ABSTRACT
Asexual sporulation is the most common reproduction mode of fungi. Most filamentous fungi have two conidiation patterns, normal conidiation and microcycle conidiation, which may be regulated by nutritional conditions. Nitrogen source can affect the fungal conidiation pattern, but the regulatory mechanism is not fully understood. In this study, we report a C2H2 zinc finger protein, MaNCP1, which has typical transcription factor characteristics and is screened from the subtractive library regulated by nitrate in the entomopathogenic fungus Metarhizium acridum. MaNCP1 and its N-terminal play critical roles in the conidiation pattern shift. Further study shows that MaNCP1 interacts with MaNmrA, which also contributes to the conidiation pattern shift and is involved in the reductive pathway of nitric oxide (NO) synthesis. Intriguingly, the conidiation pattern of the MaNCP1-disruption strain (ΔMaNCP1) can be restored to microcycle conidiation when grown on the microcycle conidiation medium, SYA, supplemented with NO donor or overexpressing MaNmrA in ΔMaNCP1. Here, we reveal that MaNCP1 governs the conidiation pattern shift through regulating the reductive synthesis of NO by physically targeting MaNmrA in M. acridum. This work provides new mechanistic insights into how changes in nitrogen utilization are linked to the regulation of fungal morphological changes.
IMPORTANCE Fungal conidia play important roles in the response to environmental stimuli and evasion of the host immune system. The nitrogen source is one of the main factors affecting shifts in fungal conidiation patterns, but the regulatory mechanism involved is not fully understood. In this work, we report that the C2H2 zinc finger protein, MaNCP1, governs the conidiation pattern shift in M. acridum by targeting the MaNmrA gene, thereby altering the regulation of the reductive pathway for NO synthesis. This work provides further insights into how the nutritional environment can regulate the morphogenesis of filamentous fungi.
KEYWORDS: Metarhizium acridum, C2H2 zinc finger protein, MaNCP1, conidiation pattern, MaNmrA, nitric oxide
INTRODUCTION
Entomopathogenic fungi, such as Metarhizium spp., are fungi that can infect and cause the death of their insect hosts; they play important roles in the control of agricultural pests (1). Conidia generated by asexual reproduction play an important role in the process of fungal distribution due to their resistance to adverse environmental conditions, host immune attack and proliferation ability (2–4). Moreover, conidia are the main infective vectors of entomopathogenic fungi (1) and the main effective unit of insecticidal fungal pesticides. The production of conidia is therefore a prerequisite for the large-scale industrial production of biocontrol fungi (1, 5–7).
Most filamentous fungi, including the model entomopathogenic fungus M. acridum, have two conidiation patterns: normal conidiation and microcycle conidiation (8). Furthermore, it has been shown that the microcycle conidiation exhibits a greater potential for use in biological pest control than normal conidiation in M. acridum (9). Normal conidiation occurs in the typical life cycle of filamentous fungi, which comprises conidiation after a period of vegetative growth (10). Microcycle conidiation bypasses the vegetative hyphae and produces conidia directly from the germinated conidia (8). Usually, shifting from the normal conidiation to the microcycle conidiation is a survival mechanism for fungi to adapt to adverse environmental factors, including temperature (10), pH (11), salt concentration (12) and nutrients (13). Nutrition is one of the most important influencing factors and the two conidiation patterns are interconvertible under different nutrition conditions in many fungi (14). Changing the content of nitrogen source (nitrate) in the microcycle conidiation medium, SYA, can shift the conidiation pattern of M. acridum to normal (15), although the underlying mechanism is still unclear. Therefore, elucidating how changes in nutrient nitrate conditions can drive shifts in the conidiation pattern should provide further insights into the regulatory roles of nitrogen utilization in fungal morphogenesis.
Nitrogen is necessary for the growth of fungi, which preferentially use nitrogen sources such as ammonium and glutamine, but can use alternative sources, such as nitrate, urea, guanidine and pyrimidine where ammonium and glutamine are absent (16). This nitrogen source selection, called nitrogen catabolite repression (NCR), is mainly mediated by AreA, a GATA transcription factor in A. nidulans (17). NmrA, a member of the short-chain dehydrogenase reductase superfamily, is a core regulator gene in the NCR pathway that affects fungal nitrogen metabolism (18, 19) by interacting with and inhibiting the activity of AreA (20). The expression level of NmrA increases with sufficient nitrogen availability but decreases under nitrogen starvation (19). NmrA is also regulated by the bZIP transcription factor MeaB (21), which can work with AreA synergistically or independently in the NCR pathway (19). Three NmrA homologous proteins, Nmr1, Nmr2 and Nmr3 have been characterized in Magnaporthe oryzae, which can bind to NADP+ and are regulated by the NADPH sensor protein Tps1 (22), which could trigger carbon catabolite repression (CCR) via the inactivation of Nmr1-3 in a mechanism independent of Nut1, an AreA homologous protein (23). In Aspergillus nidulans, NmrA, together with AreA and AreB (another GATA transcription factor), negatively regulate arginine catabolism in response to nitrogen and carbon source (24). Furthermore, NmrA appears to be involved in sclerotia formation, conidiation, virulence and aflatoxin biosynthesis in A. flavus (25).
Nitric oxide (NO), as a signaling molecule, plays important roles in many biological processes (26). NO can be produced via the reductive pathway, in which nitrate is reduced by nitrate reductase (NR), or the oxidative pathway, where L-arginine is oxidized by nitric oxide synthase (NOS) (26). However, no clear homologs to NOS have been identified in fungi where the NO production apparently mainly occurs via the reductive pathway (26, 27). NO can be detected in many filamentous fungi, such as Neurospora crassa (28), Phycomyces blakesleeanus (29), Colletotrichum coccodes (30), Blastocladiella emersonii (31), Coniothyrium minitans (32) and Fusarium graminearum (33). Previous studies have shown that exogenous NO can inhibit the light-induced conidiation in N. crassa (28) and A. nidulans (34). In C. minitans, NO is accumulated heavily during the formation of conidia (35) and regulates the conidiation through the cGMP signaling pathway (31, 35). However, the regulation of NO production during fungal conidiation remains largely uncharacterized.
To reveal the molecular components involved in transducing nitrate effects on the conidiation pattern in M. acridum, a differential expression library regulated by nitrate was constructed (15). A C2H2 zinc finger protein MAC_04326 with transcriptional factor activity was identified from this library and denominated MaNCP1 (M. acridum nitrate-related conidiation pattern shift regulatory factor 1). In M. oryzae, MGG_07339 (the homologue of MaNCP1) is involved in regulating the conidiation and virulence on rice seedlings (36). In this study, we found that MaNCP1 governs the conidiation pattern shift through regulating the reductive pathway for NO synthesis via physical interaction with the MaNmrA gene in M. acridum. These results provide insights into the regulatory molecular network linking changes in nitrate metabolism with shifts in conidiation patterns of filamentous fungi.
RESULTS
MaNCP1 contributes to the conidiation pattern shift.
To explore the regulatory roles of genes mediated by nitrate in regulating the conidiation pattern shift, eight putative transcriptional factor genes were screened from the differential expression library regulated by nitrate (15) and further verified by quantitative reverse-transcription PCR (qRT-PCR). The results showed that MAC_02692, MAC_04326 and MAC_03846 were specifically upregulated in SYA+N medium (Fig. S1A), which were consistent with the transcriptome data. However, no further evidence for a role for either MAC_02692 or MAC_03846 in the conidiation pattern shift was observed (Fig. S1B). In contrast, the deletion of MAC_04326 (MaNCP1) to produce the strain ΔMaNCP1 led to the normal conidiation of M. acridum, which could be restored to microcycle conidiation by reintroducing the MaNCP1 cassette into ΔMaNCP1 on SYA medium (Fig. S1C, and S2A-C). In addition, the deletion of MaNCP1 resulted in an increasing number of septa (Fig. S3A and B), longer hyphae (Fig. S3A and C) and an abnormal distribution of Spitzenkörper in the hypha apex (arrows in Fig. S3D), indicating MaNCP1 was involved in hyphal polar growth.
MaNCP1 encodes a C2H2 zinc finger domain protein of 756 amino acid residues, with a predicted molecular mass of 84.95 kDa and an isoelectric point of 6.35. The MaNCP1 protein contains two predicted nuclear localization signals, three C2H2-type zinc fingers in the N-terminal and a further two C2H2-type zinc fingers in the C-terminal (Fig. 1A). The homologous zinc finger proteins are also found in other filamentous fungi (Fig. S4A) and the zinc fingers are highly similar to each other (Fig. S4B). Further phylogenetic analysis and multiple sequence alignments show that MaNCP1 is relatively close in evolution to filamentous fungi but far from yeast (Fig. S4C and S5). A transcriptional activity assay indicated that MaNCP1 has transcriptional activation ability (Fig. 1B). Furthermore, the MaNCP1::eGFP fusion protein locates in the nucleus (Fig. 1C). Thus, MaNCP1 has typical characteristics of a transcriptional factor.
FIG 1.
The N-terminal zinc fingers of MaNCP1 play important roles in the conidiation pattern shift. (A) Structural features of MaNCP1. (B) Autoactivation testing of MaNCP1 using the yeast two-hybrid assay. The empty vector, pGBKT7, was used as a negative control and pGBKT7-53 and pGADT7-T were used as a positive control. (C) MaNCP1::eGFP subcellular localization. (D) Conidiation patterns of the WT, ΔMaNCP1, MaNCP1ΔN (the N-terminal C2H2 zinc fingers deletion mutant), MaNCP1ΔC (The C-terminal C2H2 zinc fingers deletion mutant) and MaNCP1ΔN+C (The combined N- and C-terminal C2H2 zinc fingers deletion mutant) strains grown on SYA at 28°C for hours. (E) Conidiation pattern of the truncated protein Mut strain without the C-terminal of MaNCP1.
To further observe the influence of the different zinc finger domains on the conidiation pattern shift, the domain deletion mutants MaNCP1ΔN, MaNCP1ΔC, and MaNCP1ΔN+C were obtained (Fig. S2D and E). It showed that the conidiation pattern of the MaNCP1ΔC strain was similar to the WT, while the MaNCP1ΔN strain was similar to the ΔMaNCP1 when grown on SYA (Fig. 1D). In addition, the C-terminal truncated MaNCP1 strain (Mut) was constructed and exhibited microcycle conidiation on SYA, which was similar to the WT strain (Fig. 1E) and demonstrates a greater contribution of the N-terminal of MaNCP1 to the conidiation pattern shift than that of the C-terminal.
MaNmrA is a direct target gene of MaNCP1 during the conidiation pattern shift.
To reveal the functions of MaNCP1 in regulating the conidiation pattern shift, RNA-seq was performed to search the differentially expressed genes (DEGs) between the WT and ΔMaNCP1 strains. Although the expression level of MaNCP1 was highest at 21 h (Fig. S6A), the samples of ΔMaNCP1 and WT strains were collected at 18 h for RNA-seq analysis, the earliest time point of observable difference in the conidiation pattern. A total of 9,552 genes were mapped to the M. acridum genome and there were 95 DEGs in ΔMaNCP1-vs-WT (Fig. S7A; Table S1), and these DEGs were involved in a variety of cellular processes and metabolic pathways (Fig. S7B and S7C). Furthermore, all the randomly selected DEGs exhibited a similar expression pattern to that observed in their respective DEG data (Fig. S6D), indicating that the RNA-seq data was reliable.
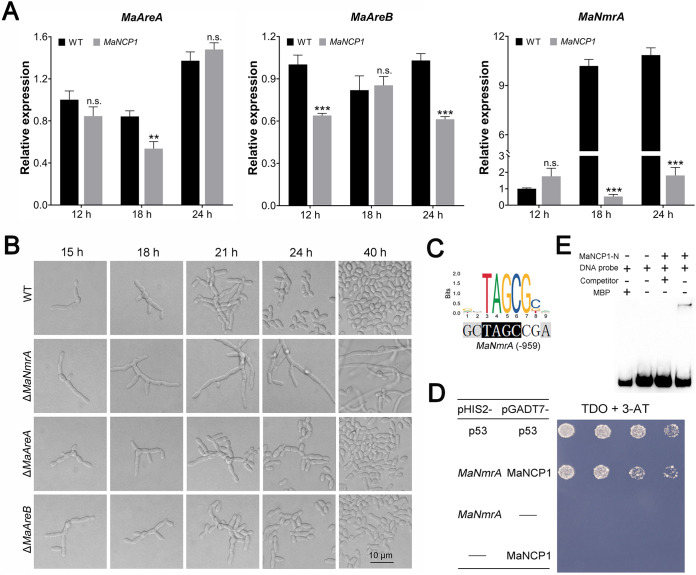
Moreover, MaNCP1 was screened from the differential expression library regulated by nitrate, and MAC_02196, an NmrA family member gene, was selected from the RNA-seq DEG data to be characterized (Fig. S7D). Unfortunately, there was no obvious difference in conidiation pattern between the ΔMAC_02196 and WT strains (Fig. S6B and C). Thus, we speculated whether other genes in the NCR pathway may be regulated by MaNCP1. Subsequently, three core genes of the NCR pathway of M. acridum, MaAreA (MAC_00939) (Fig. S8A and B), MaAreB (MAC_07065) (37) and MaNmrA (MAC_00749) (38) were selected for further analyses. A qRT-PCR analysis of the expression of these genes in ΔMaNCP1 versus WT on SYA medium (Fig. 2A) showed that the whereas the expression of MaAreA in ΔMaNCP1 was about 0.7 times of the WT at 18 h and MaAreB was about 0.8 times of the WT at 24 h, the expression of MaNmrA was reduced to ca. 0.05 times and 0.14 times of the WT at 18 h and 24 h, respectively. Furthermore, after the disruption of these three genes, only the conidiation pattern of the MaNmrA-disruption strain (ΔMaNmrA) was shifted to normal conidiation (Fig. 2B), similar to that observed in ΔMaNCP1. Moreover, the promoter of MaNmrA contained the putative binding site of MaNCP1 (Fig. 2C). Yeast one-hybrid assay showed that the recombinant yeast Y187 (pGADT7-MaNCP1×pHIS2-MaNmrA) could grow on TDO plates with the optimal concentration of 3-AT (55 mM) (Fig. S8C and Fig. 2D), indicating that MaNmrA was a direct target gene of MaNCP1. Moreover, the N-terminal of MaNCP1 (228 aa) was expressed in E. coli (Fig. S8D and E) and an electrophoretic mobility shift assay (EMSA) demonstrated that the N-terminal of MaNCP1 could specifically recognize the promoter of MaNmrA (Fig. 2E). Taken together, the data indicates that MaNCP1 may regulate the conidiation pattern by control of MaNmrA.
FIG 2.
MaNmrA is a direct target gene of MaNCP1. (A) Relative expression analysis of MaAreA, MaAreB and MaNmrA. Fungal samples for qRT-PCR were collected at 12, 18 and 24 h of culture on SYA medium at 28°C. n.s. no significant difference, P > 0.05. **, P < 0.01; ***, P < 0.001. (B) Conidiation pattern of the MaAreA, MaAreB and MaNmrA mutants grown on SYA media. (C) Prediction of the putative MaNCP1 binding site in cis-elements present in the promoters of potential downstream target genes of MaNCP1 were identified using the JASPAR 2020 database (54). (D) Yeast one-hybrid assay on TDO, SD/-Leu/-Trp/-His plates. 55 mM 3-AT was added into TDO plate. pHIS2-p53 and pGADT7-p53 acted as the positive control. pHIS2 and pGADT7-MaNCP1 or pGADT7 and pHIS2-MaNmrA acted as the negative control. (E) EMSA of MaNCP1 interaction with its putative binding site in MaNmrA. MaNCP1-N, the zinc finger cluster at the N-terminal of MaNCP1. MBP, the MBP-tag protein. Competitor, the unlabeled probe, was used in 100-fold excess to the biotin labeled probe.
MaNCP1 and MaNmrA regulate the reductive pathway of NO synthesis.
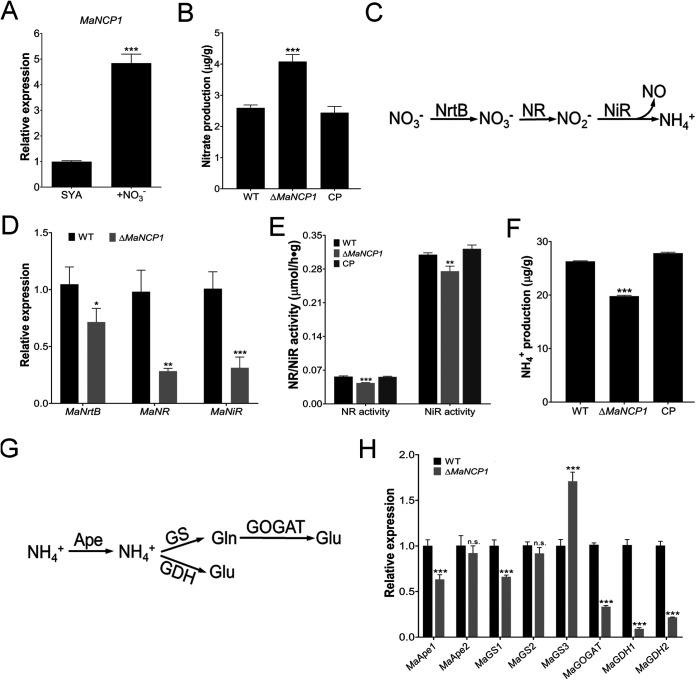
NmrA plays an important role in regulating the reductive pathway of NO synthesis, that is, the nitrate assimilation process (18, 19). Therefore, we tested whether MaNCP1 also affected the reductive synthesis of NO. The transcription level of MaNCP1 was significantly upregulated in SYA+N medium (Fig. 3A). The nitrate content in the ΔMaNCP1 strain was significantly higher than that in the WT or CP strain (Fig. 3B). qRT-PCR analysis showed that the gene expression levels of the nitrate transporter, MaNrtB (MAC_03189), nitrate reductase, MaNR (MAC_08624) and nitrite reductase, MaNiR (MAC_03493), which play important roles in the process of nitrate assimilation (Fig. 3C), were significantly lower in ΔMaNCP1 relative to the WT (Fig. 3D). Moreover, NR and NiR activities and the ammonium content in the ΔMaNCP1 strain were significantly reduced (Fig. 3E and F). The expression of a series of genes functionally involved in the process of ammonium assimilation were subsequently analyzed. Whereas no significant difference was observed in the expression levels of MaApe2 (MAC_03001) and MaGS2 (MAC_06858), the genes MaApe1 (MAC_05564), MaGS1 (MAC_01108), MaGOGAT (MAC_00032), MaGDH1 (MAC_08384) and MaGDH2 (MAC_01648), were significantly downregulated, while MaGS3 (MAC_04461) was significantly upregulated (Fig. 3G and H). Similarly, the expression of MaNmrA was also significantly upregulated in SYA+N media (Fig. 4A). Furthermore, the nitrate content in the ΔMaNmrA strain was higher than that in WT or CP-1 (the ΔMaNmrA complementary strain; Fig. 4B) and the expression levels of MaNrtB, MaNR and MaNiR, as well as the activities of NR and NiR in the ΔMaNmrA background were significantly reduced (Fig. 4C and D). However, the ammonium content in the ΔMaNmrA strain was higher (Fig. 4E) and genes involved in ammonium assimilation were also upregulated in the ΔMaNmrA background, except for MaGOGAT, which was not significantly different from that observed in the WT (Fig. 4F).
FIG 3.
Deletion of MaNCP1 affects the reductive pathway of nitrogen utilization and NO synthesis. (A) Relative expression of MaNCP1 in WT strain under SYA and SYA+N conditions. (B) Determination of the nitrate content in the WT, ΔMaNCP1 and CP strains. (C) Schematic diagram of the nitrate assimilation pathway. (D) Relative expression of MaNrtB, MaNR and MaNiR in the ΔMaNCP1 background. (E) Determination of the NR and NiR activities in the WT, ΔMaNCP1 and CP strains. (F) Determination of the ammonium content in the WT, ΔMaNCP1 and CP strains. (G) Schematic diagram of the ammonium assimilation pathway. (H) Relative expression of genes related to ammonium assimilation in the ΔMaNCP1 background. All fungal samples were grown on SYA at 28°C for 24 h. CP, the ΔMaNCP1 complementary strain. n.s. no significant difference, P > 0.05. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG 4.
Deletion of MaNmrA affects the reductive pathway of NO synthesis and nitrogen utilization. (A) Relative expression of MaNmrA in the WT strain under SYA and SYA+N conditions. (B) Determination of the nitrate content in the WT, ΔMaNmrA and the ΔMaNmrA complementary (CP-1) strains. (C) Relative expression of MaNrtB, MaNR and MaNiR in the ΔMaNmrA background. (D) Determination of the NR and NiR activities in WT, ΔMaNmrA and CP-1 strains. (E) Determination of the ammonium content in WT, ΔMaNmrA and CP-1 strains. (F) Relative expression of genes related to ammonium assimilation in the ΔMaNmrA background. All fungal samples were grown on SYA at 28°C for 24 h. n.s. no significant difference, P > 0.05. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
NO is involved in the regulation of conidiation pattern shift.
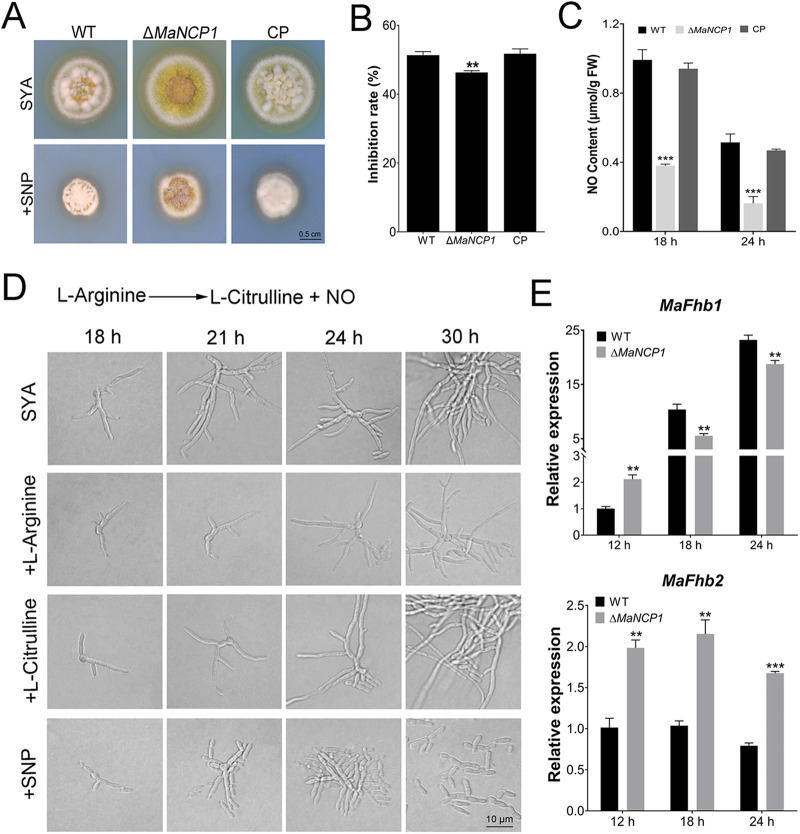
NO is an important signal molecule involved in the fungal adaptation to adverse environmental conditions (39), fungal virulence (33, 40–42), and conidial production (27, 32, 34, 35). Therefore, we tested whether the conidiation pattern shift regulated by MaNCP1 occurred with alterations to the NO content in M. acridum. The inhibition rate of the ΔMaNCP1 strain was significantly lower than that of the WT or CP strain grown on SYA supplemented with the NO donor, sodium nitroprusside (SNP) (Fig. 5A and B), and the NO content of ΔMaNCP1 strain was significantly lower than that of the WT or CP strain at 18 h and 24 h (Fig. 5C). To investigate the effect of the oxidative synthesis pathway of NO on the conidiation pattern regulated by MaNCP1, supplements L-arginine (the substrate), L-citrulline (a product) and SNP were added into SYA media, and it showed that only SNP supplements could restore microcycle conidiation in the ΔMaNCP1 strain (Fig. 5D). The flavohemoglobin gene FhbA can promote the conversion of NO to nitrate in A. nidulans (43). Disruption of MaNCP1 led to significant increases in the expression levels of two flavohemoglobin genes: MaFhb1 (MAC_06853) at 12 h and MaFhb2 (MAC_09730) at all tested time points (Fig. 5E). Similarly, the conidiation pattern of the ΔMaNmrA strain could be restored to microcycle conidiation when the ΔMaNmrA strain was grown on SYA medium with SNP (Fig. 6A), and the NO content was also significantly decreased in the ΔMaNmrA strain (Fig. 6B). The expression levels of MaFhb1 and MaFhb2 were significantly increased at the different time points in the ΔMaNmrA background (Fig. 6C).
FIG 5.
NO regulates the conidiation pattern shift in ΔMaNCP1 strain. (A, B) Growth and inhibition rates of the WT, ΔMaNCP1 and CP strains grown on SYA without or with SNP. (C) Determination of the NO content in the fungal strains on SYA medium over time. (D) Conidiation pattern of the ΔMaNCP1 strain grown on SYA medium supplemented with 2 mM L-Arginine, 2 mM L-Citrulline or 3 mM SNP. A schematic diagram of the arginine metabolism pathway is presented above. (E) Relative expression of MaFhb1 and MaFhb2 in the WT and ΔMaNCP1 strains cultured on SYA over time. n.s. no significant difference, P > 0.05. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG 6.
NO regulates the conidiation pattern shift in ΔMaNmrA strain. (A) Conidiation pattern of the ΔMaNmrA strain grown on SYA without or with 3 mM SNP. (B) Determination of the NO content in the WT, ΔMaNmrA and CP-1 strains on SYA medium over time. (C) Relative expression of MaFhb1 and MaFhb2 in the WT and ΔMaNmrA strains cultured on SYA over time. n.s. no significant difference, P > 0.05. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Overexpressing MaNmrA in the ΔMaNCP1 strain can restore the shift to microcycle conidiation on SYA medium.
To further verify whether MaNCP1 regulates the conidiation pattern shift through its binding to MaNmrA, the MaNmrA gene was overexpressed in the ΔMaNCP1 strain, ΔMaNCP1/MaNmrAOE where the conidiation pattern was observed to be similar to that of the WT strain (Fig. 7A). The expression of MaNmrA in the ΔMaNCP1/MaNmrAOE strain was significantly higher than that in the ΔMaNCP1 strain (Fig. 7B). Moreover, the NO content in the ΔMaNCP1/MaNmrAOE strain was significantly increased compared to the ΔMaNCP1 strain (Fig. 7C). Furthermore, the activities of MaNR and MaNiR (Fig. 7D) and the expression levels of MaNR and MaNiR (Fig. 7E) were significantly increased in the ΔMaNCP1/MaNmrAOE strain. Taken together, the data indicate that overexpressing MaNmrA in the ΔMaNCP1 strain can promote nitrate assimilation to increase the intracellular NO content, leading to the restoration of microcycle conidiation.
FIG 7.
MaNCP1 regulates the conidiation pattern shift by binding to MaNmrA. (A) Conidiation pattern of the ΔMaNCP1/MaNmrAOE strain grown on SYA at 28°C over time. ΔMaNCP1/MaNmrAOE refers to the strain overexpressing the MaNmrA gene in the ΔMaNCP1 background. (B) Relative expression of MaNmrA. (C) Determination of the NO content in the fungal strains. (D) Determination of the NR and NiR activities in the fungal strains. (E) Relative expression of MaNR and MaNiR. Fungal samples were collected after 24 h of culture on SYA plates at 28°C. n.s. no significant difference, P > 0.05. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In summary, MaNCP1 is activated by nitrate and binds to MaNmrA to promote nitrate assimilation and the increase in intracellular NO content, leading to microcycle conidiation of M. acridum on SYA medium (Fig. 8A). In support, MaNCP1 gene disruption leads to a significantly reduced expression of MaNmrA, with consequent impairment to nitrate assimilation and a significant decrease in NO content, resulting in the onset of normal conidiation of M. acridum on SYA medium (Fig. 8B).
FIG 8.
Schematic model of how MaNCP1 governs the conidiation pattern shift by regulating the nitrate metabolism in M. acridum. (A) MaNCP1 is involved in the nitrate assimilation pathway (the reductive pathway of NO synthesis) by binding to MaNmrA to promote the activities of key enzymes, such as nitrate reductase NR and nitrite reductase NiR, and the expression of their encoding genes to increase the intracellular NO content, leading to microcycle conidiation of M. acridum when grown on SYA medium. (B) Disruption of the MaNCP1 gene significantly reduces the expression level of MaNmrA and impairs the nitrate assimilation to significantly decrease the intracellular NO content, resulting in the normal conidiation pattern in M. acridum when grown on SYA medium. The red fork indicates the deletion of the MaNCP1 gene. The green arrows indicate positive regulation, blue arrows indicate biosynthesis and metabolic pathways. The red arrows indicate an increase or decrease in NR/NiR activity, the expression level of MaNmrA or the contents of nitrate, ammonium and NO in the absence of MaNCP1.
DISCUSSION
The conidial production of filamentous fungi is one of the most critical stages in their life cycle and plays important roles in the continued reproduction of the species and the maintenance of ecological diversity (2, 44). Here, we have studied the regulatory pathway governing the shift in conidiation pattern in response to nitrate in the model entomopathogenic fungus, M. acridum. MaNCP1, a protein with multiple C2H2 zinc finger domains, was found to mediate the reductive pathway of NO synthesis, namely, the nitrate assimilation pathway, to regulate the conidiation pattern in M. acridum. This study offers insights into novel technical means for improving the productivity of mycoinsecticides.
To date, homologs of MaNCP1 have not been reported in entomopathogenic fungi. The MaNCP1 protein contains similar domains to the mammalian Ikaros family members (Ikaros, Aiolos, Helios, Eos, and Pegasus), which all contain two C2H2 domain clusters: an N-terminal cluster composed of 3–4 zinc fingers and a C-terminal cluster containing 2 zinc fingers (45). In general, the N-terminal zinc fingers of the Ikaros family proteins are involved in binding to the DNA of target genes, while the C-terminal zinc fingers mediate protein-protein interactions (45, 46). Here, our results show that MaNCP1 has the typical characteristics of a transcription factor, and that the N-terminal of MaNCP1 protein is involved in the conidiation pattern shift. MaNmrA, a core gene in the NCR pathway, which also regulates the conidiation pattern shift of M. acridum, was identified in a screen for downstream target genes and was subsequently confirmed as a direct downstream target gene of MaNCP1. The NCR pathway is involved in regulating the expression of genes related to the assimilation and catabolism of multiple nitrogen sources (47). It has been reported that the core regulators in NCR pathway are not only involved in arginine metabolism (24), but also regulate the expression of nitrate reductase and nitrite reductase to promote nitrate assimilation (48) which, in fungi mainly involves the reductive pathway of NO synthesis.
The signaling molecule, NO, is involved in many fungal biological processes, such as in the adaptation of S. cerevisiae to adverse environmental conditions (39), the formation of appressoria in Blumeria graminis (40), and the development of conidia in N. crassa (28) and C. minitans (35). The reductive pathway of NO synthesis, namely, nitrate assimilation pathway, is a major pathway for producing NO in fungi, which has been confirmed in A. nidulans (27). In this study, we found that the transcription of the nitrate transporter encoding gene MaNrtB was significantly downregulated in ΔMaNCP1 and ΔMaNmrA strains, suggesting a reduced import of nitrate. Nevertheless, the nitrate contents in the ΔMaNCP1 and ΔMaNmrA strains were significantly higher than that in the WT or CP strains, indicating that the nitrate catabolism was significantly impaired in the absence of functional MaNCP1 or MaNmrA genes in M. acridum. Consistently, the relative expression levels of MaNR and MaNiR, the activities of NR and NiR and the NO contents were significantly decreased in both the ΔMaNCP1 and ΔMaNmrA strains. In addition, deletion of either MaNCP1 or MaNmrA affected the ammonium assimilation, but the ammonium contents and the expression trends of key genes involved in ammonium assimilation were opposite, indicating that MaNCP1 and MaNmrA played different roles in ammonium assimilation. It also indicated that the conidiation pattern shift regulated by MaNCP1 and MaNmrA may be mainly mediated by the assimilation of nitrate, rather than ammonium. Furthermore, flavohemoglobin may be a further factor responsible for initiating changes in NO contents, since flavohemoglobin can catalyze the conversion of NO to produce nitrate and/or nitrite, thereby preventing nitrooxidative stress in cells caused by excessive NO accumulation (43, 49, 50). Here, we found that the flavohemoglobin gene, MaFhb2, was upregulated in the absence of MaNCP1, while both MaFhb1 and MaFhb2 were upregulated in the absence of MaNmrA.
Furthermore, studies have shown that the synthesis of NO in filamentous fungi can be independent of the NOS oxidative pathway (51), and an arginine-dependent biochemical route of NO synthesis, similar to that involving NOS, has recently been reported to be active in A. nidulans (52). However, we found that the conidiation pattern of ΔMaNCP1 strain could not be restored when L-arginine or L-citrulline, the substrate and a product in the oxidative synthesis pathway of NO, were added to SYA medium, indicating that L-arginine metabolism plays a negligible role in regulating MaNCP1- mediated conidiation pattern shift. Nevertheless, we cannot rule out a role for an oxidative synthesis pathway in NO production during the conidiation pattern shift simply because no NOS homologues have not been identified in fungal genomes (41). Moreover, it was found that the overexpression of MaNmrA in the ΔMaNCP1 strain could restore microcycle conidiation pattern and WT levels of NO content. This indicated that MaNCP1 regulated the conidiation pattern shift through affecting the reductive synthesis of NO by binding on MaNmrA.
Due to a lack of any obvious DNA binding sequence, NmrA must bind to some transcription factors to regulate the expression of downstream target genes. Under nitrogen sufficiency, NmrA can bind to the AreA protein, a core GATA transcription factor in the NCR pathway, to inhibit the expression of genes involved in nitrate assimilation (16, 19). Under nitrogen starvation, NIT-4, a homolog of the pathway-specific transcription factor, NirA, has also been confirmed as a downstream target gene of NmrA in N. crassa (53). In addition, the M. oryzae NmrA homologues can also interact with the other GATA transcription factors, PAS1 and ASD4 (22). Therefore, the targeted transcription factors interacting with NmrA might differ under different conditions. In this study, we have confirmed that the expression of MaNmrA was significantly downregulated in the absence of MaNCP1 and the activity of key enzymes, NR and NiR, together with the expression of their encoding genes both decreased in the ΔMaNCP1 and ΔMaNmrA strains that grown on SYA media. However, the culture conditions used in this study did not provide typical nitrogen repressed or de-repressive environments. In fact, the M. acridum WT strain on SYA medium did not exhibit the typical de-repression status in the NR and NiR activity assays (Fig. S9), implying that the selection of nitrogen source for use involved a more complex regulatory mechanism. Furthermore, the deletion of MaAreA did not affect the conidiation pattern of M. acridum on SYA medium. We therefore reasoned that NmrA plays a central role in regulating conidiation pattern shift on SYA medium, and that this functions independently of AreA. Thus, we postulate that NmrA may interact with alternative transcription factor(s) to activate nitrate catabolism during the conidiation pattern shift in M. acridum. Further research is now needed to identify the transcription factor(s) involved downstream of NmrA in the regulation of the conidiation pattern shift on SYA medium.
MATERIALS AND METHODS
Strains and culture conditions.
All mutants were generated from the wild-type M. acridum strain CQMa102 (WT) and grown on 1/4 SDAY media (10‰ glucose, 5‰ yeast extract, 2.5‰ peptone and 18‰ agar, wt/vol) or SYA media (5‰ yeast extract, 0.5‰ KCl, 1‰ KH2PO4, 30‰ sucrose, 0.5‰ MgSO4, 3‰ NaNO3, 0.01‰ MnSO4, 0.01‰ FeSO4 and 18‰ agar, wt/vol) at 28°C. The Y2HGold and Y187 yeast strains (Clontech, Palo Alto, CA, USA) were used in the autoactivation and yeast one-hybrid assays, respectively. Escherichia coli DH5α (Solarbio, Beijing, China) and Agrobacterium tumefaciens AGL1 (Solarbio, Beijing, China) were used for the recombinant plasmid manipulations and fungal transformations, respectively.
Bioinformatics analyses.
All protein sequences used in this study were retrieved and downloaded from the NCBI genome database. cNLS mapper (https://nls-mapper.iab.keio.ac.jp/cgi-bin/NLS_Mapper_form.cgi) was used to predict the nuclear localization signal. Multiple sequence alignments were conducted with DNAMAN software (version 7). MEGA6.0 was used to construct the Neighbor-joining phylogenetic tree using the default settings with 1000 bootstrap replicates. The JASPAR 2020 database (http://jaspar.genereg.net/) (54) was used to analyze the putative binding sites of MaNCP1.
Constructions of the mutants.
To construct the MaNCP1-disruption vectors pK2-SM-MaNCP1-F and pK2-SM-MaNCP1-R (Fig. S2A), about 1,200-bp up- and downstream fragments of MaNCP1 were amplified from the genomic DNA of WT strain, followed by ligating into pK2-SM-F and pK2-SM-R vectors as described previously (55). The MaAreB- and MaNmrA-disruption strains were constructed previously (37, 38). The constructions of the disruption vectors of MAC_02692, MAC_03846, MAC_02196 and MaAreA were made similar to that of MaNCP1. To construct the MaNCP1 complementary vector pK2-MaNCP1-sur (Fig. S2B), a 2,482-bp DNA sequence of MaNCP1 with its adjacent 1,200-bp upstream fragment and a 1,200-bp downstream fragment were amplified to insert into pK2-Sur vector (56). To further explore the functions of the two clusters of zinc finger domains in MaNCP1, fusion PCR was used to amplify the C2H2 zinc fingers domain deletion fragments (the details were addressed in Table S2 and S3), which were ligated into pK2-SM-F, and about a 1.3 kb fragment at the 3′ flanking of MaNCP1 gene was amplified and inserted into the PK2-SM-R vector. These recombinant plasmids were used to screen the C2H2 zinc fingers domain deletion mutants (Fig. S2D). The N- and C-terminal zinc finger cluster mutants were labeled as MaNCP1ΔN and MaNCP1ΔC, respectively. The combined N- and C-terminal zinc finger cluster deletion mutant was marked as MaNCP1ΔN+C. To determine the subcellular localization of MaNCP1, the overexpression vector pK2-pgpdA-MaNCP1-eGFP-sur was constructed. A 2,479-bp DNA sequence of MaNCP1 was amplified with primers MaNCP1-OF/MaNCP1-OR and inserted into pK2-pgpdA-eGFP-sur vector, which carried a constitutive promoter pgpdA and an enhanced green fluorescent protein (eGFP). To overexpress the MaNmrA gene in the ΔMaNCP1 strain, a 1,383-bp DNA fragment of MaNmrA and pgpdA promoter were cloned and inserted into pK2-Nat (57), which harbors a Nat cassette, to form the MaNmrA overexpression vector pK2-pgpdA-MaNmrA-Nat. The recombinant plasmids were transferred into AGL1 for fungal transformation. Knockout transformants (e.g., ΔMaNCP1) were selected on Czapek-dox agar (CZA) media with 500 μg/mL glufosinate-ammonium (Sigma, St. Louis, MO, USA). The complementary (CP) and overexpression transformants were screened on CZA media with 20 μg/mL chlorimuron ethyl (Sigma, Bellefonte, PA, USA) or 75 μg/mL nourseothricin sulfate (Harveybio, Beijing, China). Transformants were identified by PCR and Southern blotting with DIG High Prime DNA Labeling and Detection Starter Kit I (Roche, Basel, Switzerland) (Fig. S2C and E). Primers used in this study are listed in Table S3.
Microscopic observation of the conidiation pattern.
The fresh and mature conidia (15-day-old) of fungal strains grown on 1/4SDAY media were collected to prepare the conidial suspensions (107 conidia/mL). An aliquot of 100 μL conidial suspension of each fungal strain was spread evenly onto SYA plate and cultured at 28°C for several hours, followed by cutting about 1 cm2 media contained the fungal cultures to observe the fungal growth. Images were taken with a digital light microscope (MOTIC, Xiamen, China).
Analyses of hyphal polar growth and subcellular localization.
To determine the number and the length of hyphal cells, the hyphae of the WT, ΔMaNCP1 and CP strains grown on SYA plates were stained with calcofluor white (CFW) after 14 h of culture, followed by incubating at room temperature for 20–30 min and photographing with a fluorescence microscope (Nikon Y-TV55, Tokyo, Japan). To observe the hyphal polar growth, hyphae were stained with FM4-64 (Invitrogen, Waltham, MA, USA) as described previously (58). For the subcellular localization of MaNCP1, the MaNCP1::eGFP-overexpression strain was cultured on SYA and visualized through fluorescence microscopy.
Transcriptional activity assay.
A 2,271-bp cDNA fragment encoding MaNCP1 was cloned with the primer pair of MaNCP1-F/MaNCP1-R (Table S3 in the online supplemental material) and ligated into the yeast expression vector pGBKT7 to form the recombinant plasmid pGBKT7-MaNCP1, followed by transforming into the yeast strain Y2HGold. The positive transformants were spread on an SD/-Trp solid plate containing 25 μg/mL X-α-Gal and 125 ng/mL Aureobasidin A (AbA) (i.e., SD/-Trp/X-α-Gal/AbA) to determine whether MaNCP1 has autoactivation activity, according to the Yeastmaker Gold Yeast Two-Hybrid System User Manual (Clontech).
Yeast one-hybrid assay.
The cDNA encoding MaNCP1 was amplified and ligated into pGADT7 vector to construct the recombinant plasmid pGADT7-MaNCP1. The promoter sequence of MaNmrA was amplified and ligated into the pHIS2 vector to generate pHIS2-MaNmrA, followed by transferring into the Y187 strain to construct Y187 (pHIS2-MaNmrA) and spreading on the selective media lacking tryptophan and histidine (SD/-Trp/-His) with different concentration of 3-Amino-1,2,4-triazole (3-AT), a competitive inhibitor of HIS3 expression products, to suppress the leakage expression of HIS3 products at the background level and eliminate false positives. Then, pGADT7-MaNCP1 and pHIS2-MaNmrA were co-transformed into the Y187 and spotted on the selective medium lacking leucine, tryptophan and histidine (SD/-Leu/-Trp/-His) with or without 3-AT to observe the growth of the yeast. The preparation and transformation of yeast competent cells were conducted according to the Yeastmaker Yeast Transformation System2 User Manual (Clontech). Y187 cells transformed with pGADT7-53 and pHIS2-53 vectors was used as the positive control. Y187 cells transformed with empty pHIS2 and pGADT7-MaNCP1 or empty pGADT7 and pHIS2-MaNmrA vectors were used as the negative control.
EMSA analysis.
The cDNA sequence of the zinc finger cluster at the N-terminal of MaNCP1 was subcloned and ligated into the expression vector pCold-MBP-TEV to construct the fusion recombinant plasmid pCold-MaNCP1-N. The expressed protein MaNCP1-N was purified by ÄKTA prime plus protein purification system (GE Healthcare, Stockholm, Sweden), followed by concentration and de-salting in a 30-kDa cutoff ultrafiltration tube (Millipore Amicon). The promoter region of MaNmrA, containing a putative binding site for MnNCP1 was amplified with primers Probe-F/Probe-R (Table S3) to produce a 110 bp probe which, was labeled with biotin via the EMSA Probe Biotin Labeling Kit (Beyotime, Shanghai, China) (59). The Chemiluminescent EMSA kit (Beyotime, Shanghai, China) was used for the EMSA (59). The unlabeled probe was added in a 100-fold excess.
qRT-PCR assay.
Total RNAs were isolated from conidia and/or hyphae of the WT or ΔMaNCP1 strain. To further confirm the expression patterns of MAC_00186, MAC_08242, MAC_02692, MAC_03472, MAC_03846, MAC_03700, MAC_04326 and MAC_06473 in the transcriptome data previously (15), samples of the WT strain were collected after growing on the 1/4SDAY, SYA, SYA+Nitrate (SYA+N), SYA+Sucrose (SYA+C) or SYA+Phosphate (SYA+P) plates at 28°C for 21 h. To analyze the expression of MaAreA, MaAreB, MaNmrA, MaNrtB, MaNR, MaNiR, MaFhb1, MaFhb2, etc., fungal samples were harvested from SYA at different time points between 0 and 24 h. Total RNA extraction and cDNA synthesis were performed as per the manufacturer’s instructions using Fungal RNA Kit (OMEGA, USA) and PrimeScript RT reagent kit with genomic DNA Eraser (TaKaRa, Dalian, China), respectively. SYBR Premix Ex Taq (TaKaRa, Dalian, China) was used for qRT-PCR. The relative expression level was quantified using the 2-ΔΔCt method with gpdh gene (MAC_09584) as the internal control gene. Each treatment was performed with three biological replicates. The primers used are listed in Table S3.
Nitrate and nitrite reductase activity assays and detection of NO, nitrate and ammonium content.
Fungal samples grown on SYA for 24 h and/or 30 h at 28°C were collected and ground into powder in liquid nitrogen. To assay the nitrate reductase (NR) activity, the nitrite reductase (NiR) activity and the ammonium content, the lysis buffer were added into the powdered samples to the mass/volume ratio specified in the operation manual of the respective detection kits (Comin, Suzhou, China). The suspensions were then centrifuged at 10,000 × g and 4°C for 15 min, and the supernatant collected for the subsequent biochemical measurements as per the manufacturer’s instructions. To assay the nitrate content, 0.1 g fresh sample was homogenized in 1 mL ddH2O at room temperature, followed by shaking at 90°C for 30 min. After cooling to room temperature, the supernatant were centrifuged at 10,000 × g for 20 min to be measured as described in the manufacturer’s manual (Comin, Suzhou, China). Fungal cultures on SYA at 28°C for hours were collected and washed three times with sterile water for NO content detection using a visible spectrophotometric NO content detection kit (Solarbio, Beijing, China) as described previously (60).
Transcriptomic analysis.
To reveal the mechanism underlying the MaNCP1 regulation of the conidiation pattern shift, RNA-seq was performed to identify the differentially expressed genes (DEGs) in WT versus ΔMaNCP1. Samples of the WT and ΔMaNCP1 strains after 18 h of culture on SYA were collected for RNA extraction. Approximately 10 μg DNA-free RNA from each fungal sample was submitted to BGISEQ-500 (BGI, Shenzhen, China) in the Beijing Genomics Institution (Wuhan, China) with three biological replicates for each fungal strain. The DEGs were defined as those displaying a log2 ΔMaNCP1/WT ratios ≥ 1 or ≤ −1 with a false-discover rate (FDR) ≤ 0.001. The DEGs were annotated according to the NCBI protein databases.
Statistical analysis.
The data, shown in mean ± SE, were analyzed by ANOVA (one-way analysis of variance) using SPSS 24.0 program (SPSS Inc, Chicago, IL, USA).
Data availability.
RNA-seq data had been uploaded to the NCBI BioProject database under the accession number PRJNA748190.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (32172479), the Venture & Innovation Support Program for Chongqing Overseas Returnees (cx2019035), Chongqing talent program of China (cstc2021ycjh-bgzxm0313).
We declare no competing financial interests.
Conceptualization, Y.X. and K.J.; methodology, Y.X. and K.J.; project administration, Y.X. and K.J.; investigation, C.L., D.X., M.H. and Q.Z.; data curation, C.L. and K.J.; writing – original draft preparation, C.L.; visualization, C.L.; writing—review and editing, Y.X. and K.J.; funding acquisition, Y.X. and K.J. All authors have read and agreed to the published version of the manuscript.
Footnotes
Supplemental material is available online only.
Contributor Information
Yuxian Xia, Email: yuxianxia@cqu.edu.cn.
Kai Jin, Email: jinkai@cqu.edu.cn.
Robert A. Arkowitz, Université Côte d'Azur, CNRS, Inserm
REFERENCES
- 1.Wang C, Wang S. 2017. Insect pathogenic fungi: genomics, molecular interactions, and genetic improvements. Annu Rev Entomol 62:73–90. doi: 10.1146/annurev-ento-031616-035509. [DOI] [PubMed] [Google Scholar]
- 2.Hawker LE, Madelin MF. 1976. The dormant spore, p 1–72. In Webber DJ, Hess WM (ed), The fungal spore. Wiley, New York. [Google Scholar]
- 3.Wolken WAM, Tramper J, van der Werf MJ. 2002. Toxicity of terpenes to spores and mycelium of Penicillium digitatum. Biotechnol Bioeng 80:685–690. doi: 10.1002/bit.10435. [DOI] [PubMed] [Google Scholar]
- 4.Tong X, Wang Y, Yang P, Wang C, Kang L. 2020. Tryptamine accumulation caused by deletion of MrMao-1 in Metarhizium genome significantly enhances insecticidal virulence. PLoS Genet 16:e1008675. doi: 10.1371/journal.pgen.1008675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahato SB, Majumdar I. 1993. Current trends in microbial steroid biotransformation. Phytochemistry 34:883–898. doi: 10.1016/s0031-9422(00)90685-x. [DOI] [PubMed] [Google Scholar]
- 6.Howell CR, Hanson LE, Stipanovic RD, Puckhaber LS. 2000. Induction of terpenoid syntheis in cotton roots and control of Rhizoctonia solani by seed treatment with Trichoderma virens. Phytopathology 90:248–252. doi: 10.1094/PHYTO.2000.90.3.248. [DOI] [PubMed] [Google Scholar]
- 7.Wolken WAM, van der Werf MJ. 2001. Geraniol biotransformation-pathway in spores of Penicillium digitatum. Appl Microbiol Biotechnol 57:731–737. doi: 10.1007/s002530100821. [DOI] [PubMed] [Google Scholar]
- 8.Jung B, Kim S, Lee J. 2014. Microcyle conidiation in filamentous fungi. Mycobiology 42:1–5. doi: 10.5941/MYCO.2014.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Peng G, Xia Y. 2010. Microcycle conidiation and the conidial properties in the entomopathogenic fungus Metarhizium acridum on agar medium. Biocontrol Sci Techn 20:809–819. doi: 10.1080/09583157.2010.482201. [DOI] [Google Scholar]
- 10.Anderson JG, Smith JE. 1971. The production of conidiophores and conidia by newly germinated conidia of Aspergillus niger (microcycle conidiation). J Gen Microbiol 69:185–197. doi: 10.1099/00221287-69-2-185. [DOI] [PubMed] [Google Scholar]
- 11.Pažout J, Schröder P. 1988. Microcycle conidiation in submerged cultures of Penicillium cyclopium attained without temperature changes. J Gen Microbiol 134:2685–2692. doi: 10.1099/00221287-134-10-2685. [DOI] [PubMed] [Google Scholar]
- 12.Vezina C, Singh K, Sehgal SN. 1965. Sporulation of filamentous fungi in submerged culture. Mycologia 57:722–736. doi: 10.2307/3757008. [DOI] [Google Scholar]
- 13.Sekiguchi J, Gaucher GM, Costerton JW. 1975. Microcycle conidiation in Penicillium urticae: an ultrastructural investigation of conidiogenesis. Can J Microbiol 21:2069–2083. doi: 10.1139/m75-296. [DOI] [PubMed] [Google Scholar]
- 14.Slade SJ, Harris RF, Smith CS, Andrews JH. 1987. Microcycle conidiation and spore-carrying capacity of Colletotrichum gloeosporioides on solid media. Appl Environ Microbiol 53:2106–2110. doi: 10.1128/aem.53.9.2106-2110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ZL, Jin K, Xia YX. 2016. Transcriptional analysis of the conidiation pattern shift of the entomopathogenic fungus Metarhizium acridum in response to different nutrients. BMC Genomics 17:586. doi: 10.1186/s12864-016-2971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marzluf GA. 1997. Genetic regulation of nitrogen metabolism in the fungi. Microbiol Mol Biol Rev 61:17–32. doi: 10.1128/mmbr.61.1.17-32.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravagnani A, Gorfinkiel L, Langdon T, Diallinas G, Adjadj E, Demais S, Gorton D, Arst HN, Scazzocchio C. 1997. Subtle hydrophobic interactions between the seventh residue of the zinc finger loop and the first base of an HGATAR sequence determine promoter-specific recognition by the Aspergillus nidulans GATA factor AreA. EMBO J 16:3974–3986. doi: 10.1093/emboj/16.13.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan H, Feng B, Marzluf GA. 1997. Two distinct protein-protein interactions between the NIT2 and NMR regulatory proteins are required to establish nitrogen metabolite repression in Neurospora crassa. Mol Microbiol 26:721–729. doi: 10.1046/j.1365-2958.1997.6041979.x. [DOI] [PubMed] [Google Scholar]
- 19.Wong KH, Hynes MJ, Todd RB, Davis MA. 2007. Transcriptional control of nmrA by the bZIP transcription factor MeaB reveals a new level of nitrogen regulation in Aspergillus nidulans. Mol Microbiol 66:534–551. doi: 10.1111/j.1365-2958.2007.05940.x. [DOI] [PubMed] [Google Scholar]
- 20.Kotaka M, Johnson C, Lamb HK, Hawkins AR, Ren J, Stammers DK. 2008. Structural analysis of the recognition of the negative regulator NmrA and DNA by the zinc finger from the GATA-type transcription factor AreA. J Mol Biol 381:373–382. doi: 10.1016/j.jmb.2008.05.077. [DOI] [PubMed] [Google Scholar]
- 21.Polley SD, Caddick MX. 1996. Molecular characterisation of meaB, a novel gene affecting nitrogen metabolite repression in Aspergillus nidulans. FEBS Lett 388:200–205. doi: 10.1016/0014-5793(96)00541-8. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RA, Gibson RP, Quispe CF, Littlechild JA, Talbot NJ. 2010. An NADPH-dependent genetic switch regulates plant infection by the rice blast fungus. Proc Natl Acad Sci USA 107:21902–21907. doi: 10.1073/pnas.1006839107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez J, Wright JD, Hartline D, Quispe CF, Madayiputhiya N, Wilson RA. 2012. Principles of carbon catabolite repression in the rice blast fungus: Tps1, Nmr1-3, and a MATE–family pump regulate glucose metabolism during infection. PLoS Genet 8:e1002673. doi: 10.1371/journal.pgen.1002673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macios M, Caddick MX, Weglenski P, Scazzocchio C, Dzikowska A. 2012. The GATA factors AREA and AREB together with the co-repressor NMRA, negatively regulate arginine catabolism in Aspergillus nidulans in response to nitrogen and carbon source. Fungal Genet Biol 49:189–198. doi: 10.1016/j.fgb.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Han X, Qiu M, Wang B, Yin WB, Nie X, Qin Q, Ren S, Yang K, Zhang F, Zhuang Z, Wang S. 2016. Functional Analysis of the Nitrogen Metabolite Repression Regulator Gene nmrA in Aspergillus flavus. Front Microbiol 7:1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Lim J, Xu J, Yu JH, Zheng W. 2020. Nitric oxide as a developmental and metabolic signal in filamentous fungi. Mol Microbiol 113:872–882. doi: 10.1111/mmi.14465. [DOI] [PubMed] [Google Scholar]
- 27.Marcos AT, Ramos MS, Marcos JF, Carmona L, Strauss J, Cánovas D. 2016. Nitric oxide synthesis by nitrate reductase is regulated during development in Aspergillus. Mol Microbiol 99:15–33. doi: 10.1111/mmi.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ninnemann H, Maier J. 1996. Indications for the occurrence of nitric oxide synthases in fungi and plants and the involvement in photoconidiation of Neurospora crassa. Photochem Photobiol 64:393–398. doi: 10.1111/j.1751-1097.1996.tb02477.x. [DOI] [PubMed] [Google Scholar]
- 29.Maier J, Hecker R, Rockel P, Ninnemann H. 2001. Role of nitric oxide synthase in the light-induced development of sporangiophores in Phycomyces blakesleeanus. Plant Physiol 126:1323–1330. doi: 10.1104/pp.126.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Higgins VJ. 2005. Nitric oxide has a regulatory effect in the germination of conidia of Colletotrichum coccodes. Fungal Genet Biol 42:284–292. doi: 10.1016/j.fgb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Vieira ALG, Linares E, Augusto O, Gomes SL. 2009. Evidence of a Ca2+-NO-cGMP signaling pathway controlling zoospore biogenesis in the aquatic fungus Blastocladiella emersonii. Fungal Genet Biol 46:575–584. doi: 10.1016/j.fgb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Li B, Fu YP, Jiang DH, Xie JT, Cheng JS, Li GQ, Hamid MI, Yi XH. 2010. Cyclic GMP as a second messenger in the nitric oxide-mediated conidiation of the mycoparasite Coniothyrium minitans. Appl Environ Microbiol 76:2830–2836. doi: 10.1128/AEM.02214-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding Y, Gardiner DM, Xiao D, Kazan K. 2020. Regulators of nitric oxide signaling triggered by host perception in a plant pathogen. Proc Natl Acad Sci USA 117:11147–11157. doi: 10.1073/pnas.1918977117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcos AT, Ramos MS, Schinko T, Strauss J, Canovas D. 2020. Nitric oxide homeostasis is required for light-dependent regulation of conidiation in Aspergillus. Fungal Genet Biol 137:103337. doi: 10.1016/j.fgb.2020.103337. [DOI] [PubMed] [Google Scholar]
- 35.Gong X, Fu Y, Jiang D, Li G, Yi X, Peng Y. 2007. L-Arginine is essential for conidiation in the filamentous fungus Coniothyrium minitans. Fungal Genet Biol 44:368–1379. [DOI] [PubMed] [Google Scholar]
- 36.Cao H, Huang P, Zhang L, Shi Y, Sun D, Yan Y, Liu X, Dong B, Chen G, Snyder JH, Lin F, Lu J. 2016. Characterization of 47 Cys2-His2 zinc finger proteins required for the development and pathogenicity of the rice blast fungus Magnaporthe oryzae. New Phytol 211:1035–1051. doi: 10.1111/nph.13948. [DOI] [PubMed] [Google Scholar]
- 37.Li CC, Zhang QP, Xia YX, Jin K. 2021. MaAreB, a GATA transcription factor, is involved in nitrogen source utilization, stress tolerances and virulence in Metarhizium acridum. JoF 7:512. doi: 10.3390/jof7070512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li CC, Zhang QP, Xia YX, Jin K. 2021. MaNmrA, a negative transcription regulator in nitrogen catabolite repression pathway, contributes to nutrient utilization, stress resistance and virulence in entomopathogenic fungus Metarhizium acridum. Biology 10:1167. doi: 10.3390/biology10111167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Domitrovic T, Palhano FL, Barja-Fidalgo C, DeFreitas M, Orlando MTD, Fernandes PMB. 2003. Role of nitric oxide in the response of Saccharomyces cerevisiae cells to heat shock and high hydrostatic pressure. FEMS Yeast Res 3:341–346. doi: 10.1016/S1567-1356(03)00039-4. [DOI] [PubMed] [Google Scholar]
- 40.Prats E, Carver TLW, Mur LAJ. 2008. Pathogen-derived nitric oxide influences formation of the appressorium infection structure in the phytopathogenic fungus Blumeria graminis. Res Microbiol 159:476–480. doi: 10.1016/j.resmic.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Samalova M, Johnson J, Illes M, Kelly S, Fricker M, Gurr S. 2013. Nitric oxide generated by the rice blast fungus Magnaporthe oryzae drives plant infection. New Phytol 197:207–222. doi: 10.1111/j.1469-8137.2012.04368.x. [DOI] [PubMed] [Google Scholar]
- 42.Hromatka BS, Noble SM, Johnson AD. 2005. Transcriptional response of Candida albicans to nitric oxide and the role of the YHB1 gene in nitrosative stress and virulence. Mol Biol Cell 16:4814–4826. doi: 10.1091/mbc.e05-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schinko T, Berger H, Lee W, Gallmetzer A, Pirker K, Pachlinger R, Buchner I, Reichenauer T, Güldener U, Strauss J. 2010. Transcriptome analysis of nitrate assimilation in Aspergillus nidulans reveals connections to nitric oxide metabolism. Mol Microbiol 78:720–738. doi: 10.1111/j.1365-2958.2010.07363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krijgsheld P, Bleichrodt R, van Veluw GJ, Wang F, Müller WH, Dijksterhuis J, Wösten HAB. 2013. Development in Aspergillus. Stud Mycol 74:1–29. doi: 10.3114/sim0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perdomo J, Holmes M, Chong B, Crossley M. 2000. Eos and pegasus, two members of the Ikaros family of proteins with distinct DNA binding activities. J Biol Chem 275:38347–38354. doi: 10.1074/jbc.M005457200. [DOI] [PubMed] [Google Scholar]
- 46.Morgan B, Sun L, Avitahl N, Andrikopoulos K, Ikeda T, Gonzales E, Wu P, Neben S, Georgopoulos K. 1997. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J 16:2004–2013. doi: 10.1093/emboj/16.8.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arst HN, Jr, Cove DJ. 1973. Nitrogen metabolite repression in Aspergillus nidulans. Mol Gen Genet 126:111–141. doi: 10.1007/BF00330988. [DOI] [PubMed] [Google Scholar]
- 48.Narendja F, Goller SP, Wolschek M, Strauss J. 2002. Nitrate and the GATA factor AreA are necessary for in vivo binding of NirA, the pathway-specific transcriptional activator of Aspergillus nidulans. Mol Microbiol 44:573–583. doi: 10.1046/j.1365-2958.2002.02911.x. [DOI] [PubMed] [Google Scholar]
- 49.Liu L, Zeng M, Hausladen A, Heitman J, Stamler JS. 2000. Protection from nitrosative stress by yeast flavohemoglobin. Proc Natl Acad Sci USA 97:4672–4676. doi: 10.1073/pnas.090083597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Jesús-Berrios M, Liu L, Nussbaum JC, Cox GM, Stamler JS, Heitman J. 2003. Enzymes that counteract nitrosative stress promote fungal virulence. Curr Biol 13:1963–1968. doi: 10.1016/j.cub.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 51.Pengkit A, Jeon SS, Son SJ, Shin JH, Baik KY, Choi EH, Park G. 2016. Identification and functional analysis of endogenous nitric oxide in a filamentous fungus. Sci Rep 6:30037. doi: 10.1038/srep30037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franco-Cano A, Marcos AT, Strauss J, Cánovas D. 2021. Evidence for an arginine-dependent route for the synthesis of NO in the model filamentous fungus Aspergillus nidulans. Environ Microbiol 23:6924–6939. doi: 10.1111/1462-2920.15733. [DOI] [PubMed] [Google Scholar]
- 53.Huberman LB, Wu VW, Kowbel DJ, Lee J, Daum C, Grigoriev IV, O’Malley RC, Glass NL. 2021. DNA affinity purification sequencing and transcriptional profiling reveal new aspects of nitrogen regulation in a filamentous fungus. Proc Natl Acad Sci USA 118:e2009501118. doi: 10.1073/pnas.2009501118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fornes O, Castro-Mondragon JA, Khan A, van der Lee R, Zhang X, Richmond PA, Modi BP, Correard S, Gheorghe M, Baranasic D, Santana-Garcia W, Tan G, Cheneby J, Ballester B, Parcy F, Sandelin A, Lenhard B, Wasserman WW, Mathelier A. 2020. JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res 48:D87–D92. doi: 10.1093/nar/gkz1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du Y, Jin K, Xia Y. 2018. Involvement of MaSom1, a downstream transcriptional factor of cAMP/PKA pathway, in conidial yield, stress tolerances, and virulence in Metarhizium acridum. Appl Microbiol Biotechnol 102:5611–5623. doi: 10.1007/s00253-018-9020-7. [DOI] [PubMed] [Google Scholar]
- 56.Jin K, Ming Y, Xia YX. 2012. MaHog1, a Hog1-type mitogen-activated protein kinase gene, contributes to stress tolerance and virulence of the entomopathogenic fungus Metarhizium acridum. Microbiology (Reading) 158:2987–2996. doi: 10.1099/mic.0.059469-0. [DOI] [PubMed] [Google Scholar]
- 57.Zhao T, Wen Z, Xia Y, Jin K. 2020. The transmembrane protein MaSho1 negatively regulates conidial yield by shifting the conidiation pattern in Metarhizium acridum. Appl Microbiol Biotechnol 104:4005–4015. doi: 10.1007/s00253-020-10523-0. [DOI] [PubMed] [Google Scholar]
- 58.Araujo-Palomares CL, Riquelme M, Castro-Longoria E. 2009. The polarisome component SPA-2 localizes at the apex of Neurospora crassa and partially colocalizes with the Spitzenkörper. Fungal Genet Biol 46:551–563. doi: 10.1016/j.fgb.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, Tian X, Zhao Q, Liu Z, Li X, Ren Y, Tang J, Fang J, Xu Q, Bu Q. 2018. The E3 ligase drought hypersensitive negatively regulates cuticular wax biosynthesis by promoting the degradation of transcription factor ROC4 in rice. Plant Cell 30:228–244. doi: 10.1105/tpc.17.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng X, Zhu L, Guo J, Sun Z, Zhao M, Zhan X. 2020. Enhancing biocompatibility and neuronal anti-inflammatory activity of polymyxin B through conjugation with gellan gum. Int J Biol Macromol 147:734–740. doi: 10.1016/j.ijbiomac.2019.12.200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.00538-22-s001.pdf, PDF file, 2.5 MB (2.6MB, pdf)
Data Availability Statement
RNA-seq data had been uploaded to the NCBI BioProject database under the accession number PRJNA748190.