Abstract
Aims
Visual information about subclinical atherosclerosis was provided to physicians and participants in the VIPVIZA trial, inclusion 2013–16 in northern Sweden, aiming to improve adherence to cardiovascular disease (CVD) prevention guidelines. Pictorial risk information may be more actionable. The aim of this study was to investigate the effect of intervention with pictorial risk information on time to first dispensing of statins.
Methods and results
Asymptomatic atherosclerotic disease was screened for by carotid ultrasound examination in 3532 participants enrolled in VIPVIZA, of those 3000 met the criteria for this study. Participants were randomly assigned to receive pictorial risk information consisting of graphical representation of atherosclerosis as compared to a control group without intervention. Time to initiation of statins was assessed during 5 years of follow-up through the National prescribed drug register. After 3 years, both groups were re-examined and received the intervention information. In the intervention group, initiation of statins increased considerably for the first 3 years and a smaller increase was also seen after re-intervention. After the cross-over, the control group showed a sharp increase in initiation of statins, almost reaching the same proportion treated at 5 years. The propensity to initiate statin treatment increased over the study period and there was no difference between men and women.
Conclusions
The pictorial information had an effect on time to initiation of statins, both as original and repeated intervention and also in the control group after single-arm cross-over. The current study supports pictorial information as a tool to shorten time to initiation of statins for CVD prevention.
The VIPVIZA study is registered with ClinicalTrials.gov, number NCT01849575.
Keywords: Statin initiation, Statins, Cardiovascular risk, Atherosclerosis, Cardiovascular disease prevention
Introduction
The use of risk scores such as European systematic coronary risk evaluation (SCORE) to communicate the risk of cardiovascular disease (CVD), may be suboptimal. Also, the risk scores are not sensitive enough to identify the large group of asymptomatic individuals that account for the major proportion of cardiovascular events; and too abstract for many patients to conceptualize their CVD risk. This study utilizes data collected during Visualization of asymptomatic atherosclerotic disease for optimum cardiovascular prevention (VIPVIZA),1 a prospective, open-label, randomized controlled trial with masked evaluators (PROBE). Asymptomatic atherosclerotic disease is identified by carotid ultrasound examination, measuring intima–media thickness as well as the presence of plaque, both associated with increased risk of CVD. VIPVIZA is integrated in the Västerbotten Intervention Program (VIP),2 a population-based CVD screening and prevention programme.
For successful initiation of a medical treatment, the patient and the physician require adequate accessible information to make decisions on rational drug use. First and foremost, physician must consider primary prevention to be important and effective.3 Statins are effective preventative measures against CVD and the current study is focused on the time to initiation of statin treatment.
Objectives
The overall aim was to study if intervention with pictorial risk information influenced time to first dispensing of statins compared with a control group during the first 3 years, and during additional 2 years after re-intervention and single-arm cross-over, respectively. The impact of gender, education, presence of plaque, and enrolment year was evaluated.
Methods
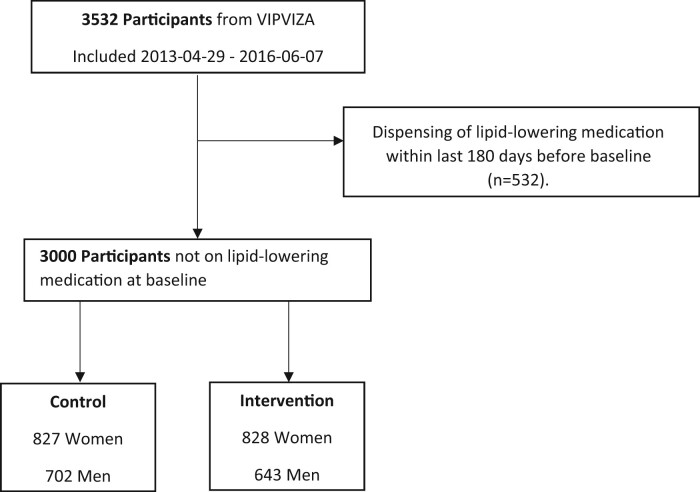
For a detailed description on the pictorial information and intervention procedure see Näslund et al.1 At the 3-year follow-up, risk factor measurements, questionnaires, and the ultrasound examination were repeated. This time the pictorial and risk factor results were given to both groups and their physicians, resulting in a single-arm cross-over. This paper focuses on time to initiation of statins in VIPVIZA, with follow-up time extended up to 5 years after baseline examination. See Table 1 for participant characteristics and Figure 1 for flow chart.
Table 1.
Baseline characteristic of 3000 participants according to control- or intervention group split by sex
| Control |
Intervention |
Overall |
||||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| (n = 702) | (n = 827) | (n = 643) | (n = 828) | (n = 1345) | (n = 1655) | |
| Hypertension (WHO) | ||||||
| Age (years) | ||||||
| 40 | 69 (9.8%) | 68 (8.2%) | 57 (8.9%) | 71 (8.6%) | 126 (9.4%) | 139 (8.4%) |
| 50 | 220 (31.3%) | 234 (28.3%) | 189 (29.4%) | 254 (30.7%) | 409 (30.4%) | 488 (29.5%) |
| 60 | 413 (58.8%) | 525 (63.5%) | 397 (61.7%) | 503 (60.7%) | 810 (60.2%) | 1028 (62.1%) |
| Body Mass Index (BMI) | ||||||
| Mean (SD) | 27.8 (4.36) | 27.4 (5.29) | 27.8 (4.28) | 27.0 (5.10) | 27.8 (4.32) | 27.2 (5.20) |
| Missing | 1 (0.1%) | 0 (0%) | 0 (0%) | 2 (0.2%) | 1 (0.1%) | 2 (0.1%) |
| LDL (mmol/L) | ||||||
| Mean (SD) | 3.70 (0.91) | 3.59 (0.87) | 3.67 (0.87) | 3.60 (0.88) | 3.68 (0.89) | 3.60 (0.88) |
| Missing | 16 (2.3%) | 6 (0.7%) | 16 (2.5%) | 4 (0.5%) | 32 (2.4%) | 10 (0.6%) |
| Total cholesterol (mmol/L) | ||||||
| Mean (SD) | 5.64 (1.00) | 5.72 (0.95) | 5.62 (0.96) | 5.70 (0.96) | 5.63 (0.98) | 5.71 (0.96) |
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.1%) | 0 (0%) | 1 (0.1%) |
| Education | ||||||
| Basic to mid-level | 492 (70.1%) | 462 (55.9%) | 454 (70.6%) | 485 (58.6%) | 946 (70.3%) | 947 (57.2%) |
| High | 205 (29.2%) | 350 (42.3%) | 185 (28.8%) | 336 (40.6%) | 390 (29.0%) | 686 (41.5%) |
| Missing | 5 (0.7%) | 15 (1.8%) | 4 (0.6%) | 7 (0.8%) | 9 (0.7%) | 22 (1.3%) |
| Systolic Blood Preasure (mm Hg) | ||||||
| Mean (SD) | 132 (15.2) | 126 (16.1) | 132 (16.5) | 127 (16.5) | 132 (15.8) | 127 (16.3) |
| Missing | 0 (0%) | 0 (0%) | 1 (0.2%) | 1 (0.1%) | 1 (0.1%) | 1 (0.1%) |
| Diastolic Blood Preasure (mm Hg) | ||||||
| Mean (SD) | 85 (10.4) | 81 (9.9) | 85 (10.8) | 81 (10.3) | 85 (10.6) | 81 (10.1) |
| Missing | 0 (0%) | 2 (0.2%) | 1 (0.2%) | 1 (0.1%) | 1 (0.1%) | 3 (0.2%) |
| Normal | 343 (48.9%) | 473 (57.2%) | 293 (45.6%) | 477 (57.6%) | 636 (47.3%) | 950 (57.4%) |
| Controlled | 85 (12.1%) | 125 (15.1%) | 68 (10.6%) | 118 (14.3%) | 153 (11.4%) | 243 (14.7%) |
| Uncontrolled | 72 (10.3%) | 74 (8.9%) | 99 (15.4%) | 74 (8.9%) | 171 (12.7%) | 148 (8.9%) |
| W/O treatment | 202 (28.8%) | 153 (18.5%) | 182 (28.3%) | 158 (19.1%) | 384 (28.6%) | 311 (18.8%) |
| Missing | 0 (0%) | 2 (0.2%) | 1 (0.2%) | 1 (0.1%) | 1 (0.1%) | 3 (0.2%) |
| SCORE | ||||||
| Low risk (<1%) | 211 (30.1%) | 678 (82.0%) | 168 (26.1%) | 675 (81.5%) | 379 (28.2%) | 1353 (81.8%) |
| Moderate risk (1–4%) | 467 (66.5%) | 147 (17.8%) | 449 (69.8%) | 149 (18.0%) | 916 (68.1%) | 296 (17.9%) |
| High risk (5–9%) | 19 (2.7%) | 0 (0%) | 22 (3.4%) | 0 (0%) | 41 (3.0%) | 0 (0%) |
| Very high risk (≥10%) | 2 (0.3%) | 0 (0%) | 1 (0.2%) | 0 (0%) | 3 (0.2%) | 0 (0%) |
| Missing | 3 (0.4%) | 2 (0.2%) | 3 (0.5%) | 4 (0.5%) | 6 (0.4%) | 6 (0.4%) |
| Framingham | ||||||
| Low risk (<5%) | 37 (5.3%) | 263 (31.8%) | 36 (5.6%) | 260 (31.4%) | 73 (5.4%) | 523 (31.6%) |
| Light risk (5–9%) | 136 (19.4%) | 354 (42.8%) | 98 (15.2%) | 363 (43.8%) | 234 (17.4%) | 717 (43.3%) |
| Moderate risk (10–19%) | 308 (43.9%) | 182 (22.0%) | 296 (46.0%) | 173 (20.9%) | 604 (44.9%) | 355 (21.5%) |
| High risk (20–39%) | 202 (28.8%) | 24 (2.9%) | 190 (29.5%) | 26 (3.1%) | 392 (29.1%) | 50 (3.0%) |
| Very high risk (≥40%) | 14 (2.0%) | 0 (0%) | 20 (3.1%) | 1 (0.1%) | 34 (2.5%) | 1 (0.1%) |
| Missing | 5 (0.7%) | 4 (0.5%) | 3 (0.5%) | 5 (0.6%) | 8 (0.6%) | 9 (0.5%) |
| VIPVIZA vascular age | ||||||
| Green | 77 (11.0%) | 52 (6.3%) | 70 (10.9%) | 41 (5.0%) | 147 (10.9%) | 93 (5.6%) |
| Yellow | 119 (17.0%) | 168 (20.3%) | 126 (19.6%) | 166 (20.0%) | 245 (18.2%) | 334 (20.2%) |
| Orange | 198 (28.2%) | 255 (30.8%) | 166 (25.8%) | 271 (32.7%) | 364 (27.1%) | 526 (31.8%) |
| Red | 308 (43.9%) | 352 (42.6%) | 281 (43.7%) | 350 (42.3%) | 589 (43.8%) | 702 (42.4%) |
| Presence of plaque | ||||||
| Non-plaque | 368 (52.4%) | 513 (62.0%) | 334 (51.9%) | 538 (65.0%) | 702 (52.2%) | 1051 (63.5%) |
| Plaque | 334 (47.6%) | 314 (38.0%) | 309 (48.1%) | 288 (34.8%) | 643 (47.8%) | 602 (36.4%) |
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | 2 (0.2%) | 0 (0%) | 2 (0.1%) |
SCORE, systematic coronary risk evaluation; VIPVIZA, visualization of asymptomatic atherosclerotic disease for optimum cardiovascular prevention.
Figure 1.
Flowchart illustrating exclusion and group eligible for analysis. From 3532 participants in VIPVIZA, 532 had at least one dispensing of lipid-lowering medication within the last 180 days prior to VIPVIZA baseline examination. The remaining 3000 patients were eligible for analysis and were further divided into control (n = 1529) and intervention (n = 1471) groups and according to sex. VIPVIZA, visualization of asymptomatic atherosclerotic disease for optimum cardiovascular prevention.

All dispensed medications to participants were available via the Swedish prescribed drug register, statins were identified by ATC codes (C10AAxx, C10B, C10BA, and C10BX).
Statistical analysis
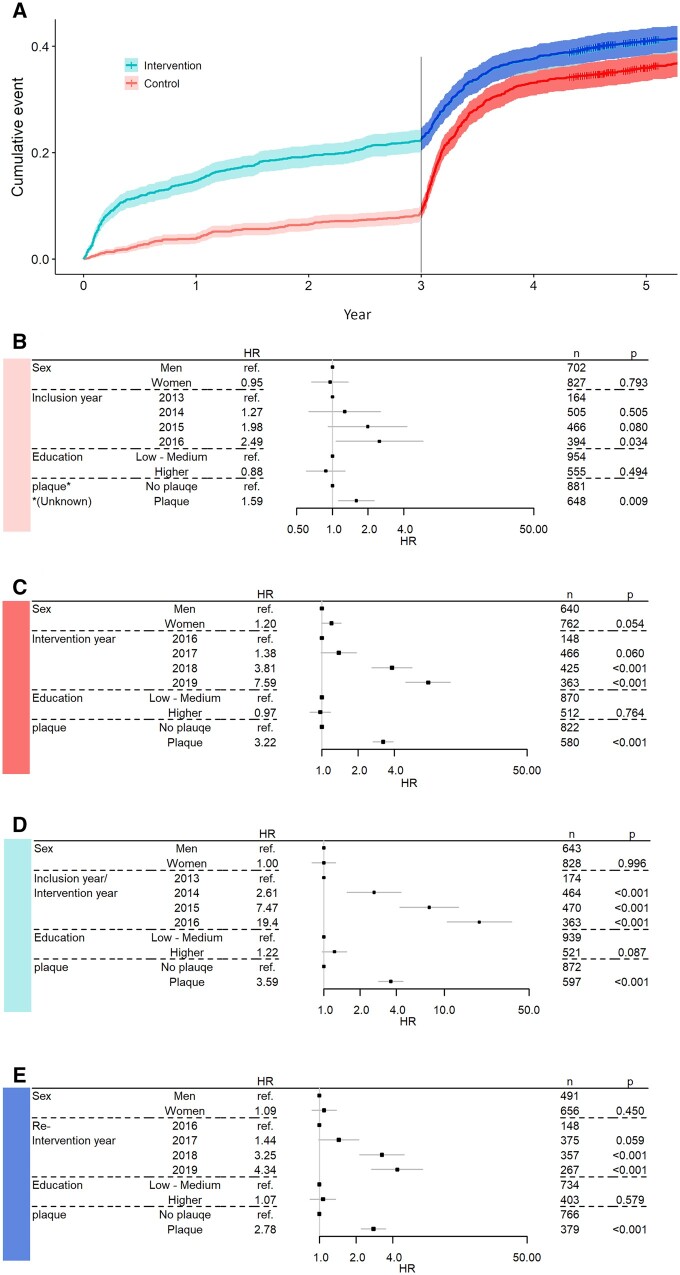
Data were stratified conditional on no statins prior to the start of the intervals (Figure 2A). Effect on time to initiation of statins for intervention year, sex, education, and presence of plaque was estimated for each stratum. Since we are focusing on time to first dispensing of statins presented results are from cox regression. The reported hazard ratios (HRs) should be viewed as the proportionality constant, of intensity of first-time dispensing.
Figure 2.
(A) Illustrating the temporal strata and cumulative proportion with statin treatment. (B–E) Forest plots with parameter estimates, number of observations in each category and P-values, based on Cox proportional hazard models. The x-axis (hazard ratio) is on log-scale. Effect on time to initiation of lipid-lowering treatment from gender, intervention year, education, and presence of plaque. (B). Control group first 3 years, n = 1509, events = 127 (20 missing, incomplete data). (C). Post 3-year follow-up intervention for individuals in control group excluding the 127 that initiated treatment prior to 3-year follow-up, n =1382, events = 462 (20 missing, incomplete data). (D). First 3 years in the intervention group, n = 1458, events = 323 (13 missing, incomplete data). (E). Post 3-year follow-up re-intervention for individuals in intervention group excluding the 323 that initiated treatment prior to 3-year follow-up, n = 1135, events = 307 (12 missing, incomplete data).
Results
In this section, B, C, D, and E refer to corresponding panels in Figure 2, where HRs are illustrated with confidence intervals (CIs) and P-values strata B–E.
Across all strata including an intervention (C, D, and E), there was an effect of being classified as plaque present. The HR for initiation of statin treatment for participants with plaque ranged from 2.8 to 3.6 across the strata, relative to participants with no plaque. The controls with plaque in stratum (B) had an HR of 1.6 (1.1–2.3, 95% CI) relative to controls without plaque, though no information regarding ultrasound results was available to the participants or their physician during that period.
No difference between men and women was observed in any of the four strata. Strata (B and D) were the only strata containing person-time from the first 3 years, while the other two strata (C and E) start at 3-year follow-up with re-intervention for the intervention group and first intervention for the group initially randomized as control.
There was a temporal effect, regarding intervention/re-intervention/inclusion year. In strata (D), participants enrolled 2014 had an HR of 2.6 (1.6–4.4, 95% CI), followed by a 7.5-fold increase (4.3–13.1, 95% CI) 2015, and an even larger increase the last inclusion year 2016, all relative 2013.
Strata (C and E) contain person-years after the 3-year follow-up from 2016 to 2019. In (E), year represents the re-intervention in the intervention group, while in (C), year represents controls first intervention after cross-over. The temporal effect is also present in (C and E) where years 2018 and 2019 have an increase in HR relative 2016 although less extreme than the increase observed in (D).
Discussion
The result in this study adds further support for the importance of improved risk communication to both physicians and patients, to improve CVD prevention by increasing propensity to initiate statin treatment. Previously, this was shown for VIPVIZA in a 1-year follow-up and lately in a 3-year follow-up.1,4 In the present study, a significant intervention effect was shown in the intervention group and the control group after single-arm cross-over. Conveying risk as pictorial presentation to participants and their respective physician in primary care, and in addition, a follow-up call to the participants tripled the average intensity for initiation of statins during the first 3 years in the intervention group. The follow-up phone call might play a key role for some participants in the intervention, giving the opportunity to answer questions and clarify the implications of the results. According to European guidelines, patients with carotid plaques are eligible for statins but have a low initiation rate in routine care. Via the ultrasound examination, people classified as low or moderate risk by SCORE, that would benefit from treatment due to presence of plaque can be identified. The pictorial information gives a more intuitive understanding of CVD risk as found in an interview study with physicians.5 The physicians experienced that with the pictorial information their risk assessment and patients’ risk perception were more accurate. Also, the intervention facilitated shared decision-making since study participants were better informed about atherosclerosis and more motivated to CVD prevention.
Important to note is the equality aspect, neither sex nor educational level had effect on time to initiation of lipid-lower treatment in any of the groups or strata, surprisingly since sex differences in statin use are well-documented.6
The effect of presence of plaque, which was unknown to physicians and participants, see Figure 2B, indicates that other risk information available to physicians from VIP also may lead to initiation of statin treatment.7 However, the effect of presence of plaque was stronger in all other strata (C, D, and E) where the ultrasound results were available to physicians and participants.
The approach to treatment of these asymptomatic participants became more active over the years this study has been ongoing (Figure 2). The strongest effect on time to initiation of statins was the year a participant was included in the study. This effect was also described in the previous evaluation of the first 465 days after the ultrasound examination8 and was observed during interviews with physicians between 2014 and 2016.5 The compiled information from VIPVIZA makes the indications for treatment more accessible for both physicians and participants, making it more likely to seek consultation of physician from the participants side and easier for the physician to access and communicate the indication for treatment.4,5,8 At the same time, a higher propensity among physicians to prescribe statins to these asymptomatic participants over time may also be due to a shift in the healthcare professionals’ attitude towards the treatment over time.
Lead author biography
Henrik Holmberg, PhD in statistics and Adjunct associate professor at Department of Public Health and Clinical Medicine, Umeå University. Since 2012, I have been working as a biostatistician in multidisciplinary research projects at the university hospital in Umeå. My research is currently focused on methods for gauging adherence to medication, by utilising data from electronic registries.
Funding
This work was supported by Region Västerbotten (Central ALF, Dnr ALFVLL-298001 and ALFVLL-643391), the Swedish Research Council (Dnr 521-2013-2708, 2016-01891, 2017-02246), the Heart and Lung Foundation (Dnr 20150369, 20170481), SKANDIA Risk & Health, and an unconditional donation from Carl Bennet Ltd, Sweden. In addition to major grants, VIP VIZA was also funded by the Swedish Society of Medicine, the Heart Foundation in Northern Sweden, ST ROKE the national association, The Swedish Insurance Society , Visare Norr (the four Northern Regions), and the Swedish and the Västerbotten Heart and Lung Associations. The funders of the study had no role in the study design, data collection, data interpretation, or writing of the report.
Data availability
The data underlying this article were provided by VIP VIZA by permission. Data will be shared on request to the corresponding author if permitted by VIP VIZA.
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Contributor Information
Henrik Holmberg, Department of Public Health and Clinical Medicine, Umeå University, 907 36 Umeå, Sweden.
Maria Sjölander, Department of Integrative Medical Biology, Umeå University, 907 36 Umeå, Sweden.
Eva-Lotta Glader, Department of Public Health and Clinical Medicine, Umeå University, 907 36 Umeå, Sweden.
Ulf Näslund, Department of Public Health and Clinical Medicine, Umeå University, 907 36 Umeå, Sweden.
Bo Carlberg, Department of Public Health and Clinical Medicine, Umeå University, 907 36 Umeå, Sweden.
Margareta Norberg, Department of Public Health and Clinical Medicine, Umeå University, 907 36 Umeå, Sweden.
Anders Själander, Department of Public Health and Clinical Medicine, Umeå University, 907 36 Umeå, Sweden.
References
- 1. Näslund U, Ng N, Lundgren A, Fhärm E, Grönlund C, Johansson H, Lindahl B, Lindahl B, Lindvall K, Nilsson SK, Nordin M, Nordin S, Nyman E, Rocklöv J, Vanoli D, Weinehall L, Wennberg P, Wester P, Norberg M; VIPVIZA trial group. Visualization of asymptomatic atherosclerotic disease for optimum cardiovascular prevention (VIPVIZA): a pragmatic, open-label, randomised controlled trial. Lancet 2019;393:133–142. [DOI] [PubMed] [Google Scholar]
- 2. Norberg M, Wall S, Boman K, Weinehall L. The Vasterbotten Intervention Programme: background, design and implications. Glob Health Action 2010;3. 10.3402/gha.v3i0.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clough JD, Martin SS, Navar AM, Lin L, Hardy NC, Rogers U, Curtis LH. Association of primary care providers’ beliefs of statins for primary prevention and statin prescription. J Am Heart Assoc 2019;8:e010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bengtsson A, Norberg M, Ng N, Carlberg B, Grönlund C, Hultdin J, Lindahl B, Lindahl B, Nordin S, Nyman E, Wennberg P, Wester P, Näslund U. The beneficial effect over 3 years by pictorial information to patients and their physician about subclinical atherosclerosis and cardiovascular risk: results from the VIPVIZA randomized clinical trial. Am J Prev Cardiol 2021;7:100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bengtsson A, Lindvall K, Norberg M, Fhärm E. Increased knowledge makes a difference!—general practitioners' experiences of pictorial information about subclinical atherosclerosis for primary prevention: an interview study from the VIPVIZA trial. Scand J Prim Health Care 2021;39:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nanna MG, Wang TY, Xiang Q, Goldberg AC, Robinson JG, Roger VL, Virani SS, Wilson PWF, Louie MJ, Koren A, Li Z, Peterson ED, Navar AM. Sex differences in the use of statins in community practice. Circ Cardiovasc Qual Outcomes 2019;12:e005562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blomstedt Y, Norberg M, Stenlund H, Nyström L, Lönnberg G, Boman K, Wall S, Weinehall L. Impact of a combined community and primary care prevention strategy on all-cause and cardiovascular mortality: a cohort analysis based on 1 million person-years of follow-up in Västerbotten County, Sweden, during 1990-2006. BMJ Open 2015;5:e009651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sjölander M, Carlberg B, Norberg M, Näslund U, Ng N. Prescription of lipid-lowering and antihypertensive drugs following pictorial information about subclinical atherosclerosis: a secondary outcome of a randomized clinical trial. JAMA Netw Open 2021;4:e2121683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article were provided by VIP VIZA by permission. Data will be shared on request to the corresponding author if permitted by VIP VIZA.
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.