Abstract
Background:
In blood and tissues, dietary and endogenously generated fatty acids (FAs) occur in free form or as part of complex lipid molecules that collectively represent the lipidome of the respective tissue. We assessed associations of plasma lipids derived from high-resolution lipidomics with incident cardiometabolic diseases and subsequently tested if the identified risk-associated lipids were sensitive to dietary fat modification.
Methods:
The EPIC Potsdam cohort study (European Prospective Investigation into Cancer and Nutrition) comprises 27 548 participants recruited within an age range of 35 to 65 years from the general population around Potsdam, Germany. We generated 2 disease-specific case cohorts on the basis of a fixed random subsample (n=1262) and all respective cohort-wide identified incident primary cardiovascular disease (composite of fatal and nonfatal myocardial infarction and stroke; n=551) and type 2 diabetes (n=775) cases. We estimated the associations of baseline plasma concentrations of 282 class-specific FA abundances (calculated from 940 distinct molecular species across 15 lipid classes) with the outcomes in multivariable-adjusted Cox models. We tested the effect of an isoenergetic dietary fat modification on risk-associated lipids in the DIVAS randomized controlled trial (Dietary Intervention and Vascular Function; n=113). Participants consumed either a diet rich in saturated FAs (control), monounsaturated FAs, or a mixture of monounsaturated and n-6 polyunsaturated FAs for 16 weeks.
Results:
Sixty-nine lipids associated (false discovery rate<0.05) with at least 1 outcome (both, 8; only cardiovascular disease, 49; only type 2 diabetes, 12). In brief, several monoacylglycerols and FA16:0 and FA18:0 in diacylglycerols were associated with both outcomes; cholesteryl esters, free fatty acids, and sphingolipids were largely cardiovascular disease specific; and several (glycero)phospholipids were type 2 diabetes specific. In addition, 19 risk-associated lipids were affected (false discovery rate<0.05) by the diets rich in unsaturated dietary FAs compared with the saturated fat diet (17 in a direction consistent with a potential beneficial effect on long-term cardiometabolic risk). For example, the monounsaturated FA-rich diet decreased diacylglycerol(FA16:0) by 0.4 (95% CI, 0.5–0.3) SD units and increased triacylglycerol(FA22:1) by 0.5 (95% CI, 0.4–0.7) SD units.
Conclusions:
We identified several lipids associated with cardiometabolic disease risk. A subset was beneficially altered by a dietary fat intervention that supports the substitution of dietary saturated FAs with unsaturated FAs as a potential tool for primary disease prevention.
Keywords: cardiovascular diseases; cholesterol; diabetes mellitus; type 2; diet, food, and nutrition; epidemiology; lipids
Clinical Perspective.
What Is New?
High-resolution lipidomics uncovered several cardiometabolic risk biomarkers across a range of lipid classes that associate with incident cardiovascular disease and type 2 diabetes independent of standard clinical biomarkers.
Several identified risk-associated lipid markers were beneficially altered by a controlled 16-week intervention when comparing a diet rich in saturated fatty acids with diets rich in monounsaturated fatty acids or a mixture of monounsaturated and n-6 polyunsaturated fatty acids.
What Are the Clinical Implications?
Identified risk-associated lipids could serve as risk biomarkers and implicate underlying disease-specific pathways.
Lipids sensitive to dietary fat modification could serve as biomarkers of intervention effects in dietary intervention trials.
Observed intervention effects on risk-associated lipids provide further evidence for the beneficial effects of exchanging dietary saturated with unsaturated fatty acids.
Plasma concentrations of total triacylglycerol (TG), high-density (high-density lipoprotein cholesterol [HDL-C]), and low-density lipoprotein cholesterol are important predictors and potential causal factors of future cardiometabolic disease risk, including myocardial infarction, stroke, and type 2 diabetes (T2D).1–3 Accordingly, these biomarkers are routinely used in clinical decision-making, and underlying molecular pathways are among the targets of first-line drugs for primary and secondary cardiometabolic disease prevention.4 Preceding a drug prescription, adopting a healthier diet is considered a cornerstone of prevention.5,6 In particular, the dietary fatty acid (FA) profile poses a plausible link to lipid metabolism and subsequent health effects.7
In blood and tissues, dietary and endogenously generated FAs occur in a free form or as part of complex lipid molecules that collectively represent the lipidome of the respective tissue (Figure 1A). Major sources of plasma lipids are adipose tissue, liver, and dietary lipids (Figure 1B). Lipid classes differ in terms of molecular structure of the headgroups, which can be largely classified into nonglycerides (eg, cholesteryl esters and sphingolipids) and glycerides (eg, phospholipids and glycerolipids; Figure 1C, Figure S1). Diversity within the lipid classes is furthermore increased by the attached FAs that exhibit different structural features (Figure 1D).
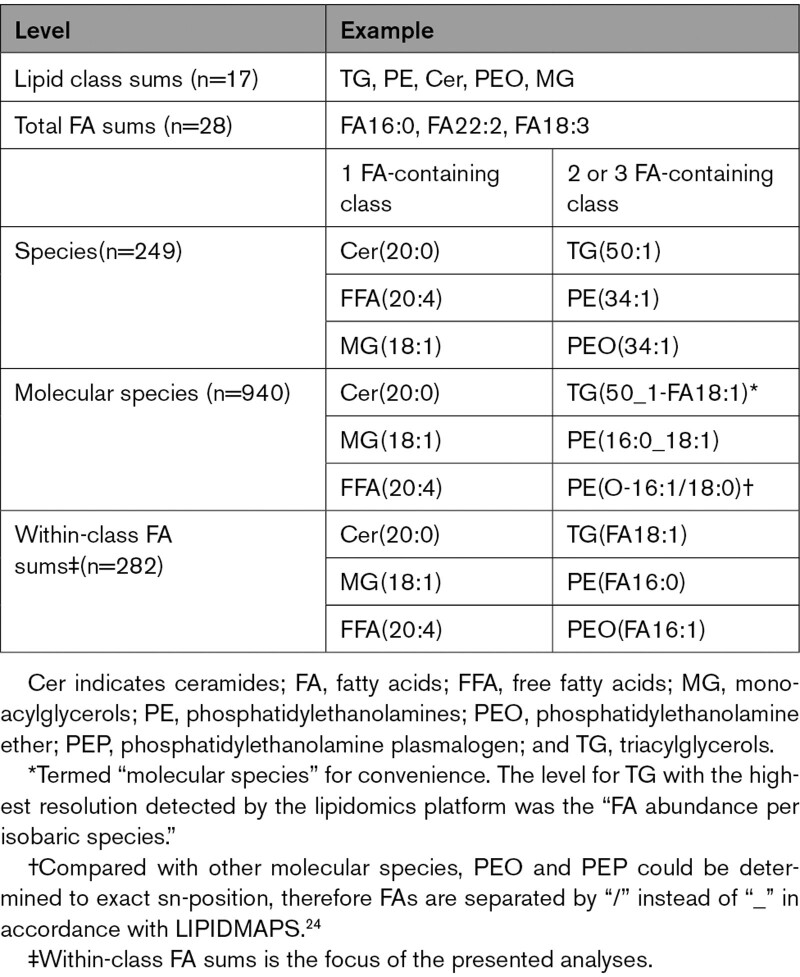
Figure 1.
Overview of lipid class occurrence in cell and plasma compartments, major sources of plasma lipids, investigated lipid classes, captured fatty acid features, and assessed lipidomics levels. A, Lipids have several functions in the organism that determine their location in cells and tissues. They make up membranes and thereby determine membrane fluidity and function, serve as energy storage, exert intracellular signaling properties, and are precursors of hormones (steroids and eicosanoids). In plasma, FFAs are mostly transported bound to albumin, whereas complex lipids are transported as part of lipoproteins. Lipids are continuously exchanged between plasma and tissues. B, The plasma lipidome largely comprises lipids ingested from the diet, released from adipose tissue, or produced by the liver. The liver, in particular, is the central hub of lipid metabolism through lipoprotein production and de novo lipogenesis. C, The lipid classes depicted here are covered by Metabolon’s Complex Lipid Panel and represent most of the major lipid classes found in the human plasma. More detailed information on the molecular differences between the lipid classes is shown in Figure S1. D, Fatty acid feature information provided by the lipidomics platform. E, Metabolon’s Complex Lipid Panel allows investigation of lipids on different aggregated levels. The main results of this publication refer to “Within-class FA sum.” More details on the different naming conventions used throughout are in Table 1. This figure was produced using smart.servier.com. CE indicates cholesterylesters; FFA, free fatty acid; PEO, phosphatidylethanolamine ether; and PEP, phosphatidylethanolamine plasmalogen.
High-throughput lipid-profiling technologies (lipidomics) generate detailed information on the (plasma) lipidome’s composition, including identification of single FAs attached to a lipid molecule.8 This enables researchers to precisely dissect lipid-disease associations and diet intervention effects. Recent studies assessed lipidomics in relation to cardiometabolic disease outcomes and elucidated connections between lipid metabolism and cardiometabolic diseases; however, low- to intermediate-level resolution of the lipidome (not determining lipid class-specific FA abundance, see Table 1), analyses targeted on few or single lipid classes (eg, only phospholipids or ceramides), or the use of patient cohorts rather than a general population sample represent shortcomings.9–19 Furthermore, even though T2D and cardiovascular diseases (CVD) are both intricately related to lipid metabolism and ectopic lipid deposition, direct comparison of the relationships with plasma lipid profiles are currently sparse and could add valuable causal insights.
Table 1.
Lipidomics Levels With Examples
Within the population-based EPIC-Potsdam study (European Prospective Investigation into Cancer and Nutrition), we therefore conducted lipidome-wide cardiometabolic disease association analyses across different lipidomics levels and tested the effect of modified dietary fat intake on identified risk-associated lipids in a separate dietary randomized controlled parallel intervention trial, the DIVAS study (Dietary Intervention and Vascular Function). Throughout the analyses, we present commonalities and differences between the investigated disease outcomes, primary composite CVD (stroke or myocardial infarction) and T2D.
Methods
EPIC-Potsdam data supporting the findings of this study are available from the corresponding author on reasonable request. Requests to access the dataset from the DIVAS study may be sent to Prof Julie Lovegrove, j.a.lovegrove@reading.ac.uk. Codes used to generate the results, figures, and tables are available on request.
Study Designs and Study Populations
EPIC-Potsdam Study
The EPIC-Potsdam study is a prospective cohort study that recruited 27 548 participants (16 644 women and 10 904 men; age range, 35–65 years) from the general population of Potsdam, Germany, and the surrounding geographical area from 1994 to 1998. Participants were then actively followed up every 2 to 3 years, by mailed questionnaires and, if necessary, by telephone. Response rates ranged between 90% and 96% per follow-up round.20 The study protocol was approved by the ethics committee of the Medical Society of the State of Brandenburg, Germany, and all participants provided a statement of written informed consent before enrollment.
Incident CVD was defined as incidence of primary nonfatal and fatal myocardial infarction and stroke (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10]) code: I21 for acute myocardial infarction, I63.0 to I63.9 for ischemic stroke, I61.0 to I61.9 for intracerebral and I60.0 to I60.9 for subarachnoid hemorrhage, and I64.0 to I64.9 for unspecified stroke). Incidence of CVD was captured by participants’ self-reports or based on information from the death certificates, which were validated by contacting the treating physicians. Inquired information included ICD-10 code, date of occurrence, and further information on symptoms and diagnostic criteria. For myocardial infarction, diagnostic criteria included clinical symptoms, ECGs, cardiac enzymes, and known coronary heart disease. For stroke, diagnosis was based on anamnesis, clinical symptoms, computed tomography/magnetic resonance imaging, angiogram, lumbar puncture, echocardiogram, Doppler, and ECG, plus imaging techniques if available. Participants with silent cardiovascular events that had not been documented within 28 days after occurrence were excluded as nonverifiable cases from all analyses.
Information on incidence of T2D was systematically acquired through self-report of a diagnosis, of T2D-relevant medication, or of dietary treatment attributable to T2D diagnosis during follow-up. Death certificates and information from tumor centers, physicians, or clinics that provided assessments for other diagnoses were screened for indication of incident T2D. For participants that were classified as potential cases on the basis of that information, a standard inquiry form was sent to the treating physician. Only physician-verified cases with a diagnosis of T2D (ICD-10 code: E11) and a diagnosis date after the baseline examination were considered confirmed incident cases of T2D.
Nested case cohorts were constructed for efficient study of molecular phenotypes. From all participants who provided blood at baseline (n=26 437), a random sample (subcohort, n=1262) was drawn, which served as a common reference population for both end points. For each end point, all incident cases that occurred in the full cohort until a specified censoring date were included in the analysis. After excluding prevalent cases of the respective outcomes, the analytic sample for T2D comprised 1886 participants, including 775 incident cases (26 cases in the subcohort) and for CVD 1671 participants, including 551 incident cases (28 cases in the subcohort). Follow-up was defined as the time between enrollment and study exit that was determined by diagnosis of the respective disease, death, dropout, or final censoring date, whichever came first. End point–specific censoring dates were November 30, 2006, for stroke and myocardial infarction and August 31, 2005, for T2D.
Anthropometric and blood pressure measurements were conducted according to a standardized protocol.21,22 Blood plasma was obtained at baseline and stored in liquid nitrogen tanks at –196 °C or in deep freezers at –80 °C until time of analysis. Baseline plasma concentrations of standard blood lipids (total cholesterol, HDL-C, and TG) were measured in 2007. Plasma samples, from which aliquots were drawn for the lipidomics measurements in 2016, were never or only once thawed and refrozen during storage (93 samples defrosted and refrozen once for aliquoting for unrelated analysis).
Detailed information on measurements of nonlipidomic biomarkers, anthropometric measures, and socioeconomic factors are provided in the Supplemental Material.
DIVAS Study
Lipidomics analysis was performed in a subset of participants (n=113 of 195) from DIVAS, a 16-week randomized controlled trial. This study recruited men and women, aged between 21 and 60 years and with estimated moderate CVD risk who were randomly assigned to either 1 of 3 isoenergetic diets: rich in saturated FAs (SFA), rich in monounsaturated FAs (MUFA), or rich in mixed unsaturated fatty acids (UFA), including both MUFA and n-6 polyunsaturated FAs. The target compositions (percent of total energy intake from total fat:SFA:MUFA:n-6 polyunsaturated FAs) were 36:17:11:4 for the SFA-rich diet (n=38), 36:9:19:4 for the MUFA-rich diet (n=39), and 36:9:13:10 for the mixed UFA-rich diet (n=36). All participants provided written informed consent; were nonsmokers; were not pregnant or lactating; had normal blood biochemistry, liver and kidney function; did not take dietary supplements, medication for hypertension, raised lipids, or inflammatory disorders; had no previous diagnosis of a myocardial infarction, stroke, or diabetes; did not consume excessive amounts of alcohol (men, <21 units/wk; women, <14 units/wk); and performed <3×30 minutes of aerobic exercise per week. The trial was single-blinded, and randomization was conducted by a study researcher using minimization stratified for sex, age, body mass index, and estimated CVD risk.23 Blood samples were taken at baseline and after 16 weeks at a similar time of day in a fasted state. Additional information on the intervention diets is provided in the Supplemental Material.
Lipidomics Profiling in EPIC-Potsdam and DIVAS
Lipidomics analysis was performed with Metabolon’s Complex Lipid Panel for EPIC-Potsdam and the DIVAS trial separately. Details on this platform are provided in the Supplemental Material.
The Complex Lipid Panel produced measurements for 15 lipid classes (free fatty acids [FFA]; cholesteryl esters [CE]; monoacylglycerols [MG]; ceramides [Cer]; dihydroceramides [dhCer]; lactosylceramides [LacCer]; hexosylceramides [HexCer]; sphingomyelins [SM]; lysophosphatidylethanolamines [LPE]; lysophosphatidylcholines [LPC]; diacylglycerols [DG]; triacylglycerols [TG]; phosphatidylcholines [PC]; phosphatidylethanolamines [PE]; phosphatidylinositol [PI]). In the case of PE, the species from the 2 subclasses phosphatidylethanolamine ether (PEO) and phosphatidylethanolamine plasmalogen (PEP) were detected and hence presented separately from PE where necessary. Measured concentrations of molecular species were used to calculate lipid class sums (by summing all molecular species from 1 class), total FA sums (by summing concentrations of all molecular species containing a specific FA), and within-class FA sums (summing all concentrations of molecular species containing a specific FA within a lipid class). Within-class FA sums are synonymous with molecular species level in lipid classes containing only one reported variable FA per molecule (1 FA–containing classes: FFA, CE, MG, Cer, dhCer, LacCer, HexCer, SM, LPE, LPC). For comparability with other studies, we further calculated the species level for those classes with >1 FA per molecule (ie, DG, TG, PC, PE, PEO, PEP, PI), by summing all species with the same total atomic mass and degree of saturation of the contained FAs (ie, isobaric species; Table 1). In total, 940 distinct lipid molecular species, 28 total FA sums, 282 within-class FA sums, and 249 species were finally available for analysis. We used the updated shorthand notations from the LIPIDMAPS initiative where applicable.24 We only refer to the shorthand notations of FAs for brevity. Respective common names of FAs are presented in Table S1.
Statistical Analysis
All lipidomics variables were log-transformed and z-scaled (mean=0, SD=1) to allow comparison of association strength across lipids and to stabilize skewed distributions.
Lipidome-Wide Association Analysis in EPIC-Potsdam
We conducted a lipidome-wide screen estimating hazard ratios for the associations between the lipid variables (class sums, total FA sums, within-class FA sums, species, molecular species) and incident CVD and T2D with Cox proportional hazards models. The case-cohort design was accounted for by assigning weights as proposed by Prentice.25 These weights are realized by counting survival time of participants of the random subcohort fully (cases and noncases) and survival time of incident cases outside the subcohort only at the date of diagnosis. Age was the underlying time variable, with entry time as age at baseline and exit time as age at event or censoring. The fully adjusted model included age, sex, waist circumference, height, leisure-time physical activity, smoking status, alcohol intake, highest achieved education level, fasting status at blood draw, total energy intake, blood pressure (systolic and diastolic), standard clinical blood lipid markers (total cholesterol, HDL-C, and TG), antihypertensive medication, lipid-lowering medication, and acetylsalicylic acid medication as covariates. Models for incident CVD were additionally adjusted for drug treatment for prevalent T2D (insulin or other) and proportion glycohemoglobin. Models for total FA sums were adjusted for the total sums of all lipid classes to disentangle FA abundances from within-class abundances. Models on within-class FA sums and (molecular) species levels were adjusted for the respective class sum to separate the association from the class sum. We accounted for multiple hypothesis testing by controlling the false discovery rate (FDR) at 5% separately for each outcome and lipidomics level.26 To check if presentation of unstratified results was warranted, we tested the potential for effect measure modification by sex by including lipid×sex interaction terms into the respective most adjusted model.
Effect of Modified Fat Intake Intervention in DIVAS
We considered all identified risk associated (FDR<0.05) within-class FA sums from EPIC-Potsdam as readouts in the DIVAS trial. We assessed the difference in postintervention within-class FA sum concentrations among the trial arms through linear regression models with trial arm coded as indicator variable (SFA-rich diet as reference) and adjusted for respective baseline concentrations in addition to age, body mass index, and sex. Similarly to the disease outcome analyses in EPIC-Potsdam, the models were further adjusted for baseline and postintervention concentrations of the respective class sums.
Software
All analyses were performed using R (version 4.1.0, package versions reported in Supplemental Material).
Results
EPIC-Potsdam Cohort Characteristics
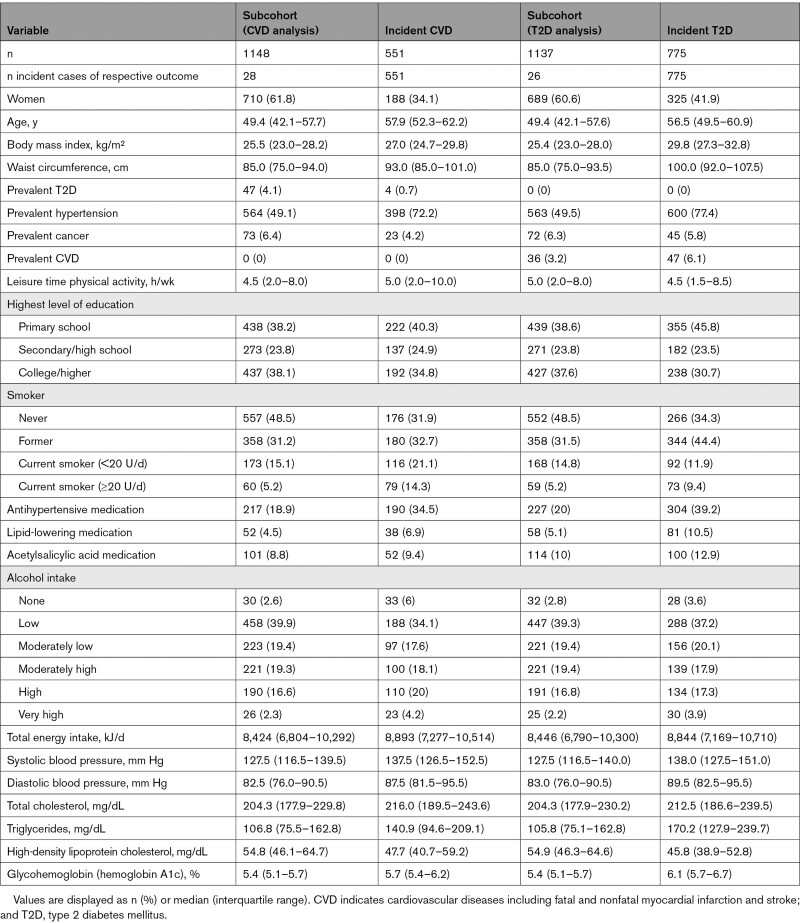
Compared with the respective subcohort participants, incident cases tended to be older, more likely to be male, current or former smokers, and on medication. On average, incident cases were characterized by higher body mass index and waist circumference, elevated total cholesterol, TG, and blood pressure, and lower HDL-C (Table 2). The median accrued follow-up time in the T2D analysis was 6.5 years (interquartile range, 6.0–8.7 years) and 8.4 years (interquartile range, 7.6–9.2 years) for the CVD analysis.
Table 2.
Baseline Characteristics of EPIC-Potsdam Participants by Outcome-Specific Subcohort Membership and Incident Cases Status
Lipid Abundances and Correlation Analyses in a General Population Sample
Overall, CE, FFA, TG, PC, and SM were the most abundant classes in plasma with average concentrations in the range of 10 to 100 µmol/L (Figure S2). The least abundant classes were Cer, dhCer, LacCer, HexCer, PEO, and LPE with close to or <0.1 µmol/L average concentrations. Most abundant FAs in total were FA16:0, FA18:0, FA18:1, FA18:2, and FA20:4 with >10 µmol/L concentrations, whereas the least abundant FAs were FA18:4, FA22:2, FA26:0, and FA26:1 with average concentrations well below 0.1 µmol/L (Figure S3). CE and MG had the widest diversity of detected FAs followed by FFA, TG, DG, and PC (Figure S3). Certain FAs were prominent in within-class profiles; for example, FA16:0 was among the most abundant within each lipid class (eg, median relative proportion in LacCer 61%; Figure S4, detailed distributions in Figure S5).
On class level, strong positive correlations were observed between structurally close classes, such as TG-DG (r=0.87, P<0.001), PEP-PEO (r=0.82, P<0.001), LPC-LPE (r=0.79, P<0.001), and PI-PC (r=0.75, P<0.001; Figure S6). Among the total FA sums, most of the stronger correlations were in line with FA elongation (eg, FA20:0–FA22:0 [r=0.63, P<0.001]) and FA desaturation steps (eg, FA22:4–FA22:5 [r=0.59, P<0.001]; Figure S7). These correlations were also present on a within-class FA sum level (Figures S8–S24).
Lipidome-Wide Cardiometabolic Risk Association Analyses
Results of the lipidome-wide screen across all levels, classes, and adjustment models are included in Table S2. For both CVD and T2D, we did not detect statistically significant (FDR<0.05) effect measure modification for the association between lipids and cardiometabolic disease risk by sex and therefore present unstratified results.
With the exception of FFA and DG, class sums of all classes were associated with at least 1 disease outcome (nominal P<0.05; Figure 2A, Table S2). All classes associated with incident CVD were positively associated. For T2D, only PE was statistically significantly positively associated, and LacCer, HexCer, LPC, LPE, and SM were inversely associated. Of note, the associations of LacCer, HexCer, LPC, and LPE were opposite for T2D and CVD (ie, higher risk observed for CVD and lower risk for T2D). However, no association remained after controlling for multiple testing. Associations of total FA sums were characterized by relatively low precision (ie, wide confidence intervals). After accounting for multiple testing, FA22:2 and FA22:4 were significantly positively associated with CVD and FA22:5 was inversely associated with T2D (Figure 2B, Table S2).
Figure 2.
Disease associations of class sums and total FA sums. Hazard ratios from models adjusted for age (as underlying time variable), sex, waist circumference, height, leisure time physical activity, smoking status, alcohol intake, highest achieved education level, fasting status at blood draw, total energy intake, blood pressure (systolic and diastolic), blood lipids (total cholesterol, high-density lipoprotein cholesterol, and standard clinical triacylglycerol), antihypertensive medication, lipid-lowering medication, and acetylsalicylic acid medication. Models for cardiovascular disease further adjusted for antidiabetic medication and proportion glycohemoglobin. A, Class sums; B, total FA sums, additionally adjusted for all class sums. CE indicates cholesteryl esters; Cer, ceramides; CVD, cardiovascular disease; DG, diacylglycerols; dhCer, dihydroceramides; FA, fatty acids; FFA, free fatty acids; HexCer, hexosylceramides; LacCer, lactosylceramides; LPC, lysophosphatidylcholines; LPE, lysophosphatidylethanolamines; MG, monoacylglycerols; PC, phosphatidylcholines; PE, phosphatidylethanolamines; PEO, phosphatidylethanolamine ether; PEP, phosphatidylethanolamine plasmalogen; PI, phosphatidylinositol; SM, sphingomyelins; and TG, triacylglycerols.
Lipidome-wide screening of all molecular species and within-class FA sums indicated that within-class FA sums largely showed similar or stronger (and more precise) risk associations compared with molecular species in 2 FA–containing classes (DG, PC, PE, PEP, PEO, PI) and TGs (Figures S25 and S26). Within-class FA sums of ≥2 FA–containing classes are therefore presented together with molecular species of 1 FA–containing classes to address the associations of FA class-specific abundances across all classes.
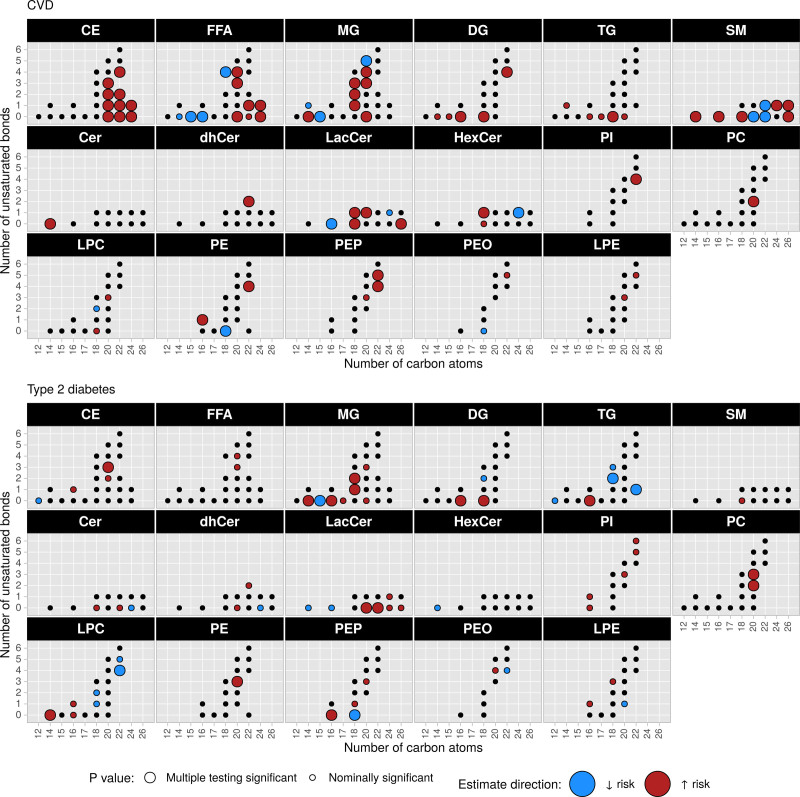
Analyses of within-class FA sums, species, and molecular species were specifically geared toward associations of FA composition within lipid classes by adjusting for the corresponding class sum. Taken together, this analysis comprised 282 distinct variables, of which in total 69 were significantly associated (FDR<0.05) with at least 1 outcome. When contrasting the disease associations, we observed lipids associated with both outcomes (n=8) and outcome-specific associations (CVD, n=49; T2D, n=12; Figure 3A and 3B). Among lipids associated with both outcomes, only MG(15:0) was inversely associated, whereas CE(20:3), MG(14:0), MG(18:1), MG(18:2), DG(FA16:0), DG(FA18:0), and PC(FA20:2) were positively associated (Figures 3 and 4). We found CEs, FFAs, and SMs nearly exclusively associated with CVD. Observed associations of CEs were all positive, whereas FFAs and SMs exhibited associations in both directions (Figures 3 and 4). Several LacCers and single other ceramides were associated. Further associations, aside from the ones associated with both outcomes, were detected among MGs and other glycero(phospho)lipid classes (Figures 3 and 4). In contrast to CVD, fewer lipids were specifically associated with T2D among which glycero(phospho)lipids represented the majority. FA16:0, in particular, was associated with higher T2D risk as part of MG, DG, TG, and PEP. Among sphingolipids only 2 positive associations (LacCer(20:0) and LacCer(22:0)) were detected.
Figure 3.
Disease associations of lipid class-specific FA abundances. P values and hazard ratios from models adjusted for age (as underlying time variable), sex, waist circumference, height, leisure time physical activity, smoking status, alcohol intake, highest achieved education level, fasting status at blood draw, total energy intake, blood pressure (systolic and diastolic), blood lipids (total cholesterol, high-density lipoprotein cholesterol, and standard clinical triacylglycerol), antihypertensive medication, lipid-lowering medication, acetylsalicylic acid medication, and respective class sum. Models for CVD further adjusted for antidiabetic medication and proportion glycohemoglobin. A, Scatter plot of P values for type 2 diabetes vs CVD; all labeled points were statistically significant after accounting for multiple testing. B, Hazard ratios (95% CI) of all significant associations after controlling for multiple testing in A. CE indicates cholesteryl esters; Cer, ceramides; CVD, cardiovascular disease; DG, diacylglycerols; dhCer, dihydroceramides; FA, fatty acids; FFA, free fatty acids; HexCer, hexosylceramides; LacCer, lactosylceramides; LPC, lysophosphatidylcholines; LPE, lysophosphatidylethanolamines; MG, monoacylglycerols; PC, phosphatidylcholines; PE, phosphatidylethanolamines; PEO, phosphatidylethanolamine ether; PEP, phosphatidylethanolamine plasmalogen; PI, phosphatidylinositol; SM, sphingomyelins; and TG, triacylglycerols.
Figure 4.
Disease associations of lipid class-specific FA abundances by FA carbon chain length and number of unsaturated bonds. P values from models adjusted for age (as underlying time variable), sex, waist circumference, height, leisure time physical activity, smoking status, alcohol intake, highest achieved education level, fasting status at blood draw, total energy intake, blood pressure (systolic and diastolic), blood lipids (total cholesterol, high-density lipoprotein cholesterol, and standard clinical triacylglycerols), antihypertensive medication, lipid-lowering medication, acetylsalicylic acid medication, and respective class sum. Models for CVD further adjusted for antidiabetic medication and proportion glycohemoglobin. CE indicates cholesteryl esters; Cer, ceramides; CVD, cardiovascular disease; DG, diacylglycerols; dhCer, dihydroceramides; FFA, free fatty acids; HexCer, hexosylceramides; LacCer, lactosylceramides; LPC, lysophosphatidylcholines; LPE, lysophosphatidylethanolamines; MG, monoacylglycerols; PC, phosphatidylcholines; PE, phosphatidylethanolamines; PEO, phosphatidylethanolamine ether; PEP, phosphatidylethanolamine plasmalogen; PI, phosphatidylinositol; SM, sphingomyelins; and TG, triacylglycerols.
Risk Associations According to FA Carbon Chain Length and Number of Double Bonds
Most species associated with higher risk contained shorter carbon chains, were saturated, or had only a few double bonds. (Figure S27). This observation was more pronounced for T2D than CVD. The species level represented a mixture of different isobaric molecular species. In most cases, one specific molecular species was statistically significantly associated with the outcome, whereas the remaining isobaric molecular species were not. For example, PEP(36_3) was positively associated with T2D risk, and the only similarly associated molecular species that represents this species was PE(P-16:0/20:3) (Figures S28 and S29). On the molecular species level, lipids containing specific FAs (ie, FA16:0 and FA18:0) were often associated with higher risk (Figures S28 and S29). Lipids that were associated with lower risk did not contain common FAs, but rather a wider range of (unsaturated) FAs and an overall absence of FA16:0 and FA18:0.
Impact of Dietary Fat Modification on Risk-Associated Lipids
From the identified 69 statistically significantly disease-associated lipids in EPIC-Potsdam, 55 were available for analysis in the DIVAS study. Among those, we found plasma concentrations of 19 significantly increased or decreased (FDR <0.05) by an UFA-rich diet relative to the SFA-rich diet (Figure 5A, Table S3). The MUFA-rich diet increased concentrations of TG(FA22:1), SM(24:1), and TG(FA18:2) and decreased DG(FA16:0), DG(FA18:0) TG(FA16:0), TG(FA18:0), DG(FA22:4), SM(18:0), SM(14:0), PEP(FA22:5), PE(FA16:1), HexCer(18:1), LPC(14:0), LacCer(20:1), and MG(20:0). The mixed UFA-rich diet decreased concentrations of DG(FA16:0), DG(FA18:0), TG(FA18:0), HexCer(18:1), PE(FA16:1), SM(14:0), PEP(FA22:5), PE(FA20:3), and LPC(14:0) and increased TG(FA22:1), TG(FA18:2), LacCer(16:0), and CE(24:0). High cardiometabolic disease risk–associated lipids were decreased and low risk–associated lipids increased by the MUFA-rich and mixed UFA-rich intervention diets (Figure 5A and 5B). Only SM(24:1) for the MUFA-rich and CE(24:0) for the mixed UFA-rich diet as high risk–associated lipids did not follow the above-mentioned pattern and were increased instead of decreased. The effects with the lowest P value (baseline concentration-adjusted difference between both UFA-rich and SFA-rich intervention arms in z scores, all P<0.001) were for the MUFA-rich diet DG(FA16:0) (–0.40 [95% CI, –0.51 to –0.30]) and TG(FA22:1) (0.53 [95% CI, 0.37–0.69]), and for mixed UFA-rich DG(FA18:0) (–0.24 [95% CI, 0.34 to –0.14]) and TG(FA18:2) (0.30 [95% CI, 0.18–0.43]).
Figure 5.
Effect of MUFA and mixed UFA-rich diets vs a SFA-rich diet on risk-associated lipids in the DIVAS trial (Dietary Intervention and Vascular Function). A, Volcano plot of effect of diet intervention on within class-FA sum concentrations from a linear regression model with postintervention concentration as dependent variable and diet type (indicator variable for MUFA-rich [yes/no], mixed UFA-rich [yes/no], SFA-rich as reference), respective lipid baseline concentration, respective baseline, and postintervention class sum concentration, age, sex, and body mass index as independent variables. Direction of associations in the EPIC-Potsdam trial (European Prospective Investigation into Cancer and Nutrition) was consistent between type 2 diabetes and CVD for all risk-associated lipids. All labeled points were statistically significant after accounting for multiple testing. B, Word clouds for overlap between change through diet type and observed risk association in EPIC-Potsdam by disease. CE indicates cholesteryl esters; Cer, ceramides; CVD, cardiovascular disease; DG, diacylglycerols; dhCer, dihydroceramides; FA, fatty acids; FFA, free fatty acids; HexCer, hexosylceramides; LacCer, lactosylceramides; LPC, lysophosphatidylcholines; LPE, lysophosphatidylethanolamines; MG, monoacylglycerols; MUFA, monounsaturated fatty acid; PC, phosphatidylcholines; PE, phosphatidylethanolamines; PEO, phosphatidylethanolamine ether; PEP, phosphatidylethanolamine plasmalogen; PI, phosphatidylinositol; SFA, saturated fatty acid; SM, sphingomyelins; TG, triacylglycerols; and UFA, unsaturated fatty acid.
Discussion
In the population-based EPIC-Potsdam cohort study, we screened the lipidome on different levels (class sums, species, molecular species, and within-class FA sums) to identify risk biomarkers, which we subsequently assessed for sensitivity to a dietary intervention aimed to modify dietary FA intakes. Class-specific FA abundances and molecular species showed strong associations that were independent of the respective class sum level associations and standard clinical blood lipid markers. From 282 distinct within-class FA sums, 69 were associated with at least 1 outcome, from which 8 were associated with both CVD and T2D, 49 with only CVD, and 12 with only T2D. We showed that 19 disease-associated lipids (12 CVD risk-specific, 5 T2D risk-specific, and 2 associated with both) were changed by substituting dietary SFA with UFA in the DIVAS trial. In general, high risk–associated lipids were lowered, whereas low risk–associated lipids were increased by the UFA interventions.
We identified several risk-associated lipids, particularly contained in DG and TG, but also in other classes, that were sensitive to the modification of the dietary FA intake. Most prominently, the high risk–associated TG(FA16:0), TG(FA18:0), DG(FA16:0), and DG(FA18:0) were decreased, whereas the low risk–associated lipids (eg, TG[FA22:1] and TG[FA18:2]) were increased with both UFA-rich diets. The achieved effects were of a magnitude that could translate into long-term cardiometabolic disease risk reduction, when considering the respective observed risk estimate sizes from EPIC-Potsdam. For example, baseline-adjusted postintervention concentrations of DG(FA16:0) were reduced by 0.4 SD units with the MUFA-rich diet versus the SFA-rich diet, whereas the respective hazard ratio for T2D was 2.8 per SD. Therefore, our results suggest that the substitution of dietary SFA with UFA could improve cardiometabolic disease risk.27
Our modeling approach in the lipidome-wide screen allowed us to distinguish between the disease risk association of lipid classes (class sum) and specific FA residues-containing lipid metabolites within a particular class (by class sum-adjusting molecular species and within-class FA sums). Others recently showed that this approach uncovers intricate disease associations.11,16
Our analyses reveal that direction, association strength, and precision of class-specific FA proportions (within-class FA sums) and FA combinations (molecular species) can diverge substantially from those of total FA sums and can vary considerably among classes. For example, our analyses showed inverse associations of TGs containing FA18:2 and FA18:3 with T2D and LPC(18:2) with CVD, but these same FAs as part of MGs were associated with higher cardiometabolic disease risk. Our results, therefore, add nuance to findings from large pooled analyses on total FA concentrations largely indicating no or inverse associations of FA18:2 and FA18:3 with CVD and T2D risk across varying lipid compartments.28–31 In a similar fashion, the association of arachidonic acid (FA20:4) with CVD was inconclusive in recent reports.29,31 In our data, FFA(20:4) and MG(20:4) were positively associated with CVD. As another example, total dihomo-γ-linoleic acid (FA20:3) in phospholipids was previously reported as strongly positively associated with incident T2D in our32 and other studies,33 although the associations with CVD outcomes were inconsistent.7 We observed statistically significant positive associations with T2D in phospholipids (PE[FA20:3] and PC[FA20:3]), but also in CE(20:3). For CVD this was the case for CE(20:3), FFA(20:3), and MG(20:3). However, in contrast to risk-associated lipids containing linoleic acid (FA18:2), lipids containing dihomo-γ-linolenic acid (FA20:3) were not increased through higher dietary UFA and lower SFA intakes, which is in line with other reports.34 This might indicate that, for those lipids, endogenous FA metabolism plays a greater role as a determinant of plasma concentrations (and hence risk associations) compared with dietary fat composition.
MGs were the class with the most lipids significantly associated with both outcomes. To our knowledge, only 1 report from the PREDIMED study (Prevención con Dieta Mediterránea) assessed the association of MGs with incident coronary heart disease, but the molecular species that were significantly associated in our analysis were not identified in their lipidomics panel.12 It is surprising that most observed significant associations in our data were positive, including molecular species containing UFAs generally considered to be beneficial or not harmful (eg, FA18:2=linoleic acid).29,31 Our finding on MG(15:0) and FFA(15:0) is in line with recent reports that found FA15:0, probably as a biomarker of dairy intake, inversely associated with T2D risk35; however, the current evidence for a relationship with CVD is inconclusive.36 We recently investigated odd-chain FAs in relation to T2D in a targeted approach and found that the association of MG(15:0) is the only consistent species between men and women in terms of direction and precision of the observed association.37 In the exploratory setting of the present analyses, we did not identify sufficient evidence for effect measure modification by sex (P>0.05 after accounting for multiple testing) to warrant deeper investigation of sex differences here.
Lipids that associated more specifically with CVD were CEs, FFAs, ceramides (especially LacCers), and SMs, whereas lipids from glycerophospholipid classes were largely T2D specific. The observed positive associations of several CEs with CVD are in line with enrichment of CEs in atherosclerotic plaques.38 However, results from PREDIMED indicated inverse associations,11,12 whereas Stegemann et al9 reported positive associations with incident CVD in the Bruneck Study. A potential explanation could be differences in study populations, because PREDIMED recruited persons at high CVD risk, whereas the Bruneck Study and EPIC-Potsdam are population based with lower baseline cardiometabolic disease risk profiles among the participants.
Sphingolipid molecular species (Cer, dhCer, HexCer, LacCer, and SM) exhibited stronger associations with CVD than with T2D, although the less precise associations from the T2D analyses were overall directionally consistent with CVD. The weaker findings in relation to incident T2D in our data seem to be in line with overall rather heterogeneous reports from other studies.17,19,39,40 A recent meta-analysis pooling longitudinal studies on various adverse cardiovascular outcomes found positive associations of Cer(d18:1/16:0), Cer(d18:1/18:0), and Cer(d18:1/24:1) with CVD risk, but reported substantial heterogeneity among the included studies.41
The possibility to determine FA abundances within lipid classes is a major advantage over previous work in similarly sized population-based cohort studies. Our findings suggest that FA carbon chain length and saturation level are not universally determinant of the direction or strength of the risk associations. Our data further allowed us to refine previously reported associations generated from lower-resolution lipidomics. For example, Rhee et al10 reported TG(52_1) as their strongest association with incident T2D with an odds ratio per SD of 1.9 (95% CI, 1.2–3.2). We replicated the high T2D risk association of TG(52_1) and further attributed this association to the isobaric species TG(52_1-FA16:0) and TG(52_1-FA18:0). Furthermore, our data suggest that the purported higher risk from shorter and less saturated species can be attributed to FA16:0 contained in most of the investigated glycerolipids. This is in line with evidence that shows that total FA16:0 concentration, irrespective of lipid compartment, is associated with T2D.28 However, the inverse association of LacCer(16:0) and FFA(16:0) with CVD suggests that the role of FA16:0 is also lipid class specific and warrants deeper investigation into class-specific FA proportions.
The plasma lipid pool is derived from different tissues and integrates modifiable (eg, habitual diet, physical activity, and fasting state) and nonmodifiable factors (eg, genetics and pathological disturbances).8 Dietary lipids from the intestine occur alongside de novo generated lipids and lipid derivatives originating from different tissues (eg, liver, adipose tissue, muscle). Plasma lipids, therefore, reflect disease-relevant metabolic disturbances (eg, hepatic glucose metabolism), habitual intake levels (eg, dietary fat quality), or specific organ damage (eg, inflammatory processes in atherosclerosis). For example, observed associations of specific CE and SM molecular species might be representative of specific lipoprotein subclasses that exhibit greater cardiovascular risk than is captured by traditional lipoprotein measurements (ie, HDL-C, low-density lipoprotein cholesterol).42,43 FA16:0, as another example, is the major product of hepatic de novo lipogenesis, a hallmark of nonalcoholic fatty liver disease, and was recently shown to be associated with incident T2D.28,43 In vivo and in vitro studies found that FA16:0 impaired intracellular insulin signaling in a range of tissues, including hepatocytes and endothelial cells, and thereby drives endothelial dysfunction, inflammation, and ectopic fat storage.44 Our data extend these results by highlighting FA16:0 in TG and DG as strongly associated with incident T2D and CVD. Our intervention analysis in DIVAS showed that TG and DG FA16:0 were sensitive to dietary fat quality, but further work is needed to determine whether the reductions of FA16:0 achieved in our study represented the different dietary FA compositions alone or reflected improved metabolic status. Plasma lipids might furthermore be reflective of cell membrane compositions and fluidity, which impact intercellular signaling and substrate flux.45 However, connecting our findings on plasma lipids to membrane compositions is not straightforward. For example, saturated phospholipids are suggested to be detrimental to membrane functionality, but we found mostly n-6 polyunsaturated FA phospholipids associated with higher risk.
When interpreting the results of our study, certain caveats apply. One, we cannot rule out that some molecular species were missing from our analysis even though Metabolon’s Complex Lipid Panel is, to our knowledge, the high-throughput platform with the largest coverage with this level of resolution. Of note, Metabolon recently removed FFAs from the Complex Lipid Panel, which made FFAs unavailable in DIVAS. Two, some associations might have been attenuated by the low reliability of specific lipids. However, in a pilot study comprising 35 EPIC-Potsdam participants, we found that 80% of the covered lipids had at least a fair, often good, or excellent reliability score over 4 months (data not shown). Three, plasma samples were stored at –80 °C at all times after sampling and processing with no or only 1 additional freeze-thaw cycle to minimize sample deterioration (eg, FA oxidation).46 In a sensitivity analysis, we did not find evidence that the additional freeze-thaw cycle of some samples (n=93) was the cause for the observed associations. Four, the platform does not resolve the n-configuration, that is, position of a double bond within a FA, not allowing exact attribution to the specific molecular isomers of FAs. Five, we modeled disease associations in the lipidome-wide screen relative to class sums. This enabled the causally relevant distinction between the cardiometabolic disease risk associations of the total lipid class plasma level versus specific FA residues within that class. However, this came at the expense of introducing higher imprecision in the associations of some lipids, which could have led to missed associations. To facilitate comparisons with previous and future reports, we provide all risk estimates from differently adjusted models, including class-unadjusted models, in the Supplemental Material. Six, lipidome-wide screening was not geared toward assessing the cardiometabolic disease risk associated with multimetabolite patterns or ratios. However, we and others have previously shown that the relationship between lipids may reflect causally critical processes in lipid metabolism.32,47,48 Seven, the lipidome-wide screen was of an exploratory nature. Further studies are needed, therefore, to judge the generalizability of our findings and to increase (combined) sample sizes to detect smaller associations, which did not withstand multiple testing adjustment in our study. Last, identifying cases by self-report could lead to cases remaining undetected. However, case verification ensured no false positives and the false negatives do not bias risk associations if this misclassification is nondifferential to the exposure of interest.49
In conclusion, lipid class–specific FA compositions available through deep lipidomics allow detailed investigation of the links between lipid metabolism and cardiometabolic diseases. We found different risk-associated lipids for CVD and T2D with minor overlap, emphasizing the differing causes, but also highlighting potential unifying pathways. Our data furthermore show sensitivity of several risk-associated lipids to a dietary fat modulation by comparing the effect of a SFA-rich diet with UFA-rich diets.
Article Information
Acknowledgments
The authors thank the Human Study Center (HSC) of the German Institute of Human Nutrition Potsdam-Rehbruecke, namely the trustee and the data hub, for data processing, and the biobank for the processing of biological samples, and M. Bergmann, the head of the HSC, for the contribution to the study design and leading the underlying processes of data generation. The authors thank the DIVAS study (Dietary Intervention and Vascular Function) investigators K. Vafeiadou, M. Weech, H. Altowaijri, S. Todd, and P. Yaqoob. Last, we thank all EPIC-Potsdam (European Prospective Investigation into Cancer and Nutrition) and DIVAS participants for their invaluable contribution to the respective studies.
Sources of Funding
The work was supported by the Federal Ministry of Science, Germany (grant 01 EA 9401) and the European Union (grant SOC 95201408 05 F02) for the recruitment phase of the EPIC-Potsdam Study (European Prospective Investigation into Cancer and Nutrition), by the German Cancer Aid (grant 70-2488-Ha I) and the European Community (grant SOC9820076905F02) for the follow-up of the EPIC-Potsdam Study, by a grant from the German Federal Ministry of Education and Research (Bundesministerium fuer Bildung und Forschung) to the German Center for Diabetes Research (DZD grant 82DZD00302), and by a grant from the European Commission and the German Federal Ministry of Education and Research within the Joint Programming Initiative A Healthy Diet for a Healthy Life, as part of the ERA-HDHL cofounded joint call Biomarkers for Nutrition and Health (01EA1704). DIVAS (Dietary Intervention and Vascular Function) was funded by the United Kingdom Food Standards Agency and Department of Health Policy Research Program (024/0036) and L. Sellem was funded by the Biotechnology and BiologicalSciences Research Council (BBSRC; BB/P028217/1) Fatty Acid Metabolism – Interlinking Diet and Cardiometabolic Health FAME as part of the ERA-HDHL: Biomarkers in Nutrition and Health.
Disclosures
Dr Lovegrove is Deputy Chair of the UK Government Scientific Advisory Committee on Nutrition (SACN) and a previous member of the SACN working group on Saturated Fats and Health. Dr Lovegrove was chair, and Dr Jackson and Sellem were members of a scientific expert committee for the International Life Sciences Institute (ILSI) on Individual Saturated Fatty acids and Cardiovascular Risk. The other authors report no conflicts.
Supplemental Material
Expanded Methods
Figures S1–S29
Tables S1–S3
Reference 50
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CE
- cholesteryl esters
- Cer
- ceramides
- CVD
- cardiovascular disease
- DG
- diacylglycerols
- dhCer
- dihydroceramides
- DIVAS
- Dietary Intervention and Vascular Function
- EPIC
- European Prospective Investigation into Cancer and Nutrition
- FA
- fatty acid
- FDR
- false discovery rate
- FFA
- free fatty acids
- HDL-C
- high-density lipoprotein cholesterol
- HexCer
- hexosylceramides
- LacCer
- lactosylceramides
- LPC
- lysophosphatidylcholines
- LPE
- lysophosphatidylethanolamines
- MG
- monoacylglycerols
- MUFA
- monounsaturated fatty acids
- PC
- phosphatidylcholines
- PE
- phosphatidylethanolamines
- PEO
- phosphatidylethanolamine ethers
- PEP
- phosphatidylethanolamine plasmalogens
- PI
- phosphatidylinositols
- SFA
- saturated fatty acids
- SM
- sphingomyelins
- T2D
- type 2 diabetes
- TG
- triacylglycerol
- UFA
- unsaturated fatty acids
Circulation is available at www.ahajournals.org/journal/circ
Supplemental Material, the podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.121.056805.
For Sources of Funding and Disclosures, see page 33.
Contributor Information
Fabian Eichelmann, Email: Fabian.Eichelmann@dife.de.
Laury Sellem, Email: L.R.Sellem@pgr.reading.ac.uk.
Clemens Wittenbecher, Email: cwittenbecher@hsph.harvard.edu.
Susanne Jäger, Email: susanne.jaeger@dife.de.
Olga Kuxhaus, Email: Olga.Kuxhaus@dife.de.
Marcela Prada, Email: Marcela.Prada@dife.de.
Rafael Cuadrat, Email: Rafael.Cuadrat@dife.de.
Kim G. Jackson, Email: k.g.jackson@reading.ac.uk.
Julie A. Lovegrove, Email: j.a.lovegrove@reading.ac.uk.
References
- 1.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 2.Hindy G, Engström G, Larsson SC, Traylor M, Markus HS, Melander O, Orho-Melander M; Stroke Genetics Network (SiGN). Role of blood lipids in the development of ischemic stroke and its subtypes: a mendelian randomization study. Stroke. 2018;49:820–827. doi: 10.1161/STROKEAHA.117.019653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, Dale CE, Padmanabhan S, Finan C, Swerdlow DI, et al. ; UCLEB consortium. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36:539–550. doi: 10.1093/eurheartj/eht571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hegele RA, Tsimikas S. Lipid-lowering agents. Circ Res. 2019;124:386–404. doi: 10.1161/CIRCRESAHA.118.313171 [DOI] [PubMed] [Google Scholar]
- 5.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, et al. ; ESC Scientific Document Group. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulze MB, Minihane AM, Saleh RNM, Risérus U. Intake and metabolism of omega-3 and omega-6 polyunsaturated fatty acids: nutritional implications for cardiometabolic diseases. Lancet Diabetes Endocrinol. 2020;8:915–930. doi: 10.1016/S2213-8587(20)30148-0 [DOI] [PubMed] [Google Scholar]
- 8.Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–3305. doi: 10.1194/jlr.M009449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stegemann C, Pechlaner R, Willeit P, Langley SR, Mangino M, Mayr U, Menni C, Moayyeri A, Santer P, Rungger G, et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation. 2014;129:1821–1831. doi: 10.1161/CIRCULATIONAHA.113.002500 [DOI] [PubMed] [Google Scholar]
- 10.Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, Yang E, Farrell L, Fox CS, O’Donnell CJ, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest. 2011;121:1402–1411. doi: 10.1172/JCI44442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razquin C, Toledo E, Clish CB, Ruiz-Canela M, Dennis C, Corella D, Papandreou C, Ros E, Estruch R, Guasch-Ferré M, et al. Plasma lipidomic profiling and risk of type 2 diabetes in the PREDIMED trial. Diabetes Care. 2018;41:2617–2624. doi: 10.2337/dc18-0840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toledo E, Wang DD, Ruiz-Canela M, Clish CB, Razquin C, Zheng Y, Guasch-Ferré M, Hruby A, Corella D, Gómez-Gracia E, et al. Plasma lipidomic profiles and cardiovascular events in a randomized intervention trial with the Mediterranean diet. Am J Clin Nutr. 2017;106:973–983. doi: 10.3945/ajcn.116.151159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razquin C, Liang L, Toledo E, Clish CB, Ruiz-Canela M, Zheng Y, Wang DD, Corella D, Castaner O, Ros E, et al. Plasma lipidome patterns associated with cardiovascular risk in the PREDIMED trial: a case-cohort study. Int J Cardiol. 2018;253:126–132. doi: 10.1016/j.ijcard.2017.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alshehry ZH, Mundra PA, Barlow CK, Mellett NA, Wong G, McConville MJ, Simes J, Tonkin AM, Sullivan DR, Barnes EH, et al. Plasma lipidomic profiles improve on traditional risk factors for the prediction of cardiovascular events in type 2 diabetes mellitus. Circulation. 2016;134:1637–1650. doi: 10.1161/CIRCULATIONAHA.116.023233 [DOI] [PubMed] [Google Scholar]
- 15.Mundra PA, Barlow CK, Nestel PJ, Barnes EH, Kirby A, Thompson P, Sullivan DR, Alshehry ZH, Mellett NA, Huynh K, et al. ; LIPID Study Investigators. Large-scale plasma lipidomic profiling identifies lipids that predict cardiovascular events in secondary prevention. JCI Insight. 2018;3:121326. doi: 10.1172/jci.insight.121326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu J, Lam SM, Wan Q, Shi L, Huo Y, Chen L, Tang X, Li B, Wu X, Peng K, et al. High-coverage targeted lipidomics reveals novel serum lipid predictors and lipid pathway dysregulation antecedent to type 2 diabetes onset in normoglycemic Chinese adults. Diabetes Care. 2019;42:2117–2126. doi: 10.2337/dc19-0100 [DOI] [PubMed] [Google Scholar]
- 17.Hilvo M, Salonurmi T, Havulinna AS, Kauhanen D, Pedersen ER, Tell GS, Meyer K, Teeriniemi AM, Laatikainen T, Jousilahti P, et al. Ceramide stearic to palmitic acid ratio predicts incident diabetes. Diabetologia. 2018;61:1424–1434. doi: 10.1007/s00125-018-4590-6 [DOI] [PubMed] [Google Scholar]
- 18.Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, Brenner H, Schöttker B, Lääperi M, Kauhanen D, Koistinen KM, et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur Heart J. 2020;41:371–380. doi: 10.1093/eurheartj/ehz387 [DOI] [PubMed] [Google Scholar]
- 19.Fretts AM, Jensen PN, Hoofnagle A, McKnight B, Howard BV, Umans J, Yu C, Sitlani C, Siscovick DS, King IB, et al. Plasma ceramide species are associated with diabetes risk in participants of the Strong Heart Study. J Nutr. 2020;150:1214–1222. doi: 10.1093/jn/nxz259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boeing H, Korfmann A, Bergmann MM. Recruitment procedures of EPIC-Germany. European Investigation into Cancer and Nutrition. Ann Nutr Metab. 1999;43:205–215. doi: 10.1159/000012787 [DOI] [PubMed] [Google Scholar]
- 21.Klipstein-Grobusch K, Georg T, Boeing H. Interviewer variability in anthropometric measurements and estimates of body composition. Int J Epidemiol. 1997;26 Suppl 1:S174–S180. doi: 10.1093/ije/26.suppl_1.s174 [DOI] [PubMed] [Google Scholar]
- 22.Schulze MB, Kroke A, Bergmann MM, Boeing H. Differences of blood pressure estimates between consecutive measurements on one occasion: implications for inter-study comparability of epidemiologic studies. Eur J Epidemiol. 2000;16:891–898. [DOI] [PubMed] [Google Scholar]
- 23.Weech M, Vafeiadou K, Hasaj M, Todd S, Yaqoob P, Jackson KG, Lovegrove JA. Development of a food-exchange model to replace saturated fat with MUFAs and n-6 PUFAs in adults at moderate cardiovascular risk. J Nutr. 2014;144:846–855. doi: 10.3945/jn.114.190645 [DOI] [PubMed] [Google Scholar]
- 24.Liebisch G, Fahy E, Aoki J, Dennis EA, Durand T, Ejsing CS, Fedorova M, Feussner I, Griffiths WJ, Köfeler H, et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J Lipid Res. 2020;61:1539–1555. doi: 10.1194/jlr.S120001025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- 27.Forouhi NG, Krauss RM, Taubes G, Willett W. Dietary fat and cardiometabolic health: evidence, controversies, and consensus for guidance. BMJ. 2018;361:k2139. doi: 10.1136/bmj.k2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imamura F, Fretts AM, Marklund M, Ardisson Korat AV, Yang WS, Lankinen M, Qureshi W, Helmer C, Chen TA, Virtanen JK, et al. ; InterAct Consortium. Fatty acids in the de novo lipogenesis pathway and incidence of type 2 diabetes: A pooled analysis of prospective cohort studies. PLoS Med. 2020;17:e1003102. doi: 10.1371/journal.pmed.1003102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marklund M, Wu JHY, Imamura F, Del Gobbo LC, Fretts A, de Goede J, Shi P, Tintle N, Wennberg M, Aslibekyan S, et al. ; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE). Biomarkers of dietary omega-6 fatty acids and incident cardiovascular disease and mortality. Circulation. 2019;139:2422–2436. doi: 10.1161/CIRCULATIONAHA.118.038908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian F, Ardisson Korat AV, Imamura F, Marklund M, Tintle N, Virtanen JK, Zhou X, Bassett JK, Lai H, Hirakawa Y, et al. ; Fatty Acids and Outcomes Research Consortium (FORCE). n-3 fatty acid biomarkers and incident type 2 diabetes: an individual participant-level pooling project of 20 prospective cohort studies. Diabetes Care. 2021;44:1133–1142. doi: 10.2337/dc20-2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu JHY, Marklund M, Imamura F, Tintle N, Ardisson Korat AV, de Goede J, Zhou X, Yang WS, de Oliveira Otto MC, Kröger J, et al. ; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE). Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017;5:965–974. doi: 10.1016/S2213-8587(17)30307-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kröger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, Döring F, Joost HG, Boeing H, Schulze MB. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr. 2011;93:127–142. doi: 10.3945/ajcn.110.005447 [DOI] [PubMed] [Google Scholar]
- 33.Forouhi NG, Imamura F, Sharp SJ, Koulman A, Schulze MB, Zheng J, Ye Z, Sluijs I, Guevara M, Huerta JM, et al. Association of plasma phospholipid n-3 and n-6 polyunsaturated fatty acids with type 2 diabetes: the EPIC-InterAct case-cohort study. PLoS Med. 2016;13:e1002094. doi: 10.1371/journal.pmed.1002094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lankinen MA, de Mello VD, Meuronen T, Sallinen T, Ågren J, Virtanen KA, Laakso M, Pihlajamäki J, Schwab U. The FADS1 genotype modifies metabolic responses to the linoleic acid and alpha-linolenic acid containing plant oils-genotype based randomized trial FADSDIET2. Mol Nutr Food Res. 2021;65:e2001004. doi: 10.1002/mnfr.202001004 [DOI] [PubMed] [Google Scholar]
- 35.Imamura F, Fretts A, Marklund M, Ardisson Korat AV, Yang WS, Lankinen M, Qureshi W, Helmer C, Chen TA, Wong K, et al. ; InterAct Consortium; Fatty Acids and Outcomes Research Consortium (FORCE). Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: a pooled analysis of prospective cohort studies. PLoS Med. 2018;15:e1002670. doi: 10.1371/journal.pmed.1002670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang J, Zhou Q, Kwame Amakye W, Su Y, Zhang Z. Biomarkers of dairy fat intake and risk of cardiovascular disease: a systematic review and meta analysis of prospective studies. Crit Rev Food Sci Nutr. 2018;58:1122–1130. doi: 10.1080/10408398.2016.1242114 [DOI] [PubMed] [Google Scholar]
- 37.Prada M, Wittenbecher C, Eichelmann F, Wernitz A, Drouin-Chartier JP, Schulze MB. Association of the odd-chain fatty acid content in lipid groups with type 2 diabetes risk: a targeted analysis of lipidomics data in the EPIC-Potsdam cohort. Clin Nutr. 2021;40:4988–4999. doi: 10.1016/j.clnu.2021.06.006 [DOI] [PubMed] [Google Scholar]
- 38.Stegemann C, Drozdov I, Shalhoub J, Humphries J, Ladroue C, Didangelos A, Baumert M, Allen M, Davies AH, Monaco C, et al. Comparative lipidomics profiling of human atherosclerotic plaques. Circ Cardiovasc Genet. 2011;4:232–242. doi: 10.1161/CIRCGENETICS.110.959098 [DOI] [PubMed] [Google Scholar]
- 39.Chew WS, Torta F, Ji S, Choi H, Begum H, Sim X, Khoo CM, Khoo EYH, Ong W-Y, Van Dam RM, et al. Large-scale lipidomics identifies associations between plasma sphingolipids and T2DM incidence. JCI Insight. 2019;4:e126925. doi: 10.1172/jci.insight.126925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yun H, Sun L, Wu Q, Zong G, Qi Q, Li H, Zheng H, Zeng R, Liang L, Lin X. Associations among circulating sphingolipids, β-cell function, and risk of developing type 2 diabetes: a population-based cohort study in China. PLoS Med. 2020;17:e1003451. doi: 10.1371/journal.pmed.1003451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mantovani A, Dugo C. Ceramides and risk of major adverse cardiovascular events: A meta-analysis of longitudinal studies. J Clin Lipidol. 2020;14:176–185. doi: 10.1016/j.jacl.2020.01.005 [DOI] [PubMed] [Google Scholar]
- 42.Holmes MV, Millwood IY, Kartsonaki C, Hill MR, Bennett DA, Boxall R, Guo Y, Xu X, Bian Z, Hu R, et al. ; China Kadoorie Biobank Collaborative Group. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J Am Coll Cardiol. 2018;71:620–632. doi: 10.1016/j.jacc.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yilmaz M, Claiborn KC, Hotamisligil GS. De novo lipogenesis products and endogenous lipokines. Diabetes. 2016;65:1800–1807. doi: 10.2337/db16-0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Q, Vijayakumar A, Kahn BB. Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol. 2018;19:654–672. doi: 10.1038/s41580-018-0044-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kröger J, Jacobs S, Jansen EH, Fritsche A, Boeing H, Schulze MB. Erythrocyte membrane fatty acid fluidity and risk of type 2 diabetes in the EPIC-Potsdam study. Diabetologia. 2015;58:282–289. doi: 10.1007/s00125-014-3421-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brenna JT, Plourde M, Stark KD, Jones PJ, Lin YH. Best practices for the design, laboratory analysis, and reporting of trials involving fatty acids. Am J Clin Nutr. 2018;108:211–227. doi: 10.1093/ajcn/nqy089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jäger S, Cuadrat R, Hoffmann P, Wittenbecher C, Schulze MB. Desaturase activity and the risk of type 2 diabetes and coronary artery disease: a mendelian randomization study. Nutrients. 2020;12:2261. doi: 10.3390/nu12082261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wittenbecher C, Cuadrat R, Johnston L, Eichelmann F, Jäger S, Kuxhaus O, Prada M, Del Greco M F, Hicks AA, Hoffman P, et al. Dihydroceramide- and ceramide-profiling provides insights into human cardiometabolic disease etiology. Nat Commun. 2022;13:936. doi: 10.1038/s41467-022-28496-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rothman KJ, Greenland S, Lash TL. Validity in epidemiologic studies. In: Rothman KJ, Greenland S, Lash TL, eds. Modern Epidemiology. 3rd ed. Lippincott Williams & Wilkins; 2008:128–147. [Google Scholar]
- 50.Ubhi BK. Direct infusion-tandem mass spectrometry (DI-MS/MS) analysis of complex lipids in human plasma and serum using the LipidyzerTM Platform. In: Methods Mol Biol. 2018;1730:227–236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.