Abstract
Aims
Rheumatic heart disease (RHD) is a major contributor to cardiac morbidity and mortality globally. This study aims to estimate the probability and predictors of progressing to non-fatal cardiovascular complications and death in young Australians after their first RHD diagnosis.
Methods and results
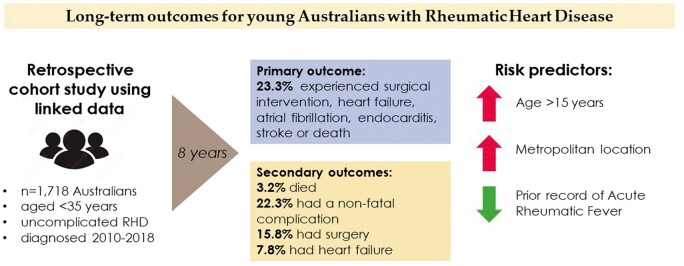
This retrospective cohort study used linked RHD register, hospital, and death data from five Australian states and territories (covering 70% of the whole population and 86% of the Indigenous population). Progression from uncomplicated RHD to all-cause death and non-fatal cardiovascular complications (surgical intervention, heart failure, atrial fibrillation, infective endocarditis, and stroke) was estimated for people aged <35 years with first-ever RHD diagnosis between 2010 and 2018, identified from register and hospital data. The study cohort comprised 1718 initially uncomplicated RHD cases (84.6% Indigenous; 10.9% migrant; 63.2% women; 40.3% aged 5–14 years; 76.4% non-metropolitan). The composite outcome of death/cardiovascular complication was experienced by 23.3% (95% confidence interval: 19.5–26.9) within 8 years. Older age and metropolitan residence were independent positive predictors of the composite outcome; history of acute rheumatic fever was a negative predictor. Population group (Indigenous/migrant/other Australian) and sex were not predictive of outcome after multivariable adjustment.
Conclusion
This study provides the most definitive and contemporary estimates of progression to major cardiovascular complication or death in young Australians with RHD. Despite access to the publically funded universal Australian healthcare system, one-fifth of initially uncomplicated RHD cases will experience one of the major complications of RHD within 8 years supporting the need for programmes to eradicate RHD.
Keywords: Rheumatic heart disease, Cardiovascular epidemiology, Competing risks analysis, Linked data, Indigenous health
Graphical Abstract
Graphical Abstract.
Introduction
Rheumatic heart disease (RHD) affects around 30 million people globally, often leading to permanent disability and 300 000 premature deaths annually.1 Rheumatic heart disease has been virtually eradicated from high-income countries, but it remains endemic in disadvantaged communities worldwide.1–4 Although Australia is not classified as having endemic RHD, disadvantaged groups remain RHD-affected. Here, RHD predominantly impacts Aboriginal and Torres Strait Islander people (hereafter Indigenous), who experience some of the highest acute rheumatic fever (ARF) and RHD incidence rates worldwide.5–8 The End RHD in Australia: Study of Epidemiology (ERASE) project determined that the Indigenous age-standardized RHD prevalence was 60 times higher than non-indigenous prevalence and that females experienced double the disease burden of males.8,9
A recent meta-analysis of RHD detected by echocardiography screening estimated that RHD progressed to complications in 7.5% while remaining stable in 60.7% of cases over 8 years.10 The REMEDY study, one of few contemporary RHD progression studies, conducted a prospective cohort study in low- and middle-income countries in Africa and Asia, with over-representation of advanced disease.11,12 Progression rates to death or complications reported by REMEDY are likely to be different from those observed in high-income countries like Australia.11–13 Previous Australian progression studies were based on register data from the Northern Territory (NT) and reported 18.6% of people progressed to heart failure (HF), 62.7% required surgical/percutaneous intervention, and 10.3% died within 10 years of RHD diagnosis.14–16 However, the data arise from a single jurisdiction, representing just 9% of the national Indigenous population.17
Understanding the probabilities and factors associated with RHD progression in Australia is critical for evaluating existing and new control policies, such as outlined by the RHD Endgame Strategy.18 For example, differences in RHD progression between age groups or geographical locations may necessitate different tailored strategies. In the absence of new policies, expenditure is projected to reach over $26.7 million to manage ARF and RHD among currently existing cases.19 Further, more than 10 000 Indigenous people are predicted to develop ARF or RHD between mid-2016 and 2031 at a cost of $317 million.
Consequently, this study investigated long-term cardiovascular outcomes following the first diagnosis of uncomplicated RHD in a contemporary young cohort (age <35 years) from multiple Australian jurisdictions covering 70% of the Australian population. In this cohort, we determined the probability of a composite of all-cause death and non-fatal RHD complication as a primary outcome over 8-year follow-up. We also determined the probability of individual and composite non-fatal cardiovascular complications as secondary outcomes, with death treated as a competing risk. Sociodemographic and clinical risk predictors for primary and secondary outcomes were also examined. These absolute, unadjusted estimates were generated for use in economic models and projections, health service planning and to inform communities/clinicians about the probability of RHD-associated outcomes in young Australians over time.
Methods
Data sources
The present retrospective cohort study was undertaken using datasets generated for the ERASE Project, described in detail elsewhere9 and registered with the Australian New Zealand Clinical Trials Registry (ACTRN12620000981921). The ERASE project identified ARF and RHD cases from linked administrative data sources collected between 2001 and 2018, namely ARF/RHD registers and inpatient hospital admissions originating from New South Wales (NSW), Queensland (Qld), South Australia (SA), Western Australia (WA), and NT (see Supplementary material online, ItemS1 for map). Hereafter, these Australian states and territories are referred to as ‘jurisdictions’. Cardiac surgery registries, including the Australian and New Zealand Society of Cardiac and Thoracic Surgery Database, confirmed facts of surgical intervention.
The data underlying this article are based on sensitive health records, obtained from Australian health services by permission from data custodians. Data will only be shared on reasonable request to the corresponding author subject to rigorous conditions, including additional ethical and data custodian approvals.
Identification of rheumatic heart disease diagnoses and cohorts
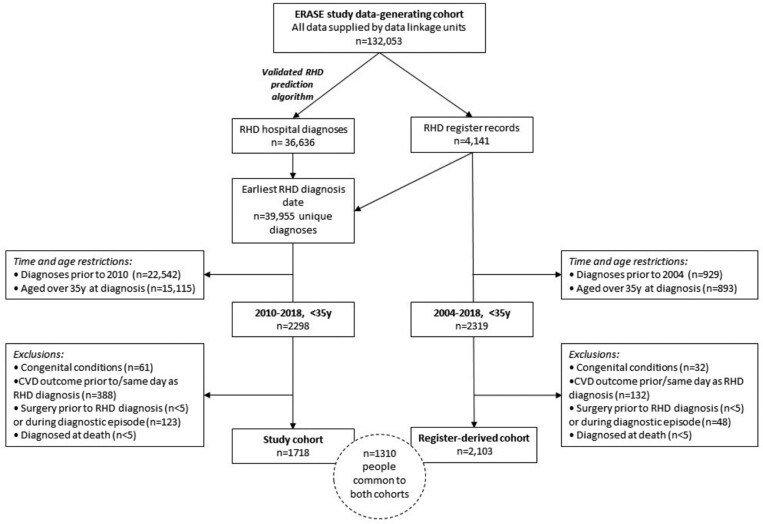
Two cohorts were defined for this study—the RHD study cohort (n = 1718, hereafter referred to as ‘study cohort’), which forms the focus of our analyses, and the register derived cohort (n = 2103, hereafter referred to as the ‘register-derived cohort’), which is used for sensitivity analyses (Figure 1). Progression estimates from the two cohorts should be interpreted together with an understanding of the overlap and strengths and limitations of each cohort.
Figure 1.
A flowchart illustrating the derivation of the main study cohort and the register-derived cohort, which formed the sensitivity analysis.
The RHD study cohort selected people aged <35 years at first diagnosis with uncomplicated RHD between 2010 and 2018, with a minimum 8.5-year ‘clearance’ period (to exclude prevalent RHD cases) and a maximum 8.9 years of follow-up. This cohort used both RHD register and hospital data to identify RHD cases. The strength of the study cohort is the inclusion of hospital-identified cases which had not previously been incorporated into analyses of RHD progression and might not be captured by register data sources.20 The limitation of this cohort was that hospitalization records were used to identify RHD diagnosis date for 32.8% of individuals, potentially resulting in some misclassification bias due to coding error. A validated RHD prediction algorithm was applied to minimize the impact of false-positive diagnoses.21,22
The register-derived cohort selected people aged <35 years diagnosed with uncomplicated RHD between 2004 and 2018, with up to 14 years of follow-up available. All cases in this cohort were clinically confirmed as first-ever RHD cases, requiring no clearance period. Additionally, results are comparable to previous NT register studies14–16 but considerably larger in size and more widely representative because of the multi-jurisdictional source. The major limitation of the register-derived cohort is that only people associated with RHD register programmes (mostly Indigenous) were included and registers are known to be incomplete.20
Defining young <35-year-old cohorts increased the likelihood that diagnoses identified were first-ever RHD diagnoses. RHD was indicated if someone had a diagnosis recorded on an RHD register or had a hospital admission deemed ‘probable RHD’ identified using the described prediction algorithm.22 When using RHD register sources, diagnosis date was the earliest date of RHD diagnosis confirmed as ‘mild, moderate, or severe’.23 Rheumatic heart disease cases identified in hospital records only were assigned a diagnosis date of the earliest hospital admission with an RHD-associated ICD-10AM code (see Supplementary material online, ItemS2 for codes). People were excluded if they had experienced surgical intervention, HF, atrial fibrillation (AF), endocarditis, or stroke on or before their first RHD diagnosis date or had a congenital condition (see Supplementary material online, ItemS2), meaning that cohorts were comprised of people with initially uncomplicated RHD and with mostly mild and moderate disease severity (Figure 1).
Cohort descriptors
A range of sociodemographic and clinical characteristics was recorded. The ‘population group’ variable broadly categorized people into three groups based on established RHD disease burdens: (i) Indigenous Australians, (ii) immigrants from low-income or lower-middle-income countries (ILIC, including Maori and Pacific Islanders), and (iii) other Australians. ILIC was assigned when a person’s country of birth was a low-income or lower-middle-income country as per the World Bank Country Income classification status (1996), or if they were New Zealand born. One-third of New Zealand-born people living in Australia identity as Pacific Islander ancestry, a group known to have high ARF/RHD burden.24,25 The remaining people were classified as ‘other Australian’.9
The remoteness of residence was categorized using Statistical Area-2 (SA2)-based Accessibility Remoteness Index of Australia (ARIA), consolidated into two groups ‘metropolitan’ (representing major cities and inner regional areas) and ‘non-metropolitan’ (representing all other areas).17 Non-metropolitan areas captured remote and very remote locations with low population densities. In the absence of individual-level data on socioeconomic status (SES), the SA2-based Index of Relative Socio-economic Disadvantage (IRDS) was used.17
Outcomes
We examined clinical and surgical outcomes likely to be associated with RHD progression12,15,18 which were designated a priori. The primary outcome of interest was time from the first RHD diagnosis to a composite of death or any non-fatal cardiovascular complication, including surgical intervention, HF, AF, endocarditis, or stroke. The four secondary outcomes of interest were time to all-cause death, time to non-fatal cardiovascular complication composite, time to surgical intervention, and time to HF.
A list of ICD-10AM diagnosis and procedure codes for the specified RHD-associated outcomes is provided in Supplementary material online, ItemS2. Surgical intervention was defined as either percutaneous valvular intervention or open valve replacement/repair surgery. The Australian Register of Deaths provided date and cause of death.9 We classified cause of death into three broad categories for descriptive analysis: RHD as the underlying cause, RHD as an associated cause, or non-RHD death.
Statistical methods
Descriptive analyses
Frequencies and unadjusted proportions summarized the demographic features of the cohort stratified by the variables described in ‘cohort descriptors’.
Estimating probability of rheumatic heart disease complication
We estimated RHD progression to a composite of fatal/non-fatal cardiovascular complications (primary outcome) and all-cause death (secondary outcome) using classical survival analyses based on the Kaplan–Meier estimator. Individuals were censored at end of available follow-up (31 December 2018).
Competing risk methods were used to estimate progression to non-fatal outcomes (secondary outcomes), to avoid overestimation arising from the inappropriate censoring of deaths with classical survival methods.26 Competing risk of death was accounted for using an Aalen–Johansen estimator implemented using the ‘survival’ package in R. Cumulative incidence (probability) and corresponding 95% confidence intervals (CIs) for specified outcomes are provided at 6 months, 1, 5, and 8 years after RHD diagnosis stratified by population group.
Risk predictors associated with rheumatic heart disease complications
Cox proportional cause-specific hazard models were fitted, to estimate the univariate and multivariable-adjusted hazard ratios (HRs) and 95% CIs for explanatory variables associated with pre-specified outcomes except for all-cause death due to small numbers. Covariates selected as potential explanatory variables in the Cox models were age group, sex, population group, remoteness of residence, IRDS, record of prior ARF, and ‘data source of RHD diagnosis’ (i.e. hospital- or register-identified). Analyses were done using SAS 9.4 and R version 3.6.1/R Studio.
Ethics
This study complies with the Declaration of Helsinki. Human Research Ethics Committees of the Health Departments (NT: Menzies School of Health Research) of WA, SA, NT, QLD, and NSW provided approval. Aboriginal Ethics Committees from WA, SA, NT, and NSW approved the study, after support letters from peak bodies of the Aboriginal Community Controlled Health Services.
Results
Cohort features
First RHD diagnoses for individuals in the study cohort occurred most commonly in the 5–14-year age group (40.3%), among females (63.2%), among Indigenous people (84.6%), and residents of very remote areas (45.1%) (Table 1). More than half of all cases (52.1%) were recorded as living in the lowest IRDS quintile areas, with a further 28.3% of people were from areas with no IRDS index available due to a very low population or poor Census data quality, and more likely to be low SES areas. By comparison, first RHD diagnoses in the register-derived cohort had a higher proportion of children in the 5–14-year age group (46.2%), were more likely Indigenous (92.5%) and resided in very remote areas (52.8%). Prior record of ARF was found for 44.4% and 50.3% of the study and register-derived cohort, respectively (Table 1).
Table 1.
Baseline descriptive statistics for the study cohort and the register-derived cohort
| Study cohort (2010–18) |
Register-derived cohort (2004–18) |
||||
|---|---|---|---|---|---|
| N | % | n | % | ||
| Total | 1718 | 100.0 | 2103 | 100.0 | |
| Age group | 0–4 | 15 | 0.9 | 20 | 1.0 |
| 5–14 | 692 | 40.3 | 972 | 46.2 | |
| 15–24 | 552 | 32.1 | 690 | 32.8 | |
| 25–34 | 459 | 26.7 | 421 | 20.0 | |
| Sex | Male | 632 | 36.8 | 830 | 39.5 |
| Female | 1086 | 63.2 | 1273 | 60.5 | |
| Population group | Indigenous | 1453 | 84.6 | 1945 | 92.5 |
| ILIC | 187 | 10.9 | 115 | 5.5 | |
| Other Australian | 78 | 4.5 | 42 | 2.0 | |
| Source of RHD diagnosis | Register | 1154 | 67.2 | 2103 | 100.0 |
| Hospital | 564 | 32.8 | 0 | 0.0 | |
| Jurisdictiona | Northern Territory (NT) | 628 | 36.6 | 913 | 43.4 |
| South Australia (SA) | 41 | 2.4 | 24 | 1.1 | |
| Queensland (Qld) | 695 | 40.5 | 867 | 41.2 | |
| Western Australia (WA) | 235 | 13.7 | 276 | 13.1 | |
| New South Wales (NSW) | 119 | 6.9 | 23 | 1.1 | |
| ARIA | Major cities | 222 | 12.9 | 110 | 5.2 |
| Inner regional | 48 | 2.8 | 29 | 1.4 | |
| Outer regional | 262 | 15.3 | 330 | 15.7 | |
| Remote | 277 | 16.1 | 338 | 16.1 | |
| Very remote | 774 | 45.1 | 1110 | 52.8 | |
| Missing/other | 135 | 7.9 | 186 | 8.8 | |
| IRDS quintile | 1 (least disadvantaged) | 38 | 2.2 | 22 | 1.0 |
| 2 | 58 | 3.4 | 43 | 2.0 | |
| 3 | 101 | 5.9 | 86 | 4.1 | |
| 4 | 140 | 8.1 | 131 | 6.2 | |
| 5 (most disadvantaged) | 895 | 52.1 | 1157 | 55.0 | |
| Data not available | 486 | 28.3 | 664 | 31.6 | |
| Previous record of ARF | Yes | 762 | 44.4 | 1058 | 50.3 |
| No | 956 | 55.6 | 1045 | 49.7 | |
ARF, acute rheumatic fever; ARIA, accessibility and remoteness index of Australia; IRDS, index of relative socioeconomic disadvantage; RHD, rheumatic heart disease.
See Supplementary material online, ItemS1 for a map of Australian jurisdictions.
In the study cohort, Indigenous people were generally diagnosed with RHD at a younger age, and more often resided in non-metropolitan areas and had a prior record of ARF (42% age 5–14 years, 94% non-metropolitan, and 48% with prior ARF history). Conversely, ILIC and other Australians were diagnosed with RHD at older ages, were more often living in metropolitan areas and had a less frequent prior record of ARF (47% age 25–34 years, 70% metropolitan, and 21% with prior ARF history).
Outcomes
Table 2 shows the number (and percentage) of individuals who progressed to a composite fatal/non-fatal outcome (primary outcome) as well as individual secondary outcomes at 6 months and end of follow-up. The median follow-up time was 3.9 years and the maximum was 8.9 years. By end of follow-up, the primary outcome (composite of fatal/non-fatal cardiovascular complications) had occurred in 16.1% (n = 276) of the cohort with 7.8% experiencing the outcome within 6 months after the first RHD diagnosis (Table 2). Surgical intervention was the most common non-fatal event (11.4%) followed by HF (5.5%), with nearly half of events occurring within the initial 6 months of follow-up. Death was recorded in 1.7% (n = 29) of the cohort over the whole follow-up period (Table 2). The proportion of cases who experienced the primary and secondary outcomes at 6 months and end of follow-up was similar between the study and register-derived cohorts (Table 2).
Table 2.
Descriptive statistics for the individual events of death, surgical intervention, heart failure, atrial fibrillation, endocarditis, or stroke in the study and register-only cohorts at 6 months and end of follow-up
| Study cohort (n = 1718) |
Register-derived cohort (n = 2103) |
|||
|---|---|---|---|---|
| 6 months after diagnosis | End of follow-up (max 8.9 years) | 6 months after diagnosis | End of follow-up (max 14 years) | |
| Death | 7 (0.4%) | 29 (1.7%) | <5 (0.0%) | 39 (1.9%) |
| Surgical intervention | 94 (5.5%) | 195 (11.4%) | 82 (3.9%) | 255 (12.1%) |
| Heart failure | 45 (2.6%) | 94 (5.5%) | 45 (2.1%) | 121 (5.8%) |
| Atrial fibrillation | 23 (1.3%) | 66 (3.8%) | 18 (0.9%) | 69 (3.3%) |
| Endocarditis | 10 (0.6%) | 21 (1.2%) | <5 (0.2%) | 22 (1.0%) |
| Stroke | <5 (0.2%) | 10 (0.6%) | <5 (0.0%) | 20 (1.0%) |
| Fatal/non-fatal eventsa | 134 (7.8%) | 276 (16.1%) | 113 (5.4%) | 347 (16.5%) |
Primary outcome: composite of death, surgical intervention, or any cardiovascular outcome as mutually exclusive events.
Probability of progression after rheumatic heart disease diagnosis
Composite of death or any non-fatal cardiovascular complication (primary outcome)
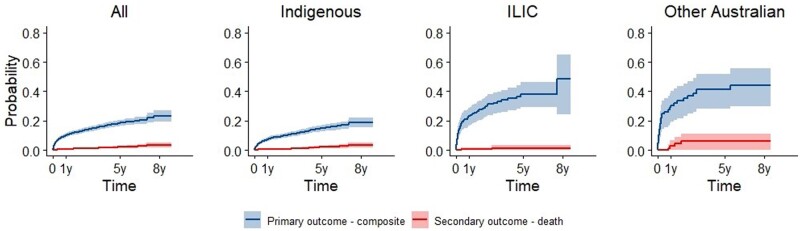
Within 6 months of RHD diagnosis, 7.9% (95% CI: 6.6–9.1%) in the study cohort had experienced the composite outcome and by 8 years this had increased to 23.3% (95% CI: 19.5–26.9%) (Figure 2, Supplementary material online, ItemS3). For the register-derived cohort, progression probabilities were 5.4% (95% CI: 4.4–6.4%) and 18.2% (95% CI: 16.2–20.2%), respectively at equivalent time points (Supplementary material online, ItemS3). Within 8 years, progression to the primary outcome in the study cohort was 18.9% (95% CI: 15.5–22.2%) for the Indigenous subgroup, 48.5% (95% CI: 24.5–64.9%) for the ILIC subgroup, and 44.2% (95% CI: 29.9–55.5%) for the other Australian population subgroup. Within 8 years, progression to the primary outcome in the study cohort was 16.2% (95% CI: 12.8–20.4%) for Indigenous males and 20.5% (95% CI: 16.3–25.7%) for Indigenous females (Supplementary material online, ItemS4).
Figure 2.
Progression from uncomplicated rheumatic heart disease to the composite of death, heart failure, surgical intervention, atrial fibrillation, infective endocarditis or stroke (primary outcome, blue), or death (secondary outcomes, red) over 8-year follow-up. Kaplan–Meier curves are presented for the whole study cohort (‘All’) and separately for Indigenous, ILIC, and other Australian population groups. Shading indicates 95% confidence intervals.
Death (secondary outcomes)
The risk of death did not exceed 6% during the follow-up period (Figure 2, Supplementary material online, ItemS3). In the study cohort, 0.4% (95% CI: 0.1–0.7%) died within 6 months and 2.1% (95% CI: 1.3–3.0%) died within 5 years of initial RHD diagnosis. For the register-derived cohort, there were no deaths in the first 6 months after diagnosis; this increased slightly to 0.8% (95% CI: 0.4–1.3%) within 5 years (Supplementary material online, ItemS3). The probability of death within 5 years in the study cohort was 2.0% (95% CI: 1.1–2.9%) for the Indigenous subgroup, 1.4% (95% CI: 0–3.2%) for the ILIC subgroup, and 5.9% (95% CI: 0.1–11.3%) for the other Australian population subgroup (Figure 2). Cause of death analyses revealed 13.8% of deaths had RHD as an underlying cause, 41.4% had RHD as an associated cause, 24.1% were non-RHD, and 20.7% had the cause missing.
Composite of any non-fatal cardiovascular complication including surgical intervention (secondary outcomes)
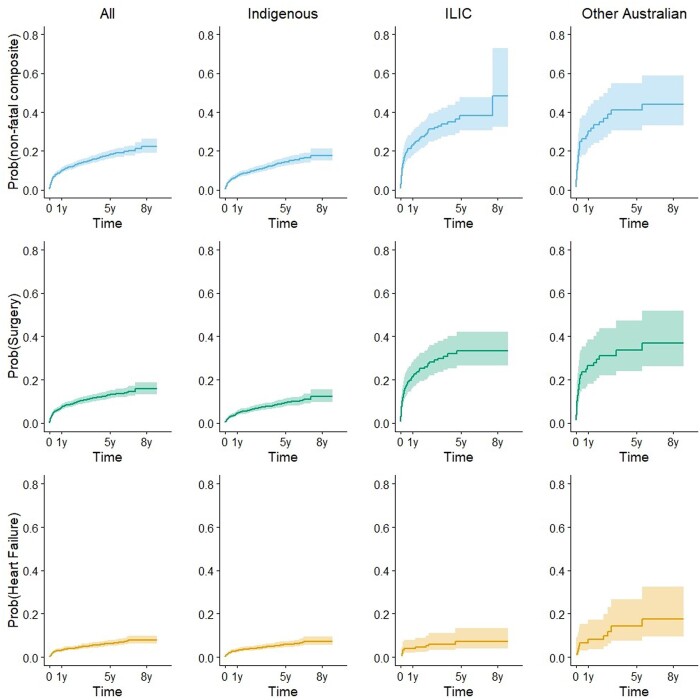
Within 6 months of RHD diagnosis, 7.7% (95% CI: 6.5–9.1%) had experienced a non-fatal cardiovascular complication and by 5 years this had increased to 18.0% (95% CI: 16.0–20.3%) in the study cohort (Figure 3). For the register-derived cohort, the progression probabilities were 5.4% (95% CI: 4.5–6.4%) at 6 months and 13.8% (95% CI: 12.3-15.5%) at 5 years (Supplementary material online, ItemS5). Progression to this outcome at 1 year was 7.0% (95% CI: 5.8–8.4) for Indigenous Australians, 23.0% (95% CI: 17.6–30.0) for ILIC, and 30.4% (95% CI: 21.6–42.8%) other Australians (Figure 3, Supplementary material online, ItemS5).
Figure 3.
Progression to secondary outcomes for the study cohort and separately for Indigenous, ILIC, and other Australian population groups is illustrated by cumulative incidence function curves for the composite of all non-fatal complications (top panel, blue), surgical intervention (middle panel, green), and heart failure (bottom panel, orange). Shading indicates 95% confidence intervals.
Surgical intervention (secondary outcomes)
In the study cohort, 12.9% (95% CI: 11.2–14.9) of people were estimated to have had surgical intervention within 5 years of RHD diagnosis. For the register-derived cohort, 13.8% (95% CI: 12.3–15.5%) had surgical intervention within 5 years (Supplementary material online, ItemS5). The proportion with surgical intervention within 5 years was 9.2% (95% CI: 7.6–11.1%) for Indigenous people, 33.4% (95% CI: 26.5–42.2%) for ILIC, and 33.6% (95% CI: 24.0–47.1%) for other Australians (Figure 3, Supplementary material online, ItemS5).
Heart failure (secondary outcomes)
In the study cohort 2.7% (95% CI: 2.0–3.5%) experienced HF within 6 months; this was 6.2% (95% CI: 5.0–7.7%) at 5 years after diagnosis (Figure 3, Supplementary material online, ItemS5). In the register-derived cohort, 2.2% (95% CI: 1.6–2.9%) experienced HF within 6 months; this was 4.9% (95% CI: 4.0–6.0%) at 5 years after diagnosis. The proportion with HF within 5 years was 5.7% (95% CI: 4.5–7.3%) for Indigenous people, 7.3% (95% CI: 4-13.3%) for ILIC, and 14.2% (95% CI: 7.6-26.6%) for other Australians (Figure 3, Supplementary material online, ItemS5).
Risk predictors of progression from rheumatic heart disease diagnosis to complications
In the univariate analyses, all potential risk predictors except sex and SES were associated with progression to the primary outcome (Supplementary material online, ItemS6). ILIC compared to Indigenous people had a 3.1-fold higher unadjusted HR of progression; for other Australians, this HR was 3.8. Metropolitan versus non-metropolitan residence was associated with a 4.1-fold higher HR of progression. Earliest identification from hospitalization versus register record was associated with a 3.0-fold higher unadjusted HR of progression. Having an ARF record prior to RHD diagnosis was associated with a 70% lower risk of progression compared to having no record.
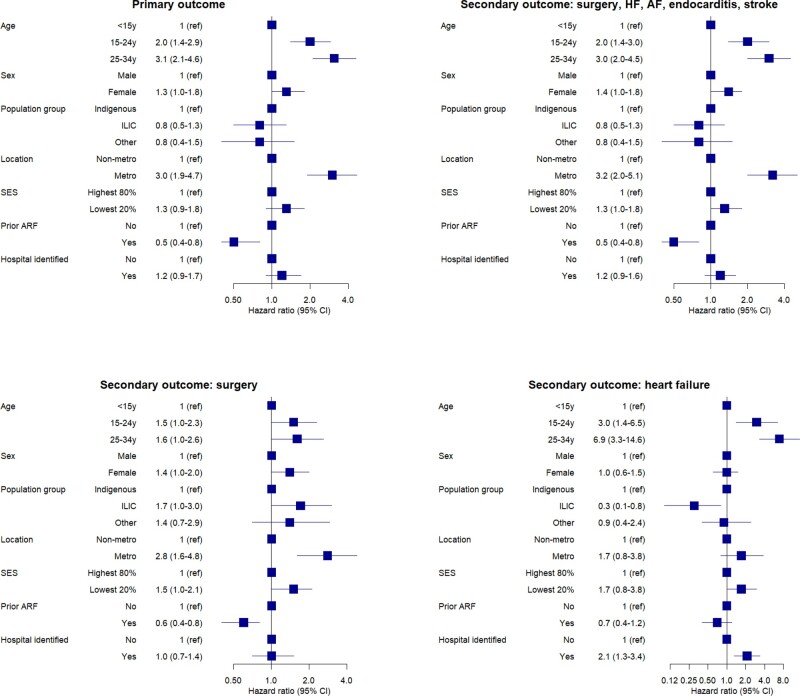
After multivariable adjustment, both older age (>14 years) and metropolitan residence were independently associated with an increased adjusted-HR, while ARF history was associated with a reduced adjusted-HR for both outcomes (Figure 4). Population group was no longer an independent predictor of the outcomes after adjustment for group differences in age, non-metropolitan residence, prior record of ARF, and other potential confounders.
Figure 4.
Forest plots illustrating risk predictors of rheumatic heart disease progression to fatal/non-fatal composite outcome (primary outcome); and secondary outcomes of cardiovascular/surgical composite outcome; surgical intervention or heart failure in the study cohorts. Hazard ratios and 95% confidence intervals are reported for multivariate analyses, univariate analyses are provided in Supplementary material online, Item S6.
The same univariate and multivariable-adjusted predictors for the primary outcome were also predictors of surgical intervention (Figure 4). Metropolitan residence was associated with increased adjusted-HR for surgical intervention (HR 2.8, 95% CI: 1.7–4.8) whereas prior record of ARF was ‘protective’ against surgical intervention (adjusted-HR 0.6, 95% CI: 0.4–0.8). The univariate and multivariable-adjusted results for HF were generally similar to those for surgical intervention although 95% CIs were wider due to lower HF event numbers compared with surgical intervention (Figure 4and Supplementary material online, ItemS6). Females had a marginally higher risk of experiencing all outcomes compared to males although the lower 95% CI of the adjusted-HR for sex was at 1.0 or below for each outcome.
A similar pattern of unadjusted and adjusted risk predictors was obtained in our sensitivity analyses using the register-only cohort; however, HRs were lower in general (Supplementary material online, ItemS6).
Discussion
This is the first multi-jurisdictional Australian study estimating the probability of RHD progression from the first diagnosis to death and/or non-fatal cardiovascular complications, such as surgical intervention. As such, this study provides the most definitive and contemporary estimates of RHD disease progression among young affected Australians. Our major finding was that one-fifth of initially uncomplicated RHD cases aged <35 years progressed to death/non-fatal complication within 8 years. Complication risk was highest within the first 6 months after RHD diagnosis. Death rates were low throughout follow-up; however, surgical intervention was required by 15.8% of the cohort and 7.8% experienced HF. Age >14 years, metropolitan residence, and prior ARF record were independent predictors of RHD progression, while sex and population group were not independently associated with progression.
Progression probabilities in this study are lower than those reported by previous international and Australian studies. The REMEDY study in low- and middle-income countries from Africa and Asia reported death rates between 12.5% and 20.8%, HF rates between 6.1% and 9.0%, and surgery rates between 3.1% and 13.0% during the 2-year follow-up of people with RHD.12 Upper-middle-income countries had the lowest rates of death and cardiovascular complications, but the highest rates of surgical intervention compared with lower-income countries. These higher progression rates were expected given the over-representation of advanced RHD in REMEDY, while the lower surgical intervention rates are likely explained by limited access to surgical or percutaneous valvular interventions.12
In this study, death estimates (2.8% within 10 years) are lower than those reported by He et al.15 (10.3% within 10 years), and Lawrence et al.14 (6.9% within 5 years) in NT register cases. Cannon et al.16 reported 10-year death estimates of 1.0–2.4% for mild-moderate RHD cases but 12.6% for severe RHD cases. The low overall death estimates in our present study cohort suggest that it comprised mostly mild-moderate RHD cases. Progression to HF in this study (7.8% within 8 years) was also considerably lower than previously reported by He et al.15 (27% within 5 years) and Lawrence et al.14 (18.6% within 10 years). However, these studies were NT-based, mostly Indigenous, and included all ages, whereas our study included multiple jurisdictions and selected a young cohort with uncomplicated RHD, which may explain the lower progression estimates. However, we cannot exclude the possibility that improvements in secondary prophylaxis have contributed to lower progression rates in established RHD cases. Nevertheless, we still found that 15.8% of our young RHD cohort required surgical intervention usually because of HF associated with severe RHD within 8 years of diagnosis.
Another important study finding was that progression risk was highest in the first 6–12 months after RHD diagnosis (Figure 2). He et al.15 also found that the development of complications was highest in the first year after RHD diagnosis, with these early complications occurring in patients with severe RHD at diagnosis. This finding highlights the importance of prompt action plans and adequate clinical surveillance after initial RHD diagnosis to prevent, detect and manage these complications in young RHD patients in both metropolitan and remote settings.
Australian RHD control efforts (including registers) have been mostly directed at Indigenous populations, capturing a higher proportion of Indigenous RHD cases.8,20 While the Indigenous burden is high in absolute terms, the average severity of cases may be lower when compared to other groups. This is supported by progression estimates in the register-derived (mostly Indigenous) cohort which had generally lower progression probabilities than the multi-jurisdictional study cohort. Despite this, one in five Indigenous people progressed to death/non-fatal complications during follow-up. This progression in young Indigenous Australians occurs on a background of socio-economic disadvantage associated with colonization, as well as challenges associated with administering treatment in remote settings and inequities in access to services.18,27 Our data suggest that ILIC/other Australians are diagnosed later in their RHD disease course than Indigenous people, leading to two in five people progressing to complicated RHD within the same time period. Additionally, ILIC/other Australians were more likely to live in metropolitan areas and were less likely to have a prior record of ARF which were found to be independent predictors of progression. Regardless of population group, the prognosis for young people after RHD diagnosis is still poor in Australia.
Risk predictors identified here align with previous Australian studies that identify urban residence as being independently associated with higher risk of experiencing adverse RHD outcomes, albeit without clear explanation.14,15 We propose that in metropolitan settings the diagnostic suspicion of ARF/RHD is lower (hence diagnoses are delayed or missed) and care for ARF/RHD is less coordinated, presenting challenges to RHD prevention and management. People with RHD migrating to Australian cities from low-income countries might also be first identified in the Australian health system. Increased risk of progression in metropolitan residents is counterintuitive from a biomedical perspective, since metropolitan areas offer the best proximal access to tertiary care; however, there remain structural and systemic barriers for many people with RHD accessing specialized cardiac services regardless of residential location.27–32 Elaboration of these barriers is provided in Supplementary material online, ItemS7. Although absolute RHD burden is low in metropolitan settings,8 we infer that only the more severe cases are reflected by hospitalization data, that registers have focussed mainly on remote areas, and that milder cases are potentially missed, irrespective of population group. It is also possible that patients with uncomplicated but more severe RHD move to metropolitan locations in order to be close to tertiary cardiac services after diagnosis. Further exploration of the metropolitan association with RHD progression is needed.
Prior ARF record was associated with a 40% lower adjusted risk of progression to composite outcomes and surgical intervention. This could indicate either earlier detection of RHD at a less severe stage in people with known ARF, or that clinical history of ARF leads to better monitoring for RHD and treatment including secondary prophylaxis. Sex was not an independent risk predictor of any RHD outcome investigated. This was unexpected, given that women with RHD are often diagnosed during health care contact for pregnancy, which is known to place increased stress on the heart.33,34 Females are also known to experience a higher absolute probability of RHD diagnosis than males8; however, this did not translate to greater disease progression.
Strengths and limitations
In addition to wider and more representative coverage of population groups across Australia, a major strength of this study is the augmentation of clinically confirmed register-derived RHD cases with hospitalization records, reducing biases resulting from studying register data alone.20 This bias is best illustrated by noting that our sensitivity analyses of register-derived individuals consistently estimated lower absolute progression probabilities to the study cohort.
The major limitation of this study is the use of administrative, rather than clinical and echocardiographic data as a source of diagnosis, disease staging, and detection of adverse outcomes. Our analyses have also omitted adjustment for secondary prophylaxis with Benzathine Penicillin G, which is facilitated by register programmes, and which may be partly responsible for the higher univariate risk associated with hospital-identified individuals. We could not fully account for internal migration; however, we believe the impact of this to be small (see Supplementary material online, ItemS1). Future research will need to investigate the increased risk of RHD-associated complications for people living in metropolitan areas, since it represents a complex combination of factors associated with poorer outcomes. Detailed assessment of the impact of register programmes and the adherence to secondary prophylaxis with Benzathine Penicillin G on ARF/RHD progression are planned.
Conclusion
This study provides the most definitive, representative, and contemporary estimates of RHD progression among young Australians. Even though Australia is a high-income country with ready access to specialized cardiac services, we observed that around one in five people aged <35 years with initially uncomplicated RHD progressed to complications within 8 years. Clinician awareness of RHD diagnosis and culturally secure management is critical in rural and remote settings where access to clinical care is challenging.27,35 However, this is also important in busy metropolitan health care settings where management of other chronic diseases is more frequent and RHD is less commonly encountered. Metropolitan RHD care could learn from practices already established in remote settings of Australia. Our data support the need for the RHD Endgame Strategy,18 which is a blueprint for eradication of RHD in Australia by 2031.
Lead author biography

Ingrid Stacey is a cardiovascular epidemiologist with a background in biostatistics. Currently, she is a Research Associate and PhD candidate within the School of Population and Global Health at the University of Western Australia, supported by a National Health and Medical Research Council postgraduate scholarship. Her PhD project uses linked administrative data from five Australian jurisdictions to investigate disease progression and complications associated with Acute Rheumatic Fever and Rheumatic Heart Disease in young Australians. This work is a sub-study of the End RHD in Australia: Study of Epidemiology (ERASE) project.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
We acknowledge that figures and other statistics represent the loss of health and human life with profound impact and sadness for people, families, community, and culture. These numbers also obscure the resilience and strengths of the people involved. We hope that the ‘numbers story’ emanating from this project can augment the ‘lived stories’ that reflect the voices of people with RHD and their families, thus jointly contributing to evidence to erase suffering from ARF and RHD in Australia.
The authors also wish to thank the staff of the data linkage units of the State and Territory governments (WA, SA-NT, NSW, and Queensland) for linkage of the data. We thank the State and Territory Registries of Births, Deaths and Marriages, the State and Territory Coroners, and the National Coronial Information System for enabling Cause of Death Unit Record File data to be used for this project. Furthermore, we thank the data custodians and data managers for the provision of the following data:
Inpatient hospital data (five states and territories).
Emergency Department data (five states and territories).
RHD registers (five states and territories).
The Australian and New Zealand Society of Cardiac and Thoracic Surgeons Cardiac Surgery Database (single registry covering five states and territories).
Royal Melbourne Children’s Hospital Paediatric Cardiac Surgery database (single data source for RHD paediatric patients from SA and NT receiving surgical intervention in Melbourne).
Primary healthcare data from NT Department of Health.
Funding
This work was supported by funding from the National Health and Medical Research Council through project grant (#114652) and seed funds from the End -RHD Centre for Research Excellence and HeartKids. I.S. was supported by an NHMRC Postgraduate Scholarship Grant (#2005398) and an Ad Hoc Postgraduate Scholarship at the University of Western Australia. J.K. was supported by a Heart Foundation Future Leader Fellowship (#102043).
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
The authors working on this article have been supported by many collaborators across Australia, including Anna Ralph, Dawn Bessarab, Lee Nedkoff, Pamela Bradshaw, Charley Budgeon, and Emma Haynes. The authors value the support/endorsement provided to the project by the following peak bodies representing the Aboriginal Community Controlled Health sector: Aboriginal Medical Services Alliance Northern Territory, Kimberley Aboriginal Medical Service (the health service serving the high-burden region of WA), Aboriginal Health Council of South Australia, and Aboriginal Health and Medical Research Council (NSW). We also received support from the Aboriginal divisions of Queensland and WA Health Departments. We are committed to providing feedback to these organizations ensuring that the findings are accessible and providing the evidence needed for policy that can reduce the burden of ARF and RHD in Australia.
References
- 1. Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, Forouzanfar MH, Longenecker CT, Mayosi BM, Mensah GA, Nascimento BR, Ribeiro ALP, Sable CA, Steer AC, Naghavi M, Mokdad AH, Murray CJL, Vos T, Carapetis JR, Roth GA.. Global, regional and national burden of rheumatic heart disease, 1990-2015. N Engl J Med 2017;377:713–722. [DOI] [PubMed] [Google Scholar]
- 2. Carapetis JR. Rheumatic heart disease in developing countries. N Engl J Med 2007;357:439–441. [DOI] [PubMed] [Google Scholar]
- 3. Carapetis JR, Steer AC, Mulholland EK, Weber M.. The global burden of group A streptococcal diseases. Lancet Infect Dis 2005;5:685–694. [DOI] [PubMed] [Google Scholar]
- 4. Seckeler MD, Hoke TR.. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin Epidemiol 2011;3:67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carapetis JR, Wolff DR, Currie BJ.. Acute rheumatic fever and rheumatic heart disease in the Top End of Australia's Northern Territory. Med J Aust 1996;164:146–149. [DOI] [PubMed] [Google Scholar]
- 6. Colquhoun SM, Condon JR, Steer AC, Li SQ, Guthridge S, Carapetis JR.. Disparity in mortality from rheumatic heart disease in Indigenous Australians. J Am Heart Assoc 2015;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rheumatic Fever and Rheumatic Heart Disease: Report of a World Health Organisation Expert Consultation Geneva, 29 October–1 November 2001. 2001. Technical Report Series 923.
- 8. Katzenellenbogen JM, Bond-Smith D, Seth RJ, Dempsey K, Cannon J, Stacey I, Wade V, de Klerk N, Greenland M, Sanfilippo FM, Brown A, Carapetis JR, Wyber R, Nedkoff L, Hung J, Bessarab D, Ralph AP.. Contemporary incidence and prevalence of rheumatic fever and rheumatic heart disease in Australia using linked data: the case for policy change. J Am Heart Assoc 2020;9:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Katzenellenbogen JM, Bond-Smith D, Seth RJ, Dempsey K, Cannon J, Nedkoff L, Sanfilippo FM, de Klerk N, Hung J, Geelhoed E, Williamson D, Wyber R, Ralph AP, Bessarab D; ERASE Collaboration Study Group. The End Rheumatic Heart Disease in Australia Study of Epidemiology (ERASE) project: data sources, case ascertainment and cohort profile. Clin Epidemiol 2019;11:997–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noubiap JJ, Agbor VN, Bigna JJ, Kaze AD, Nyaga UF, Mayosi BM.. Prevalence and progression of rheumatic heart disease: a global systematic review and meta-analysis of population-based echocardiographic studies. Sci Rep 2019;9:17022–17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karthikeyan G, Zuhlke L, Engel M, Rangarajan S, Yusuf S, Teo K, Mayosi BM.. Rationale and design of a Global Rheumatic Heart Disease Registry: the REMEDY study. Am Heart J 2012;163:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zuhlke L, Karthikeyan G, Engel ME, Rangarajan S, Mackie P, Cupido-Katya Mauff B, Islam S, Daniels R, Francis V, Ogendo S, Gitura B, Mondo C, Okello E, Lwabi P, Al-Kebsi MM, Hugo-Hamman C, Sheta SS, Haileamlak A, Daniel W, Goshu DY, Abdissa SG, Desta AG, Shasho BA, Begna DM, ElSayed A, Ibrahim AS, Musuku J, Bode-Thomas F, Yilgwan CC, Amusa GA, Ige O, Okeahialam B, Sutton C, Misra R, Abul Fadl A, Kennedy N, Damasceno A, Sani MU, Ogah OS, Elhassan TO, Mocumbi AO, Adeoye AM, Mntla P, Ojji D, Mucumbitsi J, Teo K, Yusuf S, Mayosi BM.. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: two-year follow-up of the global Rheumatic Heart Disease Registry (the REMEDY Study). Circulation 2016;134:1456–1466. [DOI] [PubMed] [Google Scholar]
- 13. Zuhlke LJ, Beaton A, Engel ME, Hugo-Hamman CT, Karthikeyan G, Katzenellenbogen JM, Ntusi N, Ralph AP, Saxena A, Smeesters PR, Watkins D, Zilla P, Carapetis J.. Group A Streptococcus, acute rheumatic fever and rheumatic heart disease: epidemiology and clinical considerations. Curr Treat Options Cardiovasc Med 2017;19:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lawrence JG, Carapetis JR, Griffiths K, Edwards K, Condon JR.. Acute rheumatic fever and rheumatic heart disease: incidence and progression in the Northern Territory of Australia, 1997 to 2010. Circulation 2013;128:492–501. [DOI] [PubMed] [Google Scholar]
- 15. He VY, Condon JR, Ralph AP, Zhao Y, Roberts K, de Dassel JL, Currie BJ, Fittock M, Edwards KN, Carapetis JR.. Long-term outcomes from acute rheumatic fever and rheumatic heart disease: a data-linkage and survival analysis approach. Circulation 2016;134:222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cannon J, Roberts K, Milne C, Carapetis JR.. Rheumatic heart disease severity, progression and outcomes: a multi-state model. J Am Heart Assoc 2017;6:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Australian Bureau of Statistics website. 2020. https://www.abs.gov.au/ (Accessed January 2020).
- 18. Wyber R, Noonan K, Halkon C, Enkel S, Cannon J, Haynes E, Mitchell AG, Bessarab DC, Katzenellenbogen JM, Bond-Smith D, Seth R, D'Antoine H, Ralph AP, Bowen AC, Brown A, Carapetis JR; END RHD CRE Investigators Collaborators. Ending rheumatic heart disease in Australia: the evidence for a new approach. Med J Aust 2020;213(Suppl 10):S3–S31. [DOI] [PubMed] [Google Scholar]
- 19. Cannon J, Bessarab DC, Wyber R, Katzenellenbogen JM.. Public health and economic perspectives on acute rheumatic fever and rheumatic heart disease. Med J Aust 2019;211:250–252. [DOI] [PubMed] [Google Scholar]
- 20. Agenson T, Katzenellenbogen JM, Seth R, Dempsey K, Anderson M, Wade V, Bond-Smith D.. Case ascertainment on Australian registers for acute rheumatic fever and rheumatic heart disease. Int J Environ Res Public Health 2020;17:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katzenellenbogen JM, Nedkoff L, Cannon J, Kruger D, Pretty F, Carapetis JR, Dempsey KE, De Dassel J, Anderson M, de Klerk N, Hung J.. Low positive predictive value of ICD-10 codes in relation to rheumatic heart disease: a challenge for global surveillance. Int Med J 2019;49:400–403. [DOI] [PubMed] [Google Scholar]
- 22. Bond-Smith D, Seth R, De Klerk N, Nedkoff L, Anderson M, Hung J, Cannon J, Griffiths K, Katzenellenbogen J.. Development and evaluation of a prediction model for ascertaining rheumatic heart disease status in administrative data. Clin Epidemiol 2020; 12:717–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. RHDAustralia (ARF/RHD writing group), National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand. The 2020 Australian Guideline for Prevention, Diagnosis and Management of Acute Rheumatic Fever and Rheumatic Heart Disease, 3rd edn. Menzies School of Health Research; 2020. https://www.rhdaustralia.org.au/ (Accessed March 2020).
- 24. Gurney JK, Stanley J, Baker MG, Wilson NJ, Sarfati D.. Estimating the risk of acute rheumatic fever in New Zealand by age, ethnicity and deprivation. Epidemiol Infect 2016;144:3058–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Webb R, Wilson N.. Rheumatic fever in New Zealand. J Paediatr Child Health 2013;49:179–184. [DOI] [PubMed] [Google Scholar]
- 26. Noordzij M, Leffondre K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ.. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant 2013;28:2670–2677. [DOI] [PubMed] [Google Scholar]
- 27. Haynes E, Walker R, Mitchell AG, Katzenellenbogen J, D'Antoine H, Bessarab D.. Decolonizing Indigenous health: generating a productive dialogue to eliminate Rheumatic Heart Disease in Australia. Soc Sci Med 2021;277:113829–113840. [DOI] [PubMed] [Google Scholar]
- 28. Haynes E, Mitchell A, Enkel S, Wyber R, Bessarab D.. Voices behind the statistics: a systematic literature review of the lived experience of rheumatic heart disease. Int J Environ Res Public Health 2020;17:1347–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haynes E, Marawili M, Marika BM, Mitchell AG, Phillips J, Bessarab D, Walker R, Cook J, Ralph AP.. Community-based participatory action research on rheumatic heart disease in an Australian Aboriginal homeland: Evaluation of the ‘On track watch’ project. Eval Program Plan 2019;74:38–53. [DOI] [PubMed] [Google Scholar]
- 30. Wyber R, Wade V, Anderson A, Schreiber Y, Saginur R, Brown A, Carapetis J.. Rheumatic heart disease in Indigenous young peoples. Lancet Child Adolesc Health 2021;5:437–446. [DOI] [PubMed] [Google Scholar]
- 31. Churchwell K, Elkind MSV, Benjamin RM, Carson AP, Chang EK, Lawrence W, Mills A, Odom TM, Rodriguez CJ, Rodriguez F, Sanchez E, Sharrief AZ, Sims M, Williams O, American HA; American Heart Association. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation 2020;142:e454–e468. [DOI] [PubMed] [Google Scholar]
- 32. Mitchell AG, Diddo J, James AD, Guraylayla L, Jinmarabynana C, Carter A, Rankin SD, Djorlom G, Coleman C, Scholes M, Haynes E, Remenyi B, Yan J, Francis JR.. Using community-led development to build health communication about rheumatic heart disease in Aboriginal children: a developmental evaluation. Aust N Z J Public Health 2021;45:212–219. [DOI] [PubMed] [Google Scholar]
- 33. Ongzalima CO, Greenland M, Vaughan G, Ng A, Fitz-Gerald JA, Sanfilippo FM, Dickinson JE, Hung J, Katzenellenbogen JM.. Rheumatic heart disease in pregnancy: profile of women admitted to a Western Australian tertiary obstetric hospital. Aust N Z J Obstet Gynaecol 2020;60:302–308. [DOI] [PubMed] [Google Scholar]
- 34. French KA, Poppas A.. Rheumatic heart disease in pregnancy: global challenges and clear opportunities. Circulation 2018;137:817–819. [DOI] [PubMed] [Google Scholar]
- 35. Tujague NA, Ryan KL.. Ticking the box of ‘cultural safety’ is not enough: why trauma-informed practice is critical to Indigenous healing. Rural Remote Health 2021;21:6411–6416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.