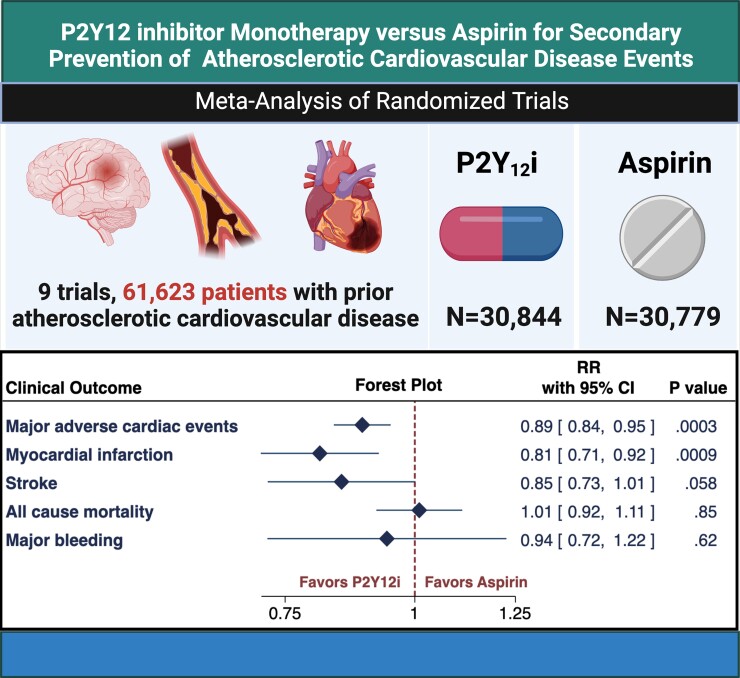
Abstract
Aim
To compare the efficacy and safety of P2Y12 inhibitor or aspirin monotherapy for secondary prevention in patients with atherosclerotic cardiovascular disease (ASCVD).
Methods and results
Medline, Embase, and Cochrane Central databases were searched to identify randomized trials comparing monotherapy with a P2Y12 inhibitor versus aspirin for secondary prevention in patients with ASCVD (cardiovascular, cerebrovascular, or peripheral artery disease). The primary outcome was major adverse cardiac events (MACE). Secondary outcomes were myocardial infarction (MI), stroke, all-cause mortality, and major bleeding. A random-effects model was used to calculate risk ratios (RR) and the corresponding 95% confidence interval (CI) and heterogeneity among studies was assessed using the Higgins I2 value. A total of 9 eligible trials (5 with clopidogrel and 4 with ticagrelor) with 61 623 patients were included in our analyses. Monotherapy with P2Y12 inhibitors significantly reduced the risk of MACE by 11% (0.89, 95% CI 0.84–0.95, I2 = 0%) and MI by 19% (0.81, 95% CI 0.71–0.92, I2 = 0%) compared with aspirin monotherapy. There was no significant difference in the risk of stroke (0.85, 95% CI 0.73–1.01), or all-cause mortality (1.01, 95% CI 0.92–1.11). There was also no significant difference in the risk of major bleeding with P2Y12 inhibitor monotherapy compared with aspirin (0.94, 95% CI 0.72–1.22, I2 = 42.6%). Results were consistent irrespective of the P2Y12 inhibitor used.
Conclusion
P2Y12 inhibitor monotherapy for secondary prevention is associated with a significant reduction in atherothrombotic events compared with aspirin alone without an increased risk of major bleeding.
Keywords: Atherosclerotic cardiovascular disease, Myocardial infarction, Stroke, Antiplatelet agents, Aspirin, P2Y12 inhibitors
Graphical Abstract
Graphical Abstract.
A meta-analysis of 9 randomized trials (61 623 patients) was conducted to compare P2Y12 inhibitor monotherapy versus aspirin monotherapy for secondary prevention of cardiovascular events in patients with established atherosclerotic cardiovascular disease (coronary, cerebrovascular, or peripheral artery disease). The included studies had follow-up periods between 3 and 36 months. Monotherapy with P2Y12 inhibitors (clopidogrel or ticagrelor) significantly reduced the risk of MACE by 11% (0.89, 95% CI 0.84–0.95, I2 = 0%) and MI by 19% (0.81, 95% CI 0.71–0.92, I2 = 0%) compared with aspirin monotherapy. There was no significant difference in the risk of stroke, all-cause mortality, or major bleeding. Subgroup analysis revealed that the reduction in MACE with P2Y12 inhibitors was driven by a reduction in recurrence of the qualifying event. CI = confidence interval, P2Y12i = P2Y12 inhibitor, RR = risk ratio.
Introduction
Antiplatelet therapy is the cornerstone for the prevention and treatment of atherothrombosis.1–3 Aspirin is the most widely used antiplatelet agent for the prevention of cardiovascular events in patients with atherosclerotic cardiovascular disease.4,5 P2Y12 inhibitors (e.g. clopidogrel, prasugrel, and ticagrelor), when combined with aspirin, provide greater antiplatelet effect and higher efficacy at preventing atherothrombotic events in patients with acute coronary syndromes or in those undergoing percutaneous coronary interventions (PCI).6–9 In the chronic phase of secondary prevention (i.e. after guideline-recommended duration of dual antiplatelet therapy is completed), P2Y12 inhibitors are often discontinued, and aspirin monotherapy is continued for long-term prevention of cardiovascular events. This preferential use of aspirin stems at least partly from insufficient evidence about the risks and benefits of P2Y12 inhibitor monotherapy compared with aspirin monotherapy.
Accordingly, we undertook a systematic review and meta-analysis of randomized trials to compare the efficacy and safety of P2Y12 inhibitor monotherapy versus aspirin monotherapy for secondary prevention of cardiovascular events in patients with atherosclerotic cardiovascular disease, including coronary, cerebrovascular, or peripheral arterial disease.
Methods
Search strategy and study characteristics
We conducted a comprehensive literature search of multiple electronic databases (Medline, EMBASE, and Cochrane Central) from inception to June 12, 2021. We used the following search terms: ‘clopidogrel’, ‘ticagrelor’, ‘prasugrel’, ‘thienopyridine’, ‘antiplatelet’, ‘aspirin’, ‘acetylsalicylic acid’, ‘prevention’, and ‘monotherapy’ to capture relevant citations. No language restrictions were applied. Presentations at major national cardiovascular meetings and bibliographies of relevant articles were also reviewed. All citations were imported into Covidence10 and duplicate citations were removed prior to title and abstract review. All studies were screened by 2 reviewers (D.A. and Z.C.) and relevant studies were identified for the full-text review. If there was discordance among reviewers regarding the inclusion of a study in the final analysis, a third reviewer was consulted to reach a consensus (A.Q.). All randomized trials reporting clinical outcomes comparing P2Y12 inhibitor monotherapy with aspirin monotherapy for secondary prevention in patients with the established atherosclerotic vascular disease were included in this analysis. Trials with a sample size below 100 participants, trials using ticlopidine in the P2Y12 inhibitor arm, and trials with a duration of monotherapy of less than 30 days were excluded. Observational or registry studies were also excluded. Full-texts of all the included trials were then reviewed for inclusion in the final meta-analysis. This review was registered with PROSPERO (CRD 42021260714).
Outcome measures
The primary efficacy outcome of interest was major adverse cardiovascular events (MACE). In the majority of studies, MACE was defined as a composite of stroke, myocardial infarction (MI), or death. The primary safety endpoint was major bleeding. Supplementary material online, Tables S1 and S2 describe the definitions of MACE and major bleeding outcomes used across the trials. Secondary outcomes assessed included MI, stroke (ischemic/hemorrhagic), and all-cause mortality. Data for the primary and secondary outcomes were extracted by two authors (D.A. and Z.C.) independently using pre-specified electronic forms. Additionally, data on the duration of monotherapy, dosage of aspirin/P2Y12 inhibitor, qualifying events, and baseline characteristics of the trial participants were extracted individually.
Statistical analysis
Pooled risk ratios (RR) and the corresponding 95% confidence intervals (CI) were calculated for the primary and secondary outcomes using the DerSimonian and Laird random-effects model.11 We also performed pre-specified subgroup analysis based on the P2Y12 inhibitor used and the qualifying atherothrombotic disease (cardiovascular, cerebrovascular, or peripheral artery disease). Additionally, among studies where the qualifying atherothrombotic disease was stable coronary disease or prior acute coronary syndrome, we evaluated the effect of prior revascularization (PCI, coronary artery bypass grafting [CABG], or mixed) on the thrombotic and safety outcomes. Significant differences between the various subgroups were evaluated using the Qb statistic.12 Heterogeneity among studies was assessed using the Higgins I2 value.12 I2 values of <25%, 25–75%, and >75% were considered to represent low, moderate, and high levels of heterogeneity, respectively. A random-effects meta-regression analysis using the empirical Bayes method was conducted to evaluate the association of trial-level variables with the primary efficacy and safety outcomes. Meta-regression model variables were selected a priori and included the duration of follow-up with monotherapy, dosage of aspirin, and the baseline risk in the aspirin arm (expressed as a ratio of event/non-event). Among trials reporting a range of aspirin dose, the higher end of the dose range was considered for the regression model. We also conducted a leave-one-out sensitivity analysis to evaluate the effect of individual trials on the pooled primary and secondary endpoints and to exclude the possibility of a single trial disproportionately affecting the overall outcome. Publication bias and small study bias were assessed visually with funnel plots and Egger’s regression test. All p-values were two-tailed with statistical significance specified at 0.05 and CI reported at 95% level. Stata version 16 (StataCorp, College Station, Texas) and R package, Metafor, version 3.6.2 (R Foundation) were used for all statistical analyses. The number needed to treat (NNT) was calculated using the pooled RRs. The risk of bias and study quality was assessed using the revised Cochrane risk-of-bias tool.13 Two authors independently assessed (K.B. and V.J.) each study using 5 domains of bias: (i) randomization process, (ii) deviations from intended interventions, (iii) missing outcomes data, (iv) measurement of the outcome, and (v) selection of the reported results. Each individual trials’ overall bias was reported as low risk, some concern, or high risk.
Results
Study characteristics
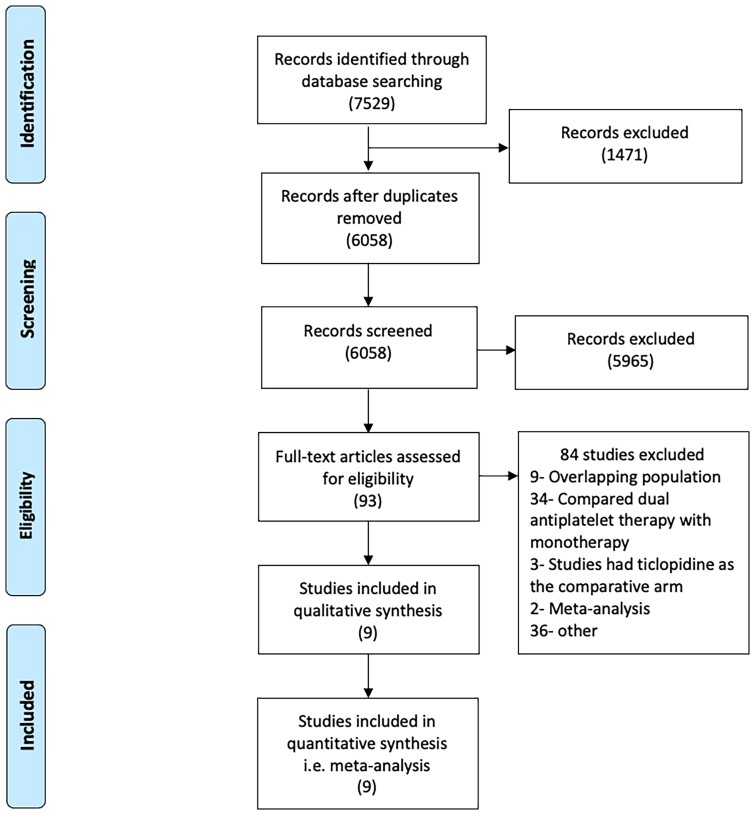
Of the 6058 results identified in the initial search, 9 randomized trials were selected for the analyses after step-wise review (Figure 1).14–22 The design and baseline characteristics of the individual trials are described in Table 1. Five studies compared aspirin with clopidogrel while 4 studies compared aspirin with ticagrelor. Six trials enrolled patients with coronary artery disease including 1 study that randomized patients with previous MI, one with chronic coronary syndromes, 2 with patients after PCI with drug-eluting stent placement, and 2 after CABG. Two studies enrolled patients after a stroke or transient ischemic attack and only 1 study, the Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial,14 included patients with a history of ischemic stroke, prior MI, or symptomatic peripheral artery disease. The included studies had follow-up periods between 3 and 36 months. The GLOBAL LEADERS trial, a clinical study comparing 2 forms of antiplatelet therapy after stent implantation trial21 was designed to compare dual antiplatelet therapy durations of 1 month versus 12 months. However, in the follow-up period from 12 to 24 months, the control group (dual antiplatelet therapy for 1 year) received aspirin whereas the experimental group (dual antiplatelet therapy for 1 month) received ticagrelor. Similarly, the Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trial20 compared dual antiplatelet therapy for 21 days followed by clopidogrel with aspirin monotherapy for 90 days. For these 2 trials, we only included outcomes from the period with monotherapy with P2Y12 inhibitors or aspirin in the treatment arms.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow chart for the study. Search of Medline, EMBASE, and Cochrane Central databases revealed 6058 citations. Of these, 9 studies met the inclusion criteria and were included in the analyses.
Table 1.
Study design and baseline characteristics of the included trials.
| Trial name | CAPRIE | ASCET | HOST EXAM | TICAB | GLOBAL LEADERS | CADET | DACAB | SOCRATES | CHANCE |
|---|---|---|---|---|---|---|---|---|---|
| Study design | |||||||||
| Total patients | 19185 | 1001 | 5438 | 1859 | 15968 | 184 | 332 | 13199 | 5170a |
| Study design | Double blind | Double blind | Open label | Double blind | Open label | Double blind | Open label | Double blind | Double blind |
| Year of publication | 1996 | 2012 | 2021 | 2019 | 2018 | 2004 | 2018 | 2016 | 2013 |
| Qualifying event | Stroke, CAD, PAD | Stable CAD | CAD patients post-PCI | CAD patients post-CABG | CAD patients post-PCI | CAD | CAD patients post-CABG | Stroke or high-risk TIA | Stroke or high-risk TIA |
| Multicentre (Yes/No) | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Country | Multinational | Norway | South Korea | Multinational | Multinational | United Kingdom | China | Multinational | China |
| Treatment arm | Clopidogrel (75 mg once daily) | Clopidogrel (75 mg once daily) | Clopidogrel (75 mg once daily) | Ticagrelor (90 mg twice daily) | Ticagrelor (90 mg twice daily) | Clopidogrel (75 mg once daily) | Ticagrelor (90 mg twice daily) | Ticagrelor (90 mg twice daily) | Clopidogrel (75 mg once daily) |
| Comparison | Aspirin (325 mg once daily) | Aspirin (75 mg once daily) | Aspirin (100 mg once daily) | Aspirin (100 mg once daily) | Aspirin (75–100 mg once daily) | Aspirin (75 mg once daily) | Aspirin (100 mg once daily) | Aspirin (100 mg once daily) | Aspirin (75 mg once daily) |
| Duration of monotherapy | 36 months | 24 months | 24 months | 12 months | 12 monthsb | 6 months | 12 months | 3 months | 68 daysc |
| Duration of follow-up | 36 months | 24 months | 24 months | 12 months | 24 months | 6 months | 12 months | 3 months | 3 months |
| Baseline characteristics | |||||||||
| Mean age (SD) | 62.5 | 62.4 | 63.5 (10.7) | 66.7 | 64.5 (10.3) | 62.6 | 63.6 | 65.8 | 62 |
| Females | 28.1% | 21.8% | 25.5% | 15.1% | 23.3% | 19.1% | 17.2% | 41.6% | 33.8% |
| Hypertension | 51.5% | 55.4% | 61.4% | 89.9% | 73.6% | – | 72.8% | 73.7% | 65.7% |
| Diabetes mellitus | 20.0% | 19.9% | 34.2% | 35.9% | 25.3% | – | 42.7% | 24.3% | 21.1% |
| Dyslipidemia | 41.0% | – | 69.3% | 81.7% | 69.6% | – | 73.1% | 38.0% | 11.1% |
| Current or previous smoker | 78.5% | 20.4% | 20.7% | 55.3% | 26.1% | 74.5% | 48.5% | – | 43.0% |
| CKD | – | 12.7% | 7.0% | 13.7% | – | 0.9% | – | – | |
| Previous stroke/TIA | 40 | – | 4.7% | 8.9% | 2.6% | – | 10.5% | 100% | 23.3% |
| Prior MI | 44% | 43.7% | 16.0% | 22.7% | 23.3% | 100% | 31% | 4.1% | 1.9 |
| PAD | 38% | 5.4% | – | 9.1% | 6.4% | – | 16.9% | – | – |
| Prior PCI | – | 73% | – | 20.2% | 32.7% | – | – | – | – |
| Prior CABG | – | 18.5% | – | 0.8% | 5.9% | – | 24.7% | – | – |
| Baseline Medication use | |||||||||
| Statins | – | 98.3% | – | 83.6% | – | 78.8% | 94.0% | – | 42.0% |
| Beta-blockers | – | 75.8% | – | 66.8% | – | 81.0% | 89.8% | – | – |
| ACEi/ARB | – | 25.2% | – | 76.9% | – | 51.1% | 60.8% | – | – |
| PPI | – | 11% | – | 30.6% | – | – | 64.2% | – | 0.9% |
CHANCE—Total study population was 5170. The population included in our analysis is 4696, as per patient-level meta-analysis by Pan et al.
GLOBAL LEADERS—Monotherapy with aspirin or ticagrelor during months 13–24 of the study period.
CHANCE—Monotherapy with aspirin from day 1 to 90 and with clopidogrel from day 22 to 90.
ACEi = angiotensin-converting-enzyme inhibitor, ARB = angiotensin receptor blockers, CABG = coronary artery bypass grafting, CKD = chronic kidney disease, MI = myocardial infarction, PAD = peripheral arterial disease, PCI = percutaneous coronary intervention, PPI = proton-pump inhibitors, SD = standard deviation, TIA = transient ischemic attack.
Study population
A total of 61 623 patients were included in these analyses. Three studies (CAPRIE, GLOBAL LEADERS, Acute Stroke or Transient Ischemic Attack Treated with Aspirin or Ticagrelor and Patient Outcomes [SOCRATES]) accounted for more than three-quarters of all patients. The qualifying event for enrolment was stroke in 24 326 (39.5%) patients and acute coronary syndrome in 18 445 (29.9%). 12 400 (20.1%) patients were included due to the presence of chronic coronary syndromes, and 6452 (10.5%) patients were included due to the presence of peripheral artery disease. The mean age of patients was between 62 and 67 years. The proportion of females ranged between 15% and 42%. Although the majority of patients had hypertension, the prevalence of diabetes, chronic kidney disease, and smoking in the different studies was wide-ranging. The most commonly used medications at baseline were statins and beta-blocking agents. Discontinuation rates and/or the proportion of patients lost to follow-up across the studies were generally higher in the P2Y12 inhibitor group (see Supplementary material online, Table S3).
Outcomes
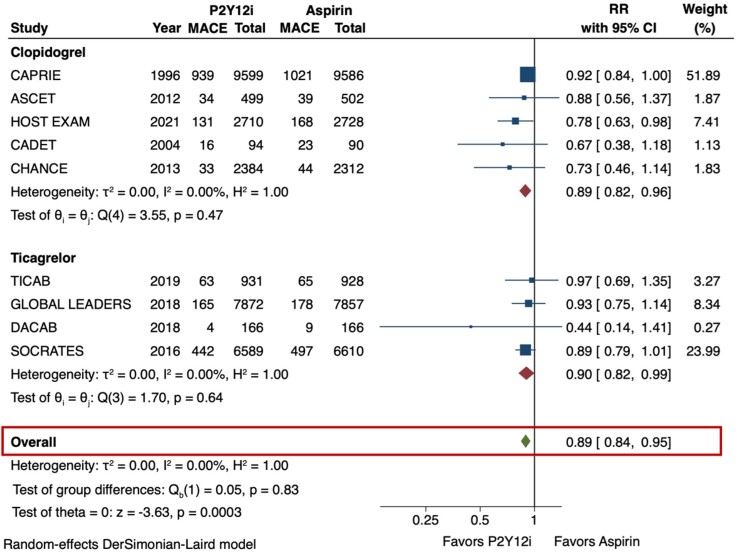
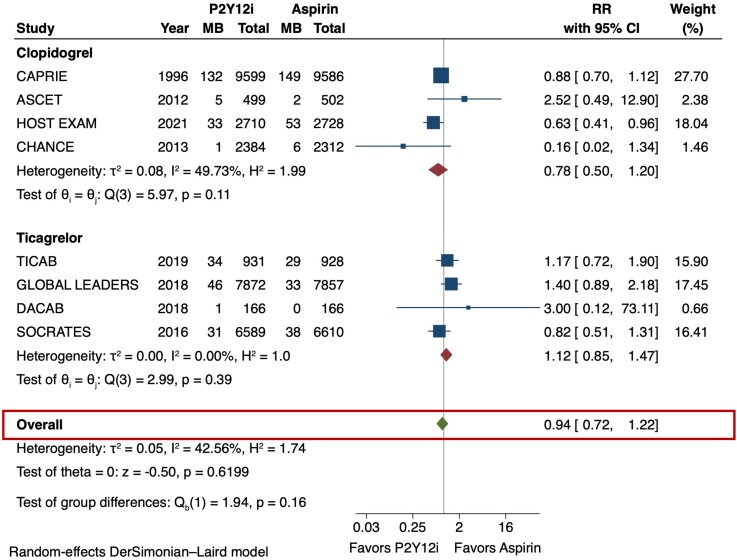
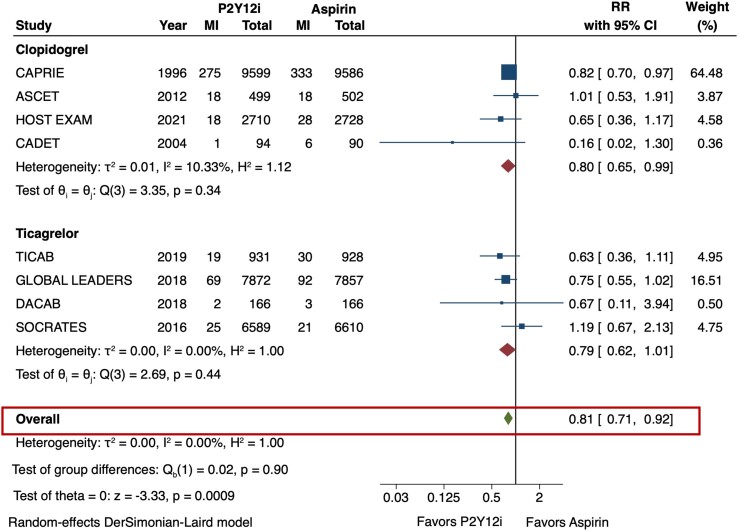
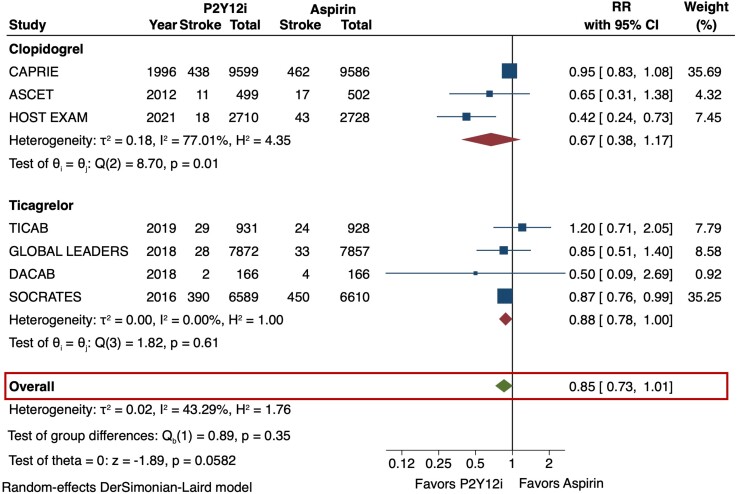
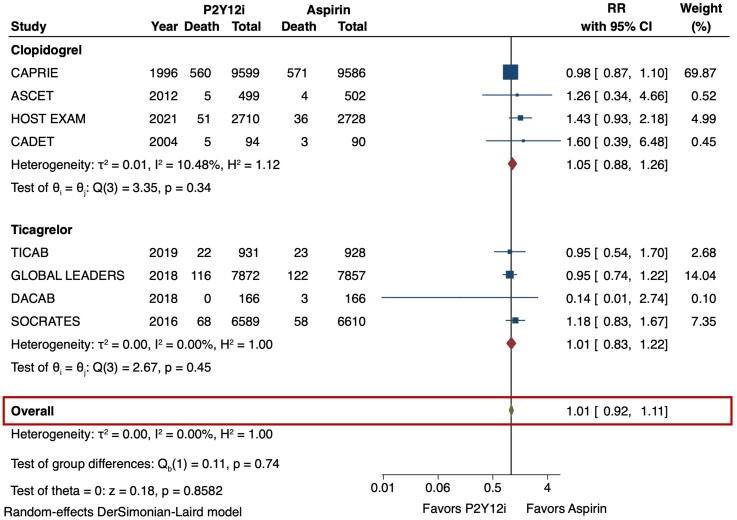
The primary efficacy outcome (MACE) and safety outcome (major bleeding) are depicted in Figures 2 and 3. The risk of MACE was significantly reduced with the use of P2Y12 inhibitor monotherapy as compared with aspirin (RR 0.89 [95% CI 0.84–0.95], I2 = 0%, NNT 141). This result was consistent irrespective of the P2Y12 inhibitor used (p-interaction = 0.83). The use of P2Y12 inhibitors was also associated with a reduced risk of MI as compared with aspirin (RR 0.81 [95% CI 0.71–0.92], I2 = 0%, NNT 273) (Figure 4). No significant difference was observed in the risk of stroke (RR 0.85 [95% CI 0.73–1.01], I2 = 43.3%) (Figure 5) or all-cause death (RR 1.01 [95% CI 0.92–1.11], I2 = 0%) (Figure 6). The risk of major bleeding (RR 0.94 [95% CI 0.72–1.22), I2 = 42.6%) (Figure 3) and any bleeding (RR 1.07 [95% CI 0.88–1.30], I2 = 60.5%) were similar between P2Y12 inhibitor monotherapy and aspirin monotherapy treatment groups. Bleeding outcomes are expanded in Supplementary material online, Table S4.
Figure 2.
Major adverse cardiovascular events (MACE) in P2Y12 inhibitor monotherapy versus aspirin monotherapy. The primary efficacy outcome of interest was MACE, which was defined as a composite of stroke, myocardial infarction (MI), or death in the majority of studies. The DerSimonian and Laird random-effects model was used to examine the risk ratios (RR). All 9 trials were included for this analysis. P2Y12 inhibitor monotherapy reduced the risk of MACE by 11% as compared with aspirin monotherapy (RR 0.89 [95% CI 0.84-0.95], I2 = 0%). This result was consistent irrespective of the P2Y12 inhibitor used (p-interaction = 0.83). CI = confidence interval, MACE = major adverse cardiovascular events, P2Y12i = P2Y12 inhibitor, RR = risk ratio.
Figure 3.
Major bleeding in P2Y12 inhibitor monotherapy versus aspirin monotherapy. The primary safety outcome of interest was major bleeding was evaluated using the DerSimonian and Laird random-effects model. There was no significant difference in the risk of major bleeding between P2Y12 inhibitor and aspirin monotherapy (RR 0.94 [95% CI 0.72–1.22], I2 = 42.6%). CI = confidence interval, MB = major bleeding, P2Y12i = P2Y12 inhibitor, RR = risk ratio.
Figure 4.
Myocardial infarction (MI) in P2Y12 inhibitor monotherapy versus aspirin monotherapy. Pooled risk ratio (RR) of MI was calculated from 8 of the 9 included trials. The use of P2Y12 inhibitors was associated with a 19% risk reduction of MI as compared with aspirin (RR 0.81 [95% CI 0.71–0.92], I2 = 0%). CI = confidence interval, MI = myocardial infarction, P2Y12i = P2Y12 inhibitor, RR = risk ratio.
Figure 5.
Stroke in P2Y12 inhibitor monotherapy versus aspirin monotherapy. The occurrence of stroke (ischemic/hemorrhagic) was reported in 7 trials. No significant difference was observed in the risk of stroke (RR 0.85 [95% CI 0.73–1.01], I2 = 43.3%). CI = confidence interval, P2Y12i = P2Y12 inhibitor, RR = risk ratio.
Figure 6.
All-cause mortality in P2Y12 inhibitor monotherapy versus aspirin monotherapy. All-cause death was analyzed as a secondary outcome from the pooled analysis of 8 studies. Monotherapy with a P2Y12 inhibitor or aspirin had similar risks of all-cause death (RR 1.01 [95% CI 0.92–1.11], I2 = 0%). CI = confidence interval, P2Y12i = P2Y12 inhibitor, RR = risk ratio.
Subgroup analyses based on the qualifying event (see Supplementary material online, Figures S1–S3) revealed that the overall reduction in MACE with P2Y12 inhibitors was driven by a reduction in recurrence of the primary event. The risk of recurrent stroke or transient ischemic attack was lower with P2Y12 inhibitors as compared with aspirin (RR 0.89 [95% CI 0.81-0.98], I2 = 0%). Similarly, in patients with coronary artery disease, the risk of MI was lower with P2Y12 inhibitors as compared with aspirin (RR 0.83 [95% CI 0.71–0.98], I2 = 2.6%). As compared with aspirin, P2Y12 inhibitors significantly reduced the risk of MI in patients treated with PCI (RR 0.73 [95% CI 0.55–0.96], I2 = 0%) (see Supplementary material online, Figure S4). The risk of major bleeding remained similar across all subgroups (see Supplementary material online, Figure S5).
Sensitivity analysis showed that the reductions in MACE (see Supplementary material online, Figure S6) and MI (see Supplementary material online, Figure S7) were consistent after eliminating the included studies one-by-one. Additionally, meta-regression analyses did not find any significant interaction with the duration of follow-up. The degree of heterogeneity between the studies was low to moderate for all outcomes, except the secondary safety outcome of any bleeding. Funnel plots did not show any significant publication bias (see Supplementary material online, Figure S8). The risk of bias assessment of the individual studies was graded between low to some concern (see Supplementary material online, Table S5).
Discussion
We conducted an updated meta-analysis of studies comparing P2Y12 inhibitor monotherapy with aspirin monotherapy for secondary prevention in patients with established atherosclerotic cardiovascular disease. Our analysis showed that compared with aspirin monotherapy, P2Y12 inhibitor monotherapy (with clopidogrel or ticagrelor) significantly reduced the risk of MACE by 11% and MI by 19%. The reduction in MACE was consistent irrespective of the P2Y12 inhibitor used and no significant interaction was found between MACE or MI and the qualifying disease/event. We found no significant difference in the risk of major bleeding with P2Y12 inhibitor monotherapy compared with aspirin monotherapy. Notably, pre-specified analysis based on the qualifying event showed a greater reduction in the recurrence of the primary event/disease with P2Y12 inhibitors. However, the reduction in MACE and recurrent events with P2Y12 inhibitor monotherapy did not translate into reduction in all-cause mortality. This may be because of the short duration of follow-up in the majority of trials and may evolve as extended periods of monotherapy with aspirin and P2Y12 inhibitors are compared.
Over the past few years, trials demonstrating the feasibility of abbreviated periods of dual antiplatelet therapy after PCI have been under the spotlight.23–25 However, the evidence regarding the preferred antiplatelet monotherapy to be used following dual antiplatelet therapy has been limited. The CAPRIE trial was the first and largest trial comparing aspirin with a P2Y12 inhibitor monotherapy in patients with recent MI, ischemic stroke, or symptomatic peripheral arterial disease.14 The observed reduction in the occurrence of composite ischemic outcomes with clopidogrel with a lower rate of hospitalization for gastrointestinal bleeding26 led to its acceptance as an alternative to aspirin. Other small trials that compared aspirin with clopidogrel in patients with chronic ischemic heart disease showed no difference in outcomes.15,16 Similarly, in patients who underwent CABG, ticagrelor monotherapy was found to be equivalent to aspirin monotherapy in terms of venous graft patency, revascularization, or bleeding.17,18 Residual risk of recurrent stroke with aspirin monotherapy prompted the SOCRATES trial, a double-blind trial with 13 199 patients that showed similar outcomes with ticagrelor and aspirin.19 Indirect evidence from a network meta-analysis demonstrated no significant difference in ischemic or bleeding outcomes with aspirin versus P2Y12 inhibitor monotherapy after a short course of dual antiplatelet therapy in patients post-PCI.27
The HOST-Extended Antiplatelet Monotherapy (HOST-EXAM) trial,22 published in 2021, was the first randomized trial directly comparing aspirin with clopidogrel monotherapy after event-free completion of dual antiplatelet therapy for 6–18 months following PCI with drug-eluting stents. The key finding from this study was lower incidence of the composite outcome of all-cause mortality, MI, stroke, readmission for the acute coronary syndrome, and major bleeding with clopidogrel. However, the short follow-up period (24 months), open-label design, and an exclusively East Asian population limit the generalizability of these results to routine clinical practice. Regardless, this trial has re-energized the debate about the optimal agent for antiplatelet monotherapy for secondary prevention of atherosclerotic cardiovascular events.
A recent meta-analysis by Chiarito et al reported a marginal reduction in the risk of MI with P2Y12 inhibitor (clopidogrel, ticlopidine, ticagrelor) monotherapy compared with aspirin but found no difference in all-cause mortality, concluding that the available evidence did not support a change in practice away from aspirin.28 Our study builds on this analysis by (i) including the HOST-EXAM trial, (ii) studying MACE events, and (iii) excluding trials with ticlopidine, hence limiting the analysis to P2Y12 inhibitors used in the present day.29 We chose MACE as the primary outcome since the goal of antiplatelet therapy is the prevention of thrombotic events in all vascular beds and not just coronary, cerebrovascular, or peripheral arterial disease events individually. Moreover, the majority of the included trials were powered for detecting differences in MACE.
Given the observed reduction in the risk of MACE and MI, our analysis may support the preferential use of clopidogrel or ticagrelor over aspirin monotherapy in patients with established atherosclerotic cardiovascular disease. The population to whom our results are most applicable includes patients who have successfully completed 6 to 18 months of dual antiplatelet therapy, patients with chronic coronary syndromes, peripheral arterial disease, and recent ischemic stroke or TIA. Current practice guidelines recommend the use of P2Y12 inhibitors as effective alternatives to aspirin monotherapy, but aspirin is still considered the default agent in these scenarios.30–33 As the evidence demonstrating the equivalency and indeed, the superiority of P2Y12 inhibitors is now established, it is reasonable to prefer P2Y12 inhibitor monotherapy over aspirin monotherapy. Personalized approach for the choice of P2Y12 inhibitor to be used for antiplatelet monotherapy should be considered. While routine pharmacogenomic testing for response to clopidogrel is currently not recommended, among patients with established suboptimal response to clopidogrel, other P2Y12 inhibitors such as ticagrelor or prasugrel should be favored.34 Genotype-guided personalized antiplatelet therapy and de-escalation using platelet function testing are options for a more tailored approach, although feasibility and cost-effectiveness are barriers to their widespread use.35,36 Notably, the cost of ticagrelor may be a barrier to its use in many countries, however, this issue is expected to improve after its patent expires in 2024.
Our study has limitations that should be considered when interpreting the pooled results. The population included in our analysis varied widely and included patients with chronic coronary syndromes, recent MI, cerebrovascular disease, or peripheral arterial disease. To account for this inter-study variability, subgroup analyses were conducted and suggested no significant interaction of qualifying diagnosis with primary or secondary outcomes. However, given the limited number of trials included, the stratified analysis may lack sufficient statistical power to demonstrate possible differences. Similarly, the results from the meta-regression analysis evaluating the role of underlying baseline risk and duration of follow-up might also be limited by the low number of studies included in the pooled analysis. Due to the lack of patient-level data, we were also unable to investigate the effect of background therapies such as statins on the endpoints. The definition of MACE varied marginally between studies but included MI, stroke, and death. While death should ideally be classified as cardiovascular and non-cardiovascular death to capture the potential off-target effects of either drug class, this was not feasible as non-cardiovascular death was reported separately in only 2 studies. Additionally, these findings do not generalize to patients with recent drug-eluting stents requiring dual antiplatelet therapy, patients who had ischemic or bleeding events while on dual antiplatelet therapy, patients with a severe disabling stroke, and patients requiring chronic anticoagulation. The CHANCE trial was designed to compare the outcomes of initial dual antiplatelet therapy for 21 days followed by clopidogrel with aspirin monotherapy after a minor stroke or transient ischemic attack. Although we included outcomes from day 22 onwards, it is possible that the events in the clopidogrel arm were influenced by the lingering effects of dual antiplatelet therapy. We also extracted data selectively from the latter half of the GLOBAL LEADERS trial. We recognize that derivation of data in part may raise doubts about the validity and the decision to include the study. However, the results were consistent upon exclusion of the GLOBAL LEADERS and CHANCE trials (see Supplementary material online, Table S6), confirming the robustness of the analyses. Future research directly comparing the outcomes of monotherapy with aspirin versus P2Y12 inhibitors for specified indications and head-to-head comparison between different P2Y12 inhibitors will help provide definitive evidence.
In conclusion, in this meta-analysis of randomized trials, P2Y12 inhibitor monotherapy for chronic secondary prevention was associated with lower risk of MACE and MI compared with aspirin monotherapy in select patients with established atherosclerotic cardiovascular disease. Dedicated randomized trials comparing the 2 strategies and individual P2Y12 agents are needed to further establish the optimal antiplatelet therapy for secondary prevention in patients with atherosclerotic cardiovascular disease.
Lead author biography

Devika Aggarwal completed medical school at Maulana Azad Medical College in New Delhi, India and is currently a resident in Internal Medicine at Beaumont Hospital-Royal Oak in Michigan, USA. She is passionate about pursuing a fellowship in cardiovascular disease followed by a career in academic cardiology. Her current areas of interest include coronary artery disease, antithrombotic therapies, and lipid management.
Supplementary Material
Acknowledgements
None
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
None.
Conflict of interest: Dr. Furtado reports research grants and personal fees from AstraZeneca, Bayer, Biomm, and Servier, and research grants from Pfizer, EMS, Aché, CytoDin, Brazilian Ministry of Health, University Health Network (received from his institution), and Lemann Foundation Research Fellowship. Dr. Navarese reports research grants from Abbott, Amgen, and lecture fees/honoraria from Amgen, AstraZeneca, Bayer, Pfizer, and Sanofi-Regeneron, outside the submitted work. Dr. Banerjee is a consultant for Medtronic, Kaneka, and Cordis, and receives institutional research grants from Boston Scientific Corporation, and Chiesis. Dr. Bhatt discloses the following relationships—Advisory Board: Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Novartis, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Lexicon, Lilly, Medtronic, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, 89Bio; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Philips, Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Takeda. Dr. Qamar reports receiving institutional grant support from Novo Nordisk, NorthShore Auxiliary Research Scholar Fund, NorthShore CardioDiabetes Pilot Grant and fees for educational activities from the American College of Cardiology, Society for Vascular Medicine, Society for Cardiovascular Angiography and Interventions, Janssen and Janssen, Pfizer, Medscape, and Clinical Exercise Physiology Association. All other authors have no disclosures to report.
Data availability
Our data is derived from trials available in the public domain.
References
- 1. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, Neumann F-J, Sechtem U, Banning AP, Bonaros N, Bueno H, Bugiardini R, Chieffo A, Crea F, Czerny M, Delgado V, Dendale P, Flachskampf FA, Gohlke H, Grove EL, James S, Katritsis D, Landmesser U, Lettino M, Matter CM, Nathoe H, Niessner A, Patrono C, Petronio AS, Pettersen SE, Piccolo R, Piepoli MF, Popescu BA, Räber L, Richter DJ, Roffi M, Roithinger FX, Shlyakhto E, Sibbing D, Silber S, Simpson IA, Sousa-Uva M, Vardas P, Witkowski A, Zamorano JL, Achenbach S, Agewall S, Barbato E, Bax JJ, Capodanno D, Cuisset T, Deaton C, Dickstein K, Edvardsen T, Escaned J, Funck-Brentano C, Gersh BJ, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Prescott E, Saraste A, Storey RF, Svitil P, Valgimigli M, Windecker S, Aboyans V, Baigent C, Collet J-P, Dean V, Delgado V, Fitzsimons D, Gale CP, Grobbee D, Halvorsen S, Hindricks G, Iung B, Jüni P, Katus HA, Landmesser U, Leclercq C, Lettino M, Lewis BS, Merkely B, Mueller C, Petersen S, Petronio AS, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa-Uva M, Touyz RM, Benkhedda S, Metzler B, Sujayeva V, Cosyns B, Kusljugic Z, Velchev V, Panayi G, Kala P, Haahr-Pedersen SA, Kabil H, Ainla T, Kaukonen T, Cayla G, Pagava Z, Woehrle J, Kanakakis J, Tóth K, Gudnason T, Peace A, Aronson D, Riccio C, Elezi S, Mirrakhimov E, Hansone S, Sarkis A, Babarskiene R, Beissel J, Maempel AJC, Revenco V, de Grooth GJ, Pejkov H, Juliebø V, Lipiec P, Santos J, Chioncel O, Duplyakov D, Bertelli L, Dikic AD, Studenčan M, Bunc M, Alfonso F, Bäck M, Zellweger M, Addad F, Yildirir A, Sirenko Y, Clapp B. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 2. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–2236. [DOI] [PubMed] [Google Scholar]
- 3. Aboyans V, Ricco J-B, Bartelink M-LEL, Björck M, Brodmann M, Cohnert T, Collet J-P, Czerny M, De Carlo M, Debus S, Espinola-Klein C, Kahan T, Kownator S, Mazzolai L, Ross Naylor A, Roffi M, Röther J, Sprynger M, Tendera M, Tepe G, Venermo M, Vlachopoulos C, Desormais I, ESC Scientific Document Group . 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763–816. [DOI] [PubMed] [Google Scholar]
- 4. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Carla Roncaglioni M, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet Lond Engl 2009;373:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antithrombotic Trialists’ Collaboration . Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Storey RF, Husted S, Harrington RA, Heptinstall S, Wilcox RG, Peters G, Wickens M, Emanuelsson H, Gurbel P, Grande P, Cannon CP. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol 2007;50:1852–1856. [DOI] [PubMed] [Google Scholar]
- 7. Wallentin L, Varenhorst C, James S, Erlinge D, Braun OO, Jakubowski JA, Sugidachi A, Winters KJ, Siegbahn A. Prasugrel achieves greater and faster P2Y12receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur Heart J 2008;29:21–30. [DOI] [PubMed] [Google Scholar]
- 8. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F-J, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 9. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 10. Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org.
- 11. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steering Committee CAPRIE . A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348:1329–1339. [DOI] [PubMed] [Google Scholar]
- 15. Pettersen A-ÅR, Seljeflot I, Abdelnoor M, Arnesen H. High On-Aspirin Platelet Reactivity and Clinical Outcome in Patients With Stable Coronary Artery Disease: Results From ASCET (Aspirin Nonresponsiveness and Clopidogrel Endpoint Trial). J Am Heart Assoc 2012;1:e000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Woodward M, Lowe GDO, Francis LMA, Rumley A, Cobbe SM, CADET Study Investigators . A randomized comparison of the effects of aspirin and clopidogrel on thrombotic risk factors and C-reactive protein following myocardial infarction: the CADET trial. J Thromb Haemost JTH 2004;2:1934–1940. [DOI] [PubMed] [Google Scholar]
- 17. Schunkert H, Boening A, von Scheidt M, Lanig C, Gusmini F, de Waha A, Kuna C, Fach A, Grothusen C, Oberhoffer M, Knosalla C, Walther T, Danner BC, Misfeld M, Zeymer U, Wimmer-Greinecker G, Siepe M, Grubitzsch H, Joost A, Schaefer A, Conradi L, Cremer J, Hamm C, Lange R, Radke PW, Schulz R, Laufer G, Grieshaber P, Pader P, Attmann T, Schmoeckel M, Meyer A, Ziegelhöffer T, Hambrecht R, Kastrati A, Sandner SE. Randomized trial of ticagrelor vs. aspirin in patients after coronary artery bypass grafting: the TiCAB trial. Eur Heart J 2019;40:2432–2440. [DOI] [PubMed] [Google Scholar]
- 18. Zhao Q, Zhu Y, Xu Z, Cheng Z, Mei J, Chen X, Wang X. Effect of ticagrelor plus aspirin, ticagrelor alone, or aspirin alone on saphenous vein graft patency 1 year after coronary artery bypass grafting: A randomized clinical trial. JAMA 2018;319:1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, Jonasson J, Minematsu K, Molina CA, Wang Y, Wong KSL. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med 2016;375:35–43. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, Jia J, Dong Q, Xu A, Zeng J, Li Y, Wang Z, Xia H, Johnston SC. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369:11–19. [DOI] [PubMed] [Google Scholar]
- 21. Vranckx P, Valgimigli M, Jüni P, Hamm C, Steg PG, Heg D, van Es GA, McFadden EP, Onuma Y, van Meijeren C, Chichareon P, Benit E, Möllmann H, Janssens L, Ferrario M, Moschovitis A, Zurakowski A, Dominici M, Van Geuns RJ, Huber K, Slagboom T, Serruys PW, Windecker S, Abdellaoui M, Adlam D, Akin I, Albarran Gonzalez-Trevilla A, Almeida M, Alves Lemos Neto P, Aminian A, Anderson R, Andreae R, Angioi M, Asano T, Barbato E, Barlis P, Barraud P, Benit E, Bertrand O, Beygui F, Bolognese L, Botelho R, Bouwman C, Bressers M, Brunel P, Buszman P, Buysschaert I, Canas da Silva P, Carrie D, Cequier A, Chichareon P, Chin Chang C, Chowdhary S, Collet C, Colombo A, Cotton J, Cruz Ferreira R, Curello S, Curzen N, de Bot J, de Vreede T, Delle Karth G, Dijksma L, Dominici M, Édes I, Eeckhout E, Eitel I, Faluközy J, Fath-Ordoubadi F, Ferrario M, Fontos G, Francisco Diaz J, Freitas Quintella E, Frey B, Friedrich G, Galasko G, Galuszka G, Gama Ribeiro V, Garg S, Gargiulo G, Geisler T, Gelev V, Ghandilyan A, Goicolea J, Gori T, Gragnano F, Guimarães A, Hamm C, Haude M, Heg D, Heijke P, Hernández Antolin RA, Hildick-Smith D, Hillen D, Hoekman I, Hofma S, Holmvang L, Hoole S, Horváth I, Huber K, Hugense A, Ibrahim K, Iñiguez A, Isaaz K, Jambrik Z, Janssens L, Jasionowicz P, Jonk J, Jung W, Jüni P, Katagiri Y, Kogame N, Koh TH, Koning R, Konteva M, Kőszegi Z, Krackhardt F, Kreuger Y, Kukreja N, Ladan B, Lantelme P, Leandro S, Leibundgut G, Liebetrau C, Lindeboom W, Macaya Miguel C, Mach F, Magro M, Maillard L, Manavifar N, Mauri L, McFadden E, Merkely B, Miyazaki Y, Młodziankowski A, Moccetti T, Modolo R, Möllman H, Morelle J-F, Moschovitis A, Munndt Ottesen M, Muurling M, Naber CK, Neumann F-J, Oldroyd K, Ong P, Onuma Y, Palsrok S, Petrov I, Plante S, Prokopczuk J, Rademaker-Havinga T, Raffel C, Rensing B, Roffi M, Royaards K-J, Sabate M, Schächinger V, Seidler T, Serra Peñaranda A, Serruys P, Sikarulidze L, Slagboom T, Soliman OI, Sousa A, Spitzer E, Stables R, Steg G, Steinwender C, Subkovas E, Suryapranata H, Takahashi K, Talwar S, Teiger E, ter Weele A, Teurlings E, Thury A, Tijssen J, Tonev G, Trendafilova-Lazarova D, Tumscitz C, Umans V, Ungi I, Valkov V, van der Harst P, van Geuns RJ, van Meijeren C, Vassilev D, Velchev V, Velthuizen E, Verheugt F, Vlcek N, vom Dahl J, Vrolix M, Walsh S, Werner N, Windecker S, Witsenburg M, Zaman A, Żmudka K, Zrenner B, Zurakowski A, Zweiker R. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet Lond Engl 2018;392:940–949. [DOI] [PubMed] [Google Scholar]
- 22. Koo B-K, Kang J, Park KW, Rhee T-M, Yang H-M, Won K-B, Rha S-W, Bae J-W, Lee NH, Hur S-H, Yoon J, Park T-H, Kim BS, Lim SW, Cho YH, Jeon DW, Kim S-H, Han J-K, Shin E-S, Kim H-S, Koo B-K, Kang J, Park KW, Rhee T-M, Lee H, Yang H-M, Won K-B, Rha S-W, Bae J-W, Lee NH, Hur S-H, Yoon J, Park T-H, Kim BS, Lim SW, Cho YH, Jeon DW, Kim S-H, Han J-K, Shin E-S, Kim H-S, Han K-R, Moon K-W, Oh SK, Kim U, Rhee M-Y, Kim D-I, Kim S-Y, Lee S-Y, Lee SU, Kim S-W, Kim SY, Jeon H-K, Cha KS, Jo S-H, Ryu JK, Suh I-W, Choi H-H, Woo S-I, Chae I-H, Shin W-Y, Kim D-K, Oh JH, Jeong MH, Kim YH. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet Lond Engl 2021;397:2487–2496. [DOI] [PubMed] [Google Scholar]
- 23. Hahn J-Y, Song YB, Oh J-H, Chun WJ, Park YH, Jang WJ, Im E-S, Jeong J-O, Cho BR, Oh SK, Yun KH, Cho D-K, Lee J-Y, Koh Y-Y, Bae J-W, Choi JW, Lee WS, Yoon HJ, Lee SU, Cho JH, Choi WG, Rha S-W, Lee JM, Park TK, Yang JH, Choi J-H, Choi S-H, Lee SH, Gwon H-C. Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: The SMART-CHOICE randomized clinical trial. JAMA 2019;321:2428–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, Džavík V, Escaned J, Gil R, Gurbel P, Hamm CW, Henry T, Huber K, Kastrati A, Kaul U, Kornowski R, Krucoff M, Kunadian V, Marx SO, Mehta SR, Moliterno D, Ohman EM, Oldroyd K, Sardella G, Sartori S, Shlofmitz R, Steg PG, Weisz G, Witzenbichler B, Han Y-l, Pocock S, Gibson CM. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N Engl J Med 2019;381:2032–2042. [DOI] [PubMed] [Google Scholar]
- 25. Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, Ohya M, Suwa S, Takagi K, Nanasato M, Hata Y, Yagi M, Suematsu N, Yokomatsu T, Takamisawa I, Doi M, Noda T, Okayama H, Seino Y, Tada T, Sakamoto H, Hibi K, Abe M, Kawai K, Nakao K, Ando K, Tanabe K, Ikari Y, Hanaoka KI, Morino Y, Kozuma K, Kadota K, Furukawa Y, Nakagawa Y, Kimura T. Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. JAMA 2019;321:2414–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhatt DL, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Reduction in the need for hospitalization for recurrent ischemic events and bleeding with clopidogrel instead of aspirin. Am Heart J 2000;140:67–73. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002870300789897 [DOI] [PubMed] [Google Scholar]
- 27. Kuno T, Ueyama H, Takagi H, Bangalore S. P2Y12 inhibitor monotherapy versus aspirin monotherapy after short-term dual antiplatelet therapy for percutaneous coronary intervention: Insights from a network meta-analysis of randomized trials. Am Heart J 2020;227:82–90. [DOI] [PubMed] [Google Scholar]
- 28. Chiarito M, Sanz-Sánchez J, Cannata F, Cao D, Sturla M, Panico C, Godino C, Regazzoli D, Reimers B, De Caterina R, Condorelli G, Ferrante G, Stefanini GG. Monotherapy with a P2Y12 inhibitor or aspirin for secondary prevention in patients with established atherosclerosis: a systematic review and meta-analysis. Lancet Lond Engl 2020;395:1487–1495. [DOI] [PubMed] [Google Scholar]
- 29. Bhatt DL, Bertrand ME, Berger PB, L’Allier PL, Moussa I, Moses JW, Dangas G, Taniuchi M, Lasala JM, Holmes DR, Ellis SG, Topol EJ. Meta-Analysis of Randomized and Registry Comparisons of Ticlopidine With Clopidogrel After Stenting. J Am Coll Cardiol 2002;39:9–14. [DOI] [PubMed] [Google Scholar]
- 30. Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd-Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol 201129;58:2432–2446. [DOI] [PubMed] [Google Scholar]
- 31. Collet J-P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM, ESC Scientific Document Group . 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 32. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, Lennon O, Meschia JF, Nguyen TN, Pollak PM, Santangeli P, Sharrief AZ, Smith SC Jr, Turan TN, Williams LS. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021;52:e364–e467. [DOI] [PubMed] [Google Scholar]
- 33. Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FGR, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RAG, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Eileen Walsh M. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e726–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tantry US, Bonello L, Aradi D, Price MJ, Jeong Y-H, Angiolillo DJ, Stone GW, Curzen N, Geisler T, ten Berg J, Kirtane A, Siller-Matula J, Mahla E, Becker RC, Bhatt DL, Waksman R, Rao SV, Alexopoulos D, Marcucci R, Reny J-L, Trenk D, Sibbing D, Gurbel PA. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol 2013;62:2261–2273. [DOI] [PubMed] [Google Scholar]
- 35. Sibbing D, Aradi D, Jacobshagen C, Gross L, Trenk D, Geisler T, Orban M, Hadamitzky M, Merkely B, Kiss RG, Komócsi A, Dézsi CA, Holdt L, Felix SB, Parma R, Klopotowski M, Schwinger RHG, Rieber J, Huber K, Neumann F-J, Koltowski L, Mehilli J, Huczek Z, Massberg S, Parma R, Parma Z, Lesiak M, Komosa A, Huczek Z, Koltowski L, Kowara M, Rymuza B, Klopotowski M, Malek L, Aradi D, Veress G, Dézsi AD, Merkely B, Lux Á, Kiss RG, Papp J, Kovács A, Dézsi CA, Amer S, Ruzsa Z, Róna S, Komócsi A, Ili R, Ungi I, Nagy F, Zweiker R, Tóth-Gayor G, Huber K, Haller P, von Scheidt W, Blüthgen A, Neumann F-J, Trenk D, Leggewie S, Kreider-Stempfle HU, Remp T, Kara K, Mügge A, Wutzler A, Fichtlscherer S, Zeiher AM, Seeger F, Hinterseer M, König A, Lederle S, Jacobshagen C, Czepluch F, Maier L, Schillinger W, Sossalla S, Hummel A, Felix S, Karakas M, Sydow K, Rudolph T, Halbach M, Gori T, Münzel T, May A, Gerstenberg C-M, Pilecky D, Rieber J, Deichstetter M, Sibbing D, Mehilli J, Gross L, Kääb S, Löw A, Orban M, Orban M, Sattler S, Deuschl S, Teupser D, Holdt L, Mudra H, Räder T, Schütz T, Vahldiek F, Divchev D, Ince H, Nienaber CA, Radunski H, Boekstegers P, Horstkotte J, Mueller R, Geisler T, Müller K, Schwinger R, Rasp O. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet Lond Engl 2017;390:1747–1757. [DOI] [PubMed] [Google Scholar]
- 36. Pereira NL, Farkouh ME, So D, Lennon R, Geller N, Mathew V, Bell M, Bae J-H, Jeong MH, Chavez I, Gordon P, Abbott JD, Cagin C, Baudhuin L, Fu Y-P, Goodman SG, Hasan A, Iturriaga E, Lerman A, Sidhu M, Tanguay J-F, Wang L, Weinshilboum R, Welsh R, Rosenberg Y, Bailey K, Rihal C. Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs Conventional Clopidogrel Therapy on Ischemic Outcomes After Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial. JAMA 202025;324:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our data is derived from trials available in the public domain.