ABSTRACT
Analysis of Leptospira dissemination and colonization of sex organs in rodents is of significant value as it queries the possibility of mammal-to-mammal venereal transmission. The aim of our study was to evaluate the presence and viability of Leptospira interrogans in testes of mice using models of infection that we previously developed. Using sublethal and lethal doses of bioluminescent strains of L. interrogans serovars Manilae and Copenhageni, we visualized the presence of leptospires in testes of C57BL/6 mice as early as 30 min and up to days 3–4 postinfection. This was confirmed by qPCR for the Copenhageni serovar after lethal infection of C3H/HeJ mice. In this model, no histopathological changes were noticed in testis. We further studied persistence of serovar Copenhageni in C3H/HeJ testes after lethal and sublethal infection, with different doses of leptospires. No viable leptospires were recovered from testes of lethally infected mice. However, we found live culturable Leptospira in testes of 19/19 (100%) sublethally infected mice at the acute phase but not at 15 days postinfection, which corresponds to the chronic phase of renal colonization. The data suggest that colonization of testes with live and potentially infectious leptospires is transient and limited to the spirochetemic phase of infection. Further studies are necessary to evaluate if presence of Leptospira in testes of mice leads to excretion in semen and to venereal transmission to female mice.
IMPORTANCE Analysis of venereal transmission of Leptospira is important to determine if direct animal to animal transmission occurs, which could impact measures to prevent and treat leptospirosis. The goal of this study was to determine if live Leptospira colonize mouse testes. We found that colonization of mouse testes with live Leptospira was transient and limited to the acute spirochetemic phase of infection and that transient colonization of the testes was insufficient to cause histopathological changes.
KEYWORDS: L. interrogans, Leptospira, culture, leptospirosis, lethal infection, testes
INTRODUCTION
Leptospirosis, caused by several pathogenic species of a spirochete named Leptospira (1, 2), is a zoonotic infection affecting about 1 million people annually, of which approximately 60,000 perish. The disease is prevalent in regions of Southeast Asia, Africa, and South America and, also, to some extent, in developing countries (2). Slum dwellers are at high risk of exposure to leptospirosis due to substandard sanitation living conditions (2, 3). Rats and mice are asymptomatic reservoir host carriers of this pathogen and shed it in urine. Humans, wild and domestic animals acquire the disease after exposure of abraded skin and mucosa to contaminated water and soil (1, 2, 4).
Previous studies have reported detection of Leptospira in reproductive organs of cows and boars (5–7). In females, genital leptospirosis has been linked to stillbirth, abortion and weak progeny (6–9). Detection of leptospiral DNA in vaginal fluid of cows (10) and genital tract of sheep (11, 12) suggested sexual transmission may occur by venereal route. In bulls, L. interrogans Pomona infection was associated with lesions in testis and epididymis. However, in that particular case the semen may have been contaminated with urine containing Leptospira (13). In rams, Leptospira was detected in testis and epididymis by culture but no Leptospira was microscopically observed (14). In humans, Leptospira disseminates through blood to various organs and after colonization of the kidney, Leptospira is shed in urine (1, 2, 4). A possible human-to-human sexual transmission case of leptospirosis was reported (15). However, human-to-human transmission of Leptospira has not been established.
The goal of this study was to determine if live Leptospira colonize mouse testes. Analysis of venereal transmission of Leptospira is important to determine if direct animal to animal transmission occurs which could impact measures to prevent and treat leptospirosis.
RESULTS
Dissemination of bioluminescent L. interrogans in male and female C57BL/6J mice.
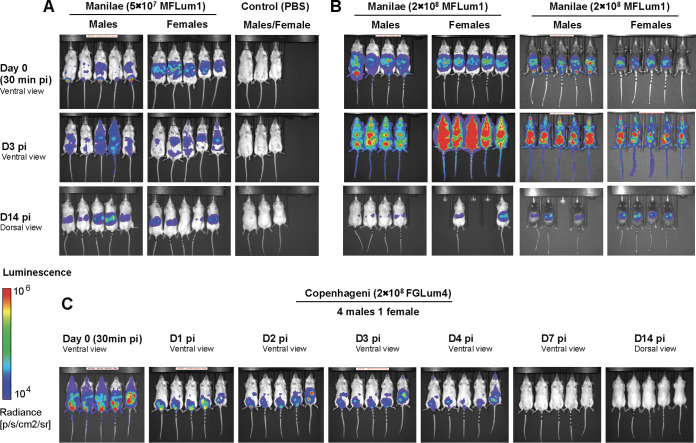
We recently showed that male hamsters are more susceptible to lethal infection with L. interrogans than females (16). However, it had not been reported in mice, likely because most of the studies were performed in females. Therefore, we infected both male and female albino C57BL/6J mice with 5 × 107, (a sublethal dose) and 2 × 108 (a potentially lethal dose) of MFLum1, a bioluminescent derivative of L. interrogans serovar Manilae L495. Live imaging was performed 10 min after injection of the luciferin substrate in mice. Only metabolically active live bioluminescent leptospires can glow since emission of bioluminescence requires ATP and oxygen (17). As previously described, at 30 min postinfection (p.i) in female mice, bioluminescent leptospires exiting from the peritoneal cavity were observed concentrated in a round shape (Fig. 1A and B). Then at day 3 p.i, leptospires disseminated in blood, with a complete systemic dissemination in mice infected with the highest dose, and at day 14 p.i leptospires were observed only in kidneys, as expected (17). However, in males we observed a different pattern of dissemination: at 30 min p.i we observed a less defined shape of bioluminescence than in females, and a striking high amount of light in the inguinal area that was not observed in females, and which we hypothesize to be the testes. Although renal colonization was observed at day 14 in 17/18 infected mice, the levels of light in kidneys were rather different between mice within a group. However, no obvious differences were observed between males and females. We also infected black C57BL6/J mice and observed the same differences between females and males harboring MFLum1 in the inguinal area (Fig. 1B).
FIG 1.
Live imaging tracking over time of bioluminescent L. interrogans injected intra-peritoneally into albino or black, female and male C57BL/6J mice. Live imaging was performed according to (ref FVP 2020) for 5 min, 10 min after the IP administration of d-luciferin, in ventral view (days 0 and 3) and dorsal view (day 14). Data are expressed as radiance of light measured in photons/second/cm2 in and represented by colors, according to the scale, with more intense emitted light in red and less in blue. Pictures of (n = 5) male and (n = 5) female albino C57BL/6J after infection with 5 × 107 bioluminescent L. interrogans Manilae MFlum1 at Day (D) 0 30 min postinfection (p.i), day 3 p.i and day 14 p.i, compared to n = 3 males and females control mice injected with PBS (A). Pictures of n = 4 male and n = 5 female Albino C57BL/6J, and n = 5 and groups of n = 5 black C57BL/6J mice infected with 2 × 108 MFLUM1 at d0 (30 min), days 3 and 14 p.i. (B). Pictures of n = 4 male Albino and n = 1 female Albino infected with 2 × 108 L. interrogans Copenhageni FGLum4 at days 0 (30 min), 1–4, and 14 p.i. (C).
Then we wondered whether this finding could be extended to other serovars. We repeated the infection in males with 2 × 108 FGLum4, a bioluminescent derivative of L. interrogans Fiocruz L1-130, the parental strain which has been shown to cause more severe leptospirosis in male hamsters. Live imaging was performed every day at the acute phase, and at day 14. Although FGLum4 was not observed at day 14, and therefore is considered as a mutant strain unable to colonize the kidney, we found it in the inguinal area at 30 min p.i, days 1–4 p.i. (Fig. 1C), suggesting that different serovars of L. interrogans disseminate and can home to male genitals in the acute phase of leptospirosis.
To further assess the presence of leptospires in testes and study their viability as an indicator of their potential infectivity, we studied and characterized the C3H/HeJ leptospirosis model in males infected with sublethal doses of 105 and 106, and a lethal dose 108 of L. interrogans Copenhageni Fiocruz L1-130.
Weight-loss and survival differences after lethal and sublethal infection of C3H-HeJ mice.
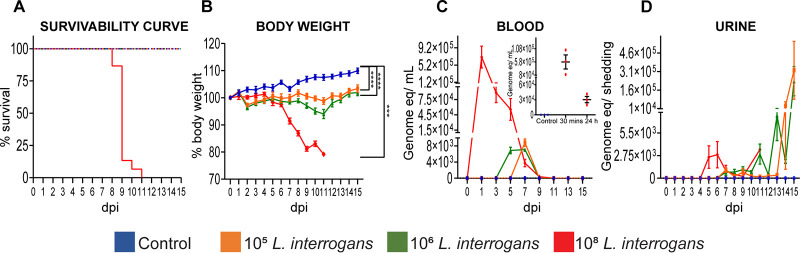
Infection with a sublethal dose of 105 and 106 L. interrogans led to survival of all mice (Fig. 2A) while causing significant weight-loss throughout the course of infection until it reached the inflection point on day 11 after which mice started gaining weight. This decline in body weight was proportional with the increase in infection dose (Fig. 2B) and it correlates with the bacteriemia peak measured by qPCR of Leptospiral DNA (days 3–9 postinfection) (Fig. 2C), whereas body weight gains correlate with the peak of shedding of Leptospira in urine (days 12–15) (Fig. 2D). Following lethal infection with 108 L. interrogans, 100% of mice met endpoint criteria between days 8–11 postinfection (Fig. 2A) which was preceded by a significant loss in weight starting on day 5 (Fig. 2B). Bacteriemia peaked on day 1, starting 30 min after infection (Fig. 2C and inset), that was followed by initiation of urine shedding on day 5 (Fig. 2D). The sublethal data were consistent with our previous results (18, 19).
FIG 2.
Weight, survival differences and dissemination in body fluids after lethal and sublethal infection of mice with L. interrogans. Survival of mice after sublethal and lethal (A) infection; body weight was measured daily until 15 days postinfection (B); blood was collected on alternate days and tested for Leptospira load by 16S rRNA qPCR (C). Urine was collected daily and tested for Leptospira load by 16S rRNA qPCR (D). qPCR data is represented as genome equivalent (eq) denoting number of leptospires. Blood was collected after early lethal infection at 30 min and 24 h (Data of one experiment, n = 3 mice per group) and qPCR was performed to determine Leptospira load by 16S rRNA qPCR. Sublethal infection is represented as data of two independent experiments; n = 8 mice. Lethal infection is represented as data of three independent experiments; n = 13 mice. Statistics was performed by unpaired t test with Welch’s correction between control and infected groups. dpi, day postinfection, ***P < 0.001, ****P < 0.0001.
Leptospira DNA was found in mouse testes after lethal and sublethal infection.
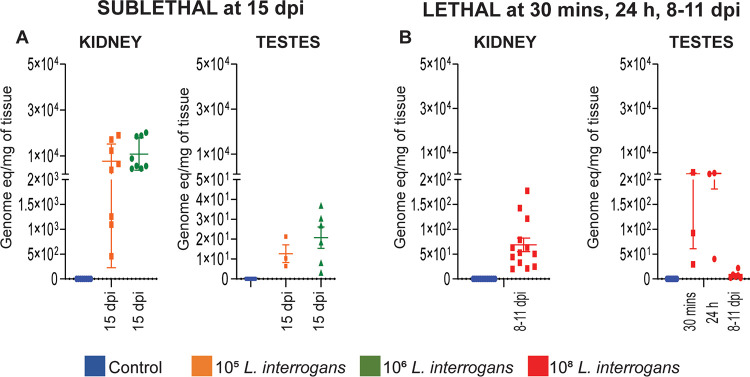
At termination after sublethal infection (day 15), all mouse kidneys tested positive for leptospiral DNA (~104 equivalent genomes/mg) which was a good measure of infectivity of the Fiocruz L1-130 strain. In testes, 3/8 (37.5%) mice tested positive for Leptospira after infection with 105 leptospires and 6/8 (75%) mice tested positive after infection with 106 leptospires, although all leptospiral loads were very low, at the limit of qPCR detection (Fig. 3A, Table 1). In contrast, after lethal infection with 108 leptospires, at 30 min p.i, 3/3 mice had 30–1000 leptospires per mg of testes tissue, and after 24h postinfection 40–740 leptospires per mg of tissue were detected in 3/3 mice (Fig. 3B). In another experiment 6/13 (46.15%) mice had very low numbers of leptospires in the testes at 8–11 days p.i (Fig. 3B, Table 1). As expected, higher leptospiral load was detected in kidneys compared to testes (Fig. 3A, B, Table 1). Lethal and sublethal infection with L. interrogans (Table 1) led to spirochetal accumulation measured by qPCR in the kidney tubules of 100% of mice.
FIG 3.
Leptospira presence in testes and kidney after sublethal and lethal infection. Testes and kidney were harvested at termination at day 15 (sublethal) and 8–11 (lethal) and Leptospira load was measured in the tissues by 16S rRNA qPCR. qPCR data is represented as genome equivalent (eq) denoting number of leptospires. Sublethal (A), n = 8 mice, two independent experiments; lethal 8–11 dpi (B), n = 13 mice, data represents three independent experiments, lethal 30 min and 24 h (B), n = 3 mice, data represents one experiment. dpi, day postinfection.
TABLE 1.
Tissues positive for Leptospira interrogans 16s rRNA after lethal and sublethal infectiona
| Kidney |
Testes |
||||
|---|---|---|---|---|---|
| LIC genome eq/mg tissue No. of positive/total (%) |
LIC genome eq/mg tissue No. of positive/total (%) |
||||
| 15 dpi |
8–11 dpi |
15 dpi |
8–11 dpi |
||
| 105 | 106 | 108 | 105 | 106 | 108 |
| 8/8 (100) | 8/8 (100) | 13/13 (100) | 3/8 (37.5) | 6/8 (75) | 6/13 (46) |
Data represents n = 8 mice, for sublethal infection, data of two independent experiments and n = 13 mice, for lethal infection, data of three independent experiments. dpi, day postinfection.
Live Leptospira was cultured from testes after sublethal and lethal L. interrogans infection.
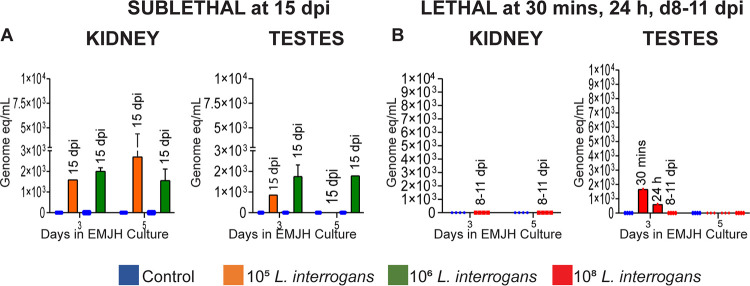
Morphology and motility of L. interrogans was checked under a dark field microscope and confirmed by qPCR in cultures from kidney and testes of mice. For sublethal infections, in mice euthanized 15 days p.i, we recovered live Leptospira in cultures from 1/8 (12.5%) testes of mice infected with 105 Leptospira and from 2/8 (25%) testes of mice infected with 106 Leptospira at 3 and 5 days after inoculation of the tissue into EMJH media. The Leptospira were motile but presented in dot form, not in spiral form. Positive dark field cultures were confirmed to species by 16S rRNA qPCR (Fig. 4A and Table 2). For lethal infections, we recovered ~1750 live Leptospira in 3 day testes cultures from mice euthanized 30 min postinfection, 500 Leptospira were detected in 3 day cultures of mice euthanized 24h postinfection and no Leptospira was recovered from mice euthanized 8–11 days postinfection. Kidney cultures, used as a positive control for infection and tissue colonization, were positive in 12.5–37.5% of mice infected with 105 and in 37.5–87.5% of mice infected with 106 Leptospira (motile spirochetal forms) on days 3 and 5 after inoculation of the tissue into EMJH media, whereas no Leptospira was cultured from kidneys from mice infected with the lethal dose (Fig. 4B and Table 2).
FIG 4.
Live Leptospira was recovered in EMJH culture of testes and kidney after sublethal but not lethal infection. 2 μL of EMJH testes and kidney culture was tested by 16S rRNA qPCR on days 3 and 5 after termination at day 15 (sublethal) and days 8–11 (lethal). qPCR data is represented as genome equivalent (eq) denoting number of leptospires. Panel A, n = 8 mice, data represents two independent sublethal experiments and Panel B n = 4 mice, data represents one of two independent lethal experiments (8–11 dpi), n = 3, data represents one lethal experiment (30 min and 24 h). dpi, day postinfection.
TABLE 2.
PCR confirmation of Leptospira interrogans in EMJH culture of testes and kidney after lethal and sublethal infectiona
| Days in culture | Kidney |
Testes |
||||||
|---|---|---|---|---|---|---|---|---|
| LIC genome eq/mL | ||||||||
| Control |
L. interrogans
|
Control |
L. interrogans
|
|||||
| 15 dpi |
8–11 dpi |
15 dpi |
8–11 dpi |
|||||
| 105 | 106 | 108 | 105 | 106 | 108 | |||
| No. of positive/total (%) | ||||||||
| Day 3 | 0/8 (0) | 1/8 (12.5) | 4/8 (50) | 0/8 (0) | 0/8 (0) | 1/8 (12.5) | 2/8 (25) | 0/8 (0) |
| Day 5 | 0/8 (0) | 2/8 (25) | 4/8 (50) | 0/8 (0) | 0/8 (0) | 0/8 (0) | 1/8 (12.5) | 0/8 (0) |
Presence of Leptospira was confirmed by 16s rRNA qPCR after placing the kidney and testes in EMJH culture and tested at days 3 and 5, n = 8 mice, data of two independent experiments. dpi, day postinfection.
Sublethal and lethal infection with L. interrogans did not cause inflammation in testes.
No mononuclear cell infiltration or tissue structure morphological changes were observed in testes tissues from mice infected with 105, 106 and 108 Leptospira after H&E staining, whereas histopathology analysis of kidney showed increased infiltration of immune cells in L. interrogans infected mice compared to control (Fig. 5).
FIG 5.
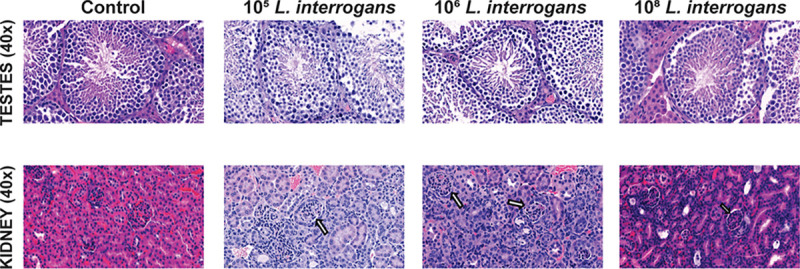
L. interrogans infection led to renal inflammation without testicular inflammation. Representative images of H & E staining for testes and kidney. Images were captured at ×40 magnification. Arrow indicates increased immune cell infiltration in kidney sections, data represents one of two independent experiments for lethal infection (108 LIC); for sublethal infection (105 and 106 LIC) data represents one experiment.
DISCUSSION
Analysis of dissemination of Leptospira to sex organs in mice is of significant value as it queries the possibility of mammal-to-mammal venereal transmission. Previous reports regarding dissemination of Leptospira to sex organs in mares, cattle, boars, sheep and rams have suggested that leptospirosis may be sexually transmitted in large animals (5–11, 13, 14). This leads to reproductive failure and pregnancy issues like abortion, stillbirth, and sick offspring (6–8). In this study, we used two serovars of pathogenic Leptospira, two strains of mice (C57BL/6 immunocompetent and immunocompromised C3H/HeJ with a mutation in TLR4) and two different techniques to monitor Leptospira dissemination after infection to determine if live Leptospira disseminates to and colonizes the testes.
Both 105 and 106 sublethal doses of L. interrogans serovar Copenhageni led to survival of all male mice whereas the 108 dose led to death of all infected animals before the term of the experiment (15 days). Both sublethal and lethal infections led to dissemination of Leptospira to blood, urine, kidney and testes (Fig. 2 and 3). A 108 dose of Leptospira had been used previously in our lab to induce sublethal infection of mice (20). Important differences between these experiments are the route of infection and the sex of the mice. In the first study we used female mice infected via eye drop (20). In the current study, as we were aiming to evaluate the presence of Leptospira in testes after sublethal and lethal infection, we inoculated male mice with a 108 dose of Leptospira as we had previously observed higher susceptibility to infection in male hamsters (16). We used an intraperitoneal route for infection as we had previously observed that male mice infected with an IP 108 dose had Leptospira in blood 24h day after infection (21). Furthermore, the IP route is the only route that allows us to administer a predetermined infectious dose to induce sublethal or lethal infection. A comparison of disease progression markers between lethal and sublethal infections in male mice led to the interesting observation that lethal infection leads to a much higher Leptospira load (~7 × 105) in blood 24h postinfection than sublethal infection which only peaked on days 5–7 at ~104. The latter was observed in our previous sublethal studies (18, 19) consistent with what has been shown in the bioluminescent model, where the peak of MFLum1 dissemination in blood at days 3 and 4 was proportional to the infectious dose, and also proportional to the level of renal colonization 1 month p.i (17). This was also observed by others in rats (22). In addition, after lethal infection body weight started to decline on day 5 as Leptospira colonizes the kidney and the mice kept losing weight (>10% on day 7) reaching the 20% endpoint between days 8–11, whereas after sublethal infection, mice lose < 10% of weight until day 11 after which they start to recover as reflected by weight gains and increased Leptospira shedding in urine on days 12–15. Increased shedding of Leptospira in urine by otherwise healthy mice signals successful kidney colonization which ensures that Leptospira continues to be shed into the environment and completes the enzootic cycle. Detection of high burdens of Leptospira in blood 24h after infection followed by a 10% weight loss and shedding of a low burden of Leptospira in urine within the first week of infection (days 5–7) predicts worse clinical outcomes, as also shown recently for C57B/6 mice (23, 24). These differences in kinetics of disease progression between lethal and sublethal infections in mice are important to design better diagnostic assays that can detect leptospirosis earlier and predict disease outcomes.
Leptospira was detected in testes of 18/18 mice as early as 30 min post lethal infection and in 19/19 mice 1–4 days postinfection by DNA qPCR and bioluminescence, respectively (Fig. 1, 3). Whether this shows a tropism of leptospires for testis or if this a passive effect due to the blood dissemination and high vascularization of testis is an interesting question that remains to be studied. Low numbers of Leptospira DNA were still detected at 8–15 days postinfection after lethal and sublethal infections in 37.5–75% of mice (Fig. 1, 3). The data conclusively shows presence of Leptospira in mouse testes and that the load of Leptospira in testes decreases as the infection progresses in time (Fig. 3). This is consistent with the bioluminescent C57BL/6 model, showing that leptospires disappear from circulation after 1 week p.i to progressively reappear in one organ, the kidney (17). Previous studies have detected Leptospiral DNA in vaginal fluid of cows (10), ovaries, uterus and uterine tubes of sheep (11, 12). Although we did not study the presence of leptospiral in these organs, we can speculate that leptospires may also infect the reproductive tract of female mice.
Another important finding in this study was detection of live Leptospira in testes of 12.5–25% of mice, 15 days after sublethal infection. Morphology and motility were checked under a dark field microscope. Leptospira were motile but did not present in spiral form and positive dark field cultures were confirmed to species by 16S rRNA qPCR. We considered mobile non spiral Leptospira viable as these forms often present in culture and then later morph into spirochetal forms if given enough time and suitable culture conditions. Viability of Leptospira in testes is further supported by bioluminescence of Leptospira visualized in testes of 19/19 mice up to 4 days postinfection with L. interrogans serovar Manilae. In contrast, Leptospira was not recovered in EMJH cultures of testes 8–11 days after lethal infection, despite renal colonization. We hypothesize that the lack of oxygen in the organs of moribund mice could have harmed or killed the remaining leptospires, that are aerobic bacteria. Earlier studies also determined viability of Leptospira in culture from testes of 13.63% infected boars and 41.67% infected rams (6, 14). Reports suggest that venereal spread of Leptospira from male to females is plausible. Cilia et al. stated that the presence of serovar Bratislava in boars could be transmitted to sows during mating season (6) and a similar probability of transmission in ovines has been reported (25, 26). Leptospira has been detected in semen (27–30) and oviducts (7, 31) of cattle. Reproductive problems have been encountered due to genital leptospirosis like early fetal death (8, 32), abortion (33, 34), lower fertility (34), estrus repetition (35) in mares, swine and cattle. The above-mentioned issues may occur due to presence of Leptospira in semen, in turn affecting the sperm viability. Alteration in spermatogenesis due to damage, affected sperm viability in 3.5% infected bulls, with no molecular detection by PCR, in the same study, viable sperm was observed with molecular detection in 1.5% infected bulls (36). These data suggest that sexual activity may be a source of transmission in infected individuals.
We also found that presence of live Leptospira in testis of infected mice did not result in inflammatory changes to this organ. This was also observed in boars infected with pathogenic Leptospira (6, 37). In another study, experimental infection with Leptospira pomona showed no testicular lesions in 33.33% bulls (13). No inflammatory triggers were observed after exposure of bovine endometrial cells to heat killed Leptospira and outer membrane Leptospiral proteins (38). We speculate that viable forms of Leptospira present in testes may not cause tissue damage which may allow them to remain undetected and a possible source of transmission.
In this study we show presence of live Leptospira in testes of mice as early as 30 min postinfection and up to 4 days postinfection in 100% of the mice tested, which is within the spirochetemic phase of infection. Given that a much lower percentage of individuals (up to 25%) were infected up to 15 days postinfection with low numbers of Leptospira 7 days after Leptospira exits the blood phase, the data suggests that colonization of testes may be transient and mostly limited to the spirochetemic phase of infection and that transient colonization is insufficient to cause histopathological changes. Further studies are necessary to evaluate if presence of Leptospira in testes of some mice leads to excretion in semen and to venereal transmission using either artificial insemination of females with spiked semen or other methods that allow for bypassing possible contamination of semen with urine of the same mouse.
MATERIALS AND METHODS
Animals.
Two- to 6-month old female and male albino C57BL/6J Tyrc-2J mice (bred in the animal facility at Institut Pasteur) and 8-week old male and female C57BL/6J mice (Janvier Labs, France) were used in this study. All protocols were reviewed by the Institut Pasteur (Paris, France), the competent authority for compliance with the French and European regulations on Animal Welfare and with Public Health Service recommendations. This project has been reviewed and approved (#2014-049) by the Institut Pasteur ethic committee (CETEA #89). C3H-HeJ male mice, 7-weeks old, were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in a pathogen-free environment at the Laboratory Animal Care Unit of the University of Tennessee Health Science Center (UTHSC). All experiments were performed in compliance with the UTHSC Institutional Animal Care and Use Committee Protocol no. 19-0062 (18, 19).
Bacteria.
Pathogenic Leptospira interrogans serovars Manilae and Copenhageni (strain Fiocruz L1-130) were cultivated in EMJH media, resuspended in PBS and spirochetes were enumerated by dark-field microscopy (Zeiss USA, Hawthorne, NY), using a Petroff-Hausser counting chamber, as previously described (39). For infection of C3H/HeJ mice, bacteria retrieved from infected hamsters were used after 2 culture passages. Two bioluminescent strains of L. interrogans obtained after chromosomal insertion of the firefly luciferase cassette by random mutagenesis using the Himar1 transposon were used for live imaging. MFLum1 is a derivative of L. interrogans serovar Manilae strain L495 (17) and FGLum4 is a derivative of L. interrogans serovar Copenhageni Fiocruz L1-130. MFLum1 is a virulent strain able to provoke severe leptospirosis and renal colonization (17) whereas FGLum4 is a mutant strain in LIC10006 (33810.1), the DNA gyrase subunit A (gyrA), and is unable to colonize the mouse kidney.
Infection of mice and study design.
For live imaging, groups of 4 to 5 albino or black C57BL/6J females and males were intraperitoneally injected with PBS (control) or with 5 × 107 or 2 × 108 L. interrogans MFLum1, or 2 × 108 L. interrogans FGLum4 in 200 μL of PBS (infected). Live imaging of mice was performed as in (17) and recently described in detail (23). Detection and kinetics of live leptospires dissemination was done by tracking the bioluminescence 10 min after injection of Luciferin (30 mg/mL in PBS).
For the C3H lethal/sublethal infection study, groups of C3H/HeJ (n = 3 to 4) were injected with PBS (control) and with 105, 106 (sublethal doses) or 108 (lethal dose) L. interrogans serovar Copenhageni intraperitoneally. Survival and body weight was monitored daily, urine was collected daily, and blood was collected on alternate days. Baseline fluid collection was done on day 0. At termination on day 15, kidney and testes were collected from both groups of mice for quantification of Leptospira load, for culture determination of bacterial viability and tissues were stored in 10% formalin for H&E staining (18, 19). For lethal infection, the endpoint criteria was weight loss reaching 20% or depressed state (ruffled fur and loss of mobility) with >15% weight loss.
Leptospira detection by q-PCR.
DNA isolation from blood, urine, kidney and testes sections were carried out using NucleoSpin tissue kit (Clontech, Mountain View, CA) according to manufacturer’s instructions. Leptospira 16S rRNA TAMRA probe CTCACCAAGGCGACGATCGGTAGC, forward primer CCCGCGTCCGATTAG and reverse primer TCCATTGTGGCCGAACAC (Eurofins Genomics, Huntsville, AL) were used for Leptospira detection using qPCR (18, 19). A standard curve of 105 to 1 L. interrogans was run with the samples for quantification. At termination, kidney and testes were placed in EMJH culture to determine viability (motility and morphology) of by dark field microscopy (20X, Zeiss USA, Howthorne) and Leptospira was quantified in culture supernatant by 16S rRNA qPCR on days 3 and 5 post tissue inoculation.
Histopathology by HandE staining.
Kidney and testes tissues were fixed in 10% formalin buffer, paraffin embedded, sectioned and stained by H&E. Histopathology was performed at the Core Histology Department, UT Methodist University Hospital, Memphis, TN. Images were captured using CaseViewer software after digitally scanning the tissues section using Pannoramic 350 Flash III (3D Histech, Hungary).
Statistical analysis.
Graphs were plotted using GraphPad Prism software. Unpaired t test with Welch’s correction was used to analyze differences between control and L. interrogans infected groups, P < 0.05 is significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Histology department from UT Methodist University Hospital for processing and staining tissues, Michelle Morrison from the Department of Pathology, UTHSC for digitally scanning tissue slides and Sheila Criswell at the Department of Diagnostic and Health Sciences, UTHSC for analysis of testes sections. This work was supported by the Public Health Service awards AI139267 (M.G.S.), AI142129 (M.G.S.), and AI155211 (M.G.S.), from the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) of the United States of America, and by a Ph.D fellowship “DimMalinf” to C.W. for Gwendoline Ratet. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of NIAID or NIH.
Conceptualization: Advait Shetty, Maria Gomes-Solecki, Frédérique Vernel-Pauillac, Catherine Werts. Experimental investigation: Advait Shetty, Suman Kundu, Frédérique Vernel-Pauillac, Gwendoline Ratet. Data analysis: Advait Shetty, Maria Gomes-Solecki, Frédérique Vernel-Pauillac, Catherine Werts. Writing original draft: Advait Shetty, Maria Gomes-Solecki, Catherine Werts. Editing: Maria Gomes-Solecki, Frédérique Vernel-Pauillac and Catherine Werts. Supervision and funding acquisition: Maria Gomes-Solecki, Catherine Werts.
Contributor Information
Maria Gomes-Solecki, Email: mgomesso@uthsc.edu.
Catherine Ayn Brissette, University of North Dakota.
REFERENCES
- 1.Potula H-H, Richer L, Werts C, Gomes-Solecki M. 2017. Pre-treatment with Lactobacillus plantarum prevents severe pathogenesis in mice infected with Leptospira interrogans and may be associated with recruitment of myeloid cells. PLoS Negl Trop Dis 11:e0005870. doi: 10.1371/journal.pntd.0005870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haake DA, Levett PN. 2015. Leptospirosis in humans. Curr Top Microbiol Immunol 387:65–97. doi: 10.1007/978-3-662-45059-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felzemburgh RDM, Ribeiro GS, Costa F, Reis RB, Hagan JE, Melendez AXTO, Fraga D, Santana FS, Mohr S, dos Santos BL, Silva AQ, Santos AC, Ravines RR, Tassinari WS, Carvalho MS, Reis MG, Ko AI. 2014. Prospective study of leptospirosis transmission in an urban slum community: role of poor environment in repeated exposures to the Leptospira agent. PLoS Negl Trop Dis 8:e2927. doi: 10.1371/journal.pntd.0002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adler B, de la Pena Moctezuma A. 2010. Leptospira and leptospirosis. Vet Microbiol 140:287–296. doi: 10.1016/j.vetmic.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Ellis WA. 2015. Animal leptospirosis. Curr Top Microbiol Immunol 387:99–137. doi: 10.1007/978-3-662-45059-8_6. [DOI] [PubMed] [Google Scholar]
- 6.Cilia G, Bertelloni F, Piredda I, Ponti MN, Turchi B, Cantile C, Parisi F, Pinzauti P, Armani A, Palmas B, Noworol M, Cerri D, Fratini F. 2020. Presence of pathogenic Leptospira spp. in the reproductive system and fetuses of wild boars (Sus scrofa) in Italy. PLoS Negl Trop Dis 14:e0008982. doi: 10.1371/journal.pntd.0008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loureiro AP, Lilenbaum W. 2020. Genital bovine leptospirosis: a new look for an old disease. Theriogenology 141:41–47. doi: 10.1016/j.theriogenology.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Hamond C, Pestana CP, Rocha-de-Souza CM, Cunha LER, Brandão FZ, Medeiros MA, Lilenbaum W. 2015. Presence of leptospires on genital tract of mares with reproductive problems. Vet Microbiol 179:264–269. doi: 10.1016/j.vetmic.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Di Azevedo MIN, Pires BC, Libonati H, Pinto PS, Cardoso Barbosa LF, Carvalho-Costa FA, Lilenbaum W. 2020. Extra-renal bovine leptospirosis: Molecular characterization of the Leptospira interrogans Sejroe serogroup on the uterus of non-pregnant cows. Vet Microbiol 250:108869. doi: 10.1016/j.vetmic.2020.108869. [DOI] [PubMed] [Google Scholar]
- 10.Loureiro AP, Pestana C, Medeiros MA, Lilenbaum W. 2017. High frequency of leptospiral vaginal carriers among slaughtered cows. Anim Reprod Sci 178:50–54. doi: 10.1016/j.anireprosci.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Silva AF, Farias PJA, Silva MLCR, Araújo Júnior JP, Malossi CD, Ullmann LS, Costa DF, Higino SSS, Azevedo SS, Alves CJ. 2019. High frequency of genital carriers of Leptospira sp. in sheep slaughtered in the semi-arid region of northeastern Brazil. Trop Anim Health Prod 51:43–47. doi: 10.1007/s11250-018-1657-9. [DOI] [PubMed] [Google Scholar]
- 12.Nogueira DB, da Costa FTR, Bezerra CdS, Silva MLCR, da Costa DF, Viana MP, da Silva JD, Júnior JPA, Malossi CD, Ullmann LS, Santos CdSAB, Alves CJ, de Azevedo SS. 2020. Use of serological and molecular techniques for detection of Leptospira sp. carrier sheep under semiarid conditions and the importance of genital transmission route. Acta Trop 207:105497. doi: 10.1016/j.actatropica.2020.105497. [DOI] [PubMed] [Google Scholar]
- 13.Sleight SD, Atallah OA, Steinbauer DJ. 1964. Experimental Leptospira Pomona Infection in Bulls. Am J Vet Res 25:1663–1668. [PubMed] [Google Scholar]
- 14.Smith RE, Reynolds IM, Clark GW. 1965. Experimental Leptospirosis in Rams. Cornell Vet 55:412–419. [PubMed] [Google Scholar]
- 15.Harrison NA, Fitzgerald WR. 1988. Leptospirosis–can it be a sexually transmitted disease? Postgrad Med J 64:163–164. doi: 10.1136/pgmj.64.748.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes CK, Guedes M, Potula H-H, Dellagostin OA, Gomes-Solecki M. 2018. Sex matters: male hamsters are more susceptible to lethal infection with lower doses of pathogenic leptospira than female hamsters. Infect Immun 86. doi: 10.1128/IAI.00369-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratet G, Veyrier FJ, Fanton d'Andon M, Kammerscheit X, Nicola M-A, Picardeau M, Boneca IG, Werts C. 2014. Live imaging of bioluminescent leptospira interrogans in mice reveals renal colonization as a stealth escape from the blood defenses and antibiotics. PLoS Negl Trop Dis 8:e3359. doi: 10.1371/journal.pntd.0003359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nair N, Guedes MS, Werts C, Gomes-Solecki M. 2020. The route of infection with Leptospira interrogans serovar Copenhageni affects the kinetics of bacterial dissemination and kidney colonization. PLoS Negl Trop Dis 14:e0007950. doi: 10.1371/journal.pntd.0007950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richer L, Potula H-H, Melo R, Vieira A, Gomes-Solecki M. 2015. Mouse model for sublethal Leptospira interrogans infection. Infect Immun 83:4693–4700. doi: 10.1128/IAI.01115-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan JP, Nair N, Potula H-H, Gomes-Solecki M. 2017. Eyedrop inoculation causes sublethal leptospirosis in mice. Infect Immun 85. doi: 10.1128/IAI.01050-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shetty A, Kundu S, Gomes-Solecki M. 2021. Inflammatory signatures of pathogenic and non-pathogenic leptospira infection in susceptible C3H-HeJ mice. Front Cell Infect Microbiol 11:677999. doi: 10.3389/fcimb.2021.677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monahan AM, Callanan JJ, Nally JE. 2008. Proteomic analysis of Leptospira interrogans shed in urine of chronically infected hosts. Infect Immun 76:4952–4958. doi: 10.1128/IAI.00511-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vernel-Pauillac F, Werts C. 2020. In vivo imaging of bioluminescent leptospires. Methods Mol Biol 2134:149–160. doi: 10.1007/978-1-0716-0459-5_14. [DOI] [PubMed] [Google Scholar]
- 24.Vernel-Pauillac F, Murray GL, Adler B, Boneca IG, Werts C. 2021. Anti-Leptospira immunoglobulin profiling in mice reveals strain specific IgG and persistent IgM responses associated with virulence and renal colonization. PLoS Negl Trop Dis 15:e0008970. doi: 10.1371/journal.pntd.0008970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Director A, Penna B, Hamond C, Loureiro AP, Martins G, Medeiros MA, Lilenbaum W. 2014. Isolation of Leptospira interrogans Hardjoprajitno from vaginal fluid of a clinically healthy ewe suggests potential for venereal transmission. J Med Microbiol 63:1234–1236. doi: 10.1099/jmm.0.065466-0. [DOI] [PubMed] [Google Scholar]
- 26.Lilenbaum W, Varges R, Brandão FZ, Cortez A, de Souza SO, Brandão PE, Richtzenhain LJ, Vasconcellos SA. 2008. Detection of Leptospira spp. in semen and vaginal fluids of goats and sheep by polymerase chain reaction. Theriogenology 69:837–842. doi: 10.1016/j.theriogenology.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 27.Masri SA, Nguyen PT, Gale SP, Howard CJ, Jung SC. 1997. A polymerase chain reaction assay for the detection of Leptospira spp. in bovine semen. Can J Vet Res 61:15–20. [PMC free article] [PubMed] [Google Scholar]
- 28.Heinemann MB, Garcia JF, Nunes CM, Morais ZM, Gregori F, Cortez A, Vasconcellos SA, Visintin JA, Richtzenhain LJ. 1999. Detection of leptospires in bovine semen by polymerase chain reaction. Aust Vet J 77:32–34. doi: 10.1111/j.1751-0813.1999.tb12422.x. [DOI] [PubMed] [Google Scholar]
- 29.Ali SA, Boby N, Preena P, Singh SV, Kaur G, Ghosh SK, Nandi S, Chaudhuri P. 2021. Microcapillary LAMP for rapid and sensitive detection of pathogen in bovine semen. Anim Biotechnol doi: 10.1080/10495398.2020.1863225. [DOI] [PubMed] [Google Scholar]
- 30.Magajevski FS, Girio RJS, Mathias LA, Myashiro S, Genovez ME, Scarcelli EP. 2005. Detection of Leptospira spp. in the semen and urine of bulls serologically reactive to Leptospira interrogans serovar hardjo. 36:43–45. [Google Scholar]
- 31.Bielanski A, Surujballi O, Golsteyn Thomas E, Tanaka E. 1998. Sanitary status of oocytes and embryos collected from heifers experimentally exposed to Leptospira borgpetersenii serovar hardjobovis. Anim Reprod Sci 54:65–73. doi: 10.1016/S0378-4320(98)00145-6. [DOI] [PubMed] [Google Scholar]
- 32.Muniz Oliveira GD, Nogueira Garcia LA, Aymée Pires Soares L, Lilenbaum W, Nunes de Souza G. 2021. Leptospirosis by Sejroe strains leads to embryonic death (ED) in herds with reproductive disorders. Theriogenology 174:121–123. doi: 10.1016/j.theriogenology.2021.08.022. [DOI] [PubMed] [Google Scholar]
- 33.Pinna A, Martins G, Hamond C, Medeiros MA, de Souza GN, Lilenbaum W. 2014. Potential differences between Leptospira serovars, host-adapted (Bratislava) and incidental (Copenhageni), in determining reproductive disorders in embryo transfer recipient mares in Brazil. Vet Rec 174:531–531. doi: 10.1136/vr.101444. [DOI] [PubMed] [Google Scholar]
- 34.Mori M, Bakinahe R, Vannoorenberghe P, Maris J, de Jong E, Tignon M, Marin M, Desqueper D, Fretin D, Behaeghel I. 2017. Reproductive disorders and Leptospirosis: a case study in a mixed-species farm (cattle and swine). Vet Sci 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Libonati HA, Santos GB, Souza GN, Brandão FZ, Lilenbaum W. 2018. Leptospirosis is strongly associated to estrus repetition on cattle. Trop Anim Health Prod 50:1625–1629. doi: 10.1007/s11250-018-1604-9. [DOI] [PubMed] [Google Scholar]
- 36.Maiolino SR, Cortez A, Langoni H, Giuffrida R, Dos Santos JR, de Nardi Júnior G, Lara GHB, Motta RG, Chacur MGM, Monteiro FM, Heinemann MB, de Souza Filho AF, de Souza Araújo Martins L, Bello TS, Ribeiro MG. 2021. Sperm viability, serological, molecular, and modified seminal plasma agglutination tests in the diagnosis of Leptospira in the semen and serum of bovine bulls. Braz J Microbiol 52:2431–2438. doi: 10.1007/s42770-021-00562-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cilia G, Bertelloni F, Cerri D, Fratini F. 2021. Leptospira fainei Detected in Testicles and Epididymis of Wild Boar (Sus scrofa). Biology (Basel) 10:193. doi: 10.3390/biology10030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molinari PCC, Nally JE, and, Bromfield JJ. 2021. Bovine endometrial cells do not mount an inflammatory response to Leptospira. Reprod Fertil 2:187–198. doi: 10.1530/RAF-21-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nair N, Gomes-Solecki M. 2020. A mouse model of sublethal leptospirosis: protocols for infection with leptospira through natural transmission routes, for monitoring clinical and molecular scores of disease, and for evaluation of the host immune response. Curr Protoc Microbiol 59:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.