Abstract
Aims
In coronavirus disease 2019 (COVID-19), myocardial injury is associated with systemic inflammation and higher mortality. Our aim was to perform a proof of concept trial with canakinumab, a monoclonal antibody to interleukin-1β, in patients with COVID-19, myocardial injury, and heightened inflammation.
Methods and results
This trial required hospitalization due to COVID-19, elevated troponin, and a C-reactive protein concentration more than 50 mg/L. The primary endpoint was time to clinical improvement at Day 14, defined as either an improvement of two points on a seven-category ordinal scale or discharge from the hospital. The secondary endpoint was mortality at Day 28. Forty-five patients were randomly assigned to canakinumab 600 mg (n = 15), canakinumab 300 mg (n = 14), or placebo (n = 16). There was no difference in time to clinical improvement compared to placebo [recovery rate ratio (RRR) for canakinumab 600 mg 1.15, 95% confidence interval (CI) 0.46–2.91; RRR for canakinumab 300 mg 0.61, 95% CI 0.23–1.64]. At Day 28, 3 (18.8%) of 15 patients had died in the placebo group, compared with 3 (21.4%) of 14 patients with 300 mg canakinumab, and 1 (6.7%) of 15 patients with 600 mg canakinumab. There were no treatment-related deaths, and adverse events were similar between groups.
Conclusion
There was no difference in time to clinical improvement at Day 14 in patients treated with canakinumab, and no safety concerns were identified. Future studies could focus on high dose canakinumab in the treatment arm and assess efficacy outcomes at Day 28.
Keywords: COVID-19, Myocardial injury, Canakinumab, Interleukin-1
Graphical Abstract
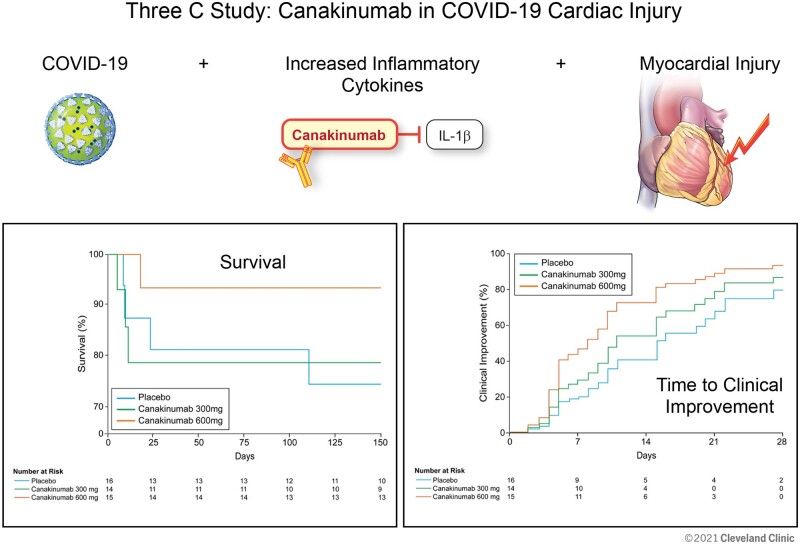
Graphical Abstract.
Introduction
Effective treatments for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), are limited.1–3 Although COVID-19 is predominantly a respiratory illness, cardiovascular complications can result in substantial morbidity and mortality.4,5 The incidence of myocardial injury, defined as a troponin above the 99th percentile upper reference limit, varies depending on the underlying comorbidities and severity of illness of the population studied but has been reported in as many as one-third of patients hospitalized with COVID-19.4–6 Furthermore, myocardial injury is associated with increased mortality.4–6
The causes of myocardial injury in COVID-19 are numerous and include critical illness with oxygen-supply demand mismatch, generation of a prothrombotic milieu, and endothelial dysfunction, among others.7 These distinct pathobiologies may result from an inappropriate systemic inflammatory response. In general, after the first week of illness, the onset of cardiac injury and heightened inflammation occurs, and immunosuppression may improve outcomes. In a randomized trial, broad immunosuppression with dexamethasone has been shown to lower mortality in patients with severe COVID-19 pneumonia,2 and observational case–control studies have suggested a potential benefit with targeted immune modulation with interleukin-1 (IL-1) antagonists.8
Canakinumab is a fully human monoclonal antibody neutralizing IL-1β with linear dose-dependent pharmacokinetics, a long elimination half-life of 26 days, and proven efficacy in auto-inflammatory syndromes as well as in patients with atherosclerotic disease and increased inflammation.9–11 In COVID-19, systemic inflammation and cardiac injury are correlated,4,5 and initial production of IL-1 results in an inappropriate feedback loop by inducing its own expression as well as the production of other proinflammatory cytokines.12 Given this mechanism of action and the putative role of an inappropriate innate immune response in COVID-19 associated cardiac injury, our hypothesis was that canakinumab would safely shorten time to recovery in patients with COVID-19, myocardial injury, and heightened inflammation. A favourable signal from this initial proof of concept trial could inform the design and conduct of larger studies.
Methods
Study design and participants
The Three C study is an investigator-initiated double-blind randomized placebo-controlled proof of concept trial conducted in five hospitals at a single institution (Cleveland Clinic) to assess for an early signal of efficacy (Supplementary material online, Appendix p1). Details of the trial design have been previously published.13 The trial was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice Guidelines, and the design and conduct were approved by the United States Food and Drug Administration. The trial was overseen by a data monitoring committee with details described in a separate charter. The data monitoring committee assessed the safety and made no formal assessment of efficacy. The protocol was approved by the Institutional Review Board, and written informed consent was provided by all patients or their legally authorized representative. Data management was centrally coordinated by the Cleveland Clinic Coordinating Center for Clinical Research (C5Research). Data were entered into a secure REDCap cloud database (https://www.redcapcloud.com), and analysis was performed by C5Research.
The study population included 45 patients with the first patient randomized on 28 April 2020 and the last patient randomized on 25 August 2020. Included patients were ≥18 years old, hospitalized for COVID-19 infection with a documented upper respiratory tract specimen positive for SARS-CoV2 RNA, had a troponin T > 99th percentile upper reference range, an NT-proBNP greater than the age-adjusted upper reference limit, and a C-reactive protein (CRP) >50 mg/L. The 5th generation Roche Troponin T assay was used (hsTnT), and a value ≥ 12 ng/L was considered abnormal. Key exclusion criteria included myocardial infarction (MI) according to the 4th universal definition of MI,14 uncontrolled systemic bacterial infection, mechanical ventilation for greater than 48 h, or haemodynamic instability requiring mechanical circulatory support (Supplementary material online, Appendix p 1–3). Patients with a prior history of MI or coronary artery disease (CAD) were included, and all patients had a 12-lead electrocardiogram and echocardiogram prior to enrolment.
Randomization and masking
Randomization was centralized through REDCap Cloud, and patients were randomized in a 1:1:1 allocation ratio to a single infusion of canakinumab 600 mg intravenous (IV), canakinumab 300 mg IV, and placebo with stratification by the hospital and whether or not the patient was intubated at the time of enrolment. One patient was randomized as non-intubated but was then intubated prior to administration of study infusion. All clinical and research personnel as well as participants were blinded to treatment assignment, except for a research pharmacist who prepared the canakinumab infusion or equal volume (250 mL) of 5% dextrose for placebo infusion. This research pharmacist did not participate in the administration of the infusion. The single intravenous infusion was given over 2 h. Enrolled patients received COVID-19 therapies considered appropriate by their clinicians, irrespective of their participation in the study.
Procedures
After randomization, the investigator was required to discontinue treatment if continuation would negatively impact a participant’s well-being. After study discontinuation, the participant would remain in the study unless consent was withdrawn. Reasons for study discontinuation could include subject or surrogate request, pregnancy, use of prohibited treatment (Supplementary material online, Appendix p 42), any safety risk to the subject, and any laboratory abnormalities that in the judgement of the investigator would prevent the subject from continuing. After randomization, adverse events and clinical status according to the ordinal scale were assessed daily until discharge and repeated at Day 14, Day 21, Day 28, Day 90, and Day 150. Concomitant medications were collected at baseline and daily thereafter until Day 7, at Day 14, Day 21, and Day 28. These included antibiotics related to non-COVID-19 infections, antivirals related to COVID-19, corticosteroids, use of convalescent plasma, and other immunosuppressive agents. Follow-up laboratory testing was performed according to clinical standard of care, in accordance with the institutional policy for caregiver safety and conservation of personal protective equipment and was therefore not uniform across all patients. Subsequent encounters after the initial visit included either in-person or telehealth encounters after the patient had been discharged.
Outcomes
The primary efficacy outcome was time to clinical improvement up to Day 14, defined as the time in days from randomization to either an improvement of two points on seven category ordinal scale or discharge from the hospital, whichever occurred first. This ordinal scale was simplified from an original model proposed by the World Health Organization by removing the category uninfected as this assessment may be difficult to document, as well as combining the categories of ventilation and ventilation plus additional organ support.15 The modified categories were: 1 not hospitalized with resumption of normal activities; 2 not hospitalized but unable to resume normal activities; 3 hospitalized, not requiring supplemental oxygen; 4 hospitalized, requiring supplemental oxygen; 5 hospitalized, requiring nasal high-flow oxygen, non-invasive mechanical ventilation, or both; 6 hospitalized, requiring extracorporeal membrane oxygenation, invasive mechanical ventilation, or both; 7 death. The secondary endpoint was all-cause mortality at Day 28. Exploratory endpoints included clinical status up to Day 28, the need for mechanical ventilation in non-intubated patients, length of hospitalization, changes in inflammatory markers, and mortality at Day 90 and Day 150. All adverse events were described according to severity (mild, moderate, and severe), relationship to study treatment, duration, whether it constitutes a serious adverse event, and any action taken (Supplementary material online, Appendix p 44–47).
Statistical analysis
Estimates of the efficacy of canakinumab in this patient population have not been established. Therefore, this trial was designed as a proof of concept study, and the sample size was based on feasibility and the intent to inform estimates and confidence intervals (CIs).16 Continuous variables were summarized using median, Q1 (25th percentile), and Q3 (75th percentile), and categorical variables were summarized using frequency and percentage. Adjustment according to intubation at randomization was performed for recovery rate ratios (RRR), as this was a stratification factor for randomization. Adjusted recovery rate ratios with 95% CIs were calculated from a Cox proportional hazards model. This RRR is similar to the hazard ratio (HR) in survival analysis, but for the beneficial outcome of clinical improvement. Therefore, a RRR greater than one indicates an improvement with canakinumab. This approach is aligned with prior randomized studies in patients with COVID-19.1 Hazard ratios for mortality were also calculated from a Cox proportional hazards model. Sub-group analyses were planned based on whether or not the patient was intubated at baseline, as intubated and non-intubated patient groups may represent distinct populations. An assessment for interaction between canakinumab and CRP, corticosteroid use, and remdesivir use with clinical improvement was also planned. Baseline was defined as clinical status at the administration of study infusion. Given the small size of the study, no estimates or intervals should be regarded as definitive for treatment effect, and no adjustments were made for multiple hypothesis testing. As noted in the statistical analysis plan (Supplementary material online, Appendix p 15), no inferential statistics are planned a priori as the intent is to provide data regarding effect size to inform feasibility of larger studies. DMB and KEW had access to raw data and performed statistical analyses. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) with graphics support from R Core Team (2018) (https://www.R-project.org Last Accessed 10 April 2021). The statistical analysis plan (Supplementary material online, Appendix p 7–21) and the study protocol (Supplementary material online, Appendix p 22–53) are available whereas other study documents, including individual patient-level data, are not. The trial is registered at clinicaltrials.gov (NCT0 4365153).
Role of the funding source
The funder of the study provided the study drug and assisted with the study design. The funder was not involved in data collection or analysis. The corresponding author (P.C.C.) had full access to all of the data and the final responsibility to submit for publication.
Results
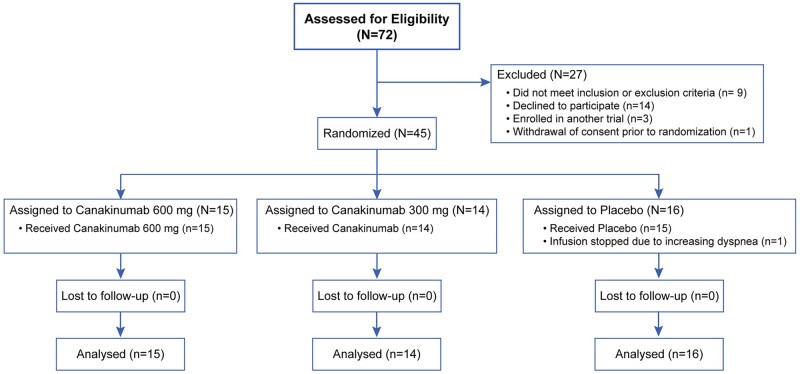
The trial population consisted of 45 patients (Figure 1). Overall, 15 subjects received 600 mg IV canakinumab, 14 subjects received 300 mg IV canakinumab, and 16 subjects received placebo. A majority were men, cardiovascular comorbidities were common, and over a third were African American (Table 1). Most patients had dyspnoea with significant hypoxaemia, and 10 patients were intubated at baseline. By chance, numerically more patients who received canakinumab 600 mg had diabetes mellitus [10 (66.7%) of 15] compared to placebo [7 (43.8%) of 16]. In addition, high-flow oxygen, non-invasive ventilation, or mechanical ventilation at baseline was more common in patients who received canakinumab 600 mg [8 (53.3%) of 15] or placebo [8 (50.0%) of 16] compared to canakinumab 300 mg [5 (35.7%) of 14]. Cardiac markers were mild to moderately elevated, whereas inflammatory markers were markedly elevated (Table 1). By chance, NT-proBNP and CRP were numerically higher whereas d-dimer was lower in placebo patients compared to patients who received canakinumab 600 mg. Prior to randomization, 21 (46.7%) of 45 patients were receiving corticosteroids including 10 (62.5%) of 16 patients who received placebo, 3 (21.4%) of 14 patients who received canakinumab 300 mg, and 8 (53.3%) of 15 patients who received canakinumab 600 mg. After randomization, an additional six patients were treated with corticosteroids including five patients who received canakinumab 300 mg and one patient who received canakinumab 600 mg. Before randomization, 21 (46.7%) of 45 patients were receiving remdesivir including 6 (37.5%) of 16 who received placebo, 5 (35.7%) of 14 who received canakinumab 300 mg, and 10 (66.7%) of 15 who received canakinumab 600 mg. After randomization, an additional five patients received remdesivir including one patient who received a placebo and four who received 300 mg of canakinumab. Four patients received convalescent plasma, including two patients who received a placebo and two patients who received 600 mg IV canakinumab.
Figure 1.
CONSORT flow diagram.
Table 1.
Baseline characteristics
| Total (n = 45) | Placebo (n = 16) | Canakinumab 300 mg (n = 14) | Canakinumab 600 mg (n = 15) | |
|---|---|---|---|---|
| Age (years) | 68.8 (61.1, 74.3) | 68.2 (56.1, 83.3) | 70.7 (64.7, 74.6) | 66.4 (63.5, 72.9) |
| Male | 33 (73.3%) | 13 (81.3%) | 9 (64.3%) | 11 (73.3%) |
| Female | 12 (26.7%) | 3 (18.8%) | 5 (35.7%) | 4 (26.7%) |
| African-American | 17 (37.8%) | 5 (31.3%) | 4 (28.6%) | 8 (53.3%) |
| Caucasian | 25 (55.6%) | 10 (62.5%) | 9 (64.3%) | 6 (40.0%) |
| Asian | 1 (2.2%) | 0 (0.0%) | 1 (7.1%) | 0 (0.0%) |
| Hispanic | 3 (6.7%) | 1 (6.3%) | 0 (0.0%) | 2 (13.3%) |
| Body mass index | 28.8 (25.3, 36.3) | 29.2 (24.0, 42.9) | 28.3 (25.8, 32.0) | 29.2 (28.0, 46.4) |
| Diabetes mellitus | 21 (46.7%) | 7 (43.8%) | 4 (28.6%) | 10 (66.7%) |
| Hypertension | 32 (71.1%) | 12 (75.0%) | 9 (64.3%) | 11 (73.3%) |
| Hyperlipidaemia | 29 (64.4%) | 9 (56.3%) | 8 (57.1%) | 12 (80.0%) |
| Coronary artery disease | 10 (22.2%) | 4 (25.0%) | 4 (28.6%) | 2 (13.3%) |
| Stroke | 2 (4.4%) | 1 (6.3%) | 1 (7.1%) | 0 (0.0%) |
| Atrial fibrillation or flutter | 6 (13.3%) | 2 (12.5%) | 2 (14.3%) | 2 (13.3%) |
| Chronic obstructive pulmonary disease | 8 (17.8%) | 2 (12.5%) | 3 (21.4%) | 3 (20.0%) |
| Chronic kidney disease | 15 (33.3%) | 7 (43.8%) | 2 (14.3%) | 6 (40.0%) |
| Current or former smoker | 15 (33.3%) | 6 (37.5%) | 4 (28.6%) | 5 (33.3%) |
| Time from symptom onset to randomization (days) | 7 (4,9) | 6 (4,10) | 9 (6,11) | 6 (3,8) |
| Dyspnoea | 36 (80.0%) | 14 (87.5%) | 11 (78.6%) | 11 (73.3%) |
| Temperature | 36.9 (36.6, 37.4) | 36.8 (36.5, 36.9) | 37.4 (36.9, 37.9) | 36.8 (36.6, 37.2) |
| Hospitalized requiring invasive mechanical ventilation | 10 (22.2%) | 3 (18.8%) | 2 (14.3%) | 5 (33.3%) |
| Hospitalized requiring nasal high-flow oxygen or non-invasive ventilation, or both | 11 (24.4%) | 5 (31.3%) | 3 (21.4%) | 3 (20.0%) |
| Hospitalized requiring supplemental oxygen | 20 (44.4%) | 6 (37.5%) | 9 (64.3%) | 5 (33.3%) |
| Hospitalized, not requiring supplemental oxygen | 4 (8.9%) | 2 (12.5%) | 0 (0%) | 2 (13.3%) |
| Baseline PaO2/FiO2 ratio | 148 (73, 204) | 117 (66, 210) | 160 (77, 246) | 148 (73, 203) |
| Baseline SOFA scores | 3 (2, 5) | 4 (2, 5) | 2 (2, 4) | 3 (2, 6) |
| Corticosteroids | 21 (46.7%) | 10 (62.5%) | 3 (21.4%) | 8 (53.3%) |
| Remdesivir | 21 (46.7%) | 6 (37.5%) | 5 (35.7%) | 10 (66.7%) |
| High-sensitivity troponin T (ng/L) (reference range <12 ng/L) | 22 (15, 37) | 32 (16, 163) | 25 (14, 36) | 21 (15, 31) |
| N-terminal pro B-type natriuretic peptide (pg/mL) (reference range <125 pg/mL) | 479 (248, 1508) | 810 (349, 10 264) | 372 (277, 1508) | 371 (181, 1401) |
| C reactive protein (mg/L) (reference range 0–4 mg/L) | 153 (121, 185) | 176 (150, 199) | 122 (64, 153) | 127 (108, 197) |
| White blood cell count (reference range 3.70–11.0 k/μL) | 7.5 (5.5 9.9) | 7.8 (6.0, 10.7) | 6.2 (5.1, 8.4) | 7.8 (5.6, 11.0) |
| Neutrophil count (reference range 1.45–7.50 k/μL) | 5.7 (4.4, 8.7) | 6.9 (4.5, 9.1) | 5.2 (4.1, 8.2) | 5.4 (4.4, 9.2) |
| Lymphocyte count | 0.7 (0.5, 1.0) | 0.5 (0.4, 0.9) | 0.7 (0.5, 1.2) | 0.8 (0.7, 1.0) |
| (reference range 1.0–4.0 k/μL) | ||||
| Neutrophil/lymphocyte | 8.9 (5.5, 14.6) | 13.5 (5.5, 22.3) | 9.6 (5.6, 10.9) | 7.5 (5.4, 11.9) |
| Ferritin (ng/mL) (reference range 14.7–205.1 ng/mL) | 1015 (589, 2111) | 1246 (768, 2355) | 998 (857, 1626) | 740 (448, 1969) |
| D-dimer (ng/mL) (reference range <500 ng/mL) | 1320 (730, 2140) | 1500 (560, 3340) | 950 (730, 1340) | 1795 (870, 3430) |
| Left ventricular ejection fraction (%) | 60 (55, 60) | 58 (43, 63) | 50 (55, 65) | 55 (45, 60) |
Expressed as n (%) or median (Q1, Q3)
SOFA, sequential organ failure assessment.
The primary endpoint of time to clinical improvement by Day 14 was not different between patients who received canakinumab 600 mg IV (RRR 1.15, 95% CI 0.46–2.91) or 300 mg IV canakinumab (RRR 0.61, 95% CI 0.23–1.64) compared with placebo (Table 2). Through Day 14, 9 (56.3%) of 16 placebo patients demonstrated clinical improvement, compared with 7 (50.0%) of 14 who received 300 mg canakinumab, and 9 (60.0%) of 15 who received 600 mg canakinumab (Figure 3). Among 10 patients intubated prior to study infusion, including 3 patients who received placebo, 2 patients who received canakinumab 300 mg, and 5 who received canakinumab 600 mg, none had achieved clinical improvement by Day 14.
Table 2.
Efficacy outcomes
| Endpoint | Placebo | Canakinumab 300 mg (n = 14) | Canakinumab 600 mg (n = 15) |
|---|---|---|---|
| (N = 16) | |||
| Primary endpoint, n (%) | |||
| Clinical improvement or discharge at Day 14 | 9 (58.3) | 7 (58.1) | 9 (60) |
| RRR (CI) | 0.61 (0.23, 1.64) | 1.15 (0.46, 2.91) | |
| Secondary endpoints, n (%) | |||
| Mortality at Day 28 | 3 (18.8) | 3 (21.4) | 1 (6.7) |
| HR (CI) | 1.21 (0.23, 5.59) | 0.20 (0.02, 1.98) | |
| Exploratory endpoint, n (%) | |||
| Clinical improvement or discharge at Day 28 | 11 (68.8) | 11 (78.6) | 14 (93.5) |
| RRR (CI) | 1.44 (0.57–3.62) | 2.10 (0.89–4.99) |
HR, hazard ratio; OR, odds ratio; RRR, recovery rate ratio.
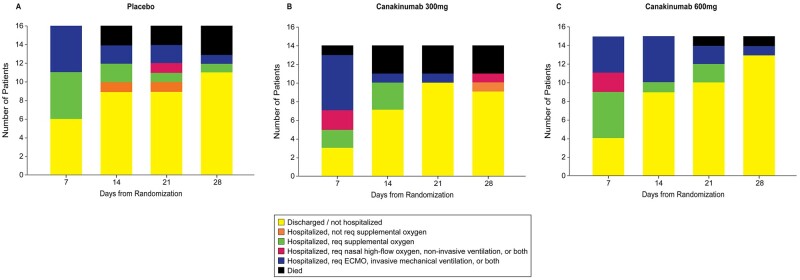
Figure 3.
Exploratory endpoint of proportion of patients in categories during follow-up at 28 days.
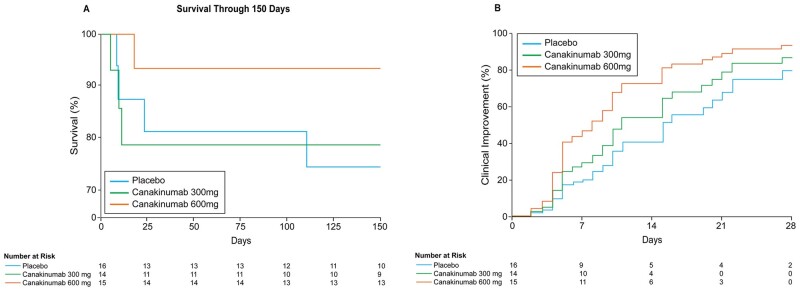
The secondary endpoint of all-cause mortality occurred in 7 (15.6%) of 45 patients by Day 28. In the placebo group, 3 (18.8%) of 15 patients died, compared with 3 (21.4%) of 14 patients who received 300 mg IV canakinumab (HR 1.12, 95% CI 0.23–5.59), and 1 (6.7%) of 15 patients who received 600 mg IV canakinumab (HR 0.20, 95% CI 0.02–1.99) (Table 2). Among patients intubated prior to study infusion, 2 (66.7%) of 3 placebo patients, 1 (50.0%) of 2 patients who received canakinumab 300 mg, and 1 (20.0%) of 5 patients who received canakinumab 600 mg died. In patients not intubated prior to study infusion, 1 (7.7%) of 13 patients who received placebo, 2 (16.7%) of 12 patients who received canakinumab 300 mg, and 0 (0.0%) of 10 patients who received canakinumab 600 mg died. At study completion at Day 150, overall mortality was similar with deaths in 4 (25.0%) of 16 patients who received placebo, 3 (21.4%) of 14 patients who received canakinumab 300 mg, and 1 (6.7%) of 15 patients who received canakinumab 600 mg (Figure 2A).
Figure 2.
Exploratory endpoints of survival at study completion (150 days) (A) and time to clinical improvement at Day 28 (B).
With respect to the exploratory endpoint of clinical status at Day 28, the RRR for patients who received 600 mg IV canakinumab compared to placebo was 2.10 (95% CI 0.89–4.99). On Day 28, the RRR for patients who received canakinumab 300 mg IV compared to placebo was 1.44 (95% CI 0.57–3.62) (Figure 2B). At Day 28, 11 (68.8%) of 16 patients who received placebo demonstrated clinical improvement, compared with 11 (78.6%) of 14 patients who received 300 mg IV canakinumab, and 14 (93.3%) of 15 patients who received 600 mg IV canakinumab (Table 2 and Figure 3). In patients intubated prior to study infusion, 0 (0.0%) of three patients who received placebo, 1 (50.0%) of two patients who received 300 mg canakinumab, and 4 (80.0%) of five patients who received canakinumab 600 mg achieved clinical improvement at Day 28. Among patients not intubated at baseline, 11 (88.5%) of 13 patients who received placebo, 10 (83.3%) of 12 patients who received canakinumab 300 mg, and 10 (100%) of 10 patients who received canakinumab 600 mg demonstrated clinical improvement at Day 28.
In patients not intubated at baseline, 3 (23.1%) of 13 patients who received placebo, 6 (50.0%) of 12 patients who received 300 mg canakinumab, and 1 (10.0%) of 10 patients who received 600 mg canakinumab subsequently required mechanical ventilation.
When assayed, CRP values declined numerically in patients who received 600 mg IV canakinumab compared with placebo. A subset of patients including eight patients who received placebo, eight patients who received 300 mg IV canakinumab, and eight patients who received 600 mg IV canakinumab had CRP values at baseline and on Day 7. At Day 7, compared with placebo (125, Q1 25, Q3 236 mg/L), patients who received canakinumab 300 mg IV (68, Q1 22, Q3 102 mg/L) and 600 mg IV canakinumab (34, Q1 16, Q3 87 mg/L) had numerically lower CRPs (Supplementary material online, Appendix p4). There was no significant interaction between baseline CRP, different canakinumab doses, and clinical improvement at 14 or 28 days (P = 0.92 and 0.95, respectively). In addition, because the study was performed during a time of limited resources due to the pandemic, patients did not return to the hospital after discharge, and laboratory testing was done according to standard of care. Therefore, changes in PaO2 to FiO2 ratios, SOFA scores, inflammatory markers, and time to negative SARS-CoV-2 RNA concentrations were not reported due to missing data.
As noted, many of the patients were treated with corticosteroids and remdesivir. There was no significant interaction between corticosteroids, different canakinumab doses, and clinical improvement at 14 or 28 days (P = 0.17 and 0.64, respectively). Likewise, there was no significant interaction between remdesivir, different canakinumab doses, and clinical improvement at 14 or 28 days (P = 0.57 and 0.59, respectively).
Adverse events were similar between the three groups. One patient who received placebo developed Candida glabrata fungemia, and one patient who received 300 mg IV canakinumab developed Staphyloccocus aureus bacteraemia. Abnormal laboratory values related to blood counts and liver chemistry tests were also similar among the groups. The infusion was completed in all patients except for one placebo patient where a clinical decision was made to stop the infusion due to increasing dyspnoea. There were no treatment-related deaths.
Discussion
The Three C Study was a proof of concept trial to assess for early efficacy regarding whether IL-1β inhibition with canakinumab would improve outcomes in patients with COVID-19, myocardial injury, and heightened inflammation. Among COVID-19 randomized controlled trials, this study is unique in that it exclusively evaluates a population at high risk, patients with elevated troponins and increased systemic inflammation. As noted, CRPs declined in patients who received canakinumab consistent with the known mechanism of action. Across all groups, 14 days outcomes were similar, which may be partly explained because no patients intubated at baseline demonstrated clinical improvement within this timeframe by Day 28, patients who received 600 mg IV canakinumab were numerically more likely to have clinical improvement. However, as emphasized, the results of this study are hypothesis-generating, and larger studies in this patient population could evaluate higher dose canakinumab with an assessment for outcomes at Day 28.
In COVID-19, the SARS-CoV2 virus and destruction of host cells leads to systemic activation of the innate immune response.17 With severe or critical COVID-19 disease, overwhelming immune activation often occurs, sometimes termed a cytokine storm. Importantly, the pathobiology of a cytokine storm begins with autoinduction of IL-1.12 IL-1β is considered the initial cytokine of the innate immune response, drives its own gene expression, and leads to further cytokine and chemokine production.18 This response leads to recruitment of inflammatory cells, pyroptosis (inflammatory-mediated cell death), and a downstream acute phase response. The consequences of this cascade are endothelial cell dysfunction with capillary leak, thrombosis, and local tissue injury. In observational studies, the association between elevated troponin, high CRP, and increased mortality is consistent with this mechanism of injury.4,5 Therefore, even though the clinical manifestations of myocardial injury in COVID-19 are protean,7 a common inciting event may be an inappropriate innate immune response.
Given the scale of the pandemic, relatively few randomized controlled trials have been published, and our current understanding of the expected disease course and timing of potential therapeutic interventions in specific populations remains limited. As an example, in a large randomized trial of remdesivir in COVID-19, the primary outcome was changed from time to recovery at 15–28 days, as data emerged that hospitalized patients with COVID-19 have a more protracted illness.1 Our results are concordant and suggest that patients with COVID-19 and myocardial injury, especially if they require mechanical ventilation, may be unlikely to demonstrate clinical improvement within 2 weeks, as none of the patients intubated at baseline in the current study demonstrated clinical improvement by Day 14. Therefore, future studies in this patient population should emphasize a longer follow-up period for the outcome of clinical improvement.
The timing of intervention in specific phases of COVID-19 is also important. Even though broad immunosuppression with dexamethasone has been shown to lower mortality in patients with COVID-19 and hypoxaemia, particularly in patients receiving mechanical ventilation,2 randomized controlled trials with IL-6 antagonists have demonstrated conflicting results.3,19–21 These results may be related to different patterns of immunopathology in COVID-19;22 however, these trials have not consistently required increased systemic inflammation for inclusion, or have excluded patients that are intubated or required more than 10 L/min of supplemental oxygen. Similarly, the CAN-COVID study of canakinumab in COVID-19 pneumonia required a CRP of only greater than 20 mg/L and excluded patients who were receiving mechanical ventilation (NCT04362813). Therefore, the extent to which these trials targeted the patient population most likely to benefit remains uncertain.
In a Phase II study of patients with periodic fever syndromes, the highest dose of canakinumab administered was 10 mg/kg IV.23 Although our study is small, no safety signals were observed with higher doses of canakinumab, and higher doses may be necessary for efficacy. In addition, high doses of anakinra (100 mg loading dose, followed by 72-h IV infusion of 2.0 mg/kg/h) have been evaluated in a Phase III study of sepsis without a difference in adverse events.24
By design, our study is small, hypothesis-generating, and subject to Type II error. In a small randomized trial, by chance, baseline characteristics may also not be well balanced. The possibility that an imbalance of baseline variables may have impacted the results, especially given between-group differences in the magnitude of respiratory support and inflammatory markers, should be noted. Moreover, when the protocol was developed, no observational data were available to inform the possible efficacy of canakinumab in COVID-19. Accordingly, no power calculations were performed. Likewise, all assessments of interaction analyses with treatment and outcome are underpowered. In addition, limited data regarding prognosis in patients with COVID-19 and cardiac injury were available during the study design. After enrolment began in our study, a large observational study demonstrated that most patients with COVID-19 associated cardiac injury have mild to moderate elevations in troponin and mortality was 18.5%,6 similar to patients in the Three C study.
Furthermore, we had initially planned exploratory analyses evaluating changes in inflammatory markers, but laboratory studies were obtained according to standard of care and varied. However, this study was conducted during a time of resource limitation with appropriate emphasis on the protection of healthcare workers and the conservation of personal protective equipment. Accordingly, patient contact, and thus blood draws, were restricted to clinical care. In addition, due to infection control considerations, patients did not return to the hospital solely for laboratory testing after discharge. Nonetheless, limited data were suggestive of a dose–response reduction in CRP with canakinumab, consistent with the known mechanism of action. Little is also known about the differential prognostic implications of acute compared to chronic non-ischemic myocardial injury in COVID-19,25 and for inclusion, we required a single abnormal troponin. In an ad hoc review of our patients, 34 had more than one hsTnT assayed, and 23 of these patients had a change in troponin >20% compatible with acute myocardial injury. Finally, COVID-19 therapies, such as corticosteroids and remdesivir, were evolving during the study and were employed heterogeneously according to changing standards of care. In future studies, standard COVID-19 therapies may be more established.
Given the high morbidity and mortality of patients with COVID-19, myocardial injury, and increased inflammation, effective treatments are required. At Day 28, patients treated with a single high dose of canakinumab were numerically more likely to have clinical improvement. Based on our results, future studies of patients with COVID-19 and myocardial injury should focus on patients with markedly elevated systemic inflammation, employ high dose canakinumab in the treatment arm, and assess efficacy outcomes at Day 28, given a longer expected duration to demonstrate clinical improvement in these severely and critically ill patients.
Lead author biography

Paul C. Cremer MD is a cardiologist and cardiovascular imager at the Cleveland Clinic where he is the associate director of the cardiac intensive care unit and the cardiovascular training program. Dr Cremer earned a Bachelor’s degree in molecular biology from Princeton University, and his medical degree from Harvard Medical School. Following completion of his internal medicine residency at Massachusetts General Hospital, he continued his postdoctoral training with a 3-year fellowship in cardiovascular medicine and a subsequent 2-year fellowship in advanced cardiovascular imaging, both at Cleveland Clinic. His clinical and research interests include auto-immune and auto-inflammatory cardiovascular disease.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
This study was funded by an investigator-initiated grant from Novartis.
Conflict of interest: Paul Cremer has served on scientific advisory committees for Sobi and Kiniksa pharmaceuticals and has received investigator-initiated grants from Kiniksa and Novartis pharmaceuticals.
Data availability: The data underlying this article will be shared on reasonable request to the corresponding author
Clinical Trial Registration
NCT04365153, IND 149328, Canakinumab in COVID-19 Cardiac Injury (The Three C Study).
Supplementary Material
Contributor Information
Paul C Cremer, Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA.
Calvin C Sheng, Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA.
Debasis Sahoo, Department of Pulmonary Medicine, Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA.
Siddharth Dugar, Department of Pulmonary Medicine, Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA.
Robier Aguillon Prada, Department of Pulmonary Medicine, Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA.
Tom Kai Ming Wang, Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA.
Ossama K Abou Hassan, Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA.
Jamie Hernandez-Montfort, Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA.
David A Wolinsky, Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA.
Daniel A Culver, Department of Pulmonary Medicine, Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA.
Prabalini Rajendram, Department of Pulmonary Medicine, Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA.
Abhijit Duggal, Department of Pulmonary Medicine, Respiratory Institute, Cleveland Clinic, Cleveland, OH, USA.
Danielle M Brennan, Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA; C5 Research, Cleveland Clinic, Cleveland, OH, USA.
Katherine E Wolski, Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA; C5 Research, Cleveland Clinic, Cleveland, OH, USA.
A Michael Lincoff, Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA.
Steven E Nissen, Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA.
Venu Menon, Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA.
References
- 1. Biegel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19: preliminary report. N Engl J Med 2020;383:1813–1826. [DOI] [PubMed] [Google Scholar]
- 2. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19: preliminary report. N Engl J Med 2020;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med 2021;384:1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, Chaudhry F, De Freitas JK, Fayad ZA, Pinney SP, Levin M, Charney A, Bagiella E, Narula J, Glicksberg BS, Nadkarni G, Mancini DM, Fuster V. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 2020;76:533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pericas JM, Hernandez-Meneses M, Sheahan TP, et al. COVID-19: from epidemiology to treatment. Eur Heart J 2020;41:2092–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ucciferri C, Auricchio A, Di Nicola M, Potere N, Abbate A, Cipollone F, Vecchiet J, Falasca K. Canakinumab in a sub-group of patients with COVID-19. Lancet Rheumatol 2020;2:e457–ee458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chakraborty A, Tannenbaum S, Rordorf C, et al. Pharmacokinetic and pharmacodynamic properties of canakinumab, a human anti-interleukin-1β monoclonal antibody. Clin Pharmacokinet 2012;51:e1–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, Gitton X, Widmer A, Patel N, Hawkins PN. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med 2009;360:2416–2425. [DOI] [PubMed] [Google Scholar]
- 11. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 12. Libby P, Luscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J 2020;41:3038–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sheng CC, Sahoo D, Dugar S, Prada RA, Wang TKM, Abou Hassan OK, Brennan D, Culver DA, Rajendram P, Duggal A, Lincoff AM, Nissen SE, Menon V, Cremer PC. Canakinumab to reduce deterioration of cardiac and respiratory function in SARS-CoV-2 associated myocardial injury with heightened inflammation (canakinumab in COVID-19 cardiac injury: the three C study). Clin Cardiol 2020;43:1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018;72:2231–2264. [DOI] [PubMed] [Google Scholar]
- 15. WHO. R&D blueprint COVID-19 therapeutic trial synopsis. http://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/ (10 April 2021).
- 16. Bacchetti P. Current sample size conventions: flaws, harms, and alternatives. BMC Med 2010;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res 2016;118:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stone JH, Frigault M, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with COVID-19. N Engl J Med 383:2333–2344. doi:10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021;384:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomized, controlled, open-label, platform trial. Lancet 397:1637–45. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jamilloux Y, Henry T, Belor A, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmune Rev 2020;19:102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood J Am Soc Hematol 2011;117:3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Charles J. Fisher, Jean-Francois A. Dhainaut, Steven M. Opal, John P. Pribble, Robert A. Balk, Gus J. Slotman, Thomas J. Iberti, Eric C. Rackow, Marc J. Shapiro, Richard L. Greenman, H. David Reines, Maire P. Shelly, Bruce W. Thompson, John F. LaBrecque, Michael A. Catalano, William A. Knaus, Jerald C. Sadoff, Mark Astiz, Charles Carpati, Roger C. Bone, Bruce Friedman, Anthony J. Mure, Collin Brathwaite, Eugenia Shapiro, Laura Melhorn Robert Taylor, Mary Keegan, Jacklyn O'Brien, Roland Schein, Maria Pena, Monina Wasserlouf, John Oropello, Ernest Benjamin, Rosanna DelGuidice, George Emmanuel, Thiam Lie, Lynn Anderson, John Marshall, Wilfred De Majo, Ori Rotstein, Debra Foster, Edward Abraham, Harold Middleton, Cindy Perry, Howard Levy, Donald E. Fry, Steven Q. Simpson, Richard E. Crowell, Mary Neidhart, Dennis Stevens, Thomas Coffman, Nagraj Narasimhan, David K. Merrick, William Bergquist, Klaus E. Matzel, Matthias Huebler, Garrett E. Foulke, Timothy E. Albertson, William F. Walby, Roblee P. Allen, Robert Baughman, Per-Olof Hasselgren, Mitchell P. Fink, Felicia Favorito, B. Taylor Thompson, Richard Corbin, G. Yovonne Shellhorse, Arnold Frazier, Sandy White, Christopher Garrard, Christine A'Court, Shirley Storer, Daniel H. Gervich, Debra Foshe, Rainer Brase, Andreas Bagdahn, Robert Cooney, J. Stanley Smith Jr, Louis F. Martin, Jean-Louis Vincent, Gilberto Friedman, Giorgio Berlot, J. Raymond Fletcher, Mark D. Williams, Theresa F. Wright, Steven Johnson, Carinda Feild, Karen Wolf, Neil MacIntyre, Howard G. Dubin, Margaret R. Durkin, Penelope K. Dubin, Karl Hermann Staubach, Alan M. Fein, Debra B. Schulman, Michael S. Niederman, Donald B. Chalfin, Paul A. M. van Leeuwen, Marja A. Boermeester, Anton J. Schneider, Joseph Bander, Amy Imm, Gordon Bernard, Loren Nelson, Mary Stroud, Karen Safcsak, Frank Cerra, Jean Rindal, Henry Mann, Neil Halpern, Jeffrey Silverstein, Margarita Alicea, William J. Sibbald, Claudio M. Martin, Frank S. Rutledge, Kathryn Petti, James A. Russell, Robert Kruger, Alana Drummond, Paul Lange, Tracy Seifert, MSN, Alain Durocher MD, Alain Tenaillon, Richard Boiteau, Thierry Lherm, Stephen F. Lowry, Susette M. Coyle, Philip S. Barie, Eric DeMaria, David R. Snydman, Steven D. Schwaitzberg, Stanley A. Nasraway Jr, Jeanne Grindlinger, Warren Summer, Benjamen de Boisblanc, Martin Wahl, Kjell Alestig, Jeffrey Grossman, Dennis Maki, Harold L. Paz, Martha Weiner, David Bihari, David Campbell, Gerard Bleichner, Michael S. Dahn, M. Patricia Lange, Jesse Hall, Anne Pohlman, Richard P. Wenzel, Mark Grosserode, Michele Costigan, William Mileski, John Weigelt, Neil Yeston, Cheryl Irizarry, Jack Ross, James Robbins, Peter Nightingale, Kate Owen, Stefan Sandstedt, Soren Berg, Gary L. Simon, Michael G. Seneff, Kathleen M. Conry, Janice L. Zimmerman, R. Phillip Dellinger, Robert Johnston Jr, Patricia Allee, Per-Olof Grande, Erling Myhre, Jean-Francois Dhainaut, Isabelle Hamy, Jean-Paul Mira, John Harmon, Jon White, Lloyd McKie, Henry Silverman, Pamela Tuma, David Bennett, J. C. Porter, Martin H. Laurell, Sidney Jacobs, Stephen Ash, David M. Stiles, Mary Jane Prior, Genell Knatterud, Michael Terrin, Joseph Kufera, Patricia Wilkens, Knut Ra, Lee Monroe, Charles Sprung, Cannon Michael, Richard Matthay, William McCabe, James Tonascia, Herbert Wiedeman, Janet Wittes, Genell Knatterud, Giles V. Campion, Carol R., Richard Lustick, Janice Lookabaugh, Gilad S. Gordon, Leslie Noe, Duane Bloedow, Smith G., Dennis Brannon, Rebecca Kush, David Ng, Elizabeth Moore, Karen Bazemore, Michael Galvan, Douglas Wagner, Frank Harrell, Dona Stablein RN Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome: results from a randomized, double-blind, placebo-controlled trial. JAMA 1994;271:1836–1843. [PubMed] [Google Scholar]
- 25. Sandoval Y, Januzzi JL, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19. J Am Coll Cardiol 2020;76:1244–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.