Abstract
Aims
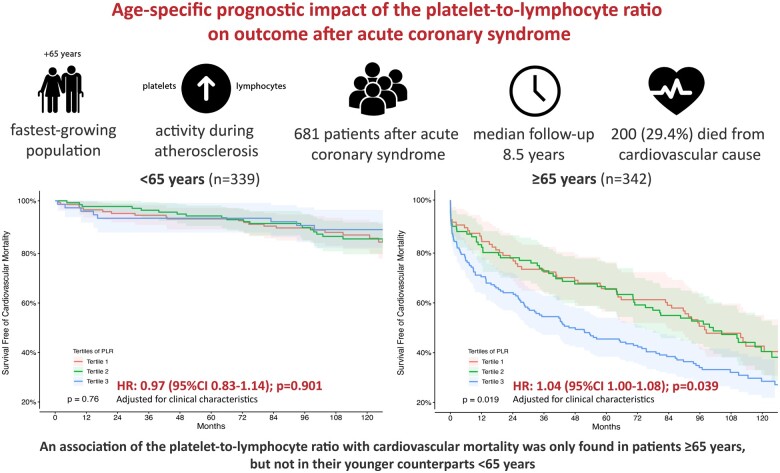
Personalized risk stratification within the ageing society after acute coronary syndrome (ACS) remains scarce but in urgent need. Increased platelet activity together with inflammatory activation play a key role during ACS. We aimed to evaluate the age-specific prognostic potential of the platelet to lymphocyte ratio (PLR) on long-term cardiovascular mortality after ACS.
Methods and results
Patients presenting with ACS admitted to the Vienna General Hospital between December 1996 and January 2010 were enrolled within a clinical registry including assessment of peripheral blood samples. The impact of the PLR on survival was assessed by Cox-regression hazard analysis. We included a total of 681 patients with a median age of 64 years (interquartile range: 45–84). Two hundred (29.4%) individuals died during the median follow-up time of 8.5 years. A strong and independent association of the PLR with cardiovascular mortality was found in the total study population [adjusted (adj.) hazard ratio (HR) per 1 standard deviation (1 SD) of 1.07 (95% confidence interval, CI: 1.03–1.10); P < 0.001]. After stratification in individuals <65 years (n = 339) and ≥65 years (n = 342), a prognostic effect of the PLR on cardiovascular mortality was solely observed in elderly patients ≥65 years [adj. HR per 1 SD of 1.04 (95% CI: 1.00–1.08); P = 0.039], but not in their younger counterparts <65 years [adj. HR per 1 SD of 0.97 (95% CI: 0.83–1.14); P = 0.901].
Conclusion
The present investigation highlights a strong and independent age-specific association of the PLR with cardiovascular mortality in patients with ACS. The PLR only allows to identify patients ≥65 years at high risk for fatal events after ACS—even from a long-term perspective.
Keywords: Acute coronary syndrome, Platelet-to-lymphocyte ratio, Prognosis
Graphical Abstract
Graphical Abstract.
Introduction
Individuals aged over 65 years represent the fastest-growing population in the western society—therefore, physicians will be confronted with novel challenges in the following decades.1,2 Age mirrors an independent and non-adjustable risk factor for adverse outcome after acute coronary syndrome (ACS). Subsequently, easily applicable strategies for the prediction of patients at high risk for fatal events are in urgent need, considering an age-specific perspective and personalized patient care.
Increased platelet activity together with inflammatory activation play a key role in the pathogenesis of atherosclerosis and subsequent plaque rupture during ACS. Platelet reactivity is associated with a pro-inflammatory response, which drives thrombus formation by further platelet activation.3 This inflammatory process can potentially lead to ACS by destabilization and rupture of atherosclerotic plaque in patients with cardiovascular disease (CVD).4
Elevated leucocyte count is an established and widely available marker for inflammation and has been associated with an increased risk for major cardiac adverse events in ACS.5,6 Considering available evidence for the interaction between platelets and lymphocytes,7 it seems intuitive that the combination of both parameters as the platelet to lymphocyte ratio (PLR) shows to be a potential new marker for outcome in patients after ACS.8 However, the age-specific modulation on the degree of platelet activation and pro-inflammatory state remains unknown. In this study, we aimed to assess the age-specific impact of the PLR as a marker for long-term cardiovascular outcome after ACS.
Materials and methods
Study design
Patients presenting with ACS admitted to the Vienna General Hospital, a university-affiliated tertiary care centre with a high-volume cardiac catheterization unit, were included in our clinical registry between December 1996 and January 2010. The presence of ACS was defined referring to the European Society of Cardiology (ESC) guidelines for the management of ACS with and without persistent ST-segment elevation.9,10 Patients with incomplete laboratory values, active malignancies under treatment, and with signs of acute infection or sepsis were excluded from the analysis to prevent interference with the platelet and lymphocyte count. The detailed protocol of this study has already been described elsewhere.11 The study complies with the declaration of Helsinki and was approved by the local ethical committee (Medical University of Vienna, No. 159/2011).
Data acquisition and laboratory analysis
Baseline characteristics and other relevant data were assessed and inserted into a predefined standardized electronic case record form at the time of hospitalization and the time of discharge via screening of patients’ medical records. Study personnel regularly performed range and consistency checks of respective electronic recorded files to overcome potential documentation bias during patient enrolment.
As a standard operating procedure at the local study centre (Division of Cardiology of the Medical University of Vienna, Austria), all patients received a standardized transthoracic echocardiography for left ventricular ejection fraction assessment during hospitalization after the acute event. Blood samples were taken immediately at the time of admission during the acute phase. Subsequent analyses of values of interest were conducted according to the laboratory standards of the General Hospital of Vienna (Medical University of Vienna—Department of Laboratory Medicine) and were performed via Sysmex XN 2000 (Sysmex Austria GmbH, Vienna, Austria).
Patient follow-up and endpoint
All patients were followed prospectively until the primary endpoint of cardiovascular mortality was reached or event-free termination occurred by end of the observation period until January 2018. Date of reaching the endpoint was determined by query of the national registry of death (Austrian Registry of Death—‘Statistik Austria’, Vienna, Austria). Cause of death was defined according to the International Classification of Disease and Related Health Problems 10th Revision.
Statistical analysis
To assess the age-specific prognostic value of PLR on long-term cardiovascular mortality the study population was stratified in a young (<65 years) and elderly (≥65 years) ACS subgroup. Age cut-off was chosen according to recent definitions of the ESC, the American College of Cardiology and American Heart Association.12,13 Baseline characteristics were compared across age groups and PLR tertiles. Platelet to lymphocyte ratio was calculated for each patient individually and log-transformed prior to regression analyses. Platelet count, lymphocyte count, the PLR, and relevant continuous variables are presented as medians with the respective interquartile ranges (IQRs) and were compared between groups or tertiles performing Mann–Whitney U test. Categorical variables are presented as counts and percentages and were compared performing χ2 test. Correlations between continuous variables and PLR were calculated using the Spearman correlation coefficient. The predictive value of PLR on cardiovascular mortality is displayed in Kaplan–Meier curves and was analysed with Cox proportional hazards models. Results of hazard models are presented as hazard ratio (HR) per 1 standard deviation (1 SD) and the respective 95% confidence interval (CI). The multivariate models were adjusted (adj.) for potential confounders: Model 1 addressing clinical patient characteristics (age, gender, hypertension, heart failure, diabetes mellitus, hypercholesterolaemia, smoking status, and family history of CVD) and Model 2 addressing procedure-related characteristics [ST-elevation myocardial infarction (STEMI), percutaneous coronary intervention with stent implantation, angioplasty, thrombolysis, and acute coronary artery bypass graft]. Tertiles of PLR were compared in Kaplan–Meier charts using log-rank test. A two-sided P-value of <0.05 was considered statistically significant in all comparisons. SPSS 26.0 (IBM SPSS, NY, USA) and R 4.0.3 (R Foundation, Vienna, Austria) were used for analyses.
Results
Baseline characteristics
A total of 681 patients were included with a median PLR of 132.5 (IQR: 98.4–182.5). The median age of the entire study population was 64 years (IQR: 45–84). While the platelet count and lymphocyte count were significantly lower, a higher PLR and rates of traditional comorbidities were observed in the subgroup of ≥65 years. Younger individuals (<65 years) with ACS were more often active smokers, had a family history of CVD and a higher body mass index compared to older individuals (≥65 years). Detailed baseline characteristics of subgroups of individuals <65 years and ≥65 years are shown in Table 1.
Table 1.
Baseline characteristics stratified by age-groups
| <65 years (n = 339) | ≥65 years (n = 342) | P-value | |
|---|---|---|---|
| Platelet-to-lymphocyte ratio (IQR) | 118.2 (91.7–154.7) | 150.2 (107.3–209.3) | <0.001 |
| Platelet count (IQR) | 247 (210–292) | 219 (178–266) | <0.001 |
| Lymphocyte count (IQR) | 2.1 (1.6–2.7) | 1.4 (1.1–2.0) | <0.001 |
| Clinical presentation | |||
| Age, years (IQR) | 45 (41–56) | 84 (73–87) | <0.001 |
| Gender (male), n (%) | 186 (54.9) | 143 (41.8) | 0.001 |
| Body mass index, kg/m2 (IQR) | 27.4 (24.7–31.1) | 25.2 (23.4–27.8) | <0.001 |
| STEMI, n (%) | 180 (53.3) | 275 (80.4) | <0.001 |
| Coronary angiography, n (%) | 293 (86.4) | 229 (67.6) | <0.001 |
| Stenting, n (%) | 276 (81.4) | 193 (56.4) | <0.001 |
| 1 vessel, n (%) | 233 (68.7) | 148 (43.3) | |
| 2 vessels, n (%) | 37 (10.9) | 33 (9.6) | |
| ≥3 vessels, n (%) | 6 (1.8) | 12 (3.5) | |
| Fibrinolysis, n (%) | 54 (16.0) | 18 (5.3) | <0.001 |
| Cardiogenic shock, n (%) | 35 (10.4) | 18 (5.3) | 0.014 |
| LVEF ≤40%, n (%) | 61 (18.0) | 110 (32.2) | <0.001 |
| Comorbidities | |||
| Hypertension, n (%) | 216 (64.1) | 269 (78.7) | <0.001 |
| Diabetes mellitus, n (%) | 53 (15.7) | 90 (26.3) | 0.001 |
| Hypercholesterolaemia, n (%) | 237 (70.3) | 218 (63.7) | 0.068 |
| Renal function failure, n (%) | 8 (2.4) | 44 (13.3) | <0.001 |
| Heart failure, n (%) | 8 (2.4) | 24 (7.3) | 0.002 |
| Current smoker, n (%) | 262 (78.4) | 98 (28.7) | <0.001 |
| Family history of CVD, n (%) | 146 (43.8) | 114 (33.3) | 0.005 |
| Laboratory analysis | |||
| Total leucocytes, G/L (IQR) | 10.3 (8.1–13.3) | 9.2 (7.0–11.5) | <0.001 |
| C-reactive protein, mg/dL (IQR) | 0.61 (0.32–1.24) | 0.82 (0.40–2.51) | 0.001 |
| Troponin T (max), µg/L (IQR) | 2.09 (0.78–4.95) | 2.18 (0.75–4.54) | 0.868 |
| Creatine kinase (max), U/L (IQR) | 910 (336–1942) | 581 (226–1352) | <0.001 |
| LDH (max), U/L (IQR) | 430 (282–698) | 419 (285–622) | 0.345 |
| Gamma-GT µkat/L (IQR) | 32 (20–52) | 28 (18–46) | 0.088 |
| Butyrylcholinesterase, U/L (IQR) | 7.4 (5.9–9.0) | 6.3 (5.5–7.6) | <0.001 |
| Total bilirubin, µmol/L (IQR) | 0.48 (0.35–0.69) | 0.64 (0.45–0.91) | <0.001 |
| Creatinine, mg/dL (IQR) | 0.98 (0.82–1.09) | 1.10 (0.93–1.36) | <0.001 |
| NT-proBNP, pg/mL (IQR) | 582 (213–1427) | 3172 (1163–7411) | <0.001 |
| Cardiovascular mortality | 34 (10.0) | 166 (48.5) | <0.001 |
Categorical data are presented as counts and percentages and analysed using χ2 test. Continuous data are presented as median and the respective interquartile range and analysed using Mann–Whitney U test.
CVD, coronary vessel disease; LDH, lactate dehydrogenase; LVEF, left ventricular ejection fraction; STEMI, ST-elevation myocardial infarction.
Significance of bold values is indicated by a p-value of <0.05.
The PLR was stratified in tertiles low [tertile 1: median 86.7 (IQR: 75.1–98.5)], intermediate [tertile 2: median 132.5 (IQR: 120.0–144.3)], and high [tertile 3: median 211.0 (IQR: 182.0–267.0)] to elucidate the association with baseline characteristics. Increasing age (spearman r = 0.244; P < 0.001), but lower rates of male patients (low PLR: 58.8% vs. high PLR: 41.0%) were observed with higher PLR values. The prevalence of STEMI (low PLR: 61.7% vs. high PLR: 71.8%) and renal function failure (low PLR: 5.3% vs. high PLR: 11.3%) increased with higher PLR tertiles, but lower rates of hypercholesteraemia (low PLR: 70.8% vs. high PLR: 58.0%) were observed. Considering relevant biomarkers, higher levels of N-terminal pro B-type natriuretic peptide (r = 0.233; P < 0.001), and C-reactive protein (CRP) (r = 0.233; P < 0.001) correlated with the PLR. Clinical presentation with cardiogenic shock, history of heart failure, hypertension, or diabetes mellitus did not differ within tertiles of PLR. Baseline characteristics including comorbidities and concentration of biomarkers stratified by PLR tertiles are shown in Table 2.
Table 2.
Baseline characteristics stratified by tertiles of platelet-to-lymphocyte ratio
| Tertile 1 (n = 227) | Tertile 2 (n = 227) | Tertile 3 (n = 227) | P-value | r-value | P-value | |
|---|---|---|---|---|---|---|
| Platelet-to-lymphocyte ratio (IQR) | 86.7 (75.1–98.5) | 132.5 (120.0–144.3) | 211.0 (182.0–267.0) | |||
| Platelet count (IQR) | 215 (172–249) | 234 (199–272) | 267 (216–323) | <0.001 | 0.366 | <0.001 |
| Lymphocyte count (IQR) | 2.5 (2.1–3.0) | 1.8 (1.5–2.1) | 1.3 (0.9–1.5) | <0.001 | −0.776 | <0.001 |
| Clinical presentation | ||||||
| Age, years (IQR) | 58 (44–73) | 60 (45–79) | 76 (56–86) | <0.001 | 0.244 | <0.001 |
| Gender (male), n (%) | 129 (56.8) | 107 (47.1) | 93 (41.0) | 0.001 | ||
| Body mass index, kg/m2 (IQR) | 27 (24–31) | 25 (23–28) | 25 (23–28) | <0.001 | −0.220 | <0.001 |
| STEMI, n (%) | 140 (61.7) | 152 (67.3) | 163 (71.8) | 0.022 | ||
| Coronary angiography, n (%) | 192 (84.6) | 169 (74.4) | 161 (70.9) | 0.003 | ||
| Stenting, n (%) | 175 (77.1) | 156 (68.7) | 138 (60.8) | <0.001 | ||
| 1 vessel, n (%) | 146 (64.3) | 124 (54.6) | 111 (48.9) | |||
| 2 vessels, n (%) | 25 (11.0) | 25 (11.0) | 20 (8.8) | |||
| ≥3 vessels, n (%) | 4 (1.7) | 7 (3.0) | 7 (3.1) | |||
| Fibrinolysis, n (%) | 22 (9.7) | 32 (14.2) | 18 (7.9) | 0.542 | ||
| Cardiogenic shock, n (%) | 12 (5.3) | 21 (9.3) | 20 (8.8) | 0.347 | ||
| LVEF ≤40%, n (%) | 47 (20.7) | 48 (21.1) | 76 (33.5) | 0.002 | ||
| Comorbidities | ||||||
| Hypertension, n (%) | 166 (73.5) | 156 (68.7) | 163 (72.1) | 0.755 | ||
| Diabetes mellitus, n (%) | 51 (22.6) | 36 (15.9) | 56 (24.8) | 0.564 | ||
| Hypercholesterolaemia, n (%) | 160 (70.8) | 164 (72.2) | 131 (58.0) | 0.004 | ||
| Renal function failure, n (%) | 12 (5.3) | 15 (6.7) | 25 (11.3) | 0.019 | ||
| Heart failure, n (%) | 9 (4.0) | 9 (4.0) | 14 (6.4) | 0.253 | ||
| Current smoker, n (%) | 149 (66.2) | 123 (54.7) | 88 (38.9) | <0.001 | ||
| Family history of CVD, n (%) | 88 (39.1) | 90 (40.0) | 82 (36.4) | 0.561 | ||
| Laboratory analysis | ||||||
| Total Leucocytes, G/L (IQR) | 10.5 (7.8–12.6) | 9.5 (7.5–12.5) | 9.6 (7.4–11.7) | 0.170 | −0.088 | 0.022 |
| C-reactive protein, mg/dL (IQR) | 0.69 (0.28–1.39) | 0.62 (0.40–1.29) | 0.82 (0.43–3.2) | 0.006 | 0.233 | <0.001 |
| Troponin T (max), µg/L (IQR) | 2.0 (0.7–4.6) | 2.5 (1.1–5.1) | 1.6 (0.5–4.4) | 0.029 | −0.044 | 0.257 |
| Creatin kinase (max), U/L (IQR) | 732 (317–1649) | 939 (404–1929) | 454 (204–1516) | 0.001 | −0.095 | 0.013 |
| LDH (max), U/L (IQR) | 422 (273–617) | 472 (306–704) | 404 (282–624) | 0.089 | −0.016 | 0.684 |
| Gamma-GT, µkat/L (IQR) | 31 (20–55) | 26 (17–42) | 29 (20–50) | 0.010 | −0.047 | 0.219 |
| Butyrylcholinesterase, U/L (IQR) | 7.3 (6.0–8.6) | 6.9 (5.7–8.5) | 6.3 (5.4–7.7) | <0.001 | −0.179 | <0.001 |
| Total bilirubin, µmol/L (IQR) | 0.55 (0.39–0.81) | 0.52 (0.38–0.77) | 0.59 (0.40–0.85) | 0.124 | 0.034 | 0.379 |
| Creatinine, mg/dL (IQR) | 1.0 (0.8–1.2) | 1.0 (0.8–1.2) | 1.0 (0.8–1.2) | 0.239 | 0.052 | 0.176 |
| NT-proBNP, pg/mL(IQR) | 840 (295–2710) | 1427 (431–3832) | 2239 (716–7788) | 0.001 | 0.233 | <0.001 |
| Cardiovascular mortality | 54 (23.8) | 59 (26.0) | 87 (38.3) | 0.001 |
Categorical data are presented as counts and percentages and analysed using χ2 test. Continuous data are presented as median and the respective interquartile range and analysed using Mann–Whitney U test. Correlation refers Spearman Rho correlation coefficient.
CVD, coronary vessel disease; LDH, lactate dehydrogenase; LVEF, left ventricular ejection fraction; STEMI, ST-elevation myocardial infarction.
Significance of bold values is indicated by a p-value of <0.05.
Association of the platelet to lymphocyte ratio on outcome
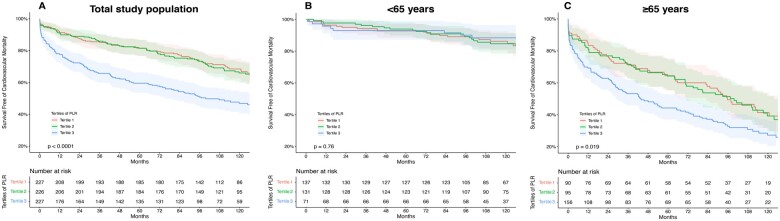
During the median follow-up time of 8.5 years, 200 (29.4%) patients died from a cardiovascular cause (Figure 1). Cox regression hazard analysis was performed to elucidate a predictive effect of the PLR on cardiovascular survival. Adjusted models for confounders showed a strong and independent association of the PLR with cardiovascular mortality in the total study population [Table 3, Model 1: adj. HR per 1 SD of 1.06 (95% CI: 1.02–1.09; P = 0.001); Model 2: adj. HR per 1 SD of 1.07 (95% CI: 1.03–1.10; P < 0.001)].
Figure 1.
Effect of platelet-to-lymphocyte ratio on long-term cardiovascular mortality stratified by age. Kaplan–Meier curves with the respective confidence intervals for the impact of platelet to lymphocyte ratio on cardiovascular mortality plotted in low (=Tertile 1), intermediate (=Tertile 2), and high (=Tertile 3) values and compared using log-rank test—total study population: P < 0.001 (A); <65years P = 0.76 (B); ≥65years: P = 0.019 (C).
Table 3.
Unadjusted and adjusted effects of platelet-to-lymphocyte ratio on outcome within the total study population and stratified according to age groups
| Crude HR (95% CI) | P-value | Model 1: Adj.HR (95% CI)a | P-value | Model 2: Adj.HR (95% CI)b | P-value | |
|---|---|---|---|---|---|---|
| Study population | 1.08 (1.01–1.09) | <0.001 | 1.06 (1.02–1.09) | 0.001 | 1.07 (1.03–1.10) | <0.001 |
| <65 years | 0.98 (0.86–1.10) | 0.677 | 1.01 (0.89–1.14) | 0.891 | 0.97 (0.83–1.14) | 0.901 |
| ≥65 years | 1.05 (1.01–1.09) | 0.016 | 1.04 (1.00–1.08) | 0.043 | 1.04 (1.00–1.08) | 0.039 |
Cox proportional hazard model, hazard ratios (HRs) for continuous variables refer to a 1 SD increase.
Multivariate model 1 was adjusted for clinical characteristics: age, gender, hypertension, heart failure, diabetes mellitus, hypercholesterolaemia, smoking status, and family history of CVD.
Multivariate model 2 was adjusted for interventions: ST-elevation myocardial infarction (STEMI), percutaneous coronary intervention with stent implantation (PCI), angioplasty, thrombolysis, and acute coronary artery bypass graft (CABG).
Significance of bold values is indicated by a p-value of <0.05.
To assess an age-dependent predictive potential, additional analyses were performed after stratification in subgroups <65 years and ≥65 years. Platelet to lymphocyte ratio proved to be an independent prognosticator for cardiovascular mortality after ACS in patients ≥65 years [Model 1: adj. HR per 1 SD of 1.04 (95% CI: 1.00–1.08; P = 0.043); Model 2: adj. HR per 1 SD of 1.04 (95% CI: 1.00–1.08; P = 0.039)], but the predictive potential was lost within the younger counterparts <65 years [Model 1: adj. HR per 1 SD of 1.01 (95% CI: 0.89–1.14; P = 0.891); Model 2: adj. HR per 1 SD of 0.97 (95% CI: 0.83–1.14); P = 0.901].
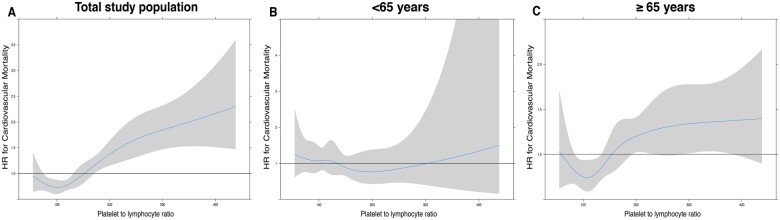
Interaction term analysis showed a strong interaction of age and PLR (P < 0.001 for interaction). Spline curves presenting HRs and the respective 95% CI for the impact of PLR on cardiovascular mortality within the entire study population, as well stratified in age-groups are presented in Figure 2.
Figure 2.
Spline curves for platelet to lymphocyte ratio levels presenting hazard ratios and the respective confidence interval for cardiovascular mortality within the entire population (A) and stratified in age groups (<65 years: B, ≥65 years: C).
Discussion
Platelet formation and inflammation are key mechanisms in the pathophysiology of coronary artery disease (CAD). Previous studies could highlight a predictive potential for the PLR as a marker for fatal adverse events in patients after ACS, but with arising evidence, the age-specific effect of this novel marker remained unknown. While initially shown without considering patients’ age, we extended the currently available evidence, demonstrating that the predictive potential of the PLR on cardiovascular mortality after ACS is confined to patients ≥65 years.
Platelets and inflammation during acute coronary syndrome
Platelet aggregation plays a crucial role in disease development and progression of atherosclerosis. Platelet activation in response to inflammatory stress (e.g. cell death, infection, smoking) has been shown to be an independent prognosticator for major adverse cardiac events in patients with ACS.14 As inflammation is a key driver for acute thromboembolic events, the interaction of platelets and leukocytes further promotes additional platelet recruitment and leukocyte activation by the release of pro-inflammatory mediators.15 Lymphocytes represent the regulatory pathway of the immune system. Repetitive activation during a chronic inflammatory state potentially drives the suppression of lymphocyte count and inflammatory exaggeration as a consequence.7 Acute coronary syndrome results from unstable atherosclerotic lesions influenced by the interaction of thrombosis and inflammation in coronary vessels, thus leading to plaque instability and rupture.3 Affected by the extent of myocardial damage, the acute myocardial ischaemic event potentially leads to a local inflammatory response with a subsequent increase of the total platelet count and increase of CRP.16 It seems that initially higher CRP values during the acute event are associated with higher no-reflow and slow-flow incidence in the respective vessel during angiography,17 thus resulting in higher rates of cardiovascular mortality in patients with higher CRP values in our study population. Compromising a prothrombotic and exaggerated proinflammatory state, the PLR was associated with an increase in cardiovascular mortality in our total study population. This is concomitant to current evidence for the predictive value of the PLR as an emerging marker for cardiovascular mortality after ACS.8,16 However, the effect was only observed in patients ≥65 years and not in the younger subgroup with patients <65 years.
Inflamm-ageing and its influence on the platelet to lymphocyte ratio
The patient group of the elderly is known to frequently present with multiple preceding comorbidities. With the accumulation of prior diseases, older patients potentially suffer from a chronic inflammatory state due to ongoing concomitant tissue damage and repair processes. Altered inflammatory processes by up-regulated tumour necrosis factor-alpha, interleukin-6, and NF-kB activation are observed in patients with chronic inflammatory conditions and also the elderly.18 T-regulatory lymphocytes as a part of the adaptive immune system play a crucial protective role in suppressing an exaggerating harming immune response, but subclinical chronic inflammation in elderly patients (also termed ‘inflamm-ageing’) leads to suppressive function of these important T-regulatory cells.19,20 While ACS causes additional pro-inflammatory activation, reduced availability of this lymphocyte subset is associated with disbalance in inflammatory homeostasis and adverse events in patients with ischaemic heart failure and reduced ejection fraction.21 Therefore, an age-specific variability needs to be considered when investigating the impact of the PLR on patient outcome. A concomitant decrease of the lymphocyte count was observed with increasing age and rate of STEMI at presentation. Lymphocytes additionally play a pivotal role in plaque stability, thus when suppressed, resulting in worse outcome and may potentially explain the higher rate of STEMI in the older subgroup.3 Platelet count is known to decrease with age, but platelet reactivity is known to increase. Platelet hyperreactivity leads to a concomitant exaggeration of an inflammatory response, which potentially has a more severe adverse impact on elderly patients with ‘inflamm-ageing’. This increased platelet activity results in a prothrombotic state and is linked to adverse outcome due to contribution to vessel no-reflow by thrombus formation and vasoconstriction.3,22 The platelet count showed a slight absolute decrease in the elderly subgroup of ≥65 years compared to younger patients <65 years, whereas the decrease of the lymphocyte count was more impressive. Therefore, it seems that the increase of the PLR and the associated increased rate of cardiovascular mortality after ACS was potentially mainly driven by less—predominantly regulatory—lymphocytes.23 Previous studies have shown an association of the lymphocyte fraction and the PLR at baseline with the severity and complexity of CAD and cardiovascular mortality after ACS.8,24 A increased risk for cardiovascular mortality was observed in patients with higher PLR in patients ≥65 years. Assuming the above mentioned hypothesized pathogenetic processes, the PLR was therefore no prognosticator in younger patients without present platelet hyperactivity and chronic inflammatory state.
Potential clinical impact
In the past decades, major risk factors for the development of CAD have been established. While factors including lifestyle or pre-existing medical conditions can be modified, factors like age, gender, or genetic predisposition are non-modifiable.
Age is a major risk factor for CVD and with increased general life expectancy, the proportion of elderly patients suffering ACS is growing. The important relation of an elevated platelet count with adverse cardiovascular outcome has been well investigated in multiple studies.3 Therefore, dual antiplatelet therapy (DAPT) is the guideline-recommended standard therapy for secondary prevention after ACS. The combination of a P2Y12 inhibitor and aspirin sufficiently inhibits thrombus formation.25 However, risk assessment and secondary prevention—especially considering an increased bleeding risk—remains a challenging field in frail and elderly patients.
Although a higher rate of STEMI was observed in the elderly subgroup, treatment strategies in these patients had a significantly lower intention to coronary angiography, stenting and thrombolysis. These data are in accordance with findings from Northern America and Europe, indicating a major undertreatment of elderly patients in terms of therapeutic strategies for coronary revascularization.26,27 Therefore, available literature—including recently published ESC guidelines of the management of ACS in patients without persistent ST-segment elevation—suggests the use of novel biomarkers in addition to established cardiovascular risk-markers to identify patients at risk for adverse events.28 Based on the observed strong discriminatory power of PLR, this novel marker might lead to improved patientcare in terms of a personalized risk stratification and outcome prognostication in elderly patients presenting with ACS. Addressing the observed underuse of coronary revascularization in elderly individuals within the present investigation, a routine measurement of PLR potentially provides prognostic value that guides clinicians through the decision to perform a coronary intervention and to provide the most possible clinical benefit in these individuals. Prolonged DAPT could be established in elderly patients with high PLR given an increased ischaemic risk after ACS. Guideline-recommended statin therapy for secondary prevention lowers low-density lipoproteins. By prevention of cholesterol accumulation and subsequent endothelial malfunction, the cardiovascular risk profile is decreased and therefore intensified statin therapy or anti-inflammatory therapy could potentially be of special interest in patients with elevated PLR and a high ‘inflamm-ageing’ state.29
Limitations
Our study is limited by its single-centre setting. However, we might overcome potential selection bias by the enrolment of a large number of individuals during an extended study period. We are not aware of any potential unknown residual confounding within the present analysis. However, based on comprehensive adjustment for a large subset of clinical patient characteristics and procedure-related characteristics, the probability of potential confounding is limited. Moreover, based on the design of the study, we did not assess an additional endpoint to cardiovascular mortality during the respective long-term patient follow-up. Therefore, a profound competing risk analysis (to re-hospitalization for ACS or urgent revascularization) was not possible to validate the effect of PLR. However, via enrolling a large sample size with a very long follow-up period—including endpoint validation via the national registry of death—we might overcome a potential detection bias.
Conclusion
In this study, we were able to highlight a strong and independent age-specific association of PLR with cardiovascular mortality in patients with ACS. Interestingly, the predictive potential was only observed in elderly patients ≥65 years, but not in their younger counterparts <65 years. The PLR is a routinely available cost-efficient and easily applicable novel parameter, which allows identifying elderly patients at high risk for fatal adverse events after ACS. Therefore, enhanced secondary prevention strategies should potentially be applied in patients ≥65 years with increased PLR after ACS in the era of personalized medicine.
Lead author biography

Niema Kazem, MD, earned a medical degree from the Medical University of Vienna. He is currently completing his residency and PhD at the Department of Cardiology at the Medical University of Vienna. His clinical and research interests include atrial fibrillation, acute coronary syndrome, and cardiovascular pharmacotherapy. Dr Kazem is an active member of the ESC working group on cardiovascular pharmacotherapy.
Conflict of interest: none declared.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, Wilkins E, Wright L, Vos R, Bax J, Blum M, Pinto F, Vardas P; ESC Scientific Document Group. European Society of Cardiology: cardiovascular disease statistics 2017. Eur Heart J 2018;39:508–579. [DOI] [PubMed] [Google Scholar]
- 2. United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing, 2019 Highlights. 2020.
- 3. Schrottmaier WC, Mussbacher M, Salzmann M, Assinger A.. Platelet-leukocyte interplay during vascular disease. Atherosclerosis 2020;307:109–120. [DOI] [PubMed] [Google Scholar]
- 4. Bentzon JF, Otsuka F, Virmani R, Falk E.. Mechanisms of plaque formation and rupture. Circ Res 2014;114:1852–1866. [DOI] [PubMed] [Google Scholar]
- 5. Koupenova M, Clancy L, Corkrey HA, Freedman Jane E.. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ Res 2018;122:337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ziegler M, Wang X, Peter K.. Platelets in cardiac ischaemia/reperfusion injury: a promising therapeutic target. Cardiovasc Res 2019;115:1178–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li N. Platelet–lymphocyte cross-talk. J Leukoc Biol 2008;83:1069–1078. [DOI] [PubMed] [Google Scholar]
- 8. Li W, Liu Q, Tang Y.. Platelet to lymphocyte ratio in the prediction of adverse outcomes after acute coronary syndrome: a meta-analysis. Sci Rep 2017;7:40426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van 't Hof A, Widimsky P, Zahger D.. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 10. Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 11. Sulzgruber P, El-Hamid F, Koller L, Forster S, Goliasch G, Wojta J, Niessner A.. Long-term outcome and risk prediction in patients suffering acute myocardial infarction complicated by post-infarction cardiac rupture. Int J Cardiol 2017;227:399–403. [DOI] [PubMed] [Google Scholar]
- 12. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 13. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 14. Iijima R, Ndrepepa G, Mehilli J, Bruskina O, Schulz S, Schömig A, Kastrati A.. Relationship between platelet count and 30-day clinical outcomes after percutaneous coronary interventions. Pooled analysis of four ISAR trials. Thromb Haemost 2007;98:852–857. [PubMed] [Google Scholar]
- 15. Hansson GK. Inflammation, Atherosclerosis, and Coronary Artery Disease. New England Journal of Medicine. 2005. Apr 21;352(16):1685–95. [DOI] [PubMed] [Google Scholar]
- 16. Kounis NG, Koniari I, Plotas P, Soufras GD, Tsigkas G, Davlouros P, Hahalis G.. Inflammation, thrombosis, and platelet-to-lymphocyte ratio in acute coronary syndromes. Angiology 2021;72:6–8. [DOI] [PubMed] [Google Scholar]
- 17. Zhang E, Gao M, Gao J, Xiao J, Li X, Zhao H, Wang J, Zhang N, Wang S, Liu Y.. Inflammatory and hematological indices as simple, practical severity predictors of microdysfunction following coronary intervention: a systematic review and meta-analysis. Angiology 2020;71:349–359. [DOI] [PubMed] [Google Scholar]
- 18. Chung HY, Sung B, Jung KJ, Zou Y, Yu BP.. The molecular inflammatory process in aging. Antioxid Redox Signal 2006;8:572–581. [DOI] [PubMed] [Google Scholar]
- 19. Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res 2012;110:159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lukas Yani S, Keller M, Melzer FL, Weinberger B, Pangrazzi L, Sopper S, Trieb K, Lobina M, Orrù V, Fiorillo E, Cucca F, Grubeck-Loebenstein B.. CD8+HLADR+ regulatory T cells change with aging: they increase in number, but lose checkpoint inhibitory molecules and suppressive function. Front Immunol 2018;9:1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hammer A, Sulzgruber P, Koller L, Kazem N, Hofer F, Richter B, Blum S, Hülsmann M, Wojta J, Niessner A.. The prognostic impact of circulating regulatory T lymphocytes on mortality in patients with ischemic heart failure with reduced ejection fraction. Mediators Inflamm 2020;2020:6079713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Biino G, Santimone I, Minelli C, Sorice R, Frongia B, Traglia M, Ulivi S, Di Castelnuovo A, Gögele M, Nutile T, Francavilla M, Sala C, Pirastu N, Cerletti C, Iacoviello L, Gasparini P, Toniolo D, Ciullo M, Pramstaller P, Pirastu M, de Gaetano G, Balduini CL.. Age- and sex-related variations in platelet count in Italy: a proposal of reference ranges based on 40987 subjects’ data. PLoS One 2013;8:e54289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao M, Ruan L, Huang Y, Wang J, Yan J, Sang Y, Li S, Wang G, Wu X.. Premature CD4+ T cells senescence induced by chronic infection in patients with acute coronary syndrome. Aging and Disease 2020;11:1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurtul A, Murat SN, Yarlioglues M, Duran M, Ergun G, Acikgoz SK, Demircelik MB, Cetin M, Akyel A, Kasapkara HA, Ornek E.. Association of platelet-to-lymphocyte ratio with severity and complexity of coronary artery disease in patients with acute coronary syndromes. Am J Cardiol 2014;114:972–978. [DOI] [PubMed] [Google Scholar]
- 25. Mackman N, Spronk HMH, Stouffer GA, ten Cate H.. Dual anticoagulant and antiplatelet therapy for coronary artery disease and peripheral artery disease patients. Arterioscler Thromb Vasc Biol 2018;38:726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Avezum A, Makdisse M, Spencer F, Gore JM, Fox KAA, Montalescot G, Eagle KA, White K, Mehta RH, Knobel E, Philippe Collet J.. Impact of age on management and outcome of acute coronary syndrome: observations from the Global Registry of Acute Coronary Events (GRACE). Am Heart J 2005;149:67–73. [DOI] [PubMed] [Google Scholar]
- 27. Zaman MJ, Stirling S, Shepstone L, Ryding A, Flather M, Bachmann M, Myint PK.. The association between older age and receipt of care and outcomes in patients with acute coronary syndromes: a cohort study of the Myocardial Ischaemia National Audit Project (MINAP). Eur Heart J 2014;35:1551–1558. [DOI] [PubMed] [Google Scholar]
- 28. Collet J-P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM; ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 29. Müller K, Chatterjee M, Rath D, Geisler T.. Platelets, inflammation and anti-inflammatory effects of antiplatelet drugs in ACS and CAD. Thromb Haemost 2015;114:498–518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.