Abstract
Type 1 diabetes (T1D) is a chronic autoimmune disease characterized by insulin deficiency and resultant hyperglycemia. Complex interactions of genetic and environmental factors trigger the onset of autoimmune mechanisms responsible for development of autoimmunity to β cell antigens and subsequent development of T1D. A potential role of virus infections has long been hypothesized, and growing evidence continues to implicate enteroviruses as the most probable triggering viruses. Recent studies have strengthened the association between enteroviruses and development of autoimmunity in T1D patients, potentially through persistent infections. Enterovirus infections may contribute to different stages of disease development. We review data from both human cohort studies and experimental research exploring the potential roles and molecular mechanisms by which enterovirus infections can impact disease outcome.
Keywords: type 1 diabetes, enterovirus, islet autoimmunity, persistent infection
INTRODUCTION
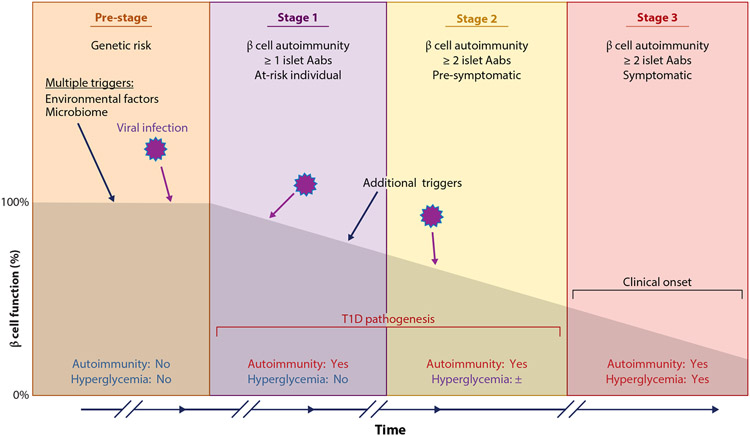
Type 1 diabetes (T1D) emerges after pancreatic islet β cells sustain prolonged autoimmune attack, leading to the loss of the majority of the β cell population. This results in a gradual loss of insulin production and disease onset (Figure 1). While genetic susceptibility has been well established in T1D, studies with monozygotic twins show only a 30–50% concordance rate, suggesting that genetics alone cannot cause disease and that environmental factors likely contribute to disease induction (1). Virus infection may contribute to the different stages of the disease (2) (Figure 1). In the actual triggering of β cell autoimmunity (defined by development of at least one islet autoantibody (IA), it is conceivable that viruses may be an etiological factor that precedes the appearance of a first islet autoantibody, the current biomarker of β cell autoimmunity. Virus infection may also contribute to the appearance of multiple autoantibodies representing stage 1 of the disease pathogenesis. It cannot be excluded that virus may promote or accelerate pathogenesis. Finally, virus detected in the pancreatic islet β cells at the time of clinical diagnosis may have contributed to insulitis, the mononuclear cell infiltration often seen in the pancreas at the time of clinical onset (Figure 1).
Figure 1.
Staging and progression of type 1 diabetes (T1D). Multiple stages are recognized in the development of T1D that are defined by the appearance of single or multiple autoantibodies (Aabs) and by dysglycemia stages. Virus infection triggers or other environmental triggers can occur in multiple stages, and multiple triggers may be commonly required to produce the clinical onset of T1D.
The highest genetic risk for T1D is conferred by the human leukocyte antigen (HLA) class II haplotypes HLA-DR3-DQ2 and HLA-DR4-DQ8 as well as genetic variants in the INS (insulin) gene (3). More than 60 single-nucleotide polymorphisms (SNPs) are associated with T1D risk (4, 5). SNPs in immune response genes such as DDX58 (DExD/H-box helicase 58), TLR2 (Toll-like receptor 2), TLR3 (Toll-like receptor 3), TLR7 (Toll-like receptor 7), TYK2 (tyrosine kinase 2), and IFIH1 (interferon induced with helicase C domain 1) are implicated in T1D disease risk (6). Genetic variants in these innate sensors may provide a key link between genetics and the environment for T1D initiation. Enteroviral infection may be a key environmental factor that contributes to any of the stages of T1D by inducing a robust antiviral response (7, 8).
Virus infection of β cells poses a particular challenge to the host. On one hand, the host must manage (and preferably clear) the infection, but on the other hand it must minimize destruction of the largely terminally differentiated, and thus irreplaceable, β cells. This balancing act between β cell death and virus clearance may in theory culminate in a compromise where viral persistence is established, allowing the host cells to remain viable. In support of this hypothesis, several risk-associated SNPs for T1D mentioned above are found in key antiviral response genes. These and other risk variants have been associated with an increased frequency of human enterovirus (HEV) infections (9). Furthermore, of the 60 or more identified candidate genes associated with T1D, 42 are expressed in human β cells, and when Ingenuity Pathway Analysis was performed on these genes, the three highest scoring canonical pathways were interferon signaling; role of JAK1, JAK2 and TYK in interferon signaling; and role of pattern recognition receptors in recognition of virus and bacteria (10). These pathways are all activated in response to virus infections and provide a possible link between genetic predisposition to T1D and host antiviral responses. It will be important to understand the possible roles of such pathways during the different stages of T1D (Figure 1).
ASSOCIATIONS BETWEEN ENTEROVIRUSES AND T1D: A LONG HISTORY
The Enterovirus genus of the Picornaviridae family comprises small nonenveloped positive sense RNA viruses. Approximately 70% of all human infections are asymptomatic, most of the remaining infections cause mostly mild febrile or cold-like symptoms, and approximately 1–2% of infections result in acute disease syndromes that range from paralysis and paresis, myocarditis, and aseptic meningitis to hand-foot-and-mouth disease. Taxonomically, the enteroviruses are now divided into four species: EV-A, EV-B, EV-C, and EV-D. Each enterovirus species contains multiple serotypes. EV-A consists of 23 serotypes of Coxsackie A virus and other enteroviruses, EV-B consists of six serotypes of Coxsackie B virus (CVB) and multiple echovirus serotypes, and EV-C contains poliovirus serotypes and others. Overall, more than 60 nonpolio enteroviruses cause disease in humans, and several of these are implicated in T1D, particularly the six serotypes of CVB viruses (2).
The link between enterovirus infections and development of T1D (formerly known as juvenile diabetes or insulin-dependent diabetes) has been studied since the 1960s, when Gamble et al. (11) reported a significant correlation between newly diagnosed diabetes patients and the annual prevalence data for Coxsackie type B4 (CVB4) virus and antibodies against CVB4 virus were found more often in diabetes patients (12). Since those early findings, significant efforts have been made to elucidate the specificity and temporality of this link. The current staging of T1D was not known at the time, nor was it understood that T1D might have been triggered years before clinical onset. In retrospect, it is not surprising that earlier serological studies inconsistently reported enterovirus antibodies to be more prevalent in T1D patients than in controls (13). However, many of the serological studies were limited by small sample sizes, enrollment of patients who were not matched for HLA alleles, or failure to differentiate between enterovirus serotypes. It was only after large prospective studies like the ones detailed below that serological evidence could be more carefully parsed in the context of the multiple variables that characterize the etiology and pathogenesis of T1D.
Enteroviruses have been found in the blood, gut, and pancreas of T1D patients in several studies, and they have also been associated with increased risk of T1D in prospective studies (14-17). Patients with T1D harbor enterovirus RNA in their peripheral blood mononuclear cells (PBMCs), and enteroviral RNA is more frequently found in the blood of patients newly diagnosed with T1D than in healthy individuals (18, 19). Studies have found that the presence of CVB RNA is associated with elevated levels of interferon α (IFN-α) in the blood of patients at various stages of T1D but not in individuals without the disease (20).
In addition to virome analyses detailed below, a few studies have found virus in gut tissue. For example, enterovirus was detected in small-intestine biopsy samples from patients with T1D, much more often than in samples from healthy individuals (21). These findings were confirmed in stools of a larger sample group by the same authors (22).
Although most data indicate associations between enterovirus and T1D outcomes, many other viruses have been implicated in T1D. Most of these older studies related findings to the time of clinical onset of the disease, not taking into account the prodrome of islet autoimmunity or the etiology of autoimmune islet disease. In addition, recent reports suggested a role for rotavirus, since introduction of routine rotavirus vaccination into the infant population was associated with a drop in the incidence of T1D (23,24). However, another meta-analysis of many other childhood vaccinations found no evidence of association between routine vaccinations and childhood T1D (25).
The COVID-19 global pandemic also raised questions concerning coronavirus involvement in syndromes involving extrapulmonary sites. Two reports examined human pancreas, islet single-cell RNA sequencing data sets, and COVID-19 autopsy material and found that expression of the viral ACE2 (angiotensin converting enzyme-2) receptor and TMPRSS2 (transmembrane protease serine-2) cofactor was absent in β cells and present in only some ductal cells (26, 27). This indicated that direct infection of pancreatic β cells was unlikely due to lack of viral entry factors on β cells. It is possible that any interactions of diabetes and COVID-19 may be due to systemic inflammation or metabolic changes in other organs.
PROSPECTIVE COHORT STUDIES
Over the last 20 years, several prospective studies have addressed the potential association between enteroviruses and T1D. Some, but not all, of the longitudinal efforts have found associations; the longitudinal studies (reviewed in 28) include DiMe, DIPP, TRIGR, BABYDIAB, DAISY, and MIDIA. Comparing these studies indicated a large range in the number of subjects followed, sample intervals used, types of virus assays, and types of study endpoints for autoimmunity seroconversion or clinical T1D (28, 29). Because of this variance, and perhaps also due to the small numbers of subjects and how they were selected at birth or later in life, the results have been controversial. More importantly, the frequency of sample collection to detect virus RNA has varied. Virus RNA is short-lived in plasma and found in stools for only 1–2 weeks after infections start in most children. A critical review of longitudinal studies completed before 2011 found inconsistent associations of enteroviruses with β cell or islet autoimmunity (defined by the appearance of one or several islet autoantibodies) or T1D and called for larger studies using frequent sampling intervals and the collection of multiple types of specimens to more accurately search for viruses (29).
The endpoints chosen to evaluate viral associations with disease outcomes are critical because there is growing recognition that both T1D and T2D may include variant forms of diabetes along a spectrum of etiologically based classification (30). These variants, often referred to as endotypes, may be distinguished on the basis of genetic risk factors, biomarker features such as autoantibodies or other plasma proteins, or distinct responder characteristics following treatment. The large TEDDY (The Environmental Determinants of Diabetes in the Young) study collected data from >8,500 newborns in a cohort followed for up to 15 years. TEDDY combined genotypic SNPs and phenotypic patient characteristics to define different T1D development pathways in children that were distinguished by the initiation of islet autoimmunity and subsequent risk of developing clinical diabetes (31). Development of islet autoantibodies (IA) usually involves one or more of several autoantibodies that commonly develop against cytoplasmic proteins in the beta cell: insulin autoantibodies (IAA), autoantibody against glutamic acid decarboxylase (GADA), autoantibody against protein tyrosine phosphatase (IA-2A), and zinc transporter 8 (ZnT8). Indeed, the TEDDY study discovered two endotypes of which autoantibody was first to be detected. In one- to four-year-olds and decreasing thereafter, IAA appeared first and primarily in children with HLA-DR4-DQ8. GADA appeared as the first autoantibody, primarily in HLA-DR3-DQ2 children, at 3–4 years of age but did not decrease with increasing age (31, 32). A key finding was distinct factors were associated with evidence of islet autoimmunity that were different for each respective autoantibody. Moreover, these factors did not necessarily correlate with those that predicted the progression from autoimmunity to diabetes. An underlying hypothesis is now emerging that seroconversion to either GADA or IAA as the first islet autoantibody seroconversion may follow independent and unique exposures to different viruses, and conversion from either of the two autoantibodies to T1D also may be independent.
TEDDY has already evaluated a number of candidate environmental triggers, including infections, probiotics, micronutrients, and microbiota. TEDDY results suggest that there are multiple pathways leading to the destruction of pancreatic β cells (33). The timing is essential. An acute virus infection may be over in 7–10 days, and neutralizing virus antibodies are usually detected with 10–12 days. Further, multiple viruses may play roles in each child, as symptomatic respiratory infections in young children are associated with increased risk of developing IA seroconversion, yet enterovirus infections often do not have respiratory symptoms (34). Very few studies collect samples with the necessary short intervals. At best, there are three-month periods between blood samples and one month between stool samples—even in the TEDDY study (35). This makes it difficult to generalize results of all human prospective studies; exposure to enteroviruses may associate with one or two outcomes, but not all, depending on the timing and many parameters of the study.
New Approaches to Test Viral Associations
To increase scientific power beyond that of earlier human studies will require more subjects, shorter sampling time, and new technology and approaches. For instance, by using insurance claims data, a Taiwanese nationwide retrospective population cohort study found a positive correlation between T1D and enterovirus infection (36). This study compared the incidence of T1D in children diagnosed with enterovirus infection with that in age- and sex-matched children without enterovirus infection in a population-based cohort. The increasing use of meta-analysis in clinical medicine affords the ability to increase the apparent numbers of patients and controls by using statistical procedures to combine data from multiple studies into a single statistical analysis. This may provide better insights linking viral infection and T1D onset when results vary from one study to the next, as described above for the many individual T1D studies. Several such studies have been completed that generally strengthen associations between viruses and T1D outcomes over those obtained from individual studies. One meta-analysis incorporated results available through 2010 and concluded that there was a clinically significant association between enterovirus infection, detected with molecular methods, and islet autoimmunity (37).
Another study of the virome in early childhood included results from 695 patients and 730 controls and identified small, significant associations between IA and stools with any enterovirus, consecutive stools positive for enterovirus (higher significance), and the number of stools positive for EV-B, similar to the TEDDY study findings (38). Yet another meta-analysis revealed an association between virus infections in pregnant women and subsequent development of IA or T1D in their children (39). There were 2,992 participants (953 offspring, 2,039 mothers) included, taken from varying study designs. The analysis showed a significant association between virus infection during pregnancy and clinical T1D during childhood, but no association with islet autoimmunity was found. The increased risk of T1D following maternal virus infection suggested a possible role of viremia involving the fetus during pregnancy. A separate study found that enterovirus infection in early pregnancy increased the risk for development of islet autoantibodies at delivery in nondiabetic mothers with HLA-DR3/3-DQ 2/2 or DR3-DQ2/X T1D risk genotypes (40). Despite higher-significance association of virus with disease outcomes in previous studies, additional larger studies with more frequent sampling and follow-up from pregnancy are required to further elucidate temporal associations between virus exposure in mothers and IA or T1D in their offspring. These novel approaches should prove useful to further understand the overall contribution of enterovirus and perhaps other viruses to T1D.
Because enterovirus is only transiently detected in stool, studies have moved to examination of multiple sequential stool samples to have a better chance of detecting virus infections in children. The DIPP study used polymerase chain reaction (PCR) combined with sequencing analysis of 4,781 longitudinal stool samples from 411 children and determined that cases had more enterovirus infections than controls (0.8 versus 0.6 infections per child) and that this excess of infections in cases occurred more than one year before the first detection of islet autoantibodies. Interestingly, the most frequent viruses were EV-A serotypes (22).
The largest multicenter TEDDY cohort study examined 8,654 sequential stools from 383 children in an age-matched cohort with metagenomic sequencing, using IA seroconversion as the outcome. The results confirmed that EV-B infections were associated with islet autoimmunity (41). More significantly, the type of enterovirus infection was important. Prolonged infection, typified by finding the same virus in sequential stools over months, was strongly associated with islet autoimmunity, whereas short EV-B infections without prolonged shedding of virus in stools were not associated (41). The stool sample observations are important to future investigations to relate prolonged infection to serum or plasma, perhaps PBMCs, viremia, nasal swabs, and possibly saliva to detail the appearance of neutralizing virus antibodies to IA seroconversion.
Evidence for Enterovirus Infection in Human Pancreas
Although in vitro infection of human islets with CVBs has been reported many times, access to human pancreas samples is very limited and dependent on pancreas tissue collected through procurement organizations such as the Network for Pancreatic Organ Donors with Diabetes (nPOD) and the Nordic Network for Islet Transplantation. However, multiple lines of evidence using these tissue sources indicate that direct infections of the pancreas by enteroviruses are not rare. Examination of human pancreatic specimens detected cells immunostained for enterovirus VP1 in multiple islets of almost 60% of 72 young patients with recent-onset of T1D compared with weak signals in a few islets in 6% of 50 individuals without the disease (42). In addition, enterovirus RNA has been detected by in situ hybridization in multiple postmortem pancreas samples from patients with T1D but not in samples from individuals without the disease (16). Viral particles and enterovirus VP1 capsid protein detected by immunocytochemistry were reported in β cells in pancreatic tissue from three of six organ donors with T1D but not in the pancreatic tissue of 26 organ donors without T1D (43). However, viable enterovirus has only rarely been recovered from pancreas samples (43), which may also result from persistence of replication-impaired virus discussed below.
The Diabetes Virus Detection (DiViD) study in Norway enrolled six adults with recent-onset T1D who agreed to undergo voluntary surgery to allow pancreatic biopsy of tissue for study (44). Examination of this explanted tissue revealed low-grade enterovirus infection in the islets in four of six patients through detection of both VP1 capsid antigen and viral RNA. Infected islets were rare; only ~2% of islets contained VP1 (45). In addition, all six patients had insulitis, but the majority of T cells were associated with peri-insulitis rather than within the islet parenchyma. Also, many IFN-stimulated genes (ISGs) were overexpressed by at least fivefold in islets affected by insulitis in the DiViD patients compared with islets from nondiabetic organ donors. Most of these overexpressed ISGs, including GBP1, TLR3, OAS1, EIF2AK2, HLA-E, IFI6, and STAT1, were overexpressed in the islet core rather than in the peri-islet area containing immune cells (46). In contrast, the T cell attractant chemokine CXCL10 was overexpressed in the peri-islet area instead of the islet, partly explaining T cell recruitment to this region. In summary, insulitic islets from the six recent-onset T1D subjects showed overexpression of ISGs, with expression patterns similar to that of islets from mice infected with virus or exposed to interferons (46-48).
Finally, both in DiViD and in other human pancreatic tissue banks, HLA class I antigens were found to be hyperexpressed throughout the islets containing at least one infected cell. This was determined by HLA detection at the protein and RNA levels, which is also associated with an increased STAT1 expression. Taken together, HLA class 1 and STAT1 expression may be interpreted as contributors to the pathogenesis of T1D (49). In addition, viral RNA was detected by in situ hybridization in islet cells from T1D patients from tissue banks (50). It is not likely that additional patients with newly diagnosed T1D will be asked to volunteer a pancreatic biopsy study in the future because of medical risks. Future investigations will therefore depend on pancreas tissue collected through procurement organizations such as nPOD and the Nordic Network for Islet Transplantation, whose activities are critically important for further progress in understanding virus infection of islets.
If it is considered that the clinical onset of T1D is the endpoint of a prodrome that has lasted for months to years, it is remarkable that enterovirus may be found in the pancreatic islets, the target organ for β cell destruction. The question is whether (a) the enterovirus presence represents a chronic infection and enterovirus has been dormant, inactivated, or slowly replicating since the time of an etiological infection, or (b) the enterovirus represents an accelerator of T1D pathogenesis promoting β cell killing, resulting in insulitis and clinical onset of the disease.
LIFESTYLE AND LOCATION MATTER: WHERE DO ENTEROVIRUSES TRIGGER ISLET AUTOIMMUNITY OR T1D?
Persistent Infections
Classically, enteroviruses are thought to cause primarily acute infections, and their replication and spread are believed to require cell lysis. This view has been seriously challenged in the last 20 years with growing awareness that persistent or chronic enterovirus infections occur much more commonly than previously thought. One report indicated that persistent infections lasting up to 30 days could be established in isolated human pancreatic islets in vitro (51). However, molecular mechanisms for viral persistence were lacking until a seminal report indicated that CVB3 could naturally generate 5′ terminal deletions ranging in size from 8 to 49 nucleotides that eliminate parts of a key RNA cloverleaf structure (52) that binds host key proteins supporting RNA replication (53). The deletion results in noncytolytic, slowly replicating virus (52). Although CVB is recognized to cause acute myocarditis, evidence of persistent virus infections was also obtained by finding virus in heart tissue from patients with dilated cardiomyopathy (54, 55). CVB with 5′ terminal cloverleaf deletion was first isolated from the heart in a fatal myocarditis case (56) and then in other myocarditis case isolates (45, 46). Terminal deletion virus populations were studied in persistent murine cardiac infections and characterized by low ratios of positive to negative-sense RNA strands, a low production of infectious particles, and low viral protein synthesis activity (52). Other studies also showed that CVB3 can persist in murine pancreas through deletion of 5′ terminal genomic sequences (57).
In addition to a mechanism for persistence in cells, the virus must have a mechanism for spreading between cells after the development of neutralizing antibodies if persistence is to be maintained longer than a few weeks. Cell lysis releases nonenveloped enterovirus, but recent discoveries indicate that enteroviruses commonly escape from infected cells without lysis by hijacking the cellular vesicular trafficking mechanisms and becoming quasi-enveloped within exosomes. Neighboring cells are constantly invaginating microvesicles, providing an efficient vehicle for cell-to-cell spread of enteroviruses without requiring cell lysis and avoiding exposure to neutralizing antibodies. Further, multiple virus progeny can gain infectious access to neighboring cells or macrophages within a single vesicle (58). The same mechanism is now known to enhance infectivity of enteroviruses and rotaviruses and possibly fecal transmissibility (59), and it has also been shown to mediate nonlytic enterovirus spread in a β cell line (60). In summary, mechanisms are now known to exist for both reduced nonlytic levels of replication and remarkably efficient cell-to-cell spread of these low levels of virus via nonlytic mechanisms.
Can Persistent Infections Lead to Islet Autoimmunity and Later T1D?
Recent observations in the DIPP and TEDDY studies indicate that the risk for a first appearing islet autoantibody was independently associated with polymorphisms in the Coxsackie and adenovirus receptor (CXADR). New scenarios may therefore be contemplated. One is that β cells are infected by enterovirus but replication is slow and nonlytic and can spread slowly between islet β cells by exosomes rather than by infectious virus particles. Although IAA was the first appearing islet autoantibody primarily in HLA-DR4-DQ8 subjects, the next step toward the clinical diagnosis of T1D is the appearance of a second autoantibody. In children with a first islet autoantibody, 60% developed a second autoantibody within one year. If IAA was first, GADA was the most common second autoantibody. If GADA was first, IAA was the most common second autoantibody (61). Interestingly enough, there was no indication that the second appearing autoantibody was associated with HLA. In children with two or more autoantibodies, 70% progressed to clinical onset of T1D within 10 years. In children with a persistent single autoantibody, only 15% progressed to T1D within 10 years. It will therefore be important in longitudinal follow-up studies to determine the presence and characteristics of virus antibodies over time (e.g., neutralizing virus antibody titers as well as antigen specificity and affinity) to test the hypothesis that the prolonged and persistent nonlytic virus infection is contributing to a slowly progressive loss of β cells.
This and other scenarios need to be investigated, perhaps with more extensive PCR-based technologies, to follow chronic virus infections of innate cells such as monocytes or natural killer (NK) cells. As insulitis does not seem to appear until T1D stage 2 (Figure 1), the final common pathway to β cell killing is likely preceded by other mechanisms. These mechanisms may include dormant enterovirus or other virus. Subsequent virus infections may accelerate the autoimmune process (29, 62). It cannot be excluded that biomarkers of infection may be more informative of these processes than biomarkers of inflammation.
Enterovirus Infection of Antigen-Presenting Cells
Although considerable research focused on virus infection of β cells and its consequences, T1D is effectively an autoimmune disease resulting from loss of tolerance for self antigens. The development or acceleration of T1D depends on the balance between autoreactive effector T cells and regulatory T cells. This balance is particularly influenced by antigen-presenting cells (APCs) such as monocytes, macrophages, and dendritic cells (DCs). There has been comparatively little research on the consequences of enterovirus infection of APCs themselves. It was first shown that enteroviruses could replicate in primary human monocytes using poliovirus, an EV-C species. Virus yield was low and only 6% of primary monocytes could support infection, but when lymphocytes were co-cultivated, virus yields increased, suggesting higher replication in activated monocytes (62). This finding was extended to macrophages and DCs, where poliovirus was also found to replicate (63). When CVB was examined, early reports indicated that human monocytes were poorly infected (64), but human monocyte-derived macrophages can be infected by CVB4 in vitro, dependent on whether macrophages were derived with M-CSF or GM-CSF (macrophage- or granulocyte-macrophage colony stimulating factor). This finding indicated that specific gene expression profiles are required in macrophages to support virus replication (65). Another report indicated that echoviruses could infect human monocyte-derived DCs more productively than CVB strains tested. However, the investigators noted a substantial difference in replication between two serotypes of echovirus; EV7 was very cytolytic and produced no DC activation whereas EV1 produced lower virus replication with significant DC activation through production of ISGs, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). CVB, in contrast, exhibited poor replication and poor activation (66).
The variable findings in the outcomes of infections of monocytes and DCs with the different enteroviruses in these studies may not be serotype dependent but rather strain dependent. This concept was illustrated when multiple clinical isolates of CVB1 were compared for replication and activation responses in human DCs and found to differ markedly in their capacity to induce innate immune responses in plasmacytoid DCs. Two of 18 strains produced strong IFN-α responses and accompanying cytokines (67).
Finally, in a different experimental concept, it was shown that phagocytosis of enterovirus-infected pancreatic β cells triggers innate immune responses and ISG induction in human DCs (68). ISG expression increased enough in DCs to produce an antiviral state. This was extended to an in vitro model for autoimmune T1D using enterovirus-infected β cells and human myeloid DC subsets. This report showed that enterovirus-infected β cells produce distinct response patterns in blood dendritic cell antigen (BDCA)1+ and BDCA3+ DCs; however, they both promoted Th1 responses that could favor the induction or maintenance of β cell autoimmune reactivity. Much more work is needed in this area.
Beyond in vitro data described above, importantly, enteroviral RNA was found in peripheral blood monocytes of 7 of 42 patients with T1D. Further, negative-strand enterovirus RNA was found in monocytes of 6 patients, indicating active replication. IFN-α mRNA was detected in blood and in monocytes in 12 of 42 patients with T1D but not in monocyte-depleted PBMCs of the same individuals. Significant plasma levels of IFN-α (≥5 IU/mL) were found in 6 patients. This suggested that monocytes of T1D patients can harbor enterovirus RNA, which may be replicating and stimulating IFN production (69). Also, both enhancing and neutralizing anti-CVB activities were examined in children, which followed from in vitro data showing an antibody enhancement of CVB infectivity of human monocytes (64). Sustained anti-CVB enhancing activity was observed in consecutive serum samples in patients with T1D in this small matched cohort. The pattern of responses differed between children who developed T1D and control children. In T1D patients, the anti–Coxsackie virus enhancing activity was predominant or even exclusive over the neutralizing activity, whereas in controls the enhancing and neutralizing activities were more balanced or the neutralizing activity was predominant (70). In summary, the impact of enterovirus infection on APC functions in immune activation or regulation is not well understood and remains a crucial open question in the search for viral triggering mechanisms.
POSSIBLE ROLES OF ENTEROVIRUS INFECTION IN β CELL DAMAGE
Molecular Mimicry
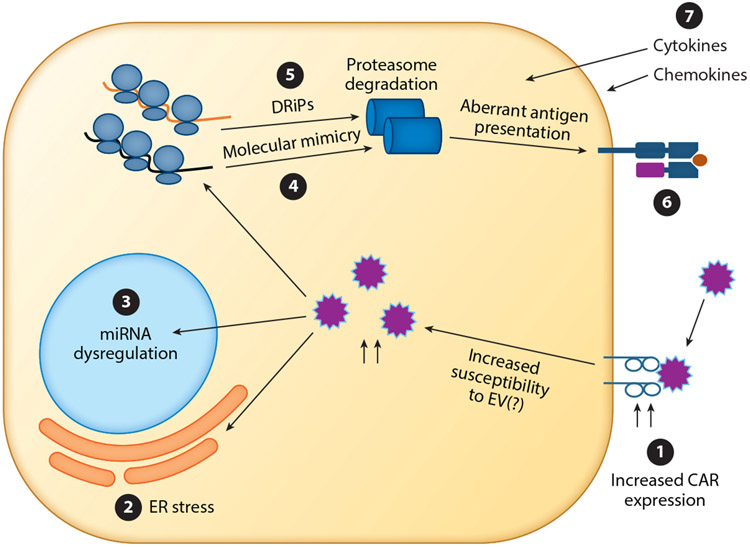
The infiltration of CD4+ and CD8+ T cells, B cells, and macrophages within the pancreatic islets and the presence of autoreactive T cells are also thought to play a role in disease development (71). The molecular basis of autoimmunity in CVB infection is proposed to be through molecular mimicry (Figure 2): The 2C nonstructural CVB protein has a shared sequence with the glutamic acid decarboxylase 65 (GAD65) enzyme, which is predominantly expressed in pancreatic β cells (72). A recent report shows a potential cross-reactivity between the 2C of CVB4 and GAD247–266 at the T cell receptor level in memory CD4 T cells in one patient (73). This suggests that exposure to CVB4 could indeed activate GAD-specific CD4 T cells via molecular mimicry. However, despite numerous studies, there is still a lack of strong evidence for this hypothesis. Also, in the TEDDY study, prolonged shedding of EV-B was associated with IAA as the first appearing autoantibody in HLA-DR4-DQ8 children (41). Levels of the metabolite γ-aminobutyric acid (GABA) were increased in these children prior to seroconversion (74). In contrast, other virus infections were likely to contribute to trigger GADA as the first appearing autoantibody, which primarily occurred in HLA-DR3-DQ2 children. Other metabolites, but not GABA, preceded GADA as the first autoantibody. Another hypothesis is that molecular mimicry does not act as a disease trigger but rather contributes to disease pathogenesis. In this context, mouse studies indicate that the expansion of previously primed autoreactive T cell populations via heterologous virus infections and molecular mimicry could lead to the acceleration of the disease in already prediabetic hosts (75). This indicates that pre-existing inflammation of the islets may be needed for the contribution of virus infections to molecular mimicry or to disease pathogenesis in general. In this case, a careful examination is needed to identify the multiple factors that may contribute to disease.
Figure 2.
Potential mechanisms in enterovirus-infected cells that may contribute to triggering islet autoimmunity. β cells of T1D patients have an increased expression of CAR ❶. This, in turn, may lead to increased susceptibility to enterovirus infection. Once inside the cell, enterovirus infection can lead to ER stress ❷ or miRNA dysregulation ❸. Translation of viral proteins can lead to the production of viral antigens that resemble self antigens ❹. Virus infection can also lead to the increased production of DRiPs ❺. These viral proteins and DRiPs, once degraded, can be presented in the context of MHC-I and lead to aberrant recognition by adaptive immunity ❻. The influx of immune cells also leads to production of cytokines, which may in turn lead to bystander damage to both infected and uninfected cells ❼. Abbreviations: CAR, Coxsackie adenovirus receptor; DRiP, defective ribosome product; ER, endoplasmic reticulum; EV, enterovirus; miRNA, microRNA; MHC-I, major histocompatibility complex class I; T1D, type 1 diabetes.
Coxsackie Adenovirus Receptor Expression Variants
The Coxsackie adenovirus receptor (CAR) is required for CVB entry into cells (Figure 2). The CAR gene produces five alternatively spliced mRNAs resulting in five isoforms of CAR, but only two have a transmembrane domain and are retained in cell membranes (designated CAR-SIV and CAR-TVV) (76, 77). The soluble CAR isoforms can inhibit CVB3 infection of cells by preventing virus attachment to membrane-bound CAR-SIV or -TVV (77).
Human β cells express CAR-SIV dominantly, but CAR is not in other islet cell types (78). Interestingly, CAR expression in β cells is heavily skewed toward insulin secretory granules, suggesting mechanisms for the sensitivity of β cells to virus infection.
In another study, CAR staining of islets was detected more frequently in patients with T1D and autoantibody-positive individuals than in controls (79). Further, the expression level of CAR was increased in explanted islets exposed to proinflammatory cytokines/chemokines produced by infected islets. These data indicate that the likelihood of CVB infection may increase in individuals with IA or T1D due to the higher induction of CAR expression by cytokines.
MicroRNA Dysregulation by Virus
MicroRNAs (miRNAs) have been investigated extensively in the context of T1D, particularly in PBMCs of T1D patients (80). In addition, miRNAs have proved important in β cell function, B cell dysfunction, and autoantigen generation, and they may have utility as biomarkers (80). Recent studies have examined the effect of enterovirus infection on miRNA expression in ductal-like PANC-1 cells and human islets. A total of 81 miRNAs were dysregulated in PANC-1 cells persistently infected with CVB4. Forty-nine of the 55 known T1D risk genes were predicted as putative targets of at least one of the dysregulated miRNAs (81). In another study, the expression of 754 miRNAs in CVB5-infected human pancreatic islets were analyzed (82). In total, 33 miRNAs were significantly dysregulated in the infected compared with control islets. These differentially expressed miRNAs were predicted to target mRNAs of 57 known T1D risk genes that collectively mediate various biological processes, including the regulation of cell proliferation, cytokine production, the innate immune response, and apoptosis (82).
Production of Novel Defective Ribosome Products
Major histocompatibility complex class I peptides are products of endogenous cellular protein degradation. These peptides are bound in the cleft between the heavy chain and β2-microglobulin and form the trimolecular complex that interacts with the T cell receptor on CD8+ T cells. Following the degradation of their source proteins—be they self-proteins or virus proteins—the trimolecular complex is used by host cells to alert the immune system during pathogen infection. Another source of these proteins can be defective ribosome products (DRiPs), which include polypeptides produced by premature translation termination and misfolding (Figure 2). The high demand for insulin may lead the β cells to produce aberrant translation products that generate diabetogenic epitopes (83). An alternative open reading frame within human insulin mRNA encodes a highly immunogenic polypeptide that is targeted by T cells in T1D patients. T1D patients can have cytotoxic CD8+ T cells directed against the N-terminal peptide of this nonconventional product that can kill human β cells and thereby may be diabetogenic. Additionally, environmental or viral factors leading to endoplasmic reticulum (ER) stress may control expression of an alternative reading frame as described for other stress-induced proteins (84). Indeed, human β cells seem exceptionally sensitive to ER stress (85). In addition to an effect on translation initiation processes, environmental stress may also have an impact on the degradation of insulin byproducts. Viruses disrupt translation at multiple levels through cleavage of translation factors by viral proteinases or by increasing or altering DRiPs (86, 87).
Inflammation-Induced Bystander Damage
Several lines of evidence suggest involvement of IFN signaling in T1D pathogenesis. Before T1D onset, a type I IFN transcriptional gene signature is detected in PBMCs (88). Blood transcriptome analysis, followed longitudinally in 400 TEDDY children and validated in Finnish DIPP children, revealed an NK signal that was present at the time of seroconversion and lasted through stage 1 and stage 2 of T1D onset until clinical diagnosis (89). T1D patients have increased IFN-α levels within the pancreata and enhanced IFN-α synthesis from peripheral blood plasmacytoid DCs compared to healthy patients (90, 91). On a cellular level, virus infection can promote the recruitment of NK cells and T cells to the islets (43) and the local production of inflammatory cytokines, particularly INF-α, INF-β, IFN-γ, TNF, and IL-1β (92). This exposure to IFN can have far-reaching consequences in the context of T1D. First, IFN directly triggers ER stress and apoptosis (92). Second, IFN can induce hyperexpression of HLA class I on β cells, which makes them more visible to cytotoxic T lymphocytes and more susceptible to destruction (93), and low-level virus infection promotes HLA hyperexpression in islets (49). Thus, infection of β cells itself may not even be needed for pathogenesis. In theory, even infection of cells adjacent to the β cells can lead to secretion of proinflammatory cytokines by DCs. It is envisioned that this could initiate bystander activation among circulating naive islet-specific T cells in pancreatic islets or lymph nodes, thus accelerating β cell destruction.
ENTEROVIRUSES AND CELIAC DISEASE
Enteroviruses were also recently associated with development of celiac disease, which is an autoimmune disease that shares HLA risk associations with T1D and is dependent on autoimmunity versus an antigen, tissue transglutaminase. A case-control study within a Norwegian birth cohort analyzed sequential monthly stools in children from age 3 to 36 months carrying the HLA genotype conferring increased risk of celiac disease (94). Both EV-A and EV-B were significantly associated with celiac disease, and long-lasting infections or higher-titer stools had a higher odds ratio (94). In another study using virus seroconversion as a marker of infection, enterovirus infections were found more frequently in case children than in controls before the appearance of celiac disease-associated tissue transglutaminase autoantibodies (95). A third study from TEDDY indicated that cumulative stool enteroviral exposures between 1 and 2 years of age in addition to higher gluten intake were associated with increased risk of seroconversion to celiac disease autoimmunity (96). Thus, emerging evidence indicates enteroviruses may be linked to autoantibody generation against antigens important in other autoimmune diseases, not exclusively those related to T1D.
CONCLUSION
T1D is a complex and multifactorial disease. In recent years, there has been increasing recognition of the potential role of enteroviruses in initiating autoimmunity in predisposed individuals, eventually leading to subsequent destruction of islet β cells. There may be a combination of mechanisms through which enterovirus infection may contribute to pathology, including ER stress and inflammation as well as direct persistent infections of β cells and potentially APCs that alter antigen presentation. With recent clinical data and the development of novel tools and human model systems, the field is now poised to start exploring the viral mechanisms that may play a role in enteroviral persistence and their contribution to both the etiology through trigger mechanisms of islet autoimmunity and the subsequent pathogenesis eventually resulting in the clinical onset of T1D in predisposed individuals.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI50237 and K124581 (R.E.L.) and DK63861 as well as the Swedish Research Council grant 2020-01537 (A.L.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Redondo MJ, Rewers M, Yu L, et al. 1999. Genetic determination of islet cell autoimmunity in monozygotic twin, dizygotic twin, and non-twin siblings of patients with type 1 diabetes: prospective twin study. BMJ 318(7185):698–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyöty H, Knip M. 2014. Developing a vaccine for Type 1 diabetes through targeting enteroviral infections. Expert Rev. Vaccines 13(8):989–99 [DOI] [PubMed] [Google Scholar]
- 3.Noble JA. 2015. Immunogenetics of type 1 diabetes: a comprehensive review. J. Autoimmun 64:101–12 [DOI] [PubMed] [Google Scholar]
- 4.Robertson CC, Rich SS. 2018. Genetics of type 1 diabetes. Curr. Opin. Genet. Dev 50:7–16 [DOI] [PubMed] [Google Scholar]
- 5.Morahan G, Mehta M, James I, et al. 2011. Tests for genetic interactions in type 1 diabetes: linkage and stratification analyses of 4,422 affected sib-pairs. Diabetes 60(3):1030–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blum SI, Tse HM. 2020. Innate viral sensor MDA5 and coxsackievirus interplay in type 1 diabetes development. Microorganisms 8(7):993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witsø E, Tapia G, Cinek O, et al. 2011. Polymorphisms in the innate immune IFIH1 gene, frequency of enterovirus in monthly fecal samples during infancy, and islet autoimmunity. PLOS ONE 6(11):e27781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witsø E, Cinek O, Tapia G, et al. 2015. Genetic determinants of enterovirus infections: polymorphisms in type 1 diabetes and innate immune genes in the MIDIA study. Viral Immunol. 28(10):556–63 [DOI] [PubMed] [Google Scholar]
- 9.Cinek O, Tapia G, Witsø E, et al. 2012. Enterovirus RNA in peripheral blood may be associated with the variants of rs1990760, a common type 1 diabetes associated polymorphism in IFIH1. PLOS ONE 7(11):e48409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marroqui L, Santos Dos RS, Fløyel T, et al. 2015. TYK2, a candidate gene for type 1 diabetes, modulates apoptosis and the innate immune response in human pancreatic β-cells. Diabetes 64(11):3808–17 [DOI] [PubMed] [Google Scholar]
- 11.Gamble DR, Kinsley ML, FitzGerald MG, et al. 1969. Viral antibodies in diabetes mellitus. BMJ 3(5671):627–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamble DR, Taylor KW, Cumming H. 1973. Coxsackie viruses and diabetes mellitus. BMJ 4(5887):260–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green J, Casabonne D, Newton R. 2004. Coxsackie B virus serology and Type 1 diabetes mellitus: a systematic review of published case-control studies. Diabetes Med. 21(6):507–14 [DOI] [PubMed] [Google Scholar]
- 14.Yin H, Berg A-K, Tuvemo T, et al. 2002. Enterovirus RNA is found in peripheral blood mononuclear cells in a majority of type 1 diabetic children at onset. Diabetes 51(6):1964–71 [DOI] [PubMed] [Google Scholar]
- 15.Oikarinen M, Tauriainen S, Oikarinen S, et al. 2012. Type 1 diabetes is associated with enterovirus infection in gut mucosa. Diabetes 61(3):687–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ylipaasto P, Klingel K, Lindberg AM, et al. 2004. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 47(2):225–39 [DOI] [PubMed] [Google Scholar]
- 17.Kim KW, Horton JL, Pang CNI, et al. 2019. Higher abundance of enterovirus A species in the gut of children with islet autoimmunity. Sci. Rep 9(1):1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hober D, Sauter P. 2010. Pathogenesis of type 1 diabetes mellitus: interplay between enterovirus and host. Nat. Rev. Endocrinol 6(5):279–89 [DOI] [PubMed] [Google Scholar]
- 19.Oikarinen S, Martiskainen M, Tauriainen S, et al. 2011. Enterovirus RNA in blood is linked to the development of type 1 diabetes. Diabetes 60(1):276–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chehadeh W, Weill J, Vantyghem MC, et al. 2000. Increased level of interferon-alpha in blood of patients with insulin-dependent diabetes mellitus: relationship with coxsackievirus B infection. J. Infect. Dis 181(6):1929–39 [DOI] [PubMed] [Google Scholar]
- 21.Oikarinen M, Tauriainen S, Honkanen T, et al. 2008. Detection of enteroviruses in the intestine of type 1 diabetic patients. Clin. Exp. Immunol 151(1):71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honkanen H, Oikarinen S, Nurminen N, et al. 2017. Detection of enteroviruses in stools precedes islet autoimmunity by several months: possible evidence for slowly operating mechanisms in virus-induced autoimmunity. Diabetologia 60(3):424–31 [DOI] [PubMed] [Google Scholar]
- 23.Harrison LC, Perrett KP, Jachno K, et al. 2019. Does rotavirus turn on type 1 diabetes? PLOS Path. 15(10):e1007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers MAM, Basu T, Kim C. 2019. Lower incidence rate oftype 1 diabetes after receipt of the rotavirus vaccine in the United States, 2001–2017. Sci. Rep 9(1):7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan E, Halliday SR, Campbell GR, et al. 2016. Vaccinations and childhood type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia 59(2):237–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coate KC, Cha J, Shrestha S, et al. 2020. SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in β cells. Cell Metab. 32(6):1028–40.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kusmartseva I, Wu W, Syed F, et al. 2020. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab. 32(6):1041–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergamin CS, Dib SA. 2015. Enterovirus and type 1 diabetes: What is the matter? World J. Diabetes 6(6):828–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stene LC, Rewers M. 2012. Immunology in the clinic review series; focus on type 1 diabetes and viruses: the enterovirus link to type 1 diabetes: critical review of human studies. Clin. Exp. Immunol 168(1):12–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balasubramanyam A. 2021. Defining and classifying new subgroups of diabetes. Annu. Rev. Med 72:63–74 [DOI] [PubMed] [Google Scholar]
- 31.Krischer JP, Liu X, Vehik K, et al. 2019. Predicting islet cell autoimmunity and type 1 diabetes: an 8-year TEDDY study progress report. Diabetes Care 42(6):1051–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Endesfelder D, zu Castell W, Bonifacio E, et al. 2019. Time-resolved autoantibody profiling facilitates stratification of preclinical type 1 diabetes in children. Diabetes 68(1):119–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rewers M, Hyöty H, Lernmark Å, et al. 2018. The environmental determinants of diabetes in the Young (TEDDY) study: 2018 update. Curr. Diabetes Rep. 18(12):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lönnrot M, Lynch KF, Elding Larsson H, et al. 2017. Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: the TEDDY study. Diabetologia 60(10):1931–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vehik K, Fiske SW, Logan CA, et al. 2013. Methods, quality control and specimen management in an international multicentre investigation of type 1 diabetes: TEDDY. Diabetes Metab. Res. Rev 29(7):557–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin J-N, Lin C-L, Yang C-H, et al. 2016. Risk of nephrotic syndrome following enteroviral infection in children: a nationwide retrospective cohort study. PLOS ONE 11(8):e0161004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeung W-CG, Rawlinson WD, Craig ME. 2011. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ 342:d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faulkner CL, Luo YX, Isaacs S, et al. 2020. The virome in early life and childhood and development of islet autoimmunity and type 1 diabetes: a systematic review and meta-analysis of observational studies. Rev. Med. Virol Dec. 30:e2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen DW, Kim KW, Rawlinson WD, et al. 2018. Maternal virus infections in pregnancy and type 1 diabetes in their offspring: systematic review and meta-analysis of observational studies. Rev. Med. Virol 28(3):e1974. [DOI] [PubMed] [Google Scholar]
- 40.Rešić Lindehammer S, Honkanen H, Nix WA, et al. 2012. Seroconversion to islet autoantibodies after enterovirus infection in early pregnancy. Viral Immunol. 25(4):254–61 [DOI] [PubMed] [Google Scholar]
- 41.Vehik K, Lynch KF, Wong MC, et al. 2019. Prospective virome analyses in young children at increased genetic risk for type 1 diabetes. Nat. Med 25(12):1865–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richardson SJ, Willcox A, Bone AJ, et al. 2009. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 52(6):1143–51 [DOI] [PubMed] [Google Scholar]
- 43.Dotta F, Censini S, van Halteren AGS, et al. 2007. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. PNAS 104(12):5115–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krogvold L, Edwin B, Buanes T, et al. 2014. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia 57:841–43 [DOI] [PubMed] [Google Scholar]
- 45.Krogvold L, Edwin B, Buanes T, et al. 2015. Detection of a low-grade enteroviral infection in the islets of Langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes 64(5):1682–87 [DOI] [PubMed] [Google Scholar]
- 46.Lundberg M, Krogvold L, Kuric E, et al. 2016. Expression of interferon-stimulated genes in insulitic pancreatic islets of patients recently diagnosed with type 1 diabetes. Diabetes 65(10):3104–10 [DOI] [PubMed] [Google Scholar]
- 47.Ylipaasto P, Kutlu B, Rasilainen S, et al. 2005. Global profiling of coxsackievirus- and cytokine-induced gene expression in human pancreatic islets. Diabetologia 48(8):1510–22 [DOI] [PubMed] [Google Scholar]
- 48.Eizirik DL, Sammeth M, Bouckenooghe T, et al. 2012. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLOS Genet. 8(3):e1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richardson SJ, Rodriguez-Calvo T, Gerling IC, et al. 2016. Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia 59(11):2448–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Busse N, Paroni F, Richardson SJ, et al. 2017. Detection and localization of viral infection in the pancreas of patients with type 1 diabetes using short fluorescently-labelled oligonucleotide probes. Oncotarget 8(8):12620–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chehadeh W, Kerr-Conte J, Pattou F, et al. 2000. Persistent infection of human pancreatic islets by coxsackievirus B is associated with alpha interferon synthesis in beta cells. J. Virol 74(21):10153–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim K-S, Tracy S, Tapprich W, et al. 2005. 5′-Terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negative-strand viral RNA. J. Virol 79(11):7024–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma N, Ogram SA, Morasco BJ, et al. 2009. Functional role of the 5′ terminal cloverleaf in Coxsackievirus RNA replication. Virology 393(2):238–49 [DOI] [PubMed] [Google Scholar]
- 54.Lévêque N, Renois F, Talmud D, et al. 2012. Quantitative genomic and antigenomic enterovirus RNA detection in explanted heart tissue samples from patients with end-stage idiopathic dilated cardiomyopathy. J. Clin. Microbiol 50(10):3378–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen Y, Renois F, Lévêque N, et al. 2013. Virus detection and semiquantitation in explanted heart tissues of idiopathic dilated cardiomyopathy adult patients by use of PCR coupled with mass spectrometry analysis. J. Clin. Microbiol 51(7):2288–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chapman NM, Kim K-S, Drescher KM, et al. 2008. 5/ terminal deletions in the genome of a coxsackievirus B2 strain occurred naturally in human heart. Virology 375(2):480–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tracy S, Smithee S, Alhazmi A, et al. 2015. Coxsackievirus can persist in murine pancreas by deletion of 5′ terminal genomic sequences. J. Med. Virol 87(2):240–47 [DOI] [PubMed] [Google Scholar]
- 58.Chen Y-H, Du W, Hagemeijer MC, et al. 2015. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell 160(4):619–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santiana M, Ghosh S, Ho BA, et al. 2018. Vesicle-cloaked virus clusters are optimal units for inter-organismal viral transmission. Cell Host Microbe 24(2):208–20.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Netanyah E, Calafatti M, Arvastsson J, et al. 2020. Extracellular vesicles released by enterovirus-infected EndoC-βH1 cells mediate non-lytic viral spread. Microorganisms 8(11):1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vehik K, Bonifacio E, Lernmark Å, et al. 2020. Hierarchical order of distinct autoantibody spreading and progression to type 1 diabetes in the TEDDY Study. Diabetes Care 43(9):2066–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eberle KE, Nguyen VT, Freistadt MS. 1995. Low levels of poliovirus replication in primary human monocytes: possible interactions with lymphocytes. Arch. Virol 140(12):2135–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wahid R, Cannon MJ, Chow M. 2005. Dendritic cells and macrophages are productively infected by poliovirus. J. Virol 79(1):401–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alidjinou EK, Sané F, Engelmann I, et al. 2013. Serum-dependent enhancement of coxsackievirus B4-induced production of IFNα, IL-6 and TNFα by peripheral blood mononuclear cells. J. Mol. Biol 425(24):5020–31 [DOI] [PubMed] [Google Scholar]
- 65.Alidjinou EK, Sané F, Trauet J, et al. 2015. Coxsackievirus B4 can infect human peripheral blood-derived macrophages. Viruses 7(11):6067–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schulte BM, Kers-Rebel ED, Prosser AC, et al. 2013. Differential susceptibility and response of primary human myeloid BDCA1+ dendritic cells to infection with different enteroviruses. PLOS ONE 8(4):e62502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hämäläinen S, Nurminen N, Ahlfors H, et al. 2014. Coxsackievirus B1 reveals strain specific differences in plasmacytoid dendritic cell mediated immunogenicity. J. Med. Virol 86(8):1412–20 [DOI] [PubMed] [Google Scholar]
- 68.Schulte BM, Kramer M, Ansems M, et al. 2010. Phagocytosis of enterovirus-infected pancreatic beta-cells triggers innate immune responses in human dendritic cells. Diabetes 59(5):1182–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alidjinou EK, Chehadeh W, Weill J, et al. 2015. Monocytes of patients with type 1 diabetes harbour enterovirus RNA. Eur. J. Clin. Investig 45(9):918–24 [DOI] [PubMed] [Google Scholar]
- 70.Sané F, Bertin A, Sioofy-Khojine A-B, et al. 2020. Enhancing and neutralizing anti-coxsackievirus activities in serum samples from patients prior to development of type 1 diabetes. Diabetes Metab. Res. Rev 36(6):e3305. [DOI] [PubMed] [Google Scholar]
- 71.Rojas M, Restrepo-Jiménez P, Monsalve DM, et al. 2018. Molecular mimicry and autoimmunity. J. Autoimmun 95:100–123 [DOI] [PubMed] [Google Scholar]
- 72.Hou J, Said C, Franchi D, et al. 1994. Antibodies to glutamic acid decarboxylase and P2-C peptides in sera from coxsackie virus B4-infected mice and IDDM patients. Diabetes 43(10):1260–66 [DOI] [PubMed] [Google Scholar]
- 73.Ashton MP, Eugster A, Walther D, et al. 2016. Incomplete immune response to coxsackie B viruses associates with early autoimmunity against insulin. Sci. Rep 6:32899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Q, Parikh H, Butterworth MD, et al. 2020. Longitudinal metabolome-wide signals prior to the appearance of a first islet autoantibody in children participating in the TEDDY study. Diabetes 69(3):465–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Christen U, Edelmann KH, McGavern DB, et al. 2004. A viral epitope that mimics a self antigen can accelerate but not initiate autoimmune diabetes. J. Clin. Investig 114(9):1290–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ifie E, Russell MA, Dhayal S, et al. 2018. Unexpected subcellular distribution of a specific isoform of the Coxsackie and adenovirus receptor, CAR-SIV, in human pancreatic beta cells. Diabetologia 61(11):2344–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dorner A, Xiong D, Couch K, et al. 2004. Alternatively spliced soluble coxsackie-adenovirus receptors inhibit coxsackievirus infection. J. Biol. Chem 279(18):18497–503 [DOI] [PubMed] [Google Scholar]
- 78.Ifie E, Russell MA, Dhayal S, et al. 2018. Unexpected subcellular distribution of a specific isoform of the Coxsackie and adenovirus receptor, CAR-SIV, in human pancreatic beta cells. Diabetologia 61(11):2344–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hodik M, Anagandula M, Fuxe J, et al. 2016. Coxsackie-adenovirus receptor expression is enhanced in pancreas from patients with type 1 diabetes. BMJ Open Diabetes Res. Care 4(1):e000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miao C, Chang J, Zhang G, et al. 2018. MicroRNAs in type 1 diabetes: new research progress and potential directions. Biochem. Cell Biol 96(5):498–506 [DOI] [PubMed] [Google Scholar]
- 81.Engelmann I, Alidjinou EK, Bertin A, et al. 2017. Persistent coxsackievirus B4 infection induces microRNA dysregulation in human pancreatic cells. Cell. Mol. Life Sci 74(20):3851–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim KW, Ho A, Alshabee-Akil A, et al. 2016. Coxsackievirus B5 infection induces dysregulation of microRNAs predicted to target known type 1 diabetes risk genes in human pancreatic islets. Diabetes 65(4):996–1003 [DOI] [PubMed] [Google Scholar]
- 83.Kracht MJL, van Lummel M, Nikolic T, et al. 2017. Autoimmunity against a defective ribosomal insulin gene product in type 1 diabetes. Nat. Med 23(4):501–7 [DOI] [PubMed] [Google Scholar]
- 84.Piganelli JD, Mamula MJ, James EA. 2020. The role of β cell stress and neo-epitopes in the immunopathology of type 1 diabetes. Front. Endocrinol 11:624590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scheuner D, Kaufman RJ. 2008. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr. Rev 29(3):317–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berglund P, Finzi D, Bennink JR, et al. 2007. Viral alteration of cellular translational machinery increases defective ribosomal products. J. Virol 81(13):7220–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rahnefeld A, Ebstein F, Albrecht N, et al. 2011. Antigen-presentation capacity of dendritic cells is impaired in ongoing enterovirus myocarditis. Eur. J. Immunol 41(9):2774–81 [DOI] [PubMed] [Google Scholar]
- 88.Ferreira RC, Guo H, Coulson RMR, et al. 2014. A type I interferon transcriptional signature precedes autoimmunity in children genetically at risk for type 1 diabetes. Diabetes 63(7):2538–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xhonneux L-P, Knight O, Lernmark Å, et al. 2021. Transcriptional networks in at-risk individuals identify signatures of type 1 diabetes progression. Sci. Transl. Med 13(587):eabd5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xia C-Q, Peng R, Chernatynskaya AV, et al. 2014. Increased IFN-α-producing plasmacytoid dendritic cells (pDCs) in human Th1-mediated type 1 diabetes: pDCs augment Th1 responses through IFN-α production. J. Immunol 193(3):1024–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang X, Yuang J, Goddard A, et al. 1995. Interferon expression in the pancreases of patients with type I diabetes. Diabetes 44(6):658–64 [DOI] [PubMed] [Google Scholar]
- 92.Marroqui L, Santos dos RS, Op de Beeck A, et al. 2017. Interferon-α mediates human beta cell HLA class I overexpression, endoplasmic reticulum stress and apoptosis, three hallmarks of early human type 1 diabetes. Diabetologia 60(4):656–67 [DOI] [PubMed] [Google Scholar]
- 93.Newby BN, Mathews CE. 2017. Type I interferon is a catastrophic feature of the diabetic islet microenvironment. Front. Endocrinol 8:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kahrs CR, Chuda K, Tapia G, et al. 2019. Enterovirus as trigger of coeliac disease: nested case-control study within prospective birth cohort. BMJ 364:l231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oikarinen M, Puustinen L, Lehtonen J, et al. 2020. Enterovirus infections are associated with the development of celiac disease in a birth cohort study. Front. Immunol 11:604529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lindfors K, Lin J, Lee HS, et al. 2020. Metagenomics of the faecal virome indicate a cumulative effect of enterovirus and gluten amount on the risk of coeliac disease autoimmunity in genetically at risk children: the TEDDY study. Gut 69(8):1416–22 [DOI] [PMC free article] [PubMed] [Google Scholar]