ABSTRACT
Although recent reports of extensively antibiotic-resistant strains have highlighted the importance of Morganella morganii as an emerging pathogen, the epidemiology of serious infections due to this organism is not well defined. The objective of this study was to determine the incidence, determinants, and outcomes of Morganella morganii bloodstream infections (BSIs). Retrospective, population-based surveillance for Morganella morganii BSIs was conducted in Queensland, Australia, in 2000 to 2019; 709 cases were identified, for an annual incidence of 9.2 cases per million population. Most cases were of community onset, with 280 (39.5%) community-associated cases and 226 (31.9%) health care-associated cases. Morganella morganii BSIs were rare in children and young adults, and the incidence increased markedly with advancing age. The most common foci of infection were skin and soft tissue (131 cases [18.5%]), genitourinary (97 cases [13.7%]), and intraabdominal (90 cases [12.7%]). Most patients (580 cases [81.8%]) had at least one comorbid medical illness, with diabetes mellitus (250 cases [35.3%]), renal disease (208 cases [29.3%]), and congestive heart failure (167 cases [23.6%]) being most prevalent. Resistance to one or more of quinolones, co-trimoxazole, aminoglycosides, or carbapenems was observed in 67 cases (9.5%), and this did not change significantly over the study. The 30-day all-cause case fatality rate was 21.2%, and increasing age, nonfocal infection, heart failure, dementia, and cancer were independently associated with increased risk of death. Morganella morganii BSIs are increasing in our population, and elderly male subjects and individuals with comorbidities are at highest risk. Although antibiotic resistance is not a major contributor to the current burden in Queensland, ongoing surveillance is warranted.
IMPORTANCE Recent reports of extensively antibiotic-resistant strains have highlighted the importance of Morganella morganii as an emerging pathogen. Despite its present and evolving importance as an agent of human disease, there is a limited body of literature detailing the epidemiology of serious infections due to Morganella morganii. Therefore, the objectives of this study were to examine the incidence and determinants of Morganella morganii BSIs and to examine risk factors for death in a large Australian population in 2000 to 2019.
KEYWORDS: bloodstream infection, epidemiology, Morganella morganii
INTRODUCTION
Morganella morganii with Proteus and Providencia species comprise the tribe Proteeae and are occasional causes of bloodstream infections (BSIs) (1). Morganella morganii has chromosomally encoded blaAmpC, which confers resistance to cephalosporins and penicillins (2). As a result, treatment with quinolones, aminoglycosides, co-trimoxazole, or carbapenems is usually recommended (3–6). However, the potential for Morganella morganii to acquire other resistance determinants has been increasingly recognized (7). Recent studies have found extensively resistant strains in sewage samples and among a range of animal sources (8–13). As a result of its ability to cause invasive disease, the presence of blaAmpC and virulence factors, and its propensity to acquire resistance determinants, Morganella morganii has been labeled an emerging “superbug” (6).
Despite its present and evolving importance as an agent of human disease, there is a limited body of literature detailing the epidemiology of serious infections due to Morganella morganii. Although there have been several reported case series of patients with BSIs, these have been limited by small sample size and reporting from single hospitals that are at risk of selection biases (14–18). The objectives of this study were to examine the incidence and determinants of Morganella morganii BSIs and to examine risk factors for death in a large Australian population.
RESULTS
A total of 709 incident Morganella morganii BSIs were identified among 705 Queensland residents. Four patients had second incident episodes. The majority of the BSIs were of community onset, with 280 (39.5%) community-associated BSIs and 226 (31.9%) health care-associated BSIs. Of the 203 (28.6%) hospital-onset cases, the median time from admission to diagnosis was 8 days (interquartile range [IQR], 5 to 17 days).
Demographic determinants.
The median age was 75.2 years (IQR, 64.2 to 83.0 years), and two-thirds (463 [65.3%]) of the episodes occurred among male patients. Morganella morganii BSIs were rare in children and young adults, and the incidence increased significantly with older age and male sex, as shown in Fig. 1. Overall, males were at twice the risk for development of Morganella morganii BSIs (10.8 versus 5.7 cases per million; incidence rate ratio [IRR], 1.89 [95% confidence interval [CI], 1.62 to 2.22]; P < 0.0001). However, this excess risk among males was due to those 50 years of age and older (32.5 versus 15.8 cases per million; IRR, 2.04 [95% CI, 1.73 to 2.42]; P < 0.0001), with no significant excess risk observed among younger individuals (1.40 versus 1.01 cases per million; IRR, 1.38 [95% CI, 0.84 to 2.29]; P = 0.18).
FIG 1.
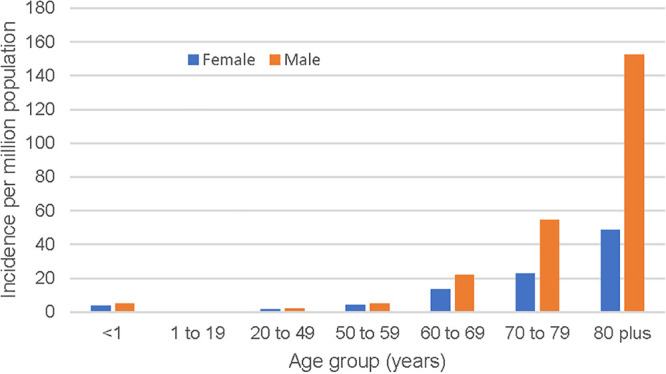
Incidence of Morganella morganii BSIs by age and sex in Queensland, Australia, in 2000 to 2019.
Incidence.
The overall age- and sex-standardized incidence was 9.2 cases per million annually, with 8.3 and 0.9 cases per million annually for antimicrobial-susceptible and -resistant isolates, respectively. Moderate year-to-year variability was observed in the incidence of Morganella morganii BSIs, as shown in Fig. 2. There was an overall standardized incidence increase of 0.23 cases per million per year, which was associated with an increased blood culture sampling rate during the study (Fig. 2). No significant trend in the incidence of resistant isolates was observed.
FIG 2.
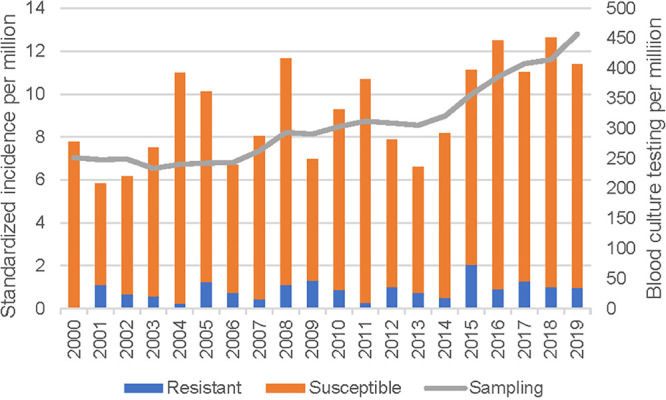
Age- and sex-standardized incidence of Morganella morganii BSIs in Queensland, Australia, in 2000 to 2019.
Clinical features.
The clinical characteristics of Morganella morganii BSIs varied considerably by onset category, as shown in Table 1. There was a progressive decrease in age associated with an increasing degree of health care exposure. Community-associated cases had the greatest proportion of males and the lowest median comorbidity scores. The proportions of cases with no comorbid disease varied by onset category and were 35 cases (17.2%), 19 cases (8.4%), and 75 cases (26.8%) for hospital-onset, health care-associated, and community-associated cases, respectively (P < 0.001 overall and P < 0.02 for each pairwise comparison).
TABLE 1.
Clinical characteristics of Morganella morganii BSIs according to onset classification
| Factor | Data for: |
P | |||
|---|---|---|---|---|---|
| Hospital-onset cases (n = 203) | Health care-associated cases (n = 226) | Community-associated cases (n = 280) | All cases (n = 709) | ||
| Age (median [IQR]) (yr) | 71.3 (61.2–80.6) | 75.2 (63.6–83.1) | 78.0 (67.9–84.6) | 75.2 (64.2–83.0) | <0.001 |
| Male sex (no. [%]) | 125 (61.6) | 135 (59.7) | 203 (72.5) | 0.005 | |
| Charlson score (median [IQR]) | 3 (1–5) | 4 (2–6) | 2 (0–3) | 2 (1–5) | <0.001 |
| Focus (no. [%]) | <0.001 | ||||
| No focus/primary | 119 (58.6) | 90 (39.8) | 116 (41.4) | 325 (45.8) | |
| Skin/soft tissue | 13 (6.4) | 55 (24.3) | 63 (22.5) | 131 (18.5) | |
| Bone/joint | 10 (4.9) | 10 (4.4) | 5 (1.8) | 25 (3.5) | |
| Head and neck | 0 | 1 (0.4) | 0 | 1 (0.1) | |
| Lower respiratory tract | 6 (3.0) | 16 (7.1) | 12 (4.3) | 34 (4.8) | |
| Endovascular | 3 (1.4) | 1 (0.4) | 1 (0.4) | 5 (0.7) | |
| Central nervous system | 0 | 0 | 1 (0.4) | 1 (0.1) | |
| Intraabdominal | 36 (17.7) | 19 (8.4) | 35 (12.5) | 90 (12.7) | |
| Genitourinary | 16 (7.9) | 34 (15.0) | 47 (16.8) | 97 (13.7) | |
| Polymicrobial (no. [%]) | 72 (35.5) | 76 (33.6) | 91 (32.5) | 239 (33.7) | 0.8 |
| Resistance to ≥1 class (no. [%]) | 27 (13.3) | 20 (8.9) | 20 (7.1) | 67 (9.5) | 0.08 |
| Resistance (no./total no. tested [%]) | |||||
| Ciprofloxacin | 15/193 (7.8) | 8/219 (3.7) | 5/268 (1.9) | 28/680 (4.1) | 0.008 |
| Gentamicin | 16/200 (8.0) | 7/224 (3.1) | 6/279 (2.2) | 29/703 (4.1) | 0.007 |
| Tobramycin | 10/196 (5.1) | 5/223 (2.2) | 1/272 (0.4) | 16/691 (2.3) | 0.003 |
| Meropenem | 1/172 (0.6) | 1/208 (0.5) | 0/246 | 2/626 (0.3) | 0.5 |
| Trimethoprim-sulfamethoxazole | 17/197 (8.6) | 12 (5.4) | 15 (5.4) | 44/698 (6.3) | 0.3 |
The most common foci of infection were skin and soft tissue (131 cases [18.5%]), genitourinary (97 cases [13.7%]), and intraabdominal (90 cases [12.7%]). Significant differences were observed for the foci of infection in relation to onset category (Table 1). Hospital-onset infections were most likely to be nonfocal and least likely to be of genitourinary or skin and soft tissue focus, whereas the greatest proportion of lower respiratory tract infections was among health care-associated cases, as shown in Table 1.
Microbiology.
Overall, 239 episodes (33.7%) were associated with polymicrobial etiology, although this proportion did not vary significantly among onset categories (Table 1). There were no significant differences in sex, median age, Charlson score, or distribution of foci of infection among those with monomicrobial versus polymicrobial infections. Among the polymicrobial episodes, 239 had one coisolate, 88 had two, 30 had three, 9 had four, and 1 had five. Escherichia coli (74 cases [20.2%]), Enterococcus faecalis (52 cases [14.2%]), Proteus mirabilis (39 cases [10.6%]), Pseudomonas aeruginosa (29 cases [7.9%]), and Klebsiella pneumoniae (23 cases [6.3%]) were the most common species among the 367 coisolated organisms.
Resistance to one or more of the quinolones, co-trimoxazole, aminoglycosides, or carbapenems was observed in 67 cases (9.5%). A trend to increased prevalence of resistance was observed for hospital-onset cases (Table 1). However, the proportions of cases with resistance to one (32 cases [4.5%]), two (15 cases [2.1%]), or three (20 cases [2.8%]) classes did not differ according to onset (P = 0.127). Resistance to ceftriaxone, cefepime, ceftazidime, and piperacillin-tazobactam was found in 52/627 (8.3%), 10/556 (1.8%), 118/652 (18.1%), and 21/541 (3.9%) isolates tested, respectively.
Outcomes.
Ninety-eight percent of cases (695 cases) were admitted to the hospital for management at the time of or within 2 days after BSI diagnosis. The median duration of the hospital stay was 11 days (6 to 28 days). A total of 150 patients died within 30 days after the Morganella morganii BSI, for an all-cause case fatality rate of 21.2%. A number of factors were found to be associated with adverse outcomes, as shown in Table 2. A logistic regression model (n = 705) was developed that had good fit (P = 0.2) and an area under the receiver operator characteristic curve of 0.7271. As shown in Table 3, older age, nonfocal infections, and comorbidities, including congestive heart failure, dementia, and cancer, were independently associated with increased risk of death.
TABLE 2.
Factors associated with 30-day all-cause case fatality due to Morganella morganii BSIs
| Factor | Data for patients who: |
P | ||
|---|---|---|---|---|
| Survived (n = 559) | Died (n = 150) | Overall (n = 709) | ||
| Age (median [IQR]) (yr) | 73.3 (62.6–82.0) | 79.7 (71.2–86.5) | 75.2 (64.2–83.0) | <0.0001 |
| Male sex (no. [%]) | 365 (65.3) | 98 (65.3) | 463 (65.3) | 0.5 |
| Infection onset (no. [%]) | 0.6 | |||
| Hospital | 156 (27.9) | 47 (31.3) | 203 (28.6) | |
| Community associated | 177 (31.7) | 49 (32.7) | 226 (31.9) | |
| Health care associated | 226 (40.4) | 54 (36.0) | 280 (39.5) | |
| Charlson score (median [IQR]) | 2 (1–4) | 4 (2–6) | 2 (1–5) | <0.0001 |
| Myocardial infarction (no. [%]) | 66 (11.8) | 23 (15.3) | 89 (12.6) | 0.2 |
| Congestive heart failure (no. [%]) | 118 (21.1) | 49 (32.7) | 167 (23.6) | 0.005 |
| Peripheral vascular disease (no. [%]) | 71 (12.7) | 27 (18.0) | 98 (13.8) | 0.1 |
| Cerebrovascular disease (no. [%]) | 46 (8.2) | 17 (11.3) | 63 (8.9) | 0.2 |
| Dementia (no. [%]) | 40 (7.2) | 23 (15.3) | 63 (8.9) | 0.003 |
| Chronic pulmonary disease (no. [%]) | 65 (11.6) | 25 (16.7) | 90 (12.7) | 0.1 |
| Rheumatic disease (no. [%]) | 15 (2.7) | 5 (3.3) | 20 (2.8) | 0.4 |
| Peptic ulcer disease (no. [%]) | 8 (1.4) | 5 (3.3) | 13 (1.8) | 0.2 |
| Liver disease (no. [%]) | 32 (5.7) | 9 (6.0) | 41 (5.8) | 0.8 |
| Diabetes mellitus (no. [%]) | 203 (36.3) | 47 (31.3) | 250 (35.3) | 0.3 |
| Plegia (no. [%]) | 43 (7.7) | 8 (5.3) | 51 (7.2) | 0.4 |
| Renal disease (no. [%]) | 155 (27.7) | 53 (35.3) | 208 (29.3) | 0.09 |
| Malignancy (no. [%]) | 91 (16.3) | 45 (30.0) | 136 (19.2) | <0.001 |
| Metastatic cancer (no. [%]) | 31 (5.6) | 23 (15.3) | 54 (7.6) | <0.001 |
| HIV positive (no. [%]) | 1 (0.2) | 0 | 1 (0.1) | 1 |
| Focus of infection (no. [%]) | 0.016 | |||
| No focus | 229 (41.4) | 89 (59.7) | 318 (45.3) | |
| Soft tissue | 109 (19.7) | 22 (14.8) | 131 (18.7) | |
| Bone and joint | 22 (4.0) | 3 (2.0) | 25 (3.6) | |
| Head and neck | 1 (0.2) | 0 | 1 (0.1) | |
| Lower respiratory tract | 28 (5.1) | 6 (4.0) | 34 (4.8) | |
| Endovascular | 5 (0.9) | 0 | 5 (0.7) | |
| Central nervous system | 1 (0.2) | 0 | 1 (0.1) | |
| Abdominal | 73 (13.2) | 17 (11.4) | 90 (12.8) | |
| Urinary/pelvic | 85 (15.4) | 12 (8.1) | 97 (3.8) | |
| Polymicrobial (no. [%]) | 185 (33.1) | 54 (36.0) | 239 (33.7) | 0.5 |
| Resistant (no. [%]) | 53 (9.5) | 14 (9.3) | 67 (9.5) | 1 |
TABLE 3.
Logistic regression modeling of factors associated with 30-day case fatality
| Factor | Odds ratio (95% CI) | P |
|---|---|---|
| Age (per yr) | 1.04 (1.01–1.05) | <0.001 |
| Congestive heart failure | 1.75 (1.14–2.69) | 0.011 |
| Dementia | 1.98 (1.10–3.58) | 0.023 |
| No cancer | 1 | |
| Cancer without metastases | 1.85 (1.06–3.25) | 0.032 |
| Cancer with metastases | 4.02 (2.17–7.44) | <0.001 |
| No focus of infection | 2.28 (1.55–3.37) | <0.001 |
DISCUSSION
This study identifies that Morganella morganii is a relatively infrequent but important cause of BSIs in our population. Older individuals, especially males and those with comorbidities, are at highest risk of developing Morganella morganii BSIs. One-fifth of patients who develop a Morganella morganii BSI will not survive past 30 days, and older patients, patients with nonfocal infections, and patients with a number of comorbidities are at highest risk of death. Although antimicrobial resistance did not increase during the study or increase the risk of death, the potential for this to add to the significant burden associated with these infections in future years merits ongoing surveillance.
The incidence rate of 9.2 cases per million population we observed is a novel observation. A previous population-based laboratory surveillance study investigating tribe Proteeae isolates in Calgary, Canada, found that Morganella morganii was associated with an overall incidence rate of 77 cases per million annually, with an incidence of bacteremia of approximately 3 cases per million per year (1). Other studies examining etiologies of BSIs in nonselected populations have not reported specific rates for Morganella morganii, to our knowledge (19, 20). It is also notable that the incidence we observed during surveillance increased over time. While there may be many explanations for this observation, it is likely that this reflects at least in part increased overall sampling in the population (21).
Previous hospital-based studies have reported on the clinical features and outcomes associated with Morganella morganii BSIs. Erlanger et al. most recently reported on 136 adults with Morganella morganii bacteremia who were admitted to a large university hospital in Israel in 1997 to 2014 (14). They found that 91 cases (68%) were acquired in a hospital or health care facility, that most patients were debilitated or highly comorbid, and that the 30-day case fatality rate was 52% (14). They further reviewed the literature and identified three other studies, including 109 cases from a medical center in Taiwan in 2003 to 2012 (16), 61 cases in a large tertiary hospital in South Korea in 1991 to 2000 (17), and 73 cases from another Taiwan medical center in 2002 and 2003 (18). Another study was also included in their review but was not limited to bacteremic cases (15). Those studies showed marked heterogeneity in results, with average ages ranging from 52 to 75 years, community-acquired infections ranging from 30% to 75%, and case fatality rates ranging from 15% to 42%. While these differences could in part reflect the definitions used and true differences in the populations under study, it is likely that selection bias arising from the conduct of these reviews at single hospitals played a significant role (22). Our study benefits from the inclusion of patients from all institutions, both small and large, and in urban and nonurban settings.
While our study benefits from a population-based design and includes a large cohort of patients surveyed over an extended period, there are some limitations. First, we did not have any data regarding the antimicrobial therapies prescribed; therefore, we could not examine this as a determinant of outcomes. However, we observed a relatively low rate of resistance and noted that its presence was not associated with survival outcomes. Second, we included only cases identified within the public system, such that patients managed in private institutions were not included in surveillance. Our results should thus be interpreted in this context, and the true rate of Morganella morganii BSIs is higher than we observed. Third, because this was a retrospective study, we were limited to the available data existing in our source databases. We did not have details regarding procedures to which patients might have been exposed, such as urinary tract instrumentation, which could be risk factors for both acquisition and adverse outcomes.
In conclusion, this novel study defines the epidemiology of Morganella morganii BSIs in a large population and identifies risk factors associated with adverse outcomes. While we observed that the incidence was increasing, antibiotic resistance was not. Given the current burden of disease and potential to increase, ongoing surveillance is warranted.
MATERIALS AND METHODS
A retrospective, population-based, laboratory surveillance, cohort design was utilized. All residents of Queensland, Australia, who had BSIs due to Morganella morganii identified within the publicly funded health care system between 1 January 2000 and 31 December 2019 were included. This study was approved by the human research ethics committee at Royal Brisbane and Women’s Hospital, and a waiver of the requirement for individual consent was granted (LNR/2020/QRBW/62494).
All blood cultures for Morganella morganii during the surveillance period, including those obtained from community and institutional collection sites statewide, were first identified by Pathology Queensland. The BacT/Alert 3D system (bioMérieux, Durham, NC) was used throughout the study period with the exception that, as of 2018, the BacT/Alert VIRTUO system (bioMérieux) was used at the main central laboratory that manages culture submissions from the Greater Brisbane area and several rural Queensland sites. At Pathology Queensland, blood cultures are routinely incubated for 5 days before being discarded for no growth. BacT/Alert FA Plus (aerobic), FN Plus (anaerobic), and PF Plus (pediatric) medium bottles were used for culture. Species identification methods included the Vitek GN ID card, the API 20E test, and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). Antibiotic susceptibility testing was performed using both an automated method (i.e., Vitek AST card) and the disc diffusion method according to recognized standards (CLSI or EUCAST) at the time of testing.
Once positive blood cultures were identified, linkages to statewide hospital admissions and death registries were performed to obtain clinical and outcome information. A series of algorithms and previously validated definitions were then applied to classify BSI episodes (23, 24). Incident episodes were defined by the first isolation of Morganella morganii per patient; repeat isolates within 30 days were deemed to represent the same incident episode. When one or more organisms were coisolated with Morganella morganii within a 48-h period, the BSI was classified as polymicrobial (25). Resistant organisms were defined as those nonsusceptible to one or more of ciprofloxacin/norfloxacin, co-trimoxazole, gentamicin/tobramycin, or imipenem/meropenem.
Admissions to any private or public institutions within the state were identified, and discharge diagnostic codes (ICD-10AM) were obtained. The index hospitalization included all encounters associated with the management of the BSI, including interhospital transfers within the state. The Registry of General Deaths as of 31 December 2020 was queried to confirm deaths in any setting within Queensland.
Hospital-onset BSIs were those for which the index blood culture was drawn more than 2 calendar days after admission or within 2 calendar days after hospital discharge (26). Community-onset BSIs were those for which the index culture was drawn in the community or within the first 2 days of hospital admission. Health care-associated BSIs were community-onset BSIs that occurred among nursing home residents or patients who had encounters at a health care institution within 30 days and/or admission to a hospital for more than 2 days within the 90 days prior to the index blood culture (27). Community-associated BSIs were community-onset BSIs that were not health care associated. Comorbid medical illnesses were defined using the Charlson Comorbidity Index (28, 29). A clinical focus was assigned based on review of diagnosis-related group and primary diagnosis hospital discharge codes.
Data were analyzed using Stata 17 (StataCorp, College Station, TX, USA). The primary unit of analysis was incident BSI episodes, and results were reported as age- and sex-standardized (to the 2019 Queensland population) annual rates per million population. Nonresidents of Queensland were excluded. Denominator data were stratified by age, sex, and hospital, and the health service area was obtained from Queensland Health using data available from the Australian Bureau of Statistics (30). The total annual number of sets of blood cultures performed by Pathology Queensland was obtained (21). IRRs with exact 95% CIs were calculated for group comparisons.
A multivariable logistic regression model was developed to examine factors associated with all-cause 30-day case fatality rates. Age, gender, onset classification, resistance, comorbidities, polymicrobial infection, and focus of infection were included in the initial model. Stepwise backward variable elimination was performed in order to develop the most parsimonious model. Calibration and discrimination were assessed using the Hosmer-Lemeshow test and the area under the receiver operator characteristic curve, respectively. P values of <0.05 were deemed to represent statistical significance for all comparisons.
Data availability.
Data cannot be shared publicly due to institutional ethics, privacy, and confidentiality regulations. Data release for the purposes of research under Section 280 of the Public Health Act 2005 requires application to the Director-General (pha@health.qld.gov.au).
Contributor Information
Kevin B. Laupland, Email: kevin.laupland@qut.edu.au.
Krisztina M. Papp-Wallace, Louis Stokes Cleveland VAMC
REFERENCES
- 1.Laupland KB, Parkins MD, Ross T, Pitout JD. 2007. Population-based laboratory surveillance for tribe Proteeae isolates in a large Canadian health region. Clin Microbiol Infect 13:683–688. doi: 10.1111/j.1469-0691.2007.01715.x. [DOI] [PubMed] [Google Scholar]
- 2.Power P, Galleni M, Ayala JA, Gutkind G. 2006. Biochemical and molecular characterization of three new variants of AmpC β-lactamases from Morganella morganii. Antimicrob Agents Chemother 50:962–967. doi: 10.1128/AAC.50.3.962-967.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizrahi A, Delerue T, Morel H, Le Monnier A, Carbonnelle E, Pilmis B, Zahar JR. 2020. Infections caused by naturally AmpC-producing Enterobacteriaceae: can we use third-generation cephalosporins? A narrative review. Int J Antimicrob Agents 55:105834. doi: 10.1016/j.ijantimicag.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Stewart AG, Paterson DL, Young B, Lye DC, Davis JS, Schneider K, Yilmaz M, Dinleyici R, Runnegar N, Henderson A, Archuleta S, Kalimuddin S, Forde BM, Chatfield MD, Bauer MJ, Lipman J, Harris-Brown T, Harris PNA, MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN). 2021. Meropenem versus piperacillin-tazobactam for definitive treatment of bloodstream infections caused by AmpC β-lactamase-producing Enterobacter spp, Citrobacter freundii, Morganella morganii, Providencia spp, or Serratia marcescens: a pilot multicenter randomized controlled trial (MERINO-2). Open Forum Infect Dis 8:ofab387. doi: 10.1093/ofid/ofab387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.da Cunha Ferreira T, Martins IS. 2021. Risk factors of death in bloodstream infections caused by AmpC β-lactamase-producing Enterobacterales in patients with neoplasia. Infect Drug Resist 14:3083–3097. doi: 10.2147/IDR.S312920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandy A. 2020. Ringing bells: Morganella morganii fights for recognition. Public Health 182:45–50. doi: 10.1016/j.puhe.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Xiang G, Lan K, Cai Y, Liao K, Zhao M, Tao J, Ma Y, Zeng J, Zhang W, Wu Z, Yu X, Liu Y, Lu Y, Xu C, Chen L, Tang YW, Chen C, Jia W, Huang B. 2021. Clinical molecular and genomic epidemiology of Morganella morganii in China. Front Microbiol 12:744291. doi: 10.3389/fmicb.2021.744291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan J, Mann D, Li S, Deng X, Kassem II. 2021. First report of the mobile colistin resistance gene, mcr-9.1, in Morganella morganii isolated from sewage in Georgia, USA. J Glob Antimicrob Resist S2213-7165(21)00267-8. doi: 10.1016/j.jgar.2021.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Xiang R, Li M. 2021. Identification of Tn6835 and a novel genomic island, MMGI-1, in a pan-resistant Morganella morganii strain. Antimicrob Agents Chemother 65:e02524-20. doi: 10.1128/AAC.02524-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocha MFG, Diógenes EM, Carvalho VL, Marmontel M, da Costa MO, da Silva VMF, de Souza Amaral R, Gravena W, do Carmo NAS, Marigo J, Ocadaque CJ, Freitas AS, Pinheiro RM, de Lima-Neto RG, de Aguiar Cordeiro R, de Aquino Pereira-Neto W, de Melo Guedes GM, Sidrim JJC, de Souza Collares Maia Castelo-Branco D. 2021. One Health implications of antimicrobial resistance in bacteria from Amazon river dolphins. Ecohealth 18:383–396. doi: 10.1007/s10393-021-01558-4. [DOI] [PubMed] [Google Scholar]
- 11.Yeh H, Gao SY, Lin CC. 2021. Wound infections from Taiwan cobra (Naja atra) bites: determining bacteriology, antibiotic susceptibility, and the use of antibiotics: a Cobra BITE study. Toxins (Basel) 13:183. doi: 10.3390/toxins13030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmieri N, Hess C, Hess M, Alispahic M. 2020. Sequencing of five poultry strains elucidates phylogenetic relationships and divergence in virulence genes in Morganella morganii. BMC Genomics 21:579. doi: 10.1186/s12864-020-07001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngo ND, Le QX, Pham AQ, Nguyen NT, Ha HT, Dinh MMQ, Le TQ. 2020. Clinical features, bacteriology, and antibiotic treatment among patients with presumed Naja bites in Vietnam. Wilderness Environ Med 31:151–156. doi: 10.1016/j.wem.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Erlanger D, Assous MV, Wiener-Well Y, Yinnon AM, Ben-Chetrit E. 2019. Clinical manifestations, risk factors and prognosis of patients with Morganella morganii sepsis. J Microbiol Immunol Infect 52:443–448. doi: 10.1016/j.jmii.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Falagas ME, Kavvadia PK, Mantadakis E, Kofteridis DP, Bliziotis IA, Saloustros E, Maraki S, Samonis G. 2006. Morganella morganii infections in a general tertiary hospital. Infection 34:315–321. doi: 10.1007/s15010-006-6682-3. [DOI] [PubMed] [Google Scholar]
- 16.Lin TY, Chan MC, Yang YS, Lee Y, Yeh KM, Lin JC, Chang FY. 2015. Clinical manifestations and prognostic factors of Morganella morganii bacteremia. Eur J Clin Microbiol Infect Dis 34:231–236. doi: 10.1007/s10096-014-2222-8. [DOI] [PubMed] [Google Scholar]
- 17.Kim BN, Kim NJ, Kim MN, Kim YS, Woo JH, Ryu J. 2003. Bacteraemia due to tribe Proteeae: a review of 132 cases during a decade (1991–2000). Scand J Infect Dis 35:98–103. doi: 10.1080/0036554021000027015. [DOI] [PubMed] [Google Scholar]
- 18.Lee IK, Liu JW. 2006. Clinical characteristics and risk factors for mortality in Morganella morganii bacteremia. J Microbiol Immunol Infect 39:328–334. [PubMed] [Google Scholar]
- 19.Skogberg K, Lyytikainen O, Ollgren J, Nuorti JP, Ruutu P. 2012. Population-based burden of bloodstream infections in Finland. Clin Microbiol Infect 18:E170–E176. doi: 10.1111/j.1469-0691.2012.03845.x. [DOI] [PubMed] [Google Scholar]
- 20.Gradel KO, Schønheyder HC, Arpi M, Knudsen JD, Ostergaard C, Søgaard M. 2014. The Danish Collaborative Bacteraemia Network (DACOBAN) database. Clin Epidemiol 6:301–308. doi: 10.2147/CLEP.S66998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laupland KB, Niven DJ, Pasquill K, Parfitt EC, Steele L. 2018. Culturing rate and the surveillance of bloodstream infections: a population-based assessment. Clin Microbiol Infect 24:910.e1–910.e4. doi: 10.1016/j.cmi.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Steckelberg JM, Melton LJ, III, Ilstrup DM, Rouse MS, Wilson WR. 1990. Influence of referral bias on the apparent clinical spectrum of infective endocarditis. Am J Med 88:582–588. doi: 10.1016/0002-9343(90)90521-E. [DOI] [PubMed] [Google Scholar]
- 23.Leal JR, Gregson DB, Church DL, Henderson EA, Ross T, Laupland KB. 2016. The validation of a novel surveillance system for monitoring bloodstream infections in the Calgary Zone. Can J Infect Dis Med Microbiol 2016:2935870. doi: 10.1155/2016/2935870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenz R, Leal JR, Church DL, Gregson DB, Ross T, Laupland KB. 2012. The distinct category of healthcare associated bloodstream infections. BMC Infect Dis 12:85. doi: 10.1186/1471-2334-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leal J, Gregson DB, Ross T, Flemons WW, Church DL, Laupland KB. 2010. Development of a novel electronic surveillance system for monitoring of bloodstream infections. Infect Control Hosp Epidemiol 31:740–747. doi: 10.1086/653207. [DOI] [PubMed] [Google Scholar]
- 26.Laupland KB, Gregson DB, Church DL. 2015. Validity of calendar day-based definitions for community-onset bloodstream infections. BMC Res Notes 8:123. doi: 10.1186/s13104-015-1051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ. 2002. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. 2005. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 30.Queensland Government. 2003. Queensland population projections 2002 to 2026. https://www.qgso.qld.gov.au/statistics/theme/population/population-projections/regions. Accessed September 22, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared publicly due to institutional ethics, privacy, and confidentiality regulations. Data release for the purposes of research under Section 280 of the Public Health Act 2005 requires application to the Director-General (pha@health.qld.gov.au).


