Abstract
Endometriosis is a chronic gynaecological disease affecting 1 in 10 reproductive-age women. It is defined as the presence of endometrium-like tissue outside the uterus. Beyond this placid anatomical definition, endometriosis is a complex, hormonal, inflammatory, and systemic condition that poses significant familial, psychological, and economic burden. The interaction between the cardiovascular system and endometriosis has become a field of interest as the underlying mutual mechanisms become better understood. On the basis of accumulating fundamental and clinical evidence, it is likely that there exists a close relationship between endometriosis and the cardiovascular system. Therefore, investigating the endometriosis—cardiovascular interaction is highly clinically significant. In this review, we highlight our current understanding of the pathophysiology of endometriosis with systemic hormonal, pro-inflammatory, pro-angiogenic, immunologic, and genetic processes beyond the peritoneal microenvironment. Additionally, we provide current clinical evidence about how endometriosis interacts with cardiovascular risk factors and cardiovascular disease (CVD). To date, only small associations between endometriosis and CVD have been reported in observational studies, inherently limited by the potential influence of unmeasured confounding. Cardiovascular disease in women with endometriosis remains understudied, under-recognized, and underdiagnosed. More detailed study of the cardiovascular-endometriosis interaction is needed to fully understand its clinical relevance, underlying pathophysiology, possible means of early diagnosis and prevention.
Keywords: Endometriosis, Cardiovascular disease, Heart disease, Women
Graphical Abstract
Introduction
Endometriosis is a chronic gynaecological disease estimated to affect 10% of reproductive-age women.1 Recent insights have linked endometriosis to several pathological mechanisms ranging from systemic inflammation, pro-atherogenic lipid profile, and enhanced oxidative stress to endothelial dysfunction.2 Considered as a systemic disease, the pathogenesis of endometriosis and the impact of the disease remain poorly understood. Robust knowledge is restricted to data from women surgically diagnosed with endometriosis limiting the development of multidisciplinary approaches to ameliorate the substantial cumulative burden of this condition.
A focused update consensus document on gynaecological and obstetric conditions that impact cardiovascular risk in women was released in March 2021.3 The scarcity of a strong dataset limited the ability to provide clear evidence regarding the pathophysiology, pathogenesis, and prognosis of endometriosis in cardiovascular disease (CVD). Here, we review relevant evidence regarding the relationship between endometriosis and CVD.
The purpose of this review article is to further our understanding of the shared mechanisms underlying endometriosis and atherosclerotic CVD, by contextualizing biological pathways common to both diseases, addressing the association between cardiovascular risk factors and endometriosis, and finally, highlighting key clinical evidence that link endometriosis with adverse cardiovascular events.
Endometriosis: overview
Endometriosis: epidemiology, symptoms, diagnosis, and treatment
Endometriosis is estimated to affect 10% of reproductive-age women. The true prevalence of endometriosis remains unknown since historically, diagnoses have only been made using laparoscopy and more recently multimodal imaging techniques. Misdiagnosis remains common, and diagnosis is often largely delayed by years. Currently, the prevalence ranges from 2% to 11% among asymptomatic women, 5% to 50% among infertile women, and 5% to 21% among women with pelvic pain.1,4,5
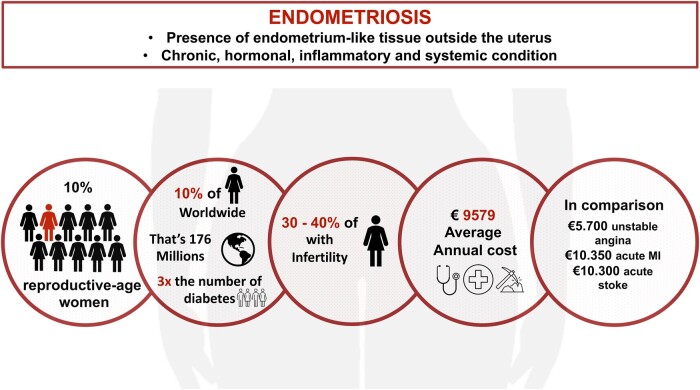
The most common signs and symptoms of endometriosis include chronic pelvic pain, dyspareunia, dysmenorrhoea, and infertility, although asymptomatic cases can arise.6 Subsequently, decreased quality of life, fatigue, depression, high analgesic consumption, and reduced work productivity may occur. Endometriosis poses a major economic burden, similar to diabetes, with an estimated annual cost per patient of €95797 (Figure 1). The condition presents with four phenotypes: superficial peritoneal lesions, ovarian endometriomas, deep infiltrating endometriosis, and extragenital areas including such as peripheral nerves as well as rectal, diaphragmatic, and pleural locations.6,8,9
Figure 1.
Epidemiology and economic burden of endometriosis.
Because endometriosis manifests in a wide spectrum of severity, from asymptomatic cases to severe conditions, diagnosis is challenging and often delayed. Classical symptoms such as chronic pelvic pain, dysmenorrhoea, and dyspareunia are non-specific and can overlap with other conditions involving the urinary or digestive tract. Key features of endometriosis can be identified during patient interviews through a family history of endometriosis, including a cyclic nature of pelvic pain, poor or no response to analgesics, severe primary dysmenorrhoea during adolescence and infertility.6 Although a normal physical examination does not rule out endometriosis, pelvic and rectal examination may detect infiltration and palpable sensitive areas involving the pelvic cavity (vagina, rectovaginal septum, uterosacral ligaments, and pouch of Douglas). The low diagnostic performance of physical examinations may improve during menstruation10,11 but a key step in diagnosis now involves non-invasive imaging techniques with transvaginal ultrasound12,13 and magnetic resonance imaging.14,15 Diagnosis of endometriosis should be based on patient interviews, examination and imaging, and surgery should be restricted to diagnostic uncertainty and persistent symptoms despite an optimal medical therapy.6 Currently available therapeutic approaches include medical treatment with non-hormonal (non-steroidal anti-inflammatory drugs) and hormonal treatments (combined oral contraceptives, progestins, and gonadotropin-releasing hormone analogues), surgery (conservative and definitive), and assisted reproductive technologies in patients with endometriosis-related infertility.
The pathobiology of endometriosis
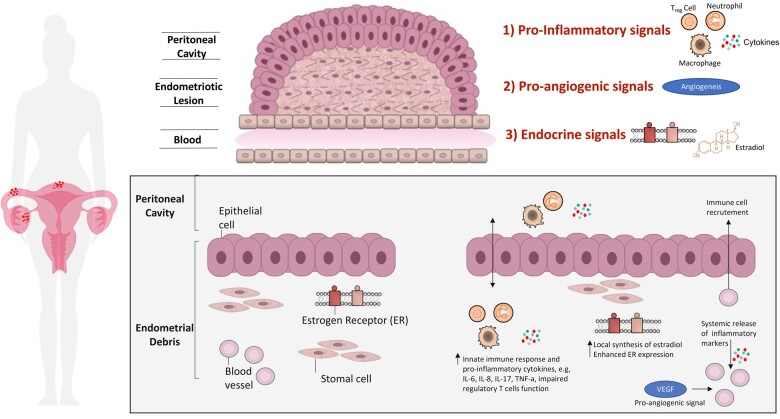
The pathobiology of endometriosis is complex, and synergistic biological pathways are required to facilitate the establishment and persistence of endometriotic debris within the peritoneal cavity and ectopic regions. Endometriotic debris is characterized by epithelial, stromal, endothelial, glandular, and immune cell components with altered immunoinflammatory profiles compared with normal endometrium.16,17 A pro-inflammatory, pro-angiogenic, and aberrant immune-endocrine environment is required to facilitate the growth and survival of endometriotic lesions (Figure 2).
Figure 2.
Molecular pathways underpinning endometriosis. Endometriotic lesions are characterized by a unique environment. The interplay between pro-inflammatory signals, pro-angiogenic signals, and a unique endocrine signature contribute to the pathogenesis of endometriosis. This schematic representation of the endometriotic lesion identifies three distinct molecular pathways that facilitate lesion growth in endometriosis. (1) Pro-inflammatory signals: increased neutrophil infiltration in the peritoneal fluid; increased macrophage populations in the peritoneal fluid and eutopic endometrium; elevated cytokine levels (IL-6, IL-8, IL-17, IL-33, TNF-a, etc.) in both the peritoneal fluid and plasma; at sites of inflammation, macrophages and mast cells drive neutrophil recruitment through the release of chemokines; impair regulatory T cells function. (2) Pro-angiogenic signals: Damage-associated molecular patterns: high-mobility group box 1, elevated IL-33 level, etc.; vascular endothelial growth factor. (3) Endocrine signals: local synthesis of oestradiol by endometriotic lesions; enhanced oestrogen receptor expression. ILs, interleukins; OR, oestrogen receptor; Treg cells, regulatory T cells; VEGF, vascular endothelial growth factor.
Immune cells are predominantly implicated in endometriosis pathogenesis, including neutrophils, macrophages, monocytes, and regulatory T cells. Intense neutrophil and macrophages infiltration has been observed in eutopic endometrium and the peritoneal fluid.18 At sites of inflammation, macrophages and mast cells drive neutrophil recruitment through the release of chemokines. A localized and systemic inflammatory response is observed in endometriosis, with elevated cytokine levels (IL-6, IL-8, IL-17, IL-33, TNF-a, etc.) in both the peritoneal fluid and plasma. Impaired T cell, B cell, mast cell, dendritic cell, and natural killer cell function influences disease establishment and progression.19 Collectively, this promotes aberrant local and systemic chronic inflammation. Angiogenesis is supported by damage-associated molecular patterns, such as high-mobility group box 1, IL-33 and other cytokines, and high VEGF expression. Local synthesis of oestradiol from endometriotic lesions combined with enhanced oestrogen receptor (OR) expression in endometriotic tissues contributes to disease progression. Enhanced OR expression has been associated with enhanced inflammatory activity through cytokine release, anti-apoptotic signalling, angiogenesis, and subsequent lesion growth.20,21 Endothelial cell damage, endothelial dysfunction, increased oxidative stress,22 and increased levels of microvesicles23 have been reported both at the site of endometriosis and in peripheral blood.
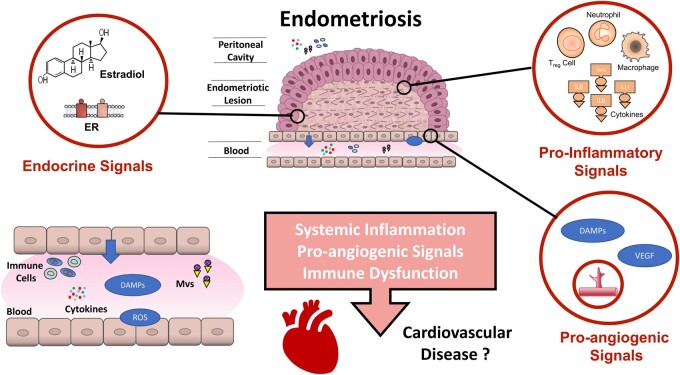
Most processes and biomarkers discussed in the pathobiology of endometriosis demonstrate compelling associations with atherosclerosis and CVD development in general. Future research is necessary to clarify the mechanistic roles of these intercellular communication mediators in endometriosis relative to facilitating the development of other comorbid conditions (Figure 3).
Figure 3.
Summary of proposed mechanisms for endometriosis–cardiovascular interaction. Cardiovascular dysfunction in endometriosis may be caused by several mechanisms, including pro-inflammatory, pro-angiogenic, and aberrant immune-endocrine function. (? indicates that future studies are warranted.) DAMPs, damage-associated molecular patterns; ILs, interleukins; MVs, microvesicles; OR, oestrogen receptor; ROS, reactive oxygen species; Treg cells, regulatory T cells; VEGF, vascular endothelial growth factor.
Endometriosis and atherosclerosis
Endometriosis and atherosclerosis: shared pathophysiological mechanisms
The most widely accepted pathophysiological hypothesis for endometriosis is based on retrograde menstruation, which occurs in most patients.24 Menstrual blood containing endometrial cells flows back through the fallopian tubes and into the pelvic cavity, where the cells implant, develop, continue to thicken, and bleed over the course of each menstrual cycle. Alternative theories involving endometrial stem cells, stem cells from bone marrow, lymphovascular emboli of endometrial cells, and coelomic metaplasia have been proposed to explain the unusual locations of endometriosis.
Endometriosis and atherosclerosis are traditionally viewed as distinct entities, with endometriosis typically affecting young reproductive-age women, while atherosclerosis is an aging-related process. Recent insights have unveiled cellular and molecular overlaps between the two conditions. Chronic inflammation, enhanced oxidative stress, endothelial dysfunction, and cellular proliferation are the hallmarks of both atherosclerosis and endometriosis.24–28 The full spectrum of the pathogenesis and pathophysiology of endometriosis has been extensively detailed elsewhere,2,24,29 and it is now widely acknowledged that endometriosis is a multifactorial condition involving hormonal, pro-inflammatory, pro-angiogenic, immunologic, and genetic processes. Briefly, the hormone-dependent process and proliferation of endometrial fragments require oestradiol provided by systemic hormones and favoured by disrupted hormonal signalling pathways.30,31 Significant macrophage recruitment combined with intense activation of cytokines and pro-angiogenic factors facilitates neovascularization and ectopic lesion growth32,33 in an enhanced local and systemic pro-inflammatory environment.34,35 Building on this biological overlap between atherosclerosis and endometriosis, recent research has provided evidence supporting an association between atherosclerotic CVD and endometriosis. Increased arterial stiffness36 and impaired flow-mediated dilation,37,38 a surrogate marker of endothelial dysfunction potentially reversible after surgical treatment,39 were associated with endometriosis.
Shared genetic susceptibility to endometriosis and atherosclerosis
Recent advances have identified variants at CDKN2BAS on chromosome 9p21 as a susceptibility locus for endometriosis40 and atherosclerotic phenotypes, such as intracranial aneurysm, abdominal aortic aneurysm, peripheral vascular disease, diabetes, and stroke.41 Similarly, locus 7q22 has been associated with endometriosis and CVD.42 CDKN2B-AS transcript levels are known to correlate with the severity of atherosclerosis,43 and a recently published genome-wide association study (GWAS) identified that rs10965235 single-nucleotide polymorphism (SNP) in the CDKN2B-AS gene is associated with endometriosis.44 Advances in GWASs and the application of SNPs in the identification of increased genetic susceptibility to CVD in women with endometriosis may be of particular interest in tomorrow’s research.
Endometriosis and cardiovascular risk factors
Cardiovascular risk factors are being increasingly recognized as having a significant association with endometriosis.
Hypertension
Pioneer work by Mu et al.45 found a strong association between endometriosis and hypertension. In a prospective cohort of 116 430 nurses between 25 and 42 years of age followed for 20 years, 4244 women were diagnosed with laparoscopically confirmed endometriosis. After adjusting for cofounders, the relative risk (RR) for the development of hypertension was 1.14 [95% confidence interval (CI), 1.09–1.18] among women with endometriosis (Table 1). Conversely, the RR of developing laparoscopically confirmed endometriosis was 1.29 (95% CI 1.18–1.41) among hypertensive women. Several hypotheses for such associations have been proposed, starting with the inflammatory process surrounding endometriosis known for decades as a key component in the pathogenesis of hypertension.71 Overall, 30% of the reported association between endometriosis and hypertension was accounted for by treatment effects, namely hysterectomy/oophorectomy and earlier age of surgery. A decline in the production of sex hormones either in postmenopausal women or after ovariectomy is known to significantly increase the risk of hypertensive disorders.72,73 Non-steroidal anti-inflammatory drugs aimed to reduce pelvic pain are known to increase blood pressure and may be an important co-founder. Okoth et al.47 found an adjusted odds ratio (aOR) of 1.12 (95% CI 1.07–1.17) for hypertension among 56 090 women with endometriosis compared with 223 669 matched controls. Finally, endometriosis is an independent and significant risk factor for the occurrence of gestational hypertension-pre-eclampsia.46,48–50
Table 1.
Summary of observed associations between endometriosis and cardiovascular risk factors
| Author(s) | Study name | Location | Population | Design | Method of data collection | Ascertainment period (year) | Findings |
|---|---|---|---|---|---|---|---|
| Hypertension and gestational hypertension-pre-eclampsia | |||||||
| Mu et al.45 | NHSII (Nurses’ Health Study II) | USA | 116 430 female nurses aged 25–42. 4244 women with laparoscopically confirmed endometriosis and 91 554 control women | Prospective cohort study | Self-completed questionnaire | 1989–2009 | Hypertension (RR 1.14, 95% CI 1.09–1.18) |
| Nagai et al.46 | JNHS (Japan Nurses’ Health Study) | Japan | 49 927 female nurses aged 41.2 ± 7.9. Estimated age at peak incidence: 36 years of age and cumulative incidence at 50 years of age: 7.4% | Prospective cohort study | Self-completed questionnaire | 2001–07 | Hypertension (OR 1.26, 95% CI 1.07–1.47) |
| Okoth et al.47 | UK | Women aged between 16 and 50. 56 090 women with endometriosis and 223 669 matched control women | Population-based cohort study | Electronic health records | 1995–2018 | Hypertension (aHR 1.12, 95% CI 1.07–1.17) | |
| Pan et al.48 | Taiwan | 6300 women with endometriosis and 10 312 matched control women | Population-based cohort study | Electronic health records | 1998–2012 | Gestational hypertension and/or pre-eclampsia (aOR 2.27, 95% CI 1.76–2.93) | |
| Lalani et al.49 | 33 studies; 280 488 patients | Meta-analysis of observational studies | 1990–2017 | Pre-eclampsia (OR 1.18, 95% CI 1.01–1.39); Gestational hypertension and/or pre-eclampsia (OR 1.21, 95% CI 1.05–1.39) | |||
| Farland et al.50 | NHSII (Nurses’ Health Study II) | USA | 116 430 female nurses aged 25 to 42. 4244 women with laparoscopically confirmed endometriosis and 91 554 control women. 196 722 reported pregnancies | Prospective cohort study | Self-completed questionnaire | 1989–2009 | Gestational hypertension and/or pre-eclampsia (RR 1.30, 95% CI 1.16–1.45) |
| Dyslipidaemia | |||||||
| Mu et al.45 | NHSII (Nurses’ Health Study II) | USA | 116 430 female nurses aged 25 to 42. 4244 women with laparoscopically confirmed endometriosis and 91 554 control women | Prospective cohort study | Self-completed questionnaire | 1989–2009 | Hypercholesterolaemia (RR 1.25, 95% CI 1.21–1.30) |
| Nagai et al.46 | JNHS (Japan Nurses’ Health Study) | Japan | 49 927 female nurses aged 41.2 ± 7.9. Estimated age at peak incidence: 36 years of age and cumulative incidence at 50 years of age: 7.4% | Prospective cohort study | Self-completed questionnaire | 2001–07 | Hypercholesterolaemia (OR 1.30, 95% CI 1.15–1.47) |
| Tani et al.36 | Japan | 28 women with laparoscopically confirmed endometriosis. 21 control women | Case–control study | Laboratory data | August 2010 to May 2013 | Lower HDL-C (P < 0.01). No difference in TG; LDL-C | |
| Santoro et al.38 | Italy | 37 women with laparoscopically confirmed endometriosis. 31 control women | Cross-sectional study | Laboratory data | July 2010 to June 2011 | Higher HDL-C (P = 0.045). No difference in TG; LDL-C | |
| Melo et al.51 | Brazil | 40 women with laparoscopically confirmed endometriosis. 80 control women | Cross-sectional study | Laboratory data | Higher LDL-C (P < 0.001); higher TG (P = 0.02); higher HDL-C (P = 0.008) | ||
| Crook et al.52 | UK | 29 women with laparoscopically confirmed endometriosis. 29 controls | Case–control study | Laboratory data | Higher TG level (P < 0.02) and LP(a) level (P < 0.01). No difference in LDL-C; HDL-C | ||
| Hopeman et al.53 | USA | 24 women with endometriosis. 181 infertile controls with in vitro fertilization | Cross-sectional study | Laboratory data | Lower eicosapentaenoic acid (P = 0.009) | ||
| Kinugasa et al.37 | Japan | 41 women with laparoscopically confirmed endometriosis. 28 control women | Cross-sectional study | Laboratory data | April 2009 to March 2010 | Higher asymmetric dimethylarginine (ADMA) level (P = 0.04). No difference in TG; HDL-C; LDL-C | |
| Pretta et al.54 | Italy | 66 women with laparoscopically confirmed endometriosis. 66 matched control women | Case–control study | Laboratory data | No difference in TG; HDL-C; LDL-C | ||
| Verit et al.55 | Turkey | 47 women with laparoscopically confirmed endometriosis. 40 matched control women | Case–control study | Laboratory data | November 2006 to May 2007 | Higher TG; LDL-C (P < 0.0001); lower HDL-C (P < 0.0001) | |
| Obesity | |||||||
| Ferrero et al.56 | Italy | 366 women with laparoscopically confirmed endometriosis. 248 control women | Prospective cohort study | Electronic health records | August 2000 to February 2004 | Lower BMI (21.17 ± 2.86 vs. 22.33 ± 3.68, P < 0.001). No difference in BMI according to endometriosis stages and severity | |
| Holdsworth-Carson et al.57 | Australia | 357 women with laparoscopically confirmed endometriosis. 152 control women | Retrospective case–control study | Electronic health records | May 2012 to March 2016 | Lower BMI (25.0 ± 0.3 vs. 27.2 ± 0.5, P < 0.001). Inverse relationship between BMI and frequency of endometriosis (P < 0.001) | |
| Shah et al.58 | NHSII (Nurses’ Health Study II) | USA | 5504 incident cases of endometriosis during 1 299 349 woman-years (incidence rate = 385 per 100 000 woman-years) | Prospective cohort study | Self-completed questionnaire | September 1989 to June 2011 | BMI at age 18 and current BMI: each significantly inversely associated with endometriosis (P < 0.0001) |
| Missmer et al.59 | NHSII (Nurses’ Health Study II) | USA | 1721women with laparoscopically confirmed endometriosis | Prospective cohort study | Self-completed questionnaire | September 1989 to June 1999 | Inverse relation with BMI at age 18 years (for BMI of >30 vs. 19–20.4 kg/m2: RR 0.8, 95% CI 0.6–1.1; P = 0.004) |
| Hediger et al.60 | USA | 32 women with laparoscopically confirmed endometriosis. 52 control women | Prospective cohort study | Self-completed questionnaire | April 1999 to January 2000 | Lower BMI (23.7 ± 3.8 vs. 26.9 ± 6.2, 95% CI P = 0.006). For every unit increase in BMI, 12–14% decrease in the likelihood of being diagnosed with endometriosis | |
| Lafay Pillet et al.61 | France | 238 women with laparoscopically confirmed endometriosis. 238 matched control women | Case–control study | February 2005 to October 2008 | Lower BMI (21.70 ± 3.7 vs. 23.29 ± 4, 95% CI P < 0.001) | ||
| Farland et al.62 | E3N (Teachers Health Study) | France | 98 995 female teachers. 2416 women with laparoscopically confirmed endometriosis and 61 208 control women. | Prospective cohort study | Self-completed questionnaire | Odds of endometriosis were lower among women with a large vs. lean body size at 8 years (P = 0.003), at menarche (P < 0.0001) and at ages 20–25 years (P < 0.0001) | |
| Liu et al.63 | 11 studies; 9298women with endometriosis | Meta-analysis of observational studies | 33% reduction in the risk of endometriosis for each 5 kg/m2 increase in BMI (RR = 0.67, 95% CI 0.53–0.84); with statistically significant heterogeneity across the studies (P < 0.001, I2 = 86.9%) | ||||
| Tang et al.64 | China | 709 women with laparoscopically confirmed endometriosis. 807 matched control women | Retrospective case–control study | January 2018 to August 2019 | No difference in BMI (21.1 vs. 20.9, 95% CI P = 0.223). OR 1.979% (95% CI 1.15–3.52, P = 0.0185) for endometriosis in obese (BMI > 27.50) | ||
| Tobacco smoking | |||||||
| Cramer et al.65 | USA | 317 women with laparoscopically confirmed endometriosis. 4023 control women | Prospective cohort study | January 1981 to June 1983 | Decreased risk for endometriosis among smokers who began before age 17 years and ≥one pack a day | ||
| Calhaz-Jorge et al.66 | Portugal | 488 women with laparoscopically confirmed endometriosis. 591 control women | Prospective cohort study | 1993–2000 |
|
||
| Bravi et al.67 | 38 studies; 13 129 women diagnosed with endometriosis | Meta-analysis of observational studies | Publications up to September 2014 |
|
|||
| Air pollution exposure | |||||||
| Mahalingaiah et al.68 | NHSII (Nurses’ Health Study II) | USA | 2486 women with laparoscopically confirmed endometriosis, over 710 230 person-years of follow-up | Geographic information system software | 1993–2007 | No association between endometriosis risk and distance to road or exposure to particulate matter | |
| Diabetes and gestational diabetes | |||||||
| Farland et al.69 | NHSII (Nurses’ Health Study II) | USA | 112 037 female nurses. 5242 women with laparoscopically confirmed endometriosis and 106 795 control women | Prospective cohort study | Self-completed questionnaire | 1989 to June 2017 | No association between endometriosis and risk of type 2 diabetes in multivariable confounder-adjusted models (HR 1.06; 95% CI 0.98–1.13) or models accounting for potential mediating factors (HR 0.94; 95% CI 0.87–1.00) |
| Pérez-López et al.70 | 12 studies; 48 762 pregnancies including 3461 with endometriosis | Meta-analysis of observational studies | Publications up to April 2017 | No significant effect on gestational diabetes risk (OR = 1.14; 95% CI 0.86–1.51; P = 0.35, I2 = 56%, Egger’s test P = 0.45) | |||
aHR, adjusted hazard ratio; BMI, body mass index; HDL-C, high-density lipoprotein (HDL) cholesterol; LDL-C, low-density lipoprotein (LDL) cholesterol; Lp(a), Lipoprotein(a); OR, odds ratio; RR, relative risk; TG, triglycerides.
Dyslipidaemia
Observational studies have suggested a strong association between endometriosis and an enhanced atherogenic lipid profile. The most convincing evidence was provided by Mu et al.45 in Nurses’ Health Study II (NHSII; n = 116 430), with a 25% increased risk (95% CI 1.21–1.30) to develop hypercholesterolaemia in endometriosis and a 22% increased risk (95% CI 1.15–1.31) of developing laparoscopically confirmed endometriosis in women with hypercholesterolaemia. The Japan Nurses’ Health Study, a cross-sectional survey of 49 927 women (2001–07), confirmed a 30% increased odds of hypercholesterolaemia (95% CI 1.15–1.47) in women with endometriosis although there was no adjustment for confounding factors.46
Focusing on the lipid profile, in a cross-sectional study of 120 women, 40 of whom had laparoscopically proven endometriosis, Melo et al.51 reported higher levels of total cholesterol, LDL-cholesterol, triglycerides, and HDL-cholesterol. The altered lipid profile in endometriosis has been further investigated by several reports and yielded inconsistent results. Tan et al.74 effectively addressed these varying results in a recent analysis of nine studies24,30,37,51–55,75 investigating the RRs of dyslipidaemia among women with endometriosis. Overall, these studies were limited by a small number of patients, differences in both parameters measured, and timing of measurements; therefore, these results should be interpreted with caution.
A preclinical study demonstrated altered serum lipid profiles in mice with endometriosis.76 Later reports confirmed that dysregulated phospholipid and sphingolipid metabolism plays a key role in endometriosis pathogenesis.77–80 Sphingolipids serve as biologically active components of cell membranes and are involved in many processes, such as proliferation, maturation, and apoptosis. Lee et al.78 provided evidence of altered sphingolipid metabolism flux in the serum, peritoneal fluid, and endometrial tissue of women with endometriosis. They observed up-regulation of specific sphingolipid enzymes (sphingomyelin synthase 1, sphingomyelinase 3, and glucosylceramide synthase) in endometriotic debris, with corresponding increased glucosylceramide, decreased sphingomyelin levels, and decreased apoptosis in the endometrium. Ceramides act as second messengers in activating the apoptotic cascade and precursors for many other sphingolipids. Elevated ceramide levels were evidenced in the peritoneal fluid of infertile endometriotic women, triggering reactive oxygen species formation, and leading to oocytotoxicity.81 Similarly, several investigations have suggested a pivotal role for sphingolipids in the pathogenesis of myocardial infarction, hypertension, stroke, and diabetes.82 This set of evidence suggests that sphingolipids may function as mediators of interorgan and intercellular communication. Future studies are warranted to investigate the role of sphingolipids in endometriosis and investigate whether endometriosis-induced sphingolipids promote a systemic pro-inflammatory and pro-oxidant cascade, which then leads to dysfunction of other organs including those associated with the cardiovascular system.
Obesity
An inverse relationship between endometriosis and body mass index (BMI) is commonly acknowledged,56–63 although some studies of limited impact have reported otherwise.64,83 Reduction in the adipose stem cell population,84 an anorexigenic effect through altered hepatic gene expression,85 and promotion of lipid dysfunction and fat loss76 are key mechanisms thought to be involved in the clinically low BMI phenotype observed in women with endometriosis.
Smoking, air pollution exposure, and diabetes
While tobacco smoking is an established risk factor for ischaemic heart disease, its relationship with endometriosis is puzzling. Indeed, intense variation and conflicting data regarding the association between smoking and endometriosis have been reported. Several studies have suggested that smoking may be associated with a decreased risk of endometriosis,65,66,86 but recently published data dismissed any link between endometriosis and tobacco smoking habits.67 Similarly, the association between endometriosis and pollution exposures remains speculative.87,88 Only one study from NHSII showed no increased risk for endometriosis with regards to air pollution exposure.68
Despite the potential overlap in the molecular pathways between endometriosis and diabetes, namely type 1, there is no robust evidence for an association between diabetes and endometriosis to date.89 Indeed, no association between laparoscopically confirmed endometriosis and risk of type 2 diabetes risk in a multivariable analysis could be evidenced in the NHSII.69 The risk of gestational diabetes mellitus among women with endometriosis remains controversial with discordant data across studies and meta-analyses.49,50,70
Endometriosis and cardiovascular disease
Endometriosis and ischaemic burden (coronary artery disease and stroke)
The first hint suggesting a possible association between ischaemic heart disease and inflammation of the upper genital tract came with evidence that women with pelvic inflammatory disease are more likely to have myocardial infarction and stroke than the general population.90,91 A limited number of studies suggested an association between endometriosis, coronary artery disease (CAD), and stroke.47,53,92–94 Only two large population-based cohort studies have shown an association between ischaemic heart disease and endometriosis (Table 2). Okoth et al.47 conducted a retrospective study of 279 759 women (1995–2018) and found an aOR of 1.24 (95% CI 1.13–1.37) for a composite endpoint of ischaemic heart disease, heart failure (HF), and cerebrovascular disease if endometriosis was present. In addition, endometriosis was associated with a 1.40-fold (95% CI 1.22–1.61) increased risk for ischaemic heart disease and 1.19-fold (95% CI 1.04–1.36) increased risk for cerebrovascular disease. Similarly, NHSII included 116 430 US women (1989–2009) and found a 52% greater risk for myocardial infarction (RR 1.52; 95% CI 1.17–1.98) in laparoscopically confirmed endometriosis.92 Laparoscopically confirmed endometriosis was independently associated with a 1.62-fold (95% CI 1.39–1.89) increased risk of the composite of myocardial infarction, angiographically confirmed angina, and revascularization either by coronary artery bypass graft surgery or by percutaneous coronary intervention. It is worth noting that co-occurring endometriosis among women with myocardial infarction did not translate into worse in-hospital outcomes.94
Table 2.
Description of large-scale studies on observed associations between endometriosis and cardiovascular disease
| Author(s) | Study name | Location | Population | Design | Method of data collection | Ascertainment period (year) | Findings |
|---|---|---|---|---|---|---|---|
| Okoth et al.47 | UK | Women aged between 16 and 50. 56 090 women with endometriosis and 223 669 matched control women | Population-based cohort study | Electronic health records | 1995–2018 | Endometriosis composite outcome: IHD, HF, cerebrovascular disease (aHR, 1.24 ; 95% CI 1.13–1.37) IHD (aHR 1.40; 95% CI 1.22–1.61); cerebrovascular disease (aHR, 1.19; 95% CI 1.04–1.36); arrhythmia (aHR, 1.26; 95% CI 1.11–1.43) | |
| Mu et al.92 | NHSII (Nurses’ Health Study II) | USA | 116 430 female nurses aged 25–42. 4244 women with laparoscopically confirmed endometriosis and 91 554 control women | Prospective cohort study | Self-completed questionnaire | 1989–2009 | Myocardial infarction (RR, 1.52, 95% CI 1.17–1.98); angiographically confirmed angina (RR 1.91, 95% CI 1.59–2.29); CABG/coronary angioplasty procedure/stent (RR 1.35, 95% CI 1.08–1.69) |
| Chiang et al.93 | Taiwan | Women aged between 18 and 50. 17 543 women with endometriosis women and 70 172 matched control women | Retrospective population-based cohort | Electronic health records | 1997–2013 | Composite outcome: Myocardial infarction, HF, stroke (aHR 1.17, 95% CI 1.05–1.29) | |
| Sugiura-Ogasawara et al.95 | Japan Environment and Children’s Study (JECS) | Japan | 103 070 pregnancies | Prospective cohort study | Self-completed questionnaire | January 2011 to March 2014 | Endometriosis: independent predictors for VTE (OR 2.70, 95% CI 1.21–6.00) |
aHR, adjusted hazard ratio; CABG, coronary artery bypass graft; HF, heart failure; IHD, ischaemic heart disease; OR, odds ratio; RR, relative risk; VTE, venous thrombo-embolic events.
The association of endometriosis with the prevalence and incidence of CVD appears likely from the aforementioned studies, but after considering the advances all these studies bring to the field, their limitations must also be considered. First, the populations studied were primarily Caucasian Europeans, limiting the generalizability of the evidence to other ethnicities. Despite efforts to harmonize these datasets, there are inevitable inconsistencies, including known and unknown factors, across these reports. For instance, the definitions of endometriosis (e.g. surgically confirmed, clinical suspicion, etc.) and CAD (e.g. acute myocardial infarction, coronary stenosis, etc.) varied widely across studies. This inhomogeneity among definition added uncertainty to the cardiovascular endpoints, particularly because the pathophysiology of acute myocardial infarction (plaque rupture and thrombosis) is not the same as the process of stable CAD. Furthermore, some observational cohorts inconsistently shared control groups.
Second, hysterectomy in women aged 50 years or younger has been associated with a significantly increased risk of ischaemic heart disease, with oophorectomy linked to an increased risk of both CAD and stroke.96,97 Similar risk was evidenced in the NHSII cohort,92 as 42% of the association between CAD and endometriosis was potentially explained by the greater frequency of hysterectomy/oophorectomy and earlier age at surgery following an endometriosis diagnosis. Accordingly, the adverse outcome portended by hysterectomy regardless of oophorectomy status was associated with the adverse risk factor profile of women who underwent hysterectomy.98
Finally, the role of hormonal treatment strategies for endometriosis, including combined oral contraceptives, progestins, and gonadotrophin-releasing hormone (GnRH) analogues, has been highly questioned regarding a potentially enhanced lipid profile,99 cardiovascular risk profile, and weight gain.100–102
Endometriosis and venous thrombo-embolic events
Systemic inflammation is known to up-regulate procoagulant factors, increase platelet reactivity, and favour thrombo-embolic events.103 As such, endometriosis was initially defined as a hormonal-dependent and inflammatory disease to perfectly link inflammation and thrombosis; however, the current picture differs substantially from this simplistic model. Basic research and evidence reporting an enhanced prothrombotic and inflammatory state in endometriosis did not translate into a clinically increased risk of venous thrombo-embolic events (VTE).
A review of the literature discloses several studies reporting an association between endometriosis and a procoagulant state. High tissue factor expression,104 increased procoagulant microparticle levels,39 and modest systemic changes of coagulation parameters105 have been reported, but these variables did not translate into increased VTE rates. To date, no studies have shown that endometriosis is associated with an increased risk of VTE except during pregnancy. Only anecdotal case series reported episodes of VTE mainly favoured by extrinsic compression, pelvic mass, and immobilization.106–109 However, careful attention should be paid to pregnant women with endometriosis. In a nationwide Japanese birth cohort study of 103 070 pregnancies, the frequency of VTE was 7.5 per 10 000 women during the pregnancy and post-partum period. Endometriosis and recurrent pregnancy loss were identified as novel independent risk factors for VTE.95
Regarding pregnancy, these results indicate that there is either no or a weak, direct interaction between VTE and endometriosis. Assisted reproductive techniques and, most notably, exogenous hormones are nonetheless key indirect contributors to the risk of venous thrombosis and ischaemic endpoints (Myocardial infarction, stroke, etc.) in endometriosis.110,111 Although successful fertility therapy was not associated with an increased risk of CVD later in life in the general population,111 recent updated studies reported assisted reproductive techniques increased the risk of venous thrombosis in women with endometriosis.112
Endometriosis, arrhythmias, and heart failure
The literature is limited regarding HF and arrhythmias in endometriosis. Only one study conducted by Okoth et al.47 examined the association between endometriosis and several cardiovascular outcomes including HF and arrhythmia, in a large UK retrospective matched cohort study. They found that endometriosis was associated with a higher risk of arrhythmia (adjusted HR 1.26; 95% CI 1.11–1.43; P = 0.001). The noxious impact of chronic inflammation has been proposed by the authors to explain the association between endometriosis and arrhythmia. These results should be interpreted with caution, as no reference is made to the type of arrhythmia and several well-known cofactors associated with arrhythmia onset were omitted from the model (left ventricular ejection fraction, sleep disorders, treatment strategies in particular hormonal treatments, hysterectomy/oophorectomy status, dietary patterns, physical activity, inflammatory biological burden, etc.). Furthermore, cancer antigen-125, a well-established tumour biomarker related to ovarian cancer, has been associated with endometriosis113 and more recently with HF114,115 and new-onset atrial fibrillation (AF).116,117 To date, there is insufficient evidence to support a causal relationship between endometriosis or arrhythmias and HF.
Endometriosis and cardiovascular pharmacology
Several cardiovascular medications have been tested in animal models, with promising results on the establishment and maintenance of endometriotic lesions. However, their translation into human clinical trials has been very limited.
Statins, classically known for their roles in lowering cholesterol and anti-inflammatory properties, have been shown to play a role in endometriosis by modifying cell signalling in preclinical studies using human endometriotic stromal cell cultures. Statins therapy lead to increased apoptosis, decreased proliferation, and impaired cell adhesion and motility.118,119 Additionally, statins inhibited stromal cell invasion and reduced angiogenesis.120–122 Recent findings showed that platelets play important roles in the development of endometriosis in general and in fibrogenesis in particular.123,124 Antiplatelet treatment was demonstrated to impede the progressive epithelial-mesenchymal transition, fibroblast-to-myofibroblast transdifferentiation, and smooth muscle metaplasia, resulting in reduced lesion size of endometriotic lesions and fibrogenesis.123 Low-dose aspirin, used in a preclinical setting, has lately been proposed for its ability to down-regulate progesterone resistance and a target for endometriosis-related infertility.125
Women with endometriosis tend to have higher levels of psychological stress, which is known to promote tumour growth and metastasis. Β-Adrenergic receptor blockade in a mouse model of endometriosis completely abolished the promotional effect of chronic stress, through suppression of ADRB2 and CREB activation, thus suppressing angiogenesis and proliferation.126 Additionally, perioperative use of propranolol in a mouse model of endometriosis significantly decelerated the growth of residual lesions that were intentionally left out during the primary surgery.127 Similarly, telmisartan inhibited vascularization, immune cell content, and growth of murine endometriosis-like lesions.128,129
Summary and authors’ perspectives
Cardiovascular disease remains the leading cause of mortality among women, causing 1 in 3 deaths each year,130 whereas endometriosis affects 1 in 10 reproductive-age women. This unequivocal epidemiological observation should be considered in the field of future cardiovascular research in women. Current clinical evidence is insufficient to implicate a strong association and potential role for endometriosis in CVD.
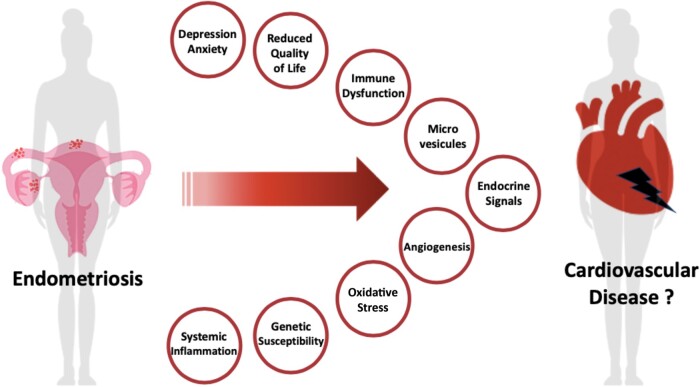
The literature is sparse regarding the association between CVD and endometriosis (Graphical Abstract). The epidemiologically observed associations between the two should be interpreted with caution for several reasons. Current evidence is limited by small sample sizes, observational designs, and the specific characteristics of the population from which the samples are derived (high-income countries, cohort study of hospital-based healthcare workers, primarily Caucasian Europeans, etc.). Only small associations between endometriosis and CVD have been reported in the literature, although inconsistently. Representation of participants with endometriosis in cardiovascular clinical trials and registries is challenging, as endometriosis remains under-recognized. Endometriosis is usually diagnosed in higher socioeconomic groups and likely related to inequities in access to health care. In addition to socioeconomic impact, multiple factors can be identified as barriers to inclusion of women with endometriosis in CV studies: misdiagnosis is common; as diagnosis remains challenging and largely delayed by years; unawareness about ongoing trials, combined cardiac and gynaecologic care, and monitoring in clinical trials, etc. The chronic and heterogeneous nature of endometriosis (e.g. disease stage and lesion type) and the confounding influence of hormonal, non-hormonal, and pain-related interventions further complicate the cause-and-effect relationship in CV endpoints.
Graphical Abstract.
Cardiovascular disease risk estimation remains challenging, especially in women with endometriosis. Further research is needed to better understand how endometriosis should be incorporated into risk prediction models alongside well-established risk factors. Indeed, the reduced quality of life and psychosocial risk factors such as depression and anxiety may overlap and help to explain the predisposition for CVD. In considering women with endometriosis, there is a need to better understand the potential associations with CVD and risk factors and to clarify the pathophysiology of the female heart at a broader level (Graphical Abstract).
Inclusivity is therefore mandatory, and specific registries, studies, and trials are urgently needed to address the underlying biological mechanisms potentially shared between CVD and endometriosis, clarify whether endometriosis can cause CVD; assess the prognosis of endometriosis in cardiovascular patients, and finally, advance potential innovative solutions and tailored management of CVD in women with endometriosis.
Multicentre case-controlled and cross-sectional studies may be of particular interest in women admitted to cardiology departments to ascertain the association between endometriosis and various CVDs (ischaemic cardiomyopathy, HF, AF, etc.) and risk factors. However, the benefits of such study designs are limited by the fact that (i) endometriosis is greatly underdiagnosed, (ii) there are asymptomatic cases, and (iii) the assessment of specific CVD phenotypes and risk factors distributions among endometriosis patients necessitates a large sample. Only international and/or national levels initiatives may have the power to address the prevalence, links, impact, and prognosis of endometriosis and CVD by analysing data from large-scale surveys. Strong evidence regarding the association between these two conditions obtained from large-scale cohorts or large population-based studies, may contribute to a change in the prevention, screening, early detection, and treatment of CV risk factors among women with endometriosis.
Lead author biography
Dr Benjamin Marchandot is a general cardiologist in the Cardiac Care Unit (CCU) at Strasbourg University Hospital, France. He is a member of Strasbourg Cardiovascular Research Group—GERCA (Groupe pour l’Enseignement et la Recherche Cardiovasculaire en Alsace), a multidisciplinary research team involved in the field of CVD, thrombosis, and haemostasis.
Conflict of interest: The authors declare that there is no conflict of interest regarding the publication of this article. O.M. received institutional research grants from Fondation Coeur et Vaisseaux, AstraZeneca and Boehringher Ingelheim. K.M. received a grant from Edwards Lifesciences (THV-F20-142). The manuscript has been read and approved for submission by all authors. The authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Funding
This work was supported by Groupe pour l Enseignement, la Pr赥ntion et la Recherche Cardiologique en Alsace (GERCA).
Data availability: No new data were generated or analysed in support of this research.
Contributor Information
Benjamin Marchandot, Division of Cardiovascular Medicine, Nouvel Hopital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France.
Anais Curtiaud, Division of Cardiovascular Medicine, Nouvel Hopital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France.
Kensuke Matsushita, Division of Cardiovascular Medicine, Nouvel Hopital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France; INSERM (French National Institute of Health and Medical Research), UMR 1260, Regenerative Nanomedicine, FMTS, Strasbourg, France.
Antonin Trimaille, Division of Cardiovascular Medicine, Nouvel Hopital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France; INSERM (French National Institute of Health and Medical Research), UMR 1260, Regenerative Nanomedicine, FMTS, Strasbourg, France.
Aline Host, Department of Obstetrics and Gynecology, Hautepierre Hospital, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France; ENDOALSACE, Strasbourg Expert Center for Endometriosis, Hautepierre Hospital, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France.
Emilie Faller, Department of Obstetrics and Gynecology, Hautepierre Hospital, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France; ENDOALSACE, Strasbourg Expert Center for Endometriosis, Hautepierre Hospital, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France.
Olivier Garbin, Department of Obstetrics and Gynecology, Hautepierre Hospital, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France; ENDOALSACE, Strasbourg Expert Center for Endometriosis, Hautepierre Hospital, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France.
Chérif Akladios, Department of Obstetrics and Gynecology, Hautepierre Hospital, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France; ENDOALSACE, Strasbourg Expert Center for Endometriosis, Hautepierre Hospital, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France.
Laurence Jesel, Division of Cardiovascular Medicine, Nouvel Hopital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France; INSERM (French National Institute of Health and Medical Research), UMR 1260, Regenerative Nanomedicine, FMTS, Strasbourg, France.
Olivier Morel, Division of Cardiovascular Medicine, Nouvel Hopital Civil, Strasbourg University Hospital, 1 place de l’Hôpital, 67000 Strasbourg, France; INSERM (French National Institute of Health and Medical Research), UMR 1260, Regenerative Nanomedicine, FMTS, Strasbourg, France.
References
- 1. Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, Missmer SA. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol 2018;51:1–15. [DOI] [PubMed] [Google Scholar]
- 2. Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet 2021;397:839–852. [DOI] [PubMed] [Google Scholar]
- 3. Maas AHEM, Rosano G, Cifkova R, Chieffo A, van Dijken D, Hamoda H, Kunadian V, Laan E, Lambrinoudaki I, Maclaran K, Panay N, Stevenson JC, van Trotsenburg M, Collins P. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: a consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur Heart J 2021;42:967–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med 2020;382:1244–1256. [DOI] [PubMed] [Google Scholar]
- 5. Parazzini F, Esposito G, Tozzi L, Noli S, Bianchi S. Epidemiology of endometriosis and its comorbidities. Eur J Obstet Gynecol Reprod Biol 2017;209:3–7. [DOI] [PubMed] [Google Scholar]
- 6. Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol 2019;15:666–682. [DOI] [PubMed] [Google Scholar]
- 7. Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T, Falcone T, Graham B, Halis G, Horne A, Kanj O, Kjer JJ, Kristensen J, Lebovic D, Mueller M, Vigano P, Wullschleger M, D'Hooghe T. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012;27:1292–1299. Erratum in: Hum Reprod. 2014;29(9):2073. [DOI] [PubMed] [Google Scholar]
- 8. Johnson NP, Hummelshoj L, Adamson GD, Keckstein J, Taylor HS, Abrao MS, Bush D, Kiesel L, Tamimi R, Sharpe-Timms KL, Rombauts L, Giudice LC; World Endometriosis Society Sao Paulo Consortium. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod 2017;32:315–324. [DOI] [PubMed] [Google Scholar]
- 9. Menni K, Facchetti L, Cabassa P. Extragenital endometriosis: assessment with MR imaging. A pictorial review. Br J Radiol 2016;89:20150672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril 2017;108:886–894. [DOI] [PubMed] [Google Scholar]
- 11. Koninckx PR, Meuleman C, Oosterlynck D, Cornillie FJ. Diagnosis of deep endometriosis by clinical examination during menstruation and plasma CA-125 concentration. Fertil Steril 1996;65:280–287. [PubMed] [Google Scholar]
- 12. Guerriero S, Condous G, van den Bosch T, Valentin L, Leone FP, Van Schoubroeck D, Exacoustos C, Installé AJ, Martins WP, Abrao MS, Hudelist G, Bazot M, Alcazar JL, Gonçalves MO, Pascual MA, Ajossa S, Savelli L, Dunham R, Reid S, Menakaya U, Bourne T, Ferrero S, Leon M, Bignardi T, Holland T, Jurkovic D, Benacerraf B, Osuga Y, Somigliana E, Timmerman D. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol 2016;48:318–332. [DOI] [PubMed] [Google Scholar]
- 13. Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol 2018;51:16–24. [DOI] [PubMed] [Google Scholar]
- 14. Bazot M, Bharwani N, Huchon C, Kinkel K, Cunha TM, Guerra A, Manganaro L, Buñesch L, Kido A, Togashi K, Thomassin-Naggara I, Rockall AG. European society of urogenital radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur Radiol 2017;27:2765–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guerriero S, Saba L, Pascual MA, Ajossa S, Rodriguez I, Mais V, Alcazar JL. Transvaginal ultrasound vs magnetic resonance imaging for diagnosing deep infiltrating endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;51:586–595. [DOI] [PubMed] [Google Scholar]
- 16. Ahn SH, Khalaj K, Young SL, Lessey BA, Koti M, Tayade C. Immune-inflammation gene signatures in endometriosis patients. Fertil Steril 2016;106:1420–1431.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Symons LK, Miller JE, Kay VR, Marks RM, Liblik K, Koti M, Tayade C. The immunopathophysiology of endometriosis. Trends Mol Med 2018;24:748–762. [DOI] [PubMed] [Google Scholar]
- 18. Tariverdian N, Siedentopf F, Rücke M, Blois SM, Klapp BF, Kentenich H, Arck PC. Intraperitoneal immune cell status in infertile women with and without endometriosis. J Reprod Immunol 2009;80:80–90. [DOI] [PubMed] [Google Scholar]
- 19. Saunders PTK, Horne AW. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021;184:2807–2824. [DOI] [PubMed] [Google Scholar]
- 20. Han SJ, Jung SY, Wu SP, Hawkins SM, Park MJ, Kyo S, Qin J, Lydon JP, Tsai SY, Tsai MJ, DeMayo FJ, O'Malley BW. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell 2015;163:960–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, Tokunaga H, Su EJ. Role of estrogen receptor-β in endometriosis. Semin Reprod Med 2012;30:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scutiero G, Iannone P, Bernardi G, Bonaccorsi G, Spadaro S, Volta CA, Greco P, Nappi L. Oxidative stress and endometriosis: a systematic review of the literature. Oxid Med Cell Longev 2017;2017:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Munrós J, Martínez-Zamora MA, Tàssies D, Coloma JL, Torrente MA, Reverter JC, Carmona F, Balasch J. Total circulating microparticle levels are increased in patients with deep infiltrating endometriosis. Hum Reprod 2017;32:325–331. [DOI] [PubMed] [Google Scholar]
- 24. Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers 2018;4:9. [DOI] [PubMed] [Google Scholar]
- 25. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004;109(Suppl 1):III27–III32. [DOI] [PubMed] [Google Scholar]
- 26. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012;32:2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, Tokgözoğlu L, Lewis EF. Atherosclerosis. Nat Rev Dis Primers 2019;5:56. [DOI] [PubMed] [Google Scholar]
- 28. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril 2012;98:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014;10:261–275. [DOI] [PubMed] [Google Scholar]
- 30. Marquardt RM, Kim TH, Shin JH, Jeong JW. Progesterone and estrogen signaling in the endometrium: what goes wrong in endometriosis? Int J Mol Sci 2019;20:3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chantalat E, Valera MC, Vaysse C, Noirrit E, Rusidze M, Weyl A, Vergriete K, Buscail E, Lluel P, Fontaine C, Arnal JF, Lenfant F. Estrogen receptors and endometriosis. Int J Mol Sci 2020;21:2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Capobianco A, Rovere-Querini P. Endometriosis, a disease of the macrophage. Front Immunol 2013;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bacci M, Capobianco A, Monno A, Cottone L, Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S, Panina-Bordignon P, Manfredi AA, Rovere-Querini P. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol 2009;175:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harada T, Iwabe T, Terakawa N. Role of cytokines in endometriosis. Fertil Steril 2001;76:1–10. [DOI] [PubMed] [Google Scholar]
- 35. Samimi M, Pourhanifeh MH, Mehdizadehkashi A, Eftekhar T, Asemi Z. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: basic science and new insights based on gene expression. J Cell Physiol 2019;234:19384–19392. [DOI] [PubMed] [Google Scholar]
- 36. Tani A, Yamamoto S, Maegawa M, Kunimi K, Matsui S, Keyama K, Kato T, Uemura H, Kuwahara A, Matsuzaki T, Yasui T, Kamada M, Soeki T, Sata M, Irahara M. Arterial stiffness is increased in young women with endometriosis. J Obstet Gynaecol 2015;35:711–715. [DOI] [PubMed] [Google Scholar]
- 37. Kinugasa S, Shinohara K, Wakatsuki A. Increased asymmetric dimethylarginine and enhanced inflammation are associated with impaired vascular reactivity in women with endometriosis. Atherosclerosis 2011;219:784–788. [DOI] [PubMed] [Google Scholar]
- 38. Santoro L, D'Onofrio F, Campo S, Ferraro PM, Tondi P, Campo V, Flex A, Gasbarrini A, Santoliquido A. Endothelial dysfunction but not increased carotid intima-media thickness in young European women with endometriosis. Hum Reprod 2012;27:1320–1326. [DOI] [PubMed] [Google Scholar]
- 39. Santoro L, D'Onofrio F, Campo S, Ferraro PM, Flex A, Angelini F, Forni F, Nicolardi E, Campo V, Mascilini F, Landolfi R, Tondi P, Santoliquido A. Regression of endothelial dysfunction in patients with endometriosis after surgical treatment: a 2-year follow-up study. Hum Reprod 2014;29:1205–1210. [DOI] [PubMed] [Google Scholar]
- 40. Uno S, Zembutsu H, Hirasawa A, Takahashi A, Kubo M, Akahane T, Aoki D, Kamatani N, Hirata K, Nakamura Y. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nat Genet 2010;42:707–710. [DOI] [PubMed] [Google Scholar]
- 41. Napoli C, Crudele V, Soricelli A, Al-Omran M, Vitale N, Infante T, Mancini FP. Primary prevention of atherosclerosis: a clinical challenge for the reversal of epigenetic mechanisms? Circulation 2012;125:2363–2373. [DOI] [PubMed] [Google Scholar]
- 42. Webb TR, Erdmann J, Stirrups KE, Stitziel NO, Masca NGD, Jansen H, Kanoni S, Nelson CP, Ferrario PG, König IR, Eicher JD, Johnson AD, Hamby SE, Betsholtz C, Ruusalepp A, Franzén O, Schadt EE, Björkegren JLM, Weeke PE, Auer PL, Schick UM, Lu Y, Zhang H, Dube M-P, Goel A, Farrall M, Peloso GM, Won H-H, Do R, van Iperen E, Kruppa J, Mahajan A, Scott RA, Willenborg C, Braund PS, van Capelleveen JC, Doney ASF, Donnelly LA, Asselta R, Merlini PA, Duga S, Marziliano N, Denny JC, Shaffer C, El-Mokhtari NE, Franke A, Heilmann S, Hengstenberg C, Hoffmann P, Holmen OL, Hveem K, Jansson J-H, Jöckel K-H, Kessler T, Kriebel J, Laugwitz KL, Marouli E, Martinelli N, McCarthy MI, Van Zuydam NR, Meisinger C, Esko T, Mihailov E, Escher SA, Alver M, Moebus S, Morris AD, Virtamo J, Nikpay M, Olivieri O, Provost S, AlQarawi A, Robertson NR, Akinsansya KO, Reilly DF, Vogt TF, Yin W, Asselbergs FW, Kooperberg C, Jackson RD, Stahl E, Müller-Nurasyid M, Strauch K, Varga TV, Waldenberger M, Zeng L, Chowdhury R, Salomaa V, Ford I, Jukema JW, Amouyel P, Kontto J, Nordestgaard BG, Ferrières J, Saleheen D, Sattar N, Surendran P, Wagner A, Young R, Howson JMM, Butterworth AS, Danesh J, Ardissino D, Bottinger EP, Erbel R, Franks PW, Girelli D, Hall AS, Hovingh GK, Kastrati A, Lieb W, Meitinger T, Kraus WE, Shah SH, McPherson R, Orho-Melander M, Melander O, Metspalu A, Palmer CNA, Peters A, Rader DJ, Reilly MP, Loos RJF, Reiner AP, Roden DM, Tardif J-C, Thompson JR, Wareham NJ, Watkins H, Willer CJ, Samani NJ, Schunkert H, Deloukas P, Kathiresan S; Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators. Systematic evaluation of pleiotropy identifies 6 further loci associated with coronary artery disease. J Am Coll Cardiol 2017;69:823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ou M, Li X, Zhao S, Cui S, Tu J. Long non-coding RNA CDKN2B-AS1 contributes to atherosclerotic plaque formation by forming RNA-DNA triplex in the CDKN2B promoter. EBioMedicine 2020;55:102694. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44. Lee GH, Choi YM, Hong MA, Yoon SH, Kim JJ, Hwang K, Chae SJ. Association of CDKN2B-AS and WNT4 genetic polymorphisms in Korean patients with endometriosis. Fertil Steril 2014;102:1393–1397. [DOI] [PubMed] [Google Scholar]
- 45. Mu F, Rich-Edwards J, Rimm EB, Spiegelman D, Forman JP, Missmer SA. Association between endometriosis and hypercholesterolemia or hypertension. Hypertension 2017;70:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nagai K, Hayashi K, Yasui T, Katanoda K, Iso H, Kiyohara Y, Wakatsuki A, Kubota T, Mizunuma H. Disease history and risk of comorbidity in women's life course: a comprehensive analysis of the Japan Nurses' Health Study baseline survey. BMJ Open 2015;5:e006360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Okoth K, Wang J, Zemedikun D, Thomas GN, Nirantharakumar K, Adderley NJ. Risk of cardiovascular outcomes among women with endometriosis in the United Kingdom: a retrospective matched cohort study. BJOG 2021;128:1598–1609. [DOI] [PubMed] [Google Scholar]
- 48. Pan ML, Chen LR, Tsao HM, Chen KH. Risk of gestational hypertension-preeclampsia in women with preceding endometriosis: a nationwide population-based study. PLoS One 2017;12:e0181261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lalani S, Choudhry AJ, Firth B, Bacal V, Walker M, Wen SW, Singh S, Amath A, Hodge M, Chen I. Endometriosis and adverse maternal, fetal and neonatal outcomes, a systematic review and meta-analysis. Hum Reprod 2018;33:1854–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Farland LV, Prescott J, Sasamoto N, Tobias DK, Gaskins AJ, Stuart JJ, Carusi DA, Chavarro JE, Horne AW, Rich-Edwards JW, Missmer SA. Endometriosis and risk of adverse pregnancy outcomes. Obstet Gynecol 2019;134:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Melo AS, Rosa e, Silva JC, Rosa e, Silva AC, Poli-Neto OB, Ferriani RA, Vieira CS. Unfavorable lipid profile in women with endometriosis. Fertil Steril 2010;93:2433–2436. [DOI] [PubMed] [Google Scholar]
- 52. Crook D, Howell R, Sidhu M, Edmonds DK, Stevenson JC. Elevated serum lipoprotein(a) levels in young women with endometriosis. Metabolism 1997;46:735–739. [DOI] [PubMed] [Google Scholar]
- 53. Hopeman MM, Riley JK, Frolova AI, Jiang H, Jungheim ES. Serum polyunsaturated fatty acids and endometriosis. Reprod Sci 2015;22:1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pretta S, Remorgida V, Abbamonte LH, Anserini P, Ragni N, Del Sette M, Gandolfo C, Ferrero S. Atherosclerosis in women with endometriosis. Eur J Obstet Gynecol Reprod Biol 2007;132:226–231. [DOI] [PubMed] [Google Scholar]
- 55. Verit FF, Erel O, Celik N. Serum paraoxonase-1 activity in women with endometriosis and its relationship with the stage of the disease. Hum Reprod 2007;23:100–104. [DOI] [PubMed] [Google Scholar]
- 56. Ferrero S, Anserini P, Remorgida V, Ragni N. Body mass index in endometriosis. Eur J Obstet Gynecol Reprod Biol 2005;121:94–98. [DOI] [PubMed] [Google Scholar]
- 57. Holdsworth-Carson SJ, Dior UP, Colgrave EM, Healey M, Montgomery GW, Rogers PAW, Girling JE. The association of body mass index with endometriosis and disease severity in women with pain. J Endometriosis Pelvic Pain Disord 2018;10:79–87. [Google Scholar]
- 58. Shah DK, Correia KF, Vitonis AF, Missmer SA. Body size and endometriosis: results from 20 years of follow-up within the Nurses' Health Study II prospective cohort. Hum Reprod 2013;28:1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol 2004;160:784–796. [DOI] [PubMed] [Google Scholar]
- 60. Hediger ML, Hartnett HJ, Louis GM. Association of endometriosis with body size and figure. Fertil Steril 2005;84:1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pillet M-CL, Schneider A, Borghese B, Santulli P, Souza C, Streuli I, de Ziegler D, Chapron C. Deep infiltrating endometriosis is associated with markedly lower body mass index: a 476 case-control study. Hum Reprod 2012;27:265–272. [DOI] [PubMed] [Google Scholar]
- 62. Farland LV, Missmer SA, Bijon A, Gusto G, Gelot A, Clavel-Chapelon F, Mesrine S, Boutron-Ruault MC, Kvaskoff M. Associations among body size across the life course, adult height and endometriosis. Hum Reprod 2017;32:1732–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu Y, Zhang W. Association between body mass index and endometriosis risk: a meta-analysis. Oncotarget 2017;8:46928–46936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tang Y, Zhao M, Lin L, Gao Y, Chen GQ, Chen S, Chen Q. Is body mass index associated with the incidence of endometriosis and the severity of dysmenorrhoea: a case-control study in China? BMJ Open 2020;10:e037095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cramer DW, Wilson E, Stillman RJ, Berger MJ, Belisle S, Schiff I, Albrecht B, Gibson M, Stadel BV, Schoenbaum SC. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA 1986;255:1904–1908. [PubMed] [Google Scholar]
- 66. Calhaz-Jorge C, Mol BW, Nunes J, Costa AP. Clinical predictive factors for endometriosis in a Portuguese infertile population. Hum Reprod 2004;19:2126–2131. [DOI] [PubMed] [Google Scholar]
- 67. Bravi F, Parazzini F, Cipriani S, Chiaffarino F, Ricci E, Chiantera V, Viganò P, La Vecchia C. Tobacco smoking and risk of endometriosis: a systematic review and meta-analysis. BMJ Open 2014;4:e006325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mahalingaiah S, Hart JE, Laden F, Aschengrau A, Missmer SA. Air pollution exposures during adulthood and risk of endometriosis in the Nurses' Health Study II. Environ Health Perspect 2014;122:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Farland LV, Degnan WJ, Harris HR, Tobias DK, Missmer SA. A prospective study of endometriosis and risk of type 2 diabetes. Diabetologia 2021;64:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pérez-López FR, Martínez-Domínguez SJ, Viñas A, Pérez-Tambo R, Lafita A, Lajusticia H, Chedraui P; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Endometriosis and gestational diabetes mellitus risk: a systematic review and meta-analysis. Gynecol Endocrinol 2018;34:363–369. [DOI] [PubMed] [Google Scholar]
- 71. De Miguel C, Rudemiller NP, Abais JM, Mattson DL. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr Hypertens Rep 2015;17:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res 2002;53:688–708. [DOI] [PubMed] [Google Scholar]
- 73. Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 1999;340:1801–1811. [DOI] [PubMed] [Google Scholar]
- 74. Tan J, Taskin O, Iews M, Lee AJ, Kan A, Rowe T, Bedaiwy MA. Atherosclerotic cardiovascular disease in women with endometriosis: a systematic review of risk factors and prospects for early surveillance. Reprod Biomed Online 2019;39:1007–1016. [DOI] [PubMed] [Google Scholar]
- 75. Santoro L, D’Onofrio F, Flore R, Gasbarrini A, Santoliquido A. Endometriosis and atherosclerosis: what we already know and what we have yet to discover. Am J Obstet Gynecol 2015;213:326–331. [DOI] [PubMed] [Google Scholar]
- 76. Dutta M, Anitha M, Smith PB, Chiaro CR, Maan M, Chaudhury K, Patterson AD. Metabolomics reveals altered lipid metabolism in a mouse model of endometriosis. J Proteome Res 2016;15:2626–2633. [DOI] [PubMed] [Google Scholar]
- 77. Feider CL, Woody S, Ledet S, Zhang J, Sebastian K, Breen MT, Eberlin LS. Molecular imaging of endometriosis tissues using desorption electrospray ionization mass spectrometry. Sci Rep 2019;9:15690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lee YH, Tan CW, Venkatratnam A, Tan CS, Cui L, Loh SF, Griffith L, Tannenbaum SR, Chan JK. Dysregulated sphingolipid metabolism in endometriosis. J Clin Endocrinol Metab 2014;99:E1913–E1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li J, Gao Y, Guan L, Zhang H, Sun J, Gong X, Li D, Chen P, Ma Z, Liang X, Huang M, Bi H. Discovery of phosphatidic acid, phosphatidylcholine, and phosphatidylserine as biomarkers for early diagnosis of endometriosis. Front Physiol 2018;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Letsiou S, Peterse DP, Fassbender A, Hendriks MM, van den Broek NJ, Berger R, O DF, Vanhie A, Vodolazkaia A, Van Langendonckt A, Donnez J, Harms AC, Vreeken RJ, Groothuis PG, Dolmans M-M, Brenkman AB, D'Hooghe TM. Endometriosis is associated with aberrant metabolite profiles in plasma. Fertil Steril 2017;107:699–706. [DOI] [PubMed] [Google Scholar]
- 81. Lee YH, Yang JX, Allen JC, Tan CS, Chern BSM, Tan TY, Tan HH, Mattar CNZ, Chan JKY. Elevated peritoneal fluid ceramides in human endometriosis-associated infertility and their effects on mouse oocyte maturation. Fertil Steril 2018;110:767–777.e5. [DOI] [PubMed] [Google Scholar]
- 82. Levade T, Augé N, Veldman RJ, Cuvillier O, Nègre-Salvayre A, Salvayre R. Sphingolipid mediators in cardiovascular cell biology and pathology. Circ Res 2001;89:957–968. [DOI] [PubMed] [Google Scholar]
- 83. Teng SW, Horng HC, Ho CH, Yen MS, Chao HT, Wang PH; Taiwan Association of Gynecology Systematic Review Group. Women with endometriosis have higher comorbidities: analysis of domestic data in Taiwan. J Chin Med Assoc 2016;79:577–582. [DOI] [PubMed] [Google Scholar]
- 84. Zolbin MM, Mamillapalli R, Nematian SE, Goetz TG, Taylor HS. Adipocyte alterations in endometriosis: reduced numbers of stem cells and microRNA induced alterations in adipocyte metabolic gene expression. Reprod Biol Endocrinol 2019;17:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Goetz TG, Mamillapalli R, Taylor HS. Low body mass index in endometriosis is promoted by hepatic metabolic gene dysregulation in mice. Biol Reprod 2016;95:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Aban M, Ertunc D, Tok EC, Tamer L, Arslan M, Dilek S. Modulating interaction of glutathione-S-transferase polymorphisms with smoking in endometriosis. J Reprod Med 2007;52:715–721. [PubMed] [Google Scholar]
- 87. Bellelis P, Podgaec S, Brão MM. Environmental factors and endometriosis. Rev Assoc Med Bras 2011;57:448–452. [DOI] [PubMed] [Google Scholar]
- 88. Mahalingaiah S, Lane KJ, Kim C, Cheng JJ, Hart JE. Impacts of air pollution on gynecologic disease: infertility, menstrual irregularity, uterine fibroids, and endometriosis: a systematic review and commentary. Curr Epidemiol Rep 2018;5:197–204. [Google Scholar]
- 89. Simmen RCM, Brown DM, Quick CM, Alhallak I, Rose TK, Liu S, Kelley AS. Co-morbidity of type 1 diabetes and endometriosis: bringing a new paradigm into focus. J Endocrinol 2019;243:R47–R57. https://doi.org/10.1530/JOE-19-0248. [DOI] [PubMed] [Google Scholar]
- 90. Chen PC, Tseng TC, Hsieh JY, Lin HW. Association between stroke and patients with pelvic inflammatory disease: a nationwide population-based study in Taiwan. Stroke 2011;42:2074–2076. [DOI] [PubMed] [Google Scholar]
- 91. Liou T-H, Wu C-W, Hao W-R, Hsu M-I, Liu J-C, Lin H-W. Risk of myocardial infarction in women with pelvic inflammatory disease. Int J Cardiol 2013;167:416–420. [DOI] [PubMed] [Google Scholar]
- 92. Mu F, Rich-Edwards J, Rimm EB, Spiegelman D, Missmer SA. Endometriosis and risk of coronary heart disease. Circ Cardiovasc Qual Outcomes 2016;9:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chiang HJ, Lan KC, Yang YH, Chiang JY, Kung FT, Huang FJ, Lin YJ, Su YT, Sung PH. Risk of major adverse cardiovascular and cerebrovascular events in Taiwanese women with endometriosis. J Formos Med Assoc 2021;120:327–336. [DOI] [PubMed] [Google Scholar]
- 94. Akinjero A, Adegbala O, Akinyemiju T. Abstract P320: is co-occurring endometriosis among women with myocardial infarction associated with worse in-hospital outcomes? findings from the nationwide inpatient sample. Circulation 2017;135:AP320. [Google Scholar]
- 95. Sugiura-Ogasawara M, Ebara T, Matsuki T, Yamada Y, Omori T, Matsumoto Y, Kato S, Kano H, Kurihara T, Saitoh S, Kamijima M; the Japan Environment & Children's Study (JECS) Group. Endometriosis and recurrent pregnancy loss as new risk factors for venous thromboembolism during pregnancy and post-partum: the JECS birth cohort. Thromb Haemost 2019;119:606–617. [DOI] [PubMed] [Google Scholar]
- 96. Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J 2011;32:745–750. [DOI] [PubMed] [Google Scholar]
- 97. Laughlin-Tommaso SK, Khan Z, Weaver AL, Smith CY, Rocca WA, Stewart EA. Cardiovascular and metabolic morbidity after hysterectomy with ovarian conservation: a cohort study. Menopause 2018;25:483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Howard BV, Kuller L, Langer R, Manson JE, Allen C, Assaf A, Cochrane BB, Larson JC, Lasser N, Rainford M, Van Horn L, Stefanick ML, Trevisan M; Women's Health Initiative. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women's Health Initiative Observational Study. Circulation 2005;111:1462–1470. [DOI] [PubMed] [Google Scholar]
- 99. Farish E, Fletcher CD, Barnes JF, Mack A, Gray CE, Hart DM. Reversible menopause induced by the GnRH analogue buserelin: effects on lipoprotein metabolism. Acta Endocrinol (Copenh) 1992;127:123–126. [DOI] [PubMed] [Google Scholar]
- 100. Ferrero S, Evangelisti G, Barra F. Current and emerging treatment options for endometriosis. Expert Opin Pharmacother 2018;19:1109–1125. [DOI] [PubMed] [Google Scholar]
- 101. Berlanda N, Somigliana E, Viganò P, Vercellini P. Safety of medical treatments for endometriosis. Expert Opin Drug Saf 2016;15:21–30. [DOI] [PubMed] [Google Scholar]
- 102. Yim SF, Lau TK, Sahota DS, Chung TK, Chang AM, Haines CJ. Prospective randomized study of the effect of. ‘add-back’ hormone replacement on vascular function during treatment with gonadotropin-releasing hormone agonists. Circulation 1998;98:1631–1635. [DOI] [PubMed] [Google Scholar]
- 103. Esmon CT. Inflammation and thrombosis. J Thromb Haemost 2003;1:1343–1348. [DOI] [PubMed] [Google Scholar]
- 104. Epelboin S, Labrosse J, Fauque P, Levy R, Gervoise-Boyer MJ, Devaux A, Bergère M, de Vienne C, Jonveaux P, De Mouzon J, Pessione F. Endometriosis and assisted reproductive techniques independently related to mother-child morbidities: a French longitudinal national study. Reprod Biomed Online 2021;42:627–633. [DOI] [PubMed] [Google Scholar]
- 105. Ottolina J, Bartiromo L, Dolci C, Salmeri N, Schimberni M, Villanacci R, Viganò P, Candiani M. Assessment of coagulation parameters in women affected by endometriosis: validation study and systematic review of the literature. Diagnostics (Basel) 2020;10:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Li M, Chen K, Fong YF. A rare case of endometriosis invading external iliac vein causing deep vein thrombosis. Am J Obstet Gynecol 2019;220:113–114. [DOI] [PubMed] [Google Scholar]
- 107. Ianieri MM, Buca DIP, Panaccio P, Cieri M, Francomano F, Liberati M. Retroperitoneal endometriosis in postmenopausal woman causing deep vein thrombosis: case report and review of the literature. Clin Exp Obstet Gynecol 2017;44:148–150. [PubMed] [Google Scholar]
- 108. Chiaramonte R, Castorina S, Castorina EG, Panarello A, Antoci SA. Thrombosis of iliac vessels, a rare complication of endometriosis: case report and review of literature. J Adv Res 2017;8:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sharma RP, Delly F, Marin H, Sturza S. Endometriosis causing lower extremity deep vein thrombosis—case report and review of the literature. Int J Angiol 2009;18:199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rosendaal FR, Helmerhorst FM, Vandenbroucke JP. Female hormones and thrombosis. Arterioscler Thromb Vasc Biol 2002;22:201–210. [DOI] [PubMed] [Google Scholar]
- 111. Krikun G, Lockwood CJ, Paidas MJ. Tissue factor and the endometrium: from physiology to pathology. Thromb Res 2009;124:393–396. [DOI] [PubMed] [Google Scholar]
- 112. Udell JA, Lu H, Redelmeier DA. Long-term cardiovascular risk in women prescribed fertility therapy. J Am Coll Cardiol 2013;62:1704–1712. [DOI] [PubMed] [Google Scholar]
- 113. Nisenblat V, Bossuyt PM, Shaikh R, Farquhar C, Jordan V, Scheffers CS, Mol BW, Johnson N, Hull ML. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016;2016:CD012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. D’Aloia A, Faggiano P, Aurigemma G, Bontempi L, Ruggeri G, Metra M, Nodari S, Dei Cas L. Serum levels of carbohydrate antigen 125 in patients with chronic heart failure: relation to clinical severity, hemodynamic and Doppler echocardiographic abnormalities, and short-term prognosis. J Am Coll Cardiol 2003;41:1805–1811. [DOI] [PubMed] [Google Scholar]
- 115. Li X, He M, Zhu J, Yao P, Li X, Yuan J, Min X, Lang M, Yang H, Hu FB, Wu T, Wei S. Higher carbohydrate antigen 125 levels are associated with increased risk of coronary heart disease in elderly Chinese: a population-based case-control study. PLoS One 2013;8:e81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yucel H, Kaya H, Zorlu A, Yıldırımlı K, Sancakdar E, Gunes H, Kurt R, Ozgul U, Turgut OO, Yilmaz MB. Cancer antigen 125 levels and increased risk of new-onset atrial fibrillation. Herz 2015;40:119–124. [DOI] [PubMed] [Google Scholar]
- 117. Sekiguchi H, Shimamoto K, Takano M, Kimura M, Takahashi Y, Tatsumi F, Watanabe E, Jujo K, Ishizuka N, Kawana M, Hagiwara N. Cancer antigen-125 plasma level as a biomarker of new-onset atrial fibrillation in postmenopausal women. Heart 2017;103:1368–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nasu K, Yuge A, Tsuno A, Narahara H. Simvastatin inhibits the proliferation and the contractility of human endometriotic stromal cells: a promising agent for the treatment of endometriosis. Fertil Steril 2009;92:2097–2099. [DOI] [PubMed] [Google Scholar]
- 119. Sokalska A, Wong DH, Cress A, Piotrowski PC, Rzepczynska I, Villanueva J, Duleba AJ. Simvastatin induces apoptosis and alters cytoskeleton in endometrial stromal cells. J Clin Endocrinol Metab 2010;95:3453–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Esfandiari N, Khazaei M, Ai J, Bielecki R, Gotlieb L, Ryan E, Casper RF. Effect of a statin on an in vitro model of endometriosis. Fertil Steril 2007;87:257–262. [DOI] [PubMed] [Google Scholar]
- 121. Sharma I, Dhawan V, Mahajan N, Saha SC, Dhaliwal LK. In vitro effects of atorvastatin on lipopolysaccharide-induced gene expression in endometriotic stromal cells. Fertil Steril 2010;94:1639–1646.e1. [DOI] [PubMed] [Google Scholar]
- 122. Soares SR, Martínez-Varea A, Hidalgo-Mora JJ, Pellicer A. Pharmacologic therapies in endometriosis: a systematic review. Fertil Steril 2012;98:529–555. [DOI] [PubMed] [Google Scholar]
- 123. Zhang Q, Liu X, Guo SW. Progressive development of endometriosis and its hindrance by anti-platelet treatment in mice with induced endometriosis. Reprod Biomed Online 2017;34:124–136. [DOI] [PubMed] [Google Scholar]
- 124. Guo SW, Ding D, Liu X. Anti-platelet therapy is efficacious in treating endometriosis induced in mouse. Reprod Biomed Online 2016;33:484–499. [DOI] [PubMed] [Google Scholar]
- 125. Wang L, Zhang J, Zhang H, Li R, Li C, Zhao X, Li M. Low-dose aspirin can downregulate progesterone resistance and increase the expression of LIF in endometriosis during the implantation window. Gynecol Endocrinol 2021;37:725–729. [DOI] [PubMed] [Google Scholar]
- 126. Long Q, Liu X, Qi Q, Guo SW. Chronic stress accelerates the development of endometriosis in mouse through adrenergic receptor β2. Hum Reprod 2016;31:2506–2519. [DOI] [PubMed] [Google Scholar]
- 127. Long Q, Zheng H, Liu X, Guo SW. Perioperative intervention by β-blockade and NF-κB suppression reduces the recurrence risk of endometriosis in mice due to incomplete excision. Reprod Sci 2019;26:697–708. [DOI] [PubMed] [Google Scholar]
- 128. Nenicu A, Körbel C, Gu Y, Menger MD, Laschke MW. Combined blockade of angiotensin II type 1 receptor and activation of peroxisome proliferator-activated receptor-γ by telmisartan effectively inhibits vascularization and growth of murine endometriosis-like lesions. Hum Reprod 2014;29:1011–1024. [DOI] [PubMed] [Google Scholar]
- 129. Nenicu A, Gu Y, Körbel C, Menger MD, Laschke MW. Combination therapy with telmisartan and parecoxib induces regression of endometriotic lesions. Br J Pharmacol 2017;174:2623–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang NY, Tsao CW; On behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]