Abstract
Fifteen filamentous strains, morphologically classified as Eikelboom type 021N bacteria, were isolated from bulking activated sludges. Based on comparative 16S ribosomal DNA (rDNA) sequence analysis, all strains form a monophyletic cluster together with all recognized Thiothrix species (88.3 to 98.7% 16S rDNA sequence similarity) within the gamma-subclass of Proteobacteria. The investigated Eikelboom type 021N isolates were subdivided into three distinct groups (I to III) demonstrating a previously unrecognized genetic diversity hidden behind the uniform morphology of the filaments. For in situ detection of these bacteria, 16S rRNA-targeted oligonucleotide probes specific for the entire Eikelboom type 021N-Thiothrix cluster and the Eikelboom type 021N groups I, II, and III, respectively, were designed, evaluated, and successfully applied in activated sludge.
Activated sludge systems are used worldwide for wastewater treatment. One of the major operational problems of these systems is the excessive growth of filamentous bacteria (7, 14, 32). This causes poor settlement of activated sludge flocs, a problem commonly referred to as bulking. On the other hand, it has been suggested that a certain number of filamentous bacteria are required for proper floc formation (27). Thus, depending upon their identity and abundance, filamentous bacteria can be either beneficial or detrimental for efficient separation of the treated wastewater from the biomass in the settling tanks. Therefore, the ability to unambiguously identify filamentous bacteria is crucial for proper control of wastewater treatment systems. Since cultivation of most filamentous bacteria is difficult and time-consuming, their classification is traditionally achieved by microscopic observation of morphological traits and simple staining reactions of the filaments within activated sludge using the keys proposed by Eikelboom (6). Since the majority of filamentous bacteria in activated sludge are morphologically different from previously identified bacteria, these filamentous bacteria were provisionally classified by type numbers (6). Keeping in mind that the morphology of bacteria can vary significantly depending upon the environmental conditions, more-reliable tools for in situ identification of filamentous bacteria are urgently required.
Eikelboom type 021N bacteria have frequently been described as causative agents for sludge bulking (7, 16, 22, 25, 28, 32, 35, 36). Recently, an oligonucleotide probe for in situ detection of Eikelboom type 021N bacteria has been designed by Wagner et al. (31) and used for in situ detection of this microorganism within bulking sludges (22, 24). Their in situ hybridization results suggest that Eikelboom type 021N bacteria comprise a variety of phylogenetically and physiologically different microorganisms. Additionally, a recent report also indicated that bacteria of the Eikelboom type 021N morphotype encompass several genotypically different microorganisms (12).
In this study, we analyzed the phylogenetic characteristics of 15 strains of Eikelboom type 021N bacteria, most of which have been newly isolated in the course of the present study. These strains could be divided into three phylogenetic groups, and we subsequently designed oligonucleotide probes specific for each group and an additional probe which targets a 16S rRNA signature sequence of all 15 Eikelboom type 021N isolates and the recognized Thiothrix species (T. eikelboomii, T. nivea, T. unzii, T. fructosivorans, and T. defluvii).
MATERIALS AND METHODS
Bacterial strains.
All Eikelboom type 021N isolates analyzed in this study were recovered from bulking activated sludge of different wastewater treatment plants (WWTPs) listed in Table 1. Four of the studied Eikelboom type 021N strains, KR-A, T1-4, T2-1, and SNR-3, were isolated previously by Kohno (16). Eikelboom type 021N strain AP3 (T. eikelboomii, ATCC 49788) (11, 12, 34) was purchased from the American Type Culture Collection. In addition, 10 Eikelboom type 021N strains were isolated from WWTPs in Japan in the course of this study.
TABLE 1.
Origins of Eikelboom type 021N strains used in this study and GenBank accession numbers of 16S rDNA sequences determined in this study
| Strain | Origin | Accession no. |
|---|---|---|
| B3-1 | Soy sauce factory WWTP | AB042532 |
| B4-1 | Municipal WWTP | AB042533 |
| B2-7 | Pickle manufacturer WWTP | AB042534 |
| SCM-A | Chicken butchering factory WWTP | AB042535 |
| B5-1 | Pickle manufacturer WWTP | AB042536 |
| B2-8 | Pickle manufacturer WWTP | AB042537 |
| OS-F | Municipal WWTP in Kofu City | AB042538 |
| KR-A | Municipal WWTP in Kyoto City | AB042539 |
| T1-4 | Tofu (soybean curd) factory WWTP in Ishikawa Prefecture | AB042540 |
| T2-1 | Tofu (soybean curd) factory WWTP in Ishikawa Prefecture | AB042541 |
| COM-A | Industrial WWTP (details unknown) | AB042542 |
| AP3 | ATCC 49788 | AB042819 |
| SNR-3 | Freeze-dried tofu factory WWTP in Nagano prefecture | AB042543 |
| EJ1M-B | Industrial WWTP (details unknown) | AB042544 |
| EJ2M-B | Industrial WWTP (details unknown) | AB042545 |
Paraformaldehyde-fixed cells of Legionella pneumophila were kindly provided by Dorothee Grimm (Würzburg, Germany).
Culture media.
Medium IAM used for the isolation of Eikelboom type 021N bacteria contained 0.15 g of sodium acetate, 0.15 g of glucose, 5 ml of vitamins mixture, 10 ml of salts solution A, and 1 ml of phosphate solution A per liter. Vitamins mixture contained 20 mg of calcium pantothenate, 20 mg of nicotinic acid, 1 mg of biotin, 1 mg of cyanocobalamin, 1 mg of folic acid, 20 mg of pyridoxine hydrochloride, 20 mg of p-aminobenzoic acid, 20 mg of inositol, 20 mg of thiamine hydrochloride, and 20 mg of riboflavin per liter. Salts solution A contained 10 g of (NH4)2SO4, 5.0 g of KCl, 5.0 g of MgSO4 · 7H2O, 2.0 g of CaCl2 · 2H2O, 2.0 g of CaCO3 and 0.05 g of Fe3Cl · 6H2O per liter. Phosphate solution A was prepared with 124 g of Na2HPO4 and 15.4 g of NaH2PO4 per liter, and the pH was adjusted to 7.2 with 1 N HCl. Medium EGGC, which was used for the maintenance of Eikelboom type 021N isolates, contained 0.3 g of sodium acetate, 0.3 g of Casamino Acids, 0.6 g of glucose, 10 ml of salts solution A, 5 ml of vitamins mixture, and 2 ml of phosphate solution A per liter. For the preparation of medium EGGC, sodium acetate and Casamino Acids were dissolved in distilled water and mixed with salts solution A, and the pH was adjusted to 7.2 with 1 N HCl. This mixture was autoclaved separately, and sterile-filtered solutions of the remaining components were added. For agar plates and slants, media were solidified by addition of 15 g of Bacto Agar (Difco) per liter. Medium TA, which was used for the sulfur oxidation test, contained 0.082 g of sodium acetate, 10 ml of salts solution T, 5 ml of vitamins mixture, 4 ml of phosphate solution T, and 7.5 ml of freshly prepared thiosulfate solution (100 g of Na2S2O3 · 5H2O per liter) per liter. Salts solution T contained 16 g of NH4NO3, 5.0 g of MgSO4 · 7H2O, 2.0 g of CaCO3, 0.05 g of Fe3Cl · 6H2O, and 8.4 g of NaHCO3 per liter. Phosphate solution T contained 66 g of K2HPO4 and 16.6 g of KH2PO4 per liter (pH 7.2). For the preparation of medium TA, sodium acetate was dissolved in distilled water and mixed with salts solution T, and the pH was adjusted to 7.2 with 1 N HCl. This mixture was autoclaved separately, and the remaining components were added.
Isolation of Eikelboom type 021N strains from activated sludge.
Filamentous bacteria were collected from the activated sludge samples using a bundle of 5 nichrome wires (0.5 by 50 mm), each bent at the end to form a hook, and transferred into sterile distilled water. Following homogenization of this suspension by a blender for 5 min (Auto Cell Master CM-200; Iuchi, Osaka, Japan), filamentous bacteria were again collected using a bundle of 12 stainless steel thin wires (0.15 by 40 mm), each bent at the end to form a hook, and transferred into sterile distilled water (16). This suspension was diluted to a concentration of 500 to 3,000 bacterial filaments or cells per ml, and 0.1-ml aliquots were spread on IAM plates and incubated for 3 weeks at 25 or 30°C. Fingerprint-like colonies (characteristic of Eikelboom type 021N bacteria [16, 34]) were examined under a phase-contrast microscope. Only isolates that were classified as Eikelboom type 021N bacteria by the Eikelboom keys (6, 7) were selected for further analysis. Eikelboom type 021N isolates were streaked on IAM agar plates repeatedly for further purification and maintained on EGGC agar plates or slants.
Morphological characteristics and staining.
Eikelboom type 021N isolates grown on EGGC agar for 3 to 5 days at 30°C were morphologically characterized by phase-contrast microscopy and several staining procedures. Gram staining was performed with a FAVOR-G SET-S kit (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan). Neisser stain and poly-β-hydroxybutyrate (PHB) stain techniques were performed as described by Jenkins (13).
Quinone analysis.
Cells grown in EGGC liquid medium were harvested by centrifugation and stored at −20°C. Quinones were extracted from 0.5 to 1 g (wet weight) of cells with chloroform-methanol (2:1, vol/vol), purified by column chromatography using Sep-Pak Cartridges (Waters Co.), and analyzed by a reverse-phase high-performance liquid chromatography (HPLC) (29). The HPLC system was equipped with a Beckman 126 Solvent Module, a Zorbax ODS column (4.6 [inner diameter] by 250 mm) (Du Pont Co.) in a column oven (30°C), a Beckman 168 diode array detector, and a Beckman System Gold for data analysis. A mixture of methanol-isopropyl ether (9:2, vol/vol) was used as eluent at a flow rate of 1 ml/min (10).
Oxidation of reduced sulfur.
Cells grown on EGGC agar plates for 5 days at 30°C were inoculated in 5 ml of TA medium in sterile test tubes (17 by 160 mm) and shaken at 200 rpm at 30°C for 12 days. Observation of sulfur granules was performed on cultures after incubation for 1 day and 12 days. The concentrations of thiosulfate and sulfate in the cultures at day 0 and day 12 were determined by an HPLC system (Shimadzu LC-6A) equipped with a CDD-6A detector (Shimadzu) and a column (4.5 by 150 mm) of Shim-pack IC-A3 (Shimadzu). HPLC analysis was performed at 40°C by using 8 mM p-hydroxybenzoic acid and 3.2 mM bis-tris as the eluent at a flow rate of 1.2 ml/min. The injection volume of the samples was 20 μl.
Sequencing of 16S rDNA and phylogenetic analysis.
Cells grown on EGGC agar plates were suspended in sterilized distilled water and stored at −20°C. Crude lysates of the stored cells were prepared by proteinase K (20 U/ml) digestion (20 min, 60°C), heat treatment (95°C for 10 min), and centrifugation (16,000 × g, 5 min) (9). Almost complete DNA coding for 16S rRNA (rDNA) was amplified by PCR directly from the crude lysates using the bacterial consensus primers Eu8f (Escherichia coli positions 8 to 27) and Eu1492r (E. coli positions 1510 to 1492) (33). PCR was performed using a PCR thermal cycler MP (Takara Shuzo Co., Kyoto, Japan) with an initial denaturation at 95°C for 9 min followed by 35 cycles of denaturation at 95°C for 1 min, primer annealing at 50°C for 1 min, and extension at 72°C for 2 min. PCR products were extracted with chloroform-isoamyl alcohol (24:1, vol/vol), purified by MicroSpin S-400 HR columns (Amersham Pharmacia Biotech, Uppsala, Sweden), and directly sequenced with an ABI 377 automated DNA sequencer (Perkin-Elmer Applied Biosystems) using sequencing primers which correspond to positions 536 to 518, 821 to 803, 1111 to 1093, 1406 to 1389, and 1094 to 1112 of the E. coli 16S rRNA. The obtained 16S rDNA sequences were subjected to BLAST search (http://www.ncbi.nlm.nih.gov/BLAST/) to determine 16S rDNA similarities with sequences deposited in GenBank, EMBL, and GSDB databases. The retrieved sequences were aligned by using the CLUSTAL W program, version 1.6 (30). Subsequently, the alignment was refined by visual inspection. Evolutionary distances were calculated by using the program MEGA, version 1.0 (17). Phylogenetic trees were constructed based on the distance matrix data obtained with the neighbor-joining method (26). Robustness of the tree topology was evaluated by (i) bootstrap resampling analysis (8) with 100 replicates and (ii) applying maximum-parsimony and maximum-likelihood methods using the ARB program package (encompassing more than 16,000 published and unpublished homologous small subunit rDNA primary structures; Strunk et al., unpublished data [program available at http://www.mikro.biologie.tu-muenchen.de]). The composition of the data sets varied with respect to the reference sequences and the alignment positions included. Variability of the individual alignment positions was determined using the respective tool of the ARB package and was used as the criterion to remove or include variable positions for phylogenetic analyses.
Determination of DNA base composition.
Cells grown on EGGC agar plates were suspended in potassium phosphate buffer and stored at −20°C. Crude lysates were prepared from the stored cells as described above. DNA was purified from crude lysates by the method of Marmur (21). Purified DNA was subsequently hydrolyzed by P1 nuclease (GC kit; Yamasa Shoyu Co., Choshi, Japan) followed by alkaline phosphatase (from E. coli) (Wako Pure Chemicals Industry, Ltd., Osaka, Japan) as described by Kamagata and Mikami (15). The G+C contents were determined by a reversed-phase HPLC (Shimadzu SCL-6B) using a CLC-ODS column (6.0 by 150 mm; Shimadzu). Separation was achieved at 40°C by using 5% methanol in 10 mM phosphate buffer (pH 3.5) as the mobile phase at a flow rate of 1 ml/min. Each deoxyribonucleoside was detected by a UV-visible light spectrophotometric detector (SPD-6AV; Shimadzu) determining A270. An equimolar mixture of deoxyribonucleosides (GC kit; Yamasa Shoyu Co.) was used as the standard.
Oligonucleotide probes and fluorescence in situ hybridization.
The following oligonucleotide probes were used for in situ hybridization of pure cultures of Eikelboom type 021N strains: (i) EUB 338 (5′-GCTGCCTCCCGTAGGAGT-3′), targeting most but not all members of the domain Bacteria (2, 5); (ii) 21N (5′-TCCCTCTCCCAAATTCTA-3′), previously designed to specifically target a signature region on the 16S rRNA of the Eikelboom type 021N isolate II-26 (31); and (iii) TNI (5′-CTCCTCTCCCACATTCTA-3′), targeting the 16S rRNA of T. nivea (31). In addition, the following oligonucleotide probes (probe designations according to Alm et al. [1]) were designed in this study using the probe design-probe match tools of the ARB software package: (i) G1B (S-*-021Ng1-1029-a-A-18), specific for the group I isolates of Eikelboom type 021N; (ii) G2M (S-*-021Ng2-842-a-A-18), specific for the group II isolates of Eikelboom type 021N; (iii) G3M (S-*-021Ng3-996-a-A-18), specific for the group III isolates of Eikelboom type 021N; and (iv) G123T (S-G-Thioth-697-a-A-18), specific for the 15 Eikelboom type 021N isolates (group I, II, and III) and the recognized Thiothrix species, T. eikelboomii, T. nivea, T. unzii, T. fructosivorans, and T. defluvii. In order to ensure probe specificity, all available 16S and 23S rDNA sequences included in the ARB database were checked for the presence of the probe target sites. Oligonucleotides were synthesized and directly 5′ labeled with 5(6)-carboxyfluorescein-N-hydroxysuccinimideester (FLUOS), the hydrophilic sulfoindocyanine fluorescent dyes Cy3 and Cy5, or tetramethylrhodamine-5-isothiocyanate. For in situ hybridization activated sludge samples from 24 WWTPs in Japan and Europe as well as the Eikelboom type 021N isolates grown on medium EGGC for 3 days at 30°C were fixed with paraformaldehyde and hybridized as described elsewhere (3). To enhance the specificity of probe G123T it was used together with an equimolar amount of the unlabeled competitor oligonucleotide G123T-C (5′-CCTTCCGATCTCTACGCA-3′) in all experiments. Slides were examined using an Olympus AX80 fluorescence microscope equipped with an IPLab-spectrum image analyzing system (Signal Analysis) and a color charge-coupled device camera (Hamamatsu Photonics, Hamamatsu, Japan) or a Zeiss confocal laser scanning microscope LSM510. Optimal hybridization stringency was determined for each probe by quantification of filament fluorescence resulting from different formamide concentrations in the hybridization buffer using the equipment and procedures described previously (5). For screening of the activated sludge samples the newly designed probes (labeled with different fluorescent dyes) were applied together in different combinations under stringent hybridization conditions. Simultaneous hybridization with probes requiring different stringency was realized by a successive hybridization procedure (31).
Nucleotide accession numbers.
The 16S rDNA sequences determined in this study have been deposited in DDBJ under accession numbers AB042532 through AB042545 and AB042819.
RESULTS
Phylogenetic analysis of 16S rRNA sequences.
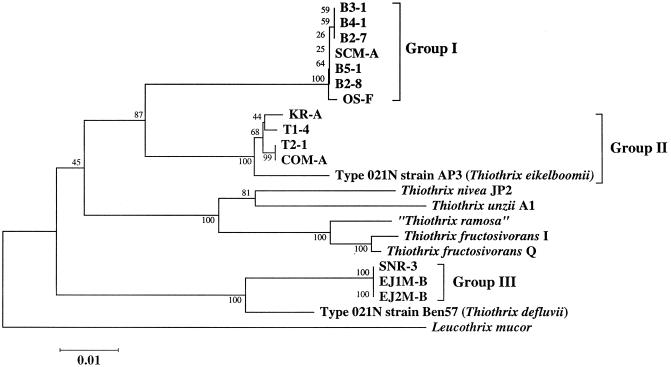
For the 15 Eikelboom type 021N isolates, including T. eikelboomii AP3, near full-length 16S rRNA gene fragments (∼1,420 bp) were successfully amplified and directly sequenced. Subsequent comparative 16S rDNA sequence analysis revealed that the investigated Eikelboom type 021N isolates are affiliated with the gamma-subclass of Proteobacteria and show the highest 16S rDNA sequence similarities with members of the genus Thiothrix. Consistently, the 15 Eikelboom type 021N isolates shared the characteristic deletion in helix 18 of the 16S rRNA secondary structure (corresponding to E. coli positions 455 to 477) previously reported by Howarth et al. (12) for members of the Eikelboom type 021N-Thiothrix group. Phylogenetic analysis revealed that the retrieved 16S rDNA sequences could be placed into three distinct groups (Fig. 1), each comprising highly similar sequences (99.6 to 100% for group I, 98.5 to 100% for group II, and 99.9 to 100% for group III). Independent of the data set and treeing method used, the group I isolates B3-1, B4-1, B2-7, SCM-A, B5-1, B2-8, and OS-F formed a monophyletic lineage which is clearly separated from all recognized Thiothrix species and the other Eikelboom type 021N isolates investigated in this study. The nearest neighbor of the group I isolates is T. eikelboomii (94.0 to 94.3% 16S rDNA sequence similarity). The group II isolates KR-A, T1-4, T2-1, and COM-A always clustered together with T. eikelboomii (which was consequently included in group II in this study), with 98.5 to 98.7% 16S rDNA sequence similarity, and the group III isolates SNR-3, EJ1M-B, and EJ2M-B, formed a stable association with T. defluvii (96.8 to 97.0% 16S rDNA sequence similarity). The other Thiothrix species, T. nivea, T. unzii, T. ramosa, and T. fructosivorans, formed a fourth monophyletic grouping, which was supported by all treeing methods (Fig. 1).
FIG. 1.
Phylogenetic tree derived from 16S rDNA sequences showing the position of the Eikelboom type 021N strains. The tree was calculated using the neighbor-joining method. The bar represents 1% estimated sequence divergence. Numbers at nodes represent percentage bootstrap values. The GenBank accession numbers of the reference strains used in the phylogenetic analysis are as follows: T. defluvii, AF127020; T. fructosivorans I, L79963; T. fructosivorans Q, L79962; T. unzii, L79961; T. ramosa, U32940; T. nivea JP2, L40993; Leucothrix mucor, X87277.
G+C contents of DNA and quinones.
The determined G+C moles percent of the DNA of all Eikelboom type 021N isolates were within a very narrow range (Table 2). Those of groups I, II, and III were 43.9 to 44.7, 44.1 to 46.1, and 44.0 to 44.4, respectively. All Eikelboom type 021N isolates contained an eight-isoprene ubiquinone as the major quinone.
TABLE 2.
Morphological characteristics and DNA G+C content of the Eikelboom type 021N strains
| Type 021N group | Strain | Cell diam (μm) | Cell length (μm) | G+C content (mol%) |
|---|---|---|---|---|
| I | B3-1 | 1.5–4.0 | 0.5–3.0 | 44.6 |
| B4-1 | 1.2–2.0 | 0.5–2.0 | 44.6 | |
| B2-7 | 1.9–2.8 | 0.5–2.0 | 44.2 | |
| SCM-A | 1.9–4.0 | 0.5–2.5 | 44.3 | |
| B5-1 | 1.2–2.5 | 0.5–2.5 | 44.7 | |
| B2-8 | 1.9–4.0 | 0.5–3.0 | 43.9 | |
| OS-F | 1.9–2.5 | 0.5–2.0 | 44.4 | |
| II | KR-A | 0.8–1.2 | 0.5–4.0 | 44.1 |
| T1-4 | 0.8–1.2 | 1.0–2.0 | 45.6 | |
| T2-1 | 0.8–1.5 | 0.8–2.0 | 46.1 | |
| COM-A | 1.2–2.8 | 1.0–8.0 | 44.9 | |
| AP3 | 1.2–8.0 | 1.0–5.0 | 46.0 | |
| III | SNR-3 | 1.0–2.0 | 0.8–2.5 | 44.4 |
| EJ1M-B | 2.0–4.0 | 0.5–5.5 | 44.1 | |
| EJ2M-B | 2.0–4.0 | 0.5–4.0 | 44.0 |
Morphological characteristics.
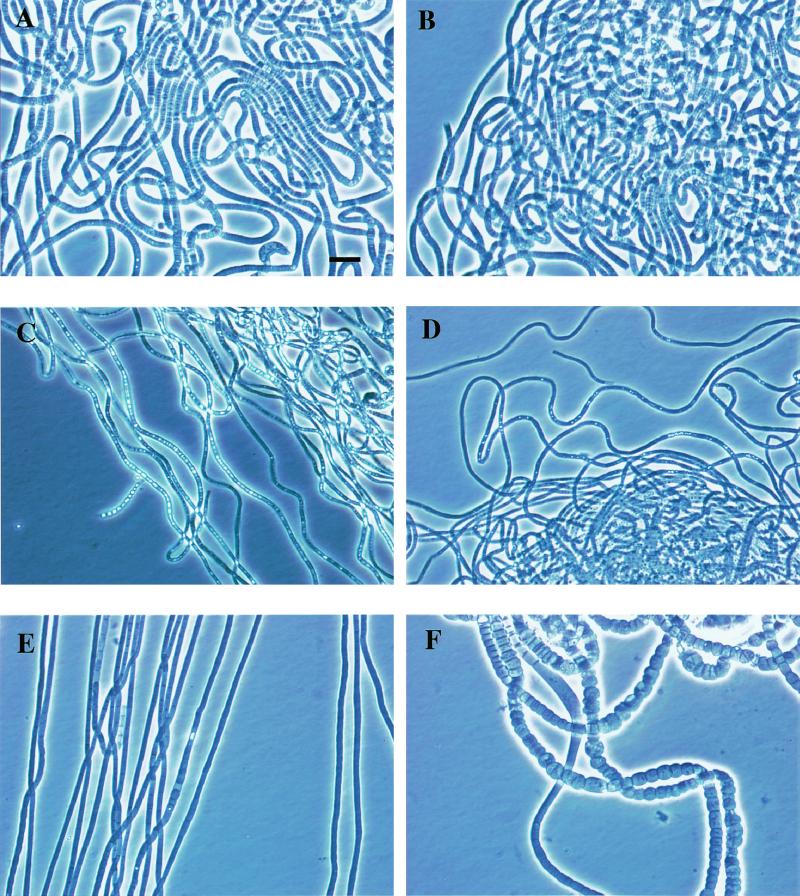
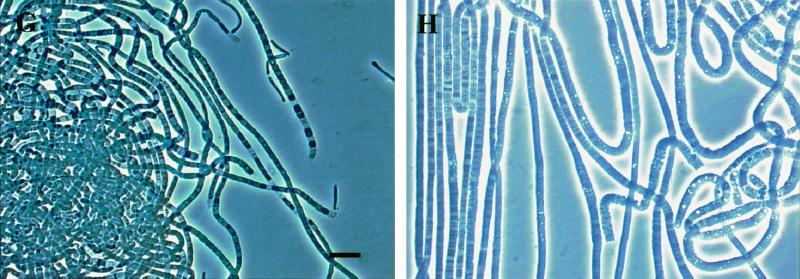
The colonies of the investigated Eikelboom type 021N isolates were colorless and showed the fingerprint-like structure that is characteristic of Eikelboom type 021N bacteria (16, 34). The filaments grown on EGGC agar plates were very long (more than 0.5 mm), unbranched, sheathless, nonmotile, slightly curled, and multicellular (Fig. 2). Single cells within the filaments were not uniform in size and showed a barrel-like to ovoid shape, and the septa between individual cells were clearly visible. Overall, filaments of the majority of the investigated Eikelboom type 021N isolates were highly similar, and thus differentiation between the respective isolates of the three different phylogenetic groups by morphological characteristics is not possible (Table 2). However, it should be noted that two isolates of the Eikelboom type 021N group II significantly differed in their morphology from all the other isolates. Particularly, individual cells of Eikelboom type 021N isolate AP3 (T. eikelboomii) were highly diverse in shape and size (1.2 to 8.0 μm in width and 1.0 to 5.0 μm in length) while the Eikelboom type 021N isolate COM-A showed an exceptional length variation of individual cells (1.0 to 8.0 μm) but did not vary extensively in width.
FIG. 2.
Phase-contrast photomicrographs of Eikelboom type 021N strains in pure culture. Two strains of each Eikelboom type 021N group and additional two strains, COM-A and AP3, which significantly differed in morphology, are shown. The bar in photo A indicates 10 μm and magnification was ×1,000 for all photos. (A) Group I strain B3-1; (B) group I strain OS-F; (C) group II strain KR-A; (D) group II strain T1-4; (E) group II strain COM-A; (F) group II strain AP3 (characteristic part is shown); (G) group III strain SNR-3; (H) group III strain EJ2M-B.
Staining characteristics.
Gram and Neisser staining was negative for all Eikelboom type 021N isolates. However, differences between the groups within the investigated Eikelboom type 021N isolates could be demonstrated using PHB staining. While distinct PHB granules were observed in the majority of the cells of the Eikelboom type 021N isolates belonging to groups I and III, cells of the group II isolates were stained completely and no individual granules were visible.
Oxidation of reduced sulfur.
No sulfur granules were detected in the cells grown on IAM agar medium or on EGGC agar medium. However, when the cells were incubated with 3 mM Na2S2O3, high numbers of sulfur granules were observed inside and outside of the cells of the group I and II isolates after 1 day and 12 days of incubation. On the other hand, only a few sulfur granules were present in the cells of the group III isolates after 1 day and no sulfur granules were present after 12 days. A significant amount of sulfuric acid was produced by the group I and II isolates, whereas there was no production or only slight production of this compound by the group III isolates.
Fluorescence in situ hybridization.
Previously, Wagner et al. (31) designed the 16S rRNA-targeted probes 21N and TNI for specific in situ detection of Eikelboom type 021N bacteria and T. nivea in environmental samples, respectively. In the present study, we reevaluated the specificity and sensitivity of these probes in the light of the new 16S rRNA sequences obtained from the 15 Eikelboom type 021N isolates and the recently published 16S rRNA sequences of additional Thiothrix species (12). Interestingly, only the 16S rRNA of the group II isolate T1-4 is fully complementary to probe 21N, while all the other Eikelboom type 021N isolates possess one or two mismatches with this probe (Table 3). Consequently, in situ hybridization of isolate T1-4 with probe 21N yielded a strong signal, while very weak or no hybridization signals could be observed for other group II, group I, and group III isolates (Table 4). T. eikelboomii and T. defluvii show a single mismatch with probe 21N, while all other described Thiothrix species have three or more mismatches with this probe and will thus not be detected with this probe. The 16S rRNA sequences of all Eikelboom type 021N isolates included in this study showed two or three mismatches with probe TNI (Table 3). While all group I isolates shared one centrally located strong C-C mismatch and one distal weak T-G mismatch with probe TNI, all group II isolates (except for isolate T1-4, which has three mismatches with probe TNI) and all group III isolates exhibited two distal mismatches (a weak T-G and a strong C-A mismatch) with the 5′ end of probe TNI (Table 3). Considering that a mismatch at the 5′ or 3′ end of an oligonucleotide probe is less destabilizing for the probe-target hybrid compared to a centrally located mismatch, hybridization with probe TNI yielded the expected results: No signal could be observed for group I isolate OS-F and group II isolate T1-4, while strong signals were detectable for group II isolate KR-A and group III isolate SNR-3 (Table 4).
TABLE 3.
Difference alignment of the target region of the 16S rRNA of Eikelboom type 021N isolates for the previously designed probes 21N and TNIa
| Organism(s) | Target sequenced |
|---|---|
| Eikelboom type 021N strain II-26b (5′-3′) | UAGAAUUUGGGAGAGGGA |
| Group I strains | ------C----------G |
| Strain T1-4 of group II | ------------------ |
| Other group II strains including T. eikelboomii | ------G----------- |
| Group III strains and T. defluvii | ------G----------- |
| T. niveac | ------G---------AG |
| T. unzii | ------G---------AG |
| T. ramosa | -----GG---------AG |
| T. fructosivorans | -----GG---------AG |
See reference 31 for details.
Target organism of probe 21N.
Target organism of probe TNI.
–, identical to the target sequence of probe 21N.
TABLE 4.
Fluorescence in situ hybridization of members of the three 021N groups
| Probe | Fluorescence of straina:
|
|||
|---|---|---|---|---|
| OS-F (I) | T1-4 (II) | KR-A (II) | SNR-3 (III) | |
| EUB | ++ | ++ | ++ | ++ |
| 21N | − | ++ | − | + |
| TNI | − | − | ++ | ++ |
Strain group is indicated in parentheses. Symbols: −, no signal; +, very weak signal; ++, strong signal.
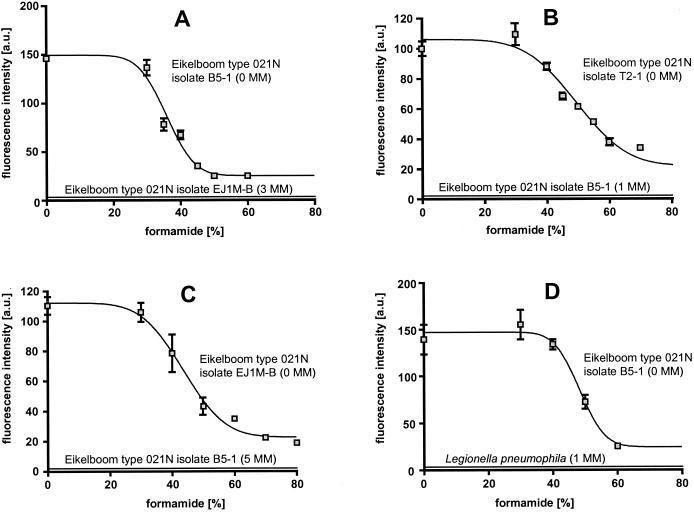
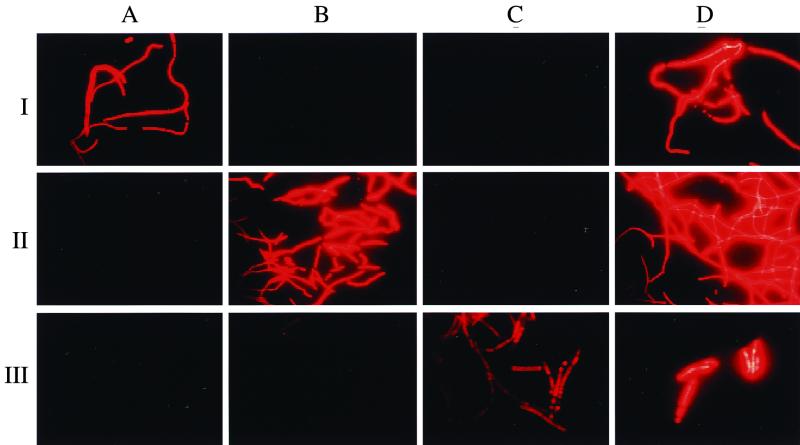
To extend the probe set for in situ detection of Thiothrix spp. and Eikelboom type 021N bacteria, four additional 16S rRNA-targeted oligonucleotide probes were designed (Table 5). Probe G1B specifically targets all Eikelboom type 021N group I isolates and displays at least three mismatches with all other available 16S rRNA sequences, including the Eikelboom type 021N group II and group III isolates (Table 6). Probe G2M was designed to specifically detect the group II isolates (including T. eikelboomii) and displays one central strong C-A mismatch to the group I isolates and at least three mismatches with all other available 16S rRNA sequences, including all Eikelboom type 021N group III isolates. Probe G3M was designed to specifically detect the group III isolates and displays one weak, putatively nondiscriminative G-U mismatch with T. defluvii, at least two mismatches to all other available 16S rRNA sequences, and at least five mismatches to Eikelboom type 021N group I and II isolates. In addition, G123T was designed to specifically hybridize to a signature region on the 16S rRNA of all recognized Thiothrix species, including all Eikelboom type 021N isolates analyzed in this study. Probe G123T has a relatively weak T-G mismatch with a few other microorganisms (mainly Legionella sp.) which are not members of the Eikelboom type 021N-Thiothrix clade. Thus, to enhance specificity this probe should only be applied in combination with the competitor probe G123T-C. Optimal hybridization stringency was determined for the four probes by increasing the formamide concentration in the hybridization buffer in increments of 5 or 10% at a constant hybridization temperature of 46°C. Probe-conferred signals remained at the same level following the addition of formamide up to 30% for probe G1B, 35% for probe G2M, 30% for probe G3M, and 40% for probe G123T, and then decreased rapidly (Fig. 3). Using these stringent hybridization conditions, the application of the group-specific probes allowed us to specifically discriminate between the three Eikelboom type 021N groups, while G123T was successfully used to visualize all Eikelboom type 021N isolates analyzed in this study (Fig. 4). For each of the probes no signals were observed with the tested nontarget organisms if the stringent hybridization conditions were applied (Fig. 3).
TABLE 5.
Eikelboom type 021N group specific probe sequences, target sites, and formamide concentration in the hybridization buffer required for specific in situ hybridization
| Probe name (OPDa name) | Target group | Sequence (5′→3′) | Target site (16S rRNA position)b | Formamide concn (%) |
|---|---|---|---|---|
| G1B (S-*-021Ng1-1029-a-A-18) | 021N group I | TGTGTTCGAGTTCCTTGC | 1029–1046 | 30 |
| G2M (S-*-021Ng2-842-a-A-18) | 021N group II | GCACCACCGACCCCTTAG | 842–859 | 35 |
| G3M (S-*-021Ng3-996-a-A-18) | 021N group III | CTCAGGGATTCCTGCCAT | 996–1013 | 30 |
| G123T (S-G-Thioth-697-a-A-18) | 021N-Thiothrix | CCTTCCGATCTCTATGCA | 697–714 | 40 |
OPD, oligonucleotide probe database.
From E. coli numbering of Brosius et al. (4).
TABLE 6.
Difference alignment of the target region of the 16S rRNA of Eikelboom type 021N isolates for various probes
| Probe and organism or target | Target sequencea |
|---|---|
| G1B | |
| Target sequence (5′-3′) | GCAAGGAACUCGAACACA |
| Type 021N group I | ------------------ |
| Type 021N group II | UUCG-----G-U------ |
| Type 021N group III | ---------CU-C----- |
| T. nivea | UUCG-----A-UG----- |
| T. unzii | UUCG-----A-U------ |
| T. ramosa | UUCG-----A-U------ |
| T. fructosivorans I | UUCG-----A-U------ |
| T. fructosivorans Q | UUCG-----A-U------ |
| T. defluvii | ---------CUAC----- |
| G2M | |
| Target sequence (5′-3′) | CUAAGGGGUCGGUGGUGC |
| Type 021N group II | ------------------ |
| Type 021N group I | ------A----------- |
| Type 021N group III | U-U---AU--A------- |
| T. nivea | U-U----U-U-------- |
| T. unzii | UCU--A------------ |
| T. ramosa | U-U----U-U-------G |
| T. fructosivorans I | U-U--A-U-U-------G |
| T. fructosivorans Q | U-U--A-U-U-------G |
| T. defluvii | --U--AAU---------- |
| G3M | |
| Target sequence (5′-3′) | AUGGCAGGAAUCCCUGAG |
| Type 021N group III | ------------------ |
| Type 021N group I | ---U-GA-------GU-- |
| Type 021N group IIb | ---UAGC-------GC-- |
| T. eikelboomii AP3 | GA----C---AG-G--G- |
| T. nivea | UC--A------A--A-U- |
| T. unzii | UC--A-----CA--A-U- |
| T. ramosa | GA----C---AG-G--G- |
| T. fructosivorans I | G---AGCA------A--A |
| T. fructosivorans Q | GA----C---AG-G--G- |
| T. defluvii | ----U------------- |
| G123T | |
| Target sequence (5′-3′) | UGCAUAGAGAUCGGAAGG |
| Type 021N group I | ------------------ |
| Type 021N group II | ------------------ |
| Type 021N group III | ------------------ |
| T. nivea | ------------------ |
| T. unzii | ------------------ |
| T. ramosa | ------------------ |
| T. fructosivorans I | ------------------ |
| T. fructosivorans Q | ------------------ |
| T. defluvii | ------------------ |
–, identical to the target sequence.
Except T. eikelboomii AP3.
FIG. 3.
Probe dissociation curves of newly designed probes under increasingly stringent hybridization and washing conditions. For each data point mean fluorescence intensity values (arbitrary units [a.u.]) of at least 100 cells of the filaments and suitable nontarget reference organisms were determined. Error bars indicate the standard deviation. (A) probe G1B, specific for group I; (B) probe G2M, specific for group II; (C) probe G3M, specific for group III; (D) probe G123T, specific for the Eikelboom Type 021N-Thiothrix cluster. For each microorganism used, the number of mismatches (MM) with the respective probe is given in parentheses. It should be mentioned that the nontarget organisms with one mismatch (strain B5-1 and L. pneumophila) were slightly visible to the human eye after nonstringent hybridization with the respective probes. However, due to constraints of the eight-bit digital image analysis software, these weak signals could not be recorded with the confocal microscope during the probe dissociation curve experiments.
FIG. 4.
Whole-cell hybridization of Eikelboom type 021N strains in pure culture with newly designed probes. Rows: I, group I strain B5-1; II, group II strain T2-1; III, group III strain EJ1M-B. Columns: A, Probe G1B; B, probe G2M; C, probe G3M; D, probe G123T.
Subsequently, the developed probes were used in different combinations for an in situ diversity survey of Eikelboom type 021N bacteria in bulking and nonbulking activated sludge samples from 24 municipal and industrial WWTPs in Japan and Europe (Table 7). Eikelboom type 021N bacteria of group I were detected in five Japanese and one Swedish WWTP. Eikelboom type 021N bacteria of group II could be visualized in seven Japanese and two European WWTPs (Fig. 5) while Eikelboom type 021N bacteria of group III were not found in any of the investigated samples. No signals were detected with the three Eikelboom type 021N bacterial group-specific probes in 12 sludge samples from German WWTPs. Except for the Swedish WWTP all filaments detected with the group-specific probe G1B or G2M also hybridized simultaneously with probe G123T that is specific for the entire Eikelboom type 021N-Thiothrix cluster. In the Swedish plant a minor fraction of the filaments identified as Eikelboom type 021N bacteria of group II did not hybridize with probe G123T.
TABLE 7.
Application of developed probes for in situ survey of filamentous bacteria of the Eikelboom type 021N in activated sludge from different WWTPs
| WWTP | PEa | Sewage type | Bulkingb | Probeb
|
|||
|---|---|---|---|---|---|---|---|
| G1B | G2M | G3M | G123T | ||||
| Kyoto A, Japan | 210,000 | Municipal | + | + | − | − | + |
| Kagawa A, Japan | 35,500 | Municipal | + | + | + | − | + |
| Hokkaido A, Japan | 68,000 | Municipal | + | − | + | − | + |
| Tokyo A1, Japan | 400,000 | Municipal | − | − | − | − | + |
| Tokyo A2, Japan | 400,000 | Municipal | − | − | + | − | + |
| Tokyo A3, Japan | 400,000 | Municipal | − | + | + | − | + |
| Tokyo B1, Japan | 220,000 | Municipal | − | − | + | − | + |
| Tokyo B2, Japan | 220,000 | Municipal | − | + | + | − | + |
| Tokyo C, Japan | 80,000 | Municipal | − | − | − | − | − |
| Tokyo D, Japan | 500,000 | Municipal | + | + | + | − | + |
| Malmö, Sweden (aerobic basin) | 150 | Municipal | + | + | + | − | + |
| Munich I, Germany (high-load basin) | 2,000,000 | Municipal | − | − | + | − | + |
| Munich I, Germany (low-load basin for nitrification) | 2,000,000 | Municipal | − | − | − | − | − |
| Plattling, Germany | 26,000 | Rendering plant effluent | − | − | − | − | − |
| Munich II, Germany (high-load basin) | 1,000,000 | Municipal | − | − | − | − | − |
| Munich II, Germany (low-load basin for nitrification) | 1,000,000 | Municipal | − | − | − | − | − |
| Kraftisried, Germany | 6,000 | Rendering plant effluent | − | − | − | − | − |
| Stephanskirchen, Germany | 80,000 | Municipal | − | − | − | − | − |
| Poing, Germany (high-load basin) | 110,000 | Municipal | − | − | − | − | − |
| Poing, Germany (low-load basin for nitrification) | 110,000 | Municipal | − | − | − | − | − |
| Freising, Germany | 110,000 | Municipal | − | − | − | − | − |
| Erding, Germany | NDc | Municipal | − | − | − | − | + |
| Schongau, Germany | 185,000 | Municipal | − | − | − | − | − |
| Oberding, Germany | ND | Rendering plant effluent | + | − | − | − | − |
PE, population equivalents.
Bulking and reactions to probes were scored as positive (+) or negative (−).
ND, not determined.
FIG. 5.
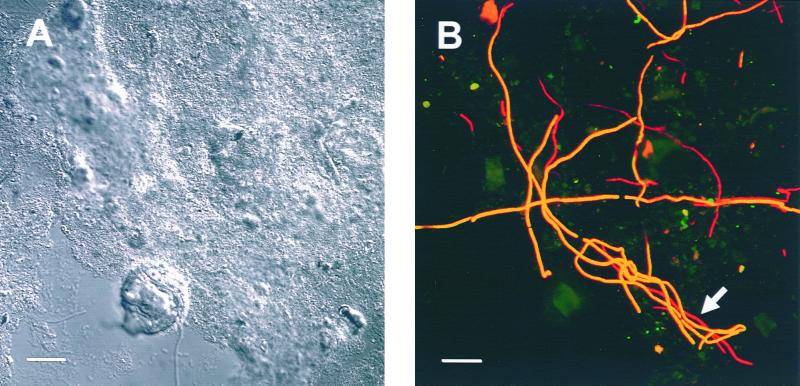
In situ detection of Eikelboom type 021N bacteria in activated sludge from the WWTP Großlappen (Munich, Germany). (A) An interference contrast image of a sludge floc. (B) Fluorescence in situ hybridization of the same microscopic field using probes G2M (labeled with Cy3, colored in green) and G123T (labeled with Cy5, colored in red). Yellow filaments did hybridize with both probes and were thus identified as members of group II. Red filaments (arrow) only bound probe G123T and are most likely members of the genus Thiothrix. Bar, 20 μm.
DISCUSSION
The 15 isolates of filamentous bacteria analyzed in this study showed highly similar morphological, staining, and physiological characteristics, which are indicative of classification as Eikelboom type 021N bacteria. Comparative 16S rRNA sequence analysis revealed that these isolates can be divided into three distinct evolutionary groups which, together with recognized Thiothrix species, form a monophyletic grouping within the gamma-subclass of the class Proteobacteria. It should be noted that the investigated Eikelboom type 021N isolates were clearly different from Thiothrix species described in Bergey's Manual of Systematic Bacteriology (18) in the shape of colonies, the length of filaments, sheath formation, and sulfur granule production. Furthermore, the G+C contents of Eikelboom type 021N isolates analyzed here are much lower (43.9 to 46.1%) than the G+C contents reported for T. nivea (52%) (19) and T. ramosa (51.0 to 52.4%) (23). In addition, 16S rRNA sequence similarities of the investigated Eikelboom type 021N isolates with T. nivea and T. ramosa are lower than 92%. However, recently, Howarth et al. (12) have proposed a change of the definition of the genus Thiothrix and suggested the inclusion of Eikelboom type 021N strains into this genus. Consequently, Eikelboom type 021N strain AP3 was designated as T. eikelboomii, and the newly isolated Eikelboom type 021N strain Ben 57 was classified as T. defluvii. In our study we showed a previously unrecognized diversity within the Eikelboom type 021N strains, and these findings underline the necessity of obtaining more phenotypic and genotypic information of Eikelboom type 021N isolates to clarify their taxonomic position.
Based on the extended 16S rRNA data set for members of the Eikelboom type 021N-Thiothrix group, the specificity and sensitivity of the previously published 16S rRNA-targeted probes TNI and 21N (31) was reevaluated. Results demonstrated that, using the hybridization conditions recommended by Wagner et al. (31), probe TNI also targets the Eikelboom type 021N group II, including T. eikelboomii (except for strain T1-4), and the Eikelboom type 021N group III, including T. defluvii. Of the newly analyzed strains, only strain T1-4 of the Eikelboom type 021N group II is targeted by probe 21N. Thus, members of the Eikelboom type 021N group I, for the first time described in this study, cannot be detected in environmental samples by application of these probes. This might explain the findings of Nielsen et al. (22), who used the oligonucleotide probes 21N and TNI for in situ analysis of bulking activated sludges in industrial and domestic WWTPs dominated by filamentous bacteria morphologically identified as Eikelboom type 021N bacteria. The authors reported that the dominant filamentous bacteria in some treatment plants did not hybridize with probe 21N but hybridized with probe TNI and that the dominant filamentous bacteria in other treatment plants did not hybridize with either probe 21N or TNI. Nielsen and coworkers thus hypothesized that the Eikelboom type 021N morphotype comprises different species of filamentous bacteria which are dominating in different bulking sludges. Our results confirm this hypothesis and suggest that previous reports on sludge bulking caused by Eikelboom type 021N bacteria (7, 13, 14, 16, 25, 32, 35, 36) were descriptions of the same phenomenon caused by different bacterial species which share the same morphology.
New 16S rRNA-targeted probes were designed and evaluated for three subgroups of Eikelboom type 021N bacteria and for all recognized Eikelboom type 021N-Thiothrix species and subsequently successfully applied in a diversity survey of Eikelboom type 021N bacteria in various Japanese and European WWTPs. The results of the survey showed that members of group I and group II Eikelboom type 021N bacteria do occur in WWTPs on both continents. In contrast, group III Eikelboom type 021N bacteria could not be detected in situ in any of the analyzed WWTPs. Future studies will have to show whether this is caused by low in situ abundance (below the detection limit of the in situ hybridization method) or by restricted occurrence of these filaments in particular WWTPs. The finding of Eikelboom type 021N bacteria which hybridize with probe G2M but not with probe G123T in one Swedish WWTP suggests the existence of additional, not-yet-recognized diversity of Eikelboom type 021N bacteria.
Currently we are using the developed probes to investigate (i) the in situ physiology of the different groups of Eikelboom type 021N bacteria by combining fluorescence in situ hybridization with microautoradiography (20) and (ii) more generally, the links between the different Eikelboom type 021N bacteria and bulking of activated sludge.
ACKNOWLEDGMENTS
M.H. was supported by a grant of the Sonderforschungsbereich 411 from the Deutsche Forschungsgemeinschaft (Research Center for Fundamental Studies of Aerobic Biological Wastewater Treatment) to M.W. We thank Yasuyo Ashizawa and Yoko Ueda for quinone and the G+C content determination.
REFERENCES
- 1.Alm E W, Oerther D B, Larsen N, Stahl D A, Raskin L. The oligonucleotide probe database. Appl Environ Microbiol. 1996;62:3557–3559. doi: 10.1128/aem.62.10.3557-3559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann R I, Binder B J, Olson R J, Chisholm S W, Devereux R, Stahl D A. Combination of 16S rRNA-targeted oligonucleotide probe with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann R I. In situ identification of micro-organisms by whole cell hybridization with rRNA-targeted nucleic acid probes. In: Akkermans A D L, Elsas J D, Bruij F J, editors. Molecular microbial ecology manual, 3.3.6. London, United Kingdom: Kluwer; 1995. pp. 1–15. [Google Scholar]
- 4.Brosius J, Dull T L, Sleeter D D, Noller H F. Gene organization and primary structure of a ribosomal operon from Escherichia coli. J Mol Biol. 1981;148:107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- 5.Daims H, Brühl A, Amann R, Schleifer K-H, Wagner M. The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol. 1999;22:434–444. doi: 10.1016/S0723-2020(99)80053-8. [DOI] [PubMed] [Google Scholar]
- 6.Eikelboom D H. Filamentous organisms observed in activated sludge. Water Res. 1975;9:365–388. [Google Scholar]
- 7.Eikelboom D H, van Buijsen H J J. Microscopic sludge investigation manual. Report A94a. Delft, The Netherlands: IMG-TNO; 1981. [Google Scholar]
- 8.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 9.Hiraishi A. Direct automated sequencing of 16S rRNA amplified by polymerase chain reaction from bacterial cultures without DNA purification. Lett Appl Microbiol. 1992;15:210–213. doi: 10.1111/j.1472-765x.1992.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 10.Hiraishi A, Ueda Y, Ishihara J, Mori T. Comparative lipoquinone analysis of influent sewage and activated sludge by high-performance liquid chromatography and photodiode array detection. J Gen Appl Microbiol. 1996;42:457–469. [Google Scholar]
- 11.Howarth R, Head M, Unz R F. Phylogenetic assessment of five filamentous bacteria isolated from bulking activated sludges. Water Sci Technol. 1998;37(4–5):303–306. [Google Scholar]
- 12.Howarth R, Unz R F, Seviour E M, Seviour R J, Blackall L L, Pickup R W, Jones J G, Yaguchi J, Head I M. Phylogenetic relationships of filamentous sulfur bacteria (Thiothrix spp. and Eikelboom type 021N bacteria) isolated from wastewater-treatment plants and description of Thiothrix eikelboomii sp. nov., Thiothrix unzii sp. nov., Thiothrix fructosivorans sp. nov. and Thiothrix defluvii sp. nov. Int J Syst Bacteriol. 1999;49:1817–1827. doi: 10.1099/00207713-49-4-1817. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins D. Towards a comprehensive model of activated sludge bulking and foaming. Water Sci Technol. 1992;25(6):215–230. [Google Scholar]
- 14.Jenkins D, Richard M G, Daigger G T. Manual on the causes and control of activated sludge bulking and forming. 2nd ed. Chelsea, Mich: Lewis Publishers; 1993. [Google Scholar]
- 15.Kamagata Y, Mikami E. Isolation and characterization of novel thermophilic Methanosaeta strain. Int J Syst Bacteriol. 1991;41:191–196. doi: 10.1099/00207713-42-3-463. [DOI] [PubMed] [Google Scholar]
- 16.Kohno T. Morphology, physiology, and nutrition of a sulfur-oxidizing filamentous organism isolated from activated sludge. Water Sci Technol. 1988;20(11–12):241–247. [Google Scholar]
- 17.Kumar S, Tamura K, Nei M. MEGA; molecular evolutionary genetics analysis, version 1.0. University Park: The Pennsylvania State University; 1993. [Google Scholar]
- 18.Larkin J M. Genus II. Thiothrix. In: Staley J P, Bryant M P, Pfennig N, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 3. Baltimore, Md: The Williams & Wilkins Co.; 1989. pp. 2098–2101. [Google Scholar]
- 19.Larkin J M, Shinabarger D L. Characterization of Thiothrix nivea. Int J Syst Bacteriol. 1983;33:841–846. [Google Scholar]
- 20.Lee N, Nielsen P H, Andreasen K H, Juretschko S, Nielsen J L, Schleifer K-H, Wagner M. Combination of fluorescent in situ hybridization and microautoradiography: a new tool for structure-function analyses in microbial ecology. Appl Environ Microbiol. 1999;65:1289–1297. doi: 10.1128/aem.65.3.1289-1297.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marmur J. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 22.Nielsen P H, Andeasen K, Wagner M, Blackall L L, Lemmer H, Seviour R J. Variety of type 021N in activated sludge as determined by in situ substrate uptake pattern and in situ hybridization with fluorescent rRNA targeted probes. Water Sci Technol. 1998;37(4–5):423–430. [Google Scholar]
- 23.Odintsova E V, Dubinina G A. Thiothrix ramosa nov. sp., a new species of colourless filamentous sulfur bacteria. Mikrobiologii. 1990;59:637–646. [Google Scholar]
- 24.Pernelle J, Cotteux E, Duchene P. Effectiveness of oligonucleotide probes targeted against Thiothrix nivea and type 021N 16S rRNA for in situ identification and population monitoring in activated sludges. Water Sci Technol. 1998;37(4–5):431–440. [Google Scholar]
- 25.Richard M G, Shimizu G P, Jenkins D. The growth physiology of the filamentous organism type 021N and its significance to activated sludge bulking. J Water Pollut Control Fed. 1985;57:1152–1161. [Google Scholar]
- 26.Saitou N, Nei M. The neighbor joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 27.Sezgin M, Jenkins D, Parker D S. A unified theory of filamentous activated sludge bulking. J Water Pollut Control Fed. 1978;50:362–381. [Google Scholar]
- 28.Strom F, Jenkins D. Identification and significance of filamentous microorganisms in activated sludge. J Water Pollut Control Fed. 1984;57:449–459. [Google Scholar]
- 29.Tamaoka K, Katayama-Fujimura Y, Kuraishi H. Analysis of bacterial menaquinone mixtures by high performance liquid chromatography. J Appl Bacteriol. 1983;54:31–36. [Google Scholar]
- 30.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W; improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner M, Amann R, Kaempeer P, Assmus B, Hartmann A, Hutzler P, Springer N, Schleifer K-H. Identification and in situ detection of Gram-negative filamentous bacteria in activated sludge. Syst Appl Microbiol. 1994;17:405–417. [Google Scholar]
- 32.Wanner J. Activated sludge bulking and foaming control. Lancaster, Pa: Technomic Publishing Company; 1994. [Google Scholar]
- 33.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams T M, Unz R F. Filamentous sulfur bacteria of activated sludge: Characterization of Thiothrix, Beggiatoa, and Eikelboom type 021N strains. Appl Environ Microbiol. 1985;49:887–898. doi: 10.1128/aem.49.4.887-898.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams T M, Unz R F. Isolation and characterization of filamentous bacteria present in bulking activated sludge. Appl Microbiol Biotechnol. 1985;22:273–282. [Google Scholar]
- 36.Ziegler M, Lange M, Dott W. Isolation and morphological and cytological characterization of filamentous bacteria from bulking sludge. Water Res. 1990;24:1437–1451. [Google Scholar]