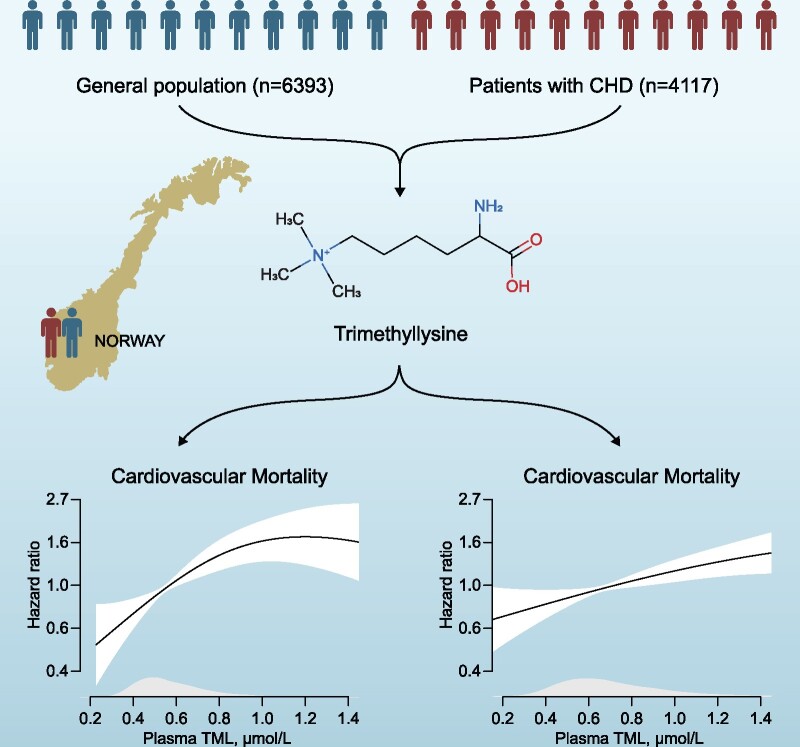
Abstract
Aims
Trimethyllysine (TML) is involved in carnitine synthesis, serves as a precursor of trimethylamine N-oxide (TMAO) and is associated with cardiovascular events in patients with established coronary heart disease (CHD). We prospectively examined circulating TML as a predictor of all-cause and cardiovascular mortality in community-dwelling adults and patients with CHD.
Methods and results
By Cox regression modelling, risk associations were examined in 6393 subjects in the community-based Hordaland Health Study (HUSK). A replication study was conducted among 4117 patients with suspected stable angina pectoris in the Western Norway Coronary Angiography Cohort (WECAC). During a mean follow-up of 10.5 years in the HUSK-cohort, 884 (13.8%) subjects died, of whom 287 from cardiovascular causes. After multivariable adjustments for traditional cardiovascular risk factors, the hazard ratio (HR) [95% confidence interval (95% CI)] for all-cause mortality comparing the 4th vs. 1st TML-quartile was 1.66 (1.31–2.10, P < 0.001). Particularly strong associations were observed for cardiovascular mortality [HR (95% CI) 2.04 (1.32–3.15, P = 0.001)]. Corresponding risk-estimates in the WECAC (mean follow-up of 9.8 years) were 1.35 [1.10–1.66, P = 0.004] for all-cause and 1.45 [1.06–1.98, P = 0.02] for cardiovascular mortality. Significant correlations between plasma TML and TMAO were observed in both cohorts (rs ≥ 0.42, P < 0.001); however, additional adjustments for TMAO did not materially influence the risk associations, and no effect modification by TMAO was found.
Conclusions
Elevated TML-levels were associated with increased risk of all-cause and cardiovascular mortality both in subjects with and without established CHD.
Keywords: Epidemiology, Risk factor, Cardiovascular outcomes, Mortality
Graphical Abstract
Graphical Abstract.
Introduction
Growing evidence indicates that intestinal microbiota-derived metabolites contribute to atherosclerosis, thrombosis, and systemic inflammation.1 Particular interest has been given trimethylamine N-oxide (TMAO) as a potential mediator of cardiovascular disease (CVD).2 Trimethylamine N-oxide is generated in the liver from trimethylamine, which is produced by various gut microbes from dietary precursors such as phosphatidylcholine and L-carnitine.2 Dose-dependent associations of TMAO with cardiovascular outcomes have been observed in several high-risk patient cohorts;3,4 however, the potential causative role of TMAO in atherothrombosis remains unclear.5
The TMAO-precursor trimethyllysine (TML) was recently identified as a potential CVD risk marker in an untargeted metabolomics approach.6 Systemic TML is partly diet-derived from various plant- and animal sources.6,7 Also, TML is produced endogenously during post-translational modifications of proteins, particularly histone methylation,8 which is a central epigenetic regulation process. In addition to its conversion to TMAO, TML is involved in several TMAO-independent pathways associated with CVD progression.9 Of note, observational clinical studies limited to patients with established CVD have found TML to be a predictor of future diabetes10 and cardiovascular events6,11,12 beyond traditional risk factors.
The ability of TML to predict adverse outcomes in the general population is unknown. We therefore prospectively explored associations between circulating TML and all-cause and cardiovascular mortality in a large Norwegian cohort of community-dwelling adults. Further, we sought to validate the results in a higher-risk cohort of patients undergoing coronary angiography for stable angina pectoris.
Methods
Study design
Two independent cohorts of subjects with or without CHD were examined. The Hordaland Health study (HUSK) is a prospective, community-based cohort study of participants living in Western Norway and has been described in detail previously.13 A total of 7051 subjects (born during 1925–27 or 1950–51) who underwent baseline surveys during 1997–99 were included. HUSK was the primary cohort in the current study.
The Western Norway Coronary Angiography Cohort (WECAC) is a prospective observational cohort study.14 It includes 4164 patients who during 2000–04 underwent elective coronary angiography for suspected stable angina pectoris at two University Hospitals in Western Norway. Approximately two-third of the participants were enrolled in the Western Norway B-vitamin Intervention Trial (WENBIT; clinicaltrials.gov: NCT00354081), a randomized trial investigating effects of B-vitamin treatment on CVD outcomes and mortality.15
Subjects with missing data on plasma TML or covariables included in the risk models were excluded, yielding 6393 individuals in HUSK and 4117 patients in WECAC eligible for final analyses.
The study protocols for both HUSK and WECAC were approved by the Regional Committee for Medical and Health Research Ethics and the Norwegian Data Inspectorate. All study subjects provided written informed consent.
Baseline characteristics and biochemical analyses
The collection of baseline and biochemical data for both cohorts has been described in detail previously.13,14 Hypertension was defined by pre-existing diagnosis. Smoking was classified according to self-reported smoking habits and/or plasma cotinine concentrations ≥ 85 nmol/L (available in WECAC only). Diabetes mellitus included both types 1 and 2.
In HUSK, non-fasting venous blood samples were drawn at the baseline survey. In WECAC, blood samples were collected either 1–3 days before or immediately after the baseline angiography. Plasma TML and plasma TMAO were measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS).16 Study-specific analyses for both HUSK and WECAC were performed at Bevital AS, Bergen, Norway (www.bevital.no), as previously described.17
Follow‐up and clinical endpoints
For both cohorts, we obtained data on study endpoints from the Cause of Death Registry at Statistics Norway (http://www.ssb.no/en).18 A unique 11-digit personal identification number for each study subject was used to link to the registry. The primary outcomes were all-cause and cardiovascular mortality, whereas non-cardiovascular mortality was considered as a secondary outcome. The study participants were followed from enrolment until death or throughout 2012.
Statistical analysis
Categorical variables are presented as percentages and continuous variables as means [standard deviations (SD)]. Baseline characteristics of both cohorts were summarized across TML quartiles; trends were tested by linear regression for continuous variables, and logistic regression for ordinal and binary variables. Baseline associations between TML and TMAO were additionally evaluated using Spearman rank correlations and scatter-plots. Survival was visualized using Kaplan–Meier curves, and differences across TML-quartiles were estimated with the log-rank test. In addition, potential non-linear relationships between plasma TML, all-cause and cardiovascular mortality were visualized using a generalized additive model. Univariate, age- and sex-adjusted (Model 1) and multivariable (Model 2) Cox regression models were applied to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). The multivariable model was adjusted for age (continuous), sex (binary), smoking (binary), diabetes mellitus (binary), hypertension (binary), BMI (continuous), and total cholesterol (continuous). Additional adjustments for TMAO were performed in an extended model. Potential effect modifications by TMAO and risk factors in Model 2 were examined by adding interaction product terms to Model 1. Subgroups were created using existing categorical variables or by separating continuous variables at the median level. As recently recommended for observational studies, we applied E-value analyses to assess robustness to potential unmeasured or uncontrolled confounding.19
In the primary cohort (HUSK-cohort), we additionally assessed model fit when adding plasma TML to Model 2 by comparing the Akaikes’ information criterion.20 Further, we tested model discrimination by calculating areas under receiver operator characteristics curves (ROC-AUC) and the integrated discrimination improvement (IDI). Reclassification of subjects was evaluated by calculating the continuous net reclassification improvement (NRI > 0).21
Results
Baseline characteristics (HUSK-cohort)
Baseline characteristics of the study participants according to plasma TML quartiles are presented in Table 1. HUSK consisted of 55.5% women, mean (SD) age at baseline was 59 (12) years. Mean (SD) plasma TML and TMAO were 0.62 (0.30) and 9.11 (16.8) µmol/L, respectively.
Table 1.
Baseline characteristics of community-based subjects (HUSK-cohort) and patients with coronary heart disease (WECAC) according to quartiles of plasma trimethyllysine
| HUSK-cohort (n = 6393) | |||||
|---|---|---|---|---|---|
| Quartile 1 (<0.46) | Quartile 2 (0.46–0.55) | Quartile 3 (0.55–0.68) | Quartile 4 (>0.68) | P trend | |
| Plasma TML (µmol/L | 0.41 (0.04) | 0.50 (0.03) | 0.61 (0.04) | 0.94 (0.43) | … |
| TMAO (µmol/L | 4.53 (4.37) | 5.78 (6.51) | 7.93 (11.6) | 18.2 (28.7) | <0.001 |
| Age (years) | 54 (11) | 58 (12) | 60 (12.2) | 62 (12.1) | <0.001 |
| Female sex (%) | 85.0 | 62.4 | 41.8 | 33.9 | <0.001 |
| BMI (kg/m2) | 24.8 (3.8) | 25.5 (3.9) | 26.0 (3.8) | 26.5 (3.7) | <0.001 |
| Hypertension (%) | 11.0 | 15.6 | 18.8 | 26.8 | <0.001 |
| Diabetes mellitus (%) | 2.2 | 3.0 | 3.9 | 5.5 | <0.001 |
| Current smoking (%) | 31.8 | 27.4 | 22.5 | 20.1 | <0.001 |
| Creatinine (µmol/L) | 80.7 (8.6) | 86.9 (9.6) | 92.7 (11.0) | 99.8 (17.3) | <0.001 |
| eGFR (mL/min per 1.73 m2) | 75 (11) | 72 (12) | 70 (13) | 66 (14) | <0.001 |
| Total cholesterol (mmol/L) | 5.9 (1.1) | 6.0 (1.1) | 6.0 (1.1) | 5.9 (1.1) | 0.53 |
|
| |||||
| WECAC (n = 4117) | |||||
|
| |||||
| Quartile 1 (<0.54) | Quartile 2 (0.54–0.67) | Quartile 3 (0.67–87) | Quartile 4 (>0.87) | P trend | |
|
| |||||
| Plasma TML (µmol/L) | 0.46 (0.06) | 0.61 (0.04) | 0.76 (0.06) | 1.28 (0.70) | … |
| TMAO (µmol/L) | 5.15 (4.15) | 6.92 (6.10) | 8.28 (7.30) | 15.75 (21.12) | <0.001 |
| Age (years) | 60 (10) | 61 (10) | 62 (10) | 64 (10) | <0.001 |
| Female sex (%) | 47.1 | 30.6 | 18.7 | 16.3 | <0.001 |
| BMI (kg/m2) | 25.9 (4.1) | 26.2 (4.0) | 26.3 (3.9) | 26.8 (4.0) | <0.001 |
| Hypertension (%) | 41.4 | 44.0 | 45.6 | 55.6 | <0.001 |
| Diabetes mellitus (%) | 12.5 | 10.5 | 11.3 | 13.0 | 0.63 |
| Current smoking (%) | 34.8 | 32.7 | 29.2 | 30.2 | 0.01 |
| Creatinine (µmol/L) | 81.9 (10.9) | 87.8 (11.8) | 93.0 (12.7) | 107.0 (51.7) | <0.001 |
| eGFR (mL/min per 1.73 m2) | 95 (13) | 91 (14) | 87 (15) | 79 (21) | <0.001 |
| Total cholesterol (mmol/L) | 5.1 (1.1) | 5.2 (1.3) | 5.0 (1.1) | 5.0 (1.2) | 0.01 |
| Prior CVD (%) | |||||
| AMI | 31.9 | 40.1 | 41.3 | 47.3 | <0.001 |
| PAD | 6.4 | 8.1 | 9.9 | 11.6 | <0.001 |
| PCI | 14.9 | 18.9 | 20.1 | 22.4 | <0.001 |
| CABG | 8.7 | 10.2 | 11.5 | 15.6 | <0.001 |
| Medications after angiography (%) | |||||
| Aspirin | 79.4 | 81.0 | 82.1 | 83.4 | 0.02 |
| Statins | 77.4 | 79.5 | 82.3 | 81.3 | 0.01 |
| CCB | 20.5 | 20.4 | 21.6 | 27.5 | <0.001 |
| β-Blocker | 69.3 | 71.4 | 73.9 | 74.8 | 0.002 |
| ACEI | 15.9 | 18.8 | 20.9 | 26.7 | <0.001 |
Continuous variables are presented as means (SD), and categorical variables are reported as percentages.
ACEIs, angiotensin-converting-enzyme inhibitors; AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass grafting; CCB, calcium channel blockers; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; TMAO, trimethylamine-N-oxide; TML, trimethyllysine.
There was a positive relationship between incremental TML quartiles and age, BMI, and creatinine. A higher proportion of subjects in the upper TML quartiles had hypertension and diabetes mellitus, whereas a lower proportion were current smokers. No relationship was found with total cholesterol. Spearman’s correlation analysis showed a significant relationship between plasma TML and TMAO (rs = 0.44, P < 0.001) (Supplementary material online, Figure S1).
Plasma trimethyllysine and risk of mortality (HUSK-cohort)
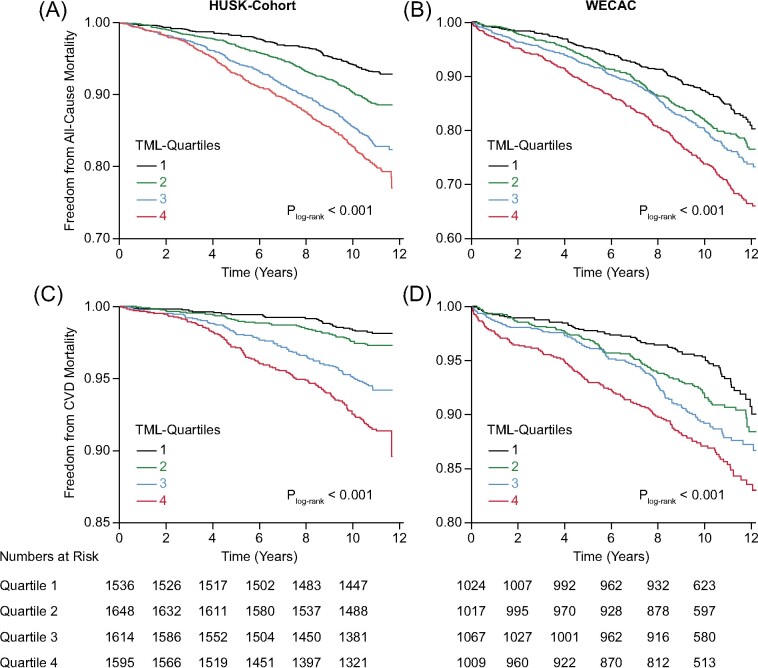
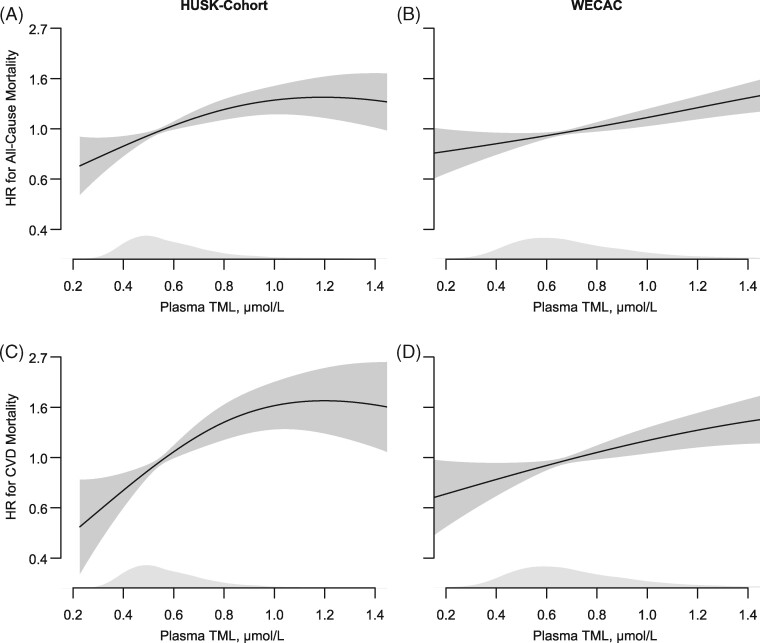
A total of 884 (13.8%) subjects died over a mean (SD) follow-up of 10.5 (1.9) years, of whom 287 (32.4%) from cardiovascular and 597 (67.5%) from non-cardiovascular causes. Kaplan–Meier plots of event-free survival are presented in Figure 1. Using Cox regression analyses, we found a graded positive association between plasma TML and mortality, particularly cardiovascular mortality (Table 2 and Figure 2). The multivariable HRs (95% CI) comparing the 4th vs. 1st TML quartile were 1.66 (1.31–2.10, P < 0.001) and 2.04 (1.32–3.15, P = 0.001) for all-cause and cardiovascular mortality, respectively. Notably, the risk relationships remained essentially unaltered after additional adjustments for TMAO [multivariable HRs (95% CI) of 1.72 (1.35–2.19 P < 0.001) and 2.23 (1.43–3.48, P < 0.001) for all-cause and cardiovascular mortality, respectively]. Similarly, inclusion of creatinine in the multivariable model only slightly attenuated the risk estimates [HRs (95% CI) of 1.61 (1.25–2.07), P < 0.001] for all-cause and 1.88 (1.19–2.98, P = 0.01) for cardiovascular mortality.
Figure 1.
Kaplan–Meier curves showing event-free survival according to quartiles of plasma trimethyllysine in the Hordaland Health Study-cohort (A and C) and Western Norway Coronary Angiography Cohort (B and D).
Table 2.
Risk associations between plasma trimethyllysine and mortality in community-based subjects (HUSK-cohort)
| Unadjusted |
Model 1a |
Model 2b |
|||||
|---|---|---|---|---|---|---|---|
| Events (%) | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| All-cause mortality | |||||||
| Plasma TML | |||||||
| Quartiles | |||||||
| Q1 | 6.9 | Reference | Reference | Reference | |||
| Q2 | 11.2 | 1.67 (1.31–2.11) | <0.001 | 1.17 (0.92–1.49) | 0.19 | 1.20 (0.94–1.53) | 0.14 |
| Q3 | 16.9 | 2.59 (2.07–3.24) | <0.001 | 1.47 (1.17–1.86) | 0.001 | 1.59 (1.26–2.01) | <0.001 |
| Q4 | 20.1 | 3.15 (2.53–3.92) | <0.001 | 1.51 (1.19–1.90) | 0.001 | 1.66 (1.31–2.10) | <0.001 |
| Trend | 1.44 (1.35–1.53) | <0.001 | 1.14 (1.07–1.22) | <0.001 | 1.18 (1.10–1.27) | <0.001 | |
| Per 1-SDc | 1.31 (1.24–1.38) | <0.001 | 1.10 (1.03–1.18) | 0.01 | 1.11 (1.04–1.19) | 0.002 | |
| CVD mortality | |||||||
| Quartiles | |||||||
| Q1 | 1.8 | Reference | Reference | Reference | |||
| Q2 | 2.5 | 1.47 (0.91–2.36) | 0.12 | 0.98 (0.61–1.59) | 0.94 | 0.98 (0.61–1.58) | 0.92 |
| Q3 | 5.4 | 3.13 (2.05–4.79) | <0.001 | 1.65 (1.06–2.56) | 0.03 | 1.66 (1.07–2.58) | 0.02 |
| Q4 | 8.1 | 4.79 (3.18–7.21) | <0.001 | 2.06 (1.34–3.19) | 0.001 | 2.04 (1.32–3.15) | 0.001 |
| Trend | 1.72 (1.53–1.93) | <0.001 | 1.34 (1.18–1.52) | <0.001 | 1.33 (1.17–1.51) | <0.001 | |
| Per 1-SDc | 1.44 (1.33–1.56) | <0.001 | 1.24 (1.11–1.38) | <0.001 | 1.19 (1.07–1.32) | 0.002 | |
| Non-CVD mortality | |||||||
| Quartiles | |||||||
| Q1 | 5.1 | Reference | Reference | Reference | |||
| Q2 | 8.6 | 1.74 (1.32–2.30) | <0.001 | 1.25 (0.95–1.66) | 0.11 | 1.30 (0.98–1.72) | 0.07 |
| Q3 | 11.5 | 2.40 (1.84–3.12) | <0.001 | 1.42 (1.07–1.86) | 0.01 | 1.58 (1.19–2.08) | 0.001 |
| Q4 | 12.0 | 2.56 (1.97–3.33) | <0.001 | 1.28 (0.97–1.70) | 0.08 | 1.49 (1.12–1.98) | 0.01 |
| Trend | 1.33 (1.23–1.43) | <0.001 | 1.07 (0.98–1.16) | 0.13 | 1.12 (1.03–1.22) | 0.01 | |
| Per 1-SDc | 1.24 (1.16–1.32) | <0.001 | 1.03 (0.95–1.12) | 0.47 | 1.07 (0.98–1.16) | 0.12 | |
CI, confidence interval; HR, hazard ratio; Q1, first quartile; Q4, fourth quartile; SD, standard deviation; TML, trimethyllysine.
Adjusted for age and sex.
Adjusted for age, sex, BMI, diabetes mellitus, current smoking, hypertension, and total cholesterol.
Log-transformed.
Figure 2.
Dose–response associations between plasma trimethyllysine and risk of total and cardiovascular mortality in the Hordaland Health Study-cohort (A and C) and Western Norway Coronary Angiography Cohort (B and D), adjusted for age and sex. The shaded areas covering the splines depicts 95% confidence intervals. Kernel density plots show the distribution of trimethyllysine.
Sensitivity analysis, model discrimination, and reclassification (HUSK-cohort)
Application of E-value formula to Model 2 revealed E-values of 2.71 and 1.95 for the effect estimate and lower CI, when comparing the 4th vs. 1st plasma TML quartile in relation to all-cause mortality (Supplementary material online, Table S1). For CVD mortality, corresponding E-values were 3.50 for the effect estimate and 1.97 for the lower CI.
As shown in Supplementary material online, Table S2, the risk-relationship between plasma TML and all-cause mortality was not modified by conventional risk factors included in the multivariable model (pinteraction ≥ 0.10), or by plasma TMAO (pinteraction = 0.90).
For both total and cardiovascular mortality, the addition of plasma TML to the multivariable model improved goodness of fit (Table 3). As shown in Table 3, TML also improved model discrimination and reclassification of patients at risk.
Table 3.
Model fit, discrimination, and reclassification in community-based subjects (HUSK-cohort)
| AIC | P-value | NRI (95% CI) | P-value | IDI | P-value | ROC–AUC | P-value | |
|---|---|---|---|---|---|---|---|---|
| All-cause mortality | ||||||||
| Modela without TML | 4035 | 0.82 | ||||||
| Modela with TML | 4016 | <0.001 | 0.16 (0.09–0.23) | <0.001 | 0.0046 | <0.001 | 0.83 | 0.01 |
| CVD mortality | ||||||||
| Modela without TML | 1894 | 0.84 | ||||||
| Modela with TML | 1878 | <0.001 | 0.35 (0.23–0.46) | <0.001 | 0.0063 | <0.001 | 0.85 | 0.03 |
AIC, Akaike’s information criterion; IDI, integrated discrimination index; NRI, net reclassification improvement.
Multivariable model (Model 2) adjusted for age, sex, BMI, diabetes mellitus, current smoking, hypertension, and total cholesterol.
Plasma trimethyllysine and risk prediction among patients with coronary heart disease (WECAC)
Baseline characteristics of the WECAC are presented in Table 1. Mean (SD) circulating TML was 0.77 (0.47) µmol/L. Similar to HUSK, incremental TML quartiles were positively associated with BMI and age, and patients in the upper TML-quartiles more likely had hypertension. In contrast to in the HUSK-cohort, no relationship was found with diabetes. TML and TMAO were significantly correlated (rs = 0.42, P < 0.001) (Supplementary material online, Figure S2).
During a mean (SD) follow-up of 9.8 (2.6) years, 901 (21.9%) patients died, of whom 410 (45.5%) from cardiovascular causes and 491 (54.5%) from non-cardiovascular causes. Multivariable HRs (95% CI) comparing the 4th vs. 1st TML quartile were 1.35 (1.10–1.66, P = 0.004) and 1.45 (1.06–1.98, P = 0.02) for all-cause and cardiovascular mortality, respectively (Table 4 and Figure 1). The addition of TMAO to Model 2 slightly attenuated the risk estimates [multivariable HRs (95% CI) 1.29 (1.04–1.59), P = 0.02 and 1.37 (0.99–1.89), P = 0.06] for all-cause and cardiovascular mortality, respectively]. Additional inclusion of creatinine attenuated the risk-associations to a greater extent [multivariable HRs (95% CI) 1.22 (0.99–1.50), P = 0.06] for all-cause and 1.30 (0.95–1.79, P = 0.10) for cardiovascular mortality]. No effect modification by TMAO was observed (pinteraction = 0.24).
Table 4.
Risk associations between plasma trimethyllysine and mortality in patients with stable angina pectoris (WECAC)
| Events (%) | Unadjusted |
Model 1a |
Model 2b |
||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| All-cause mortality | |||||||
| Plasma TML | |||||||
| Quartiles | |||||||
| Q1 | 15.1 | Reference | Reference | Reference | |||
| Q2 | 20.4 | 1.36 (1.11–1.68) | 0.004 | 1.10 (0.89–1.35) | 0.39 | 1.10 (0.89–1.36) | 0.38 |
| Q3 | 22.7 | 1.56 (1.28–1.91) | <0.001 | 1.13 (0.92–1.39) | 0.25 | 1.14 (0.93–1.41) | 0.21 |
| Q4 | 29.4 | 2.12 (1.74–2.57) | <0.001 | 1.38 (1.13–1.69) | 0.002 | 1.35 (1.10–1.66) | 0.004 |
| Trend | 1.27 (1.20–1.35) | <0.001 | 1.11 (1.04–1.18) | 0.001 | 1.10 (1.04–1.18) | 0.002 | |
| Per 1-SDc | 1.25 (1.18–1.33) | <0.001 | 1.14 (1.07–1.22) | <0.001 | 1.12 (1.05–1.19) | 0.001 | |
| CVD mortality | |||||||
| Quartiles | |||||||
| Q1 | 6.3 | Reference | Reference | Reference | |||
| Q2 | 8.9 | 1.42 (1.04–1.96) | 0.03 | 1.15 (0.83–1.58) | 0.40 | 1.14 (0.82–1.57) | 0.44 |
| Q3 | 11.0 | 1.80 (1.33–2.43) | <0.001 | 1.31 (0.96–1.79) | 0.09 | 1.31 (0.96–1.79) | 0.09 |
| Q4 | 13.6 | 2.32 (1.72–3.11) | <0.001 | 1.51 (1.11–2.05) | 0.01 | 1.45 (1.06–1.98) | 0.02 |
| Trend | 1.31 (1.20–1.43) | <0.001 | 1.15 (1.04–1.26) | 0.004 | 1.13 (1.03–1.24) | 0.01 | |
| Per 1-SDc | 1.28 (1.18–1.39) | <0.001 | 1.17 (1.07–1.29) | 0.001 | 1.14 (1.04–1.25) | 0.01 | |
| Non-CVD mortality | |||||||
| Quartiles | |||||||
| Q1 | 8.8 | Reference | Reference | Reference | |||
| Q2 | 11.4 | 1.32 (1.00–1.74) | 0.05 | 1.06 (0.80–1.40) | 0.69 | 1.07 (0.81–1.42) | 0.64 |
| Q3 | 11.7 | 1.40 (1.06–1.83) | 0.02 | 1.00 (0.76–1.32) | 0.99 | 1.02 (0.77–1.35) | 0.87 |
| Q4 | 15.9 | 1.97 (1.53–2.56) | <0.001 | 1.29 (0.98–1.68) | 0.07 | 1.28 (0.98–1.68) | 0.07 |
| Trend | 1.24 (1.14–1.34) | <0.001 | 1.08 (0.99–1.18) | 0.07 | 1.08 (0.99–1.18) | 0.08 | |
| Per 1-SDc | 1.23 (1.13–1.33) | <0.001 | 1.11 (1.02–1.22) | 0.02 | 1.10 (1.01–1.20) | 0.03 | |
CI, confidence interval; HR, hazard ratio; Q1, first quartile; Q4, fourth quartile; SD, standard deviation; TML, trimethyllysine.
Adjusted for age and sex.
Adjusted for age, sex, BMI, diabetes mellitus, current smoking, hypertension, and total cholesterol.
Log-transformed.
In a sensitivity analysis excluding patients (n = 1034) without any significant (>50%) stenotic coronary arteries at the baseline coronary angiography, we found essentially similar risk-associations [HRs (95% CI) of 1.42 (1.14–1.78, P = 0.002) for total and 1.49 (1.07–2.06, P = 0.017) for cardiovascular mortality].
Discussion
In this large prospective two-cohort study including more than 10 000 subjects with or without established CHD, elevated circulating TML was reproducibly associated with a graded increased risk of long-term mortality, in particular cardiovascular mortality.
Trimethyllysine and cardiovascular disease
Existing data examining relationships of circulating TML with clinical outcomes are scarce and limited to high-risk patient populations. We previously demonstrated plasma TML as a predictor of future myocardial infarction12 in the WECAC. In a small study of patients with carotid atherosclerosis, TML was associated with an increased 5-year risk of cardiovascular mortality.22 Similarly, positive associations of TML with cardiovascular events have recently been reported in patients with suspected chronic6 and acute11 coronary syndromes. The present study extends previous findings among patients with CVD and for the first time identifies TML as a predictor of all-cause and cardiovascular mortality in the general population. Thus, our results could suggest a role of TML in both primary and secondary risk prediction. However, a potential clinical usefulness of the biomarker needs further exploration in future studies.
Trimethyllysine, trimethylamine N-oxide, and gut microbiota
Our observation of a strong association between plasma TML and TMAO in a community-based population validates findings from a recent report among patients presenting with the acute coronary syndrome.11 Trimethyllysine contains a trimethylamine moiety and can be utilized as a substrate for microbiota-dependent TMAO-generation.6 However, its conversion to TMAO appears considerably lower compared to precursors such as choline and carnitine.6 Notably, a microbiota-independent endogenous pathway generating TMAO from TML has also been suggested22 but currently lacks experimental evidence. In the present study, the risk estimates between circulating TML and mortality were only slightly attenuated after additional adjustments for TMAO, and there were no effect modifications by TMAO. Thus, the TML-mortality relationship seems unlikely to be mediated solely through TMAO.
Intriguingly, a very recent animal study 23 unravelled an alternative gut-dependent metabolism of TML to N,N,N-trimethyl-5-aminovaleric acid (TMAVA), which is implicated in the pathogenesis of the non-alcoholic fatty liver disease, a prevalent CVD risk factor.24 Accordingly, elevated TML-concentrations are present in patients with diagnosed liver steatosis and have been associated with increased risk of fatty liver.23 Hence, several microbiota-dependent pathways may mediate the adverse relationship between TML and mortality.
Trimethyllysine, epigenetic regulation, and carnitine biosynthesis
Endogenous TML generation results from post-translational modifications of proteins, such as histone methylation. This histone modification is dynamic and plays a potentially important regulatory role in pathways linked to CVD,25 including endothelial dysfunction.26 In this context, it is interesting that circulating TML predicted angiographic progression of atherosclerosis in a sub-cohort of WECAC.27 Of note, a strong correlation between free TML and protein-bound TML was reported in a small sample of both healthy subjects and subject with CHD,6 indicating that circulating free TML may partly reflect levels of protein-bound TML in the vasculature.
Trimethyllysine-availability regulates the rate of carnitine biosynthesis, with y-butyrobetaine as an intermediate.28 Carnitines play an essential role for maintaining the mitochondrial function, and disruptions of carnitine homeostasis have been linked to decreased NO-signalling and endothelial dysfunction.29 In line with data showing accumulation of carnitine pathway metabolites in obesity,10 TML was positively associated with BMI in both cohorts. Notably, impaired carnitine synthesis from TML has been suggested in the pathophysiology of both type 2-diabetes and atherothrombosis,10,12 both major risk factors for mortality. However, the TML-carnitine-relationship is complex, as only about one-fourth of body carnitine sources are thought to come from de novo biosynthesis.10 Also, whether TML utilized for carnitine generation predominantly originates from endogenous or nutritional sources remains unclear.7,28
Trimethyllysine and renal function
Similar to TMAO, clearance of TML depends on renal excretion, and the kidney is an important regulator of carnitine biosynthesis.28 Additional adjustment for creatinine only mildly affected risk estimates in the HUSK-cohort. Among patients in WECAC, however, the risk estimates were attenuated to a greater extent numerically, in particular for cardiovascular mortality. Renal disease is a major CVD risk factor;30 hence, kidney function may limit the utility of TML as a prognostic marker in this patient population. As most subjects of both cohorts had adequate kidney function,31 further studies among subjects with chronic kidney disease could further evaluate interactions of TML, renal function, and adverse outcomes.
Trimethyllysine and non-cardiovascular mortality
An interesting finding across both cohorts was the positive, albeit considerably weaker, relation of plasma TML with long-term non-cardiovascular mortality. Of note, proteins involved in both synthesis, removal and recognition of TML have been identified to contribute towards cancer development and several other diseases in humans.8 Also, the related metabolite TMAO has recently been linked to carcinogenesis,32 cognitive decline,33 and adverse prognosis in patients with pulmonary disease.34 Our findings need further replication and could motivate investigations on potential relationships of TML with non-cardiovascular disease outcomes, particularly in the general population.
Strengths and limitations
Major strengths of these prospective cohort studies include the large sample sizes, the replication of results in two independent populations with different health profiles, the long-term follow-up and endpoint data obtained from a national registry. We are aware of several limitations. First, both cohorts only included single measurements of plasma TML, which may have resulted in underestimation of risk associations due to regression dilution bias.35 Second, recognized challenges with using registry data to categorize causes of death include differences in certification practices between physicians, use of unspecific medical codes, a low proportion of deaths in which medical autopsies are undertaken, and changes in coding practices over time.18 However, a Norwegian autopsy study from 2011 showed a substantial agreement between mortality statistics and findings from autopsies for both coronary deaths and fatal strokes36 Third, inherent to any observational study, the possibility of residual or unmeasured confounding cannot be ruled out. Of note, we found particularly high E-values for the association of TML with all-cause and cardiovascular mortality in the HUSK-cohort, indicating robustness to potential unmeasured confounding.19 Fourth, both cohorts included mainly white subjects from a limited geographical area in Western Norway. As shown for TMAO, both geographical37 and ethnic38 differences may considerably affect associations of microbiota-related metabolites with clinical outcomes. Our results, therefore, should be replicated in regions with different demographics and nutritional profiles.
Conclusion
In two large prospective cohorts of subjects with or without established CHD, we observed consistent relationships of TML with long-term all-cause and cardiovascular mortality. Further research is needed to clarify mechanisms determining systemic TML-concentrations.
Lead author biography

Espen Ø. Bjørnestad, MD, is a cardiology resident and researcher at the Department of Cardiology at Stavanger University Hospital. He has particular interest in biomarkers for cardiovascular risk prediction.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Supplementary Material
Acknowledgements
We thank the recruiting study personnel and other co-workers at Stavanger University Hospital, Stavanger, Norway and Haukeland University Hospital, Bergen, Norway. We are grateful to the staff of Statistics Norway and the Cause of Death Registry for providing outcome data on the study participants.
Contributor Information
Espen Ø Bjørnestad, Department of Cardiology, Stavanger University Hospital, Gerd-Ragna Bloch Thorsens gate 8, 4011 Stavanger, Norway.
Indu Dhar, Mohn Nutrition Research Laboratory, Department of Clinical Science, University of Bergen, Postboks 7804, 5020 Bergen, Norway.
Gard F T Svingen, Department of Cardiology, Haukeland University Hospital, Jonas Lies vei 65, 5021 Bergen, Norway.
Eva R Pedersen, Department of Cardiology, Haukeland University Hospital, Jonas Lies vei 65, 5021 Bergen, Norway; Department of Clinical Science, University of Bergen, Postboks 7804 NO-5020 Bergen, Norway.
Mads M Svenningsson, Department of Cardiology, Haukeland University Hospital, Jonas Lies vei 65, 5021 Bergen, Norway.
Grethe S Tell, Department of Global Public Health and Primary Care, University of Bergen, Årstadveien 17, 5020 Bergen, Norway.
Per M Ueland, Department of Clinical Science, University of Bergen, Postboks 7804 NO-5020 Bergen, Norway.
Stein Ørn, Department of Cardiology, Stavanger University Hospital, Gerd-Ragna Bloch Thorsens gate 8, 4011 Stavanger, Norway.
Gerhard Sulo, Centre for Disease Burden, Division of Mental and Physical Health, Norwegian Institute of Public Health, Zander Kaaesgate 7, 5015 Bergen, Norway.
Reijo Laaksonen, Finnish Cardiovascular Research Center, University of Tampere, Tampere University Hospital, Arvo Ylpön Katu 34, 33520 Tampere, Finland.
Ottar Nygård, Mohn Nutrition Research Laboratory, Department of Clinical Science, University of Bergen, Postboks 7804, 5020 Bergen, Norway; Department of Cardiology, Haukeland University Hospital, Jonas Lies vei 65, 5021 Bergen, Norway.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
The data from WECAC that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of interest: none declared.
References
- 1. Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res 2020;127:553–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:2089–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scvishhiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J 2017;38:2948–2956. [DOI] [PubMed] [Google Scholar]
- 4. Yao M-E, Liao P-D, Zhao X-J, Wang L. Trimethylamine-N-oxide has prognostic value in coronary heart disease: a meta-analysis and dose-response analysis. BMC Cardiovasc Disord 2020;20:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zeisel SH, Warrier M. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr 2017;37:157–181. [DOI] [PubMed] [Google Scholar]
- 6. Li XS, Wang Z, Cajka T, Buffa JA, Nemet I, Hurd AG, Gu X, Skye SM, Roberts AB, Wu Y, Li L, Shahen CJ, Wagner MA, Hartiala JA, Kerby RL, Romano KA, Han Y, Obeid S, Lüscher TF, Allayee H, Rey FE, DiDonato JA, Fiehn O, Tang WHW, Hazen SL. Untargeted metabolomics identifies trimethyllysine, a TMAO-producing nutrient precursor, as a predictor of incident cardiovascular disease risk. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Servillo L, Giovane A, Cautela D, Castaldo D, Balestrieri ML. Where does N(ε)-trimethyllysine for the carnitine biosynthesis in mammals come from? PLoS One 2014;9:e84589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maas MN, Hintzen JCJ, Porzberg MRB, Mecinović J. Trimethyllysine: from carnitine biosynthesis to epigenetics. Int J Mol Sci 2020;21:9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schiattarella GG, Trimarco B. Microbial metabolites as predictive biomarkers: a paradigm shift for cardiovascular risk stratification. Eur Heart J 2019;40:2710–2712. [DOI] [PubMed] [Google Scholar]
- 10. Strand E, Rebnord EW, Flygel MR, Lysne V, Svingen GFT, Tell GS, Løland KH, Berge RK, Svardal A, Nygård O, Pedersen ER. Serum carnitine metabolites and incident type 2 diabetes mellitus in patients with suspected stable angina pectoris. J Clin Endocrinol Metab 2018;103:1033–1041. [DOI] [PubMed] [Google Scholar]
- 11. Li XS, Obeid S, Wang Z, Hazen BJ, Li L, Wu Y, Hurd AG, Gu X, Pratt A, Levison BS, Chung Y-M, Nissen SE, Tang WHW, Mach F, Räber L, Nanchen D, Matter CM, Lüscher TF, Hazen SL. Trimethyllysine, a trimethylamine N-oxide precursor, provides near- and long-term prognostic value in patients presenting with acute coronary syndromes. Eur Heart J 2019;40:2700–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bjørnestad E, Olset H, Dhar I, Løland K, Pedersen EKR, Svingen GFT, Svardal A, Berge RK, Ueland PM, Tell GS, Nilsen DWT, Nordrehaug JE, Nygaard E, Nygård O. Circulating trimethyllysine and risk of acute myocardial infarction in patients with suspected stable coronary heart disease. J Intern Med 2020;288:446–456. [DOI] [PubMed] [Google Scholar]
- 13. Refsum H, Nurk E, Smith AD, Ueland PM, Gjesdal CG, Bjelland I, Tverdal A, Tell GS, Nygård O, Vollset SE. The Hordaland Homocysteine Study: a community-based study of homocysteine, its determinants, and associations with disease. J Nutr 2006;136:1731s–1740s. [DOI] [PubMed] [Google Scholar]
- 14. Svingen GF, Ueland PM, Pedersen EK, Schartum-Hansen H, Seifert R, Ebbing M, Løland KH, Tell GS, Nygård O. Plasma dimethylglycine and risk of incident acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol 2013;33:2041–2048. [DOI] [PubMed] [Google Scholar]
- 15. Ebbing M, Bleie Ø, Ueland PM, Nordrehaug JE, Nilsen DW, Vollset SE, Refsum H, Pedersen EK, Nygård O. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA 2008;300:795–804. [DOI] [PubMed] [Google Scholar]
- 16. Midttun Ø, Kvalheim G, Ueland PM. High-throughput, low-volume, multianalyte quantification of plasma metabolites related to one-carbon metabolism using HPLC-MS/MS. Anal Bioanal Chem 2013;405:2009–2017. [DOI] [PubMed] [Google Scholar]
- 17. Bjørnestad E, Borsholm RA, Svingen GFT, Pedersen ER, Seifert R, Midttun Ø, Ueland PM, Tell GS, Bønaa KH, Nygård O. Neopterin as an effect modifier of the cardiovascular risk predicted by total homocysteine: a prospective 2-cohort study. J Am Heart Assoc 2017;6:e006500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pedersen AG, Ellingsen CL. Data quality in the causes of death registry. Tidsskr nor Laegeforen 2015;135:768–770. [DOI] [PubMed] [Google Scholar]
- 19. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167:268–274. [DOI] [PubMed] [Google Scholar]
- 20. Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr 1974;19:716–723. [Google Scholar]
- 21. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skagen K, Trøseid M, Ueland T, Holm S, Abbas A, Gregersen I, Kummen M, Bjerkeli V, Reier-Nilsen F, Russell D, Svardal A, Karlsen TH, Aukrust P, Berge RK, Hov JE, Halvorsen B, Skjelland M. The carnitine-butyrobetaine-trimethylamine-N-oxide pathway and its association with cardiovascular mortality in patients with carotid atherosclerosis. Atherosclerosis 2016;247:64–69. [DOI] [PubMed] [Google Scholar]
- 23. Zhao M, Zhao L, Xiong X, He Y, Huang W, Liu Z, Ji L, Pan B, Guo X, Wang L, Cheng S, Xu M, Yang H, Yin Y, Garcia-Barrio MT, Chen YE, Meng X, Zheng L. TMAVA, a metabolite of intestinal microbes, is increased in plasma from patients with liver steatosis, inhibits γ-butyrobetaine hydroxylase, and exacerbates fatty liver in mice. Gastroenterology 2020;158:2266–2281.e27. [DOI] [PubMed] [Google Scholar]
- 24. Cai J, Zhang X-J, Ji Y-X, Zhang P, She Z-G, Li H. Nonalcoholic fatty liver disease pandemic fuels the upsurge in cardiovascular diseases. 2020;126:679–704. [DOI] [PubMed] [Google Scholar]
- 25. Udali S, Guarini P, Moruzzi S, Choi SW, Friso S. Cardiovascular epigenetics: from DNA methylation to microRNAs. Mol Aspects Med 2013;34:883–901. [DOI] [PubMed] [Google Scholar]
- 26. Kumar A, Kumar S, Vikram A, Hoffman TA, Naqvi A, Lewarchik CM, Kim YR, Irani K. Histone and DNA methylation-mediated epigenetic downregulation of endothelial Kruppel-like factor 2 by low-density lipoprotein cholesterol. Arterioscler Thromb Vasc Biol 2013;33:1936–1942. [DOI] [PubMed] [Google Scholar]
- 27. Løland KH, Bleie O, Borgeraas H, Strand E, Ueland PM, Svardal A, Nordrehaug JE, Nygård O. The association between progression of atherosclerosis and the methylated amino acids asymmetric dimethylarginine and trimethyllysine. PLoS One 2013;8:e64774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vaz FM, Wanders RJ. Carnitine biosynthesis in mammals. Biochem J 2002;361:417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharma S, Black SM. Carnitine homeostasis, mitochondrial function, and cardiovascular disease. Drug Discov Today Dis Mech 2009;6:e31–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation 2007;116:85–97. [DOI] [PubMed] [Google Scholar]
- 31. Zuo H, Svingen GFT, Tell GS, Ueland PM, Vollset SE, Pedersen ER, Ulvik A, Meyer K, Nordrehaug JE, Nilsen DWT, Bønaa KH, Nygård O. Plasma concentrations and dietary intakes of choline and betaine in association with atrial fibrillation risk: results from 3 prospective cohorts with different health profiles. J Am Heart Assoc 2018;7(8):e008190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan CWH, Law BMH, Waye MMY, Chan JYW, So WKW, Chow KM. Trimethylamine-N-oxide as one hypothetical link for the relationship between intestinal microbiota and cancer - where we are and where shall we go? J Cancer 2019;10:5874–5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vogt NM, Romano KA, Darst BF, Engelman CD, Johnson SC, Carlsson CM, Asthana S, Blennow K, Zetterberg H, Bendlin BB, Rey FE. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimer's Res Ther 2018;10:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ottiger M, Nickler M, Steuer C, Bernasconi L, Huber A, Christ-Crain M, Henzen C, Hoess C, Thomann R, Zimmerli W, Mueller B, Schuetz P. Gut, microbiota-dependent trimethylamine-N-oxide is associated with long-term all-cause mortality in patients with exacerbated chronic obstructive pulmonary disease. Nutrition 2018;45:135–141.e1. [DOI] [PubMed] [Google Scholar]
- 35. Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol 1999;150:341–353. [DOI] [PubMed] [Google Scholar]
- 36. Gulsvik AK, Gulsvik A, Svendsen E, Mæhle BO, Thelle DS, Wyller TB. Diagnostic validity of fatal cerebral strokes and coronary deaths in mortality statistics: an autopsy study. Eur J Epidemiol 2011;26:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yazaki Y, Salzano A, Nelson CP, Voors AA, Anker SD, Cleland JG, Lang CC, Metra M, Samani NJ, Ng LL, Suzuki T. Geographical location affects the levels and association of trimethylamine N-oxide with heart failure mortality in BIOSTAT-CHF: a post-hoc analysis. Eur J Heart Fail 2019;21:1291–1294. [DOI] [PubMed] [Google Scholar]
- 38. Yazaki Y, Aizawa K, Israr MZ, Negishi K, Salzano A, Saitoh Y, Kimura N, Kono K, Heaney L, Cassambai S, Bernieh D, Lai F, Imai Y, Kario K, Nagai R, Ng LL, Suzuki T. Ethnic differences in association of outcomes with trimethylamine N-oxide in acute heart failure patients. ESC Heart Fail 2020;7:2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from WECAC that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of interest: none declared.