Abstract
Background
Obstructive sleep‐disordered breathing (oSDB) is a condition that encompasses breathing problems when asleep, due to an obstruction of the upper airways, ranging in severity from simple snoring to obstructive sleep apnoea syndrome (OSAS). It affects both children and adults. In children, hypertrophy of the tonsils and adenoid tissue is thought to be the commonest cause of oSDB. As such, tonsillectomy ‐ with or without adenoidectomy ‐ is considered an appropriate first‐line treatment for most cases of paediatric oSDB.
Objectives
To assess the benefits and harms of tonsillectomy with or without adenoidectomy compared with non‐surgical management of children with oSDB.
Search methods
We searched the Cochrane Register of Studies Online, PubMed, EMBASE, CINAHL, Web of Science, Clinicaltrials.gov, ICTRP and additional sources for published and unpublished trials. The date of the search was 5 March 2015.
Selection criteria
Randomised controlled trials comparing the effectiveness and safety of (adeno)tonsillectomy with non‐surgical management in children with oSDB aged 2 to 16 years.
Data collection and analysis
We used the standard methodological procedures expected by The Cochrane Collaboration.
Main results
Three trials (562 children) met our inclusion criteria. Two were at moderate to high risk of bias and one at low risk of bias. We did not pool the results because of substantial clinical heterogeneity. They evaluated three different groups of children: those diagnosed with mild to moderate OSAS by polysomnography (PSG) (453 children aged five to nine years; low risk of bias; CHAT trial), those with a clinical diagnosis of oSDB but with negative PSG recordings (29 children aged two to 14 years; moderate to high risk of bias; Goldstein) and children with Down syndrome or mucopolysaccharidosis (MPS) diagnosed with mild to moderate OSAS by PSG (80 children aged six to 12 years; moderate to high risk of bias; Sudarsan). Moreover, the trials included two different comparisons: adenotonsillectomy versus no surgery (CHAT trial and Goldstein) or versus continuous positive airway pressure (CPAP) (Sudarsan).
Disease‐specific quality of life and/or symptom score (using a validated instrument): first primary outcome
In the largest trial with lowest risk of bias (CHAT trial), at seven months, mean scores for those instruments measuring disease‐specific quality of life and/or symptoms were lower (that is, better quality of life or fewer symptoms) in children receiving adenotonsillectomy than in those managed by watchful waiting:
‐ OSA‐18 questionnaire (scale 18 to 126): 31.8 versus 49.5 (mean difference (MD) ‐17.7, 95% confidence interval (CI) ‐21.2 to ‐14.2); ‐ PSQ‐SRBD questionnaire (scale 0 to 1): 0.2 versus 0.5 (MD ‐0.3, 95% CI ‐0.31 to ‐0.26); ‐ Modified Epworth Sleepiness Scale (scale 0 to 24): 5.1 versus 7.1 (MD ‐2.0, 95% CI ‐2.9 to ‐1.1).
No data on this primary outcome were reported in the Goldstein trial.
In the Sudarsan trial, the mean OSA‐18 score at 12 months did not significantly differ between the adenotonsillectomy and CPAP groups. The mean modified Epworth Sleepiness Scale scores did not differ at six months, but were lower in the surgery group at 12 months: 5.5 versus 7.9 (MD ‐2.4, 95% CI ‐3.1 to ‐1.7).
Adverse events: second primary outcome
In the CHAT trial, 15 children experienced a serious adverse event: 6/194 (3%) in the adenotonsillectomy group and 9/203 (4%) in the control group (RD ‐1%, 95% CI ‐5% to 2%).
No major complications were reported in the Goldstein trial.
In the Sudarsan trial, 2/37 (5%) developed a secondary haemorrhage after adenotonsillectomy, while 1/36 (3%) developed a rash on the nasal dorsum secondary to the CPAP mask (RD ‐3%, 95% CI ‐6% to 12%).
Secondary outcomes
In the CHAT trial, at seven months, mean scores for generic caregiver‐rated quality of life were higher in children receiving adenotonsillectomy than in those managed by watchful waiting. No data on this outcome were reported by Sudarsan and Goldstein.
In the CHAT trial, at seven months, more children in the surgery group had normalisation of respiratory events during sleep as measured by PSG than those allocated to watchful waiting: 153/194 (79%) versus 93/203 (46%) (RD 33%, 95% CI 24% to 42%). In the Goldstein trial, at six months, PSG recordings were similar between groups and in the Sudarsan trial resolution of OSAS (Apnoea/Hypopnoea Index score below 1) did not significantly differ between the adenotonsillectomy and CPAP groups.
In the CHAT trial, at seven months, neurocognitive performance and attention and executive function had not improved with surgery: scores were similar in both groups. In the CHAT trial, at seven months, mean scores for caregiver‐reported ratings of behaviour were lower (that is, better behaviour) in children receiving adenotonsillectomy than in those managed by watchful waiting, however, teacher‐reported ratings of behaviour did not significantly differ.
No data on these outcomes were reported by Goldstein and Sudarsan.
Authors' conclusions
In otherwise healthy children, without a syndrome, of older age (five to nine years), and diagnosed with mild to moderate OSAS by PSG, there is moderate quality evidence that adenotonsillectomy provides benefit in terms of quality of life, symptoms and behaviour as rated by caregivers and high quality evidence that this procedure is beneficial in terms of PSG parameters. At the same time, high quality evidence indicates no benefit in terms of objective measures of attention and neurocognitive performance compared with watchful waiting. Furthermore, PSG recordings of almost half of the children managed non‐surgically had normalised by seven months, indicating that physicians and parents should carefully weigh the benefits and risks of adenotonsillectomy against watchful waiting in these children. This is a condition that may recover spontaneously over time.
For non‐syndromic children classified as having oSDB on purely clinical grounds but with negative PSG recordings, the evidence on the effects of adenotonsillectomy is of very low quality and is inconclusive.
Low‐quality evidence suggests that adenotonsillectomy and CPAP may be equally effective in children with Down syndrome or MPS diagnosed with mild to moderate OSAS by PSG.
We are unable to present data on the benefits of adenotonsillectomy in children with oSDB aged under five, despite this being a population in whom this procedure is often performed for this purpose.
Plain language summary
Tonsillectomy with or without adenoidectomy versus no surgery for obstructive sleep‐disordered breathing in children
Review question
This review compared the benefits and harms of surgical removal of the tonsils (tonsillectomy) with or without removal of the adenoids (adenoidectomy) against non‐surgical management in children with disturbed sleep caused by breathing problems due to blockage of the upper airways (called obstructive sleep‐disordered breathing; oSDB). We included any studies in which children were randomly allocated to surgery or no surgery published up to March 2015.
Background
oSDB can occur in both children and adults. It ranges in severity from simple snoring to obstructive sleep apnoea syndrome (OSAS), where episodes of complete blockage of the upper airways and restricted breathing can cause oxygen levels in the blood to drop, waking the child from sleep. Enlargement of the tonsils and adenoids is thought to be the most common cause in children. As such, tonsillectomy with or without adenoidectomy ((adeno)tonsillectomy) is considered a valuable first‐line treatment for most children.
Study characteristics
We included three studies, with a total of 562 children. Two were at moderate to high risk and one at low risk of bias. We did not combine the results of the studies because the trials differed substantially; they evaluated three different groups of children: those with mild to moderate OSAS (453 children aged five to nine years; CHAT trial), those who had symptoms and signs suggestive of oSDB (29 children aged two to 14 years; Goldstein) and children with Down syndrome or mucopolysaccharidosis and mild to moderate OSAS (80 children aged six to 12 years; Sudarsan). The studies compared: adenotonsillectomy versus no surgery (CHAT trial and Goldstein) or adenotonsillectomy versus a breathing mask (continuous positive airway pressure; CPAP) during sleep (Sudarsan).
Key results
In the largest trial with lowest risk of bias (CHAT trial), at seven months, mean scores for disease‐specific quality of life and/or symptoms were lower (meaning better quality of life or fewer symptoms) in children receiving adenotonsillectomy than in those managed by watchful waiting.
In the Sudarsan trial, the mean OSAS quality of life score at 12 months did not differ significantly between the adenotonsillectomy and CPAP groups. The mean modified Epworth Sleepiness Scale score did not differ at six months, but was lower in the surgery group at 12 months.
Adverse events
In the CHAT trial, 15 children experienced a serious adverse event: 6/194 (3%) in the adenotonsillectomy group and 9/203 (4%) in the control group. No major complications were reported by Goldstein. In the Sudarsan trial, 2/37 children (5%) developed a postoperative bleed in the surgery group and 1/36 (3%) in the CPAP group developed a rash due to the breathing mask.
Secondary outcomes
In the CHAT trial, at seven months, mean scores for general quality of life were higher in children receiving adenotonsillectomy than those managed by watchful waiting.
In the CHAT trial, at seven months, more children in the surgery group had normalisation of overnight sleep study findings than those assigned to watchful waiting. At six months, sleep study recordings were similar between groups in the Goldstein trial and resolution of OSAS based on overnight sleep study findings did not significantly differ between the adenotonsillectomy and CPAP groups in the Sudarsan trial.
In the CHAT trial, at seven months, neurocognitive performance and attention and executive function scores were similar in both groups.
In the CHAT trial, at seven months, mean scores for caregiver‐reported ratings of behaviour were lower (meaning better behaviour) in children receiving adenotonsillectomy than in those managed by watchful waiting. However, teacher‐reported ratings of behaviour did not significantly differ.
Quality of the evidence
Moderate quality evidence is available that children without a syndrome who have been diagnosed with mild to moderate OSAS do benefit from early adenotonsillectomy in terms of quality of life, symptoms and behaviour as reported by caregiver and high quality evidence in terms of overnight sleep study findings. The evidence on the effects of adenotonsillectomy in children without a syndrome who were diagnosed as having oSDB but who had a normal overnight sleep study is of very low quality. The evidence for children with Down syndrome or MPS diagnosed with mild to moderate OSAS is of low quality.
Summary of findings
for the main comparison.
| (Adeno)tonsillectomy compared with non‐surgical management for obstructive sleep‐disordered breathing in children | |||||
|
Patient or population: children with obstructive sleep‐disordered breathing Settings: secondary or tertiary care Intervention: adenotonsillectomy Comparison: no surgery (including CPAP in one trial) | |||||
| Outcomes | No surgery | Adenotonsillectomy | RR (95% CI) or MD (95%CI) | No of participants (studies) | Quality of the evidence (GRADE) |
| Children diagnosed with mild to moderate OSAS by PSG (comparator: no surgery) | |||||
| Disease‐specific quality of life or symptoms [mean total scores at 7 months] | OSA‐18: 49.5 (SD 10.3) PSQ‐SRBD: 0.5 (SD 0.2) Epworth Sleepiness Scale: 7.1 (SD 5.1) |
OSA‐18: 31.8 (SD 14.9) PSQ‐SRBD: 0.2 (SD 0.2) Epworth Sleepiness Scale: 5.1 (SD 4.4) |
MD ‐17.7 (‐21.2 to ‐14.2) MD ‐0.3 (‐0.31 to ‐0.26) MD ‐2.0 (‐2.9 to ‐1.1) |
395 (1) 396 (1) 398 (1) |
⊕⊕⊕⊝ moderate1 |
|
Adverse events, complications and morbidity associated with (adeno)tonsillectomy and comparators Expressed as the proportion of children experiencing a serious adverse event [7 months] |
9/203 (4%) | 6/194 (3%) | RR 0.70 (0.25 to 1.92) | 397 (1) | ⊕⊕⊕⊝ moderate2 |
| Respiratory events during sleep as measured by the AHI using PSG [mean AHI at 7 months] | 5.9 (SD 10.1) | 1.6 (SD 3.0) | MD ‐4.3 (‐5.7 to ‐2.9) | 407 (1) | ⊕⊕⊕⊕ high |
| Neurocognitive performance [7 months] | The General Conceptual Ability score from the DAS‐II did not change significantly in either group (crude data not reported) | n/a | 397 (1) 3 | ⊕⊕⊕⊕ high | |
| Attention [mean NEPSY at 7 months] | 106.2 (SD 15.0) | 108.6 (SD 15.5) | MD 2.4 (‐0.6 to 5.4) | 397 (1) | ⊕⊕⊕⊕ high |
| Behaviour [mean scores at 7 months] | CR Conners: 52.4 (SD 10.5) TR Conners: 53.7 (SD 12.2) CR BRIEF: 50.5 (SD 11.9) TR BRIEF: 55.4 (SD 13.5) |
CR Conners: 49.6 (SD 10.8) TR Conners: 51.6 (SD 12.0) CR BRIEF: 46.8 (SD 11.6) TR BRIEF: 54.2 (SD 13.6) |
MD ‐2.8 (‐4.9 to ‐0.7) MD ‐2.1 (‐5.3 to 1.2) MD ‐3.7 (‐6.0 to ‐1.4) MD ‐1.2 (‐4.9 to 2.5) |
392 (1) 212 (1) 392 (1) 207 (1) |
⊕⊕⊕⊝ moderate4 |
| Children with a clinical diagnosis of oSDB but negative PSG recordings (comparator: no surgery) | |||||
| Adverse events, complications and morbidity associated with (adeno)tonsillectomy and comparators [6 months] | 0/9 | 0/11 | n/a | 20 (1) | ⊕⊝⊝⊝ very low1 |
| Respiratory events during sleep as measured by the AHI using PSG [median AI at 6 months] | 0 (range 0 to 8.4) | 0.4 (range 0 to 3.1) | n/a (P value = 1.0) | 20 (1) | ⊕⊝⊝⊝ very low1 |
| Children with Down syndrome and MPS diagnosed with mild to moderate OSAS by PSG (comparator: CPAP) | |||||
| Disease‐specific quality of life or symptoms [mean score at 12 months] | OSA‐18: 75.0 (SD 2.5) Epworth Sleepiness Scale: 7.9 (SD 1.7) |
OSA‐18: 73.6 (SD 4.1) Epworth Sleepiness Scale: 5.5 (SD 1.4) |
MD ‐1.4 (‐3.0 to 0.2) MD ‐2.4 (‐3.1 to ‐1.7) |
73 (1) 73 (1) |
⊕⊕⊝⊝ low6 |
|
Adverse events, complications and morbidity associated with (adeno)tonsillectomy and comparators Expressed as the proportion of children experiencing adverse events [12 months] |
1/36 (rash) | 2/37 (secondary haemorrhage) | RR 1.95 (0.18 to 20.5) | 73 (1) | ⊕⊝⊝⊝ very low7 |
| Respiratory events during sleep as measured by the AHI using PSG [mean AHI at 12 months] | 1.1 (SD 0.6) | 1.1 (SD 0.7) | MD 0.0 (‐0.3 to 0.3) | 73 (1) | ⊕⊕⊝⊝ low6 |
| AHI: Apnoea/Hypopnoea Index; BRIEF: Behavior Rating Inventory of Executive Function (caregiver‐rated scores range from 28 to 101, and teacher‐rated scores range from 37 to 131, with higher scores indicating worse functioning); CAS: clinical assessment score; Conners: Conners Rating Scale Revised: Long Version Global Index (CR T‐scores range from 38 to 90, and TR T‐scores range from 40 to 90, with higher scores indicating worse functioning); CPAP: continuous positive airway pressure; CR: caregiver‐rated; DAS: Differential Ability Scales (scores range from 30 to 170, with higher scores indicating better functioning); Epworth Sleepiness Scale: Epworth Sleepiness Scale modified for children (scores range from 0 to 24, with higher scores indicating greater daytime sleepiness); MD: mean difference; MPS: mucopolysaccharidosis; NEPSY: Developmental Neuropsychological Assessment (scores range from 50 to 150, with 100 representing the population mean and higher scores indicating better functioning); OSA‐18: Obstructive Sleep Apnoea‐18 (scores range from 18 to 126, with higher scores indicating worse quality of life); OSAS: obstructive sleep apnoea syndrome; oSDB: obstructive sleep‐disordered breathing; PedsQL: Pediatric Quality of Life Inventory (scores range from 0 to 100, with higher scores indicating better quality of life); PSG: polysomnography; PSQ‐SRBD: Paediatric Sleep Questionnaire Sleep‐Related Breathing Disorder Scale (scores range from 0 to 1, with higher scores indicating greater severity); RR: risk ratio; SD: standard deviation; TR: teacher‐rated | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1We downgraded the evidence from high to moderate quality due to risk of detection bias (subjective outcome measures based on parental observation while parents were not blinded to treatment allocation).
2We downgraded the evidence from high to moderate quality due to imprecision of the effect estimate.
3This is the number quoted in the study for the number of participants that completed the primary analysis.
4 We downgraded the evidence from high to moderate quality due to risk of detection bias (subjective outcome measures based on parental and teacher observation while parents were not blinded to treatment allocation and it was unclear whether teachers were blinded).
5We downgraded the evidence from high to very low quality due to the small sample size, the high rate of attrition leading to a high risk of bias and the uncertainty as to whether the treatment received in the control group was adequate and represented current practice.
6We downgraded the evidence from high to low quality due to uncertainties around the method of randomisation and allocation concealment, and the unblinded outcome assessment leading to a high risk of bias.
7We downgraded the evidence from high to very low quality due to uncertainties around the method of randomisation and allocation concealment, the unblinded outcome assessment leading to a high risk of bias, and imprecision of the effect estimate.
Background
Description of the condition
Obstructive sleep‐disordered breathing (oSDB) is a condition that encompasses problems breathing when asleep due to an obstruction of the upper airways and ranges in severity from simple snoring to obstructive sleep apnoea syndrome (OSAS). It affects both children and adults. Simple snoring, the mildest expression of oSDB, is not associated with arousal from sleep or episodes of low oxygen saturation in arterial blood. In contrast, OSAS, the most severe expression of oSDB, involves repeated episodes of restricted breathing (hypopnoea) and/or complete obstruction (apnoea) with reduction in the normal levels of oxygen saturation in arterial blood and arousal during sleep (Nespoli 2013).
oSDB is a common condition in the paediatric population, with an estimated prevalence of primary snoring in children ranging from 8% to 27% and of OSAS from 1% to 5% (Marcus 2012; Shine 2005). Obesity is a well‐established risk factor for oSDB (Shelton 1993; Shine 2005). Since childhood obesity rates are rising in many Western countries, the prevalence of oSDB is expected to increase in the coming years.
In children, hypertrophy of the tonsils and adenoid tissue is thought to be the most common cause of oSDB; it causes narrowing of the airway, which is a particular problem during sleep when the muscles of the pharynx relax, leading to partial or complete obstruction of the airway (Marcus 2005).
An overnight sleep study (polysomnography; PSG) is considered the most comprehensive investigation for diagnosing OSAS (Marcus 2012). In many countries, however, this test is not routinely performed in children with a suspected diagnosis of OSAS because of its high cost and limited availability (Friedman 2013; Marcus 2012; Pringle 2013). Moreover, the correlation between clinical parameters including quality of life scores and PSG parameters is poor (Baldassari 2014). Clinical signs and symptoms are unable to accurately predict paediatric OSAS as diagnosed by PSG (Certal 2012), while a recent study found that clinical parameters such as demographics, physical examination findings and parent‐reported questionnaires do not robustly discriminate between different levels of OSAS based on PSG parameters (Mitchell 2015). Therefore, in everyday practice the severity of oSDB is usually assessed with a clinical history and examination, with some clinicians relying on overnight pulse oximetry (Pringle 2013).
oSDB may have a considerable impact on children's quality of life, comparable in some aspects to that of juvenile rheumatoid arthritis (Baldassari 2008), and has been linked with behavioural and neurocognitive morbidities (Beebe 2006; Owens 2009; Sedky 2014; Tauman 2011). Cognitive assessments of children with oSDB (either based on symptoms or on PSG) have shown a six‐point lower score on the Wechsler Preschool and Primary Scale Intelligence IQ test compared with those without oSDB (Gottlieb 2004). Children with oSDB have also been shown to be more likely to suffer from behavioural problems such as hyperactivity, emotional lability and aggression than children without oSDB (Rosen 2004). Furthermore, children with untreated OSAS, the most severe form of oSDB, are at risk of severe health problems, including failure to thrive and cardiovascular diseases such as hypertension, cor pulmonale and left ventricular hypertrophy (Marcus 2001).
Description of the intervention
Intervention
Surgical removal of the palatine tonsils with or without removal of the adenoids, called (adeno)tonsillectomy, is a common surgical procedure in children (Erickson 2009; Patel 2014). By tonsillectomy, the palatine tonsils are removed from their investing tissue in the oropharynx. The operation can be performed by various techniques including blunt dissection, guillotine knife, bipolar electrocautery, laser, microdebrider or coblation, according to the surgeon's preference. Adenoidectomy involves the removal of the adenoids (pharyngeal tonsil) from the nasopharynx; common techniques include curettage or suction cautery. The operation involves a general anaesthetic and can be performed as a day case or with an overnight stay (Cooper 2013; Lalakea 1999; Marcus 2012). Certain children undergoing surgery for oSDB are at increased risk of peri‐ and postoperative respiratory compromise (Baugh 2011; Fung 2010; Robb 2009; Schwengel 2009; Statham 2006). Guidelines from the American Academy of Pediatrics (Marcus 2012) and the UK Royal College of Paediatrics and Child Health (Royal College of Paediatrics and Child Health 2009) therefore recommend overnight observation for high‐risk cases such as young children (below four years of age), those with certain comorbidities (cardiovascular, craniofacial, neuromuscular conditions) or children with severe OSAS (e.g. an oxygen saturation level in arterial blood of 80% or lower or an Apnoea/Hypopnoea Index (AHI) greater than 24).
Throat pain and reduced oral intake are common following (adeno)tonsillectomy with over 50% of children still experiencing pain three days after the operation despite analgesia. Vomiting and nausea occur less frequently with one in 10 children reporting vomiting several days postoperatively (Stanko 2013). An important complication is postoperative bleeding, which may occur in up to 5% of children (Baugh 2011). A recent retrospective study reviewing the case notes of children presenting to the Accident & Emergency Department within four weeks of (adeno)tonsillectomy suggested that the secondary bleed rate may be higher among those operated because of OSAS than among those receiving surgery because of recurrent tonsillitis (Achar 2015). Over the past decade there has been increasing interest in partial removal of the tonsils, known as tonsillotomy, which may be associated with lower postoperative morbidity and fewer complications than complete removal of the tonsils (tonsillectomy). Several randomised controlled trials (RCTs) have compared tonsillectomy and tonsillotomy for oSDB in children, but this comparison will be addressed in a separate Cochrane review (Blackshaw 2014).
Comparator
We included all types of non‐surgical management of oSDB that are commonly used in daily clinical practice.
Lifestyle interventions: dietary advice, exercise programmes.
Medical management: intranasal and oral corticosteroids, leukotriene receptor antagonists.
Mechanical interventions: continuous positive airway pressure (CPAP).
Watchful waiting: observation and monitoring.
Recent evidence has suggested that children with OSAS have raised local and systemic inflammatory markers, which causes proliferation of lymphoid tissue within the tonsils and adenoids (Kim 2009). Intranasal and oral corticosteroids aim to increase airway patency by reducing the inflammatory response occurring in the oropharynx. Leukotriene levels have also been shown to be higher in the adenotonsillar tissue of children with OSAS compared to those with tonsillitis (Goldbart 2004). This is why the use of leukotriene receptor antagonists such as montelukast has been suggested to have beneficial effects in children with oSDB (Friedman 2011). Other non‐surgical management options for oSDB involve non‐invasive ventilatory support (e.g. CPAP or nasal insufflation) and reducing the effort of breathing by weight loss regimes.
How the intervention might work
In children, hypertrophy of the tonsils and adenoid tissue is thought to be the most common cause of oSDB. Therefore, surgical removal of the adenoid tissue and palatine tonsils, i.e. adenotonsillectomy, is widely considered an effective treatment for oSDB in children. Uncontrolled and non‐randomised studies have shown improvements in objective and subjective measures of sleep, behaviour, cognition and quality of life (Garetz 2008). A 2009 systematic review, however, showed that (adeno)tonsillectomy may not be curative, with only two out of three children achieving complete polysomnographic resolution (Friedman 2009).
Why it is important to do this review
There is substantial evidence of an association between childhood oSDB and adverse health outcomes, particularly in those with OSAS. Consequently, the identification and implementation of an effective treatment for this condition should prevent those outcomes and improve health. In uncontrolled and non‐randomised studies (adeno)tonsillectomy demonstrates significant improvements in sleep as measured by subjective measures (e.g. caregiver reporting) and objective measures such as respiratory events during sleep as measured by the AHI using PSG. The operation is nowadays considered a valuable first‐line treatment for most cases of oSDB in children. However, the potential benefits of (adeno)tonsillectomy in children, a surgical procedure performed under a general anaesthetic, should be carefully balanced against the risks, including the risk of adverse events. A systematic review of the current literature to identify and summarise the results of randomised controlled trials comparing the clinical effectiveness and/or safety of (adeno)tonsillectomy with non‐surgical management in children with oSDB is therefore highly warranted.
Objectives
To assess the benefits and harms of tonsillectomy with or without adenoidectomy compared with non‐surgical management of children with oSDB.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing the effectiveness of (adeno)tonsillectomy with non‐surgical management in children with oSDB.
Types of participants
Children aged two years up to the age of 16 years with oSDB. We included RCTs where the diagnosis of oSDB is based upon clinical history and examination alone as well as those where overnight pulse oximetry and/or PSG is carried out to confirm the diagnosis. We excluded RCTs in children with central SDB (e.g. SDB related to neurological conditions or brain injury) and in children with combinations of central and obstructive SDB.
Types of interventions
Intervention
(Adeno)tonsillectomy, irrespective of the surgical technique used.
Comparator
Non‐surgical management. We included all types of non‐surgical management of oSDB such as lifestyle interventions, including those aimed at weight reduction, medical treatments such as intranasal and oral corticosteroids and leukotriene receptor antagonists, mechanical interventions including CPAP, and no treatment (watchful waiting).
Types of outcome measures
We analysed the primary and secondary outcomes listed below in this review, but we did not use these outcomes as a basis for including or excluding studies.
Primary outcomes
Disease‐specific quality of life (using any validated instrument, such as Obstructive Sleep Apnoea 18 (OSA‐18) or Obstructive Sleep Disorders 6‐survey (OSD‐6) ‐ see the Spruyt 2011 review for a comprehensive list) and/or a disease‐specific symptom score (using any validated instrument, such as the Paediatric Sleep Questionnaire ‐ see the Spruyt 2011 review for a comprehensive list).
Adverse events, complications and morbidity associated with (adeno)tonsillectomy and comparators. We extracted data on intraoperative and (severity of) postoperative bleeding (requiring attention and/or intervention and/or hospitalisation), (severity of) postoperative infection (requiring attention and/or intervention and/or hospitalisation), (severity of) postoperative dehydration (requiring attention and/or intervention and/or hospitalisation), (severity of) postoperative pain (using a validated instrument) and days until no longer requiring analgesia.
Secondary outcomes
Generic quality of life (using any validated instrument ‐ see the Hullmann 2011 review for comprehensive list).
Respiratory events during sleep as measured by the AHI using PSG.
Other measures of respiratory events during sleep (e.g. Respiratory Disturbance Index (RDI), oxygen desaturations, respiratory event‐related arousals).
Cardiovascular disease, including hypertension, right and left ventricular dysfunction, pulmonary hypertension.
Neurocognitive performance (using a validated instrument).
Attention (using a validated instrument).
Behaviour (using a validated instrument).
School performance.
Absence from school.
Weight changes.
Search methods for identification of studies
The Cochrane ENT Trials Search Co‐ordinator (TSC) conducted systematic searches for randomised controlled trials. There were no language, publication year or publication status restrictions. The date of the search was 5 March 2015.
Electronic searches
The TSC searched:
the Cochrane Register of Studies Online (searched 5 March 2015);
-
Ovid MEDLINE (1946 to March Week 1 2015)
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations);
PubMed (as a top up to searches in Ovid MEDLINE);
Ovid EMBASE (1974 to 2015 week 9);
EBSCO CINAHL (1982 to 5 March 2015);
LILACS (searched 5 March 2015);
KoreaMed (searched 5 March 2015);
IndMed (searched 5 March 2015);
PakMediNet (searched 5 March 2015);
Web of Knowledge, Web of Science (1945 to 5 March 2015);
CNKI (searched via Google Scholar 5 March 2015);
ClinicalTrials.gov, www.clinicaltrials.gov (searched via the Cochrane Register of Studies 5 March 2015);
ICTRP (searched 5 March 2015);
ISRCTN, www.isrctn.com (searched 5 March 2015);
Google Scholar (searched 5 March 2015);
Google (searched 5 March 2015).
The TSC modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011)). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the TSC searched Ovid MEDLINE, TRIP database, The Cochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The TSC conducted a PubMed related articles search and Science Citation Index search for the already included studies (Goldstein 2004; Marcus 2013; Sudarsan 2014), to identify any additional articles of relevance.
Data collection and analysis
Selection of studies
Two review authors (RPV, BJH) independently screened the titles and abstracts obtained from the database searches and citations of relevant systematic reviews to assess their potential relevance for full review. The same review authors (RPV, BJH) independently reviewed the full text of potentially relevant titles and abstracts against the pre‐defined inclusion and exclusion criteria. Disagreements were resolved by discussion with a third review author (DC). We documented the exclusion of any studies from the review and described the reasons for exclusion in the Characteristics of excluded studies table.
Data extraction and management
Two review authors (RPV, BJH) independently extracted data from the included studies using standardised forms. We extracted the following information from each study:
characteristics of trials: setting, design, method of data analysis;
participants: study population, number of participants in each group, patient characteristics such as age, gender, ethnicity, body mass index (BMI) and the way a diagnosis of oSDB was made;
interventions: type of surgical procedure including technique and pre‐ and postoperative treatment, type of non‐surgical management;
outcomes: primary and secondary outcomes recorded, time points, treatment failure, adverse events associated with treatment and comparator.
We extracted data in a manner that would allow us to perform an intention‐to‐treat (ITT) analysis.
Assessment of risk of bias in included studies
Two review authors (RPV, BJH) independently assessed the methodological quality of the included trials. Any disagreements were resolved by discussion with a third review author (DC). We performed 'Risk of bias' assessment using the 'Risk of bias' tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). We judged the following domains as high, low or unclear risk of bias:
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective outcome reporting (reporting bias); and
other sources of bias.
We presented the results in a 'Risk of bias' summary figure and a 'Risk of bias' graph.
Measures of treatment effect
We proposed to express dichotomous outcomes as risk ratios (RR), risk differences (RD) with accompanying 95% confidence intervals (CI) and number needed to treat to benefit (NNTB) and continuous outcome variables either as mean differences (MD) if reported on the same scale or as standardised mean differences (SMD) if different continuous scales had been used, with accompanying 95% CIs.
Unit of analysis issues
We identified no studies with non‐standard designs, such as cross‐over and cluster‐randomised trials.
Dealing with missing data
In the case of missing data, we contacted the trial authors to request further data or conducted an available case analysis where necessary. In primary analyses, we analysed the available data based on the ITT principle, whereby participants are analysed in the groups to which they were randomised. For continuous outcomes, we calculated missing statistics, such as standard deviations (SDs), from other available statistics (e.g. P values) according to the methods described in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
We proposed to assess the impact of incomplete data reporting on our meta‐analyses findings by performing scenario analyses (best‐case and worst‐case scenarios) for dichotomous data, but the available data did not allow us to perform such analyses (see Assessment of heterogeneity section).
Assessment of heterogeneity
We assessed the level of clinical diversity between trials by reviewing the differences in the types of participants recruited, the way a diagnosis of oSDB was made, the interventions used and the outcomes measured between trials.
We found the clinical heterogeneity between the included trials to be substantial since the study populations varied from children with a clinical diagnosis of oSDB but negative PSG parameters (Goldstein 2004) to syndromic (Sudarsan 2014) and non‐syndromic children (Marcus 2013) diagnosed with mild to moderate OSAS by PSG. As such, we considered that pooling of the trial results was not justified and decided to refrain from performing meta‐analyses.
Assessment of reporting biases
For each study, we searched the internet and ClinicalTrials.gov (www.clinicaltrials.gov) for available study protocols. Whenever possible, we assessed whether the outcomes reported in the publications of the trials were listed in the registered trial protocol. More formal assessments using funnel plots would have been conducted if sufficient studies had been available.
Data synthesis
Performing a meta‐analysis of individual trials is only meaningful and justified when trials show satisfactory clinical homogeneity in terms of study population, setting, intervention and comparator, and outcome measures. However, since we decided to refrain from pooling the trial results because of substantial clinical diversity between the trials (see Assessment of heterogeneity section), we reported the effect estimates as presented by the individual trials.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses for the following characteristics, if sufficient data were available:
oSDB severity (OSAS versus less severe oSDB);
body weight (obese versus non‐obese children);
age (younger than three, three to seven and above seven years);
race (African‐American versus other).
Sensitivity analysis
To assess the robustness of the review findings we planned to perform a sensitivity analysis including only RCTs classified as having a low risk of bias. If sufficient data were available we planned to assess whether the following variations are factors affecting the outcome:
oSDB definition (clinical diagnosis alone or SDB diagnosis based on respiratory events during sleep as measured by polysomnography);
type of surgery (tonsillectomy with or without adenoidectomy);
type of non‐surgical management (lifestyle intervention, corticosteroid or leukotriene receptor antagonist treatment, CPAP, no treatment or watchful waiting).
GRADE approach and 'Summary of findings' for the main comparison
We used the GRADE approach to rate the overall quality of evidence for each outcome. There are four possible ratings: high, moderate, low and very low. A 'high quality of evidence' rating implies that we feel confident about the effect estimate and that further research is very unlikely to change our confidence in the effect estimate. In contrast, a 'very low quality of evidence' rating implies that our confidence in the effect estimate is very uncertain.
Evidence from RCTs that do not have serious limitations are rated as 'high quality'. However, several factors can contribute to downgrading of the evidence to moderate, low or very low. The degree of downgrading depends on each of the following factors:
study limitations (risk of bias);
indirectness of evidence;
imprecision;
inconsistency; and
publication bias.
We included a 'Summary of findings' table for the main comparison (Table 1), constructed according to the description in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
We included the following outcomes in the 'Summary of findings' table:
disease‐specific quality of life and/or disease‐specific symptom score;
adverse events, complications and morbidity associated with (adeno)tonsillectomy and comparators;
respiratory events during sleep as measured by the AHI using PSG;
neurocognitive performance;
attention;
behaviour.
Results
Description of studies
For details of the included trials see the 'Characteristics of included studies' table. The main reasons for excluding studies from the review are shown in the 'Characteristics of excluded studies' table. Details of ongoing studies are presented in the 'Characteristics of ongoing studies' table.
Results of the search
We retrieved a total of 1660 records from our electronic database searches and we identified eight records through other sources. Removing duplicates left 866 unique records. After screening titles and abstracts we identified 13 potentially eligible publications. We obtained the full text of these papers and excluded one study since it was a quasi‐randomised trial (Xie 2010), one paper as it was a summary of the results of the Marcus 2013 trial (Witmans 2013), and five papers that were commentaries on the Marcus 2013 trial (Brouillette 2013; Ebell 2013; Ramsden 2014; Rodriguez 2014; Schilder 2014). Three papers reported on additional findings of the Marcus 2013 trial (Katz et al 2014; Quante et al 2014; Garetz et al 2015) and we included them in this review as part of the Marcus 2013 trial. This left three trials eligible for inclusion (Goldstein 2004; Marcus 2013; Sudarsan 2014). We identified no additional eligible trials after screening the reference lists of the full‐text papers and relevant systematic reviews. We identified four ongoing studies (ChiCTR‐TRC‐10001136; NCT01918007; NCT02315911; POSTA Child Study).
A flow chart of the number of studies found in the original searches and those included or excluded in the review process is shown in Figure 1.
1.
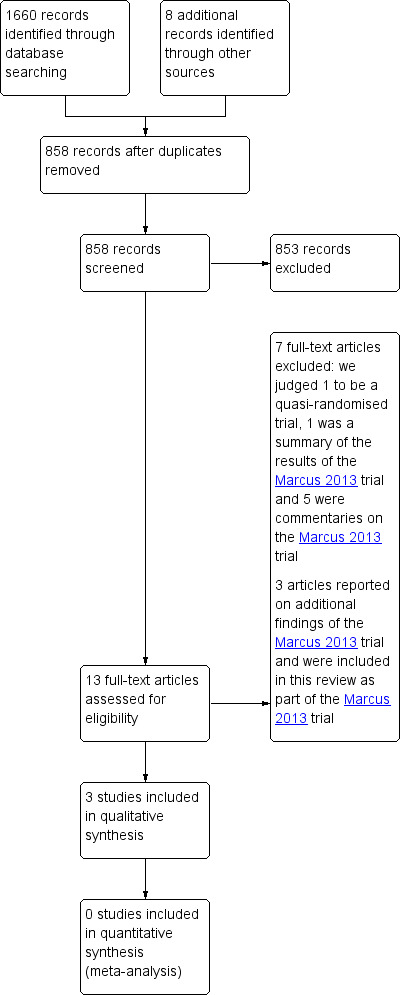
Process for sifting search results and selecting studies for inclusion.
Included studies
Details of the methods, participants, interventions and outcomes of the included studies are presented in the 'Characteristics of included studies' table.
Design
We included three parallel‐group RCTs in the review (Goldstein 2004; Marcus 2013; Sudarsan 2014). Two were investigator‐blinded trials (Goldstein 2004; Marcus 2013), while one was an open‐label trial (Sudarsan 2014).
Participants and setting
In the first trial, children with OSAS defined as an AHI score of 2 or more events per hour or an Obstructive Apnoea Index (OAI) score of 1 or more events per hour as assessed by overnight PSG, aged five to nine years, were recruited from six US clinical sites, each headed by an experienced paediatric sleep specialist or otolaryngologist (Marcus 2013). A total of 463 children were randomised; the mean age was 6.5 years, 49% of the children were boys, 34% were obese, 53% were African‐American and the mean AHI score was 6.7. The study was supported by grants from the National Institute of Health.
In the second trial, children with a clinical diagnosis of oSDB (clinical assessment score of 40 or more) but negative PSG recordings, aged 2 to 14 years, were recruited from paediatric otolaryngology private offices and clinics of a tertiary care centre and otolaryngology and paediatric pulmonary clinics of a secondary care centre in the USA (Goldstein 2004). A total of 29 children were randomised; the mean age was 5.8 years, 48% of the children were boys and 76% were African‐American. Within the surgery group 20% of children had a BMI ≥ 95th percentile compared with 14% of patients in the no surgery group. The study was supported by a research grant from the National Institute of Child Health and Human Development.
In the most recent trial, syndromic children (Down syndrome and mucopolysaccharidoses (MPS)) with OSAS defined as an AHI score of 1 or more events per hour as assessed by overnight PSG, aged 6 to 12 years, were recruited from the MPS support and DS Society, Chennai India, along with individual referral cases (Sudarsan 2014). A total of 80 children were randomised; the mean age was 8.3 years, 66% of the children were boys, 44% had MPS and the mean AHI score was 3.6 (76% mild OSAS and 24% moderate OSAS).
Interventions and comparators
In Marcus 2013, 226 children were randomly allocated to early adenotonsillectomy (surgery within four weeks after randomisation; method of surgical technique used not specified) and 227 children to the watchful waiting with supportive care group (WWSC, comprising conservative medical management). In both groups children received (referral for) treatment for comorbidities such as asthma and allergic rhinitis, education regarding general sleep hygiene and healthy behaviours, and nasal saline spray as needed. In the surgical group, 19 children used intranasal corticosteroids compared with eight children in the WWSC group while montelukast was used in seven and eight children, respectively.
In Goldstein 2004, 15 children were randomly allocated to adenotonsillectomy (method of surgical technique used not specified) and 14 children to no surgery (no further details were provided on whether children received any other treatment in this group).
In Sudarsan 2014, 40 children were randomly allocated to adenotonsillectomy (coblation) and 40 children to CPAP.
Outcomes
Reassessment of children occurred at seven months in the Marcus 2013 trial, at six months in the Goldstein 2004 trial, and at six and 12 months in the Sudarsan 2014 trial. Whether the included trials did (or did not) report on our pre‐specified outcomes can be found in Table 2.
1. Overview of the outcomes reported in the included studies.
| Marcus 2013 | Goldstein 2004 | Sudarsan 2014 | |
| Disease‐specific quality of life and/or symptom score (validated) | x | x | |
| OSA‐18 | x | x | |
| PSQ‐SRBD | x | ||
| Epworth Sleepiness Scale | x | x | |
| Adverse events | x | x | x |
| Generic quality of life (validated) | x | ||
| PedsQL | x | ||
| PSG ‐ AHI | x | x | x |
| PSG ‐ other parameters | x | x | |
| ODI | x | ||
| % of sleep time with CO2 > 50 mm Hg | x | ||
| RDI | x | ||
| Median % of the night with O2 < 90% | x | ||
| Cardiovascular disease | x | x | |
| Neurocognitive performance | x | ||
| DAS | x | ||
| Attention | x | ||
| NEPSY | x | ||
| Behaviour | x | ||
| CR‐Conners | x | ||
| TR‐Conners | x | ||
| CR BRIEF | x | ||
| TR BRIEF | x | ||
| School performance | |||
| Absence from school | |||
| Weight changes | x | x |
AHI: Apnoea/Hypopnoea Index; BRIEF: Behavior Rating Inventory of Executive Function; Conners: Conners Rating Scale Revised: Long Version Global Index; CR: caregiver‐rated; DAS: Differential Ability Scales‐II; NEPSY: attention and executive function scores on the Developmental Neuropsychological Assessment; ODI: Oxygen Desaturation Index; OSA‐18: obstructive sleep apnoea‐18; PedsQL: Pediatric Quality Of Life Inventory; PSG: polysomnography; PSQ‐SRBD: Pediatric Sleep Questionnaire Sleep‐Related Breathing Disorder Scale; QoL: quality of life; RDI: Respiratory Disturbance Index; TR: teacher‐rated
Excluded studies
After reviewing the full text, we excluded seven articles; see also 'Characteristics of excluded studies' table. Furthermore, we identified three papers reporting on additional findings of the Marcus 2013 trial (Katz 2014; Quante 2014; Garetz 2015) and we included these in this review as part of the Marcus 2013 trial.
Ongoing studies
We identified four ongoing studies (ChiCTR‐TRC‐10001136; NCT01918007; NCT02315911; POSTA Child Study); see 'Characteristics of ongoing studies' table.
Risk of bias in included studies
Summary assessment of risk of bias
We judged the risk of bias to be low in one trial (Marcus 2013), and moderate to high in two trials (Goldstein 2004; Sudarsan 2014). Details of the 'Risk of bias' assessment of the included trials are presented in a 'Risk of bias' graph (Figure 2) and a 'Risk of bias' summary figure (Figure 3). While participants and personnel cannot be blinded in any trial comparing surgery with non‐surgical management (performance bias), assessors can be blinded (detection bias).
2.
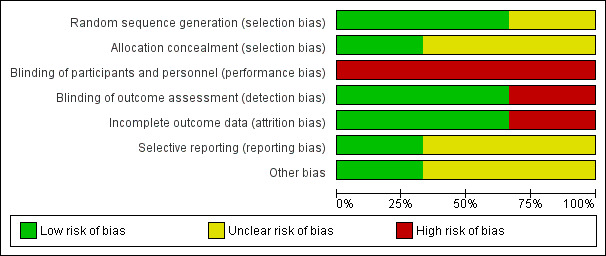
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
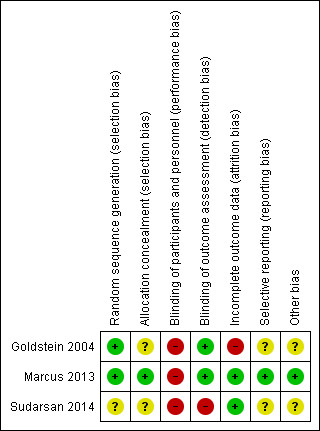
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The method of random sequence generation was adequately described in two trials (Goldstein 2004; Marcus 2013), while this was unclear in one trial (Sudarsan 2014). Allocation concealment was adequately described in one trial (Marcus 2013), and unclear in two trials (Goldstein 2004; Sudarsan 2014).
Blinding
Due to the nature of the interventions and the comparators, surgery versus no surgery, participants and personnel were not blinded. In two trials follow‐up assessments were performed by investigators who were blinded to the treatment group assignment of trial participants (Goldstein 2004; Marcus 2013), while this was not the case in one trial (Sudarsan 2014). In one trial teachers were asked to score several of the outcome measures and it was unclear whether they were blinded (Marcus 2013).
Incomplete outcome data
All trials reported the number of participants who failed to complete the trial and the number of participants that were excluded (Goldstein 2004; Marcus 2013; Sudarsan 2014). In Goldstein 2004, nine of the 29 (31%) randomised children were not included in the analyses; in Marcus 2013 and Sudarsan 2014 these percentages were 12% (56/453) and 9% (7/80), respectively. In the Marcus 2013 trial, a sensitivity analysis was performed on the primary outcome to assess the possible effect of these missing data and the results remained unchanged. No such analysis was reported in Goldstein 2004 and Sudarsan 2014.
Selective reporting
We had access to the study protocol for one trial (Marcus 2013). All outcomes reported in the publication were listed in the registered trial protocol. As such, we judged the risk of bias from selective reporting to be low for this trial. We had no access to the study protocol for the other trials (Goldstein 2004; Sudarsan 2014), so we judged the risk of bias from selective reporting to be unclear for these trials. As we could only include three studies, we refrained from conducting more formal methods of selective reporting assessment using funnel plots.
Other potential sources of bias
Baseline characteristics appeared to be balanced in the Marcus 2013 trial, while (slight) imbalances for baseline characteristics were observed in the trials of Goldstein 2004 and Sudarsan 2014. Intention‐to‐treat analyses was performed in one trial (Marcus 2013), but not in Goldstein 2004, while this was unclear in the Sudarsan 2014 trial.
There is a risk of underestimating the effects of surgery when a large number of patients in the non‐surgery group also undergo surgery during the course of the trial. In the Marcus 2013 trial, 16 children (7%) in the control group underwent surgery, while 16 children (7%) allocated to the surgical group did not undergo adenotonsillectomy. In the Goldstein 2004 trial, one child underwent adenoidectomy in the control group, but this child was excluded from analyses. No information on the number of children that underwent surgery in the control group was reported in the Sudarsan 2014 trial.
Effects of interventions
See: Table 1
The study populations differed substantially between the trials, i.e. non‐syndromic (Marcus 2013) and syndromic (Down syndrome and mucopolysaccharidosis (MPS)) (Sudarsan 2014) children diagnosed with mild to moderate obstructive sleep apnoea syndrome (OSAS) by polysomnography (PSG) and non‐syndromic children clinically classified as having obstructive sleep‐disordered breathing (oSDB) but with negative PSG recordings (Goldstein 2004). We therefore present the results of the studies separately. The available data did not allow us to assess whether variations in surgery (tonsillectomy with or without adenoidectomy) or variations in non‐surgical management affected the outcome. We carefully looked for and, where possible, extracted subgroup data for oSDB severity, body weight, age or race for each of the individual trial outcomes.
Children diagnosed with mild to moderate OSAS by PSG
The Marcus 2013 trial included 397 of the 453 randomised children (194 in the surgery group and 203 in the no surgery group) in their primary analyses and reported on the following outcomes:
Disease‐specific quality of life and/or symptom score
At baseline, mean total OSA‐18, PSQ‐SRBD and modified Epworth Sleepiness Scale scores were comparable in the adenotonsillectomy and watchful waiting with supportive care (WWSC) groups: 53.2 (standard deviation (SD 17.7)) versus 54.1 (SD 19.2), 0.5 (SD 0.2) versus 0.5 (SD 0.2), and 7.1 (SD 4.6) versus 7.4 (SD 5.1) respectively.
At seven months, mean scores for those instruments measuring disease‐specific quality of life and/or symptoms were lower (that is, better quality of life or fewer symptoms) in children receiving adenotonsillectomy than in those managed by watchful waiting. Respectively they were:
OSA‐18 questionnaire (scale 18 to 126): 31.8 (SD 14.9) versus 49.5 (SD 20.3) (mean difference (MD) ‐17.7, 95% confidence interval (CI) ‐21.2 to ‐14.2);
PSQ‐SRBD questionnaire (scale 0 to 1): 0.2 (SD 0.2) versus 0.5 (SD 0.2) (MD ‐0.3, 95% CI ‐0.34 to ‐0.26);
Modified Epworth Sleepiness Scale (scale 0 to 24): 5.1 (SD 4.4) versus 7.1 (SD 5.1) (MD ‐2.0, 95% CI ‐2.9 to ‐1.1).
Subgroup analyses revealed no interaction between OSAS severity, obesity or age and treatment with respect to any of the disease‐specific quality of life or symptom score instruments. An interaction was found between race and treatment for PSQ‐SRBD scores. The relative improvement associated with surgery was significantly lower in African‐American children compared with children of other races. The change from baseline was ‐0.24 (SD 0.19) versus ‐0.04 (SD 0.19) for adenotonsillectomy versus WWSC in African‐American children, and ‐0.32 (SD 0.16) versus ‐0.02 (SD 0.18) for others; interaction between race and treatment (P value < 0.01).
Quality of the evidence
We judged the evidence for this outcome to be of moderate quality; we downgraded it from high to moderate quality due to risk of detection bias (subjective outcome measures based on parental observation while parents were not blinded to treatment allocation).
Adverse events, complications and morbidity associated with adenotonsillectomy and comparators
During the seven‐month follow‐up period, a total of 15 children experienced a serious adverse event: 6/194 (3%) in the adenotonsillectomy group (three had tonsillar bleeding, one had postoperative pain, one had a lower respiratory tract illness and one suffered from vomiting/dehydration) versus 9/203 (4%) in the WWSC group (one had tonsillar bleeding, one had postoperative pain, three had an asthma exacerbation, one had an upper respiratory tract illness, one suffered from vomiting/dehydration, one had hypersomnolence and one had hypertension) (risk difference (RD) ‐1%, 95% CI ‐5% to 2%). In eight of the 236 children (3%) that underwent adenotonsillectomy during follow‐up, serious perioperative complications occurred (defined as bleeding, dehydration or pain requiring an additional surgical procedure, hospitalisation or prolonged hospitalisation). A total of nine children were classified as treatment failures (defined as serious change in clinical status potentially related to inadequately treated OSAS that might require additional intervention); all of these nine children were randomised to WWSC (9/203 (4%)) and were recommended for early adenotonsillectomy because of increased problems with sleep quality or sleepiness (three children), school behavioural problems (one child), morning headaches (one child), asthma exacerbation (one child), hypertension (one child) and bacterial infections (two children).
Quality of the evidence
We judged the evidence for this outcome to be of moderate quality; we downgraded it from high to moderate quality due to imprecision of the effect estimate.
Generic quality of life
Generic quality of life as assessed by the mean total caregiver‐rated PedsQL scores at baseline was 77.3 (SD 15.3) in the adenotonsillectomy group and 76.5 (SD 15.7) in the WWSC group. At seven months, children receiving adenotonsillectomy had a significantly higher mean total caregiver‐rated PedsQL score at seven months than those managed with WWSC (with higher scores indicating better quality of life): 83.3 (SD 15.1) versus 77.4 (SD 14.9) (MD 5.9, 95% CI 3.0 to 8.8).
Subgroup analysis for generic quality of life results revealed no interaction between OSAS severity, obesity, age or race.
Quality of the evidence
We judged the evidence for this outcome to be of moderate quality; we downgraded it from high to moderate quality due to risk of detection bias (subjective outcome measures based on parental observation while parents were not blinded to treatment allocation).
Respiratory events during sleep as measured by the Apnoea/Hypopnoea Index (AHI) using PSG
At baseline, mean AHI scores were 6.9 (SD 5.7) in the surgery group and 6.6 (SD 5.6) in the control group. At seven months, the mean AHI score was significantly lower in the surgery group compared with the control group: 1.6 (SD 3.0) versus 5.9 (SD 10.1) (MD ‐4.3, 95% CI ‐5.7 to ‐2.9).
Subgroup analyses revealed no interaction between obesity, age or race and treatment for AHI scores. AHI scores of children with more severe OSAS at baseline who underwent adenotonsillectomy improved more compared with those with less severe OSAS at baseline (interaction P value < 0.05).
More children in the early adenotonsillectomy group had normalisation of respiratory events during sleep as measured by PSG at seven months than those allocated to WWSC: 153/194 (79%) versus 93/203 (46%) (RD 33%, 95% CI 24% to 42%).
PSG recordings of African‐American children, obese children and children with more severe OSAS at baseline normalised less frequently irrespective of the assigned treatment.
Quality of the evidence
We judged the evidence for this outcome to be of high quality.
Other measures of respiratory events during sleep
In the adenotonsillectomy and WWSC groups, the mean Oxygen Desaturation Index (ODI) scores at baseline were 8.6 (SD 7.6) and 8.2 (SD 7.2), respectively. At seven months, the mean ODI score was significantly lower in the children receiving surgery compared with those allocated to control treatment: 3.88 (SD 4.1) versus 7.2 (SD 10.7) (MD ‐3.4, 95% CI ‐5.0 to ‐1.8).
At baseline, the mean percentage of sleep time with CO2 above 50 mm Hg was 12.0 (SD 19.9) in the surgery group and 9.0 (SD 19.1) in the control group. At seven months, the mean percentage of sleep time with CO2 above 50 mm Hg was lower in children who underwent adenotonsillectomy compared with those allocated to WWSC: 7.3 (SD 14.6) versus 9.5 (SD 18.5) (MD ‐2.2, 95% CI ‐6.0 to 1.6).
The relative improvements in mean ODI score and mean percentage of sleep time with CO2 above 50 mm Hg associated with adenotonsillectomy were significantly larger in children with more severe OSAS at baseline compared with those children with less severe OSAS at baseline (interaction terms P value < 0.01 and P value < 0.05, respectively). Subgroup analyses revealed no interaction between obesity, age or race and treatment for mean ODI scores and mean percentage of sleep time with CO2 above 50 mm Hg.
Quality of the evidence
We judged the evidence for this outcome to be of high quality.
Cardiovascular disease
There was no significant change in blood pressure and heart rate during the seven months follow‐up period in children receiving surgery compared with those allocated to watchful waiting. Baseline OSAS severity was associated with higher overnight heart rate (average increase in heart rate of three beats per minute for an AHI of 2 versus 10).
Quality of the evidence
We judged the evidence for this outcome to be of high quality.
Neurocognitive performance
Generalised intellectual functioning as measured by the General Conceptual Ability score from the Differential Ability Scales‐II (DAS) did not change significantly in either the adenotonsillectomy group or in the WWSC group (crude data not reported in the manuscript).
Quality of the evidence
We judged the evidence for this outcome to be of high quality.
Attention
At baseline, mean attention and executive function scores on the Developmental Neuropsychological Assessment (NEPSY) at baseline were comparable between the adenotonsillectomy and WWSC group (101.5 (SD 15.9) versus 101.1 (SD 14.6) respectively) and did not substantially differ from the normative mean (100 (SD 15)). Children in the surgery group had a higher mean NEPSY score at seven months than those in the no surgery group (with higher scores indicating better functioning) but the difference was not statistically significant: 108.6 (SD 15.5) versus 106.2 (SD 15.0) (MD 2.4, 95% CI ‐0.6 to 5.4).
No interaction between obesity, age or race and treatment for mean NEPSY scores was observed in subgroup analyses.
Quality of the evidence
We judged the evidence for this outcome to be of high quality.
Behaviour
At baseline, mean Conners Rating Scale scores as rated by caregivers and teachers did not substantially differ between the adenotonsillectomy and WWSC group: 52.5 (SD 11.6) versus 52.6 (SD 11.7) and 56.4 (SD 14.4) versus 55.1 (SD 12.8), respectively.
At seven months, mean scores for those instruments measuring caregiver‐reported ratings of behaviour were lower (that is better behaviour) in children receiving adenotonsillectomy than in those managed by watchful waiting:
Conners Rating Scale scores (scale 38 to 90): 49.6 (SD 10.8) versus 52.4 (SD 10.5) (MD ‐2.8, 95% CI ‐4.9 to ‐0.7);
BRIEF (scale 40 to 90): 46.8 (SD 11.6) versus 50.5 (SD 11.9) (MD ‐3.7, 95% CI ‐6.0 to ‐1.4).
However, at seven months, mean scores for those instruments measuring teacher‐reported ratings of behaviour did not significantly differ between the two groups:
Conners Rating Scale scores (scale 38 to 90): 51.6 (SD 12.0) versus 53.7 (SD 12.2) (MD ‐2.1, 95% CI ‐5.3 to 1.2);
BRIEF (scale 40 to 90): 54.2 (SD 13.6) versus 55.4 (SD 13.5) (MD ‐1.2, 95% CI ‐4.9 to 2.5).
Subgroup analyses revealed no interaction between OSAS severity, obesity or age and treatment with respect to any of the behaviour scores. An interaction between race and treatment was found for Conners Rating Scale and BRIEF scores as completed by caregivers. The relative improvement associated with adenotonsillectomy was significantly lower in African‐American children than in children of other race: Conners Rating Scale scores ‐1.06 (SD 10.85) versus ‐0.98 (SD 9.53) for adenotonsillectomy versus WWSC in African‐American children, and ‐4.84 (SD 9.49) versus 0.61 (SD 9.22) for others (interaction between race and treatment P value < 0.01) and BRIEF scores ‐1.82 (SD 8.86) versus ‐0.30 (SD 9.27) for adenotonsillectomy versus WWSC in African‐American children, and ‐4.98 (SD 7.69) versus 1.17 (SD 8.29) for others (interaction P value < 0.05).
Quality of the evidence
We judged the evidence for this outcome to be of moderate quality; we downgraded it from high to moderate quality due to risk of detection bias (subjective outcome measures based on parental and teacher observation while parents were not blinded to treatment allocation and it was unclear whether teachers were blinded).
Weight changes
Mean weight and body mass index (BMI) were comparable at baseline in the adenotonsillectomy and WWSC groups: 31.2 kg (SD 13.0) versus 30.5 kg (SD 12.4) and 19.1 kg/m2 (SD 5.0) versus 18.9 kg/m2 (SD 4.8), respectively. At seven months, mean weight and BMI were larger in the surgery group than in the no surgery group but the differences were not statistically significant: 34.6 kg (SD 14.1) versus 32.8 kg (SD 12.6) (MD 1.8, 95% CI ‐0.8 to 4.4) and 20.0 kg/m2 (SD 5.3) versus 19.3 kg/m2 (SD 4.7) (MD 0.7, 95% CI ‐0.3 to 1.7), respectively.
Subgroup analyses revealed no interaction between baseline weight status, age or race and treatment with respect to mean weight and BMI at follow‐up.
Quality of the evidence
We judged the evidence for this outcome to be of high quality.
Children with a clinical diagnosis of oSDB but negative PSG recordings
The Goldstein 2004 trial included 20 of the 29 randomised children (11 in the surgery group and nine in the no surgery group) in the final analyses and did report on the following outcomes:
Adverse events, complications and morbidity associated with adenotonsillectomy and comparators
No major complications, including postoperative respiratory complications, postoperative bleeding or readmissions to the hospital, were reported.
Respiratory events during sleep as measured by the AHI using PSG
At baseline, median AHI scores did not substantially differ between children receiving adenotonsillectomy and those allocated to no surgery: 0.5 (range 0 to 3.6) versus 0.6 (range 0 to 2.0). At six months, no significant difference was observed between the groups in median AHI scores: 0.4 (range 0 to 3.1) versus 0 (range 0 to 8.4) with a P value of 1.00.
Other measures of respiratory events during sleep
At baseline PSG, median Respiratory Disturbance Index (RDI) scores and median percentages of the night with oxygen saturation levels below 90% were comparable for the adenotonsillectomy and control group: 1.5 (range 0 to 4.7) versus 1.3 (range 0 to 2.6) and 0 (range 0 to 5.6) versus 0 (range 0 to 0.7), respectively. At six months, median RDI scores and median percentages of the night with oxygen saturation levels below 90% did not differ between the groups: 0.6 (range 0 to 4.2) versus 1.2 (range 0 to 13) and 0 (range 0 to 0.5) versus 0 (range 0 to 0.5), respectively.
Cardiovascular disease
Systemic hypertension was found initially in one of the 11 children (9%) in the adenotonsillectomy group compared with one the nine children (11%) in the non‐surgical group. At six months follow‐up none of the 20 children had hypertension. No children had echocardiograms suggestive of pulmonary hypertension at initial assessment or at follow‐up.
Weight changes
At baseline, mean body mass index (BMI) scores did not substantially differ between the adenotonsillectomy and the no surgery group: 2.2 (standard deviation (SD) 3.0) versus 2.0 (SD 3.0). Compared to baseline, mean BMI scores at follow‐up were higher in both groups, but there was no significant difference between the two groups at follow‐up: 3.1 (SD 3.0) versus 2.4 (SD 3.0) (MD 0.7, 95% CI ‐1.9 to 3.3). The number of children defined as obese (BMI greater or equal to 95th percentile) increased by 9% in the adenotonsillectomy group compared with no increase in the control group.
Quality of the evidence
We judged the evidence for these outcomes to be of very low quality; we downgraded it from high to very low quality due to the small sample size, the rate of attrition leading to a high risk of bias and the uncertainty as to whether the treatment received in the control group was adequate and represented current practice.
Children with Down syndrome and MPS diagnosed with mild to moderate OSAS by PSG
The Sudarsan 2014 trial included 73 of the 80 randomised children (37 in the surgery group and 36 in the no surgery group) in the final analyses and did report on the following outcomes:
Disease‐specific quality of life and/or symptom score
At baseline, mean total OSA‐18 and modified Epworth Sleepiness Scale scores were comparable in the adenotonsillectomy and continuous positive airway pressure (CPAP) group: 117.0 (SD 2.3) versus 116.9 (SD 1.3) and 13.8 (SD 1.3) versus 14.4 (SD 2.2), respectively.
The mean total OSA‐18 score at 12 months did not significantly differ between the adenotonsillectomy and CPAP groups: 73.6 (SD 4.1) versus 75.0 (SD 2.5) (MD ‐1.4, 95% CI ‐3.0 to 0.2). The mean modified Epworth Sleepiness Scale scores did not differ at six months: 11.0 (SD 0.9) versus 10.9 (SD 1.6) (MD 0.1, 95% CI ‐0.5 to 0.7), but were lower in the surgery group at 12 months: 5.5 (SD 1.4) versus 7.9 (SD 1.7) (MD ‐2.4, 95% CI ‐3.1 to ‐1.7).
Adverse events, complications and morbidity associated with adenotonsillectomy and comparators
During follow‐up, 2/37 (5%) developed a secondary haemorrhage after adenotonsillectomy, while 1/36 (3%) developed a rash on the nasal dorsum secondary to the CPAP mask (RD ‐3%, 95% CI ‐6% to 12%).
Respiratory events during sleep as measured by the AHI using PSG
At baseline, mean AHI scores were 3.8 (SD 1.4) in the surgery group and 3.5 (SD 1.5) in the control group. At six months, the mean AHI score of children undergoing early adenotonsillectomy was significantly higher than those of children allocated to CPAP: 2.6 (SD 0.9) versus 1.1 (0.6) (MD 1.5, 95% CI 1.2 to 1.9), but no differences were observed between the groups in mean AHI scores at 12 months: 1.1 (SD 0.7) versus 1.1 (SD 0.6) (MD 0.0, 95% CI ‐0.3 to 0.3).
Resolution of OSAS (AHI score below 1) as measured by PSG at 12 months was observed in 34/37 (92%) in the surgery group versus 31/36 (86%) managed by CPAP (RD 6%, 95% CI ‐9% to 20%).
Quality of the evidence
We judged the evidence for the adverse events outcome to be of very low quality and for all other outcomes to be of low quality; we downgraded it mainly due to the uncertainties around the method of randomisation and allocation concealment, and the unblinded outcome assessment leading to a high risk of bias. We further downgraded the evidence for the adverse events outcome due to imprecision of the effect estimate.
Discussion
Summary of main results
This review includes three trials comparing the effectiveness of adenotonsillectomy with non‐surgical management in children with obstructive sleep‐disordered breathing (oSDB). The studies evaluated three different groups of children: those who had been diagnosed with mild to moderate obstructive sleep apnoea syndrome (OSAS) based on the findings of an overnight sleep study (453 children aged five to nine years; low risk of bias; Marcus 2013), those who had symptoms and signs suggestive of oSDB but normal findings during an overnight sleep study (29 children aged two to 14 years; moderate to high risk of bias; Goldstein 2004), and children with Down syndrome or mucopolysaccharidosis (MPS) diagnosed with mild to moderate OSAS based on the findings of an overnight sleep study (80 children aged six to 12 years; moderate to high risk of bias; Sudarsan 2014). The studies included two different comparisons: adenotonsillectomy versus no surgery (Goldstein 2004; Marcus 2013), or adenotonsillectomy versus a breathing mask (continuous positive airway pressure; CPAP) during sleep (Sudarsan 2014).
For otherwise healthy children without a syndrome and of older age (five to nine years) diagnosed with mild to moderate OSAS by PSG there is moderate quality evidence that they benefit from early adenotonsillectomy in terms of quality of life, symptoms and behaviour as rated by caregivers and high quality evidence that they benefit in terms of PSG parameters, but not in terms of objective measures of attention and neurocognitive performance compared with watchful waiting. Furthermore, PSG recordings of almost half of the children managed non‐surgically had normalised by seven months.
For non‐syndromic children classified as having oSDB on purely clinical grounds but with negative recordings on PSG, the evidence on the effects of adenotonsillectomy is of very low quality and is inconclusive.
Low‐quality evidence suggests that adenotonsillectomy and CPAP may be equally effective in children with Down syndrome or MPS diagnosed with mild to moderate OSAS by PSG.
Overall completeness and applicability of evidence
We identified only three trials comparing the effectiveness of adenotonsillectomy with no surgery in children with oSDB. Based on one large, methodologically rigorous trial (Marcus 2013), there is moderate to high quality evidence that immediate adenotonsillectomy confers overall beneficial effects compared with watchful waiting in older (five to nine years), non‐syndromic children diagnosed with mild to moderate OSAS by PSG. It is, however, uncertain whether the results of this latter trial can be easily applied to daily clinical practice:
Children under five years were excluded, despite this being a population in whom this procedure is often performed for this purpose.
The trial included children with mild to moderate OSAS based on PSG recordings. However, in daily ENT practice PSG is not routinely performed in children with signs and symptoms suggestive of oSDB (Friedman 2013; Pringle 2013), and the decision for surgery is generally made on the basis of concerns over signs and symptoms, whether or not complemented by results of overnight pulse oximetry. A national UK case study survey performed in 2005 and repeated in 2011 showed that less than 2% of UK ENT surgeons would use PSG in assessing the child and approximately 70% would proceed with management of the child with no form of sleep study (Pringle 2013).
53% of the children were of African‐American race, which hampers the applicability of trial results to other populations, especially since African‐American children had lower rates of normalisation of PSG recordings irrespective of assigned treatment in the Marcus 2013 trial.
Children with severe OSAS were excluded because of ethical considerations and trial findings may therefore not be extrapolated to this specific group.
The trial did not include lifestyle interventions, medical treatments or mechanical interventions as comparators to adenotonsillectomy. There is some evidence supporting the use of anti‐inflammatory medications for the treatment of OSAS. A 2011 Cochrane review focusing on the effectiveness of anti‐inflammatory medications for OSAS in children found one small trial suitable for inclusion (Kuhle 2011). This trial randomly allocated 13 children diagnosed with mild to moderate OSAS by PSG to intranasal corticosteroids (fluticasone nasal spray) for six weeks and 12 children to placebo, and found intranasal corticosteroids spray to be superior in terms of AHI improvements over time (Brouillette 2001). A more recent double‐blind, placebo‐controlled trial performed in 46 children diagnosed with non‐severe OSAS by PSG showed montelukast (leukotriene receptor antagonist) for 12 weeks to be superior in terms of improvement in Obstructive Apnoea Index (OAI), symptoms and adenoid size over time (Goldbart 2012).
Neurocognitive performance expressed as mean attention and executive function score (NEPSY) at study enrolment did not substantially differ from the normative mean in the surgery and no surgery group. It is, however, unknown whether the mean NEPSY score is sensitive enough to detect neurocognitive impairment in children with mild to moderate OSAS or that the condition does not impact on neurocognitive functioning at all. Furthermore, the duration of follow‐up (i.e. seven months) may be too short to detect any significant change in NEPSY scores between the surgery and no surgery group and children on medication for Attention Deficit Hyperactivity Disorder (ADHD) were excluded.
Children with craniofacial disorders, genetic conditions such as Down syndrome and severe health problems were excluded, which hampers applicability of the trial findings to these complex patients.
Current evidence on the effects of surgery for children with a clinical diagnosis of oSDB but with negative PSG recordings is derived from one trial. We judged the quality of the evidence to be very low and insufficient to draw any meaningful conclusion (Goldstein 2004).
The third trial, with moderate to high risk of bias, compared the effects of surgery with CPAP in a specific population diagnosed with mild to moderate OSAS by PSG, i.e. children with Down syndrome and MPS (Sudarsan 2014). As such, it is unknown whether their findings also apply to non‐syndromic children.
Quality of the evidence
For non‐syndromic children diagnosed with mild to moderate OSAS by PSG, we judged the data on quality of life, symptoms, behaviour and adverse events to be of moderate quality, and the other outcome data to be of high quality. For non‐syndromic children with a clinical diagnosis of oSDB but with negative PSG recordings, we judged the evidence to be of very low quality and insufficient to draw any meaningful conclusion. For children with Down syndrome and MPS diagnosed with mild to moderate OSAS by PSG, we judged the evidence for the adverse events outcome to be of very low quality and for all other outcomes to be of low quality.
Potential biases in the review process
We strictly adhered to the pre‐specified review protocol, Venekamp 2014, and made only minor changes to this protocol when drafting the full review (see Differences between protocol and review section).
We are confident that we have included all relevant randomised controlled trials in our review since we did not identify any relevant publications based on our iterative search strategy, including a broad internet search and reviewing of the reference lists of all identified studies and systematic reviews.
There was substantial clinical heterogeneity between the included trials in terms of types of participants recruited, i.e. non‐syndromic (Marcus 2013) and syndromic (Down syndrome and MPS) (Sudarsan 2014) children diagnosed with mild to moderate OSAS by PSG and non‐syndromic children clinically classified as having oSDB but with negative PSG recordings (Goldstein 2004). As such, we refrained from performing meta‐analyses.
Agreements and disagreements with other studies or reviews
The effects of (adeno)tonsillectomy in children with oSDB have been assessed in several systematic reviews and meta‐analyses (Baldassari 2008; Costa 2009; Friedman 2009; Garetz 2008; Jeyakumar 2011; Marcus 2012; Sedky 2014; Teo 2013).
A comprehensive systematic review related to the 2012 clinical practice guideline recommendations on the management of paediatric OSAS by the American Academy of Pediatrics (AAP) synthesised the available evidence on the effects of adenotonsillectomy in children with oSDB (Marcus 2012). It was concluded that grade B quality evidence (moderate risk of bias) is available to conclude that "adenotonsillectomy is very effective in treating OSAS" and that "adenoidectomy or tonsillectomy alone may not be sufficient because residual lymphoid tissue may contribute to persistent obstruction". The 2012 AAP guideline therefore states that "if a child is determined to have OSAS, has a clinical examination consistent with adenotonsillar hypertrophy, and does not have a contraindication to surgery, the clinician should recommend adenotonsillectomy as the first line of treatment" and "if the child has OSAS but does not have adenotonsillar hypertrophy, other treatment should be considered" (Marcus 2012). This recommendation is in agreement with the overall findings of our review based on the findings of the Marcus 2013 trial, which can be considered as level A evidence (low risk of bias).
With our search we also identified a large number of other systematic reviews of non‐randomised or uncontrolled studies and individual studies (that were not cited in these reviews) assessing the effects of (adeno)tonsillectomy in children with oSDB (Table 3). In general, these studies support the high quality evidence derived from the Marcus 2013 trial. However, a few remarkable observations deserve further attention. First, 79% of the children undergoing adenotonsillectomy had normalisation of seven‐month PSG parameters (defined as a reduction in both the AHI score to less than 2 events per hour and the OAI score to less than 1 event per hour) in the Marcus 2013 trial. A 2009 systematic review and meta‐analysis of nine observational studies (526 children with oSDB) reported a postoperative "cure" rate (defined as an AHI score of less than 1 per hour) of approximately 60% (Friedman 2009). These discrepancies may be caused by differences in PSG parameters used to define "cure". Besides, the study populations are also different with OSAS being less severe in the Marcus 2013 trial compared with the children that were included in the systematic review of Friedman 2009 (mean AHI score before surgery: 6.7 versus 6.9 to 34.1, respectively). Second, 49% of children in the non‐surgical group had normalisation of seven‐month PSG parameters in the Marcus 2013 trial. This is higher than observed in the Burstein 2013 study, which investigated 16 children undergoing adenotonsillectomy for oSDB with 16 matched non‐surgery controls. At follow‐up PSG (1.4 to 2 years after the initial PSG), 44% of the adenotonsillectomy group had an AHI of less than 1 compared with 25% of the non‐surgical group. The reason for this low recovery rate may be the high prevalence of obese (75%) and African‐American children (91%) in the Burstein 2013 study. Finally, baseline mean attention and executive function based on the NEPSY scores in both groups was comparable with the normative mean in the Marcus 2013 trial. Although mean NEPSY scores improved in both groups, no statistically significant difference between the two groups was observed for the change in mean NEPSY scores from baseline. A recent systematic review examined the relationship between attention deficit hyperactivity disorder (ADHD) and paediatric oSDB by performing a meta‐analysis of 18 studies including 529 children and found a medium relationship between ADHD symptoms and PSG‐confirmed oSDB (Sedky 2014). The same review included 12 studies assessing pre‐ versus post‐adenotonsillectomy ADHD symptoms and found a medium decrease in ADHD symptoms at 2 to 13 months after surgery (Sedky 2014).
2. Agreements and disagreements with other studies and reviews.
| Systematic reviews | ||||||
| No. of studies | Participants | Intervention | Comparison | Outcomes | Results | |
| Baldassari 2008 | 9 | N = 1470 Age: 1 to 18 OSAS |
AT | n/a | QoL 1 to 6 mo (7 studies) | Significant improvement in OSA‐18 scores after AT |
| QoL 6 to 16 mo (2 studies) | Significant improvement in OSA‐18 scores after AT No significant differences between OSA‐18 scores after AT in short and long term |
|||||
| Garetz 2008 | 25 | N = 19 to 297 (range) Age: 0 to 18 oSDB |
AT | Children without SDB symptoms | QoL 6 and 9 to 23 mo (13 studies) | Significant improvements in OSD‐6, OSA‐18 and CHQPF‐28 scores after AT |
| Behaviour (12 studies) | Larger improvement on Conners scale in AT versus control children Significant improvement in CBCL and BASC scores after AT |
|||||
| Neurocognitive functioning (9 studies) | CPT, DAS and K‐ABC scores improved significantly after AT versus matched control children scores. Only NEPSY verbal scores were lower versus controls and these improved after surgery | |||||
| Costa 2009 | 4 | N = 110 Age: 0 to 18 Obese OSAS |
AT | n/a | AHI | Mean AHI decrease after AT: 18.3 events/hour |
| Cure rate using the individual study criteria (AHI < 5 or AHI ≤ 2) | 38.5% | |||||
| Friedman 2009 | 23 | N = 1079 Age: 0 to 20 OSAS |
AT | Different control groups used in individual studies | Treatment success as defined per each individual study (23 studies) | 66.3% |
| Treatment success defined as AHI < 1 (9 studies) | 59.8% | |||||
| Treatment success defined as AHI < 5 (16 studies) | 66.2% | |||||
| PSG cure rate in uncomplicated children (19 studies) | 73.8% | |||||
| PSG cure rate in children with co‐morbidities (e.g. obesity, severe OSAS) or in high‐risk populations (e.g. age < 3) (9 studies) | 38.7% | |||||
| Jeyakumar 2011 | 9 | N = 795 Age: 0 to 18 Normal weight or overweight Surgery for any reason |
T or AT | n/a | Change in BMI (3 studies) | BMI increase after surgery of 7% |
| Change in weight (3 studies) | Increase in standardised weight scores after surgery: 46% to 100% | |||||
| % weight gain (3 studies) | 50% to 75% gained weight, 28% lost weight and 22% to 31% unchanged after surgery | |||||
| Teo 2013 | 14 | N = 418 Age: 2 to 12 oSDB |
AT | n/a | Blood pressure (3 studies) | Improvement after AT |
| Mean pulmonary artery pressure (6 studies) | Improvement after AT | |||||
| Echocardiographic findings (7 studies) | Improvement after AT | |||||
| Pulse rate and pulse rate variability (1 study) | Decrease after AT | |||||
| Sedky 2014 | 12 | N = 529 Age: 0 to 18 oSDB |
AT | n/a | ADHD symptoms | Medium improvement in ADHD symptoms after AT |
| Individual studies not cited in systematic reviews | ||||||
| Study design | Study population | Intervention | Comparison | Outcomes | Results | |
| Arrarte 2007 | Non‐controlled observational study | Brazil N = 27 Age: 2 to 10 Respiratory obstructive symptoms during sleep |
AT | n/a | Nocturnal pulse oximetry (ODI) | Significant decrease in ODI after AT |
| oSDB symptoms | 92.6% of children noticed symptom improvement after AT | |||||
| Apostolidou 2008 | Prospective controlled study | Greece N = 70 Age: 0 to 16 OSAS Habitual snoring Adenoidal and/or tonsillar hypertrophy |
AT in obese children | AT in non‐obese children | OAHI < 1 | No differences between the 2 groups before and after AT |
| Mitchell 2009 | Prospective controlled study | USA N = 89 Age: 3 to 18 OSAS (AHI >2) |
AT in obese children | AT in non‐obese children | OSA‐18 | Most OSA‐18 scores were higher in obese versus non‐obese children after AT |
| BASC | No significant difference between groups after AT | |||||
| Attia 2010 | Prospective cohort study | Egypt N = 87 Age: 2 to 16 OSAS |
AT | Healthy children | AHI | Significant improvement in AHI after AT with postoperative values matching the control group |
| Ezzat 2010 | Cohort study | Egypt N = 184 Age: 3 to 16 OSAS symptoms with positive OPO |
AT | ‐ Healthy children ‐ AT for other reasons ‐ No ENT surgery |
IQ (S‐BIS) | Significant improvement in IQ after AT with postoperative values matching the control groups |
| Parental symptom questionnaire (not validated) | 99% reported symptom improvements after AT | |||||
| Fung 2010 | Case‐control study | Canada N = 98 Age ≤ 17 oSDB symptoms with positive OPO |
T or AT in obese children | T or AT in non‐obese children | Postoperative respiratory complications | Obese children had more complications than non‐obese children |
| Mean length of stay in hospital | Obese versus non‐obese children: 18 versus 8 hours | |||||
| Randhawa 2011 | Prospective cohort study | UK N = 258 Age: 6 to 16 OSAS (AHI ≥1) |
AT | Healthy children | CHQPF‐28 (4 years) | Significant improvements after AT in 9/13 domains |
| Goldstein 2012 | Non‐controlled observational study | USA N = 100 Age: 2 to 12 Snoring and disruptive sleep for 3 mo OSAS |
AT | n/a | CAS‐15 (not validated) | Significant improvement after AT |
| OSA‐18 | Significant improvement after AT | |||||
| PedsQL | Significant improvement after AT | |||||
| CBCL | Significant improvement after AT | |||||
| AHI | Mean AHI decrease after AT: 15.9 events/hour | |||||
| Tagaya 2012 | Non‐controlled observational study | Japan N = 49 Age: 1 to 10 OSAS (AHI ≥ 5) Normal weight |
AT | n/a | AHI (1.5 years) | Pre‐ and postoperative AHI were significantly higher in symptomatic versus asymptomatic children |
| Abreu 2013 | Prospective controlled study | Brazil N = 60 Age: 6 to 17 Symptoms of airway obstruction |
AT | Other paediatric surgery | TAVIS‐3 visual attention test | Significantly greater improvements in AT group versus other surgery group |
| Modified Epworth Sleepiness scale | Marked reduction in daytime sleepiness in AT group versus other surgery group | |||||
| Burstein 2013 | Matched, historical cohort study | USA N = 33 Age: 1 to 12 OSAS |
AT | No treatment | CAS‐15 (not validated) | Mean CAS‐15 was significantly lower in AT group |
| CBCL | Mean CBCL scores were significantly lower in AT group | |||||
| AHI | Significantly greater decrease in AHI among the AT group versus control group | |||||
| Huang 2014 | Non‐controlled observational study | Taiwan N = 88 Age: 8.9 (SD 2.7) OSAS |
AT | n/a | AHI (0 mo) | Mean AHI 13.5 (SD 7.2) |
| AHI (6 mo) | Mean AHI 3.5 (SD 8.1) | |||||
| AHI >1 (6 mo) | 53% | |||||
| AHI (36 mo) | Mean AHI 6.5 (SD 5.6) Residual OSAS after AT was associated with BMI, AHI, enuresis and allergic rhinitis before surgery |
|||||
| AHI >1 (36 mo) | 68% | |||||
| Kang 2014 | Non‐controlled observational study | Taiwan N = 119 Age: 6.9 (SD 3.3) OSAS |
AT | n/a | AHI (3 mo) | Mean AHI decrease after AT: 13.8 events/hour |
| OSA‐18 (3 mo) | Significant improvement after AT | |||||
| Kobayashi 2014 | Non‐controlled observational study | Japan N = 45 Age < 13 OSAS |
AT | n/a | AHI (3 to 6 mo) | Significant improvement after AT |
| OSA‐18 (3 to 6 mo) | Significant improvement after AT | |||||
| Lee 2014 | Non‐controlled observational study | Taiwan N = 144 Age: 2 to 18 Primary snoring (AHI < 1) and OSAS (AHI > 1) |
AT | n/a | OSA‐18 (3 mo) | Improvement in mean OSA‐18 scores after AT increased as disease severity increased and was not affected by gender, age or adiposity |
| Volsky 2014 | Prospective non‐randomised controlled study | USA N = 64 Age: 3 to 16 OSAS (AHI 1 to 5) and tonsillar hypertrophy |
AT | Observation | OSA‐18 (3 mo) | Mean OSA‐18 significantly improved in AT group versus no significant change in observation group |
| OSA‐18 (8 mo) | No statistically significant difference between the 2 groups | |||||
| CHQPF‐28 (3 mo) | No statistical difference between the 2 groups | |||||
| CHQPF‐28 (8 mo) | No statistically significant difference between the 2 groups | |||||
| Feng 2015 | Prospective cohort study | China N = 35 Age: 4 to 8 OSAS |
A and AT | Healthy children | OSA‐18 | Significant improvement after AT with postoperative values matching the control groups |
| C‐WISC | Significant improvement after AT with postoperative values matching the control groups | |||||
| Hamada 2015 | Non‐controlled observational study | Japan N = 147 Age: 11 mo to 6 years OSAS |
AT | n/a | AHI in infants and toddlers (N = 50) | Mean AHI before AT: 13.5 (SD 7.1); Mean AHI after AT: 4.7 (SD 3.4). |
| AHI in preschool children (N = 97) | Mean AHI before AT: 16.0 (SD 10.2); Mean AHI after AT: 4.4 (SD 2.2) |
|||||
| Lee 2015 | Non‐controlled observational study | Taiwan N = 144 Age: 2 to 18 Primary snoring (AHI < 1) and OSAS (AHI > 1) |
AT | n/a | OSA‐18 (3 mo) | Significant improvement after AT |
| OSA‐18 (6 mo) | Significant improvement after AT. No differences between 3 and 6 mo mean total OSA‐18 scores |
|||||
A: adenoidectomy; ADHD: attention deficit hyperactivity disorder; AHI: Apnoea/Hypopnoea Index; AT: adenotonsillectomy; BASC: Behavioural Assessment System for Children test; BMI: body mass index; CAS‐15: Clinical Assessment Score‐15; CBCL: Child Behavior Checklist; CHQPF‐28: Child Health Questionnaire Parent Form‐28; Conners: Conners rating scale; CPT: continuous performance test; C‐WISC: Chinese Wechsler Intelligence Scale For Children; DAS: Differential Abilities Scale; ENT: ear, nose and throat; K‐ABC: Kaufman Assessment Battery For Children; mo: months; n/a: not applicable; N: number; NEPSY: Developmental Neuropsychological Assessment; OAHI: Obstructive Apnoea/Hypopnoea Index; ODI: Oxygen Desaturation Index; OPO: overnight pulse oximetry; OSA‐18: Obstructive Sleep Apnoea‐18; OSAS: obstructive sleep apnoea syndrome; OSD‐6: Obstructive Sleep Disorders 6‐Survey; oSDB: obstructive sleep‐disordered breathing; PedsQL: Pediatric Quality of Life Inventory; PSG: polysomnography; QoL: quality of life; S‐BIS: Stanford‐Binet Intelligence Scales; SD: standard deviation; T: tonsillectomy; TAVIS‐3: 3rd version of a computerised test of visual attention
Authors' conclusions
Implications for practice.
For otherwise healthy children without a syndrome and of older age (five to nine years) diagnosed with mild to moderate obstructive sleep apnoea syndrome (OSAS) by polysomnography (PSG) there is moderate quality evidence that children benefit from early adenotonsillectomy in terms of quality of life, symptoms and behaviour as rated by caregivers and high quality evidence that they benefit in terms of PSG parameters, but not in terms of objective measures of attention and neurocognitive performance, compared with watchful waiting.
It is uncertain how these findings apply to children with obstructive sleep‐disordered breathing (oSDB) encountered in everyday ENT practices in which PSG is not routinely performed in children with signs and symptoms suggestive of oSDB when deciding whether or not to perform surgery. Adenotonsillectomy involves specific surgical risks (including bleeding and infection), the risks associated with a general anaesthetic (including an increased risk of intra‐ and postoperative respiratory compromise in certain children with oSDB), a period in hospital and a postoperative recovery period (with over 50% of children still experiencing pain three days after the operation despite analgesia). With these risks in mind physicians and parents should carefully weigh the benefits and risks of adenotonsillectomy against watchful waiting in older children (five to nine years) diagnosed with mild to moderate OSAS by PSG since the condition may recover spontaneously over time.
Implications for research.
While moderate quality evidence is available that early adenotonsillectomy confers benefit in terms of quality of life, symptoms and behaviour as rated by caregivers and high quality evidence that this procedure is beneficial in terms of PSG parameters in otherwise healthy children without a syndrome and of older age (five to nine years) diagnosed with mild to moderate OSAS by PSG, there are still important gaps in the evidence that need to be addressed in future research. It is currently unknown whether adenotonsillectomy is also effective in children younger than five years of age, an important group presenting to ENT surgeons and sleep physicians, and in those with a clinical diagnosis of oSDB rather than OSAS based on PSG recordings. Furthermore, the role of medical management (as an alternative or an adjunct to surgery) in children with oSDB has not been established.
Ongoing trials will address some of these gaps, including a wider age range of children and a range of diagnostic criteria for oSDB (NCT01918007; NCT02315911; POSTA Child Study).
The ongoing Australian POSTA Child Study (POSTA 1) includes children aged three to five years diagnosed with OSAS by PSG (defined as a pre‐operative Apnoea/Hypopnoea Index (AHI) score of 1 to 10 events per hour), has an intellectual test (of cognitive ability) as the primary outcome and follow‐up is up to two years (POSTA Child Study). The second Australian POSTA Child Study (POSTA 2), which is at the development stage, aims to randomise children who display symptoms of oSDB during PSG (e.g. snoring and flow limitation) but have an AHI score of less than 1.
A Swedish trial will randomise children aged between 2 and 4.9 years diagnosed with mild to moderate OSAS by PSG (defined as a pre‐operative AHI score of 2 to 10 events per hour) to adenotonsillectomy or active observation for six months (NCT02315911). The primary outcome is AHI at six months by PSG and further PSGs will be performed at three and 10 years follow‐up.
The Greek Chania Community Oximetry‐Based Study investigates whether adenotonsillectomy confers benefit over no surgery in children with a clinical diagnosis of snoring and adenotonsillar hypertrophy and abnormalities in oxygenation detected by overnight pulse oximetry (NCT01918007). The primary outcomes are change in the proportion of children with a McGill oximetry score of 1 between baseline and three months follow‐up assessment and the proportion of children who achieved a desaturation index (≥ 3% drop) of < 2 episodes per hour at three months, if they had a desaturation index of ≥ 3.5 episodes per hour at baseline. These trials will produce important evidence of the benefits of surgery in children with oSDB encountered in daily clinical practice.
However, areas of uncertainty will continue to exist around the diagnosis, prognosis and management of children with oSDB and how best to organise health services for children suffering from this condition. Important questions that deserve further investigations include 'how can parents, GPs, sleep physicians and ENT surgeons distinguish simple snoring from OSAS?', 'what role does PSG play in the diagnosis of oSDB and how does this impact on treatment decisions?', 'should PSG be repeated after medical or surgical treatment, and at which time points?', 'what is the effectiveness of medical management in children with oSDB in comparison or in addition to surgical management?', 'what is the impact of weight loss in children with oSDB?', 'what is the role of CPAP in children with oSDB, especially in those with special needs?', 'how compliant are families with this treatment at home?', 'which subgroups of children benefit most from various management strategies?', and, importantly, 'what are the concerns of parents of children with oSDB?', and 'which outcomes matter most to the parents of children with oSDB?'.
Notes
Split from 'Adenotonsillectomy for obstructive sleep apnoea in children' (Lim 2009) (to be withdrawn on completion of this review).
Acknowledgements
Dr Michael McKean was a co‐author of the original review.
We gratefully acknowledge the assistance received from the staff at the Cochrane ENT Disorders Group editorial base and thank Samantha Faulkner for her support with the search strategy and searches.
We would like to thank the external peer reviewers (Cristina Baldassari and Craig Derkay) and the editors for their valuable feedback.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Cochrane ENT Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
| Cochrane Register of Studies (Online) | Ovid MEDLINE | EMBASE (Ovid) |
| #1 MESH DESCRIPTOR Sleep Apnea Syndromes #2 MESH DESCRIPTOR Sleep Apnea, Obstructive EXPLODE ALL TREES #3 MESH DESCRIPTOR Snoring EXPLODE ALL TREES #4 (sleep* near (apnea* or hypopnea* or apneahypopnea* or apnoea* or hypopnoea* or apnoeic)):TI,AB,KY #5 (sleep* near3 disorder* near3 breath*):TI,AB,KY #6 (OSA or OSAS or OSAHS or SDB or SRBD or OSDB or SAHS):TI,AB,KY #7 ((hypertroph* or hyperplasia or obstructive) near3 (tonsil* or adenoid* or adenotonsil*)):TI,AB,KY #8 snoring:TI,AB,KY #9 ((nighttime or sleep* or "night time") near (((breath* or airway*) near (obstruct* or restric*)) or (mouth near3 breath*))):TI,AB,KY #10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 #11 MESH DESCRIPTOR Tonsillectomy EXPLODE ALL TREES #12 MESH DESCRIPTOR Palatine Tonsil EXPLODE ALL TREES WITH QUALIFIERS SU #13 (tonsillectom* or tonsilectom* or adenotonsillectom*):TI,AB,KY #14 MESH DESCRIPTOR Palatine Tonsil EXPLODE ALL TREES #15 (tonsil* or adenotonsil*):TI,AB,KY #16 #14 OR #15 #17 MESH DESCRIPTOR Surgical Procedures, Operative EXPLODE ALL TREES #18 (surg* or laser* or extract* or resect* or excis* or operat* or dissect* or remov* or coblat* or ablat*):TI,AB,KY #19 #17 OR #18 #20 #16 AND #19 #21 #11 OR #12 OR #13 OR #20 #22 #10 AND #21 |
1 Sleep Apnea Syndromes/ 2 exp Sleep Apnea, Obstructive/ 3 exp Snoring/ 4 (sleep* adj5 (apnea* or hypopnea* or apneahypopnea* or apnoea* or hypopnoea* or apnoeic)).ab,ti. 5 (sleep* adj3 disorder* adj3 breath*).ab,ti. 6 (OSA or OSAS or OSAHS or SDB or SRBD or OSDB or SAHS).ab,ti. 7 ((hypertroph* or hyperplasia or obstructive) adj3 (tonsil* or adenoid* or adenotonsil*)).ab,ti. 8 snoring.ab,ti. 9 ((nighttime or sleep* or "night time") adj3 (((breath* or airway*) adj5 (obstruct* or restric*)) or (mouth adj3 breath*))).ab,ti. 10 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 11 exp Tonsillectomy/ 12 exp Palatine Tonsil/su [Surgery] 13 (tonsillectom* or tonsilectom* or adenotonsillectom*).ab,ti. 14 exp Palatine Tonsil/ 15 (tonsil* or adenotonsil*).ab,ti. 16 14 or 15 17 exp Surgical Procedures, Operative/ 18 (surg* or laser* or extract* or resect* or excis* or operat* or dissect* or remov* or coblat* or ablat*).ab,ti. 19 17 or 18 20 16 and 19 21 11 or 12 or 13 or 20 22 10 and 21 |
1 Sleep Apnea Syndromes/ 2 exp Sleep Apnea, Obstructive/ 3 (sleep* adj3 (apnea* or hypopnea* or apneahypopnea* or apnoea* or hypopnoea* or apnoeic)).ab,ti. 4 (sleep* adj3 disorder* adj3 breath*).ab,ti. 5 (OSA or OSAS or OSAHS or SDB or SRBD or OSDB or SAHS).ab,ti. 6 ((hypertroph* or hyperplasia or obstructive) adj3 (tonsil* or adenoid* or adenotonsil*)).ab,ti. 7 ((nighttime or sleep* or "night time") adj3 (((breath* or airway*) adj5 (obstruct* or restric*)) or (mouth adj3 breath*))).ab,ti. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 exp *tonsillectomy/ 10 exp Palatine Tonsil/su [Surgery] 11 (tonsillectom* or tonsilectom* or adenotonsillectom*).ab,ti. 12 exp Palatine Tonsil/ 13 (tonsil* or adenotonsil*).ab,ti. 14 exp Surgical Procedures, Operative/ 15 (12 or 13) and 14 16 (surg* or laser* or extract* or resect* or excis* or operat* or dissect* or remov* or coblat* or ablat*).ab,ti. 17 (14 or 16) and 12 18 ((tonsil* or adenotonsil*) adj5 (surg* or laser* or extract* or resect* or excis* or operat* or dissect* or remov* or coblat* or ablat*)).ab,ti. 19 15 or 17 or 18 20 9 or 10 or 11 or 19 21 8 and 20 |
| CINAHL (EBSCO) | Web of Science (web of Science) | Trial Registries |
| S22 S10 AND S21 S21 S11 OR S12 OR S13 OR S20 S20 S16 AND S19 S19 S17 OR S18 S18 TX surg* or laser* or extract* or resect* or excis* or operat* or dissect* or remov* or coblat* or ablat* S17 (MH "Surgery, Operative+") S16 S14 OR S15 S15 TX tonsil* or adenotonsil* S14 (MH "Tonsil") S13 TX tonsillectom* or tonsilectom* or adenotonsillectom* S12 (MH "Tonsil/SU") S11 (MH "Tonsillectomy") S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 S9 TX (nighttime or sleep* or "night time") N5 (((breath* or airway*) N5 (obstruct* or restric*)) or (mouth N3 breath*)) S8 TX snoring S7 TX (hypertroph* or hyperplasia or obstructive) N3 (tonsil* or adenoid* or adenotonsil*) S6 TX OSA or OSAS or OSAHS or SDB or SRBD or OSDB or SAHS S5 TX sleep* N3 disorder* N3 breath* S4 TX sleep* N5 (apnea* or hypopnea* or apneahypopnea* or apnoea* or hypopnoea* or apnoeic) S3 (MH "Snoring") S2 (MH "Sleep Apnea, Obstructive") S1 (MH "Sleep Apnea Syndromes") |
#1 TOPIC: (sleep* near/5 (apnea* or hypopnea* or apneahypopnea* or apnoea* or hypopnoea* or apnoeic)) #2 TOPIC: (sleep* near/3 disorder* near/3 breath*) #3 TOPIC: (OSA or OSAS or OSAHS or SDB or SRBD or OSDB or SAHS) #4 TOPIC: ((hypertroph* or hyperplasia or obstructive) near/3 (tonsil* or adenoid* or adenotonsil*)) #5 TOPIC: (snoring) #6 TOPIC: (((nighttime or sleep* or "night time") near/5 (((breath* or airway*) near/5 (obstruct* or restric*)) or (mouth near/3 breath*)))) #7 #6 OR #5 OR #4 OR #3 OR #2 OR #1 #8 TOPIC: (tonsillectom* or tonsilectom* or adenotonsillectom*) #9 TOPIC: (((tonsil* or adenotonsil*) near/5 (surg* or laser* or extract* or resect* or excis* or operat* or dissect* or remov* or coblat* or ablat*))) #10 #9 OR #8 #11 #10 AND #7 |
ClinicalTrials.gov sleep AND (apnea OR hypopnea OR apneahypopnea OR apnoea OR hypopnoea OR apnoeic OR (disordered AND breathing)) AND (tonsilectomy OR adenotonsillectomy OR tonsillotomy) ICTRP sleep AND disorder* AND breath* AND tonsil* OR sleep AND apnea* AND tonsil* OR sleep AND apnoea* AND tonsil* OR sleep AND hypopnea* AND tonsil* OR sleep AND hypopnoea* AND tonsil* |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Goldstein 2004.
| Methods | Allocation: randomised; blocks of 2 Design: parallel groups | |
| Participants |
Number: 29 children (78 children were screened for eligibility; 59 were eligible, but in 3 children no PSG could be obtained and 27 children had positive PSG recordings)
Age: 2 to 14 years
Gender: 48% boys, 52% girls
Setting: paediatric otolaryngology private offices and clinics at the University Hospital of Brooklyn, SUNY Downstate Medical Center, and the otolaryngology and paediatric pulmonary clinics at the Kings County Hospital Center in Brooklyn (USA). Children were referred to the specialty offices by their GPs for evaluation of snoring and breathing difficulties during the night between March 1999 and May 2001
Eligibility criteria: children with a clinical assessment score of 40 or more and negative PSG recordings The clinical assessment score was based on the presence or absence of the following items: night‐time symptoms, daytime symptoms, history of mouth breathing, chronic rhinorrhoea, recurrent tonsillitis combined with findings at physical examination (including body mass index, blood pressure and tonsil size) and findings of further investigations (sleep tape, lateral neck radiography and echocardiogram) Symptoms highly suggestive for OSAS (e.g. pauses, gasping, daytime sleepiness) contributed more than non‐specific symptoms such as morning headache and rhinorrhoea. The more severe the symptom or the findings of physical examination or further investigation, the higher the score. Further details on the clinical assessment score can be found in Table 1 of the publication. PSG was considered positive for OSAS when the Respiratory Disturbance Index (number of obstructive apnoeas plus obstructive hypopnoeas per hour of sleep) was at least 5, or when oxygen saturation levels below 90% were present for at least 10% of the night. Obstructive apnoea was defined as the cessation of oronasal airflow with continued respiratory effort for at least 2.5 times the typical breath interval, while obstructive hypopnoea was defined as a decrease in oronasal airflow amplitude of at least 50% without a decrease in respiratory effort for the same duration Exclusion criteria: children with craniofacial syndromes, neuromuscular disorders or known cranial nerve palsies |
|
| Interventions |
Intervention group: adenotonsillectomy (method not specified); n = 15 Comparator group: no surgery (no further details provided); n = 14 Use of additional interventions: none described |
|
| Outcomes |
Primary outcome: change in clinical assessment score
Secondary outcomes: comparison of initial and final PSG recordings including Apnoea Index, Respiratory Disturbance Index and percentage of the night with oxygen saturation levels below 90%; proportion of patients with final clinical assessment score below 20 and below 40 According to protocol children would be reassessed at 6 months |
|
| Funding sources | The study was supported by a research grant from the National Institute of Child Health and Human Development | |
| Declarations of interest | No information provided | |
| Notes |
Participants lost to follow‐up total: 9/29 children (31%) Participants lost to follow‐up adenotonsillectomy group: 4/15 children (27%); 2 refused surgery and were lost to follow‐up, 2 had surgery but were lost to follow‐up) Participants lost to follow‐up no surgery group: 5/14 children (36%); 1 child received adenoidectomy and was excluded, 4 were lost to follow‐up) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by using a computerized list generated by biostatistician in blocks of 2." |
| Allocation concealment (selection bias) | Unclear risk | Not described Comment: small block size (2 participants) potentially allows prior knowledge of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "T&A was performed … by a pediatric otolaryngologist who was not 1 of the investigators." Quote: "The follow‐up assessments were performed by the investigators who were blinded to whether the child had had surgery." Comment: clinical assessment score was partly based on presence or absence of symptoms. Participants and parents were unblinded for treatment allocation and this might have had an impact on reporting of symptoms and as such might have introduced detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significant number of patients not included in analyses (31%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of low or high risk |
| Other bias | Unclear risk | Baseline characteristics: imbalance for gender, race and total clinical assessment score Did not use intention‐to‐treat analysis According to sample size calculation a total of 22 children (11 children in both treatment groups) were needed to detect a statistically significant difference in clinical assessment scores between the treatment and control group. In the final analyses, 20 children were included (11 children in the adenotonsillectomy group and 9 children in the no surgery group) |
Marcus 2013.
| Methods | Allocation: randomised; stratified by site, age, race and weight Design: parallel groups | |
| Participants |
Number: 453 children (10,519 children were screened for eligibility; 594 were eligible based on polysomnographic screening, but 130 were excluded owing to failure to meet other inclusion criteria and 11 were excluded owing to site withdrawal)
Age: 5 to 9 years
Gender: 49% boys, 51% girls
Comorbidities: 1 child in each group had hypertension Setting: 6 clinical sites each headed by an experienced paediatric sleep specialist or otolaryngologist (Children's Hospital of Pennsylvania; Cincinnati Children's Medical Center; Rainbow Babies and Children's Hospital, Cleveland; Children's Hospital Boston; Cardinal Glennon Children's Hospital, St Louis; Montefiore Medical Center, New York) Initially, paediatric sleep centres/sleep laboratories and paediatric ENT clinics were the primary recruitment sources. However, due to a slow accrual rate recruitment was broadened to general paediatric clinics and to public advertising in the general community. Recruitment period: January 2008 through September 2011 Eligibility criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention group: adenotonsillectomy (method not specified) within 4 weeks after randomisation; n = 226 Comparator group: watchful waiting with supportive care (WWSC, comprising conservative medical management); n = 227 Use of additional interventions: treatment or referral for treatment for comorbidities such as asthma and allergic rhinitis, education regarding general sleep hygiene and healthy behaviours, and use of nasal saline spray as needed |
|
| Outcomes |
Primary outcome: change in the attention and executive function scores on the Developmental Neuropsychological Assessment (NEPSY)
Secondary outcomes: caregiver and teacher ratings of behaviour as assessed by Conners Rating Scale Revised, behaviour as assessed by the Behaviour Rating Inventory of Executive Function (BRIEF); OSAS symptoms as assessed by the Pediatric Sleep Questionnaire Sleep‐Related Breathing Disorder scale (PSQ‐SRBD); sleepiness as assessed by the Epworth Sleepiness Scale modified for children; global quality of life as assessed by the caregiver‐rated total score from the Pediatric Quality of Life Inventory (PedsQL); disease‐specific quality of life as assessed by the total score on the 18‐item Obstructive Sleep Apnoea‐18 assessment tool; generalised intellectual functioning as assessed by the General Conceptual Ability score from the Differential Ability Scales‐II (DAS); PSG recordings According to protocol children would be reassessed at 7 months |
|
| Funding sources | The study was supported by research grants from the National Institutes of Health | |
| Declarations of interest | Declarations of interest provided by the authors at the end of the manuscript | |
| Notes |
Participants lost to follow‐up total: 56/453 children (12%) Participants lost to follow‐up adenotonsillectomy group: 32/226 children (14%) Participants lost to follow‐up no surgery group: 24/227 children (11%) Participants that received no surgery in treatment group: 16/226 (7%) Participants that received surgery in control group: 16/227 (7%) Trial has been registered at ClinicalTrials.gov: http://clinicaltrials.gov/show/NCT00560859 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation, stratified by ... is performed using a web‐based procedure maintained by the DCC." |
| Allocation concealment (selection bias) | Low risk | Quote: "Clinics do not have access to the randomisation schedule, so the standard of allocation concealment is met." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Thus, an usual single blinded situation is created, whereby subjects and parents are unblinded but the sleep physicians, some of whom are responsible for the overall conduct of pediatric sleep medicine at their site, are blinded. At each site, a research coordinator is identified who is unblinded, while other staff, such as those who perform neuropsychological testings, are blinded." Comment: parents and children were told not to discuss their treatment with study personnel. However, a risk of unblinding still exists. Study personnel documented all episodes in which parents or children discussed their treatments with study personnel. Furthermore, risk of bias due to unblinding is high for the subjective outcomes (Conners Rating Scale Revised, PedsQL, BRIEF, PSQ‐SRBD, OSA‐18) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12% of patients not included in analysis; a sensitivity analysis was performed on the primary outcome to assess the possible effect of these missing data and the results remained unchanged |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all outcomes reported in the manuscript were included in the study protocol |
| Other bias | Low risk | No other biases detected Intention‐to‐treat analysis was performed |
Sudarsan 2014.
| Methods | Allocation: randomised; no further information provided Design: parallel groups | |
| Participants |
Number: 80 children (124 children were screened for eligibility; 80 were eligible, 20 were excluded because of negative PSG recordings, 17 declined study participation and 7 children had previous craniofacial corrective surgeries)
Age: 6 to 12 years
Gender: 66% boys, 34% girls
Setting: participants were recruited from the MPS support and the DS Society, Chennai (India) along with individual referral cases Eligibility criteria: 1. MPS and DS syndromic children aged 6 to 12 years 2. Complaints of snoring and mouth breathing, daytime hyperactivity, urinary incontinence, restless sleep 3. Obstructive adenoids and tonsils (grade > 2) 4. OSAS based on positive PSG recordings (AHI > 1) Exclusion criteria: previous history of adenotonsillectomy and/or using CPAP, history of craniofacial reconstruction surgeries/other OSAS surgeries, central apnoea, unfit/unwilling for surgery/medications |
|
| Interventions |
Intervention group: adenotonsillectomy (coblation); n = 40 Children were monitored postoperatively in an intensive care unit (ICU) setup for a minimum of 24 hours and then discharged Comparator group: CPAP; n = 40 Children were admitted to the Sleep Lab of the hospital for an overnight stay for demonstration/trial and fitting of CPAP machine Use of additional interventions: none described |
|
| Outcomes |
Outcomes: 1. Cure defined as resolution of OSAS (AHI < 1) at 12 months 2. Mean AHI at 6 and 12 months 3. Parent/caregiver reported Epworth Sleepiness Scale‐Children (ESS‐C) with an ESS‐C > 10 set to be suggestive of OSAS at 6 and 12 months 4. Quality of life assessed by the parent/caregiver reported OSA‐18 questionnaires at 12 months 5. Complications |
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes |
Participants lost to follow‐up total: 7/80 children (9%) Participants lost to follow‐up adenotonsillectomy group: 3/40 children (8%) Participants lost to follow‐up no surgery group: 4/40 children (10%) No information provided on CPAP adherence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants, parents and outcome assessors were unblinded for treatment allocation and this might have had an impact on reporting of symptoms and as such might have introduced detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9% of children not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of low or high risk |
| Other bias | Unclear risk | Baseline characteristics: imbalance for gender and number of children with MPS Unclear whether they used intention‐to‐treat analysis No formal sample size calculations were performed No information provided on CPAP adherence |
ADHD: attention deficit hyperactivity disorder; AHI: Apnoea/Hypopnoea Index; BMI: body mass index; CPAP: continuous positive airway pressure; DCC: Data Co‐ordination Centre; DS: Down syndrome; GP: general practitioner; MPS: mucopolysaccharidosis; OAI: Obstructive Apnoea Index; OSAS: obstructive sleep apnoea syndrome PSG: polysomnography; WWSC: watchful waiting with supportive care
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brouillette 2013 | This article was a commentary on the Marcus 2013 trial |
| Ebell 2013 | This article was a commentary on the Marcus 2013 trial |
| Ramsden 2014 | This article was a commentary on the Marcus 2013 trial |
| Rodriguez 2014 | This article was a commentary on the Marcus 2013 trial |
| Schilder 2014 | This article was a commentary on the Marcus 2013 trial |
| Witmans 2013 | This article was a summary of the results of the Marcus 2013 trial |
| Xie 2010 | ALLOCATION We judged this study to be a quasi‐randomised trial as 30 children with odd numbers were assigned to group A (treatment group) while 30 children with even numbers were assigned to group B (control group) |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐TRC‐10001136.
| Trial name or title | 'Effect of adenotonsillectomy on behavioural problems in children with mild obstructive sleep apnoea: a randomized controlled trial' |
| Methods | Parallel, open randomised controlled trial |
| Participants | Hong Kong Chinese prepubertal children aged between 6 and 11 years with mild OSA confirmed by nocturnal PSG (AHI between 1 and 5) and parental report of habitual snoring (at least 3 nights per week) |
| Interventions |
Intervention: adenotonsillectomy Comparison: no surgery; parents of children allocated to this group will be given instructions to allow close monitoring of their child for any disease deterioration. They will also be provided with direct contact to the research team and an earlier follow‐up appointment will be scheduled if necessary |
| Outcomes | Primary outcome: behavioural and psychosocial changes assessed by Child Behaviour Checklist (CBCL) at 6 months Secondary outcomes: polysomnographic findings at 6 months; 24‐hour blood pressure at 6 months; attention assessed by Conners Continuous Performance Test II (CPT‐II) at 6 months; symptoms of attention deficiency hyperactivity disorder (ADHD) assessed by ADHD rating scale‐IV parents version at 6 months; daytime sleepiness assessed by the modified Epworth Sleepiness Scale (ESS) at 6 months; fasting insulin and glucose, serum lipid profile and serum inflammation |
| Starting date | December 2010 |
| Contact information | Dr. Albert Martin Li, Department of Paediatrics, Prince of Wales Hospital, Shatin, NT |
| Notes |
http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR‐TRC‐10001136 http://www.nhs.uk/Conditions/Snoring/Pages/clinical‐trial‐details.aspx?TrialId=ChiCTR‐TRC‐10001136&Condition=Snoring˜Children&pn=1&Rec=0&CT=0 |
NCT01918007.
| Trial name or title | 'Adenotonsillectomy for obstructive sleep‐disordered breathing in childhood: The Chania Community Oximetry‐Based Study' |
| Methods | Parallel, investigator‐blinded randomised controlled trial |
| Participants | Children with snoring and adenotonsillar hypertrophy and abnormalities in oxygenation detected by nocturnal pulse oximetry |
| Interventions |
Intervention: adenotonsillectomy Comparison: no adenotonsillectomy for 3 months after the baseline study evaluation |
| Outcomes |
Primary outcomes:
Secondary outcomes: somatic growth; abnormalities predisposing OSA and OSA symptoms; sleepiness; behavioural abnormalities; enuresis; quality of life; cardiovascular effects; systemic inflammation; effects on sympathetic nervous system activation; improvement in baseline oxygen saturation |
| Starting date | June 2013 |
| Contact information | Chania General Hospital 'St. George' |
| Notes | http://clinicaltrials.gov/show/NCT01918007 |
NCT02315911.
| Trial name or title | 'Randomized control trials of surgery for pediatric OSA' |
| Methods | Parallel randomised controlled trials |
| Participants | Children aged between 2 and 4.9 years with:
|
| Interventions | Children with mild to moderate OSAS: Intervention: adenotonsillectomy Comparison: expectant observation for 6 months Children with severe OSAS: Intervention: adenotonsillectomy Comparison: adenotonsillectomy and suturing of the tonsillar pillars (adeno‐pharyngoplasty) |
| Outcomes |
Primary outcome: AHI at 6 months PSG Secondary outcomes: ODI at 6 months PSG, postoperative pain, number of days until normal diet, speech or swallowing difficulties after surgery, per‐ and postoperative bleeding, disease‐specific quality of life (SDQ and OSA‐18), innate lymphoid cells in tonsils, PSG findings at 3 and 10 years follow‐up |
| Starting date | December 2014 |
| Contact information | Danielle Friberg, Assistant Professor, Karolinska University Hospital (Sweden) |
| Notes | https://clinicaltrials.gov/ct2/show/NCT02315911 |
POSTA Child Study.
| Trial name or title | 'The POSTA Child Study (Preschool Obstructive Sleep Apnoea (OSA) Tonsillectomy, Adenoidectomy Child Study)' |
| Methods | Parallel, open randomised controlled trial |
| Participants | Preschool aged children (age range 3 to 5 years) with mild to moderate OSA (defined as a pre‐operative AHI score of 1 to 10 events per hour), enlarged adenoids and tonsils and suitable for adenotonsillectomy |
| Interventions |
Intervention: adenotonsillectomy within 2 months of randomisation Comparison: delayed intervention group (usual 12 months waiting list for adenotonsillectomy) |
| Outcomes | Main outcomes: IQ assessed by neurocognitive and behavioural testing |
| Starting date | July 2010 |
| Contact information | Prof Karen Waters, Department of Respiratory Medicine, The Children's Hospital at Westmead Locked Bag 4001 Westmead NSW 2145, Australia |
| Notes |
http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12611000021976 https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611000021976 |
AHI: Apnoea/Hypopnoea Index; ODI: Oxygen Desaturation Index; OSA: obstructive sleep apnoea; OSAS: obstructive sleep apnoea syndrome; PSG: polysomnography; SDQ: Sleep Disorders Questionnaire.
Differences between protocol and review
Recently, our group has published a Cochrane review protocol on tonsillectomy or tonsillotomy for children with oSDB (Blackshaw 2014). To maximise agreement between these reviews we made the following changes to our protocol (Venekamp 2014):
we changed the age range of included children from "children up to the age of 16 years" to "children aged two years up to the age of 16 years";
we further specified the definition used in our second primary outcome ("adverse events, complications and morbidity associated with (adeno)tonsillectomy and comparators");
we defined "race (African‐American versus other)" as the fourth subgroup of interest.
Finally, we originally stated that three independent review authors would perform data extraction and 'Risk of bias' assessment of included studies, but for our full review two review authors (RPV, BJH) independently completed these tasks and any disagreements were resolved by discussion with a third review author (DC).
Contributions of authors
Protocol drafted by: RPV, BJH, DC, AGMS Screening search results: RPV, BJH Extracting data: RPV, BJH Assessing risk of bias: RPV, BJH Entering data into RevMan: RPV Carrying out analysis: RPV, BJH Interpreting the analysis: RPV, BJH, DC, HB, AGMS Writing the review: all authors General advice on the review: all authors
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
Professor Anne Schilder's team is supported by a NIHR Research Professorship Award
-
National Institute for Health Research, UK.
Infrastructure funding for Cochrane ENT
-
National Institute for Health Research, UK.
NIHR/Cochrane Incentive Award 2014
Declarations of interest
AGMS is joint Co‐ordinating Editor of the Cochrane ENT Group. RPV is an Editor of the Cochrane Acute Respiratory Infections (ARI) and ENT Group. BJH, DC, JL and HB declare no conflicts of interests in the current work.
New
References
References to studies included in this review
Goldstein 2004 {published data only}
- Goldstein NA, Pugazhendhi V, Rao SM, Weedon J, Campbell TF, Goldman AC, et al. Clinical assessment of pediatric obstructive sleep apnea. Pediatrics 2004;114(1):33‐4. [DOI] [PubMed] [Google Scholar]
Marcus 2013 {published data only}
- Garetz SL, Mitchell RB, Parker PD, Moore RH, Rosen CL, Giordani B, et al. Quality of life and obstructive sleep apnea syndrome after pediatric adenotonsillectomy. Pediatrics 2015;135(2):e477‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ES, Moore RH, Rosen CL, Mitchell RB, Armin R, Arens R, et al. Growth after adenotonsillectomy for obstructive sleep apnea: an RCT. Pediatrics 2014;132(2):282‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus CL, Moore RH, Rosen CL, Giordani B, Garetz SL, Mitchell RB, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. New England Journal of Medicine 2013;368(25):2366‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quante M, Wang R, Weng J, Rosen CL, Amin R, Garetz SL, et al. The effect of adenotonsillectomy for childhood sleep apnea on cardiometabolic measures. Sleep 2014 Dec 10 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Redline S, Amin R, Beebe D, Chervin RD, Garetz SL, Giordani B, et al. The Childhood Adenotonsillectomy Trial (CHAT): rationale, design, and challenges of a randomized controlled trial evaluating a standard surgical procedure in a pediatric population. Sleep 2011;34(11):1509‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sudarsan 2014 {published data only}
- Sudarsan SS, Paramasivan VK, Arumugam SV, Murali S, Kameswaran M. Comparison of treatment modalities in syndromic children with obstructive sleep apnea‐‐a randomized cohort study. International Journal of Pediatric Otorhinolaryngology 2014;78(9):1526‐33. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Brouillette 2013 {published data only}
- Brouillette RT. Adenotonsillectomy in childhood obstructive sleep apnea syndrome improves polysomnographic measures of breathing and sleep, but not attention and executive function. Journal of Pediatrics 2013;163(5):1530‐1. [DOI] [PubMed] [Google Scholar]
Ebell 2013 {published data only}
- Ebell MH. Early adenotonsillectomy and watchful waiting are both options for children with sleep apnea. American Family Physician 2013;88(7):468. [Google Scholar]
Ramsden 2014 {published data only}
- Ramsden JD. Symptoms of obstructive sleep apnoea are treated by adenotonsillectomy, but without change in neurocognitive outcome. Evidence Based Medicine 2014;19(2):62. [DOI] [PubMed] [Google Scholar]
Rodriguez 2014 {published data only}
- Rodriguez H. A randomized trial of adenotonsillectomy for childhood sleep apnea. Archivos Argentinos De Pediatria 2014;112(1):106‐17. [Google Scholar]
Schilder 2014 {published data only}
- Schilder AG. Early adenotonsillectomy for obstructive sleep apnoea improved quality of life and symptoms but not attention or executive function. Archives of Disease in Childhood – Education and Practice 2014;99(5):199. [DOI] [PubMed] [Google Scholar]
Witmans 2013 {published data only}
- Witmans M, Shafazand S. Adenotonsillectomy or watchful waiting in the management of childhood obstructive sleep apnea. Journal of Clinical Sleep Medicine 2013;15(9):1225‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xie 2010 {published data only}
- Xie L‐S, Huang Q. Efficacy of adenoidectomy and tonsillectomy for children with obstructive sleep apnea syndrome. Journal of Otolaryngology and Ophthalmology of Shandong University 2010;24(5):21‐3. [Google Scholar]
References to ongoing studies
ChiCTR‐TRC‐10001136 {published data only}
- ChiCTR‐TRC‐10001136. Effect of adenotonsillectomy on behavioural problems in children with mild obstructive sleep apnoea: a randomized controlled trial. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR‐TRC‐10001136 (accessed 11 November 2014).
NCT01918007 {published data only}
- NCT01918007. Adenotonsillectomy for obstructive sleep‐disordered breathing in childhood:The Chania Community Oximetry‐Based Study. http://clinicaltrials.gov/show/NCT01918007 (accessed 11 November 2014).
NCT02315911 {published data only}
- NCT02315911. Randomized control trials of surgery for pediatric OSA. https://clinicaltrials.gov/ct2/show/NCT02315911(accessed 12 March 2015).
POSTA Child Study {published data only}
- The POSTA Child Study. Preschool Obstructive Sleep Apnoea (OSA) Tonsillectomy, Adenoidectomy child study. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12611000021976 (accessed 11 November 2014).
Additional references
Abreu 2013
- Abreu CB, Fuchs SC, Pascoto GR, Weber R, Guedes MC, Pignatari SS, et al. Effect of adenotonsillectomy on visual attention tests among children with sleep‐disordered breathing: a controlled prospective cohort study. Clinical Otolaryngology 2013;38(6):487‐93. [DOI] [PubMed] [Google Scholar]
Achar 2015
- Achar P, Sharma RK, de S, Donne AJ. Does primary indication for tonsillectomy influence post‐tonsillectomy haemorrhage rates in children?. International Journal of Pediatric Otorhinolaryngology 2015;79(2):246‐50. [DOI] [PubMed] [Google Scholar]
Apostolidou 2008
- Apostolidou MT, Alexopoulos EI, Chaidas K, Ntamagka G, Karathanasi A, Apostolidis TI, et al. Obesity and persisting sleep apnea after adenotonsillectomy in Greek children. Chest 2008;134(6):1149‐55. [DOI] [PubMed] [Google Scholar]
Arrarte 2007
- Arrarte J, Lubianca Neto JF, Fischer GB. The effect of adenotonsillectomy on oxygen saturation in children with sleep breathing disorders. International Journal of Pediatric Otorhinolaryngology 2007;71(6):973‐8. [DOI] [PubMed] [Google Scholar]
Attia 2010
- Attia G, Ahmad MA, Saleh AB, Elsharkawy A. Impact of obstructive sleep apnea on global myocardial performance in children assessed by tissue Doppler imaging. Pediatric Cardiology 2010;31(7):1025‐36. [DOI] [PubMed] [Google Scholar]
Baldassari 2008
- Baldassari CM, Mitchell RB, Schubert C, Rudnick EF. Pediatric obstructive sleep apnea and quality of life: a meta‐analysis. Otolaryngology ‐ Head and Neck Surgery 2008;138(3):265‐73. [DOI] [PubMed] [Google Scholar]
Baldassari 2014
- Baldassari CM, Alam L, Vigilar M, Benke J, Martin C, Ishman S. Correlation between REM AHI and quality‐of‐life scores in children with sleep‐disordered breathing. Otolaryngology ‐ Head and Neck Surgery 2014;151(4):687‐91. [DOI] [PubMed] [Google Scholar]
Baugh 2011
- Baugh RF, Archer SM, Mitchell RB, Rosenfeld RM, Amin R, Burns JJ, et al. Clinical practice guideline: tonsillectomy in children. Otolaryngology ‐ Head and Neck Surgery 2011;144:S1‐30. [DOI] [PubMed] [Google Scholar]
Beebe 2006
- Beebe DW. Neurobehavioral morbidity associated with disordered breathing during sleep in children: a comprehensive review. Sleep 2006;29(9):1115‐34. [DOI] [PubMed] [Google Scholar]
Blackshaw 2014
- Blackshaw H, Zhang LY, Venekamp RP, Wang B, Chandrasekharan D, Schilder AGM. Tonsillectomy versus tonsillotomy for sleep‐disordered breathing in children. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD011365] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brouillette 2001
- Brouillette RT, Manoukian JJ, Ducharme FM, Oudjhane K, Earle LG, Ladan S, et al. Efficacy of fluticasone nasal spray for pediatric obstructive apnea. Journal of Pediatrics 2001;138(6):838‐44. [DOI] [PubMed] [Google Scholar]
Burstein 2013
- Burstein DH, Jackson A, Weedon J, Graw‐Panzer KD, Fahmy S, Goldstein NA. Adenotonsillectomy for sleep‐disordered breathing in a predominantly obese pediatric population. International Journal of Pediatric Otorhinolaryngology 2013;77(4):525‐9. [DOI] [PubMed] [Google Scholar]
Certal 2012
- Certal V, Catumbela E, Winck JC, Azevedo I, Teixeira‐Pinto A, Costa‐Pereira A. Clinical assessment of pediatric obstructive sleep apnea: a systematic review and meta‐analysis. Laryngoscope 2012;122(9):2105‐14. [DOI] [PubMed] [Google Scholar]
Cooper 2013
- Cooper L, Ford K, Bajaj Y. Paediatric adenotonsillectomy as a daycase for obstructive sleep apnoea: how we do it in a tertiary unit. International Journal of Pediatric Otorhinolaryngology 2013;77(11):1877‐80. [DOI] [PubMed] [Google Scholar]
Costa 2009
- Costa DJ, Mitchell R. Adenotonsillectomy for obstructive sleep apnea in obese children: a meta‐analysis. Otolaryngology ‐ Head and Neck Surgery 2009;140(4):455‐60. [DOI] [PubMed] [Google Scholar]
Erickson 2009
- Erickson BK, Larson DR, Sauver JL, Meverden RA, Orvidas LJ. Changes in incidence and indications of tonsillectomy and adenotonsillectomy, 1970‐2005. Otolaryngology ‐ Head and Neck Surgery 2009;140(6):894‐901. [DOI] [PubMed] [Google Scholar]
Ezzat 2010
- Ezzat WF, Fawaz S, Abdelrazek Y. To what degree does adenotonsillectomy affect neurocognitive performance in children with obstructive sleep apnea hypopnea syndrome due to adenotonsillar enlargement. ORL; Journal of Oto‐Rhino‐Laryngology and Its Related Specialties 2010;72(4):215‐9. [DOI] [PubMed] [Google Scholar]
Feng 2015
- Feng HW, Jiang T, Zhang HP, Wang Z, Zhang HL, Zhang H, et al. Comparisons of thyroid hormone, intelligence, attention, and quality of life in children with obstructive sleep apnea hypopnea syndrome before and after endoscopic adenoidectomy. BioMed Research International 2015;2015:523716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedman 2009
- Friedman M, Wilson M, Lin HC, Chang HW. Updated systematic review of tonsillectomy and adenoidectomy for treatment of pediatric obstructive sleep apnea/hypopnea syndrome. Otolaryngology ‐ Head and Neck Surgery 2009;140(6):800–8. [DOI] [PubMed] [Google Scholar]
Friedman 2011
- Friedman BC, Goldman RD. Anti‐inflammatory therapy for obstructive sleep apnea in children. Canadian Family Physician 2011;57(8):891‐3. [PMC free article] [PubMed] [Google Scholar]
Friedman 2013
- Friedman NR, Perkins JN, McNair B, Mitchell RB. Current practice patterns for sleep‐disordered breathing in children. Laryngoscope 2013;123(4):1055‐8. [DOI] [PubMed] [Google Scholar]
Fung 2010
- Fung E, Cave D, Witmans M, Gan K, El‐Hakim H. Postoperative respiratory complications and recovery in obese children following adenotonsillectomy for sleep‐disordered breathing: a case‐control study. Otolaryngology ‐ Head and Neck Surgery 2010;142(6):898‐905. [DOI] [PubMed] [Google Scholar]
Garetz 2008
- Garetz S. Behavior, cognition, and quality of life after adenotonsillectomy for pediatric sleep‐disordered breathing: summary of the literature. Otolaryngology ‐ Head and Neck Surgery 2008;138(1):S19‐26. [DOI] [PubMed] [Google Scholar]
Goldbart 2004
- Goldbart AD, Goldman JL, Li RC, Brittian KR, Tauman R, Gozal D. Differential expression of cysteinyl leukotriene receptors 1 and 2 in tonsils of children with obstructive sleep apnea syndrome or recurrent infection. Chest 2004;126(1):13‐8. [DOI] [PubMed] [Google Scholar]
Goldbart 2012
- Goldbart AD, Greenberg‐Dotan S, Tal A. Montelukast for children with obstructive sleep apnea: a double‐blind, placebo‐controlled study. Pediatrics 2012;130(3):e575‐80. [DOI] [PubMed] [Google Scholar]
Goldstein 2012
- Goldstein NA, Stefanov DG, Graw‐Panzer KD, Fahmy SA, Fishkin S, Jackson A, et al. Validation of a clinical assessment score for pediatric sleep‐disordered breathing. Laryngoscope 2012;122(9):2096‐104. [DOI] [PubMed] [Google Scholar]
Gottlieb 2004
- Gottlieb DJ, Chase C, Vezina RM, Heeren TC, Corwin MJ, Auerbach SH, et al. Sleep‐disordered breathing symptoms are associated with poorer cognitive function in 5‐year‐old children. Journal of Pediatrics 2004;145(4):458‐64. [DOI] [PubMed] [Google Scholar]
Hamada 2015
- Hamada M, Iida M, Nota J, Matsumoto N, Sawada S, Mukushita N, et al. Safety and efficacy of adenotonsillectomy for obstructive sleep apnea in infants, toddlers, and preschool children. Auris Nasus Larynx 2015;42(3):208‐12. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Huang 2014
- Huang YS, Guilleminault C, Lee LA, Lin CH, Hwang FM. Treatment outcomes of adenotonsillectomy for children with obstructive sleep apnea: a prospective longitudinal study. Sleep 2014;37(1):71‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hullmann 2011
- Hullmann SE, Ryan JL, Ramsey RR, Chaney JM, Mullins LL. Measures of general pediatric quality of life. Arthritis Care and Research 2011;63(S11):S420‐30. [DOI] [PubMed] [Google Scholar]
Jeyakumar 2011
- Jeyakumar A, Fettman N, Armbrecht ES, Mitchell R. A systematic review of adenotonsillectomy as a risk factor for childhood obesity. Otolaryngology ‐ Head and Neck Surgery 2011;144(2):154‐8. [DOI] [PubMed] [Google Scholar]
Kang 2014
- Kang KT, Weng WC, Lee CH, Lee PL, Hsu WC. Discrepancy between objective and subjective outcomes after adenotonsillectomy in children with obstructive sleep apnea syndrome. Otolaryngology ‐ Head and Neck Surgery 2014;151(1):150‐8. [DOI] [PubMed] [Google Scholar]
Kim 2009
- Kim J, Bhattacharjee R, Dayyat E, Snow AB, Kheirandish‐Gozal L, Goldman JL, et al. Increased cellular proliferation and inflammatory cytokines in tonsils derived from children with obstructive sleep apnea. Pediatric Research 2009;66(4):423‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kobayashi 2014
- Kobayashi R, Miyazaki S, Karaki M, Hoshikawa H, Nakata S, Hara H, et al. Evaluation of adenotonsillectomy and tonsillectomy for pediatric obstructive sleep apnea by rhinomanometry and the OSA‐18 questionnaire. Acta Oto‐Laryngologica 2014;134(8):818‐23. [DOI] [PubMed] [Google Scholar]
Kuhle 2011
- Kuhle S, Urschitz MS. Anti‐inflammatory medications for obstructive sleep apnea in children. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD007074.pub2] [DOI] [PubMed] [Google Scholar]
Lalakea 1999
- Lalakea ML, Marquez‐Biggs I, Messner AH. Safety of pediatric short‐stay tonsillectomy. Archives of Otolaryngology ‐ Head and Neck Surgery 1999;125(7):749‐52. [DOI] [PubMed] [Google Scholar]
Lee 2014
- Lee CH, Kang KT, Weng WC, Lee PL, Hsu WC. Quality of life after adenotonsillectomy for children with sleep‐disordered breathing: a linear mixed model analysis. International Journal of Pediatric Otorhinolaryngology 2014;78(8):1374‐80. [DOI] [PubMed] [Google Scholar]
Lee 2015
- Lee CH, Kang KT, Weng WC, Lee PL, Hsu WC. Quality of life after adenotonsillectomy in children with obstructive sleep apnea: short‐term and long‐term results. International Journal of Pediatric Otorhinolaryngology 2015;79(2):210‐5. [DOI] [PubMed] [Google Scholar]
Lim 2009
- Lim J, McKean MC. Adenotonsillectomy for obstructive sleep apnoea in children. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD003136.pub2] [DOI] [PubMed] [Google Scholar]
Marcus 2001
- Marcus CL. Sleep‐disordered breathing in children. American Journal of Respiratory and Critical Care Medicine 2001;164(1):16‐30. [DOI] [PubMed] [Google Scholar]
Marcus 2005
- Marcus CL, Katz ES, Lutz J, Black CA, Galster P, Carson KA. Upper airway dynamic responses in children with the obstructive sleep apnea syndrome. Pediatric Research 2005;57(1):99‐107. [DOI] [PubMed] [Google Scholar]
Marcus 2012
- Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AG, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012;130(3):576‐84. [DOI] [PubMed] [Google Scholar]
Mitchell 2009
- Mitchell RB, Boss EF. Pediatric obstructive sleep apnea in obese and normal‐weight children: impact of adenotonsillectomy on quality‐of‐life and behavior. Developmental Neuropsychology 2009;34(5):650‐61. [DOI] [PubMed] [Google Scholar]
Mitchell 2015
- Mitchell RB, Garetz S, Moore RH, Rosen CL, Marcus CL, Katz ES, et al. The use of clinical parameters to predict obstructive sleep apnea syndrome severity in children: the Childhood Adenotonsillectomy (CHAT) study randomized clinical trial. JAMA Otolaryngology ‐ Head and Neck Surgery 2015;141(2):130‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nespoli 2013
- Nespoli L, Caprioglio A, Brunetti L, Nosetti L. Obstructive sleep apnea syndrome in childhood. Early Human Development 2013;89(1):33‐7. [DOI] [PubMed] [Google Scholar]
Owens 2009
- Owens JA. Neurocognitive and behavioral impact of sleep disordered breathing in children. Pediatric Pulmonology 2009;44(5):417‐22. [DOI] [PubMed] [Google Scholar]
Patel 2014
- Patel HH, Straight CE, Lehman EB, Tanner M, Carr MM. Indications for tonsillectomy: a 10 year retrospective review. International Journal of Pediatric Otorhinolaryngology 2014;78(12):2151‐5. [DOI] [PubMed] [Google Scholar]
Pringle 2013
- Pringle MB, Natesh BG, Buchanan EM. National UK survey on the assessment and surgical management of suspected paediatric obstructive sleep apnoea syndrome. International Journal of Pediatric Otorhinolaryngology 2013;77(10):1689‐96. [DOI] [PubMed] [Google Scholar]
Randhawa 2011
- Randhawa PS, Cetto R, Chilvers G, Georgalas C, Narula AA. Long‐term quality‐of‐life outcomes in children undergoing adenotonsillectomy for obstructive sleep apnoea: a longitudinal study. Clinical Otolaryngology 2011;36(5):475‐81. [DOI] [PubMed] [Google Scholar]
Robb 2009
- Robb PJ, Bew S, Kubba H, Murphy N, Primhak R, Rollin A‐M, et al. Tonsillectomy and adenoidectomy in children with sleep related breathing disorders: consensus statement of a UK multidisciplinary working party. Clinical Otolaryngology 2009;34(1):61‐3. [DOI] [PubMed] [Google Scholar]
Rosen 2004
- Rosen CL, Storfer‐Isser A, Taylor HG, Kirchner HL, Emancipator JL, Redline S. Increased behavioral morbidity in school‐aged children with sleep‐disordered breathing. Pediatrics 2004;114(6):1640‐8. [DOI] [PubMed] [Google Scholar]
Royal College of Paediatrics and Child Health 2009
- Royal College of Paediatrics and Child Health. Working Party on Sleep Physiology and Respiratory Control Disorders in Childhood; Standards for Services for Children with Disorders of Sleep Physiology 2009. http://www.rcpch.ac.uk/sites/default/files/asset_library/Research/Clinical%20Effectiveness/Endorsed%20guidelines/Sleep%20Physiology%20Disorders%20%28RCPCH%29/Report%20TextC.pdf 2009.
Schwengel 2009
- Schwengel DA, Sterni LM, Tunkel DE, Heitmiller ES. Perioperative management of children with obstructive sleep apnea. Anesthesia & Analgesia 2009;109(1):60‐75. [DOI] [PubMed] [Google Scholar]
Sedky 2014
- Sedky S, Bennett DS, Carvalho KS. Attention deficit hyperactivity disorder and sleep disordered breathing in pediatric populations: a meta‐analysis. Sleep Medicine Reviews 2014;18(4):349‐56. [DOI] [PubMed] [Google Scholar]
Shelton 1993
- Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. American Review of Respiratory Disease 1993;148(2):462‐6. [DOI] [PubMed] [Google Scholar]
Shine 2005
- Shine NP, Coates HL, Lannigan FJ. Obstructive sleep apnea, morbid obesity, and adenotonsillar surgery: a review of the literature. International Journal of Pediatric Otorhinolaryngology 2005;69(11):1475‐82. [DOI] [PubMed] [Google Scholar]
Spruyt 2011
- Spruyt K, Gozal D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: a review of currently available instruments. Sleep Medicine Reviews 2011;15(1):19‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanko 2013
- Stanko D, Bergesio R, Davies K, Hegarty M, Ungern‐Sternberg BS. Postoperative pain, nausea and vomiting following adeno‐tonsillectomy ‐ a long‐term follow‐up. Pediatric Anesthesia 2013;23(8):690‐6. [DOI] [PubMed] [Google Scholar]
Statham 2006
- Statham MM, Elluru RG, Buncher R, Kalra M. Adenotonsillectomy for obstructive sleep apnea syndrome in young children: prevalence of pulmonary complications. Archives of Otolaryngology ‐‐ Head and Neck Surgery 2006;132(5):476‐80. [DOI] [PubMed] [Google Scholar]
Tagaya 2012
- Tagaya M, Nakata S, Yasuma F, Mitchell RB, Sasaki F, Miyazaki, S, et al. Children with severe or moderate obstructive sleep apnoea syndrome show a high incidence of persistence after adenotonsillectomy. Acta Oto‐Laryngologica 2012;132(11):1208‐14. [DOI] [PubMed] [Google Scholar]
Tauman 2011
- Tauman R, Gozal D. Obstructive sleep apnea syndrome in children. Expert Review of Respiratory Medicine 2011;5(3):425‐40. [DOI] [PubMed] [Google Scholar]
Teo 2013
- Teo DT, Mitchell RB. Systematic review of effects of adenotonsillectomy on cardiovascular parameters in children with obstructive sleep apnea. Otolaryngology ‐ Head and Neck Surgery 2013;148(1):21‐8. [DOI] [PubMed] [Google Scholar]
Volsky 2014
- Volsky PG, Woughter MA, Beydoun HA, Derkay CS, Baldassari CM. Adenotonsillectomy vs observation for management of mild obstructive sleep apnea in children. Otolaryngology ‐ Head and Neck Surgery 2014;150(1):126‐32. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Venekamp 2014
- Venekamp RP, Hearne BJ, Chandrasekharan D, Blackshaw H, Lim J, Schilder AGM. Tonsillectomy or adenotonsillectomy versus non‐surgical management for sleep‐disordered breathing in children. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD011165] [DOI] [PMC free article] [PubMed] [Google Scholar]


