Abstract
Objective
To investigate the relationship between the incidence of contrast-induced acute kidney injury (CI-AKI) and the levels of the systemic immune-inflammatory index (SII, platelet × neutrophil/lymphocyte ratio) and high-sensitivity C-reactive protein (hsCRP) in patients with ST-segment elevation myocardial infarction (STEMI) undergoing percutaneous coronary intervention (PCI), to analyze further the predictive value of the combination of SII and hsCRP for CI-AKI.
Methods
Retrospectively analyze the clinical data of STEMI patients who underwent PCI in our cardiology department from November 2019 to March 2021. Restricted cubic splines were used to determine the correlation between SII and hsCRP and the risk of CI-AKI. Patients were divided into the CI-AKI group (n=71) and the non-CI-AKI group (n=344) according to postoperative creatinine changes. Logistic regression was used to analyze the factors influencing CI-AKI. ROC curves were used to evaluate the predictive value of SII, hsCRP, and their combined levels on CI-AKI.
Results
Restricted cubic spline analysis showed that when SII>653.73×109/L and hsCRP>5.52mg/dl, there was a positive correlation with the incidence of CI-AKI. And the incidence of CI-AKI rose with the inflammation status. The receiver operating characteristic curve of SII combined with hsCRP was 0.831, which was higher than SII or hsCRP alone. The logistic regression analysis showed that high-risk factors of CI-AKI were diabetes mellitus, platelet count, and highly elevated SII and hsCRP.
Conclusion
Within a certain range, elevated inflammatory biomarkers SII and hsCRP were risk factors for CI-AKI after PCI in patients with STEMI. This study suggests that the combination of SII and hsCRP predicts the risk of CI-AKI more accurately than either biomarker alone.
Keywords: restricted cubic spline, systemic immune-inflammatory index, high-sensitivity C-reactive protein, contrast-induced acute kidney injury, ST-segment elevation myocardial infarction, percutaneous coronary intervention
Introduction
A large and increasing number of procedures using radiocontrast media are performed each year worldwide.1 The use of iodinated contrast media has previously been linked to acute kidney injury (AKI), occurring within days after their administration and typically referred to as contrast-induced acute kidney injury (CI-AKI). It is associated with long-term impairment of kidney function, the need for renal replacement therapy, and subsequent all-cause deaths.2 Several studies have shown that the proportion of ACS patients with renal function injury is as high as 30%.3 The incidence of CI-AKI in ST-segment elevation myocardial infarction (STEMI) patients is four times higher than that in patients with stable angina pectoris.4 Furthermore, AKI was an independent prognostic factor for long-term mortality of patients with STEMI complicated by cardiogenic shock and treated with primary percutaneous coronary intervention (PCI).5 Therefore, early recognition of high-risk groups is particularly crucial for active prevention and treatment of CI-AKI.
Although the underlying pathophysiology of CI-AKI has not been fully understood, it has been found that inflammation plays a central role in acute renal injury and CI-AKI.6 Various studies have shown that systemic inflammatory response severity associated with poor prognosis in cardiovascular diseases could be measured from peripheral blood-based parameters. Of them, Systemic immune-inflammatory index (SII = neutrophil count × platelet count/lymphocyte count), high-sensitivity C-reactive protein (hsCRP), and procalcitonin are more closely associated with CI-AKI.
The interest in the association between SII and hsCRP and the risk of cardiovascular events in patients undergoing angiography or PCI is rapidly growing. Multiple studies have established an association of SII and hsCRP with the risk of CI-AKI in STEMI patients.7,8 Liu et al9 have shown that hsCRP is a useful and independent predictor of CI-AKI and was also significantly associated with in-hospital mortality in STEMI patients undergoing primary PCI. Simultaneously, studies on kidney disease have shown that SII is significantly associated with the risk of death in patients with kidney transplantation and metastatic renal cell carcinoma.10 It has become a new biochemical indicator to predict death and prognosis in kidney disease.
However, the utility of their combined in predicting CI-AKI in STEMI has not been studied. Thus, this retrospective study investigated the value of SII combined with hsCRP for predicting CI-AKI after PCI in STEMI patients.
Materials and Methods
Subjects
A retrospective analysis was performed on 563 patients with STEMI who underwent PCI in our hospital from November 2019 to March 2021. Patients were included in the study if they met the following criteria: (1) diagnosed with STEMI, (2) underwent PCI, and, (3) had follow-up creatinine levels in hospital for at least 72 hours. The exclusion criteria were those patients with estimated glomerular filtration rate (eGFR) < 15mL/(min·1.73m2) or severe liver dysfunction, inflammation (including febrile disease, autoimmune disease, acute or chronic inflammatory disease, or recent history of infection), malignancy, hematologic disorders, missing preprocedural or postprocedural SCr, surgery or trauma within two months; allergy to artificial contrast agents and exposure to contrast medium within previous seven days. Patients who died during PCI were also excluded. Finally, the remaining 415 patients were enrolled in the study. The mean age was 62.70±12.92. According to the occurrence of CI-AKI after PCI, patients were divided into the CI-AKI group (n=71) and non-CI-AKI group (n=344). The study protocol has been approved by the Medical Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (Ethics number: XYFY2022-KL122-01). All selected patients provided written informed consent for PCI.
Percutaneous Coronary Interventions
The study populations were recruited from coronary intervention cases that included coronary stenting or balloon angioplasty. All patients who planned to undergo PCI were routinely received a single dose of oral aspirin (300 mg), 300–600 mg of clopidogrel just before the procedure, and 100 U/kg of intravenous heparin (additional boluses were given as appropriate to achieve activated clotting time > 250 ms). PCI was performed according to standard clinical practice using standard catheters, guide wires and balloon catheters via the radial approach. Nonionic, low-osmolar contrast medium was administered during the procedure (ioversol, Yangtze River Pharmaceutical Group). The contrast dose, medication, and intra-aortic balloon pump (IABP) support were left to the discretion of the interventional cardiologist according to patient need. High-risk patients should be administered 0.9% saline by intravenous infusion at a rate of approximately 1 mL/kg per hour, beginning 6–12 hours before the procedure and continuing for up to 12–24 hours after the radiographic examination, if diuresis is appropriate and cardiovascular condition allows it. In patients with left ventricular ejection fraction (LVEF) less than 40% or heart failure, the hydration rate should be reduced to 0.5 mL·kg−1·h−1.11 Intravenous hydration was continued longer in cases who developed CI-AKI at the discretion of the attending physicians.
Study Method
All patients received venous blood collection at room temperature before PCI on admission. Relevant demographic variables were gathered from the medical record systems for all patients, including age, gender, systolic and diastolic blood pressure on admission, history of smoking, drinking, hypertension, and diabetes mellitus. Serum biomarkers include fasting blood glucose, serum creatinine (SCr), blood urea, serum uric acid, cystatin C, eGFR, albumin, monocyte count, neutrophil, and lymphocyte count, platelet count, Hemoglobin, hsCRP, N-terminal pro-brain natriuretic peptide (NT-proBNP) on admission, SII. Monitor blood lipids, including cholesterol levels on admission (small dense Low-Density Lipoprotein (sd-LDL), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol, triglycerides, lipoprotein-a (Lp (a)), total cholesterol). The data collected during PCI included the main diseased vessels (left coronary artery, left anterior descending coronary artery, left circumflex coronary artery, and right coronary artery), the total length of the implanted stent, the average diameter of the implanted stent, the number of implanted stents, contrast medium volume, and contact time. Serum creatinine level measurement was repeated at 48 and 72 hours after contrast medium exposure in all patients. All blood samples were tested in the central laboratory of our hospital. Left ventricular ejection fraction was recorded by echocardiography to evaluate left ventricular systolic function.
Definition
The primary outcome for this study was the occurrence of CI-AKI, which was defined as an absolute serum creatinine increase ≥ 44 mol/L or a relative increase in serum creatinine ≥ 25% occurring within 48–72 h after the coronary procedure, in accordance with the European Society of Urogenital Radiology (ESUR) Guidelines.12 The rates of CI-AKI were calculated using pre- and post-procedural serum creatinine measurements. Related definitions and diagnostic criteria included the following: Current smoking is those who have smoked regularly in the past six months. The SII was defined as follows: SII=P×N/L, where P, N, and L were the preprocedural peripheral platelet, neutrophil, and lymphocyte counts, respectively.
Statistical Analysis
Natural logarithm transformed values were used for the statistical analyses of some variables as the original values were skewed. Continuous variables with normal distribution were expressed as the mean ± SD and compared using the Independent sample t-test. Continuous variables without normal distribution were expressed as the median (25th-75th interquartile range) and compared using the Mann–Whitney U-test. Categorical variables are expressed as counts and percentages (%) and compared using the Chi-square test. Multiple binary logistic regression analysis, Backward Wald method, was used to define the independent predictors of CI-AKI. Restricted cubic spline (RCS) analyses were used to explore the nonlinear correlation between the SII, hsCRP, and the prevalence of CI-AKI, respectively. The red area represents the 95% confidence interval. In addition, receiver operating characteristic (ROC) curves were conducted to calculate the sensitivity and specificity and determine the cutoff value. At the same time, a Pairwise comparison of the ROC between the SII, hsCRP, and the combined was performed using DeLong’s test. Furthermore, multiple logistic regression analysis was carried out to evaluate whether SII and hsCRP were associated with the incidence of CI-AKI after controlling possible confounding variables (diabetes mellitus, diuretics, sd-LDL, fasting plasma glucose, serum creatinine, eGFR, ln(NT-proBNP), hsCRP, neutrophil count, lymphocyte count, platelet count, NLR, PLR, and SII). All statistical analyses were performed using SPSS version 26.0 (SPSS Inc., Chicago, IL, USA), and the statistical software package R, version 4.0.3 (https://cran.r-project.org). All tests were two-tailed, and P-value < 0.05 was considered statistically significant.
Results
Different Characteristics Between Non-CI-AKI and CI-AKI Groups
A total of 415 patients were enrolled after applying inclusion-exclusion criteria in this study, and there was 71 (17.1%) developed CI-AKI. The baseline demographic characteristics, demographic data, previous medical history, vital signs on admission, laboratory indices, angiographic variables, and medication use at discharge, are listed in Tables 1 and 2. The patients in the CI-AKI group had significantly a higher prevalence of diabetes mellitus, fasting plasma glucose, serum creatinine, sd-LDL, ln(NT-proBNP), hsCRP, neutrophil granulocyte count, platelet count, NLR, PLR, preprocedural SII level and diuretic use, and lower eGFR, lymphocyte count than those in the non-CI-AKI group. No significant differences in the frequency of smoking, hypertension, contrast medium volume, and other indicators between the groups.
Table 1.
Baseline Demographic and Clinical Characteristics of the Study Groups
| Variable | Non-CI-AKI Group (n=344) | CI-AKI Group (n=71) | χ2/t Value | P value |
|---|---|---|---|---|
Age (year,  ±s) ±s) |
62.72±12.90 | 67.03±12.34 | 0.068 | 0.946 |
| Age>75, n (%) | 56(16.27) | 13(18.31) | 0.175 | 0.676 |
| Men, n (%) | 271(78.78) | 53(74.65) | 0.587 | 0.444 |
| Hypertension, n (%) | 144(41.86) | 30(42.25) | 0.004 | 0.951 |
| Diabetes mellitus, n (%) | 94(27.33) | 33(46.48) | 10.166 | 0.001 |
| Smoking, n (%) | 145(42.15) | 28(39.44) | 0.450 | 0.502 |
| Drinking, n (%) | 94(27.33) | 18(25.35) | 0.116 | 0.733 |
Systolic pressure (mmHg,  ±s) ±s) |
126.78±20.90 | 127.82±22.56 | −0.375 | 0.708 |
| LVEF< 40%, n (%) | 64(18.60) | 17(23.94) | 1.068 | 0.301 |
Diastolic pressure (mmHg,  ±s) ±s) |
78.31±14.22 | 79.61±14.87 | −0.691 | 0.490 |
| Aspirin, n (%) | 344(100) | 71(100) | – | 1 |
| Clopidogrel/Ticagrelor, n (%) | 344(100) | 71(100) | – | 1 |
| Beta-blockers, n (%) | 280(81.40) | 60(84.51) | 0.385 | 0.535 |
| ACEI/ARB, n (%) | 226(65.70) | 51(71.83) | 0.997 | 0.318 |
| CCB, n (%) | 40(11.63) | 6(8.45) | 0.603 | 0.438 |
| Diuretics, n (%) | 86(25.00) | 26(36.62) | 4.033 | 0.045 |
| Statins, n (%) | 319(92.73) | 65(91.55) | 0.119 | 0.730 |
| Nitrates, n (%) | 250(72.67) | 49(69.01) | 0.392 | 0.531 |
| Criminal artery vessels, n (%) | ||||
| Left anterior descending, n (%) | 140(40.70) | 33(46.48) | 0.809 | 0.368 |
| Left circumflex, n (%) | 91(26.45) | 16(22.54) | 0.472 | 0.492 |
| Right coronary artery, n (%) | 107(31.10) | 19(26.76) | 0.525 | 0.469 |
| Left main, n (%) | 6(1.74) | 3(4.23) | 1.708 | 0.191 |
| Stent number per patient, n (%) | ||||
| 1 (n, %) | 303(88.08) | 59(83.10) | 1.312 | 0.252 |
| ≥2 (n, %) | 41(11.92) | 12(16.90) | 1.312 | 0.252 |
| Stent length (mm) | 16.19 ± 3.21 | 16.83 ± 2.74 | −1.730 | 0.086 |
| Stent diameter (mm) | 3.17 ± 0.89 | 3.23 ± 0.77 | 0.551 | 0.582 |
| Contrast exposure time (min) | 37.96±11.26 | 40.23±13.65 | 1.488 | 0.138 |
| Contrast medium>100 (mL) | 182(52.91) | 46(64.79) | 3.356 | 0.067 |
Abbreviations: CI-AKI, contrast-induced acute kidney injury; LVEF, left ventricular ejection fraction; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor inhibitor; CCB, calcium channel blockers.
Table 2.
Hematological and Biochemical Measurements of the Study Population
| Variable | Non-CI-AKI Group (n=344) | CI-AKI Group (n=71) | t/Z value | P value |
|---|---|---|---|---|
| Triglycerides (mmol/L) | 1.20(0.88,1.70) | 1.26(0.90,1.92) | −0.839 | 0.401 |
| Total cholesterol (mmol/L) | 4.28 ± 0.99 | 4.26 ± 0.99 | −0.086 | 0.931 |
| High-density lipoprotein (mmol/L) | 0.99 ± 0.23 | 1.01 ± 0.24 | 0.670 | 0.503 |
| Low-density lipoprotein (mmol/L) | 2.61 ± 0.81 | 2.60 ± 0.80 | −0.081 | 0.936 |
| sd-LDL | 0.87 ± 0.45 | 1.24 ± 0.43 | 6.294 | <0.001 |
| Albumin (g/L) | 39.42 ± 5.08 | 38.67 ± 5.57 | −1.119 | 0.264 |
| Fasting plasma glucose (mmol/L) | 5.58 (5.01,6.64) | 6.33 (5.35,8.94) | −3.186 | 0.001 |
| Blood urea (mmol/L) | 5.74 ± 1.98 | 5.60 ± 2.08 | −0.545 | 0.586 |
| Serum creatinine (µmol /L) | 67.35 ± 15.62 | 75.96 ± 18.97 | 4.069 | <0.001 |
| Uric acid (µmol/L) | 318.97 ± 92.38 | 307.13 ± 87.43 | −0.992 | 0.322 |
| cystatin C (mg/L) | 1.06 ± 0.35 | 1.05 ± 0.40 | −0.235 | 0.815 |
| eGFR (mL/min) | 119.72 ± 33.42 | 104.14 ± 33.78 | −3.572 | <0.001 |
| ln(NT-proBNP) | 7.11 ± 1.17 | 8.09 ± 0.90 | −7.899 | <0.001 |
| Lipoprotein (A) (mg/L) | 260.85 ± 171.83 | 281.61 ± 157.83 | 0.939 | 0.348 |
| Total bilirubin (µmol/L) | 16.52 ± 8.32 | 16.18 ± 7.25 | −0.318 | 0.751 |
| Direct bilirubin (µmol /L) | 6.03 ± 3.17 | 5.97 ± 2.98 | −0.161 | 0.872 |
| hsCRP (mg/L) | 3.70(1.10,6.70) | 9.30(6.00,11.20) | −7.129 | <0.001 |
| Hemoglobin (g/L) | 135.29 ± 17.55 | 137.56 ± 19.03 | 0.980 | 0.328 |
| Monocyte count (109/L) | 0.53 ± 0.63 | 0.45 ± 0.24 | −1.052 | 0.294 |
| Neutrophil count (109/L) | 6.15 ± 2.68 | 6.93 ± 3.02 | 2.186 | 0.029 |
| Lymphocyte count (109/L) | 1.74 ± 0.88 | 1.45 ± 0.76 | −2.641 | 0.009 |
| Platelet count (109/L) | 175.00 ± 48.00 | 248.83 ± 57.41 | 11.392 | <0.001 |
| NLR | 4.41 ± 3.36 | 6.19 ± 4.24 | 3.882 | 0.001 |
| PLR | 33.85 ± 17.78 | 41.56 ± 18.75 | 3.295 | 0.001 |
| SII | 769.52 ± 677.30 | 1571.92 ± 1280.18 | 5.135 | <0.001 |
| Red blood cell distribution width (%) | 17.26 ± 11.03 | 15.96 ± 9.39 | −0.931 | 0.352 |
| Platelet distribution width (%) | 15.86 ± 1.55 | 15.81 ± 1.44 | −0.264 | 0.792 |
Abbreviations: eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro-B type natriuretic peptide; hsCRP, high-sensitivity C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
SII and hsCRP Level Before PCI and CI-AKI
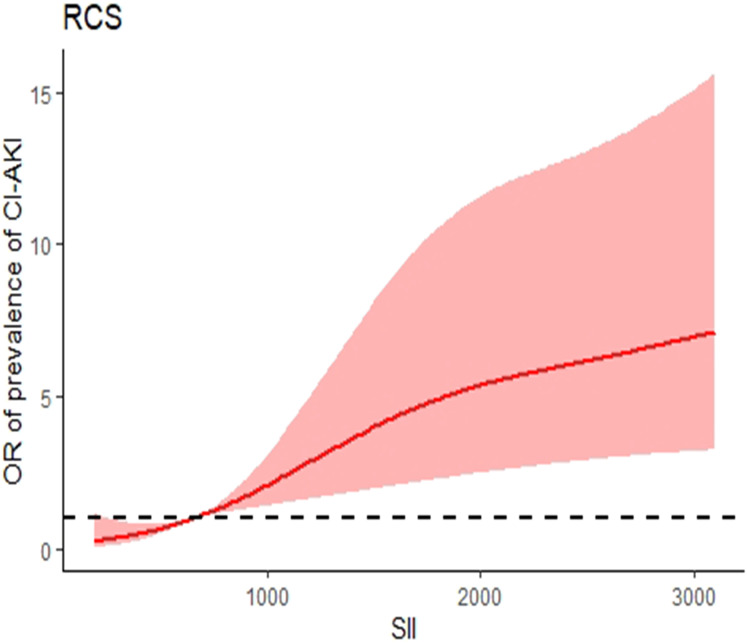
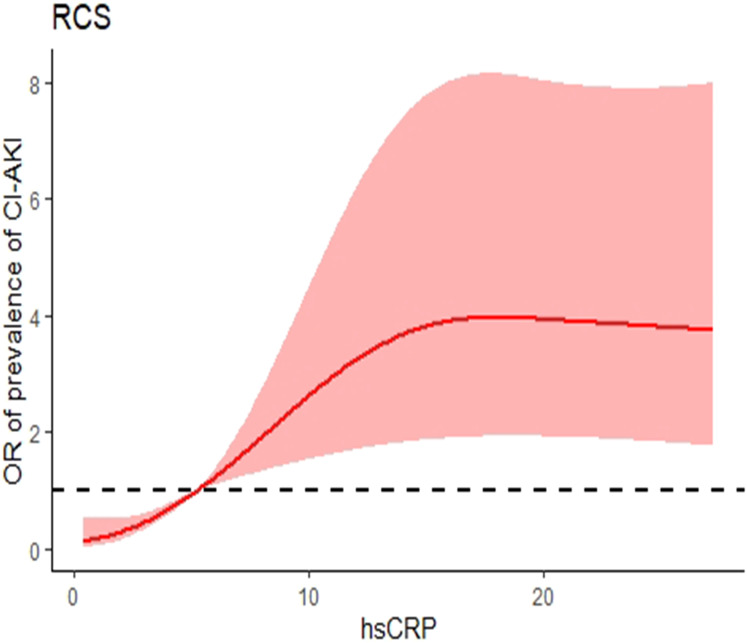
After adjustment for all potential confounders in this study, when the SII concentration was greater than 653.73×109/L, the prevalence of CI-AKI increased with SII concentration. When the SII concentration was less than 653.73×109/L, the prevalence of CI-AKI reached a plateau. When the hsCRP was less than 5.20 mg/L, it was a plateau for the prevalence of CI-AKI; when the hsCRP was greater than 5.20 mg/L, there was a positive correlation between the prevalence of CI-AKI and the hsCRP (Figures 1 and 2).
Figure 1.
Nonlinear associations between SII concentration and the prevalence of CI-AKI. When the SII concentration was greater than 653.73×109/L, the prevalence of CI-AKI increased with SII concentration. When the SII concentration was less than 653.73×109/L, the prevalence of CI-AKI reached a plateau.
Abbreviations: RCS, restricted cubic spline; SII, systemic immune-inflammation index; CI-AKI, contrast-induced acute kidney injury.
Figure 2.
Nonlinear associations between hsCRP concentration and the prevalence of CI-AKI. When the hsCRP was less than 5.20 mg/L, it was a plateau for the prevalence of CI-AKI; when the hsCRP was greater than 5.20 mg/L, there was a positive correlation between the prevalence of CI-AKI and the hsCRP.
Abbreviations: RCS, restricted cubic spline; hsCRP, high-sensitivity C-reactive protein; CI-AKI, contrast-induced acute kidney injury.
Associations Between the Prevalence of CI-AKI in Different Subgroups According to SII and Serum hsCRP Levels
According to RCS analysis results, patients were further stratified as preprocedural low or high SII (< 653.73×109 or ≥ 653.73×109/L, respectively) and low or high hsCRP (< 5.20 or ≥ 5.20 mg/L, Table 3). The rates of CI-AKI were higher in the high SII or hsCRP groups relative to the low groups. Because SII and hsCRP are biomarkers of inflammation, the patients were also stratified into low-, medium-, or high-inflammation groups. Specifically, low inflammation was defined as low SII and low hsCRP. Medium inflammation was considered the combination low SII and high hsCRP, or high SII and low hsCRP. In the high-inflammation group, both SII and hsCRP were high. The incidence rates of CI-AKI rose with the inflammation status (P < 0.001).
Table 3.
Incidence Rates of CI-AKI Varied According to Low or High SII and hsCRP
| Non-CI-AKI | CI-AKI | χ2/Z value | P | ||
|---|---|---|---|---|---|
| Subjects, n | 344 | 71 | |||
| SII | Low | 193 | 13 | 33.629 | <0.001 |
| High | 151 | 58 | |||
| hsCRP | Low | 199 | 10 | 45.059 | <0.001 |
| High | 145 | 61 | |||
| Inflammation | Low | 118 | 1 | −8.252 | <0.001 |
| Medium | 156 | 21 | |||
| High | 70 | 49 | |||
Notes: Low SII is defined as SII < 653.73×109L; high SII is ≥ 653.73×109/L. Low hsCRP is considered < 5.2 mg/L; high hsCRP is ≥ 5.2 mg/L. low inflammation was defined as low SII and low hsCRP. Medium inflammation was considered the combination low SII and high hsCRP, or high SII and low hsCRP. In the high-inflammation group, both SII and hsCRP were high.
Value of SII and hsCRP in Predicting CI-AKI by ROC
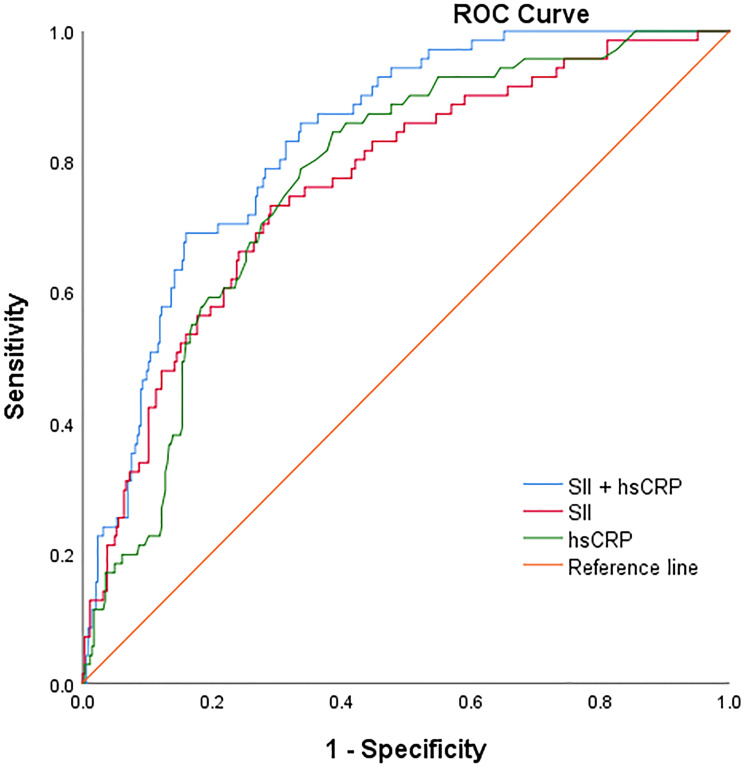
The ability of SII, hsCRP and combined SII with hsCRP to predict CI-AKI As shown in Figure 3 and Table 4, we calculated ROC curves to illustrate the performance of SII, hsCRP and the combination of both factors to discriminate CI-AKI from non-CI-AKI in all participants. We calculated the cutoff point as 831.05×109/L for SII and 5.55 mg/L for hsCRP to estimate the presence of CI-AKI with a sensitivity of 73.2% and 70.9% as well as a specificity of 84.5% and 61.3%, respectively. The AUC was calculated to assess the performance of CI-AKI discrimination for each biomarker. Pairwise comparison of ROC curves by the DeLong method indicated that the models of SII (AUC: 0.764; 95% CI: 0.704–0.825; P = 0.001) and hsCRP alone (AUC: 0.769; 95% CI: 0.714–0.823; P < 0.001) produced similar degrees of discrimination of CI-AKI (z = −0.093, P = 0.926), and both were inferior to the combined SII with the hsCRP model (AUC: 0.831; 95% CI: 0.786–0.876; P < 0.001).
Figure 3.
ROC curve of CI-AKI predicted by SII, hsCRP and combined index. The receiver operating characteristic curve of SII combined with hsCRP was 0.831, which was higher than SII or hsCRP alone.
Abbreviations: ROC, receiver operating characteristic; AUC, area under the ROC curve; CI-AKI, contrast-induced acute kidney injury; SII, Systemic immune-inflammation index; hsCRP, high-sensitivity C-reactive protein.
Table 4.
Predictive Values of SII and hsCRP for CI-AKI by ROC Curves
| Variable | AUC | Cut-off value | Sensitivity | Specificity | 95% CI | P |
|---|---|---|---|---|---|---|
| SII | 0.764 | 831.05×109/L | 73.2 | 70.9 | 0.704–0.825 | 0.001 |
| hsCRP | 0.769 | 5.55 mg/L | 84.5 | 61.3 | 0.714–0.823 | <0.001 |
| SII+hsCRP | 0.831 | 1741.80 | 80.7 | 71.4 | 0.786–0.876 | <0.001 |
Abbreviations: ROC, receiver operating characteristic; AUC, area under the ROC curve; CI, Confidence interval.
Multiple Logistic Regression Analysis of Risk Factors for CI-AKI
The multiple logistic regression results showed that diabetes mellitus, platelet count, and inflammatory biomarkers were high-risk factors of CI-AKI (Table 5). The incidence of CI-AKI was higher in patients in whom both SII and hsCRP were elevated (the high inflammation group), compared with elevations of either SII or hsCRP alone (low or medium inflammation). The OR value of CI-AKI was 16.676 with 95.0% CI 1.825–152.389.
Table 5.
Multile Logistic Regression Analysis of Risk Factors for CI-AKI
| β | SE | Wald | P | Exp(B) | 95.0% CI for Exp(B) | |
|---|---|---|---|---|---|---|
| Low | Reference | |||||
| High SII or hsCRP | 2.614 | 1.099 | 5.660 | 0.017 | 13.659 | 1.585–117.723 |
| High SII + hsCRP | 2.814 | 1.129 | 6.214 | 0.013 | 16.676 | 1.825–152.389 |
| DM | 0.846 | 0.330 | 6.567 | 0.010 | 2.330 | 1.220–4.451 |
| PLT | 0.026 | 0.006 | 20.134 | <0.001 | 1.027 | 1.015–1.038 |
Abbreviations: DM, diabetes mellitus; PLT, platelet count.
Discussion
In this retrospective study, we investigated whether the combined values of the inflammatory biomarkers SII and hsCRP may predict the development of CI-AKI after PCI in STEMI patients. Of the 415 patients, 17.1% (71/415) developed CI-AKI. The study demonstrated that patients with preprocedural high levels of either SII or hsCRP were more likely to develop CI-AKI, compared with patients with lower values. Notably, preprocedural high levels of both SII and hsCRP were significantly more predictive of CI-AKI, relative to that of lower values of either. The multiple logistic regression analysis confirmed that elevated preprocedural levels of SII together with hsCRP were a risk factor for CI-AKI after PCI. The study also found that diabetes mellitus and platelet count were independent risk factors for CI-AKI. According to these results, the combination of SII and hsCRP can be used to predict an increased risk of CI-AKI after PCI and is more accurate than either biomarker alone.
CI-AKI is a well-recognized risk factor for worse patient survival and renal outcomes regardless of the baseline renal function, including prolonged length of hospital stay and the associated costs, which have been important limitations of angiographic procedures with radiocontrast media, especially in STEMI patients.3,4,13,14 Hence, early identification of patients with a high CI-AKI risk is crucial to allow the necessary interventions, and its occurrence is currently included as one of the metrics for quality outcomes of PCI.
Although the pathophysiological mechanisms that cause CI-AKI are not fully and correctly defined. However, previous studies have demonstrated that an inflammatory response and an exacerbated thrombotic state occur during the development of CI-AKI and that mediators reflecting these responses may be a marker of CI-AKI.15–17 Moreover, various studies have shown that systemic inflammatory response severity associated with poor prognosis in cardiovascular diseases could be measured from peripheral blood-based parameters.
SII is a recently derived biomarker that was shown to reflect inflammatory and immune balance in various cancer types and cardiovascular diseases. Previous experimental and clinical research suggested that patients with elevated SII had a greater risk of poor clinical outcomes.18–21 The use of SII in cardiovascular diseases has expanded even more after Jesse Fest et al provides its reference values for the first time.22 Subsequently, several studies have found that a high level of SII is associated with increased short- and long-term mortality of patients with heart failure and cardiogenic shock.23,24 In a recent study, Ali Bag˘cı et al observed that the determination of pre-procedure SII may help predict the risk of CI-AKI in patients with STEMI.25 Compared with NLR and PLR as well as neutrophil and lymphocyte counts, it is thought that SII can indicate the inflammatory status in patients more comprehensively.20 In our study, the patients in the high SII group had a higher incidence of CI-AKI. It could be that increased SII level was accompanied by increased inflammatory activity, leading to further deterioration of renal inflammation and tissue damage.26 The advantage of SII is that it reflects both the overactive clotting pathway and the inflammatory pathway, both of which are potential mechanisms of CI-AKI, and SII can be considered a more sensitive predictor of inflammation in the body.
High-sensitivity C-reactive protein (hsCRP), as a marker of systemic inflammation, has been proven to be associated with increased relative risks of cardiovascular events in patients with coronary stenting.27,28 In this study, compared with the non-CI-AKI group, the level of hsCRP in the CI-AKI group was significantly increased, and hsCRP was closely related to the incidence of CI-AKI. Liu et al demonstrated that the increase in hsCRP level is a significant and independent predictor of CI-AKI in STEMI patients treated with primary PCI, and those with higher hsCRP were associated with in-hospital mortality and composite MACEs closely related.9,29 Recent studies showed that the hsCRP had a stronger association with heart failure, and higher hsCRP predicted all-cause mortality during long-term follows-up.30 It is well known that C-reactive protein increases with age and in patients with renal insufficiency, factors that highly influence the survival of STEMI patients.27,31 These findings alone could explain the worse clinical long-term outcome observed in patients with high hsCRP values. Although the underlying hyperinflammatory state and the specific mechanisms underlying the occurrence of CI-AKI are unclear. Several studies have shown that medications with beneficial effects on the modulation of systemic inflammation and endothelial function, such as statins, reduce circulating hsCRP concentrations and significantly reduce the risk of CI-AKI.32,33 Given the relatively low costs of CRP measurements, preprocedural risk stratification with hsCRP as an adjunct to established clinical risk factors may be useful and should be given due attention in those patients with elevated preprocedural hsCRP.
Taken together, SII and hsCRP, as indicators of systemic inflammatory response activity, are relatively common and easy to obtain in clinical practice. It is closely related to the incidence of coronary heart disease and renal insufficiency. However, few studies have explored the value of the combination of hsCRP and SII in predicting CI-AKI and the nonlinear correlation between inflammatory markers and the pathogenesis of CI-AKI. Our studies confirmed that SII and hsCRP levels, within certain ranges, were positively correlated with the prevalence of CI-AKI after PCI in STEMI patients. The combination of these 2 biomarkers had a higher predictive value for CI-AKI than did either by itself, which is of great significance for determining individualized monitoring and optimizing treatment strategies to improve prognosis.
Limitations
Our study has several limitations that need to be considered. Firstly, it was a single-centered and retrospective cross-sectional study, some patients were excluded due to missing variables, and there might be selection bias. Second, we calculated SII and hsCRP only once at admission and did not monitor changes in these inflammatory biomarkers during the study period. Finally, due to limited data from the electronic medical record system, there are also some causes of hospitalization-related CI-AKI that we did not evaluate (eg, perioperative hydration, antihypertensive and hypoglycemic medications, etc.), the clinical value of SII and hsCRP in patients with STEMI after PCI should be further confirmed by multi-center and enlarged sample size.
Conclusions
We conclude that elevated levels of the pre-procedural inflammatory biomarkers SII and hsCRP are risk factors for CI-AKI in patients with STEMI undergoing PCI. The combination of elevated SII and hsCRP can better predict a high risk of CI-AKI compared with either of these alone. Our study may provide a simple and easy reference index for clinicians to predict the occurrence of CI-AKI, and identify and screen high-risk patients with CI-AKI. Easily and accurately assess an individual’s risk of CI-AKI before PCI and make the right clinical decision to plan and initiate the most appropriate disease management on time.
Acknowledgments
We thank Min Zhang contributed some of the data for the manuscript, and Tongda Xu contributed to the review and approval of the final version of the manuscript.
Funding Statement
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval and Informed Consent
The study was conducted in accordance with the Declaration of Helsinki and has been approved by the Medical Ethics Committee of the Affiliated Hospital of Xuzhou Medical University. Due to the study being a retrospective analysis, the review committee waived the requirement for written informed consent. Confidential patient information was removed from the entire data set prior to analysis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Mettler FA Jr, Mahesh M, Bhargavan-Chatfield M, et al. Patient exposure from radiologic and nuclear medicine procedures in the United States: procedure volume and effective dose for the period 2006–2016. Radiology. 2020;295(2):418–427. doi: 10.1148/radiol.2020192256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rear R, Bell RM, Hausenloy DJ. Contrast-induced nephropathy following angiography and cardiac interventions. Heart. 2016;102(8):638–648. doi: 10.1136/heartjnl-2014-306962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abe D, Sato A, Hoshi T, et al. Clinical predictors of contrast-induced acute kidney injury in patients undergoing emergency versus elective percutaneous coronary intervention. Circ J. 2014;78(1):85–91. doi: 10.1253/circj.cj-13-0574 [DOI] [PubMed] [Google Scholar]
- 4.Marenzi G, Cosentino N, Bartorelli AL. Acute kidney injury in patients with acute coronary syndromes. Heart. 2015;101(22):1778–1785. doi: 10.1136/heartjnl-2015-307773 [DOI] [PubMed] [Google Scholar]
- 5.Hayıroğlu Mİ, Bozbeyoglu E, Yıldırımtürk Ö, Tekkeşin Aİ, Pehlivanoğlu S. Effect of acute kidney injury on long-term mortality in patients with ST-segment elevation myocardial infarction complicated by cardiogenic shock who underwent primary percutaneous coronary intervention in a high-volume tertiary center. Turk Kardiyol Dern Ars. 2020;48(1):1–9. doi: 10.5543/tkda.2019.84401 [DOI] [PubMed] [Google Scholar]
- 6.Hossain MA, Costanzo E, Cosentino J, et al. Contrast-induced nephropathy: pathophysiology, risk factors, and prevention. Saudi J Kidney Dis Transpl. 2018;29(1):1–9. doi: 10.4103/1319-2442.225199 [DOI] [PubMed] [Google Scholar]
- 7.Bağcı A, Aksoy F, Baş HA. Systemic immune-inflammation index may predict the development of contrast-induced nephropathy in patients with ST-segment elevation myocardial infarction. Angiology. 2022;73(3):218–224. doi: 10.1177/00033197211030053 [DOI] [PubMed] [Google Scholar]
- 8.Ortolani P, Marzocchi A, Marrozzini C, et al. Predictive value of high sensitivity C-reactive protein in patients with ST-elevation myocardial infarction treated with percutaneous coronary intervention. Eur Heart J. 2008;29(10):1241–1249. doi: 10.1093/eurheartj/ehm338 [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Tan N, Zhou YL, et al. High-sensitivity C-reactive protein predicts contrast-induced nephropathy after primary percutaneous coronary intervention. J Nephrol. 2012;25(3):332–340. doi: 10.5301/jn.5000007 [DOI] [PubMed] [Google Scholar]
- 10.Ozbek E, Besiroglu H, Ozer K, Horsanali MO, Gorgel SN. Systemic immune inflammation index is a promising non-invasive marker for the prognosis of the patients with localized renal cell carcinoma. Int Urol Nephrol. 2020;52(8):1455–1463. doi: 10.1007/s11255-020-02440-y [DOI] [PubMed] [Google Scholar]
- 11.Mehran R, Dangas GD, Weisbord SD. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146–2155. doi: 10.1056/NEJMra1805256 [DOI] [PubMed] [Google Scholar]
- 12.Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR contrast media safety committee guidelines. Eur Radiol. 2011;21(12):2527–2541. doi: 10.1007/s00330-011-2225-0 [DOI] [PubMed] [Google Scholar]
- 13.Sato A, Aonuma K, Watanabe M, et al. Association of contrast-induced nephropathy with risk of adverse clinical outcomes in patients with cardiac catheterization: from the CINC-J study. Int J Cardiol. 2017;227:424–429. doi: 10.1016/j.ijcard.2016.11.019 [DOI] [PubMed] [Google Scholar]
- 14.Gupta R, Gurm HS, Bhatt DL, Chew DP, Ellis SG. Renal failure after percutaneous coronary intervention is associated with high mortality. Catheter Cardiovasc Interv. 2005;64(4):442–448. doi: 10.1002/ccd.20316 [DOI] [PubMed] [Google Scholar]
- 15.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi: 10.1155/2009/137072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun KH. Contrast-induced acute kidney injury and inflammation. Korean Circ J. 2018;48(1):84–85. doi: 10.4070/kcj.2017.0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagareddy P, Smyth SS. Inflammation and thrombosis in cardiovascular disease. Curr Opin Hematol. 2013;20(5):457–463. doi: 10.1097/MOH.0b013e328364219d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442 [DOI] [PubMed] [Google Scholar]
- 19.Jin Z, Wu Q, Chen S, et al. The associations of two novel inflammation indexes, SII and SIRI with the risks for cardiovascular diseases and all-cause mortality: a ten-year follow-up study in 85,154 individuals. J Inflamm Res. 2021;14:131–140. doi: 10.2147/JIR.S283835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang YL, Wu CH, Hsu PF, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50(5):e13230. doi: 10.1111/eci.13230 [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Zhang Q, Wang R, et al. Systemic immune-inflammatory index predicts clinical outcomes for elderly patients with acute myocardial infarction receiving percutaneous coronary intervention. Med Sci Monit. 2019;25:9690–9701. doi: 10.12659/MSM.919802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fest J, Ruiter R, Ikram MA, Voortman T, van Eijck C, Stricker BH. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: a population-based prospective cohort study. Sci Rep. 2018;8(1):10566. doi: 10.1038/s41598-018-28646-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Y, Huang W, Shi Z, Chen Y, Ma J. Positive association between systemic immune-inflammatory index and mortality of cardiogenic shock. Clin Chim Acta. 2020;511:97–103. doi: 10.1016/j.cca.2020.09.022 [DOI] [PubMed] [Google Scholar]
- 24.Hayıroğlu Mİ, Çınar T, Çinier G, et al. Evaluating systemic immune-inflammation index in patients with implantable cardioverter defibrillator for heart failure with reduced ejection fraction. Pacing Clin Electrophysiol. 2022;45(2):188–195. doi: 10.1111/pace.14436 [DOI] [PubMed] [Google Scholar]
- 25.Bağcı A, Aksoy F, Baş HA. Systemic immune-inflammation index may predict the development of contrast-induced nephropathy in patients with st-segment elevation myocardial infarction. Angiology. 2021;33197211030053. doi: 10.1177/00033197211030053 [DOI] [PubMed] [Google Scholar]
- 26.Jansen MP, Emal D, Teske GJ, Dessing MC, Florquin S, Roelofs JJ. Release of extracellular DNA influences renal ischemia reperfusion injury by platelet activation and formation of neutrophil extracellular traps. Kidney Int. 2017;91(2):352–364. doi: 10.1016/j.kint.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 27.Wang CH, Zhang SY, Fang Q, et al. Renal dysfunction and hsCRP predict long-term outcomes of percutaneous coronary intervention in acute myocardial infarction. Am J Med Sci. 2015;349(5):413–420. doi: 10.1097/MAJ.0000000000000430 [DOI] [PubMed] [Google Scholar]
- 28.Chen R, Liu C, Zhou P, et al. Both low and high postprocedural hsCRP associate with increased risk of death in acute coronary syndrome patients treated by percutaneous coronary intervention. Mediators Inflamm. 2020;2020:9343475. doi: 10.1155/2020/9343475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jian-Wei Z, Yu-Jie Z, Shu-Jun C, Qing Y, Shi-Wei Y, Bin N. Impact of preprocedural high-sensitivity C-reactive protein on contrast-induced nephropathy in patients undergoing primary percutaneous coronary intervention. Angiology. 2014;65(5):402–407. doi: 10.1177/0003319713482177 [DOI] [PubMed] [Google Scholar]
- 30.Çinier G, Hayıroğlu Mİ, Kolak Z, et al. The value of C-reactive protein-to-albumin ratio in predicting long-term mortality among HFrEF patients with implantable cardiac defibrillators. Eur J Clin Invest. 2021;51(8):e13550. doi: 10.1111/eci.13550 [DOI] [PubMed] [Google Scholar]
- 31.Ribeiro DR, Ramos AM, Vieira PL, et al. High-sensitivity C-reactive protein as a predictor of cardiovascular events after ST-elevation myocardial infarction. Arq Bras Cardiol. 2014;103(1):69–75. doi: 10.5935/abc.20140086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xinwei J, Xianghua F, Jing Z, et al. Comparison of usefulness of simvastatin 20 mg versus 80 mg in preventing contrast-induced nephropathy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am J Cardiol. 2009;104(4):519–524. doi: 10.1016/j.amjcard.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 33.Peruzzi M, De Luca L, Thomsen HS, et al. A network meta-analysis on randomized trials focusing on the preventive effect of statins on contrast-induced nephropathy. Biomed Res Int. 2014;2014:213239. doi: 10.1155/2014/213239 [DOI] [PMC free article] [PubMed] [Google Scholar]