Abstract
Aims
A risk score, CERT2, based on distinct ceramide and phosphatidylcholine lipid species, has shown robust performance in predicting cardiovascular risk in secondary prevention. Here, our aim was to investigate the predictive value of CERT2 in primary prevention compared to classical lipid biomarkers and its compatibility with clinical characteristics used in the SCORE risk chart.
Methods and results
Four ceramides [Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/24:0), Cer(d18:1/24:1)] and three phosphatidylcholines [PC(14:0/22:6), PC(16:0/22:5), PC(16:0/16:0)] were analysed by targeted tandem liquid chromatography–mass spectrometry method in FINRISK 2002, which is a population-based risk factor survey investigating men and women aged 25–74 years. Primary prevention subjects (N = 7324) were followed up for 10 years for the following outcomes: incident coronary heart disease (CHD), cardiovascular disease (CVD), major adverse cardiovascular event (MACE), stroke, and heart failure. Hazard ratios per standard deviation obtained from adjusted Cox proportional hazard models were significant for all these endpoints, and the highest for fatal ones, i.e. fatal CHD [1.45 (95% confidence interval 1.07–1.97)], CVD [1.39 (1.06–1.83)], and MACE [1.39 (1.07–1.80)]. The categorical net reclassification improvement was 0.051 for the 10-year risk of incident CVD. Incidence of fatal events was over 10-fold more frequent in the highest CERT2 category compared to the lowest risk category and modified SCORE risk charts, utilizing CERT2 and diabetes mellitus, increased granularity of risk assessment compared to a chart utilizing total cholesterol.
Conclusion
CERT2 is a significant predictor of incident cardiovascular outcomes and risk charts utilizing this score provide an easy tool to estimate relative and absolute risk for incident CVD.
Keywords: Ceramide, Phospholipid, Cardiovascular, Primary prevention, Risk, Death
Graphical Abstract
Graphical Abstract.
Introduction
Lipid biomarkers such as total cholesterol (TC), LDL-cholesterol (LDL-C), and HDL-cholesterol (HDL-C) are widely used in cardiovascular disease (CVD) risk assessment and treatment stratification. Other lipid markers, beyond these routinely used cholesterol markers, have recently been evaluated: we have focused on sphingo- and phospholipid metabolites, as these bioactive lipids have repeatedly shown a significant association with atherosclerotic vascular disease and its clinical manifestations.1–4 We have recently published CERT2, an updated version of the ceramide-based cardiovascular risk test CERT1 and showed its performance in various cardiovascular secondary prevention studies.5–7 In addition to ceramides, CERT2 incorporates phosphatidylcholine lipids and, based on these, utilizes three lipid ratios and concentration of an individual lipid. Previous results have indicated that the CERT2 risk score (scale 0–12) provides more granular risk stratification than conventional lipid biomarkers in secondary prevention.5–7
The performance of CERT2 has not yet been investigated in primary prevention. Thus, our aim in the present study was to investigate the performance of CERT2 in a large primary prevention study and to compare its risk stratification capability with TC, which is the lipid biomarker currently used in the SCORE risk charts that are recommended for CVD risk assessment in the latest European ESC/EAS Guidelines for the Management of Dyslipidemias.8 This evaluation was further motivated by recent publication of the large-scale Copenhagen General Population Study, which revealed a U-shaped association of TC with total mortality, and lack of association between CVD mortality and both TC and LDL-C.9 This, together with the fact that in the SCORE risk charts CVD death is used as the phenotype of interest,8 calls for investigations on whether other biomarkers instead of TC can improve performance of the risk evaluation tools.
In addition to TC, SCORE incorporates age, sex, current smoking status, and systolic blood pressure to estimate the absolute 10-year risk for CVD death.8 Since the current practise to determine individual’s absolute risk is often mainly age driven, our other aim was to develop a new relative risk chart. In older individuals (>70 years), risk calculators including age as one of the risk markers indicate high absolute risk, which may sometimes lead to unnecessary treatments. Relative risk assessment may be useful in the identification of younger individuals (e.g. between 45 and 55 years) at relatively high risk, but not reaching absolute risk level that would trigger preventive measures. Finally, to further increase risk granularity, we also included diabetes mellitus in the risk charts.
Methods
Study cohort
The FINRISK Study, including a questionnaire and health examination with blood draw, has been performed every 5 years since 1972, mainly to monitor trends in cardiovascular and other non-communicable disease risk factors in the Finnish population.10 The FINRISK 2002 Study is a stratified random sample of 13 498 men and women aged 25–74 years from five geographical areas of Finland, of whom 8798 participated (participation rate 65.5%).3 After exclusion of participants who had permanently moved abroad, those with prevalent MACE (n = 393) and those with missing data or serum sample (n = 1081), 7324 subjects were included in the current analysis. Study population, data collection protocol, and methods of laboratory analysis have been described in detail in earlier publications.3,10–12 The study protocol was approved by the Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital District, and all participants gave a written informed consent.
Individuals with incident cardiovascular events during the follow-up were identified by record linkage of the FINRISK 2002 data with the countrywide electronic health registers on the basis of the personal ID code, unique to every permanent resident of Finland. Registers included the Causes-of-Death Register, Hospital Discharge Register, and Drug Reimbursement Registers. Definitions of the endpoints were as follows: stroke: stroke (intracerebral haemorrhage, cerebral infarction), excluding subarachnoid haemorrhage: I61, I63, I64 except I63.6 (ICD-10) or 431, 4330A, 4331A, 4339A, 4340A, 4341A, 4349A, 436 (ICD-9) as the cause of death or as the main or side diagnosis at hospital discharge; coronary heart disease (CHD): I20–I25, I46, R96 or R98 (ICD-10), or 410–414 or 798 (ICD-9) as cause of death, or I200, I21–I22 (ICD-10) or 410, 4110 (ICD-9) as the main diagnosis at hospital discharge, or coronary bypass surgery or coronary angioplasty at hospital discharge or identified from the specific countrywide register of invasive cardiac procedures; CHD death: death due to CHD; cardiovascular disease (CVD) event: CHD or stroke; CVD death: death due to CVD; heart failure (HF): I50, I110, I30, I132 (ICD-10) or 4029B, 4148, 428 (ICD-9); major adverse cardiovascular event (MACE): CVD or HF; and MACE death: death due to MACE. Baseline examination date was used to distinguish disease and events prior to the date as ‘prevalent’ or post examination date as ‘incident’. A person was determined to be on lipid-lowering treatment at baseline investigation, if the subject had purchased statin and/or ezetimibe during the previous 180 days before the blood draw.
Analytical methods for mass spectrometry
Serum samples stored for 17 years in −70°C were used. Lipids were extracted using a modified Folch extraction13 using Hamilton MICROLAB STAR system (Hamilton Robotics, Switzerland). Samples (10 µL) were aliquoted into a 96-well plate, and 10 µL of 10 mM butylated hydroxytoluene in methanol was added, followed by internal standard (IS) mixture (20 µL) containing a known amount of synthetic IS and by chloroform:methanol (2:1, v/v) (300 µL). The plate was sonicated at water bath for 10 min, incubated on an orbital shaker for 40 min, and then centrifuged for 15 min at 5700 g. About 280 µL of the upper organic phase was transferred into a new plate and evaporated under N2 until dryness. Extracted lipids were dissolved by adding 100 µL of water-saturated butanol and sonicated for 5 min. After sonication, 100 µL of methanol was added to all wells; the samples were mixed and then centrifuged for 5 min at 3500 g. Finally, 40 µL of extract was transferred to a 96-well plate for liquid chromatography-tandem mass spectrometry (LC–MS/MS) analysis.
The analysed lipids and ions used in this study are presented in Supplementary material online, Table S1. LC–MS/MS analysis was conducted on a Sciex TripleQuad 5500 mass spectrometer coupled to Sciex MPX LC system. Electrospray ionization in positive ion mode was used with multiple reaction monitoring. Instrument and data acquisition were controlled using Analyst® (version 1.7). The following settings were applied to all compounds in the analysis: curtain gas, 35; ion spray voltage, 5000 V; temperature, 300°C; gas 1 and gas 2, 50; declustering potential, 30; entrance potential, 10; collision exit potential, 20. Collision energy was set separately to each lipid (Supplementary material online, Table S1). Chromatographic separation was performed on an Acquity BEH C18 (2.1 mm × 75 mm, i.d. 1.7 µm) column. Temperature was set to 60°C. Mobile phases consisted of (A) 10 mM ammonium acetate with 0.1% formic acid and (B) 10 mM ammonium acetate in acetonitrile:2-propanol (4:3, v/v) with 0.1% formic acid. Loading pump solvent in MPX consisted of A:B (21:79%). Injection volume was 3 µL and flow rate was 500 µL/min. The following gradient was applied: A/B (22/78%) from 0 to 1.5 min and then B to 85% at 2 min and to 100% at 2.5 min. B was held at 100% from 2.5 to 4.0 min and then dropped to 78% at 4.1 min and held until 4.6 min. Both streams had the same parameters. MS analysis was performed from 1.1 to 3.6 min, which allowed multiplex to run a sample every 2.5 min. Each 96-well plate had a standard line (6 points), as well as QC (6) and blank samples to ensure analytical quality through the whole sample range. Standards and QC samples were extracted the same way as the study samples. Analytical method was validated according to FDA guideline for biological sample analyses.
Statistical methods
CERT2 score was calculated as follows: for each individual, the CERT2 score variables were compared with the whole study population, including also those with prevalent MACE, and the risk points were given based on which quartile (Q1) the individual belonged to. The points were summed up to have a scoring system of 0–12 points. The calculation of the score together with the quartile ranges is shown in Supplementary material online, Table S2. CERT1 was calculated as described previously.2,3
All analyses were performed for 10-year follow-up of the participants. Baseline characteristics of the cohorts were described using medians (interquartile range) for continuous variables, and numbers (percentages) for categorical variables. Two-group comparisons were performed by Wilcoxon rank-sum or Chi2 test, as appropriate. Uni- and multivariate Cox proportional hazard regression models with baseline age as time scale were used to determine hazard ratios (HRs) and 95% confidence intervals for the associations of CERT2 with incident events. The models were stratified by sex, and in multivariable models the adjustments were made for diabetes mellitus type 2 (DM2), current smoking, body mass index, systolic blood pressure, lipid-lowering treatment, LDL-C, HDL-C, and TG, and the effects were expressed per standard deviation (2.4 points). The Cox proportional hazards assumption validity of the models was confirmed with the R-function ‘cox.zph’ (survival package). Risk curves were constructed with ggplot2 package using loess method. C-statistics calculations for Cox regression models together with the net reclassification index (NRI) calculations were performed in FINRISK 2002 using the Hmisc package. For the CERT2 risk charts, median of the risk score category points was used to determine the risk for the group. All tests were two-sided and P < 0.05 was considered as statistically significant. R version 4.0.2 was used for all statistical analyses. The data can be requested from the Finnish Institute for Health and Welfare biobank.
Results
Basic clinical characteristics of the study
Baseline characteristics of the study population are described in Table 1. The subjects with cardiovascular endpoints had elevated levels of all the CERT2 components (Supplementary material online, Table S3). There was an increasing linear trend between CERT2 and age resulting in two score point higher mean values in 75-year-old participants compared to those with a mean age of 25 years at baseline (Supplementary material online, Figure S1). CERT2 correlated significantly with LDL-C (r = 0.30), TC (r = 0.28), HDL-C (r = −0.15), and TG (r = 0.17). Correlations of individual CERT2 components are presented in Supplementary material online, Table S4.
Table 1.
Baseline clinical characteristics of the FINRISK 2002 study cohort in persons without prevalent major adverse cardiovascular event, stratified by CERT2 risk categories
| Characteristic | CERT2: 0–3 | CERT2: 4–6 | CERT2: 7–8 | CERT2: 9–12 |
|---|---|---|---|---|
| N | 1210 | 3089 | 1870 | 1155 |
| Sex (male) (%) | 42 | 44 | 48 | 52 |
| Age (years) | 41 (32–52) | 46 (36–56) | 49 (39–58) | 55 (44–62) |
| Diabetes (%) | 4 | 4 | 6 | 7 |
| Current smoker (%) | 17 | 23 | 29 | 37 |
| Lipid treatment (%) | 6 | 6 | 4 | 4 |
| Antihypertensive treatment (%) | 8 | 13 | 14 | 17 |
| BMI (kg/m2) | 24.9 (22.5–27.5) | 26.1 (23.4–28.9) | 26.7 (24.1–30.1) | 27.4 (24.4–30.4) |
| Systolic blood pressure (mmHg) | 126 (117–138) | 131 (120–145) | 133 (121–148) | 138 (125–153) |
| TC (mmol/L) | 5.0 (4.5–5.6) | 5.4 (4.8–6.1) | 5.6 (5.0–6.4) | 6.0 (5.3–6.7) |
| LDL-C (mmol/L) | 2.9 (2.5–3.4) | 3.2 (2.7–3.8) | 3.5 (2.9–4.1) | 3.7 (3.1–4.4) |
| HDL-C (mmol/L) | 1.5 (1.3–1.8) | 1.5 (1.2–1.8) | 1.4 (1.2–1.7) | 1.4 (1.1–1.7) |
| TG (mmol/L) | 1.0 (0.8–1.4) | 1.1 (0.8–1.6) | 1.2 (0.9–1.8) | 1.3 (1.0–2.0) |
For continuous variables, median and IQR are presented.
BMI, body mass index; HDL-C, HDL-cholesterol; IQR, interquartile range; LDL-C, LDL-cholesterol; TC, total cholesterol; TG, triglycerides.
Performance of CERT2 in risk prediction
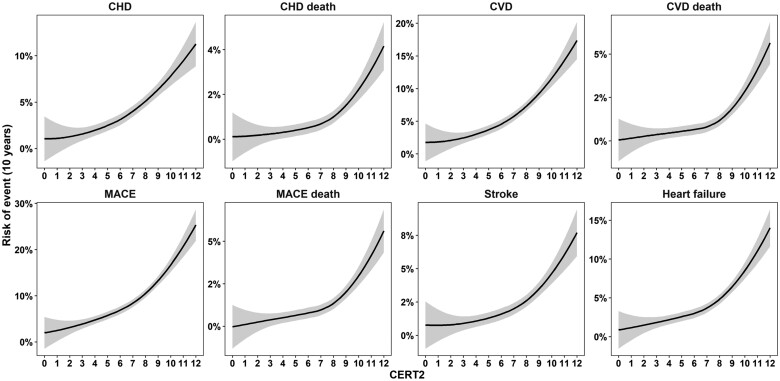
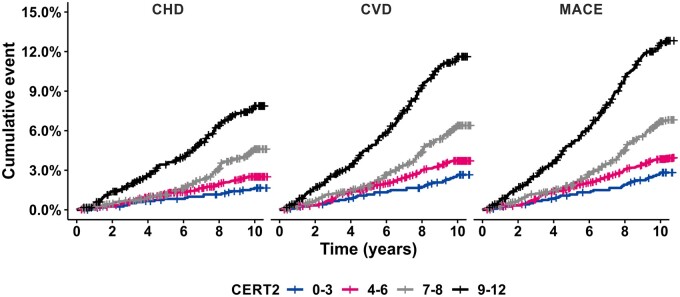
The adjusted hazard ratios for CERT2 were significant for all investigated cardiovascular endpoints, i.e. CHD, CHD death, CVD (CHD + stroke), CVD death, MACE (CVD + HF), MACE death, stroke, and HF, and the highest HRs were recorded for the fatal endpoints (Table 2). Furthermore, CERT2 associated strongly also with non-MACE deaths (Table 2). For comparison, the HRs were calculated also for clinically used lipid biomarkers for incident CVD, and CERT2 HRs appeared higher and more significant than those for routinely used lipid biomarkers as well as CERT1 score (Table 3). Also, the risk curves demonstrated increase of risk of all endpoints along with increasing CERT2 score, and for fatal endpoints the risk increased especially in subjects with a score higher than 8 points (Figure 1). People in the highest CERT2 risk score category (16% of the study population) had a 13-fold increased risk of CHD death and >10-fold increased risk of CVD and MACE deaths as compared to the lowest risk category (17% of the study population) (Table 4). The Kaplan–Meier curves also demonstrated a clear separation of the four risk categories (Figure 2).
Table 2.
Hazard ratios for incident cardiovascular events per one standard deviation increment in CERT2 score
| Endpoint | Event+ | Event− | HR (95% CI)a | P-value | HR (95% CI)b | P-value |
|---|---|---|---|---|---|---|
| CHD | 269 | 7055 | 1.52 (1.34, 1.72) | 6.5E−11 | 1.30 (1.13, 1.49) | 1.6E−04 |
| CHD death | 53 | 7271 | 1.79 (1.34, 2.40) | 9.2E−05 | 1.40 (1.02, 1.91) | 0.035 |
| CVD | 393 | 6931 | 1.49 (1.34, 1.65) | 7.6E−14 | 1.35 (1.21, 1.51) | 9.6E−08 |
| CVD death | 67 | 7257 | 1.72 (1.33, 2.22) | 4.0E−05 | 1.34 (1.02, 1.77) | 0.034 |
| MACE | 579 | 6745 | 1.46 (1.34, 1.59) | 4.4E−18 | 1.34 (1.22, 1.47) | 4.7E−10 |
| MACE death | 74 | 7250 | 1.67 (1.31, 2.14) | 3.7E−05 | 1.34 (1.04, 1.74) | 0.026 |
| Stroke | 142 | 7182 | 1.48 (1.24, 1.76) | 9.8E−06 | 1.46 (1.22, 1.76) | 4.1E−05 |
| HF | 271 | 7053 | 1.45 (1.28, 1.65) | 4.7E−09 | 1.34 (1.17, 1.53) | 2.4E−05 |
| Non-MACE death | 231 | 7093 | 1.61 (1.41, 1.85) | 7.4E−12 | 1.59 (1.38, 1.83) | 2.8E−10 |
BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; DM2, diabetes mellitus type 2; CHD, coronary heart disease; HDL-C, HDL-cholesterol; HF, heart failure; HR, hazard ratio; LDL-C, LDL-cholesterol; MACE, major adverse cardiovascular event; TG, triglycerides.
Age at baseline used as timescale, stratified for sex.
Age at baseline used as timescale, stratified for sex and adjusted for DM2, current smoking, BMI, systolic blood pressure, lipid-lowering treatment, LDL-C, HDL-C and TG.
Table 3.
Hazard ratios for incident cardiovascular disease and cardiovascular disease death per one standard deviation increment in CERT2 and other cardiovascular biomarkers
| Variable | CVD |
CVD death |
||||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI)a | P-value | HR (95% CI)b | P-value | HR (95% CI)a | P-value | HR (95% CI)b | P-value | |
| CERT2 | 1.49 (1.34, 1.65) | 7.6E−14 | 1.40 (1.26, 1.56) | 6.3E−10 | 1.72 (1.33, 2.22) | 4.0E−05 | 1.44 (1.10, 1.87) | 0.007 |
| CERT1 | 1.35 (1.22, 1.49) | 2.2E−09 | 1.26 (1.14, 1.40) | 9.8E−06 | 1.52 (1.19, 1.93) | 7.2E−04 | 1.28 (1.00, 1.64) | 0.051 |
| TC | 1.11 (1.01, 1.22) | 0.039 | 1.10 (0.99, 1.21) | 0.069 | 1.22 (0.98, 1.53) | 0.073 | 1.17 (0.94, 1.46) | 0.158 |
| LDL-C | 1.13 (1.02, 1.25) | 0.019 | 1.13 (1.02, 1.26) | 0.016 | 1.30 (1.03, 1.63) | 0.026 | 1.28 (1.01, 1.64) | 0.043 |
| HDL-C | 0.77 (0.69, 0.87) | 2.2E−05 | 0.81 (0.72, 0.91) | 5.7E−04 | 0.84 (0.63, 1.11) | 0.222 | 0.88 (0.67, 1.17) | 0.389 |
| TG | 1.21 (1.14, 1.28) | 3.1E−10 | 1.14 (1.06, 1.22) | 2.1E−04 | 1.18 (1.03, 1.37) | 0.021 | 1.09 (0.92, 1.28) | 0.331 |
| TG/HDL-C | 1.18 (1.13, 1.24) | 2.2E−12 | 1.14 (1.08, 1.20) | 2.6E−06 | 1.18 (1.05, 1.33) | 0.006 | 1.11 (0.97, 1.27) | 0.144 |
| TC/HDL-C | 1.24 (1.16, 1.33) | 1.9E−10 | 1.22 (1.13, 1.31) | 9.2E−08 | 1.25 (1.06, 1.47) | 0.009 | 1.18 (0.98, 1.41) | 0.074 |
Regarding HDL-C, models for CVD endpoint did not meet proportional hazard assumptions. Bold refers to p<0.05.
BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; DM2, diabetes mellitus type 2; HDL-C, HDL-cholesterol; HR, hazard ratio; LDL-C, LDL-cholesterol; TC, total cholesterol; TG, triglycerides.
Age at baseline used as timescale, stratified for sex.
Age at baseline used as timescale, stratified for sex and adjusted for DM2, current smoking, BMI, systolic blood pressure, and lipid-lowering treatment.
Figure 1.
Risk curves for 10 years, according to CERT2 score for cardiovascular endpoints.
Table 4.
Absolute and relative 10-year risk of cardiovascular events in the CERT2 risk categories
| Category | Population (%) | No event | Event | Risk (%) | Rel. risk | Population (%) | No event | Event | Risk (%) | Rel. risk |
|---|---|---|---|---|---|---|---|---|---|---|
| CHD | CHD death | |||||||||
|
| ||||||||||
| 0–3 | 17 | 1190 | 20 | 1.7 | 1.0 | 17 | 1208 | 2 | 0.2 | 1.0 |
| 4–6 | 42 | 3012 | 77 | 2.5 | 1.5 | 42 | 3073 | 16 | 0.5 | 3.1 |
| 7–8 | 26 | 1786 | 84 | 4.5 | 2.7 | 26 | 1860 | 10 | 0.5 | 3.2 |
| 9–12 | 16 | 1067 | 88 | 7.6 | 4.6 | 16 | 1130 | 25 | 2.2 | 13.1 |
|
| ||||||||||
| CVD | CVD death | |||||||||
|
| ||||||||||
| 0–3 | 17 | 1178 | 32 | 2.6 | 1.0 | 17 | 1207 | 3 | 0.2 | 1.0 |
| 4–6 | 42 | 2975 | 114 | 3.7 | 1.4 | 42 | 3067 | 22 | 0.7 | 2.9 |
| 7–8 | 26 | 1753 | 117 | 6.3 | 2.4 | 26 | 1859 | 11 | 0.6 | 2.4 |
| 9–12 | 16 | 1025 | 130 | 11.3 | 4.3 | 16 | 1124 | 31 | 2.7 | 10.8 |
|
| ||||||||||
| MACE | MACE death | |||||||||
|
| ||||||||||
| 0–3 | 17 | 1164 | 46 | 3.8 | 1.0 | 17 | 1207 | 3 | 0.2 | 1.0 |
| 4–6 | 42 | 2904 | 185 | 6.0 | 1.6 | 42 | 3063 | 26 | 0.8 | 3.4 |
| 7–8 | 26 | 1708 | 162 | 8.7 | 2.3 | 26 | 1858 | 12 | 0.6 | 2.6 |
| 9–12 | 16 | 969 | 186 | 16.1 | 4.2 | 16 | 1122 | 33 | 2.9 | 11.5 |
|
| ||||||||||
| Stroke | Heart failure | |||||||||
| 0–3 | 17 | 1198 | 12 | 1.0 | 1.0 | 17 | 1189 | 21 | 1.7 | 1.0 |
| 4–6 | 42 | 3048 | 41 | 1.3 | 1.3 | 42 | 2998 | 91 | 2.9 | 1.7 |
| 7–8 | 26 | 1833 | 37 | 2.0 | 2.0 | 26 | 1807 | 63 | 3.4 | 1.9 |
| 9–12 | 16 | 1103 | 52 | 4.5 | 4.5 | 16 | 1059 | 96 | 8.3 | 4.8 |
CVD, cardiovascular disease; CHD, coronary heart disease; MACE, major adverse cardiovascular event.
Figure 2.
Cumulative event rate in different CERT2 risk categories for coronary heart disease, cardiovascular disease, and major adverse cardiovascular event.
The additional value of CERT2 was investigated by adding it on top of a basic model comprising age, sex, type 2 diabetes, systolic blood pressure, and current smoking status. The C-statistics did not show statistically significant increase for any endpoint. However, CERT2 increased significantly the 10-year categorical NRI for CVD (NRI of 0.051) and stroke (NRI 0.114) but not for other investigated endpoints (Table 5). For all these endpoints, the result came primarily reclassification of events rather than non-events. For continuous NRI, also other endpoints, including CHD, CHD death, MACE, and heart failure, were significant, with the highest improvement observed for CHD (0.309). The CERT2 results for all endpoints were stronger than those observed for TC (Table 5).
Table 5.
C-statistics and net reclassification index for 10-year risk of cardiovascular events
| Endpoint | Model | C-stat | Delta | P-value | Categorical NRIb |
Continuous NRI |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| NRI (95% CI) | Event (%) | Non-event (%) | NRI (95% CI) | Event (%) | Non-event (%) | |||||
| CHD | Basic modela | 0.813 | ||||||||
| + CERT2 | 0.820 | 0.006 | 0.103 | 0.025 (−0.019; 0.069) | 3.0 | −0.5 | 0.309 (0.188; 0.429) | 15.7 | 15.2 | |
| + CERT1 | 0.816 | 0.003 | 0.343 | 0.033 (−0.008; 0.073) | 3.4 | −0.1 | 0.245 (0.123; 0.367) | 7.5 | 17.0 | |
| + TC | 0.816 | 0.002 | 0.356 | 0.026 (−0.012; 0.064) | 2.6 | 0.0 | 0.218 (0.096; 0.340) | 0.0 | 21.8 | |
| CHD death | Basic modela | 0.910 | ||||||||
| + CERT2 | 0.915 | 0.005 | 0.363 | 0.054 (−0.079; 0.187) | 5.7 | −0.3 | 0.304 (0.038; 0.570) | 17.0 | 13.4 | |
| + CERT1 | 0.914 | 0.004 | 0.331 | 0.074 (−0.029; 0.176) | 7.5 | −0.2 | 0.182 (−0.088; 0.452) | 1.9 | 16.3 | |
| + TC | 0.912 | 0.002 | 0.277 | −0.021 (−0.104; 0.062) | −1.9 | −0.2 | 0.170 (−0.100; 0.440) | −1.9 | 18.9 | |
| CVD | Basic modela | 0.815 | ||||||||
| + CERT2 | 0.819 | 0.005 | 0.149 | 0.051 (0.002; 0.100) | 5.9 | −0.8 | 0.293 (0.192; 0.394) | 13.8 | 15.5 | |
| + CERT1 | 0.817 | 0.002 | 0.475 | 0.056 (0.011; 0.100) | 5.9 | −0.3 | 0.220 (0.118; 0.321) | 4.6 | 17.4 | |
| + TC | 0.815 | 0.000 | 0.960 | 0.010 (−0.013; 0.033) | 0.8 | 0.2 | 0.144 (0.042; 0.245) | −4.1 | 18.4 | |
| CVD death | Basic modela | 0.901 | ||||||||
| + CERT2 | 0.903 | 0.002 | 0.654 | 0.072 (−0.054; 0.198) | 7.5 | −0.3 | 0.263 (0.025; 0.501) | 13.4 | 12.9 | |
| + CERT1 | 0.904 | 0.003 | 0.416 | 0.014 (−0.100; 0.127) | 1.5 | −0.1 | 0.154 (−0.086; 0.395) | −1.5 | 16.9 | |
| + TC | 0.903 | 0.002 | 0.270 | −0.031 (−0.123; 0.062) | −3.0 | −0.1 | 0.252 (0.012; 0.492) | 4.5 | 20.7 | |
| MACE | Basic modela | 0.811 | ||||||||
| + CERT2 | 0.815 | 0.004 | 0.093 | 0.034 (−0.009; 0.078) | 4.2 | −0.7 | 0.248 (0.164; 0.333) | 9.3 | 15.5 | |
| + CERT1 | 0.813 | 0.002 | 0.331 | 0.012 (−0.026; 0.050) | 1.9 | −0.7 | 0.244 (0.159; 0.328) | 5.9 | 18.5 | |
| + TC | 0.811 | 0.000 | 0.943 | −0.003 (−0.019; 0.014) | −0.2 | −0.1 | 0.135 (0.050; 0.219) | −3.8 | 17.3 | |
| MACE death | Basic modela | 0.902 | ||||||||
| + CERT2 | 0.904 | 0.002 | 0.706 | 0.105 (−0.011; 0.221) | 10.8 | −0.3 | 0.198 (−0.030; 0.426) | 8.1 | 11.7 | |
| + CERT1 | 0.904 | 0.002 | 0.482 | 0.039 (−0.070; 0.148) | 4.1 | −0.2 | 0.163 (−0.066; 0.391) | 0.0 | 16.3 | |
| + TC | 0.903 | 0.001 | 0.318 | 0.001 (−0.052; 0.054) | 0.0 | 0.1 | 0.217 (−0.012; 0.445) | 2.7 | 19.0 | |
| Stroke | Basic modela | 0.834 | ||||||||
| + CERT2 | 0.834 | 0.000 | 0.969 | 0.114 (0.040; 0.188) | 12.0 | −0.6 | 0.252 (0.086; 0.417) | 9.9 | 15.3 | |
| + CERT1 | 0.833 | −0.001 | 0.709 | 0.081 (0.014; 0.147) | 8.5 | −0.4 | 0.126 (−0.040; 0.292) | −4.2 | 16.8 | |
| + TC | 0.836 | 0.002 | 0.175 | 0.002 (−0.050; 0.054) | 0.0 | 0.2 | −0.068 (−0.234; 0.098) | 4.2 | −11.0 | |
| Heart failure | Basic modela | 0.826 | ||||||||
| + CERT2 | 0.831 | 0.005 | 0.168 | 0.049 (−0.017; 0.116) | 5.2 | −0.2 | 0.214 (0.093; 0.335) | 6.3 | 15.2 | |
| + CERT1 | 0.829 | 0.003 | 0.391 | 0.045 (−0.018; 0.109) | 4.8 | −0.3 | 0.280 (0.159; 0.400) | 8.5 | 19.5 | |
| + TC | 0.826 | 0.000 | 0.976 | 0.001 (−0.013; 0.016) | 0.0 | 0.1 | 0.109 (−0.012; 0.230) | −5.5 | 16.4 | |
CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; DM2, diabetes mellitus type 2; HF, heart failure; MACE, major adverse cardiovascular event; NRI, net reclassification index; TC, total cholesterol.
Basic model consists of DM2, current smoking, systolic blood pressure, age at baseline and sex.
Categorical NRI cut-offs were 3%, 5%, and 10% for CHD and CVD deaths. For CHD and CVD and MACE, these were multiplied by 4, for stroke by 2, and for HF by 3.
Risk charts based on CERT2
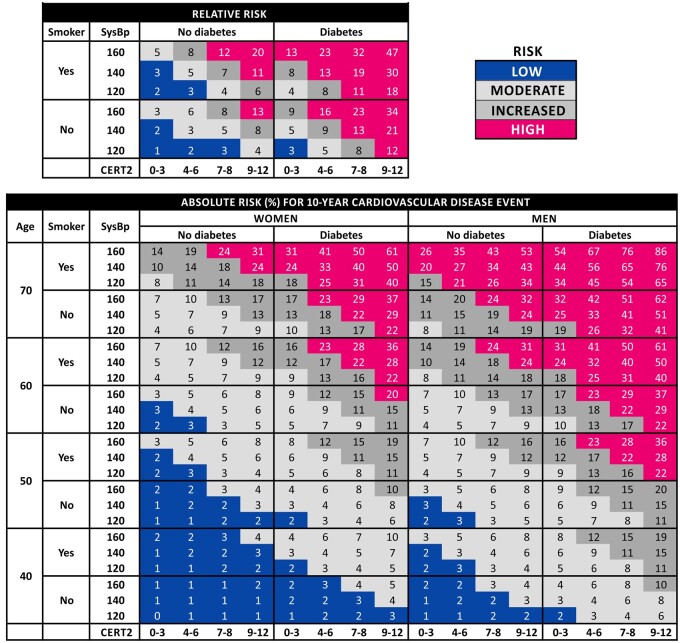
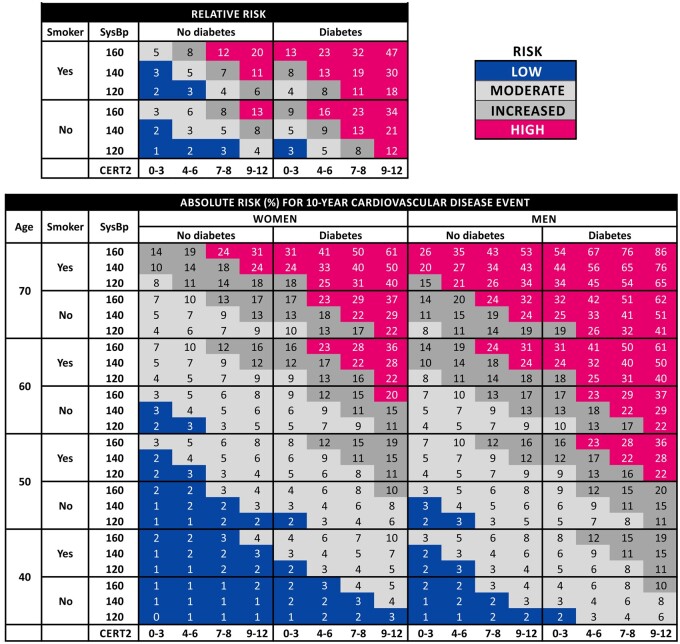
Finally, we generated risk charts including CERT2 for absolute and relative risk assessment that follow the logic of the SCORE system with the exception that also prevalent diabetes mellitus was included in the equations. Both the absolute and relative risk chart resulted in a logical presentation and the risk stratification with CERT2 appeared more granular compared to more conventional risk charts utilizing TC (Figure 3 and Supplementary material online, Figure S2) or to the previously established CERT1 score (Supplementary material online, Figure S3).
Figure 3.
Relative and absolute risk (%) of 10-year risk for incident cardiovascular disease. In the risk charts, one should round the age and systolic blood pressure to the nearest numbers in the table. In the relative risk chart, the figures are normalized against those subjects with CERT2 0–3, no diabetes, and smoking and systolic blood pressure in the lowest category. As an example, a 44-year-old smoking non-diabetic female, with CERT2 score 9 and systolic blood pressure 130 mmHg, has an absolute 10-year risk of 3% for incident cardiovascular disease. The relative risk for incident cardiovascular disease is 11 times higher than for a non-smoking, non-diabetic person with CERT2 ≤3 points and systolic blood pressure <125 mmHg.
Discussion
This study confirmed the performance of CERT2 score in primary prevention. CERT2 appeared to be a very good predictor for fatal CHD and CVD events, and significant association was also observed with non-fatal events as well as stroke and heart failure. As expected based on the previous data for sphingolipids,14 CERT2 was a predictor independent of routinely used cholesterol biomarkers, even though it showed also correlation with them. Regarding preventive measures, the highest CERT2 risk category (9–12 risk points) appears likely to be of clinical relevance. In this group incidence of non-fatal CHD, CVD and MACE events were nearly five-fold higher compared to the lowest CERT2 category. Moreover, incidence of fatal events was over 10-fold more frequent in the highest CERT2 category, which included 16% of the whole study population. Thus, CERT2 may be a useful tool for the identification of very high-risk individuals in primary prevention.
We have shown previously in several secondary prevention studies that CERT2 reaches higher hazard ratios and C-statistics than CERT1, which consists only of ceramide lipids.5,6 Here, we observed that CERT2 reaches higher hazard ratios than CERT1 also in the primary prevention setting, although the differences in C-statistics were minor. Nevertheless, the risk separation in the risk charts was again more pronounced for CERT2 and, thus, it appears that phospholipids yield additional value to ceramides in cardiovascular risk prediction.
Biomarkers, like CERT2, may be used alone or in combination with clinical characteristics and/or other biomarkers. As part of the present study, we modelled CERT2 together with clinical characteristics used in the SCORE, as well as DM2, and developed novel risk charts both for the absolute and relative CVD risk assessment. The C-statistics for the different models showed only small differences, whereas clearer results were obtained for reclassification indices, and when inspecting the risk charts. In the risk charts the outcome risk logically increased together with increasing CERT2 score values, and risk difference between low and high values was more pronounced for CERT2 than TC or CERT1. Thus, the new suggested risk charts seem to provide more granular risk assessment than the currently used SCORE charts. The increased separation in the risk may explain why the results of reclassification indices for CERT2 were more favourable than C-statistics.
The limited performance of TC is surprising given its widespread use in CVD risk assessment. However, this is in line with the recent Copenhagen General Population Study findings9 and supported further by the UK biobank analyses of 346 686 individuals without baseline CVD or statin use. The UK biobank study showed no association between TC and CVD mortality (SCORE definition), since the mean baseline TC levels in subjects experiencing fatal CVD was essentially the same than those who did not [5.9 mmol/L (228 mg/dL) in both groups] and there appeared to be a U-shaped association with the endpoint. In addition, we have recently reported that in the FINRISK 2002 cohort, analysed also in the present study, the associations of LDL-C with incident cardiovascular endpoints were weak in both middle-aged (>50 years) and older individuals.15 Taken together, it seems that the mechanisms that lead to CVD events, e.g. due to plaque rupture, may be different from those which drive the decades long atherogenic process. Thus, it is feasible that while TC and LDL-C may drive the atherosclerotic CVD process, they may also poorly predict CVD events in later life.15
A strength of the study is that FINRISK 2002 is a large, well-characterized population-based study. A limitation of the study is the lack of validation cohort and thus we are not able to report how accurate the new risk chart would be in other populations. It is likely that re-calibration is needed for absolute risk determination for countries with higher or lower risk levels compared to Finland. However, it should be noted that this limitation is not relevant for the relative risk assessment. Furthermore, this cohort study was initiated in 2002 and thus it does not fully reflect the current population health situation.
One fundamental difference between the newly proposed risk chart and SCORE is that we included risk stratification opportunity also for subjects with DM2. Earlier their risk level has been considered so high that no risk evaluation is needed. We observed a 14-fold relative risk difference between low and high risk DM2 subjects. Thus, we suggest that risk evaluation may be clinically useful also in DM2 patients as it may help more precise targeting of preventive care including lifestyle coaching and medical care. It seems reasonable to think that the CVD risk reducing sodium-glucose transport protein 2 inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1 agonists) would be particularly beneficial in DM2 with very high CVD risk.
It has earlier been shown that different components of the CERT2 score are lowered for instance by statins and PCSK9 inhibitors and thus logically CERT2 score is expected to decrease due to lipid-lowering treatments. Unpublished data (Hilvo et al., submitted) show that aggressive lipid lowering reduces CERT2 score by 2–3 points, which associates well with the expected risk reduction due to statin treatment. However, more evidence is needed to link CERT2 score lowering to risk reduction. Interestingly, in the PREDIMED trial, high ceramide concentrations were associated with CVD risk reduction in subjects on Mediterranean diet intervention, while no benefit was observed in subjects with low ceramide levels.16
In conclusion, CERT2 score associated significantly with CV death as well as with non-fatal MIs, stroke and heart failure in primary prevention. A modified SCORE risk chart with CERT2 with enhanced risk stratification was developed for both absolute and relative risk prediction including also subjects with DM2.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Author contribution statement
R.L., M.H., and P.J. contributed to the conception or design of the work. M.H., A.J., M.L., P.J., and R.L. contributed to the acquisition, analysis, or interpretation of data for the work. M.H. and R.L. drafted the manuscript. P.J., A.J., and M.L. critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Conflict of interest: Zora Biosciences Oy holds patent disclosures related for the diagnostic and prognostic use of ceramides and phospholipids in CVD. M.H., A.J., and R.L. are employees and R.L. a shareholder of Zora Biosciences Oy.
Lead author biography

Mika Hilvo holds a PhD in Medical Technology and Biotechnology from the University of Tampere, Finland. Currently, Dr Hilvo is working as Chief Scientific Officer at Zora Biosciences Oy, which is a Finnish diagnostics company. His research interests include metabolic alterations associated with cardiovascular disease and cancer and how those can be exploited in the development of novel diagnostic and prognostic tests. Dr Hilvo has extensive experience in lipidomic data analyses and cardiovascular epidemiology, as well as translation of scientific findings into products that have been taken into clinical practice.
Supplementary Material
Contributor Information
Mika Hilvo, Zora Biosciences Oy, Tietotie 2C, Espoo 02150, Finland.
Antti Jylhä, Zora Biosciences Oy, Tietotie 2C, Espoo 02150, Finland.
Mitja Lääperi, Zora Biosciences Oy, Tietotie 2C, Espoo 02150, Finland.
Pekka Jousilahti, Department of Public Health and Welfare, Finnish Institute for Health and Welfare, Helsinki, Finland.
Reijo Laaksonen, Zora Biosciences Oy, Tietotie 2C, Espoo 02150, Finland; Finnish Cardiovascular Research Center, Tampere University, Tampere, Finland.
See Commentary “An Enhanced Ceramide-Based Approach for Primary Prevention of Atherosclerotic Events” by Vasile V. and Jaffe AS.
References
- 1. Tarasov K, Ekroos K, Suoniemi M, Kauhanen D, Sylvänne T, Hurme R, Gouni-Berthold I, Berthold HK, Kleber ME, Laaksonen R, März W. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J Clin Endocrinol Metab 2014;99:E45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, Kauhanen D, Suoniemi M, Hurme R, März W, Scharnagl H, Stojakovic T, Vlachopoulou E, Lokki ML, Nieminen MS, Klingenberg R, Matter CM, Hornemann T, Jüni P, Rodondi N, Räber L, Windecker S, Gencer B, Pedersen ER, Tell GS, Nygård O, Mach F, Sinisalo J, Lüscher TF. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J 2016;37:1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Havulinna AS, Sysi-Aho M, Hilvo M, Kauhanen D, Hurme R, Ekroos K, Salomaa V, Laaksonen R. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler Thromb Vasc Biol 2016;36:2424–2430. [DOI] [PubMed] [Google Scholar]
- 4. Mundra PA, Barlow CK, Nestel PJ, Barnes EH, Kirby A, Thompson P, Sullivan DR, Alshehry ZH, Mellett NA, Huynh K, Jayawardana KS, Giles C, McConville MJ, Zoungas S, Hillis GS, Chalmers J, Woodward M, Wong G, Kingwell BA, Simes J, Tonkin AM, Meikle PJ. Large-scale plasma lipidomic profiling identifies lipids that predict cardiovascular events in secondary prevention. JCI Insight 2018;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, Brenner H, Schöttker B, Lääperi M, Kauhanen D, Koistinen KM, Jylhä A, Huynh K, Mellett NA, Tonkin AM, Sullivan DR, Simes J, Nestel P, Koenig W, Rothenbacher D, Nygård O, Laaksonen R. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur Heart J 2020;41:371–380. [DOI] [PubMed] [Google Scholar]
- 6. Hilvo M, Wallentin L, Lakic Held GT, Kauhanen C, Jylhä D, Lindbäck A, Siegbahn J, Granger A, Koenig CB, Stewart W, White RAH, Laaksonen H, Stability Investigators R. Prediction of residual risk by ceramide-phospholipid score in patients with stable coronary heart disease on optimal medical therapy. J Am Heart Assoc J Am Heart Assoc 2020;9:e015258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gencer B, Morrow DA, Braunwald E, Goodrich EL, Hilvo M, Kauhanen D, Sabatine MS, Laaksonen R, O’Donoghue ML. Plasma ceramide and phospholipid-based risk score and the risk of cardiovascular death in patients after acute coronary syndrome. Eur J Prev Cardiol 2020. [DOI] [PubMed] [Google Scholar]
- 8. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O, ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 9. Johannesen CDL, Langsted A, Mortensen MB, Nordestgaard BG. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. BMJ 2020;371:m4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borodulin K, Vartiainen E, Peltonen M, Jousilahti P, Juolevi A, Laatikainen T, Männistö S, Salomaa V, Sundvall J, Puska P. Forty-year trends in cardiovascular risk factors in Finland. Eur J Public Health 2015;25:539–546. [DOI] [PubMed] [Google Scholar]
- 11. Hilvo M, Salonurmi T, Havulinna AS, Kauhanen D, Pedersen ER, Tell GS, Meyer K, Teeriniemi A-M, Laatikainen T, Jousilahti P, Savolainen MJ, Nygård O, Salomaa V, Laaksonen R. Ceramide stearic to palmitic acid ratio predicts incident diabetes. Diabetologia 2018;61:1424–1434. [DOI] [PubMed] [Google Scholar]
- 12. Borodulin K, Tolonen H, Jousilahti P, Jula A, Juolevi A, Koskinen S, Kuulasmaa K, Laatikainen T, Männistö S, Peltonen M, Perola M, Puska P, Salomaa V, Sundvall J, Virtanen SM, Vartiainen E. Cohort profile: the national FINRISK study. Int J Epidemiol 2018;47:696696i. [DOI] [PubMed] [Google Scholar]
- 13. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 14. Poss AM, Maschek JA, Cox JE, Hauner BJ, Hopkins PN, Hunt SC, Holland WL, Summers SA, Playdon MC. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J Clin Invest 2020;130:1363–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hilvo M, Dhar I, Lääperi M, Lysne V, Sulo G, Tell GS, Jousilahti P, Nygård OK, Brenner H, Schöttker B, Laaksonen R. Primary cardiovascular risk prediction by LDL-cholesterol in Caucasian middle-aged and older adults—a joint analysis of three cohorts. Eur J Prev Cardiol, 2021. [DOI] [PubMed] [Google Scholar]
- 16. Wang DD, Toledo E, Hruby A, Rosner BA, Willett WC, Sun Q, Razquin C, Zheng Y, Ruiz-Canela M, Guasch-Ferré M, Corella D, Gómez-Gracia E, Fiol M, Estruch R, Ros E, Lapetra J, Fito M, Aros F, Serra-Majem L, Lee C-H, Clish CB, Liang L, Salas-Salvadó J, Martínez-González MA, Hu FB. Plasma ceramides, mediterranean diet, and incident cardiovascular disease in the PREDIMED Trial (Prevención con Dieta Mediterránea). Circulation 2017;135:2028–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.