11 min and then decreased to 2% at 12 min and was held for equilibration until the run ended after 15 min. The mass spectrometer (Thermo Fisher Scientific Quantiva Triple Quadrupole) was set to multiple reactions monitoring mode (SRM) with a heated electrospray ionization source (ESI) in positive mode at 3.5 kV. Drug targets were monitored for QSI-5 at transitions 309.26→105.1 m/z and 309.26→203.15 m/z at 28 and 20 V collision energy, respectively, and for SDM at transitions 311.11→156.11 m/z and 311.11→245.07 m/z at 21 and 18 V CE. The analysis of plasma samples was performed in Campus Chemical Instrumentation Center, Mass Spectrometry and Proteomics Facility (CCIC, MS&P), The Ohio State University (https://live-ccic.pantheonsite.io/MSP).
QSI-5 and SDM residue quantification in muscle, kidney, and liver.
The safety of QSI-5 and SDM was assessed by measuring the level of drug residue in the muscle, kidney, and liver using LC-MS (CCIC, MS&P Facility, OSU) as described before (34). As described above, chickens were administered with the optimized dose of QSI-5 (1 mg/L) and the therapeutic dose of SDM (495.323 mg/L) (0.05%) in drinking water daily for 7 days (starting from day 5 to day 11 of age). Tissue samples were collected individually from chickens treated with QSI-5 and SDM (five chickens per group) from experimental set up as described for floor trial at 2 DPLT, 5 DPLT, and 35 DPLT (slaughter age). All samples were weighed and extracted at a 400 mg/mL ratio of tissue in the extraction solution (50:50 H2O: ACN). Samples were homogenized using a probe sonicator 20 times and centrifuged at 13,000 × g for 30 min. Sixty μL of the supernatant was transferred into glass vials and dried in a SpeedVac for 1.5 h, and then resuspended in 120 μL of 25:25:50 H2O:ACN:MeOH. Standard calibration, LC system, mass spectrometer, and drug target monitoring were performed as described above. Calibration standard curves for measuring the accumulation of QSI-5 and SDM in the kidney, liver, and muscle are shown in Fig. S6A and S6B.
Statistical analysis.
Statistical analyses were conducted using ANOVA and the Tukey test in the GraphPad Prism 5 software (GraphPad, Inc., CA, USA). Differences in lesion scores, APEC load, BWG, and FCR between treatment and control groups were analyzed using the Mann-Whitney U test and the Kruskal-Wallis test. Differences in the OTU relative abundance between the treated and control groups were calculated using the Mann-Whitney U test. The alpha diversity was assessed using permutational multivariate analysis of variance (PERMANOVA) and the Kruskal-Wallis test. Statistically significant differences between means were determined using A P-value < 0.05. The statistical analysis of the metabolite intensity data was performed using JMP Pro14 and vegan package on Rstudio (SAS institute Inc., NC, USA). Distribution of the metabolites profile for each chicken was visualized using PCA. PERMANOVA was used to determine whether significant spatial distribution was observed between the chicken groups. Wilcoxon rank-sum test was used to identify intensity differences between QSI groups and the PC group for a designated metabolite (63). A threshold of P-value 0.001 was used to select the metabolites of interest.
Data availability.
Data from the study are included in this article and in the supplementary files. Microbiome sequence data have been deposited in the BioProject database under accession number PRJNA766869.
ACKNOWLEDGMENTS
We thank Wilbur Ouma for the assistance with the bioinformatic analysis. We thank Matthew Bernier for the assistance with the metabolomic analysis. We thank Juliette Hanson, Megan Strother, and Sara Tallmadge for assistance with chicken experiments. The research in Rajashekara laboratory is supported by the U.S. Department of Agriculture (USDA), National Institute for Food and Agriculture (NIFA) (grants number 2015-68004-23131 and 2020-6701-31401), the Technology Commercialization Office (TCO) of The Ohio State University, and the Ohio State Innovation Foundation.
We declare no competing interests.
Footnotes
Supplemental material is available online only.
Contributor Information
Gireesh Rajashekara, Email: rajashekara.2@osu.edu.
Adelumola Oladeinde, USDA-ARS.
REFERENCES
- 1.Ghunaim H, Abu-Madi MA, Kariyawasam S. 2014. Advances in vaccination against avian pathogenic Escherichia coli respiratory disease: potentials and limitations. Vet Microbiol 172:13–22. doi: 10.1016/j.vetmic.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 2.Barnes HJ, Nolan LK, Vaillancourt JF, 2008. Colibacillosis, p. 691–732. In Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE. (ed), Diseases of poultry. 12th ed. Blackwell Publishing, Ames, Iowa. [Google Scholar]
- 3.Guabiraba R, Schouler C. 2015. Avian colibacillosis: still many black holes. FEMS Microbiol Lett 362:fnv118. doi: 10.1093/femsle/fnv118. [DOI] [PubMed] [Google Scholar]
- 4.Markland SM, LeStrange KJ, Sharma M, Kniel KE. 2015. Old friends in new places: exploring the role of extraintestinal E. coli in intestinal disease and foodborne illness. Zoonoses Public Health 62:491–496. doi: 10.1111/zph.12194. [DOI] [PubMed] [Google Scholar]
- 5.Mellata M. 2013. Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog Dis 10:916–932. doi: 10.1089/fpd.2013.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tivendale KA, Logue CM, Kariyawasam S, Jordan D, Hussein A, Li G, Wannemuehler Y, Nolan LK. 2010. Avian-pathogenic Escherichia coli strains are similar to neonatal meningitis E. coli Strains and are able to cause meningitis in the rat model of human disease. Infect Immun 78:3412–3419. doi: 10.1128/IAI.00347-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutful Kabir SM. 2010. Avian colibacillosis and salmonellosis: a closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int J Environ Res Public Health 7:89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson TJ, Wannemuehler Y, Doetkott C, Johnson SJ, Rosenberger SC, Nolan LK. 2008. Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool. J Clin Microbiol 46:3987–3996. doi: 10.1128/JCM.00816-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Brito BG, Gaziri LCJ, Vidotto MC. 2003. Virulence factors and clonal relationships among Escherichia coli strains isolated from broiler chickens with cellulitis. Infect Immun 71:4175–4177. doi: 10.1128/IAI.71.7.4175-4177.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gyles CL. 2008. Antimicrobial resistance in selected bacteria from poultry. Anim Health Res Rev 9:149–158. doi: 10.1017/S1466252308001552. [DOI] [PubMed] [Google Scholar]
- 11.Zhao S, Maurer JJ, Hubert S, De Villena JF, McDermott PF, Meng J, Ayers S, English L, White DG. 2005. Antimicrobial susceptibility and molecular characterization of avian pathogenic Escherichia coli isolates. Vet Microbiol 107:215–224. doi: 10.1016/j.vetmic.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Nhung NT, Chansiripornchai N, Carrique-Mas JJ. 2017. Antimicrobial resistance in bacterial poultry pathogens: a review. Front Vet Sci 4:126. doi: 10.3389/fvets.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen RH, Bisgaard M, Löhren U, Robineau B, Christensen H. 2014. Extended-spectrum β-lactamase-producing Escherichia coli isolated from poultry: a review of current problems, illustrated with some laboratory findings. Avian Pathol 43:199–208. doi: 10.1080/03079457.2014.907866. [DOI] [PubMed] [Google Scholar]
- 14.Lima Barbieri N, Nielsen DW, Wannemuehler Y, Cavender T, Hussein A, Yan S-g, Nolan LK, Logue CM. 2017. mcr-1 identified in avian pathogenic Escherichia coli (APEC). PLoS One 12:e0172997. doi: 10.1371/journal.pone.0172997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linciano P, Cavalloro V, Martino E, Kirchmair J, Listro R, Rossi D, Collina S. 2020. Tackling antimicrobial resistance with small molecules targeting LsrK: challenges and opportunities. J Med Chem 63:15243–15257. doi: 10.1021/acs.jmedchem.0c01282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira CS, Thompson JA, Xavier KB. 2013. AI-2-mediated signalling in bacteria. FEMS Microbiol Rev 37:156–181. doi: 10.1111/j.1574-6976.2012.00345.x. [DOI] [PubMed] [Google Scholar]
- 17.Helmy YA, Deblais L, Kassem II, Kathayat D, Rajashekara G. 2018. Novel small molecule modulators of quorum sensing in avian pathogenic Escherichia coli (APEC). Virulence 9:1640–1657. doi: 10.1080/21505594.2018.1528844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Wang X, Shi W, Qian Z, Wang Y. 2019. Sensitization of avian pathogenic Escherichia coli to amoxicillin in vitro and in vivo in the presence of surfactin. PLoS One 14:e0222413. doi: 10.1371/journal.pone.0222413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dheilly A, Bouder A, Le Devendec L, Hellard G, Kempf I. 2011. Clinical and microbial efficacy of antimicrobial treatments of experimental avian colibacillosis. Vet Microbiol 149:422–429. doi: 10.1016/j.vetmic.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 20.EL-Sawah AA, Dahshan AM, El-Nahass E, Abd El-Mawgoud AI. 2018. Pathogenicity of Escherichia coliO157 in commercial broiler chickens. Beni-Suef University Journal of Basic and Applied Sciences 7:620–625. doi: 10.1016/j.bjbas.2018.07.005. [DOI] [Google Scholar]
- 21.Antão E-M, Glodde S, Li G, Sharifi R, Homeier T, Laturnus C, Diehl I, Bethe A, Philipp H-C, Preisinger R, Wieler LH, Ewers C. 2008. The chicken as a natural model for extraintestinal infections caused by avian pathogenic Escherichia coli (APEC). Microb Pathog 45:361–369. doi: 10.1016/j.micpath.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Dziva F, Stevens MP. 2008. Colibacillosis in poultry: unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol 37:355–366. doi: 10.1080/03079450802216652. [DOI] [PubMed] [Google Scholar]
- 23.Bhalodi AA, van Engelen TSR, Virk HS, Wiersinga WJ. 2019. Impact of antimicrobial therapy on the gut microbiome. J Antimicrob Chemother 74:i6–i15. doi: 10.1093/jac/dky530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Chen C, Indugu N, Werlang GO, Singh M, Kim WK, Thippareddi H. 2018. Effect of antibiotic withdrawal in feed on chicken gut microbial dynamics, immunity, growth performance and prevalence of foodborne pathogens. PLoS One 13:e0192450. doi: 10.1371/journal.pone.0192450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Der Wielen PW, Biesterveld S, Notermans S, Hofstra H, Urlings BA, van Knapen F. 2000. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl Environ Microbiol 66:2536–2540. doi: 10.1128/AEM.66.6.2536-2540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yadav S, Jha R. 2019. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J Anim Sci Biotechnol 10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Covington A, Pamer EG. 2017. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol Rev 279:90–105. doi: 10.1111/imr.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai Z, Zhang H, Li N, Bai Z, Zhang L, Xue Z, Jiang H, Song Y, Zhou D. 2016. Impact of environmental microbes on the composition of the gut microbiota of adult BALB/c mice. PLoS One 11:e0160568. doi: 10.1371/journal.pone.0160568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh P, Karimi A, Devendra K, Waldroup PW, Cho KK, Kwon YM. 2013. Influence of penicillin on microbial diversity of the cecal microbiota in broiler chickens. Poult Sci 92:272–276. doi: 10.3382/ps.2012-02603. [DOI] [PubMed] [Google Scholar]
- 30.Deblais L, Helmy YA, Kathayat D, Huang HC, Miller SA, Rajashekara G. 2018. Novel imidazole and methoxybenzylamine growth inhibitors affecting salmonella cell envelope integrity and its persistence in chickens. Sci Rep 8:13381. doi: 10.1038/s41598-018-31249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deblais L, Helmy YA, Kumar A, Antwi J, Kathayat D, Acuna UM, Huang HC, de Blanco EC, Fuchs JR, Rajashekara G. 2019. Novel narrow spectrum benzyl thiophene sulfonamide derivatives to control Campylobacter. J Antibiot (Tokyo) 72:555–565. doi: 10.1038/s41429-019-0168-x. [DOI] [PubMed] [Google Scholar]
- 32.Helmy YA, Kathayat D, Ghanem M, Jung K, Closs G, Jr, Deblais L, Srivastava V, El-Gazzar M, Rajashekara G. 2020. Identification and characterization of novel small molecule inhibitors to control Mycoplasma gallisepticum infection in chickens. Vet Microbiol 247:108799. doi: 10.1016/j.vetmic.2020.108799. [DOI] [PubMed] [Google Scholar]
- 33.Kathayat D, Closs G, Jr, Helmy YA, Deblais L, Srivastava V, Rajashekara G. 2021. In vitro and in vivo evaluation of lacticaseibacillus rhamnosus GG and bifidobacterium lactis Bb12 against avian pathogenic Escherichia coli and identification of novel probiotic-derived bioactive peptides. Probiotics Antimicrob Proteins. doi: 10.1007/s12602-021-09840-1. [DOI] [PubMed] [Google Scholar]
- 34.Kathayat D, Helmy YA, Deblais L, Srivastava V, Closs G, Jr, Khupse R, Rajashekara G. 2021. Novel small molecule growth inhibitor affecting bacterial outer membrane reduces extraintestinal pathogenic Escherichia coli (ExPEC) infection in avian model. Microbiol Spectr 9:e0000621. doi: 10.1128/Spectrum.00006-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kathayat D, Closs G, Jr, Helmy YA, Lokesh D, Ranjit S, Rajashekara G. 2021. Peptides affecting the outer membrane lipid asymmetry system (MlaA-OmpC/F) reduce avian pathogenic Escherichia coli (APEC) colonization in chickens. Appl Environ Microbiol 87:e0056721. doi: 10.1128/AEM.00567-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. 2006. Microbial ecology: human gut microbes associated with obesity. Nature 444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 37.Guo X, Xia X, Tang R, Zhou J, Zhao H, Wang K. 2008. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett Appl Microbiol 47:367–373. doi: 10.1111/j.1472-765X.2008.02408.x. [DOI] [PubMed] [Google Scholar]
- 38.Lopetuso LR, Scaldaferri F, Petito V, Gasbarrini A. 2013. Commensal clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog 5:23. doi: 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon K, Verwoolde MB, Zhang J, Smidt H, de Vries Reilingh G, Kemp B, Lammers A. 2016. Long-term effects of early life microbiota disturbance on adaptive immunity in laying hens. Poult Sci 95:1543–1554. doi: 10.3382/ps/pew088. [DOI] [PubMed] [Google Scholar]
- 40.Morrison DJ, Preston T. 2016. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez-Rubio C, Ordonez C, Abad-Gonzalez J, Garcia-Gallego A, Honrubia MP, Mallo JJ, Balana-Fouce R. 2009. Butyric acid-based feed additives help protect broiler chickens from Salmonella Enteritidis infection. Poult Sci 88:943–948. doi: 10.3382/ps.2008-00484. [DOI] [PubMed] [Google Scholar]
- 42.Eeckhaut V, Wang J, Van Parys A, Haesebrouck F, Joossens M, Falony G, Raes J, Ducatelle R, Van Immerseel F. 2016. The probiotic Butyricicoccus pullicaecorum reduces feed conversion and protects from potentially harmful intestinal microorganisms and necrotic enteritis in broilers. Front Microbiol 7:1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harijan RK, Hoff O, Ducati RG, Firestone RS, Hirsch BM, Evans GB, Schramm VL, Tyler PC. 2019. Selective inhibitors of helicobacter pylori methylthioadenosine nucleosidase and human methylthioadenosine phosphorylase. J Med Chem 62:3286–3296. doi: 10.1021/acs.jmedchem.8b01642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parveen N, Cornell KA. 2011. Methylthioadenosine/S-adenosylhomocysteine nucleosidase, a critical enzyme for bacterial metabolism. Mol Microbiol 79:7–20. doi: 10.1111/j.1365-2958.2010.07455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cornell KA, Knippel RJ, Cortright GR, Fonken M, Guerrero C, Hall AR, Mitchell KA, Thurston JH, Erstad P, Tao A, Xu D, Parveen N. 2020. Characterization of 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidases from Borrelia burgdorferi: antibiotic targets for Lyme disease. Biochim Biophys Acta Gen Subj 1864:129455. doi: 10.1016/j.bbagen.2019.129455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Namanja-Magliano HA, Evans GB, Harijan RK, Tyler PC, Schramm VL. 2017. Transition state analogue inhibitors of 5'-deoxyadenosine/5'-methylthioadenosine nucleosidase from mycobacterium tuberculosis. Biochemistry 56:5090–5098. doi: 10.1021/acs.biochem.7b00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh V, Evans GB, Lenz DH, Mason JM, Clinch K, Mee S, Painter GF, Tyler PC, Furneaux RH, Lee JE, Howell PL, Schramm VL. 2005. Femtomolar transition state analogue inhibitors of 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase from Escherichia coli. J Biol Chem 280:18265–18273. doi: 10.1074/jbc.M414472200. [DOI] [PubMed] [Google Scholar]
- 48.Devillard E, McIntosh FM, Duncan SH, Wallace RJ. 2007. Metabolism of linoleic acid by human gut bacteria: different routes for biosynthesis of conjugated linoleic acid. J Bacteriol 189:2566–2570. doi: 10.1128/JB.01359-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mudronova D, Karaffova V, Koscova J, Bartkovsky M, Marcincakova D, Popelka P, Klempova T, Certik M, Macanga J, Marcincak S. 2018. Effect of fungal gamma-linolenic acid and beta-carotene containing prefermented feed on immunity and gut of broiler chicken. Poult Sci 97:4211–4218. doi: 10.3382/ps/pey306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tabashsum Z, Peng M, Bernhardt C, Patel P, Carrion M, Biswas D. 2019. Synbiotic-like effect of linoleic acid overproducing Lactobacillus casei with berry phenolic extracts against pathogenesis of enterohemorrhagic Escherichia coli. Gut Pathog 11:41. doi: 10.1186/s13099-019-0320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hopkins D, Nesheim M. 1967. The linoleic acid requirement of chicks. Poult Sci 46:872–881. doi: 10.3382/ps.0460872. [DOI] [PubMed] [Google Scholar]
- 52.Landman WJ, van Eck JH. 2015. The incidence and economic impact of the Escherichia coli peritonitis syndrome in Dutch poultry farming. Avian Pathol 44:370–378. doi: 10.1080/03079457.2015.1060584. [DOI] [PubMed] [Google Scholar]
- 53.Levison ME, Levison JH. 2009. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect Dis Clin North Am 23:791–815. doi: 10.1016/j.idc.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.FDA. 2019. Tolerances for residues of new animal drugs in food. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=556&showFR=1.
- 55.Agunos A, Leger D, Carson C. 2012. Review of antimicrobial therapy of selected bacterial diseases in broiler chickens in Canada. Can Vet J 53:1289–1300. [PMC free article] [PubMed] [Google Scholar]
- 56.Mund MD, Khan UH, Tahir U, Mustafa B-E-, Fayyaz A. 2017. Antimicrobial drug residues in poultry products and implications on public health: A review. Int J Food Properties 20:1433–1446. doi: 10.1080/10942912.2016.1212874. [DOI] [Google Scholar]
- 57.Mangiamele P, Nicholson B, Wannemuehler Y, Seemann T, Logue CM, Li G, Tivendale KA, Nolan LK. 2013. Complete genome sequence of the avian pathogenic Escherichia coli strain APEC O78. Genome Announc 1:e0002613. doi: 10.1128/genomeA.00026-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cai XC, Xi H, Liang L, Liu JD, Liu CH, Xue YR, Yu XY. 2017. Rifampicin-resistance mutations in the rpoB gene in Bacillus velezensis CC09 have pleiotropic effects. Front Microbiol 8:178. doi: 10.3389/fmicb.2017.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peighambari SM, Julian RJ, Gyles CL. 2000. Experimental Escherichia coli respiratory infection in broilers. Avian Dis 44:759–769. doi: 10.2307/1593047. [DOI] [PubMed] [Google Scholar]
- 60.Balashova EE, Maslov DL, Lokhov PG. 2018. A metabolomics approach to pharmacotherapy personalization. J Pers Med 8. doi: 10.3390/jpm8030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, et al. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bartel J, Krumsiek J, Theis FJ. 2013. Statistical methods for the analysis of high-throughput metabolomics data. Comput Struct Biotechnol J 4:e201301009. doi: 10.5936/csbj.201301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.00286-22-s001.pdf, PDF file, 0.7 MB (728KB, pdf)
Data Availability Statement
Data from the study are included in this article and in the supplementary files. Microbiome sequence data have been deposited in the BioProject database under accession number PRJNA766869.