Abstract
Background
The phase 3 VIALE-A trial (NCT02993523) reported that venetoclax-azacitidine significantly prolonged overall survival compared with placebo-azacitidine in patients with newly diagnosed acute myeloid leukemia ineligible for intensive chemotherapy. Herein, efficacy and safety of venetoclax-azacitidine are analyzed in the Japanese subgroup of VIALE-A patients.
Methods
Eligible Japanese patients were randomized 2:1 to venetoclax-azacitidine (N = 24) or placebo-azacitidine (N = 13). Primary endpoints for Japan were overall survival and complete response (CR) + CR with incomplete hematologic recovery (CRi). Venetoclax (target dose 400 mg) was given orally once daily. Azacitidine (75 mg/m2) was administered subcutaneously or intravenously on Days 1–7 of each 28-day cycle.
Results
Median follow-up was 16.3 months (range, 1.0–20.3). Median overall survival was not reached with venetoclax-azacitidine (hazard ratio 0.409 and 95% confidence interval: 0.151, 1.109); overall survival estimate was higher with venetoclax-azacitidine than placebo-azacitidine at 12 (67 and 46%) and 18 months (57 and 31%), respectively. CR and CRi rates were 67% with venetoclax-azacitidine and 15% with placebo-azacitidine. Most common any-grade adverse events were febrile neutropenia (79 and 39%), thrombocytopenia (54 and 77%), constipation (54 and 54%) and decreased appetite (54 and 38%) in the venetoclax-azacitidine and placebo-azacitidine arms, respectively. Only 1 patient in the venetoclax-azacitidine arm, and no patients in the placebo-azacitidine arm, had grade 4 febrile neutropenia that led to treatment discontinuation.
Conclusions
This Japanese subgroup analysis of VIALE-A demonstrates comparable safety and efficacy outcomes compared with the global study and supports venetoclax-azacitidine as first-line standard-of-care for Japanese treatment-naive patients with acute myeloid leukemia who are ineligible for intensive chemotherapy.
Keywords: acute myeloid leukemia, venetoclax, azacitidine, VIALE-A, Japan
Venetoclax-azacitidine was well tolerated in the Japanese subgroup of VIALE-A (N = 37) and showed improved efficacy versus placebo-azacitidine in patients with acute myeloid leukemia ineligible for intensive chemotherapy, supporting use as first-line standard-of-care.
Introduction
Acute myeloid leukemia (AML) is the most common type of adult leukemia, with the highest incidence worldwide found in the USA, Western Europe and Australia (1). In Japan, AML is the most common leukemia, accounting for ~70% of myeloid leukemias (2,3). AML primarily affects older adults, with a third of patients in Japan being diagnosed at ≥75 years of age (4). The 5-year survival rate was 39.2% among Japanese patients with leukemias diagnosed between 2006 and 2008 (5). Current treatment for younger adults in Japan consists of intensive induction chemotherapy with idarubicin or daunorubicin plus cytarabine (2,6). In addition, patients achieving remission are advised to continue with consolidation therapy consisting of high-dose cytarabine or non–cross-resistant agents, and allogeneic stem cell transplant for eligible patients. However, many patients with newly diagnosed AML are not eligible for intensive chemotherapy because of advanced age or the presence of comorbidities (6–8). Treatment options for these patients are limited and include low-intensity therapy with the hypomethylating agents (HMAs) azacitidine or decitabine, and low-dose cytarabine [LDAC; (9,10)].
In Japan, for patients ≥65 years of age who are ineligible for standard therapy (idarubicin or daunorubicin plus cytarabine) on the basis of their performance status, comorbidities or cytogenetic abnormalities, treatment with LDAC is recommended (6). However, outcomes with less intensive regimens such as decitabine, azacitidine or LDAC remain poor, with expected rates of complete response (CR) or CR with incomplete blood count recovery (CRi) lower than 30%, and median overall survival (OS) of <1 year (11–13). More effective and well-tolerated treatment options are needed for patients with AML ineligible for intensive chemotherapy.
Venetoclax is a selective inhibitor of B-cell leukemia/lymphoma-2 (BCL2) that has been studied in several hematologic malignancies as monotherapy or in combination with other agents (14–21). Two large phase 1b/2 studies have assessed venetoclax-based therapy in combination with low-intensity regimens in older patients with previously untreated AML (21,22). When combined with azacitidine or decitabine, venetoclax therapy resulted in a CR + CRi rate of 67% and a median OS of 17.5 months (22). A subsequent confirmatory phase 3 placebo-controlled trial (VIALE-A; NCT02993523) compared the efficacy and safety of venetoclax plus azacitidine (venetoclax-azacitidine) with placebo plus azacitidine (placebo-azacitidine) in treatment-naive patients with AML ineligible for standard induction therapy (23). The venetoclax-azacitidine combination regimen significantly increased OS [14.7 and 9.6 months, respectively; hazard ratio (HR), 0.66; 95% confidence interval (CI): 0.52, 0.85 and P < 0.001] and the CR + CRi rate (66 and 28%; P < 0.001) compared with the placebo-azacitidine regimen (23).
The biologic rationale for combining venetoclax with azacitidine in AML is based on their complementary mechanisms of action. The BCL2 family members, including BCL2, BCL-XL and MCL1, mediate cancer cell survival by sequestering proapoptotic proteins. BCL2 is upregulated in AML, promotes chemoresistance, enhances the survival of leukemic progenitor and blast cells and has been associated with poor outcomes (24,25). In preclinical studies, venetoclax had additive or synergistic effects when combined with azacitidine in primary cells from patients with AML (26,27), where azacitidine was shown to downregulate MCL1 protein levels (27).
Venetoclax has been approved in the USA (28), and in >25 other countries for use in combination with azacitidine, decitabine or LDAC in patients with newly diagnosed AML who are ≥75 years of age, or those with comorbidities that preclude the use of intensive induction chemotherapy. Japan has the most aged population worldwide (29), and elderly patients with AML have limited treatment options. In March 2021, venetoclax in combination with azacitidine or LDAC was approved in Japan. The Japanese subgroup analyses aimed to provide evidence that safety and efficacy outcomes in the Japanese subgroup were comparable to the global population. Here, we present the results of the subgroup analysis of Japanese patients with AML ineligible for intensive chemotherapy who participated in the VIALE-A trial comparing venetoclax-azacitidine with placebo-azacitidine.
Materials and methods
Study design
VIALE-A (NCT02993523) is a phase 3, randomized, double-blind, placebo-controlled and multicenter study that assessed the efficacy and safety of venetoclax-azacitidine compared with placebo-azacitidine in patients with previously untreated AML who were ineligible for intensive chemotherapy (23). The dual primary endpoints for Japan were OS and CR + CRi rates. Key secondary endpoints included CR + CR with partial hematologic recovery (CRh) rates, response rates at the start of Cycle 2 and transfusion-independence rates. The study was conducted in accordance with the International Council for Harmonization requirements, Good Clinical Practice guidelines and the Declaration of Helsinki. The study protocol was reviewed and approved by an independent ethics committee/institutional review board at each study site. All patients provided written informed consent.
Patients
Full eligibility criteria have been reported previously (23). In brief, the study enrolled treatment-naive adult (≥18 years of age) patients with a confirmed diagnosis of AML [per the World Health Organization classification (30)], who were ineligible for standard induction chemotherapy because of age (≥75 years) or comorbidities. The existence of at least one of the following conditions precluded the use of intensive induction chemotherapy: Eastern Cooperative Oncology Group (ECOG) performance status 2 or 3, history of congestive heart failure requiring treatment, an ejection fraction ≤50%, chronic stable angina, diffusion capacity of the lung for carbon monoxide ≤65%, forced expiratory volume in 1 second (s) of ≤65%, creatinine clearance 0.5 ml/s to <0.75 ml/s, moderate hepatic impairment with total bilirubin >1.5 to ≤3.0 times the upper limit of normal or other comorbidities considered incompatible with standard therapy. Patients needed to have a projected life expectancy of ≥12 weeks to be included in the trial, and those who had received previous treatment for myelodysplastic syndromes or AML were excluded.
Randomization and treatment
Eligible patients were randomized 2:1 via interactive response technology to either venetoclax-azacitidine or placebo-azacitidine. Randomization was stratified by patient age (<75 and ≥75 years), region (USA, European Union, China, Japan and rest of world) and cytogenetic risk (intermediate and poor).
Venetoclax was given orally once daily. Patients were hospitalized for monitoring and tumor lysis syndrome (TLS) prophylaxis for the first 4 days of treatment in Cycle 1, until 24 h after the target dose of venetoclax was reached. This included uric acid-reducing agents, intravenous hydration and laboratory assessments. To mitigate the risk of TLS, venetoclax dosing started at 100 mg on Day 1 of Cycle 1, increasing to 200 mg on Day 2 and reaching the target dose of 400 mg on Day 3. The 400mg daily dosing was maintained until Day 28 of Cycle 1 and on Days 1–28 in all subsequent cycles. Patients in the control arm received oral placebo according to the same schedule. Azacitidine was administered to patients in both study arms at a dose of 75 mg/m2 subcutaneously or intravenously for the first 7 days of each 28-day cycle. Treatment was continued until disease progression, withdrawal of consent or other protocol-defined criteria for discontinuation were met.
Assessments
OS was defined as the time from study randomization to death due to any cause. Bone marrow assessments were performed at screening, at the end of Cycle 1 and every 3 cycles thereafter until 2 successive samples indicated CR or composite CR (CR or CR + CRi). Details on the disease evaluation criteria have been reported previously in the primary publication of this study (23). In brief, responses were assessed according to the modified International Working Group criteria for AML (31) and disease progression was defined per European LeukemiaNet criteria (32). Transfusion independence was defined as at least 56 consecutive days with no transfusion of either red blood cells (RBCs) or platelets between the first and last day of treatment.
Safety evaluations were performed throughout the study in all patients who received at least 1 dose of either venetoclax or placebo (in combination with azacitidine). Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.03. AEs were defined as those occurring between the first dose of study drug until 30 days after discontinuation of treatment.
Statistical analyses
Baseline characteristics and treatment outcomes are descriptive for this subgroup analysis.
The data cutoff for the Japanese subgroup analysis presented here was 4 January 2020, the same cutoff used for the primary analysis of VIALE-A. The efficacy analyses were performed on the full analysis set, which included the intent-to-treat population who underwent randomization. Safety analyses were performed in all patients who received at least 1 dose of study drug (venetoclax or placebo, in combination with azacitidine).
Power and sample size determinations for the global phase 3 study have been reported previously (23). OS distribution was estimated using the Kaplan–Meier methodology, and comparisons between treatment arms used the log-rank test stratified by age and cytogenetic risk. The HR between the treatment groups was estimated with the Cox proportional hazards model with the same stratification factors. Response rates and transfusion independence outcomes were compared between treatment arms using the Cochran–Mantel–Haenszel test, with age and cytogenetic risk as stratification factors. The 95% CIs were determined with the Clopper–Pearson exact method.
Results
Patients
Between 6 February 2017 and 31 May 2019, of the 48 patients screened in Japan from 15 sites, 37 (77%) were randomized to the study arms, including 24 patients assigned to venetoclax-azacitidine and 13 to placebo-azacitidine. A total of 19 patients (10 venetoclax-azacitidine and 9 placebo-azacitidine) discontinued study, all because of death. Death was related to disease progression in 84.2% (16/19) of cases.
Patient demographics and baseline characteristics of the Japanese subgroup are summarized in Table 1. The proportion of patients ≥75 years of age was higher in the venetoclax-azacitidine arm than in the placebo-azacitidine arm (79 and 69%, respectively). The venetoclax-azacitidine arm also had a higher proportion of patients with ECOG performance status 0 or 1 (75 and 62%) and intermediate-risk cytogenetics (75 and 69%). On the other hand, more patients in the placebo-azacitidine arm had ≥50% blasts in bone marrow (54 and 42%, respectively) and baseline grade 3 or 4 neutropenia (92 and 79%). The main reason for ineligibility to receive intensive chemotherapy was advanced age (≥75 years) in both treatment arms (79% with venetoclax-azacitidine and 69% with placebo-azacitidine). In general, no significant differences in baseline characteristics were observed between the venetoclax-azacitidine and placebo-azacitidine arms.
Table 1.
Patient demographics and baseline characteristics
| Characteristic | Venetoclax-azacitidine (n = 24) | Placebo-azacitidine (n = 13) |
|---|---|---|
| Age | ||
| Median, years (range) | 77.5 (68–85) | 77.0 (67–86) |
| ≥ 75 years, n (%) | 19 (79.2) | 9 (69.2) |
| Male, n (%) | 14 (58.3) | 9 (69.2) |
| AML type, n (%) | ||
| De novo | 20 (83.3) | 10 (76.9) |
| Secondary | 4 (16.7) | 3 (23.1) |
| Secondary AML type, n/N (%) | ||
| Prior MDS or CMML | 3/4 (75.0) | 3/3 (100) |
| Treatment-related AML | 1/4 (25.0) | 0/3 |
| ECOG performance status, n (%) | ||
| 0 or 1 | 18 (75.0) | 8 (61.5) |
| 2 or 3 | 6 (25.0) | 5 (38.5) |
| Blast count, n (%) | ||
| < 30% | 7 (29.2) | 4 (30.8) |
| ≥ 30% to <50% | 7 (29.2) | 2 (15.4) |
| ≥ 50% | 10 (41.7) | 7 (53.8) |
| AML with myelodysplasia-related changes, n (%) | 9 (37.5) | 5 (38.5) |
| Cytogenetic risk, n (%) | ||
| Intermediate | 18 (75.0) | 9 (69.2) |
| Poor | 6 (25.0) | 4 (30.8) |
| Somatic mutations, n/N (%) | ||
| IDH1 or IDH2 | 6/21 (28.6) | 4/13 (30.8) |
| FLT3 | 2/23 (8.7) | 2/12 (16.7) |
| NPM1 | 0/14 | 6/9 (66.7) |
| TP53 | 2/14 (14.3) | 0/9 |
| Baseline Grade 3 or 4 cytopenias, n (%) | ||
| Neutropenia | 19 (79.2) | 12 (92.3) |
| Grade 3 | 3 (12.5) | 3 (23.1) |
| Grade 4 | 16 (66.7) | 9 (69.2) |
| Anemia | 8 (33.3) | 5 (38.5) |
| Thrombocytopenia | 7 (29.2) | 4 (30.8) |
| Baseline transfusion dependence,an (%) | ||
| RBCs | 2 (8.3) | 2 (15.4) |
| Platelets | 0 | 1 (7.7) |
| ≥2 reasons for ineligibility to receive intensive therapy,bn (%) | 8 (33.3) | 7 (53.8) |
aBaseline transfusion dependence defined as RBC or platelet transfusion within 8 weeks prior to the first dose of study drug or randomization.
bPatients could report more than 1 reason.
Abbreviations: AML, acute myeloid leukemia; CMML, chronic myelomonocytic leukemia; ECOG, Eastern Cooperative Oncology Group; FLT3, fms-like tyrosine kinase 3; IDH, isocitrate dehydrogenase; MDS, myelodysplastic syndrome; NPM1, nucleophosmin; RBC, red blood cell; TP53, tumor protein 53.
Efficacy
At a median follow-up of 16.3 months (range, 1.0–20.3) median OS was not reached (NR) in the venetoclax-azacitidine arm (95% CI: 10.6, NR) and was 8.6 months (95% CI: 2.7, NR) in the placebo-azacitidine arm, with a stratified Cox proportional HR of 0.41 (95% CI: 0.15, 1.11; Fig. 1). The estimated OS was 67% (95% CI: 44.3, 81.7) with venetoclax-azacitidine and 46% (95% CI: 19.2, 69.6) with placebo-azacitidine at 12 months, and 57% (95% CI: 33.9, 74.1) and 31% (95% CI: 6.5, 60.2), respectively, at 18 months.
Figure 1.
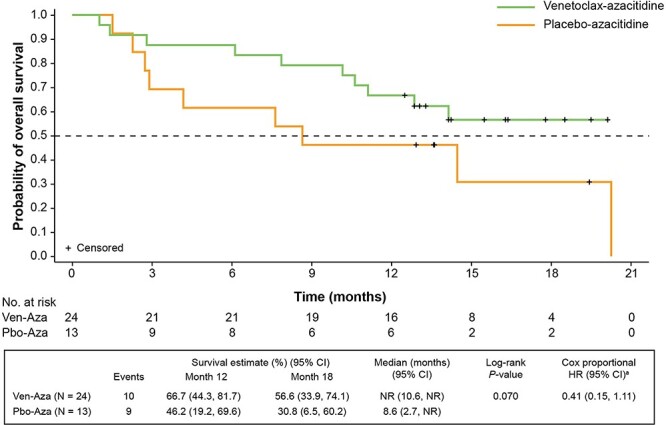
OS by treatment arm. aStratified by age (18 to <75, ≥75 years) and cytogenetic risk (intermediate, poor). Aza, azacitidine; CI, confidence interval; HR, hazard ratio; NR, not reached; OS, overall survival; Pbo, placebo; Ven, venetoclax.
Response rates and event-free survival (EFS) are shown in Fig. 2. Rates of CR and CR + CRi were higher in the venetoclax-azacitidine arm than in the placebo-azacitidine arm, with 67% of patients assigned to venetoclax-azacitidine and 15% of patients assigned to placebo-azacitidine achieving CR + CRi (Fig. 2a). The median time to first response (CR or CRi) was 1.2 months (range, 0.8–2.9) for venetoclax-azacitidine and 3.1 months (range, 3.0–3.2) for placebo-azacitidine. Half of the patients in the venetoclax-azacitidine arm achieved CR + CRi by the start of Cycle 2 compared with no patients in the placebo-azacitidine arm. The addition of venetoclax to azacitidine also resulted in a significant improvement in EFS [16.3 months (95% CI: 7.9, NR)] compared with 3.4 months (95% CI: 1.5, 14.5) with placebo-azacitidine (HR, 0.229; 95% CI: 0.088, 0.596 and P = 0.001; Fig. 2b).
Figure 2.
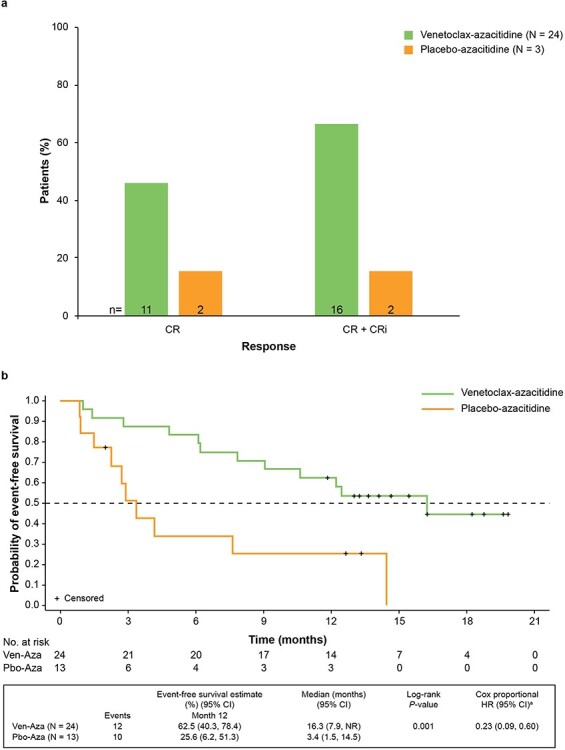
Rates of CR and CR+CRi (a), and investigator-assessed event-free survival (b). aStratified by age (18 to <75, ≥75 years) and cytogenetic risk (intermediate, poor). Aza, azacitidine; CI confidence interval; CR, complete response; CRi, CR with incomplete blood count recovery; HR, hazard ratio; Pbo, placebo; Ven, venetoclax.
Sixteen patients (67%; 95% CI: 45, 84) receiving venetoclax-azacitidine and 2 patients (15%; 95% CI: 2, 45) receiving placebo-azacitidine achieved post-baseline RBC and platelet transfusion independence while on treatment (Fig. 3). The median duration of RBC and platelet transfusion independence was 122 days (range, 57–557) for venetoclax-azacitidine, and 328 days (range, 312–344) for placebo-azacitidine. RBC transfusion independence was achieved in 75% of patients in the venetoclax-azacitidine arm (95% CI: 53.3, 90.2) and 23% in the placebo-azacitidine arm (95% CI: 5.0, 53.8). Platelet transfusion independence was achieved in 79% of patients in the venetoclax-azacitidine arm (95% CI: 57.8, 92.9) and 31% in the placebo-azacitidine arm (95% CI: 9.1, 61.4). The median number of post-baseline RBC transfusions was 11 (range, 2–47) and 8 (range, 2–45) in the venetoclax-azacitidine and placebo-azacitidine arms, respectively, with a median number of post-baseline platelet transfusions of 11 (range, 1–69) and 15 (range, 1–66) for each arm, respectively. One of 2 patients who were RBC transfusion dependent at baseline became transfusion independent during treatment with venetoclax-azacitidine, whereas neither of the 2 transfusion-dependent patients assigned to placebo-azacitidine achieved transfusion independence. Only 1 patient was dependent on platelet transfusions at baseline and remained transfusion dependent during treatment with placebo-azacitidine.
Figure 3.
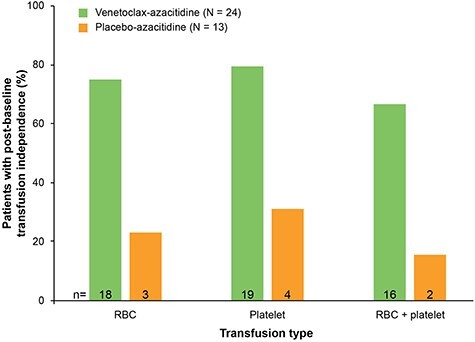
Proportion of patients with post-baseline transfusion independence. Transfusion independence was defined as a period of 56 days or more without transfusion. RBC, red blood cell.
Safety
Median duration of exposure to venetoclax/placebo was longer in the venetoclax-azacitidine arm than in the placebo-azacitidine arm [12.3 months (range, 0.9–20.0) and 1.8 months (range, 0.5–13.9)], respectively. Patients received a median of 8 treatment cycles (range, 1–19) with venetoclax-azacitidine and 2 treatment cycles (range, 1–15) with placebo-azacitidine. The most frequent reason for discontinuation of study drug within the placebo-azacitidine arm was disease progression.
All patients reported at least 1 AE of any grade. Table 2 summarizes AEs reported in ≥20% of patients in either treatment arm. The most common Grade ≥3 AEs (venetoclax-azacitidine and placebo-azacitidine, respectively) were mainly hematologic, including febrile neutropenia (79 and 39%), thrombocytopenia (50 and 77%), neutropenia (38 and 23%), leukopenia (33 and 31%) and anemia (21 and 15%; Table 2). Grade ≥3 pneumonia was observed in 21% of patients in the venetoclax-azacitidine arm and 15% of patients in the placebo-azacitidine arm. The most common nonhematologic AEs (any grade; venetoclax-azacitidine and placebo-azacitidine, respectively) included decreased appetite (54 and 31%), constipation (54 and 54%), diarrhea (46 and 46%), vomiting (42 and 23%) and nausea (38 and 31%; Table 2).
Table 2.
Adverse events reported in ≥20% of patients in either arm
| Venetoclax-azacitidine (n = 24) | Placebo-azacitidine (n = 13) | |||
|---|---|---|---|---|
| AE, n (%) | Any grade | Grade ≥3 | Any grade | Grade ≥3 |
| Any | 24 (100) | 24 (100) | 13 (100) | 12 (92.3) |
| Hematologic events | 23 (95.8) | 23 (95.8) | 11 (84.6) | 11 (84.6) |
| Thrombocytopenia | 13 (54.2) | 12 (50.0) | 10 (76.9) | 10 (76.9) |
| Neutropenia | 9 (37.5) | 9 (37.5) | 3 (23.1) | 3 (23.1) |
| Febrile neutropenia | 19 (79.2) | 19 (79.2) | 5 (38.5) | 5 (38.5) |
| Grade 3 | 18 (75.0) | 5 (38.5) | ||
| Grade 4 | 1 (4.2) | 0 | ||
| Anemia | 5 (20.8) | 5 (20.8) | 3 (23.1) | 2 (15.4) |
| Leukopenia | 8 (33.3) | 8 (33.3) | 4 (30.8) | 4 (30.8) |
| Disseminated intravascular coagulation | 1 (4.2) | 1 (4.2) | 3 (23.1) | 0 |
| Nonhematologic events | ||||
| Pneumonia | 6 (25.0) | 5 (20.8) | 2 (15.4) | 2 (15.4) |
| Nausea | 9 (37.5) | 1 (4.2) | 4 (30.8) | 0 |
| Constipation | 13 (54.2) | 0 | 7 (53.8) | 0 |
| Diarrhea | 11 (45.8) | 1 (4.2) | 6 (46.2) | 1 (7.7) |
| Vomiting | 10 (41.7) | 0 | 3 (23.1) | 0 |
| Stomatitis | 10 (41.7) | 0 | 2 (15.4) | 0 |
| Decreased weight | 6 (25.0) | 1 (4.2) | 1 (7.7) | 0 |
| Increased alanine aminotransferase | 5 (20.8) | 2 (8.3) | 1 (7.7) | 1 (7.7) |
| Hypokalemia | 6 (25.0) | 4 (16.7) | 4 (30.8) | 3 (23.1) |
| Pyrexia | 7 (29.2) | 0 | 4 (30.8) | 0 |
| Fatigue | 1 (4.2) | 0 | 1 (7.7) | 1 (7.7) |
| Decreased appetite | 13 (54.2) | 1 (4.2) | 4 (30.8) | 1 (7.7) |
| Insomnia | 6 (25.0) | 0 | 1 (7.7) | 0 |
| Malaise | 8 (33.3) | 0 | 1 (7.7) | 0 |
Abbreviation: AE, adverse event.
Serious AEs were reported in 67% of patients treated with venetoclax-azacitidine and 31% of patients treated with placebo-azacitidine; those occurring in ≥5% of patients in the venetoclax-azacitidine arm are summarized in Table 3. Serious AEs (venetoclax-azacitidine and placebo-azacitidine, respectively) included febrile neutropenia (42 and 0%), neutropenia (17 and 0%), pneumonia (13 and 8%) and leukopenia (8 and 0%). All patients were hospitalized to receive TLS prophylaxis and for monitoring, per protocol; no cases of TLS were reported in either treatment arm. To prevent the occurrence of clinically relevant infections, 79% of patients in the venetoclax-azacitidine arm and 77% of patients in the placebo-azacitidine arm received anti-infection prophylaxis.
Table 3.
Serious adverse events (any grade) occurring in ≥5% of patients
| Serious AE, n (%) | Venetoclax-azacitidine (n = 24) | Placebo-azacitidine (n = 13) |
|---|---|---|
| Any | 16 (66.7) | 4 (30.8) |
| Febrile neutropenia | 10 (41.7) | 0 |
| Neutropenia | 4 (16.7) | 0 |
| Leukopenia | 2 (8.3) | 0 |
| Pneumonia | 3 (12.5) | 1 (7.7) |
Abbreviation:AE, adverse event.
AEs led to treatment discontinuation in 4 patients: 2 (8%) patients in the venetoclax-azacitidine arm (1 patient with Grade 4 febrile neutropenia and 1 with celiac artery occlusion leading to death), and 2 (15%) patients in the placebo-azacitidine arm [1 patient with Grade 3 fatigue and Grade 4 neutropenia and 1 patient with Grade 4 acute kidney injury (AKI)]. AEs led to venetoclax/placebo dose interruptions in 16 (67%) patients receiving venetoclax-azacitidine most commonly due to febrile neutropenia (n = 9), neutropenia (n = 6) and infection (n = 6), and in 4 (31%) patients treated with placebo-azacitidine due to febrile neutropenia and thrombocytopenia (n = 1), neutropenic enterocolitis and heart failure (n = 1), AKI (n = 1) and pneumonia (n = 1).
Death within 30 days of starting study treatment occurred in 1 (4%) patient treated with venetoclax-azacitidine (due to celiac artery occlusion) and in no patients treated with placebo-azacitidine. There were no deaths due to febrile neutropenia in either arm of the study.
Discussion
This analysis in the Japanese subgroup of the VIALE-A trial in patients with untreated AML ineligible for intensive chemotherapy demonstrated similar efficacy and safety outcomes as were seen in the overall population, despite the shorter median follow-up period for the Japanese subgroup analysis [16.3 vs 20.5 months (23)]. Importantly, no AEs specific to Japanese patients were observed.
Specifically, the median OS in the Japanese subgroup (NR with venetoclax-azacitidine and 8.6 months with placebo-azacitidine) was consistent with that in the overall population, where the median OS was 14.7 and 9.6 months, respectively. The composite CR + CRi rates for the Japanese subgroup (67 and 15%, respectively), were also consistent with the response outcomes in the overall population (66 and 28%, respectively; P < 0.001), which included patients from the USA, Europe, China, Japan and the rest of the world.
Also, the higher rate of response for patients in the venetoclax-azacitidine arm in the Japanese subgroup analysis, compared with those in the placebo-azacitidine arm, correlated with a greater incidence of post-baseline transfusion independence (RBC + platelet: 67 and 15%, respectively). Responses were also reached earlier with venetoclax-azacitidine compared with placebo-azacitidine (median time to first response 1.2 and 3.1 months, respectively), with half of the patients in the venetoclax-azacitidine arm achieving a response by the start of Cycle 2.
The difference in median EFS between treatment groups was more marked in the Japanese subgroup (16.3 months with venetoclax-azacitidine and 3.4 months with placebo-azacitidine) than in the overall population (9.8 and 7.0 months, respectively). Therefore, these results support that the addition of venetoclax to HMA therapy improves the currently dismal outcomes achieved in elderly Japanese patients with AML treated with HMAs alone (11–13).
The median duration of exposure to venetoclax treatment was longer in the Japanese subgroup (12.3 months) compared with the overall population (7.6 months). Japanese patients randomized to placebo-azacitidine however, received a median of 2 treatment cycles (median duration of exposure 1.8 months), compared with a median of 4.5 treatment cycles for the overall population. Due to the double-blind design of this trial, early treatment discontinuation was at investigator discretion on the basis of lack of response, and a Japanese-specific signature cannot be confirmed.
However, the safety profile of venetoclax-azacitidine in the Japanese subgroup was consistent with safety data from the overall study population and with previous reports on the use of venetoclax in AML (22), with the most common AEs associated with venetoclax-azacitidine being hematologic or gastrointestinal. The incidence of grade 3 or 4 febrile neutropenia was higher in Japanese patients across treatment groups (79% with venetoclax-azacitidine and 39% with placebo-azacitidine) compared with the overall population (42% with venetoclax-azacitidine and 19% with placebo-azacitidine). Notably, rates of infection and treatment discontinuation or death due to febrile neutropenia were similar in the Japanese and overall populations (23). These results suggest that febrile neutropenia was manageable with appropriate monitoring and intervention, including with venetoclax dose interruptions and the use of granulocyte-colony stimulating factor (G-CSF) per investigator discretion (28).
Some key differences in baseline characteristics were noted between the overall population and the Japanese subgroup, including a preponderance of patients ≥75 years of age (Japanese subgroup and overall population, 76 and 61%, respectively), with ECOG performance status of 0 or 1 (70 and 55%), with intermediate-risk cytogenetics (73 and 63%), and with baseline Grade 3 or 4 neutropenia (84 and 69%).
Venetoclax pharmacokinetics in patients with AML have been described using a population pharmacokinetics model (33). Although Asian patients had 67% higher relative bioavailability of venetoclax compared with non-Asian patients, the range of venetoclax exposures (area under the plasma concentration-time curve at steady state) was similar in the two groups. Covariate analysis to evaluate the relationship between venetoclax exposure and safety in patients with previously untreated AML identified that Asian patients were more likely to have treatment-emergent Grade ≥3 neutropenia regardless of treatment with placebo or venetoclax in combination with an HMA. However, the predicted net effect of venetoclax on treatment-emergent Grade ≥3 neutropenia was similar both for Asian and non-Asian patients (33).
Importantly, the safety and efficacy results with venetoclax-azacitidine described here were also generally similar to those reported in a phase 1/2 trial in Japan that enrolled 6 elderly (≥75 years) patients with untreated relapsed/refractory AML [NCT02265731; (34)]. With a median exposure time to venetoclax of 12.3 months in this study and 10.3 months in the phase 1/2 study, the majority of patients in both studies experienced at least 1 Grade ≥3 treatment-emergent AE (phase 3, 100%; phase 1/2, 83%), of which the most common were hematologic (phase 3: febrile neutropenia [79%], thrombocytopenia [50%], neutropenia [38%]; phase 1/2: lymphopenia [67%], febrile neutropenia [67%], thrombocytopenia [50%] and leukopenia [50%]). Equal proportions of patients in both studies experienced serious AEs (67%). Of note, the CR rates in Japanese patients in the VIALE-A study are higher than the reported rates with LDAC, the current standard of care in Japan for elderly patients with poor-risk cytogenetics (6,8).
The small number of patients analyzed (n = 37) in this investigation of a geographic population is a limitation of this study. Nevertheless, the data indicate that venetoclax-azacitidine was effective with an expected (similar to that of the overall population) and manageable safety profile in the Japanese subgroup of VIALE-A.
The combination of venetoclax and azacitidine could be considered a first-line standard of care for Japanese patients with previously untreated AML who are not candidates for intensive induction therapy.
Acknowledgments
AbbVie and the authors thank all the trial investigators and the patients who participated in this clinical trial. Medical writing support was provided by Rebecca Crepeau, PhD from Aptitude Health, Atlanta, GA, USA, and funded by AbbVie.
Contributor Information
Kazuhito Yamamoto, Department of Hematology and Cell Therapy, Aichi Cancer Center, Nagoya, Aichi, Japan.
Atsushi Shinagawa, Department of Internal Medicine, Hitachi General Hospital, Hitachi, Ibaraki, Japan.
Courtney D DiNardo, Department of Leukemia, Division of Cancer Medicine, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Keith W Pratz, Leukemia Program, Division of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Kenichi Ishizawa, Department of Third Internal Medicine, Yamagata University Hospital, Yamagata, Japan.
Toshihiro Miyamoto, Department of Medicine and Biosystemic Science, Kyushu University Graduate School of Medical Sciences, Fukuoka, Japan.
Norio Komatsu, Department of Hematology, Juntendo University School of Medicine, Tokyo, Japan.
Yasuhiro Nakashima, Department of Hematology, Graduate School of Medicine, Osaka City University, Osaka, Japan.
Chikashi Yoshida, Department of Hematology, National Hospital Organization, Mito Medical Center, Ibaraki, Japan.
Noriko Fukuhara, Department of Hematology, Tohoku University Graduate School of Medicine, Sendai, Japan.
Kensuke Usuki, Department of Hematology, NTT Medical Center Tokyo, Tokyo, Japan.
Takahiro Yamauchi, Department of Hematology and Oncology, University of Fukui Hospital, Fukui, Japan.
Noboru Asada, Department of Hematology and Oncology, Okayama University Hospital, Okayama, Japan.
Norio Asou, Department of Hematology, Saitama Medical University International Medical Center, Hidaka, Saitama, Japan.
Ilseung Choi, Department of Hematology, National Hospital Organization, Kyushu Cancer Center, Fukuoka, Japan.
Yasushi Miyazaki, Department of Hematology, Atomic Bomb Disease Institute, Nagasaki University, Nagasaki, Japan.
Hideyuki Honda, AbbVie GK, Tokyo, Japan.
Sumiko Okubo, AbbVie GK, Osaka, Japan.
Misaki Kurokawa, AbbVie GK, Tokyo, Japan.
Ying Zhou, AbbVie, Inc., North Chicago, IL, USA.
Jiuhong Zha, AbbVie, Inc., North Chicago, IL, USA.
Jalaja Potluri, AbbVie, Inc., North Chicago, IL, USA.
Itaru Matsumura, Department of Hematology and Rheumatology, Kindai University Hospital, Osaka, Japan.
Funding
Venetoclax is being developed in a collaboration between AbbVie and Genentech. AbbVie and Genentech funded this study (NCT02993523) and participated in the study design, research, analysis, data collection, interpretation of data, reviewing and approval of the publication. All authors had access to relevant data and participated in the drafting, review and approval of this publication. No honoraria or payments were made for authorship.
Conflict of interest statements
Kazuhito Yamamoto has received honoraria from Chugai, Eisai, Mundipharma and Takeda, and has received research funding from AbbVie, Celgene, Chugai, Eisai, Incyte/IQVIA, SymBio and Zenyaku. Atsushi Shinagawa and Ilseung Choi declare that they have no conflicts of interest. Courtney D. DiNardo has received institutional research support from AbbVie, Agios, Bayer, Calithera, Celgene, Bristol-Myers Squibb, Cleave and Daiichi Sankyo, and has served as a consultant and on advisory boards for AbbVie, Agios, Celgene, Bristol-Myers Squibb, Daiichi Sankyo, Immune-Onc, Novartis, Takeda and Notable Labs. Keith W. Pratz has served in a consulting/advisory role for AbbVie, Astellas Pharma, Boston BioMedical, Bristol-Myers Squibb, Celgene and Jazz Pharmaceuticals, and has received research funding from AbbVie, Agios, Daiichi Sankyo and Millennium. Kenichi Ishizawa has received research funding from Novartis, AbbVie, Bayer and SymBio, and has served on a speakers’ bureau for Celgene, Chugai, Eisai, Novartis, Ono Pharmaceutical and Takeda. Toshihiro Miyamoto has received honoraria from AbbVie, Amgen KK, Astellas Pharma, Bristol-Myers Squibb, Celgene, Merck Sharp & Dohme, Otsuka Pharmaceutical and Takeda. Norio Komatsu has received research funding from Bristol-Myers Squibb KK, Chugai, Fujifilm Wako Pure Chemical Industries, Fuso Pharmaceutical, Kyowa Kirin, Novartis Pharma KK, Otsuka Pharmaceutical, Pfizer Japan, PharmaEssentia Japan KK, Shire Japan KK, Sumitomo Dainippon Pharma and Takeda, and has received advisory fees from AbbVie GK, Celgene KK, Japan Tobacco Inc., Novartis KK, Otsuka Pharmaceutical, PharmaEssentia Japan KK and Shire Japan KK, and has served on speakers’ bureaus for Novartis KK, Shire Japan KK and Takeda, and as a member of safety assessment committee in the M13–834 clinical trial. Yasuhiro Nakashima has received research funding from AbbVie GK, Amgen KK, Astellas Pharma, Celgene and Novartis, and has received honoraria from Amgen KK. Chikashi Yoshida has received honoraria from AbbVie GK, Astellas Pharma, Bristol-Myers Squibb, Daiichi Sankyo, Janssen Pharmaceutical KK, Meiji Seika Pharma, Nippon Shinyaku, Novartis KK and Otsuka Pharmaceutical. Noriko Fukuhara has received research funding from AbbVie, Bayer, Eisai, Gilead Sciences, Ono Pharmaceutical and Solasia Pharma, and has received honoraria from Chugai and Kyowa Kirin. Kensuke Usuki has received research funding from AbbVie, Apellis Pharmaceuticals, Astellas-Amgen-Biopharma, Astellas Pharma, Bristol-Myers Squibb, Celgene, Chugai, Daiichi Sankyo, Gilead, Janssen Pharmaceuticals, Kyowa Kirin, Mundipharma, Nippon Boehringer Ingelheim, Nippon Shinyaku, Novartis Pharma KK, Ono Pharmaceutical, Otsuka Pharmaceutical, Pfizer, Sumitomo Dainippon Pharma, SymBio and Takeda, and has served on a speakers’ bureau for Astellas Pharma, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Eisai, Kyowa Kirin, Merck Sharp & Dohme, Nippon Shinyaku, Novartis Pharma KK, Ono Pharmaceutical, Otsuka Pharmaceutical, PharmaEssentia, SymBio, Takeda and Yakult. Takahiro Yamauchi has received research funding from AbbVie, Astellas Pharma, Boehringer Ingelheim, Chugai, Janssen Pharmaceutical, Nippon Shinyaku, Ono Pharmaceutical, Otsuka Pharmaceutical, Pfizer, Solasia Pharma, Sumitomo Dainippon Pharma and Teijin Pharma. Noboru Asada has received honoraria from AbbVie, Astellas Pharma, Celgene, Chugai, Eisai, Kyowa Kirin, Novartis, Otsuka and Sanofi. Norio Asou has received research funding from AbbVie, Astellas Pharma, Chugai, Eisai and Sumitomo Dainippon Pharma, and has served on a speakers’ bureau for Asahi Kasei, Fuji Pharma, Nippon Shinyaku and Sumitomo Dainippon Pharma, and has received honoraria from Nippon Shinyaku and Novartis. Yasushi Miyazaki has received honoraria from Astellas Pharma, Celgene, Chugai, Kyowa Kirin, Nippon Shinyaku, Novartis, Otsuka Pharmaceutical and Sumitomo Dainippon Pharma, and has received research funding from Chugai and Sumitomo Dainippon Pharma. Hideyuki Honda, Sumiko Okubo, Misaki Kurokawa, Ying Zhou, Jiuhong Zha and Jalaja Potluri are employees of AbbVie and may own stock. Itaru Matsumura has received research funding from Chugai, Eisai, Kyowa Kirin, Shionogi and Sumitomo Dainippon Pharma, and has served on a speakers’ bureau for Astellas Pharma, Bristol-Myers Squibb, Daiichi Sankyo Janssen Pharmaceutical, Novartis, Otsuka Pharmaceutical and Pfizer.
Data sharing and data accessibility
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (e.g. protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
References
- 1. Deschler B, Lübbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer 2006;107:2099–107. [DOI] [PubMed] [Google Scholar]
- 2. Miyawaki S. Clinical studies of acute myeloid leukemia in the Japan Adult Leukemia Study Group. Int J Hematol 2012;96:171–7. [DOI] [PubMed] [Google Scholar]
- 3. Niino M, Matsuda T. Type distribution of myeloid leukemia from cancer incidence in five continents Vol. X. Jpn J Clin Oncol 2016;46:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cancer Information Service . Cancer Statistics in Japan; Table Download. 2. Incidence (National Estimates). https://ganjoho.jp/en/professional/statistics/table_download.html (4 August 2021, date last accessed).
- 5. Cancer Information Service . Cancer Statistics in Japan; Table Download. 5. Survival. https://ganjoho.jp/en/professional/statistics/table_download.html (4 August 2021, date last accessed).
- 6. Kiyoi H, Yamaguchi H, Maeda Y, Yamauchi T. JSH practical guidelines for hematological malignancies, 2018: I. Leukemia–1. Acute myeloid leukemia (AML). Int J Hematol 2020;111:595–613. [DOI] [PubMed] [Google Scholar]
- 7. Kantarjian H, Ravandi F, O’Brien S, et al. . Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood 2010;116:4422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tasaki T, Yamauchi T, Matsuda Y, et al. . The response to induction therapy is crucial for the treatment outcomes of elderly patients with acute myeloid leukemia: single-institution experience. Anticancer Res 2014;34:5631–6. [PubMed] [Google Scholar]
- 9. Heuser M, Ofran Y, Boissel N, et al. . Acute myeloid leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:697–712. [DOI] [PubMed] [Google Scholar]
- 10. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Acute Myeloid Leukemia. Version 3.2021. 2020. https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf (4 August 2021, date last accessed).
- 11. He PF, Zhou JD, Yao DM, et al. . Efficacy and safety of decitabine in treatment of elderly patients with acute myeloid leukemia: a systematic review and meta-analysis. Oncotarget 2017;8:41498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dombret H, Seymour JF, Butrym A, et al. . International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015;126:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kantarjian HM, Thomas XG, Dmoszynska A, et al. . Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012;30:2670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Konopleva M, Pollyea DA, Potluri J, et al. . Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov 2016;6:1106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fischer K, Al-Sawaf O, Bahlo J, et al. . Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med 2019;380:2225–36. [DOI] [PubMed] [Google Scholar]
- 16. Kumar S, Kaufman JL, Gasparetto C, et al. . Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 2017;130:2401–9. [DOI] [PubMed] [Google Scholar]
- 17. Moreau P, Chanan-Khan A, Roberts AW, et al. . Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood 2017;130:2392–400. [DOI] [PubMed] [Google Scholar]
- 18. Roberts AW, Davids MS, Pagel JM, et al. . Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 2016;374:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seymour JF, Kipps TJ, Eichhorst B, et al. . Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 2018;378:1107–20. [DOI] [PubMed] [Google Scholar]
- 20. Stilgenbauer S, Eichhorst B, Schetelig J, et al. . Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol 2016;17:768–78. [DOI] [PubMed] [Google Scholar]
- 21. Wei AH, Strickland SA Jr, Hou JZ, et al. . Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol 2019;37:1277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DiNardo CD, Pratz K, Pullarkat V, et al. . Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019;133:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DiNardo CD, Jonas BA, Pullarkat V, et al. . Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 2020;383:617–29. [DOI] [PubMed] [Google Scholar]
- 24. Del Poeta G, Venditti A, Del Principe MI, et al. . Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood 2003;101:2125–31. [DOI] [PubMed] [Google Scholar]
- 25. Lagadinou ED, Sach A, Callahan K, et al. . BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 2013;12:329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bogenberger JM, Delman D, Hansen N, et al. . Ex vivo activity of BCL-2 family inhibitors ABT-199 and ABT-737 combined with 5-azacytidine in myeloid malignancies. Leuk Lymphoma 2015;56:226–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsao T, Shi Y, Kornblau S, et al. . Concomitant inhibition of DNA methyltransferase and BCL-2 protein function synergistically induce mitochondrial apoptosis in acute myelogenous leukemia cells. Ann Hematol 2012;91:1861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. VENCLEXTA® (venetoclax tablets) . [prescribing information]. North Chicago, IL: AbbVie Inc.; 2020.
- 29. United Nations . Department of Economic and Social Affairs. World Population Ageing. 2017. https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Highlights.pdf (4 August 2021, date last accessed).
- 30. Arber DA, Orazi A, Hasserjian R, et al. . The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391–405. [DOI] [PubMed] [Google Scholar]
- 31. Cheson BD, Bennett JM, Kopecky KJ, et al. . Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid Leukemia. J Clin Oncol 2003;21:4642–9. [DOI] [PubMed] [Google Scholar]
- 32. Döhner H, Estey E, Grimwade D, et al. . Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eckert D, Brackman D, Menon R, et al. . Venetoclax exposure-efficacy and exposure-safety relationships in subjects with treatment-naïve acute myeloid leukemia who are ineligible for intensive chemotherapy. Blood 2020;136:52. 10.1182/blood-2020-136425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taniguchi S, Yamauchi T, Choi I, et al. . Venetoclax in combination with azacitidine in Japanese patients with acute myeloid leukaemia: phase 1 trial findings. Jpn J Clin Oncol 2021;51:857–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (e.g. protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.


