Abstract
Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a rare but highly morbid complication after adenoviral vector-based SARS-CoV-2 vaccination. The pre-VITT syndrome is defined as vaccine-induced immune thrombocytopenia without thrombosis typically presenting with new-onset headache. This review aims to identify at-risk patients before complications such as cerebral venous sinus thrombosis occur. We review previously published reports of 19 patients (median age 35 years, range 23–74; 16 females) who met the diagnostic criteria for a pre-VITT syndrome. Seven patients progressed to VITT, 12 patients did not. Patients who experienced VITT received delayed treatment. The median interval between the onset of headache and VITT-treatment (i.e. anticoagulation, immune globulins, or corticosteroids) was 5 days (range 1–8 days) compared with 2 days (0–5 days) in those without subsequent VITT (P = 0.033). The interval from onset of headache to anticoagulation was longer in patients with VITT (median 7 vs. 2 days; range 3–9 vs. 0–7 days; P = 0.01). Anticoagulation was safe in all patients with a pre-VITT syndrome as no haemorrhagic complications occurred after anticoagulation was started despite low platelets. The transient decline of platelet count after admission was significantly more pronounced in patients who progressed to VITT (median 67 vs. 0 × 103/µL; range 0–77 × 103/µL vs. 0–10 × 103/µL; P = 0.005). d-dimers did not differ between groups. Pre-VITT syndrome is a ‘red flag’ and allows to identify and preemptively treat patients at-risk of further progression to VITT. However, it must be distinguished from post-vaccination immune thrombocytopenia.
Keywords: Pre-VITT syndrome, VITT, TTS, Thrombocytopenia, Headache, Anticoagulation
Graphical Abstract
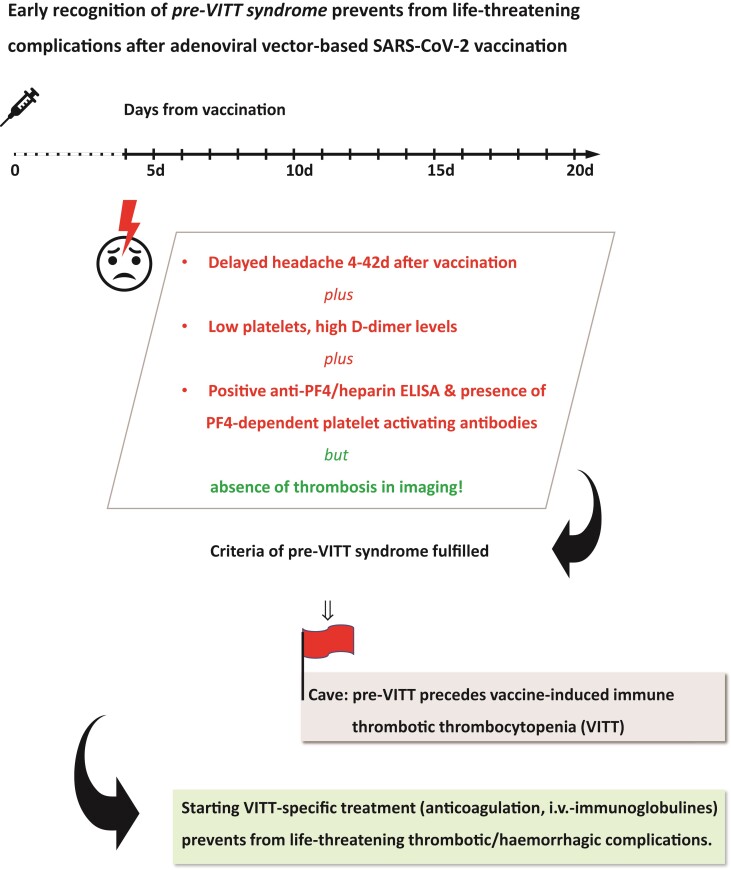
Graphical Abstract.
Introduction
Vaccine-induced immune thrombotic thrombocytopenia (VITT), currently also termed thrombosis with thrombocytopenia syndrome (TTS) and previously termed vaccine-induced prothrombotic immune thrombocytopenia (VIPIT), is a rare but highly morbid complication after adenoviral vector-based vaccination against SARS-CoV-2.1,2 Early reports of fulminant cerebral venous sinus thrombosis (CVST) in VITT patients showed mortality rates up to 60%, leading to the temporary discontinuation of Oxford-AstraZeneca’s vaccine (ChAdOx1 nCoV-19) in several European countries or Johnson & Johnson’s vaccine (AD26.COV2.S) in the USA.1,3,4 This high mortality was mainly caused by delayed start of treatment with therapeutic-dose anticoagulants and intravenous immunoglobulins (IVIG). Recent reports suggest that early recognition and immediate treatment of VITT may lower morbidity and mortality.5 At the same time, however, current guideline criteria regard the manifestation of thrombosis as a mandatory finding to diagnose VITT and consequently also as prerequisite to provide VITT-specific treatment (i.e. IVIG, therapeutic-dose anticoagulation, or corticosteroids).2,6–8
The recent description of a pre-VITT syndrome (i.e. vaccine-induced immune thrombocytopenia without thrombosis) now opens the possibility to identify patients at risk even before life-threatening complications such as CVST or other thromboses occur.9,10 Based on current data, this pre-VITT syndrome may manifest clinically as severe headache occurring 4–18 days after adenoviral vector-based SARS-CoV-2 vaccination.9 Because complete VITT may manifest within 6 weeks after adenoviral vector-based SARS-CoV-2 vaccination, it is reasonable to speculate that also pre-VITT syndrome may manifest after Day 18.6
In contrast to VITT, pre-VITT syndrome does not demonstrate thrombosis on imaging, while laboratory tests demonstrate typical thrombocytopenia (<150 × 103/µL) and elevated d-dimer levels (the pre-VITT patients identified up to now all had d-dimer levels >1.5 mg/L).9 Positive platelet factor 4 (PF4)/heparin IgG ELISA and a positive functional test for PF4-dependent platelet-activating antibodies eventually confirm the diagnosis.
Two dilemmas are clinically highly relevant for the recognition and treatment of pre-VITT. Firstly, headache is one of the most common adverse events reported after vaccination. However, these common headaches typically occur during the first 3 days following vaccination.11 Secondly, it is increasingly recognized that adenoviral vector-based SARS-CoV-2 vaccination can induce immune thrombocytopenia (ITP). These patients are at risk for major bleeding. Therefore, as headaches as well as thrombocytopenia after SARS-CoV-2 vaccination include aetiologies other than pre-VITT/VITT, the objective of this review is to introduce a clinical pathway to diagnose and manage patients presenting with delayed headache after adenoviral vector-based SARS-CoV-2 vaccination. As the further global roll-out of SARS-CoV-2 vaccines in low-to-middle-income countries will strongly rely on adenoviral vector-based vaccines,12,13 we also provide a pragmatic approach to manage patients in areas with limited access to laboratory and radiological assessments.
Methods
We used the Pubmed database to search for case reports on ‘VITT’, ‘VIPIT’, and ‘TTS’ with additional search terms ‘COVID-19 vaccination’, ‘SARS-CoV-2 vaccination’, ‘post-vaccinal headache’, and ‘post-vaccinal CVST’. We then selected published case reports and case series, if patients fulfilled the following criteria recently introduced to define the pre-VITT syndrome9:
Adenoviral vector-based SARS-CoV-2 vaccination (AstraZeneca or Johnson & Johnson) 4–42 days previously.6
Thrombocytopenia with or without elevated d-dimer level.
Absence of CVST and other thrombosis on imaging [e.g. in contrast-enhanced computed tomography (CT) angiography, magnetic resonance (MR) angiography, or digital subtraction angiography].
Positive anti-PF4/heparin IgG ELISA.
Clinical presentation with severe new-onset headache (Note: headache is the most frequent cause for hospital admission, but also other symptoms suggestive of procoagulant activity like leg pain, abdominal pain, or respiratory discomfort might potentially present as a pre-VITT syndrome).
We then compared patients with pre-VITT syndrome who subsequently progressed to VITT to patients who did not develop thrombotic complications. To identify factors potentially contributing to different outcomes in either group, we analyzed demographic data, initial laboratory findings, latency to and type of VITT-specific treatment, and clinical presentation of headache. Mann–Whitney U test was used to test for significant differences between groups (with a significance level of 0.05).
Results
To date, 20 patients fulfilling the diagnostic criteria of the pre-VITT syndrome are published in the literature.9,14–20 In one initial case series a patient was reported who developed a pre-VITT syndrome while being anticoagulated with a vitamin K antagonist.9 As this patient is not fulfilling the strict criteria for assessing the relevance of early start of anticoagulation after onset of pre-VITT, we removed this patient from this review. Consequently, 19 patients were included for further analysis (median age 35 years, range 23–74; 16 females). Demographic data, laboratory findings, treatment data, and imaging results are summarized in Supplementary material online, Table 3.
Eighteen patients (i.e. 94.7%) developed pre-VITT syndrome after a first-dose ChAdOx1 nCoV-19 vaccination (Oxford/AstraZeneca). All of these 18 patients presented with delayed headache as leading symptom. To date, only one patient was described with a pre-VITT syndrome after AD26.COV2.S vaccination (Johnson & Johnson).14 Unlike all other patients reviewed here, this patient did apparently not complain of headache, but rather of lower leg pain. Here, repeated ultrasound failed to demonstrate deep vein thrombosis and also CT of the chest, abdomen and pelvis as well as magnetic resonance imaging of the brain revealed no thrombosis. It seems reasonable to assume that a pre-VITT syndrome with delayed headache as leading symptom may also precede VITT after AD26.COV2.S vaccination. In a report by Thaler et al.,19 a female patient developed delayed headache without other symptoms, but only after hospital admission due to post-vaccination haematomas which led to the diagnosis of a pre-VITT syndrome.
One initial report described 11 patients (mean age 46 years, range 23–74 years, nine females) that presented with severe headaches at 4–18 days after ChAdOx1 nCoV-19 vaccination.9 On the first admission, none of these patients showed signs of CVST in contrast CT or MR venography, however, laboratory tests demonstrated thrombocytopenia, high d-dimer levels, and also positive PF4/heparin IgG ELISA and PF4-dependent platelet-activating antibodies. Moreover, in a subset of these patients, early initiation of VITT-specific therapy prevented progression to VITT, and patients remained free of thrombotic or haemorrhagic complications. The typical temporal evolution from pre-VITT syndrome to subsequent progression to VITT is illustrated by the following case: a 48-year-old previously healthy woman presented to a local hospital 7 days after first-dose ChAdOx1 nCOV-19 vaccination with a 2-day history of chills and severe right frontotemporal headache. Laboratory analysis showed moderate thrombocytopenia (102 × 103/µL); d-dimer levels were not measured. A cerebral CT scan including contrast venography was normal without evidence of CVST and the patient was discharged home without any treatment. Three days later, the patient was re-admitted to another hospital after being found with reduced consciousness (Glasgow Coma Scale 12), aphasia, and right-sided hemiparesis without cardiopulmonary symptoms. A CT scan revealed intracerebral haemorrhage (ICH) in the left frontotemporal lobe. Again, CT angiography showed no signs of CVST, but an incomplete thrombosis of the right jugular vein, and pulmonary embolism in the right inferior lobe. Platelet count was reduced to 35 × 103/µL and d-dimer levels were elevated to 34 mg/L. Vaccine-induced immune thrombotic thrombocytopenia was suspected and specific therapy was started with both IVIG (1 g per kg body weight per day over 2 days) and dexamethasone (40 mg/day for 4 days). Digital subtraction angiography ruled out arteriovenous malformation, aneurysms, or signs of CVST. Within a few hours, the patient’s clinical status deteriorated. A follow-up CT showed increased cerebral swelling, which led to decompressive hemicraniectomy. The next day, anticoagulation with argatroban was initiated. Vaccine-induced immune thrombotic thrombocytopenia was eventually confirmed by positive PF4/heparin IgG ELISA and the presence of PF4-dependent platelet-activating antibodies. One week later, the patient was referred to a rehabilitation facility, platelet count was normalized, and d-dimer level only slightly elevated.
In addition to the initial case series with 11 patients (including the patient who was excluded from this review due to pre-existing vitamin K antagonist treatment), a recent literature search (as of January 1, 2022) found nine additional reports of patients who met the criteria for pre-VITT syndrome (Table 1 and supplementary material online, Table 3).14–20 All 19 patients reviewed here received anticoagulation but at different times during the disease course. Among them, in 15 patients anticoagulation was combined with IVIG, and 7 of these 15 patients received corticosteroids. Overall, 7 of the 19 patients eventually progressed to VITT, while 12 patients did not. In the seven patients with subsequent VITT, four patients experienced ICH with evidence of CVST in three; six patients showed extracranial thrombosis (three pulmonary embolism, two splanchnic vein thrombosis, two limb vein thrombosis).9,14,15,17 One patient died.9 In all 18 patients who survived, platelet counts and d-dimer levels normalized following treatment and also headaches resolved within a few days. Importantly, anticoagulation in the pre-VITT syndrome appears to be safe, as no patients experienced haemorrhagic complications after initiation of anticoagulation despite low platelet counts. In fact, the degree of platelet count reduction indicates more fulminant platelet activation by the anti-PF4-antibodies and, therefore, a more prothrombotic state rather than an increased bleeding risk.21 All four patients with haemorrhagic complications due to VITT developed ICH before anticoagulation was started.9,15 In three of these patients, ICH was already established when neuroimaging showed subsequent VITT. In the remaining patient, subsequent VITT was accompanied by CVST without ICH. Only after treatment with IVIG was started without anticoagulation, the patients developed secondary ICH. Anticoagulation was then started 3 days later without further deterioration.
Table 1.
Clinical characteristics of patients with subsequent vaccine-induced immune thrombotic thrombocytopenia compared with patients without vaccine-induced immune thrombotic thrombocytopenia
| Patients with subsequent VITT | Patients without subsequent VITT | P | |
|---|---|---|---|
| n | 7 | 12 | |
| Female (n) | 4 | 12 | |
| Age (years) | 32 (24–63) | 40 (23–74) | n.s. |
| Onset of headachea (days after vaccination) | 7 (5–18) | 9 (3–14) | n.s. |
| Start of any VITT-therapya (days from onset of headache) | 5 (1–8) | 2 (0–5) | 0.033 |
| Start of anticoagulationa (days from onset of headache) | 7 (3–9) | 2 (0–7) | 0.01 |
| Start of IVIGsa (days from onset of headache) | 3 (0–5) | 3 (1–8) | n.s. |
| Initial platelet count (×103 per µL) | 97 (61–136) | 50 (12–112) | 0.014 |
| Decline of platelet count (×103 per µL) | 67 (0–77) | 0 (0–10) | 0.005 |
| Lowest platelet count (×103 per µL) | 39 (26–93) | 50 (12–105) | n.s. |
| Platelet count at discharge (×103 per µL) | 185 (130–374) | 153 (108–232) | n.s. |
| d-dimer level (mg/L) | 28.4 (4.8–36.0) | 9.8 (1.7–35.0) | n.s. |
| PF4/heparin ELISA (optical density) | 2.77 (1.45–3.47) | 3.00 (1.93–3.60) | n.s. |
Results are given by median (range). P-values are given when Mann–Whitney U test showed significant differences between groups (significance level is .05); ‘n.s.’ (not significant) is inserted where statistical analysis did not show significant differences.
Individual data were reported for n = 17 patients; for two patients detailed information was not included in the respective report.
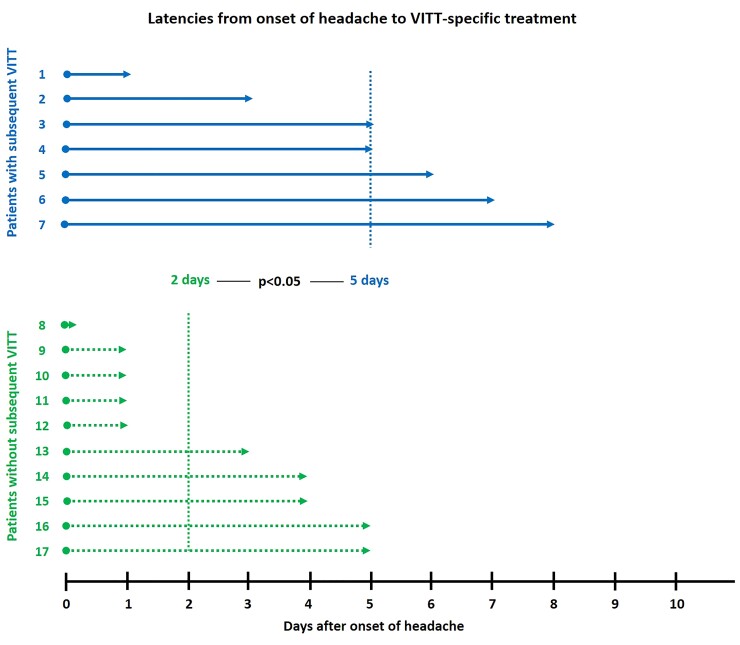
For all but two patients (Patients 18 and 19 in the supplementary material online, Table 3), detailed information on the time of onset of headache was provided in the respective reports.20 The two remaining patients are not included in the following statistical analysis of time intervals between onset of headache and start of VITT-specific treatment. Both patients remained free of thrombotic complications. Patients who progressed to VITT received delayed treatment with a median interval between onset of headache to any VITT-specific treatment (i.e. either anticoagulation, IVIG, or corticosteroids) of 5 days compared with 2 days in patients without subsequent VITT (range 1–8 vs. 0–5 days; P = 0.033; see Figure 1). Even more pronounced were the differences with regard to initiation of anticoagulation: the median time delay from onset of headache to anticoagulation was 7 days in patients with subsequent VITT compared with 2 days in patients without progression to VITT (range 3–9 vs. 0–7 days; P = 0.01). Of note, of those four patients who were treated with anticoagulation only and did not receive either IVIG or corticosteroids, three patients remained free of subsequent thrombosis. In these three patients, latency to anticoagulation was 2.3 days. In the remaining patient anticoagulation (apixaban) was started as late as 7 days after onset of headache, when she progressed to VITT with pulmonary embolism.
Figure 1.
Latencies from onset of headache to vaccine-induced immune thrombotic thrombocytopenia-specific treatment: latencies to vaccine-induced immune thrombotic thrombocytopenia-specific treatment of seven patients who developed subsequent vaccine-induced immune thrombotic thrombocytopenia are illustrated by straight arrows (blue), median time to start of vaccine-induced immune thrombotic thrombocytopenia-specific treatment was 5 days. Latencies of 10 patients who were prevented from subsequent vaccine-induced immune thrombotic thrombocytopenia are illustrated by dotted arrows (green), median time to start of VITT-specific treatment was 2 days (P < 0.05).
Surprisingly, in patients with subsequent VITT, platelet count at admission was higher compared with patients without progression to VITT (median 97 vs. 50 × 103/µL; range 61–136 × 103/µL vs. 12–112 × 103/µL; P = 0.014). However, the transient decline of platelet count after admission was significantly more extensive in patients with subsequent VITT (median 67 vs. 0 × 103/µL; range 0–77 × 103/µL vs. 0–10 × 103/µL; P = 0.005). d-dimer levels did not differ between groups (median 28.4 vs. 9.8 mg/L; range 4.8–36.0 vs. 1.7–35.0 mg/L; P = 0.088). The higher initial platelet count in those who progressed to VITT is an unexpected finding. Potentially these patients had a substantially higher baseline platelet count and overall many more platelets were activated intravascularly than in the patients who did not develop thrombosis.
Headache semiology or intensity did not discriminate well between patients with and without further development of cerebrovascular complications in pre-VITT syndrome. A more detailed description of headache phenomenology is provided in Salih et al.9 Headaches were mostly of tension-type and holocephalic with a high intensity (4–10 on a Numeric Rating Scale 1–10). In two patients, tension-type headaches were lateralized to the right hemisphere. In one of them a follow-up CT scan demonstrated ICH, but in the left hemisphere (see detailed report of the index patient above). The other patient did not show haemorrhagic or thrombotic complications on follow-up and did not develop VITT. In one patient, who also remained free of cerebrovascular complications, onset of headache was thunderclap-like. In all other patients, headache had a gradual onset including those patients who presented with ICH on follow-up.
Discussion
Clinical presentation
The prevalence of headache during the first week (i.e. Days 0–7) after AD26.COV2.S vaccination (Johnson & Johnson) was reported to be 52.2% with a peak-prevalence of 50.8% on Day 1, compared with 7.4% on Day 7.11 Headaches occurring at Days 1–3 after adenoviral vector-based SARS-COV-2 vaccination usually represent a flu-like clinical picture without specific treatment implication. In contrast, patients with delayed headache (i.e. 4–42 days) after adenoviral vector-based SARS-CoV-2 vaccination require immediate work-up to correctly diagnose pre-VITT syndrome or VITT. It should be acknowledged that because headache is common after vaccination, there is some risk to act overly presumptuous in the assessment of pre-VITT syndrome. This could not only lead to unnecessary costs but also put patients at risk from unwarranted anticoagulation therapy. Therefore, we emphasize that in addition to clinical symptoms (e.g. new-onset headache), mandatory laboratory criteria (i.e. thrombocytopenia or drop in platelets and elevated d-dimer levels) must be met to justify our proposed investigation and treatment before VITT. Finally, a positive anti-PF4 ELISA must be confirmed before pre-VITT syndrome can be diagnosed. Considering the extensive medical support required and the high morbidity and mortality in (pre-)VITT patients, we believe that our proposed pathway is justified from both a financial and risk-benefit perspective.
Based on the review of currently published case reports, headache semiology or intensity did not discriminate well between patients with and without further development of cerebrovascular complications in pre-VITT syndrome. Pre-VITT syndrome may be more frequent than VITT with CVST in patients presenting with delayed new-onset headache after adenoviral vector-based SARS-CoV-2 vaccination. Most patients with pre-VITT syndrome presented with severe headache (94.7%), while the prevalence of headache in patients with VITT and CVST was reported to be only 49.4%.10
Clinically it is highly relevant to discriminate pre-VITT from post-vaccination ITP.22–26 Immune thrombocytopenia is caused by platelet glycoprotein-specific antibodies, which induce increased phagocytosis of platelets and eventually also impaired platelet production by megakaryocytes. Immune thrombocytopenia can be triggered by adenoviral vector-based SARS-CoV-2 vaccination. However, these patients do not present with severe headache and their d-dimer levels are not strongly elevated (see Table 2). In contrast to pre-VITT patients, they can present with petechiae and haematoma other than intracranial haemorrhage.
Table 2.
Platelet count, d-dimer level, and anti-PF4 ELISA findings in pre-VITT syndrome, vaccine-induced immune thrombotic thrombocytopenia, vaccine-induced immune thrombocytopenia, and non-VITT induced thrombosis
| Pre-VITT syndrome | VITT | Vaccine-induced immune thrombocytopenia | Non-VITT induced thrombosis | |
|---|---|---|---|---|
| Platelet count | ↓ | ↓ | ↓ | ↔ |
| d-dimer level | ↑–↑↑ | ↑↑ | ↔–(↑) | ↑ |
| Anti-PF4 ELISA | ↑–↑↑ | ↑–↑↑ | ↔–(↑) | ↔ |
Low ↓; normal ↔; moderately elevated ↑; strongly elevated ↑↑.
Pathophysiology
While PF4-dependent, platelet-activating antibodies form the common pathophysiological basis of both VITT and pre-VITT syndrome,4 the specific aetiology of headache in the absence of CSVT in patients with pre-VITT syndrome remains unclear. Results of multimodal angiographies in published cases clearly argue against the hypothesis that CVST was already present but overlooked at a time when headaches first led to hospital admission. However, disseminated manifestation of microthrombosis in smaller cortical veins could cause multifocal stasis of venous blood flow. Such microthromboses could cause meningeal irritation and headaches and also potentially progress to CVST or ICH. The detection of such small thrombi in the cerebral venous and sinus system may also depend on the mode of radiological technique. While CT and MR venography have similar sensitivity to detect CVST,27 certain MR image modes like susceptibility-weighted imaging or the use of high Tesla MRI might be a more sensitive approach to detect potential microthromboses. The radiological challenge to identify early signs of small CVST is illustrated in a case reported by Ikenberg et al.15 A first brain MR was regarded void of CSVT when the patient initially presented due to headache 7 days after first-dose ChAdOx1 nCov-19 vaccination. When the patient was re-admitted 3 days later with obvious CVST of the left transverse and sigmoidal sinus, in a focused retrospective analysis of the first MRI subtle irregularities were noted at the bottom of the left transverse sinus in the contrast-enhanced T1w image which might indicate beginning thrombus formation at an early stage of CVST.15 Interestingly, in a case report of pre-VITT after the Johnson & Johnson vaccine, the patient complained of severe lower extremity pain without radiologic evidence of thrombosis on repeated ultrasound. As there are no microvessels in the large lower limb veins, we speculate that pain in the vascular bed could also result from an inflammatory process.
Vaccine-induced immune thrombotic thrombocytopenia shares common immune mechanism with heparin-induced thrombocytopenia (HIT) and other autoimmune thrombotic thrombocytopenia syndromes. The development of CVST secondary to HIT is rare,28 suggesting a specific pathophysiological factor in VITT leading to a predominantly intracranial manifestation. In antiphospholipid syndrome, multifocal stasis of venous blood flow has been discussed as pathophysiological factor contributing to headache.29 It might be speculated that VITT-associated antibodies additionally attack intracranial structures inducing inflammatory cascades in meningoencephalic tissue. Recently another hypothesis has been raised.30 Based on the finding that release of DNA from leucocytes by NETosis, i.e. the formation of neutrophil extracellular traps (NETs), is one of the main mediators of the prothrombotic state, impaired degradation of DNA can promote thrombosis formation. Preliminary findings based on systems biology and transcriptomics indicate a low DNASE1 expression in central nervous system endothelial cells that can potentially lead to increased DNA half-life and persistent procoagulant activity of NETs.31
A mouse model indicates the possibility that co-operative signalling of two platelet receptors, C-type lectin-like receptor-2 (CLEC-2) and GPIIb/IIIa, can be an underlying cause.32 C-type lectin-like receptor-2 antibody F(ab) fragments trigger within minutes a CVST-like thrombotic syndrome in mice, with platelet consumption and death. Thrombi manifest mainly in the cortical venules and transcranial intravital microscopy even showed rapidly progressing thrombosis in the superior sagittal sinus. Interfering with CLEC-2 signalling or inhibition of GPIIb/IIIa completely blocked platelet activation and CVST. Blocking GPIIb/IIIa after onset of neurological symptoms protected mice from platelet consumption, CVST, and death, which was not seen after treatment with heparin. The authors conclude that aberrant platelet activation can be a major trigger of CVST and potential target for treatment. In contrast, post-vaccination ITP is caused by increased platelet phagocytosis eventually aggravated by impaired platelet production due to autoantibodies binding to platelet glycoproteins.
We have previously reported a patient who developed a pre-VITT syndrome while being anticoagulated with a vitamin K antagonist.9 This patient did not develop CVST despite presenting with severe headache. This further supports the concept that sufficient anticoagulation during the time platelet-activating antibodies are present can prevent the development of thrombosis. However, vitamin K antagonists should never be started in VITT, because the procoagulant imbalance caused by early reduction of the anticoagulant factor protein C and delayed reduction of the procoagulant clotting factors. In our previously reported patient, however, a stable therapeutic range was already reached long before onset of the pre-VITT syndrome, with balanced low levels of prothrombotic and anticoagulatory factors, which strongly reduces the risk for new thrombotic complications. Nevertheless, vitamin K antagonists should be preferentially avoided in these patients as protein C seems to be important for the prevention of microvascular thrombosis in patients with procoagulant syndromes related to intravascular platelet activation.33–35
Assessment, diagnosis, and treatment
The number of published reports on patients presenting with a pre-VITT syndrome reviewed here is still low (n = 19). Also, two reports did not provide detailed information on time treatment was initiated after headaches started. Statistical analysis of some parameters was therefore performed only for the data of the remaining 17 patients. Thus, conclusions outlined in this review need to be interpreted with some caution. Future studies on patients with a pre-VITT syndrome might identify more specific clinical and laboratory markers defining a high risk of further progression to VITT. Although this review has a few limitations, some important conclusions can be drawn for clinical assessment, diagnosis, and treatment.
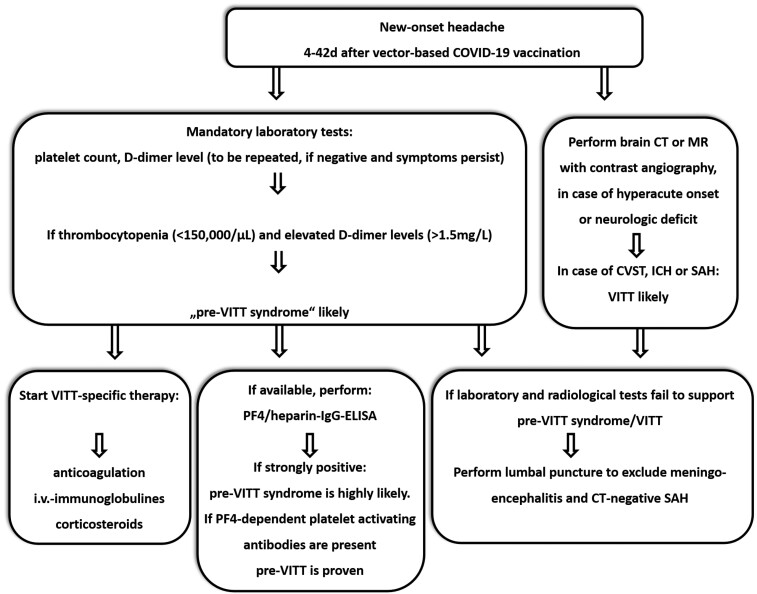
Based on current knowledge on the pre-VITT syndrome and its high potential for subsequent progression to VITT we here suggest a clinical pathway to manage patients presenting with delayed new-onset headache as leading symptom after adenoviral vector-based SARS-CoV-2 vaccination (see Figure 2). This clinical pathway should allow a reasonable diagnostic and therapeutic management of patients at-risk even in non-industrialized areas with limited health care system resources.
Figure 2.
Suggested assessment, diagnosis, and treatment of pre-vaccine-induced immune thrombotic thrombocytopenia syndrome: a diagnostic approach to the patient presenting with headache after adenoviral vector-based immunization. A detailed explanation is provided in the text.
In patients who present with new-onset, persistent headache at 4–42 days after adenoviral vector-based SARS-CoV-2 vaccination, focused laboratory testing including platelet count should be initiated. The platelet count and d-dimer level are the most important parameters to differentiate between pre-VITT, VITT, vaccine-induced ITP; and non-VITT induced thrombosis (see Table 2). If onset of headache was hyperacute (‘thunderclap’-like) or if the headache is accompanied by a neurological deficit, neuroimaging (CT or MR) with arterial and venous contrast-based angiography is mandatory. The radiological spectrum of VITT-associated cerebrovascular complications comprises CVST, ICH, and potentially also subarachnoid haemorrhages. If neuroimaging detects an alternative headache aetiology not attributable to VITT, subsequent treatment should follow according to the detected pathology and respective guidelines. In the absence of pathological findings on neuroimaging, pre-VITT syndrome remains a tentative diagnosis. If laboratory tests reveal thrombocytopenia with elevated d-dimer levels, the implications may be two-fold. Firstly, whenever possible a PF4/heparin IgG ELISA should be performed. Secondly, the immediate start of VITT-specific therapy is recommended. In centres where IVIGs are not available, therapeutic-dose anticoagulation should be started and corticosteroids might be given as adjunct. In case argatroban or fondaparinux are not available, the use of DOACs may be recommended or, alternatively, even heparin, as recent data strongly suggests that—in contrast to HIT—it can be safely used in the majority of VITT patients.4,21,36 Efforts to arrange subsequent neuroimaging and PF4/heparin IgG ELISA are recommended wherever possible. Treatment response should be evaluated by remission of headache as well as increase of platelet counts on follow-up. Elevation of d-dimer levels alone, without clinical symptoms and thrombocytopenia, are not specific enough to trigger comprehensive work-up to assess for VITT/pre-VITT syndrome.
If laboratory tests show normal platelet counts and neuroimaging fails to detect pathological findings, a lumbar puncture should be considered depending on the presence of other symptoms like altered vigilance and meningism or fever to exclude SAH, bacterial and viral meningoencephalitis, or the recently reported post-ChAdOx1-nCox19 vaccination autoimmune encephalitis.37 If cerebro-spinal fluid analysis is normal and symptoms persist, a repeated platelet count is recommended. Especially in the early phase of VITT and pre-VITT syndrome, platelets may remain within normal limits.38 Similar to HIT, a further decrease in platelets compared with absolute platelet counts may more appropriately indicate ongoing platelet consumption. In vaccination-induced ITP, however, d-dimer levels are either normal or moderately increased. Anti-PF4-antibodies are usually absent or present in low titres and do not activate platelets (see Table 2). Whether both entities pre-VITT and ITP can occur concomitantly in the same individual is currently unknown.
Conclusion
Recognition of the pre-VITT syndrome defines a window of opportunity to prevent patients from further progression to VITT, including the potentially fatal manifestation of CVST. The clinical pathway presented in this review should help to early identify patients with a pre-VITT syndrome among those presenting with post-vaccinal headache and to differentiate pre-VITT from vaccination-induced ITP. As vaccination will remain the most effective approach to contain the COVID-19 pandemic sufficient management of complications should help to maintain the acceptance of vaccinations worldwide.
Supplementary Material
Acknowledgements
We thank Dr Sascha Tafelski (Charité-Universitätsmedizin Berlin) for supporting us in performing the statistical analysis of our data.
Conflict of interest: F.S. reports personal fees from Bristol-Myers Squibb as well as MD Horizonte and AMEOS, both outside the submitted work. S.K. reports no conflicts of interests. L.S. was supported within the Gerhard-Domagk-Research-Program by the University Medicine Greifswald. T.T. reports grants from Deutsche Forschungsgemeinschaft, during the conduct of the study; personal fees, non-financial support, and other from Bristol-Myers Squibb, personal fees, non-financial support and other from Pfizer, personal fees from Bayer, personal fees, non-financial support and other from Chugai Pharma, non-financial support and other from Novo Nordisk, personal fees from Novartis, non-financial support and other from Daichii Sankyo, all of which are outside the submitted work. A.G. reports grants and non-financial support from Aspen, Boehringer Ingelheim, MSD, Bristol-Myers Squibb (BMS), Paringenix, Bayer Healthcare, Gore Inc., Rovi, Sagent, Biomarin/Prosensa, personal fees from Aspen, Boehringer Ingelheim, MSD, Macopharma. This work has been supported by Deutsche Forschungsgemeinschaft, Grant/Award Number: 374031971-TRR 240. M.E. received funding from DFG under Germany´s Excellence Strategy—EXC-2049–390688087, BMBF, DZNE, DZHK, EU, Corona Foundation, and Fondation Leducq. M.E. also reports grants from Bayer and fees paid to the Charité from AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Amgen, GSK, Sanofi, Covidien, Novartis, Pfizer, all outside the submitted work.
Contributor Information
Farid Salih, Klinik und Hochschulambulanz für Neurologie, Charité-Universitätsmedizin Berlin, Charité-Platz 1, D-10117 Berlin, Germany.
Siegfried Kohler, Klinik und Hochschulambulanz für Neurologie, Charité-Universitätsmedizin Berlin, Charité-Platz 1, D-10117 Berlin, Germany; Department of Neurology, Sana Kliniken Landkreis Biberach, Marie Curie Str. 4, 88400 Biberach, Germany; ExcellenceCluster NeuroCure, Charité-Platz 1, 10117 Berlin, Germany.
Linda Schönborn, Universitätsmedizin Greifswald, Institute of Immunology and Transfusion Medicine, Sauerbruch-Straße, 17489 Greifswald, Germany.
Thomas Thiele, Universitätsmedizin Greifswald, Institute of Immunology and Transfusion Medicine, Sauerbruch-Straße, 17489 Greifswald, Germany.
Andreas Greinacher, Universitätsmedizin Greifswald, Institute of Immunology and Transfusion Medicine, Sauerbruch-Straße, 17489 Greifswald, Germany.
Matthias Endres, Klinik und Hochschulambulanz für Neurologie, Charité-Universitätsmedizin Berlin, Charité-Platz 1, D-10117 Berlin, Germany; ExcellenceCluster NeuroCure, Charité-Platz 1, 10117 Berlin, Germany; Center for Stroke Research Berlin, Charité-Platz 1, 10117 Berlin, Germany; German Center for Neurodegenerative Diseases (DZNE), partner site Berlin, Charité-Platz 1, 10117 Berlin, Germany; German Centre for Cardiovascular Research (DZHK), partner site Berlin, Charité-Platz 1, 10117 Berlin, Germany.
Author contributions
F.S. and S.K. performed the literature search, collected, and analyzed clinical data; L.S. and T.T. collected and analyzed clinical data; A.G. and M.E. analyzed data and co-supervised the study. All authors wrote and revised the manuscript.
Lead author biography

Matthias Endres is Professor and Chair of Neurology at the Charité Hospital in Berlin. He is also on the Board of Directors of the Center for Stroke Research Berlin. His major research interests are preventive vascular mechanisms, mechanisms of cell death, regeneration and functional outcome, heart-brain interaction, post-stroke depression, and telemedicine. Matthias Endres is author of more than 500 research articles, reviews, and editorials. He is a member of the German National Academy of Sciences Leopoldina and Visiting Professor at the University of Oxford, U.K.
Data availability
The data underlying this article are available in the article and in its online Supplementary material.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
References
- 1. Long B, Bridwell R, Gottlieb M. Thrombosis with thrombocytopenia syndrome associated with COVID-19 vaccines. Am J Emerg Med 2021;49:58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19). https://www.who.int/publications/i/item/WHO-2019-nCoV-TTS-2021.1 (20 February 2022).
- 3. Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, Wiedmann M, Aamodt AH, Skattør TH, Tjønnfjord GE, Holme PA. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van de Munckhof A, Krzywicka K, de Sousa DA, van Kammen MS, Heldner MR, Jood K, Lindgren E, Tatlisumak T, Putaala J, Kremer Hovinga JA, Middeldorp S, Levi M, Arnold M, Ferro JM, Coutinho JM. Declining mortality of cerebral venous sinus thrombosis with thrombocytopenia after SARS-CoV-2 vaccination. Eur J Neurol 2022;29:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Society of Hematology . Thrombosis with thrombocytopenia syndrome. 2021. https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia.
- 7. Zazzeron L, Rosovsky RP, Bittner EA, Chang MG. Comparison of published guidelines for the diagnosis and the management of vaccine-induced immune thrombotic thrombocytopenia. Crit Care Explor 2021;3:e0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen RT. Proposed Brighton Collaboration process for developing a standard case definition for study of new clinical syndrome X, as applied to Thrombosis with Thrombocytopenia Syndrome (TTS); 2021. https://brightoncollaboration.us/wp-content/uploads/2021/04/TTS-Case-Finding-and-Definition-Process.v9.0-April-16-202115853.pdf (20 February 2022).
- 9. Salih F, Schönborn L, Kohler S, Franke C, Möckel M, Dörner T, Bauknecht HC, Pille C, Graw JA, Alonso A, Pelz J, Schneider H, Bayas A, Christ M, Kuramatsu JB, Thiele T, Greinacher A, Endres M. Vaccine-induced thrombocytopenia with severe headache. N Engl J Med 2021;385:2103–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. García-Azorín D, Do TP, Gantenbein AR, Hansen JM, Souza MNP, Obermann M, Pohl H, Schankin CJ, Schytz HW, Sinclair A, Schoonman GG, Kristoffersen ES. Delayed headache after COVID-19 vaccination: a red flag for vaccine induced cerebral venous thrombosis. J Headache Pain 2021;22:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shay DK, Gee J, Su JR, Myers TR, Marquez P, Liu R, Zhang B, Licata C, Clark TA, Shimabukuro TT. Safety Monitoring of the Janssen (Johnson & Johnson) COVID-19 vaccine – United States March April 2021. Morb Mortal Wkly Rep 2021;70:680–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization . ACT-Accelerator Strategic Plan & Budget: October 2021 to September 2022. https://www.who.int/publications/m/item/act-accelerator-strategic-plan-budget-october-2021-to-september-2022. (20 February 2022)
- 13. Gavi, the vaccine alliance . 2021. https://www.gavi.org/covax-vaccine-roll-out. (29 November 2021).
- 14. Kennedy VE, Wong CC, Hong JM, Peng T, Brondfield S, Reilly LM, Cornett P, Leavitt AD. VITT following Ad26.COV2.S vaccination presenting without radiographically demonstrable thrombosis. Blood Adv 2021;5:4662–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ikenberg B, Demleitner AF, Thiele T, Wiestler B, Götze K, Mößmer G, Lingor P. Cerebral venous sinus thrombosis after ChAdOx1 nCov-19 vaccination with a misleading first cerebral MRI scan. Stroke Vasc Neurol 2021;6:668–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khuhapinant A, Rungjirajittranon T, Suwanawiboon B, Chinthammitr Y, Ruchutrakool T. Successful venous thromboprophylaxis in a patient with vaccine-induced immune thrombotic thrombocytopenia (VITT): a case report of the first reported case in Thailand. Thromb J 2021;19:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lavin M, Elder PT, O’Keeffe D, Enright H, Ryan E, Kelly A, El Hassadi E, McNicholl FP, Benson G, Le GN, Byrne M, Ryan K, O’Connell NM, O’Donnell JS. Vaccine-induced immune thrombotic thrombocytopenia (VITT) - a novel clinico-pathological entity with heterogeneous clinical presentations. Br J Haematol 2021;195:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ryan E, Benjamin D, McDonald I, Barrett A, McHugh J, Ryan K, Enright H. AZD 1222 vaccine-related coagulopathy and thrombocytopenia without thrombosis in a young female. Br J Haematol 2021;194:553–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thaler J, Ay C, Gleixner KV, Hauswirth AW, Cacioppo F, Grafeneder J, Quehenberger P, Pabinger I, Knöbl P. Successful treatment of vaccine-induced prothrombotic immune thrombocytopenia (VIPIT). J Thromb Haemost 2021;19:1819–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thaler J, Jilma P, Samadi N, Roitner F, Mikušková E, Kudrnovsky-Moser S, Rettl J, Preiss R, Quehenberger P, Pabinger I, Knoebl P, Ay C. Long-term follow-up after successful treatment of vaccine-induced prothrombotic immune thrombocytopenia. Thromb Res 2021;207:126–130. [DOI] [PubMed] [Google Scholar]
- 21. Greinacher A, Langer F, Makris M, Pai M, Pavord S, Tran H, Warkentin TE. Vaccine-induced immune thrombotic thrombocytopenia (VITT): Update on diagnosis and management considering different resources. J Thromb Haemost 2022;20:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simpson CR, Shi T, Vasileiou E, Katikireddi SV, Kerr S, Moore E, McCowan C, Agrawal U, Shah SA, Ritchie LD, Murray J, Pan J, Bradley DT, Stock SJ, Wood R, Chuter A, Beggs J, Stagg HR, Joy M, Tsang RSM, de Lusignan S, Hobbs R, Lyons RA, Torabi F, Bedston S, O'Leary M, Akbari A, McMenamin J, Robertson C, Sheikh A. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med 2021;27:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koch M, Fuld S, Middeke JM, Fantana J, von Bonin S, Beyer-Westendorf J. Secondary immune thrombocytopenia (ITP) associated with ChAdOx1 covid-19 vaccination – a case report. TH Open 2021;5:e315–e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crickx E, Moulis G, Ebbo M, Terriou L, Briantais A, Languille L, Limal N, Guillet S, Michel M, Mahevas M, Godeau B. Safety of anti-SARS-CoV-2 vaccination for patients with immune thrombocytopenia. Br J Haematol 2021;195:703–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uaprasert N, Panrong K, Tungjitviboonkun S, Dussadee K, Decharatanachart P, Kaveevorayan P, Shoosanglertwijit R, Watanaboonyongcharoen P, Bunworasate U, Rojnuckarin P. ChAdOx1 nCoV-19 vaccine-associated thrombocytopenia: three cases of immune thrombocytopenia after 107 720 doses of ChAdOx1 vaccination in Thailand. Blood Coagul Fibrinolysis 2022;33:67–70. [DOI] [PubMed] [Google Scholar]
- 26. Kim G, Choi EJ, Park HS, Lee JH, Lee JH, Lee KH. A case report of immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. J Korean Med Sci 2021;36:e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu W, Gao L, Li T, Ramdoyal ND, Zhang J, Shao A. The performance of CT versus MRI in the differential diagnosis of cerebral venous thrombosis. Thromb Haemost 2018;118:1067–1077. [DOI] [PubMed] [Google Scholar]
- 28. Bauman MMJ, Naylor RM, Wijdicks EF. HIT in the head: a systematic review of cerebral venous sinus thrombosis in classical and autoimmune heparin-induced thrombocytopenia. J Thromb Thrombolysis 2021;52:952–961. [DOI] [PubMed] [Google Scholar]
- 29. Noureldine MHA, Haydar AA, Berjawi A, Elnawar R, Sweid A, Khamashta MA, Hughes GRV, Uthman I. Antiphospholipid syndrome (APS) revisited: would migraine headaches be included in future classification criteria? Immunol Res 2017;65:230–241. [DOI] [PubMed] [Google Scholar]
- 30. Greinacher A, Selleng K, Palankar R, Wesche J, Handtke S, Wolff M, Aurich K, Lalk M, Methling K, Völker U, Hentschker C, Michalik S, Steil L, Reder A, Schönborn L, Beer M, Franzke K, Büttner A, Fehse B, Stavrou EX, Rangaswamy C, Mailer RK, Englert H, Frye M, Thiele T, Kochanek S, Krutzke L, Siegerist F, Endlich N, Warkentin TE, Renné T. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood 2021;138:2256–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Butler LM, Hallstrom BM, Fagerberg L, Pontén Fredrik, Uhlén Mathias, Renné Thomas, Odeberg Jacob. Analysis of body-wide unfractionated tissue data to identify a core human endothelial transcriptome. Cell Syst 2016;3:287–301.e3. [DOI] [PubMed] [Google Scholar]
- 32. Stegner D, Göb V, Krenzlin V, Beck S, Hemmen K, Schuhmann MK, Schörg BF, Hackenbroch C, May F, Burkard P, Pinnecker J, Zernecke A, Rosenberger P, Greinacher A, Pichler BJ, Heinze KG, Stoll G, Nieswandt B. Foudroyant cerebral venous (sinus) thrombosis triggered through CLEC-2 and GPIIb/IIIa dependent platelet activation. Nat Cardiovasc Res 2022;1:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Warkentin TE. Ischemic limb gangrene with pulses. N Engl J Med 2015;373:642–655. [DOI] [PubMed] [Google Scholar]
- 34. Warkentin TE, Cook RJ, Sarode R, Sloane DA, Crowther MA. Warfarin-induced venous limb ischemia/gangrene complicating cancer: a novel and clinically distinct syndrome. Blood 2015;126:486–493. [DOI] [PubMed] [Google Scholar]
- 35. Warkentin TE. Venous limb gangrene during warfarin treatment of cancer-associated deep venous thrombosis. Ann Intern Med 2001;135:589–593. [DOI] [PubMed] [Google Scholar]
- 36. Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature 2021;596:565–569. [DOI] [PubMed] [Google Scholar]
- 37. Zuhorn F, Graf T, Klingebiel R, Schabitz WR, Rogalewski A. Postvaccinal encephalitis after ChAdOx1 nCov-19. Ann Neurol 2021;90:506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Page D, Zhu N, Sawler D, Sun HW, Turley E, Pai M, Wu C. Vaccine-induced immune thrombotic thrombocytopenia presenting with normal platelet count. Res Pract Thromb Haemost 2021;5:e12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary material.