Abstract
Aims
This sub-study deriving from a multicentre Italian register [Deformation Imaging by Strain in Chronic Heart Failure Over Sacubitril-Valsartan: A Multicenter Echocardiographic Registry (DISCOVER)-ARNI] investigated whether sacubitril/valsartan in addition to optimal medical therapy (OMT) could reduce the rate of implantable cardioverter-defibrillator (ICD) indications for primary prevention in heart failure with reduced ejection fraction (HFrEF) according to European guidelines indications, and its potential predictors.
Methods and results
In this observational study, consecutive patients with HFrEF eligible for sacubitril/valsartan from 13 Italian centres were included. Lack of follow-up or speckle tracking data represented exclusion criteria. Demographic, clinical, biochemical, and echocardiographic data were collected at baseline and after 6 months from sacubitril/valsartan initiation. Of 351 patients, 225 (64%) were ICD carriers and 126 (36%) were not ICD carriers (of whom 13 had no indication) at baseline. After 6 months of sacubitril/valsartan, among 113 non-ICD carriers despite having baseline left ventricular (LV) ejection fraction (EF) ≤ 35% and New York Heart Association (NYHA) class = II–III, 69 (60%) did not show ICD indications; 44 (40%) still fulfilled ICD criteria. Age, atrial fibrillation, mitral regurgitation > moderate, left atrial volume index (LAVi), and LV global longitudinal strain (GLS) significantly varied between the groups. With receiver operating characteristic curves, age ≥ 75 years, LAVi ≥ 42 mL/m2 and LV GLS ≥−8.3% were associated with ICD indications persistence (area under the curve = 0.65, 0.68, 0.68, respectively). With univariate and multivariate analysis, only LV GLS emerged as significant predictor of ICD indications at follow-up in different predictive models.
Conclusions
Sacubitril/valsartan may provide early improvement of NYHA class and LVEF, reducing the possible number of implanted ICD for primary prevention in HFrEF. Baseline reduced LV GLS was a strong marker of ICD indication despite OMT. Early therapy with sacubitril/valsartan may save infective/haemorrhagic risks and unnecessary costs deriving from ICDs.
Keywords: Sacubitril/valsartan, Heart failure, Implantable cardioverter-defibrillator, Remodelling, Left ventricular strain
Graphical Abstract
Introduction
Heart failure with reduced ejection fraction (HFrEF) is a multifactorial cardiovascular disease characterized by progressive myocardial impairment, an important burden of symptoms that limits functional capacity, and a high risk of mortality due to cardiovascular causes, such as heart failure (HF) decompensation or malignant ventricular arrhythmias. Many pharmacologic and interventional treatments were studied and approved to limit HF progression,1 among which sacubitril/valsartan, a neprilysin inhibitor/angiotensin receptor blocker, emerged in the last years for its positive effects on haemodynamic overload and myocardial remodelling, leading to lower morbidity and mortality compared with angiotensin-converting enzyme (ACE)-inhibitors.2 Sacubitril/valsartan has been traditionally indicated in HFrEF patients who are symptomatic despite optimal medical therapy (OMT) including an ACE-inhibitor, a beta-blocker, and a mineralocorticoid receptor agonist, however, the latest European Society of Cardiology (ESC) guidelines for the management of HF stated that its use as a first-line therapy instead of ACE-inhibitors may be considered.1,3,4
On the other hand, primary prevention for sudden cardiac death (SCD) due to malignant arrhythmias by implantable cardioverter-defibrillator (ICD) is recommended (class I), in patients with HFrEF and left ventricular (LV) ejection fraction (EF) ≤ 35% and New York Heart Association (NYHA) class II or III after 3 months of OMT and likely survival with good functional status >1 year, according to ESC HF guidelines1 and to the American Cardiac College/American Heart Association guidelines for the prevention of SCD.5 However, ICD implantation is hampered by infective risk and other complications for the patient,6 need for re-intervention for ICD generator replacement years after the implantation in younger subjects, and high costs for the National Health Service (NHS).
Sacubitril/valsartan has already shown its effects in providing improvement of symptoms and LVEF in HFrEF.7–9 To date, its concrete potential value in reaching LVEF improvement leading to reduce the ICD implantation rate for primary prevention has not been analysed in scientific research.
Therefore, our aim was to analyse the possible improvement of LVEF and NYHA class, entailing the loss of ICD indication, in a cohort of patients with HFrEF after 6 months of treatment with sacubitril/valsartan, deriving by a multicentre Italian registry named Deformation Imaging by Strain in Chronic Heart Failure Over Sacubitril-Valsartan: A Multicenter Echocardiographic Registry (DISCOVER)–ARNI. Moreover, possible predictors of the persistence of ICD indication at follow-up in non-ICD carriers were investigated.
Methods
Study population
In the DISCOVER-ARNI Italian multicenter register, involving 13 centres (see Supplementary material online, Table S1 for the complete list of the centres), patients with HFrEF in stable OMT in the previous 6 months, requiring treatment with sacubitril/valsartan according to the ESC guidelines1 between the years 2017 and 2019, were included. A cut-off value of LVEF <40% was adopted to define HFrEF, according to the ESC guidelines available when the study was conducted.10 At baseline, all patients underwent an ambulatory visit with echocardiographic evaluation and, after the appropriate washout (36 h) from ACE-inhibitors, started treatment with sacubitril/valsartan. Clinical, biochemical, anamnestic data, and echocardiographic measures were collected from the first visit report; then, speckle tracking echocardiography (STE) was performed offline by an independent echocardiographer on the echocardiographic images acquired by a second experienced operator. Patients with missing follow-up or with unfeasible STE data were excluded, while those patients excluded from the main study for a poor acoustic window were included in this sub-study. Data from follow-up visits were collected after 6 months of initiation of sacubitril/valsartan, including clinical and biochemical parameters, dose-adjustments, basic echocardiography, and STE. The primary endpoints of DISCOVER-ARNI were the research of predictors of LV reverse-remodelling, defined as a reduction of LV end-systolic volume or an increase in LVEF after 6 months of therapy with sacubitril-valsartan among clinical, bio-humoural, and echocardiographic parameters, with special focus on STE parameters; the secondary endpoint was the association of LV and left atrial (LA) strain with NYHA class and N-terminal pro-brain natriuretic peptide (NT-proBNP) at 6 months of follow-up. In this observational study, we operated a sub-analysis focusing on DISCOVER-ARNI patients without ICD at baseline but fulfilling ESC criteria for ICD implantation,10 with the primary endpoint of describing the percentage of persistence of ICD indication after 6 months of therapy with sacubitril/valsartan. The population was then divided, at the 6-month time point from sacubitril/valsartan initiation, into two groups according to the maintenance of indication for ICD implantation or not. Secondary endpoint was to find, among clinical, bio-humoural, and echocardiographic parameters including STE, the predictors of the persistence of ICD indications at follow-up. Each centre obtained approval from the Local Ethics Committee. All procedures were conducted in accordance with the Declaration of Helsinki.
Echocardiographic measures
Echocardiographic images were acquired by an expert imager using a commercially available system (GE Medical Systems, Horten, Norway) equipped with a 1.5–3.6-MHz transducer. All subjects were studied in the left lateral recumbent position. Standard LV diameters were measured in long-axis parasternal view. Left ventricular dimensions were calculated using standard views. Left ventricular end-diastolic and end-systolic volumes and EF, LA volume, and area were assessed from the apical four- and two-chamber views (for LVEF, the biplane Simpson method was used). Left ventricular dimensions and LA volumes were indexed to body surface area obtaining LV mass index and maximum and minimum LA volume index (LAVi), according to the European Association of Cardiovascular Imaging/American Society of Echocardiography (EACVI/ASE) recommendations.11 The trans-mitral blood flow pattern was analysed using pulsed wave Doppler with the sample position placed at the tips of the mitral leaflets; maximum early diastolic (E) and late diastolic (A) velocities were recorded, and the E/A ratio calculated. Left ventricular longitudinal function was explored using pulsed tissue Doppler imaging, placing the sample volume at the level of the mitral lateral annulus from the apical four-chamber view. The peak systolic (S′), early diastolic (E′), and late diastolic (A′) annular velocities were obtained. The E/e′ ratio was calculated as an estimate of LV filling pressures. Left ventricular diastolic function grade was assessed according to current recommendations.12 Measurements of right ventricular (RV) diameters and longitudinal function were made according to the EACVI/ASE recommendations.13 Valvular heart diseases were evaluated and graded according to ESC guidelines.14
Speckle tracking echocardiography was performed offline using 2D grey-scale apical four- and two-chamber views acquired during three consecutive cardiac cycles, with a frame rate of 40–80 frame-per-second and with a stable electrocardiographic recording, using a commercially available semiautomated two-dimensional strain software (EchoPac, GE, Milwaukee, WI, USA). The endocardial border was manually traced in apical views, delineating a region of interest (ROI), with the lowest width, composed of six segments for each view. Then, necessary manual adjustments of the ROI were performed, and the longitudinal strain curves for each segment were generated by the software. The average LV global longitudinal strain (GLS) was calculated as the average value of four-chamber, two-chamber, and three-chamber GLS curves, which was in turn measured as the negative peak of the dashed average curve of all segments.
Statistical analysis
Statistical analyses were performed using the SPSS (Statistical Package for the Social Sciences, Chicago, IL, USA) software Release 20. Variables were tested for normality via the Shapiro–Wilk test. Continuous variables were expressed as mean ± standard deviation (SD) or median and interquartile range according to the variable distribution; binary variables were expressed as counts and percentages. A P-value <0.05 was considered statistically significant.
The sub-study cohort including patients without ICD, after excluding those with LVEF = 35–40% at baseline, was divided into two groups based on the persistence or the absence of ICD implantation criteria (LVEF ≤ 35% and NYHA class = II or III) at 6 months of follow-up.
Comparisons between the groups were performed using the independent sample t-test (Mann–Whitney test for non-normally distributed variables) for continuous variables and the χ2 test for categorical variables. The covariates which showed the most significant difference between the two groups (except for LV volumes and EF, considering the intrinsic association with the outcome measure) were selected. Covariates that presented significant missing data (≥10 subjects) were excluded, then, missing data analysis with Little missing completely at random (MCAR) test and multiple imputation with regression method was performed. After imputation, the excluded variables were analysed with independent sample t-test, receiver operating characteristic (ROC) curves, and univariate regression analysis. The single continuous selected covariates were tested as predictors of persistence of ICD indication using ROC curves and their area under the curve (AUC), and were discretized using the Youden threshold of their respective ROC curve. Then, χ2 analysis with Cramer coefficient calculation and standardized residual analysis was performed to determine the association of each discretized variable, together with the selected categorical variables, with ICD indication persistence. Moreover, univariate and multivariate logistic regression analysis, with stepwise Wald method, including age, atrial fibrillation (AF), MR grade, LAVi, and LV GLS (the selected covariates), was performed to determine the contribution of the selected variables for the prediction of ICD indication persistence at follow-up.
Results
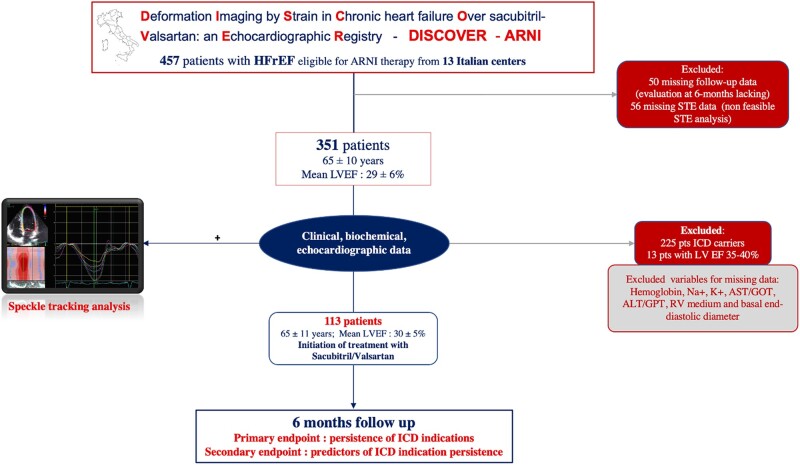
The DISCOVER-ARNI register originally included 457 patients. However, after excluding 50 patients for missing follow-up data and 56 patients for missing STE data, 351 patients were screened in the present study (mean age: 65 ± 10 years, mean LVEF: 29 ± 6%; all NYHA class > II). At baseline, 225 (64%) of these patients were already ICD carriers and then excluded; while 126 (36%) were not ICD carriers (65 ± 9 years; mean LVEF = 30 ± 6%). Of these, 13 (10%) patients did not fulfil ICD implantation criteria for LVEF > 35% at baseline. The final population then included 113 patients (Figure 1).
Figure 1.
Study design algorithm. ALT, alanine-amine-transferase; AST, aspartate-amine-transferase; GOT, glutamic-ossalacetic-transferase; GPT glutamic-piruvic-transferase; RV, right ventricular.
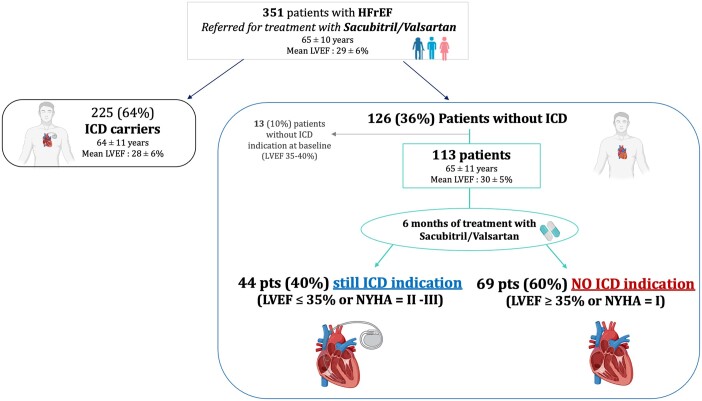
Surprisingly, after 6 months of sacubitril/valsartan therapy, of 113 patients without ICD despite having ICD criteria at baseline, 69 (60%) did not show ICD indications (having LVEF > 35% or NYHA = I); while only 44 (40%) patients still fulfilled ICD criteria for implantation (LVEF ≤ 35% or NYHA = II–III; GraphicalAbstract). No patient had NYHA = 4 at follow-up. The baseline demographic, clinical, bio-humoural, echocardiographic characteristics, and medications of the study cohort according to the two groups (presence/absence of ICD indications at follow-up) are shown in Table 1. At baseline, 78% (88) of patients started treatment with the lowest dose (24/26 mg) of sacubitril/valsartan, while 21% (24 patients) started with the intermediate dose (49/51 mg), and only 1% (1 patient) with the higher dose (97/103 mg). At 6 months, the sacubitril/valsartan dose was up-titrated in 46 patients (59% from 24/26 mg bid to 49/51 mg; 13% from 24/26 mg bid to 97/103 mg bid; 28% from 49/51 mg bid to 97/103 mg bid); 67 patients continued with the starting dose.
Graphical Abstract.
Sacubitril/valsartan saved 60% of patients with heart failure with reduced ejection fraction (HFrEF) and without ICD [despite having LVEF ≥ 35% and New York Heart Association (NYHA) class II–III at baseline] from implantable cardioverter-defibrillator (ICD) indication after 6 months of therapy.
Table 1.
Baseline demographic, clinical, bio-humoural, echocardiographic characteristics, and medications of patients without implantable cardioverter-defibrillator (ICD) indications at follow-up despite having ICD indication criteria at baseline
| Variables | Overall (n = 113) | No ICD indication at follow-up (n = 69) | ICD indication at follow-up (n = 44) | P-value | Missing data (%, 2) |
|---|---|---|---|---|---|
| Age (years) | 65 ± 11 | 62.5 ± 11 | 68 ± 11 | 0.005 | — |
| Male (%, n) | 80% (88) | 81.3% (52) | 77% (36) | 0.55 | 2 (2) |
| BMI | 27.5 ± 6 | 28 ± 6 | 27 ± 4.5 | 0.34 | — |
| sBP (mmHg) | 120 [CI: 110; 130] | 120 [CI: 110; 130] | 120 [CI: 65; 78] | 0.36 | — |
| HR (b.p.m.) | 70 [CI: 60; 76] | 67 [81.5; 250] | 72 [CI: 65; 78] | 0.27 | 2 (2) |
| Hypertension (%, n) | 57% (65) | 57% (37) | 60% (28) | 0.77 | 1 (1) |
| Diabetes mellitus (%, n) | 27% (65) | 30% (20) | 24% (11) | 0.45 | 1 (1) |
| Hypercholesterolaemia (%, n) | 48% (54) | 55% (30) | 63% (24) | 0.4 | 10 (10) |
| Smokers (%, n) | 0.95 | 5 (4.5) | |||
| Previous | 32% (36) | 38% (21) | 40% (15) | ||
| Current | 14% (16) | 18% (10) | 16% (6) | ||
| Ischaemic aetiology (%, n) | 42% (47) | 35% (23) | 51% (24) | 0.97 | 2 (2) |
| p.m. or CRT-P (%, n) | 7% (8) | 4% (3) | 10% (5) | 0.02 | 2 (2) |
| Atrial fibrillation (%, n) | 0.004 | 1 (1) | |||
| Chronic | 11% (12) | 3% (2) | 21% (10) | ||
| Paroxysmal | 11% (13) | 9% (6) | 15% (7) | ||
| ACE-inhibitors (%, n) | 59% (67) | 62% (41) | 55% (26) | 0.46 | — |
| ARB (%, n) | 22% (25) | 25% (16) | 19% (9) | 0.49 | — |
| Beta-blockers (%, n) | 96% (109) | 97% (62) | 100% (47) | 0.22 | — |
| MRA (%, n) | 65% (74) | 62% (40) | 72% (34) | 0.27 | — |
| Diuretics (%, n) | |||||
| Loop diuretics | 84% (95) | 83% (11) | 89% (5) | 0.33 | |
| Thiazides | 3% (3) | 5% (3) | — | 0.14 | |
| Sacubitril/valsartan starting dose (%, n) | 0.2 | — | |||
| 24/26 mg | 30% (33) | 37% (18) | 48% (15) | ||
| 49/51 mg | 40% (45) | 62% (30) | 48% (15) | ||
| 97/103 mg | 1% (1) | — | 3% (1) | ||
| BNP (pg/L) | 361 [CI: 113; 488] | 113 [CI: 81.5; 250] | 323 [CI: 197; 631] | 0.22 | — |
| NT-proBNP (pg/L) | 1175 [CI: 533; 3069] | 1140 [CI: 499; 2048] | 1783 [CI: 544; 4910] | 0.33 | |
| Creatinine (mg/dL) | 1.08 ± 0.32 | 1 ± 0.3 | 1.13 ± 0.3 | 0.12 | 6 (5) |
| eGFR (mL/min) | 79.5 ± 26.5 | 65.7 ± 21.5 | 0.007 | 6 (5) | |
| Maximum LAVi (mL/m2) | 44.5 ± 16 | 40.9 ± 12 | 49.5 ± 19 | 0.008 | 1 (1) |
| LVEDVi (mL/m2) | 96 ± 37 | 83 ± 23 | 115 ± 45 | <0.0001 | 1 (1) |
| LVESVi (mL/m2) | 68 ± 28 | 57 ± 16 | 84 ± 35 | <0.0001 | 1 (1) |
| LVEF (%) | 30 ± 5 | 32 ± 4 | 27 ± 5 | <0.0001 | — |
| E/A | 0.8 [0.64; 1.5] | 0.73 | 7 (6) | ||
| E/E′ | 12.7 [10; 16] | 13.5 ± 6 | 14.5 ± 6 | 0.4 | 5 (4) |
| Mitral regurgitation >2 | 38% (43) | 29% (19) | 51% (24) | 0.044 | 2 (2) |
| sPAP (mmHg) | 35 [30; 42] | 33 [30; 37] | 36 [30; 45] | 0.004 | 9 (8) |
| Tricuspid s′ (m/s) | 0.15 ± 0.2 | 0.13 ± 0.1 | 0.17 ± 0.2 | 0.39 | 10 (10) |
| TAPSE (mm) | 19 ± 3.7 | 19.5 ± 3 | 18.5 ± 4 | 0.21 | 4 (3.5) |
| LV GLS (%) | −9.8 ± 3 | −10.5 ± 3 | −8,8 ± 3 | 0.005 | 2 (2) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; CI, confidence interval; CRT-P, cardiac-resynchronization therapy-pacemaker; CRT-D, cardiac resynchronization therapy-defibrillator; ICD, implantable cardioverter-defibrillator; E/A, early diastolic wave/late diastolic wave by pulsed-wave doppler; E/E′, early diastolic wave by pulsed wave Doppler/average early diastolic wave by tissue-Doppler imaging in the three points of mitral annulus descent; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; HR, heart rate; LAVi, left atrial volume index; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end-systolic volume indexed; MRA; mineralocorticoid receptor antagonist; NT-proBNP, N-terminal-pro-BNP; p.m., pacemaker; sBP, systolic blood pressure; sPAP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic excursion. Significant p values are indicated with bold numbers.
Age, estimated glomerular filtration rate, previous/chronic AF brain natriuretic peptide (BNP) or NT-proBNP showed significant differences between the two groups.
As for echocardiographic parameters, LV volumes and EF, LAVi, mitral regurgitation severity, diastolic dysfunction, LV GLS by STE, and pulmonary artery systolic pressure showed significant differences between the two groups. These were still significantly different among the two groups at 6 months of follow-up (Table 2).
Table 2.
Demographic, clinical, bio-humoural, echocardiographic characteristics at follow-up of patients without implantable cardioverter-defibrillator (ICD) indications at follow-up despite having ICD indication criteria at baseline
| Variables | Overall (n = 113) | No ICD indication at follow-up (n = 69) | ICD indication at follow-up (n = 44) | P-value | Missing data (%, n) |
|---|---|---|---|---|---|
| BMI | 27 ± 4 | 28 ± 5 | 26 ± 4 | 0.11 | — |
| sBP (mmHg) | 120 [CI: 110; 125] | 120 [CI: 110; 125] | 110 [CI: 60; 75] | 0.36 | 2 (2) |
| HR (b.p.m.) | 65 [CI: 60; 75] | 65 [60; 75] | 68 [CI: 68; 75] | 0.27 | 3 (3) |
| BNP (pg/L) | 159 [CI: 80; 382] | 95 [CI: 51; 143] | 195 [CI: 111; 428] | 0.22 | 2 (2) |
| NT-proBNP (pg/L) | 402 [CI: 177; 860] | 300 [CI: 112; 666] | 875 [CI: 381; 5605] | 0.33 | |
| Creatinine (mg/dL) | 1.09 ± 0.34 | 1 ± 0.3 | 1.14 ± 0.4 | 0.28 | 5 (4.5) |
| eGFR (mL/min) | 74 ± 23 | 77 ± 22 | 68 ± 24 | 0.08 | 5 (4.5) |
| Maximum LAVi (mL/m2) | 43 ± 18 | 38 ± 14 | 51 ± 20 | 0.001 | 2 (2) |
| LVEDVi (mL/m2) | 87 ± 34 | 75 ± 21 | 107 ± 40 | <0.0001 | — |
| LVESVi (mL/m2) | 58 ± 28 | 45 ± 16 | 76 ± 32 | <0.0001 | — |
| LVEF (%) | 37 ± 8 | 42 ± 6 | 29 ± 5 | <0.0001 | — |
| E/A | 0.8 [0.63; 1] | 0.8 [0.63; 0.99] | 0.75 [0.6; 1.2] | 0.33 | 4 (3.5) |
| E/E′ | 11 [9; 12] | 10 [8; 12] | 11 [9; 16] | 0.03 | 4 (3.5) |
| Mitral regurgitation >2 | 33% (28) | 23% (17) | 44% (35) | 0.01 | 8 (7) |
| sPAP (mmHg) | 30 [25; 36] | 28 [24; 35] | 34 [26; 41] | 0.01 | 10 (10) |
| Tricuspid s′ (m/s) | 0.16 ± 0.2 | 0.16 ± 0.1 | 0.17 ± 0.2 | 0.7 | 10 (10) |
| TAPSE (mm) | 20 ± 3.8 | 21 ± 4 | 19 ± 4 | 0.03 | 8 (7) |
| LV GLS (%) | -11.8 ± 3 | -12.8 ± 3 | -10.2 ± 3 | <0.0001 | 2 (2) |
BMI, body mass index; BNP, brain natriuretic peptide; CI, confidence interval; E/A, early diastolic wave/late diastolic wave by pulsed-wave Doppler; E/E′, early diastolic wave by pulsed wave Doppler/average early diastolic wave by tissue-Doppler imaging in the three points of mitral annulus descent; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; HR, heart rate; LAVi, left atrial volume index; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end-systolic volume indexed; NT-proBNP, N-terminal-pro-BNP; sBP, systolic blood pressure; sPAP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic excursion. Significant p values are indicated with bold numbers.
Variables excluded for missing data (haemoglobin, transaminases, Na+, K+, RV medium, and basal diameter) were missing at random (Little MCAR test χ2 3.403, P = 0.93); after multiple imputation, their medium, and SD values were not significantly different between the study groups.
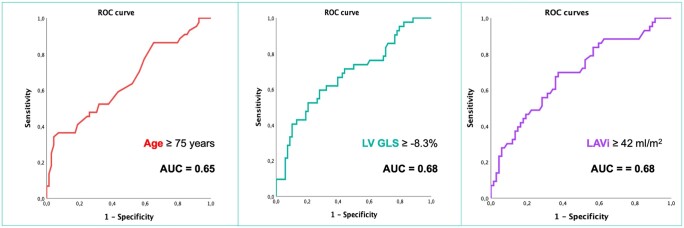
Receiver operating characteristic curves revealed that baseline age ≥75.5 years, LAVi ≥ 42 mL/m2, and LV GLS ≥ −8.3% predicted the persistence of ICD indications after 6 months of sacubitril/valsartan therapy with an AUC = 0.65, 0.68, 0.68, respectively (Figure 2). These cut-off values were then used to perform χ2 analysis, including also AF and mitral regurgitation > 2, from which LV GLS emerged as having the strongest association with ICD indications (χ2 = 10.35, Cramer’s V = 0.307, P = 0.002), together with age (χ2 = 17.74, Cramer’s V = P < 0.0001), and AF (chi-squared = 13.4, Cramer’s V = 0.34, P < 0.0001). Moreover, all the excluded variables were not significant predictors of persistence of ICD indications at univariate analysis (all P > 0.05) and ROC curves, performed after imputation, confirmed the superior predictive value of LV GLS for the persistence of ICD implantation criteria at follow-up (AUC = 0.68 vs. AUC reported in Supplementary material online, Table S2).
Figure 2.
Receiver operating curves (ROC) showing age, left ventricular global longitudinal strain (LV GLS), and left atrial volume index (LAVi) predictive value for the persistence of implantable cardioverter-defibrillator (ICD) indications at follow-up. AUC, area under the curve.
With univariate logistic regression analysis, including age, AF, MR grade, LAVi, and LV GLS, each parameter showed a significant association with the persistence of ICD indication at follow-up (Table 3). Then, when multivariate logistic analysis including all the five parameters was performed, LAVi (although with an OR very close to 1), and LV GLS remained in the final predictive model using raw numbers, with a correct classification rate = 65.5%; while, discretizing each variable using the cut-off values identified by ROC curves, only age and LV GLS remained in the final predictive model (Table 3), which showed a correct classification rate = 67.3%. Left ventricular GLS was the only parameter which remained significantly associated with the presence of ICD indication at follow-up in both multivariate models.
Table 3.
Univariate and multivariate logistic regression analysis, as raw variables (linear analysis) or using the ROC curves selected cut-off values, of possible predictors of implantable cardioverter-defibrillator (ICD) indication persistence after 6 months of therapy with sacubitril/valsartan
| Variables | Univariate OR | Multivariate model OR | Selected cut-off values | Univariate OR | Multivariate model OR |
|---|---|---|---|---|---|
| Age | 1.025 [1.004–1.046] P = 0.021 | — | ≥75 years | 5 [1.44–17.27] P = 0.011 | 6.95 [1.77–27.15] P = 0.005 |
| AF (no/current previous) | 1.42 [1.05–1.94] P = 0.023 | — | Yes/no | 5.33[0.89–30.5] P = 0.067 | — |
| MR grade | 1.45 [1.13–1.85] P = 0.03 | — | ≥2 | 1.36 [0.47–3.97] P = 0.64 | — |
| LAVi | 1.02 [1.01–1.04] P < 0.0001 | 1.02 [1.004–1.033] P = 0.011 | ≥42 mL/m2 | 0.68 [0.46–1] P = 0.056 | — |
| LV GLS | 1.2 [1.11–1.3] P < 0.0001 | 1.2 [1.10–1.28] P < 0.0001 | ≥−8.3% | 1.5 [0.76–2.9] P = 0.043 | 2.51 [1.03–6.11] P = 0.042 |
AF, atrial fibrillation; GLS, global longitudinal strain; LAVi, left atrial volume index; MR, mitral regurgitation; OR, odds ratio. Cut-off values used in the second analysis are indicated in bold, significant p-values are inicated with italic and bold numbers.
Discussion
In this sub-study of DISCOVER-ARNI, we observed that, in patients with HFrEF without ICD despite having criteria for implantation, the initiation of therapy with sacubitril/valsartan may be associated with an improvement of symptoms and LV function, expressed as NYHA class and LVEF respectively, in the 60% of subjects, thus saving the indication for ICD for arrhythmic primary prevention, according to the current criteria.1,5 Moreover, we found that a reduction of LV GLS by STE was the strongest predictor of absence of ICD indications after 6 months of sacubitril/valsartan.
In recent studies, ARNI proved to reduce the overall cardiovascular mortality and, more specifically, the risk of SCD in HFrEF patients.15 Rohde et al.16 conducted a PARADIGM2 sub-analysis (in 8399 patients), showing that sacubitril/valsartan reduced the risk of SCD in patients with an ICD [hazard ratio (HR): 0.49; 95% confidence interval (CI): 0.25–0.99] and in those who were eligible for but did not receive an ICD (HR: 0.81; 95% CI: 0.67–0.98). Moreover, Russo et al.17 studied 167 patients with dilated cardiomyopathy both with ischaemic and non-ischemic aetiology, with dual-chamber ICD on sacubitril/valsartan treatment, finding, at 12 months of follow-up, a reduction in both atrial and ventricular arrhythmias burden and an improvement in ICD electrical atrial parameters.
The precise mechanism by which ARNI may reduce SCD is not clear, however, a variety of direct and indirect hypotheses has been advanced.15 A favourable LV reverse remodelling seems to be the main responsible mechanism,18 as Martens et al.19 suggested after studying 151 patients with HFrEF, in whom they observed a lower degree of ventricular tachycardia or fibrillation, resulting in less ICD intervention rate, over a mean follow-up of 365 days. Also, Guerra et al.20 conducted a prospective study in a similar cohort treated with sacubitril/valsartan for 6 months, showing LV reverse remodelling and an improvement of LVEF from 28.3 ± 5.6% to 32.2 ± 6.5% (P < 0.001), with 5.3% arrhythmias in the first 6 months of treatment with sacubitril/valsartan.
As we know, ICD implantation is hampered by a certain risk of complications for the patient, e.g. infections, inappropriate shocks, lead dislodgement/dysfunction, pneumothorax, pocket haematoma or bleeding,5,21–23 and hospitalization. The complications were recently quantified to affect 1/13 patients over 2 years in a big Dutch cohort24 with high costs for NHS. A cost-effectiveness analysis suggested that in patients with HFrEF, treatment with sacubitril/valsartan would increase survival at lower costs compared with an ICD.25
Furthermore, the well-known protective effect of ICD for primary prevention in HFrEF patients with non-ischaemic cardiomyopathy has been debated as probably overweighed by competing risks over long-term follow-up.26 In fact, the latest update of the ESC Heart Failure Association suggests that primary prevention ICD in non-ischaemic dilated cardiomyopathy may not be considered in patients with more than 70 years or with advanced HF symptoms or with high prognostic impact comorbidities, as they are exposed to a higher probability of death from other causes.27
Moreover, current indications for ICD in primary prevention are based on clinical trials published more than 10 years ago. These recommend that patients should receive HF OMT for a minimum of 3 months before referral for prophylactic ICD, in order to allow LV reverse remodelling and improvement in HF symptoms, making ICD implantation unnecessary.1,5 Nowadays, medical therapy for HFrEF has significantly evolved with introduction of new drugs, such as ARNI or sodium-glucose-transporter 2 currently available for HFrEF, therefore, it is suggestible that the duration of this waiting period under OMT should be reconsidered.28
Left ventricular strain is a myocardial deformation index that has gained increasing evidence in the last decade and has now been fully integrated in clinical practice as a marker of LV function for diagnostic and prognostic purposes, thanks to the high availability of STE, particularly in HF setting. On the other hand, LA strain is emerging as a marker of subtle cardiac impairment and diastolic dysfunction before overt LV dysfunction and failure, as shown in different clinical settings, including HF.29–31 In fact, the last EACVI consensus document of multimodality imaging in HFpEF suggests its use in the standard evaluation of diastolic function in HFpEF, with diagnostic and prognostic impact.32,33
A study conducted on more than 2440 individuals showed that, in patients with HFrEF, LV GLS was associated not only with clinical outcome but also with the severity of HF showed by NT-proBNP, with age and AF burden.34
In previous studies, LV GLS has also shown to be a marker of LV fibrosis.30,35In fact, its reduction would probably reflect higher extent of LV fibrosis in our population, which led to lower probability of LV reverse remodelling. Accordingly, considering the well-known age-related increase of myocardial fibrosis, the strong predictive power of age in our cohort for the persistence of ICD indications would have been expectable.
In addition, LV GLS has already been shown to be a reliable predictor of LV reverse remodelling after treatment with sacubitril/valsartan.36,37 Accordingly, in our main study DISCOVER-ARNI, we found that LV GLS predicted LV reverse remodelling after 6 months of therapy with sacubitril/valsartan. In this sub-study, we found that this parameter was also a predictor of ICD indication in the same cohort and follow-up period. Therefore, our results suggest that the use of LV GLS would be useful to identify patients most prone to develop LV reverse remodelling and, consequently, with more chances to spare ICD implantation.
Limitations
Although showing promising results, this study has some limitations that should be accounted: first, the retrospective nature of the study. Then, not only the data derived from a register, in which patient may slightly differ from general population,38 and in which about 23% of patients were excluded due to missing follow-up and STE data, but also this was an Italian register study, so the study results may not be generalizable to patients across all race and ethnicities.39 Moreover, arrhythmic events during follow-up were not registered, which could have been a useful element to judge the effective need of arrhythmic primary prevention. The small number of patients with ischaemic HF did not allow us to derive significant results in this subgroup; however, this could be a starting point for future research. As regards statistical analysis, due to the lack of penalized regression tools in our software, stepwise method was used for multivariate regression, which in some case may introduce some bias to the analyses.40 Finally, the dependence of speckle tracking parameters on image quality and acquisition should be considered.
Lead author biography

Matteo Cameli, MD PhD, is Associate Professor of Cardiology at the University of Siena, where he also is the Director of Cardiovascular Fellowship Program. Moreover, he is a European Association of Cardiovascular Imaging (EACVI) Board Member since 2016, and currently is the Secretary of EACVI and the Deputy Chair of EACVI Scientific Initiatives Committee. He is the Leader of a high-level research team in University of Siena, which main research activities and expertise focuses on speckle tracking echocardiography, heart failure and transplantation, ventricular assist devices, valvular heart diseases.
Conclusions
In a cohort of patients with HFrEF, the initiation of therapy with sacubitril/valsartan may provide favourable effects on LV function and HF symptoms, saving most patients from ICD indications. Moreover, LAVi, elderly age and, in particular, lower LV GLS were strongly associated with persistence of ICD indications after 6 months and could therefore be useful to predict this improvement. These results suggest reconsidering the timing of ICD implantation in light of the new HF therapies, and to use STE as additional parameter in the baseline evaluation of patients referred for sacubitril/valsartan.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Fundings
After the acceptance of the article, a support for open access fee was provided by Novartis Farma.
Conflict of interest: none declared.
Supplementary Material
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 2. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 3. Hayman S, Atherton JJ.. Should angiotensin receptor neprilysin inhibitors replace angiotensin-converting enzyme inhibitors in heart failure with a reduced ejection fraction? Card Fail Rev 2016;2:47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bauersachs J. Heart failure drug treatment: the fantastic four. Eur Heart J 2021;42:681–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL.. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2018;72:e91–e220. Erratum in: J Am Coll Cardiol. 2018;72(14):1760. [DOI] [PubMed] [Google Scholar]
- 6. Olsen T, Jørgensen OD, Nielsen JC, Thøgersen AM, Philbert BT, Johansen JB.. Incidence of device-related infection in 97 750 patients: clinical data from the complete Danish device-cohort (1982-2018). Eur Heart J 2019;40:1862–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM, Solomon SD; PROVE-HF Investigators. Association of change in N-terminal pro-B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA 2019;322:1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Almufleh A, Marbach J, Chih S, Stadnick E, Davies R, Liu P, Mielniczuk L.. Ejection fraction improvement and reverse remodeling achieved with sacubitril/valsartan in heart failure with reduced ejection fraction patients. Am J Cardiovasc Dis 2017;7:108–113. [PMC free article] [PubMed] [Google Scholar]
- 9. Romano G, Vitale G, Ajello L, Agnese V, Bellavia D, Caccamo G, Corrado E, Di Gesaro G, Falletta C, La Franca E, Minà C, Storniolo SA, Sarullo FM, Clemenza F.. The effects of sacubitril/valsartan on clinical, biochemical and echocardiographic parameters in patients with heart failure with reduced ejection fraction: the “Hemodynamic Recovery”. J Clin Med 2019;8:2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 11. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU.. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. Erratum in: Eur Heart J Cardiovasc Imaging 2016;17(4):412. Erratum in: Eur Heart J Cardiovasc Imaging 2016;17(9):969. [DOI] [PubMed] [Google Scholar]
- 12. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD.. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 13. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB.. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713. [DOI] [PubMed] [Google Scholar]
- 14. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 15. Vecchi AL, Abete R, Marazzato J, Iacovoni A, Mortara A, De Ponti R, Senni M.. Ventricular arrhythmias and ARNI: is it time to reappraise their management in the light of new evidence? Heart Fail Rev 2022;27(1):103–110. https://doi.org/10.1007/s10741-020-09991-3. [DOI] [PubMed] [Google Scholar]
- 16. Rohde LE, Chatterjee NA, Vaduganathan M, Claggett B, Packer M, Desai AS, Zile M, Rouleau J, Swedberg K, Lefkowitz M, Shi V, McMurray JJV, Solomon SD.. Sacubitril/valsartan and sudden cardiac death according to implantable cardioverter-defibrillator use and heart failure cause: a PARADIGM-HF analysis. JACC Heart Fail 2020;8:844–855. [DOI] [PubMed] [Google Scholar]
- 17. Russo V, Bottino R, Rago A, Papa AA, Liccardo B, Proietti R, Manna V, Golino P, D’Onofrio A, Nigro G.. The effect of sacubitril/valsartan on device detected arrhythmias and electrical parameters among dilated cardiomyopathy patients with reduced ejection fraction and implantable cardioverter defibrillator. J Clin Med 2020;9:1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Casale M, Correale M, Laterra G, Vaccaro V, Morabito C, Crea P, Signorelli SS, Katsiki N, Luzza F, de Gregorio C, Dattilo G.. Effects of sacubitril/valsartan in patients with high arrhythmic risk and an ICD: a longitudinal study. Clin Drug Investig 2021;41:169–176. [DOI] [PubMed] [Google Scholar]
- 19. Martens P, Nuyens D, Rivero-Ayerza M, Van Herendael H, Vercammen J, Ceyssens W, Luwel E, Dupont M, Mullens W.. Sacubitril/valsartan reduces ventricular arrhythmias in parallel with left ventricular reverse remodeling in heart failure with reduced ejection fraction. Clin Res Cardiol 2019;108:1074–1082. [DOI] [PubMed] [Google Scholar]
- 20. Guerra F, Ammendola E, Ziacchi M, Aspromonte V, Pellegrino PL, Del Giorno G, Dell’Era G, Pimpini L, Santoro F, Floris R, Stronati G, Nigro G, Paolisso P, Guido A, Maglia G, Brunetti ND, Carbone A, Gravellone M, Antonicelli R, Cannone M, Accogli M, Dello Russo A, Palmisano P.. Effect of SAcubitril/Valsartan on left vEntricular ejection fraction and on the potential indication for Implantable Cardioverter Defibrillator in primary prevention: the SAVE-ICD study. Eur J Clin Pharmacol 2021;77:1835–1842. [DOI] [PubMed] [Google Scholar]
- 21. Ascoeta MS, Marijon E, Defaye P, Klug D, Beganton F, Perier M-C, Gras D, Algalarrondo V, Deharo J-C, Leclercq C, Fauchier L, Babuty D, Bordachar P, Sadoul N, Boveda S, Piot O.. Impact of early complications on outcomes in patients with implantable cardioverter‐defibrillator for primary prevention. Heart Rhythm 2016;13:1045–1051. [DOI] [PubMed] [Google Scholar]
- 22. Peterson PN, Varosy PD, Heidenreich PA, Wang Y, Dewland TA, Curtis JP, Go AS, Greenlee RT, Magid DJ, Normand S-LT, Masoudi FA.. Association of single‐ vs dual‐chamber ICDs with mortality, readmissions, and complications among patients receiving an ICD for primary prevention. JAMA 2013;309:2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Expósito V, Rodríguez-Mañero M, González-Enríquez S, Arias MA, Sánchez-Gómez JM, Andrés La Huerta A, Bertomeu-González V, Arce-León Á, Barrio-López MT, Arguedas-Jiménez H, Seara JG, Rodriguez-Entem F.. Primary prevention implantable cardioverter‐defibrillator and cardiac resynchronization therapy‐defibrillator in elderly patients: results of a Spanish multicentre study. Europace 2016;18:1203–1210. [DOI] [PubMed] [Google Scholar]
- 24. van Barreveld M, Verstraelen TE, van Dessel PFHM, Boersma LVA, Delnoy PPHM, Tuinenburg AE, Theuns DAMJ, van der Voort PH, Kimman GJ, Buskens E, Zwinderman AH, Wilde AAM, Dijkgraaf MGW; DO‐IT Registry Investigators. Dutch outcome in implantable cardioverter-defibrillator therapy: implantable cardioverter-defibrillator-related complications in a contemporary primary prevention cohort. J Am Heart Assoc 2021;10:e018063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zacà V. Sacubitril/valsartan or an implantable cardioverter-defibrillator in heart failure with reduced ejection fraction patients: a cost-effectiveness analysis. J Cardiovasc Med (Hagerstown) 2018;19:597–605. [DOI] [PubMed] [Google Scholar]
- 26. Schrage B, Uijl A, Benson L, Westermann D, Ståhlberg M, Stolfo D, Dahlström U, Linde C, Braunschweig F, Savarese G.. Association between use of primary-prevention implantable cardioverter-defibrillators and mortality in patients with heart failure. Circulation 2019;140:1530–1539. [DOI] [PubMed] [Google Scholar]
- 27. Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, de Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS.. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019;21:1169–1186. [DOI] [PubMed] [Google Scholar]
- 28. Wong JA, Roberts JD, Healey JS.. The optimal timing of primary prevention implantable cardioverter-defibrillator referral in the rapidly changing medical landscape. Can J Cardiol 2021;37:644–654. [DOI] [PubMed] [Google Scholar]
- 29. Venkateshvaran A, Sarajlic P, Lund LH, Fridén C, Nordgren B, Opava CH, Lundberg IE, Larsson SC, Manouras A, Bäck M.. Impaired left atrial dynamics and its improvement by guided physical activity reveal left atrial strain as a novel early indicator of reversible cardiac dysfunction in rheumatoid arthritis. Eur J Prev Cardiol 2018;25:1106–1108. [DOI] [PubMed] [Google Scholar]
- 30. Mandoli GE, Pastore MC, Benfari G, Bisleri G, Maccherini M, Lisi G, Cameli P, Lisi M, Dokollari A, Carrucola C, Vigna M, Montesi G, Valente S, Mondillo S, Cameli M.. Left atrial strain as a pre-operative prognostic marker for patients with severe mitral regurgitation. Int J Cardiol 2021;324:139–145. [DOI] [PubMed] [Google Scholar]
- 31. Cameli M, Pastore MC, Pagliaro A, Di Tommaso C, Reccia R, Curci V, Mandoli GE, Mondillo S.. Sacubitril/valsartan in an elderly patient with heart failure: a case report. Cardiology 2017;138(Suppl 1):3–6. [DOI] [PubMed] [Google Scholar]
- 32. Smiseth OA, Morris DA, Cardim N, Cikes M, Delgado V, Donal E, Flachskampf FA, Galderisi M, Gerber BL, Gimelli A, Klein Al Knuuti J, Lancellotti P, Mascherbauer J, Milicic D, Seferovic P, Solomon S, Edvardsen T, Popescu BA. . Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. 2022;23(2):e34–e61. https://doi.org/10.1093/ehjci/jeab154. [DOI] [PubMed] [Google Scholar]
- 33. Pastore MC, De Carli G, Mandoli GE, D'Ascenzi F, Focardi M, Contorni F, Mondillo S, Cameli M.. The prognostic role of speckle tracking echocardiography in clinical practice: evidence and reference values from the literature. Heart Fail Rev 2021;26:1371–1381. [DOI] [PubMed] [Google Scholar]
- 34. Tröbs S-O, Prochaska JH, Schwuchow-Thonke S, Schulz A, Müller F, Heidorn MW, Göbel S, Diestelmeier S, Lerma Monteverde J, Lackner KJ, Gori T, Münzel T, Wild PS.. Association of global longitudinal strain with clinical status and mortality in patients with chronic heart failure. JAMA Cardiol 2021;6:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cameli M, Mondillo S, Righini FM, Lisi M, Dokollari A, Lindqvist P, Maccherini M, Henein M.. Left ventricular deformation and myocardial fibrosis in patients with advanced heart failure requiring transplantation. J Card Fail 2016;22:901–907. [DOI] [PubMed] [Google Scholar]
- 36. Lu L, Guo J, Hua Y, Huang K, Magaye R, Cornell J, Kelly DJ, Reid C, Liew D, Zhou Y, Chen A, Xiao W, Fu Q, Wang BH.. Cardiac fibrosis in the ageing heart: contributors and mechanisms. Clin Exp Pharmacol Physiol 2017;44(Suppl. 1):55–63. [DOI] [PubMed] [Google Scholar]
- 37. Mazzetti S, Scifo C, Abete R, Margonato D, Chioffi M, Rossi J, Pisani M, Passafaro G, Grillo M, Poggio D, Mortara A.. Short-term echocardiographic evaluation by global longitudinal strain in patients with heart failure treated with sacubitril/valsartan. ESC Heart Fail 2020;7:964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Vecchis R, Paccone A, Di Maio M.. Sacubitril/valsartan improves left ventricular longitudinal deformation in heart failure patients with reduced ejection fraction. Minerva Cardioangiol 2019;67:456–463. [DOI] [PubMed] [Google Scholar]
- 39. Ibrahim NE, Piña IL, Camacho A, Bapat D, Felker GM, Maisel AS, Butler J, Prescott MF, Abbas CA, Solomon SD, Januzzi JL Jr; Prospective Study of Biomarkers, Symptom Improvement and Ventricular Remodeling During Entresto Therapy for Heart Failure (PROVE-HF) Study Investigators. Racial and ethnic differences in biomarkers, health status, and cardiac remodeling in patients with heart failure with reduced ejection fraction treated with sacubitril/valsartan. Circ Heart Fail 2021;13:e007829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krumholz HM. Registries and selection bias: the need for accountability. Circ Cardiovasc Qual Outcomes 2009;2:517–518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.