Abstract
Aims
Functional decline due to skeletal muscle abnormalities leads to poor outcomes in patients with acute heart failure (AHF). The 6-minute walking test (6MWT) reliably evaluates functional capacity, but its technical difficulty for the elderly often limits its benefits. Although the Short Physical Performance Battery (SPPB) is a comprehensive measure of physical performance, its role in AHF remains unclear. This study aimed to examine the prognostic significance of SPPB compared to the 6MWT in elderly patients hospitalized for AHF.
Methods and results
We retrospectively analysed 1192 elderly patients with AHF whose SPPB and 6MWT were measured during the hospitalization. The primary outcome measure was defined as a composite of all-cause death and heart failure readmission until 1 year after discharge. Patients with lower SPPB scores (0–6, n = 373) had significantly poorer outcomes than those with higher SPPB scores (7–12, n = 819) even after multivariable adjustment [adjusted hazard ratio (HR) 1.28, 95% confidence interval (CI) 1.01–1.61; P = 0.049], similar to those with shorter 6MWT (<median) than those with longer 6MWT (adjusted HR 1.61, 95% CI 1.27–2.04; P < 0.001). Although both SPPB and 6MWT [net reclassification index (NRI) 0.139, P = 0.036 and NRI 0.350, P < 0.001, respectively] exhibited incremental prognostic value over conventional risk factors of HF, the additive prognostic effect of 6MWT was superior to that of SPPB (NRI 0.300, P < 0.001).
Conclusions
Reduced functional capacity assessed by either the SPPB or 6MWT was associated with worse outcomes in hospitalized elderly patients with AHF. The incremental prognostic value over the conventional risk factors was higher in 6MWT than in SPPB.
Trial Registration
UMIN000023929
Keywords: Physical function, Functional capacity, Frailty, Elderly
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Acute heart failure (AHF) is the main cause of hospital admissions in elderly people world wide and is characterized by increased mortality, poor quality of life, and a high economic burden on the health care systems.1 Elderly patients with heart failure (HF) have substantially impaired physical function, which is accompanied by poorer clinical outcomes. Therefore, a comprehensive assessment of physical function, including the strength and endurance of peripheral muscles, is of utmost importance.2
Cardiovascular functional capacity is often estimated by indices from maximal effort tests such as peak oxygen consumption obtained from cardiopulmonary exercise tests, or a 6-minute walking test (6MWT).3,4 Owing to its clinical applicability, 6MWT has been used as a simple, reproducible, and feasible alternative to cardiopulmonary exercise testing to evaluate functional capacity in patients with HF.5 However, because of the increasing number of elderly HF patients with orthopaedic and neurological comorbidities, it is often difficult to perform exercise tests that require maximal effort.6 Recently, functional capacity was assessed using the Short Physical Performance Battery (SPPB), a comprehensive physical performance test that comprises three timed tasks: standing balance, walking speed, and chair stand tests.7 In a reflection paper from the European Medicines Agency, the SPPB was introduced as a useful instrument to assess physical performance, and a possible classification of frailty and/or pre-frailty.8 Despite the extensive literature on the prognostic value of the SPPB in various cardiovascular diseases or even in the general population,7–10 little is known regarding its role in patients hospitalized with AHF.
Therefore, we examined the prognostic significance of the SPPB in comparison to 6MWT using data from a large-scale multicentre registry focusing on hospitalized elderly patients with AHF.
METHODS
Study design and study participants
The study design and primary results of the FRAGILE-HF registry have been previously reported in detail.11 In brief, the FRAGILE-HF study was a multicentre, prospective observational cohort study that enrolled 1,332 consecutive hospitalized elderly patients (age >65 years) with AHF who could ambulate at discharge. This registry consisted of individual patients, and only the first hospitalization during the study period was registered. Heart failure decompensation was diagnosed based on the Framingham criteria.12 The exclusion criteria were1 previous heart transplantation or presence of a left ventricular assist device,2 either chronic peritoneal dialysis or haemodialysis, and3 acute myocarditis. Patients with missing brain natriuretic peptide (BNP) or N-terminal-pro BNP data and those with a BNP level <100 pg/mL or N-terminal-pro BNP level <300 pg/mL at admission were also excluded. The study was conducted in compliance with the Declaration of Helsinki and the Japanese Ethical Guidelines for Medical and Health Research involving Human Subjects from September 2016 to March 2018 at 15 centres in Japan after obtaining approval from each participating centre’s ethics committee or institutional review board (UMIN000023929). A waiver of written informed consent from each patient was granted by the institutional review board because the study met the conditions of the Japanese ethical guidelines for epidemiological studies.
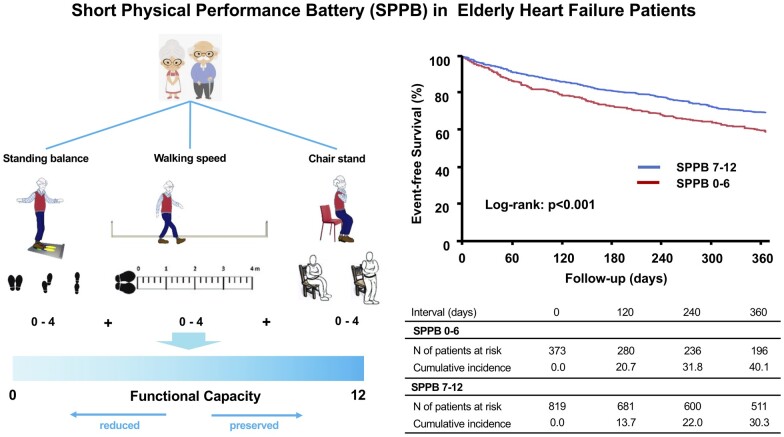
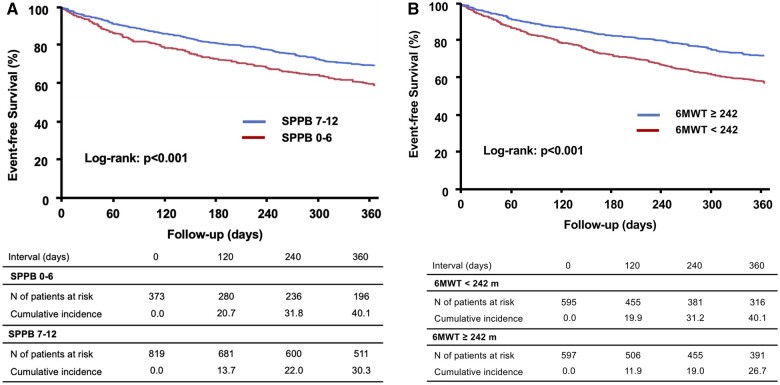
Of the 1332 patients who were enrolled, we excluded 140 patients whose data for either the SPPB score or 6-minute walking distance (6MWD) were unavailable. A total of 1192 patients were included in the analysis. Baseline patient characteristics including age, height, body weight, blood pressure, heart rate, laboratory data, and echocardiographic data were recorded or measured at the time of hospital discharge. A baseline medication was defined as medication administered at the time of discharge. Patients were divided into two groups according to the SPPB score, lower SPPB score (0–6) vs. higher SPPB score (7–12) as previously described,13 and based on the 6MWD, shorter (below the median value) vs. longer (above median value).
Short Physical Performance Battery and 6-minute walking test
The SPPB and 6MWT were evaluated by experienced physical therapists and/or HF specialists after decongestion therapy and clinical stabilization of AHF. The SPPB consists of three physical performance tests to assess the frailty domains of balance (static standing balance), gait speed (4-m walk time), and weakness (time to complete five repeated chair stands).14 Each test is scored from 0 to 4, with a total score ranging from 0 (worst performance) to 12 (best performance). For balance tests, the participants were instructed to maintain their feet in side-by-side, semi-tandem, and tandem positions for 10 s each. For the gait speed assessment test, the participants’ usual speed was timed during a 4-m walk. For the chair stand test, participants were instructed to stand up and sit down five times as quickly as possible. The 6MWT was assessed in an unobstructed hallway according to the guideline.15 Patients were instructed to walk as fast as possible between two points positioned 30 m apart and the distance walked in 6 min was recorded. Patients were allowed to use an assistive device if needed.
Outcomes
Patients were followed up at least every 3 months after discharge or according to their medical needs. For patients without follow-up in outpatient clinics, prognostic data were obtained from telephone interviews, and medical records were obtained from the other departments that took care of the patient or family. The primary outcome of interest in this analysis was a combined endpoint of all-cause death and readmission for HF within 1 year after discharge. We defined a readmission event as ‘HF readmission’ only if the criteria described in the American College of Cardiology/American Heart Association key data elements and definitions for cardiovascular endpoint events in clinical trials for HF readmissions were met.16 The first occurrence of readmission or death after discharge was considered as the outcome date.
Statistical analyses
Categorical variables are shown as numbers and percentages and were compared using the χ2 test or Fisher’s exact test, as appropriate. Continuous variables are expressed as mean and standard deviation or median and interquartile range (IQR). Based on their distribution (qualitatively judged by histogram and Q-Q plot), continuous variables were compared using Student’s t-test or Wilcoxon rank-sum test as appropriate. Two-sided P-values <0.05 were considered statistically significant. The Kaplan–Meier method was used to estimate the cumulative incidence of events, and differences were compared using the log-rank test. A Cox proportional hazards model was used to evaluate the association between each variable and the incidence of adverse events, defined as a composite of all-cause death and readmission for HF. Consistent with our previous report, we used the following conventional prognostic factors as risk-adjusting variables: age; sex; left ventricular ejection fraction; current smoking status; history of HF (de novo case or not); hypertension; diabetes mellitus; coronary artery disease; chronic obstructive lung disease; atrial fibrillation; systolic blood pressure; estimated glomerular filtration rate; haemoglobin; serum sodium level; serum albumin; log-transformed BNP; prescription of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, beta-blocker, and mineralocorticoid receptor antagonist; and New York Heart Association functional Class III/IV at discharge.11 All variables were selected a priori as they were either known predictors of outcomes in patients with HF or because of their ability to confound the association. Proportional hazard assumption violations were estimated using a generalized linear regression of scaled Schoenfeld residuals over time. Additionally, we constructed the following statistical models: Model 1, incorporating conventional prognostic factors as adjustment variables in the Cox models; Model 2, incorporating conventional prognostic factors plus the SPPB score; Model 3, incorporating conventional prognostic factors plus 6MWD; and Model 4, incorporating conventional risk factors, 6MWD, and the SPPB score. Incremental prognostic predictability was evaluated using the net reclassification improvement (NRI).
Statistical analyses were performed using the statistical software program JMP 14.0.0 (SAS Institute Inc., Cary, NC, USA) and R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient characteristics, Short Physical Performance Battery, and 6-minute walking test
The median patient age was 81 years (IQR 74–86), 57.2% were male, and the median left ventricular ejection fraction was 45% (IQR 32–60). The median SPPB score was 8 (IQR 6–11), and the median 6MWD was 242 m (IQR 150–346). The median values of each component in the SPPB score were as follows; static balance test, 4 (2–4); 4-m gait time 5.4 s (4.2–7.3); and time to complete 5 repeated chair stands, 12.8 s (9.8–16.9).
There were 373 and 819 patients in the lower SPPB scores and higher SPPB score groups, respectively. Comparisons of baseline characteristics stratified based on the SPPB scores are shown in Table 1. Patients with lower SPPB scores were older were associated with severe HF symptoms, lower estimated glomerular filtration ratio, lower serum albumin levels, and lower prescription of beta-blockers. Patients with lower SPPB scores had significantly shorter 6MWD than those with higher SPPB scores (151 ± 95 vs. 299 ± 111 m, P < 0.001). There were 595 and 597 patients in the shorter 6MWD group (<242 m), and the longer 6MWD group (greater than or equal to 242 m), respectively.
Table 1.
Baseline patients’ characteristics among those with SPPB score < 7 and ≥7
| SPPB <7 | SPPB ≥ 7 | P-value | |
|---|---|---|---|
| (n = 373) | (n = 819) | ||
| Age | 85 (80–89) | 79 (72–84) | <0.001 |
| Male | 154 (41.3) | 528 (64.5) | <0.001 |
| Body mass index | 21.3 (4.3) | 21.4 (3.5) | 0.57 |
| NYHA III or IV | 80 (21.4) | 80 (9.8) | <0.001 |
| Atrial fibrillation | 165 (44.2) | 370 (45.2) | 0.81 |
| Coronary artery disease | 137 (36.7) | 281 (34.3) | 0.46 |
| COPD | 33 (8.8) | 95 (11.6) | 0.19 |
| Diabetes mellitus | 135 (36.2) | 290 (35.4) | 0.84 |
| Hypertension | 283 (75.9) | 566 (69.1) | 0.02 |
| Laboratory data | |||
| BNP (pg/mL) | 300 (153–621) | 261 (130–459) | 0.013 |
| BUN (mg/dL) | 29 (21–41) | 25 (19–34) | <0.001 |
| Creatinine (mg/dL) | 1.38 ± 0.71 | 1.38 ± 0.88 | 0.94 |
| Haemoglobin (g/dL) | 11.3 ± 1.8 | 12.1 ± 2.0 | <0.001 |
| Albumin (g/dL) | 3.3 ± 0.5 | 3.5 ± 0.5 | <0.001 |
| Na (mEq/L) | 139.2 ± 4.0 | 139.0 ± 3.6 | 0.34 |
| K (mEq/L) | 4.3 ± 0.5 | 4.4 ± 0.5 | <0.001 |
| Medication | |||
| Beta-blocker | 257 (68.9) | 629 (76.8) | 0.005 |
| ACEI or ARB | 229 (61.4) | 586 (71.6) | 0.001 |
| MRA | 25 (6.7) | 75 (9.2) | 0.192 |
| Loop diuretics | 342 (92) | 703 (86) | 0.004 |
| Digoxin | 6 (1.6) | 28 (3.4) | 0.08 |
| Warfarin | 85 (23) | 194 (24) | 0.73 |
| DOAC | 108 (29) | 286 (35) | 0.04 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; DOAC, direct oral anticoagulant; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association functional class; SPPB, short physical performance battery.
Outcomes
During the follow-up, 138 deaths, 322 HF readmissions, and 386 primary outcome measures were observed. In the Kaplan–Meier analysis, patients with lower SPPB scores had worse outcomes than those with higher SPPB scores (P < 0.001, Figure 1A). In addition, patients with lower SPPB scores had a higher risk of adverse events compared to those with higher SPPB scores in unadjusted Cox regression analysis [hazard ratio (HR) 1.46, 95% confidence interval (CI) 1.19–1.78; P < 0.001]. Even after multivariable adjustment, lower SPPB scores were independently associated with an increased risk of adverse events (adjusted HR 1.28, 95% CI 1.01–1.61; P = 0.049, Table 2). Similarly, patients with shorter 6MWD had worse outcomes than those with longer 6MWD (P < 0.001, Figure 1B). A 6MWD less than the median value was associated with higher adverse event rates compared to that of a 6MWD greater than the median value (HR 1.32, 95% CI 1.08–1.61; P < 0.001). After multivariable adjustment, the results remained significant (adjusted HR 1.61, 95% CI 1.27–2.04; P < 0.001, Table 2). The prevalence and event rates among subgroups according to the SPPB score and 6MWD are shown in Figure 2.
Figure 1.
The Kaplan–Meier curve showing a comparison of event-free survival rates due to a composite of all-cause death and heart failure readmission between patients with (A) Short Physical Performance Battery scores lower than < 7 vs. those with scores ≥7 and (B) 6-minute walking distance less than the median value vs. greater than or equal to the median value.
Table 2.
Univariable and multivariable analyses of SPPB score and 6MWD for predicting a composite of all-cause death and heart failure readmission
| Unadjusted |
Adjusteda |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| SPPB score <7 | ||||||
| 1.46 | 1.19–1.79 | <0.001 | 1.28 | 1.01–1.61 | 0.049 | |
| 6MWD <median | ||||||
| 1.32 | 1.11–1.61 | 0.008 | 1.61 | 1.23–2.04 | <0.001 | |
6MWD, 6-minutes walking distance; CI, confidence interval; HR, hazard ratio; SPPB, Short Physical Performance Battery.
Adjusted for age, sex, left ventricular ejection fraction, current smoking status, history of heart failure, hypertension, diabetes mellitus, coronary artery disease, chronic obstructive lung disease, atrial fibrillation, systolic blood pressure, estimated glomerular filtration rate, haemoglobin, serum sodium level, serum albumin, log-transformed BNP, prescription of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, beta-blocker, and mineralocorticoid receptor antagonist and NYHA functional class.
Figure 2.
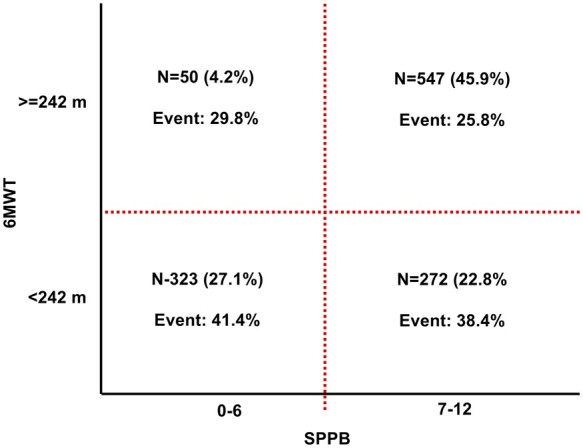
Prevalence and event rates among subgroups according to the Short Physical Performance Battery score and 6-minute walking distance.
The incremental prognostic values of SPPB scores and 6MWD over conventional prognostic factors were assessed (Table 3). Compared to Model 1, consisting of conventional prognostic factors alone, the addition of both the SPPB score (Model 2; NRI 0.139, 95% CI 0.008–0.270; P = 0.036) and 6MWD (Model 3; NRI 0.350, 95% CI 0.220–0.480; P < 0.001) showed statistically significant incremental prognostic value. However, adding 6MWD to the baseline model was associated with better risk prediction than adding the SPPB score to the model (Model 2 vs. Model 3, NRI 0.300, 95% CI 0.171–0.433; P < 0.001). In addition, 6MWD showed incremental prognostic value over the SPPB score plus conventional risk factors (Model 4 vs. Model 3, NRI 0.358, 95% CI 0.228–0.448; P < 0.001), whereas the SPPB score failed to show any additional incremental benefit over the 6MWD plus conventional risk factors (Model 4 vs. Model 2, NRI 0.048, 95% CI −0.083 to 0.180; P = 0.48).
Table 3.
Comparisons of prognostic models for predicting a composite of all-cause death and heart failure readmission
| NRI | 95% CI | P-value | |
|---|---|---|---|
| Baseline model (Model 1) | Reference | ||
| Baseline model + SPPB score (Model 2) | |||
| - vs. Model 1 | 0.139 | 0.008–0.270 | 0.036 |
| Baseline model + 6MWD (Model 3) | |||
| - vs. Model 1 | 0.350 | 0.220–0.480 | <0.001 |
| - vs. Model 2 | 0.300 | 0.171–0.433 | <0.001 |
| Baseline model + 6MWD + SPPB score (Model 4) | |||
| - vs. Model 1 | 0.338 | 0.209–0.468 | <0.001 |
| - vs. Model 2 | 0.358 | 0.228–0.488 | <0.001 |
| - vs. Model 3 | 0.048 | −0.083 to 0.180 | 0.475 |
6MWD, 6-minute walking distance; CI, confidence interval; NRI, net reclassification improvement; SPPB, Short Physical Performance Battery.
Takeshi Kitai, MD, PhD, has a staff physician in the department of Cardiovascular Medicine, at the National Cerebral and Cardiovascular Center in Japan. He received medical degree from Osaka City University in 2004 and PhD from Kyoto University Graduate School of Medicine in 2013. He completed his post-doc clinical research fellowship at the Cleveland Clinic in 2017. As a clinician-scientist and practicing heart failure/transplant cardiologist, Dr Kitai's research focuses on understanding pathophysiological mechanisms that contribute to disease progression in heart failure and other organ dysfunctions.
For sensitivity analyses, we also constructed Cox regression models and examined the prognostic values of SPPB and 6MWD using continuous values. In the univariable analysis, continuous SPPB was significantly associated with poor outcomes (HR 0.59, 95% CI 0.42–0.84; P = 0.003). However, SPPB was not statistically significant after multivariable adjustment (adjusted HR 0.97, 95% CI 0.93–1.00). In contrast, 6MWD as a continuous value remained significant both in the univariable (HR 0.81, 95% CI 0.75–0.88; P < 0.001) and multivariable analyses (adjusted HR 0.84, 95% CI 0.76–0.93; P < 0.001).
DISCUSSION
This retrospective analysis of data collected in the FRAGILE-HF registry, which enrolled hospitalized elderly patients with AHF, investigated the prognostic significance of comprehensive assessment of physical performance by the SPPB score in comparison with 6MWD. The major findings of this study were as follows:1 lower SPPB scores (<7) and shorter 6MWD (<median) were independently associated with increased post-discharge adverse event rates;2 both the SPPB score and 6MWD each had incremental prognostic values over conventional HF risk factors; and3 incremental prognostic value over the conventional risk factors was superior in 6MWD than that in SPPB score.
Physical performance and functional status have been increasingly recognized as important factors related to prognosis and quality of life, especially in elderly patients.17–19 The term ‘frailty’ has been used to characterize the state of increased vulnerability resulting from aging-associated decline in reserve and function across multiple physiologic systems.20,21 Frail patients have an increased risk of falls, disability, hospitalization, and mortality.20–23 Therefore, identifying frail patients is important for clinicians to provide appropriate care for elderly patients. Although multiple clinical models of frailty have been proposed such as the Clinical Frailty Scale and the Fried Frailty Index, there is no gold standard method that is consistently used to assess frailty.24 This may be because it is a multi-dimensional concept involving many physical, psychological, and social aspects of health.22 Given the importance of the detection of frailty and early intervention in patients with HF, many studies have addressed the potential effects of interventional exercise and nutritional supplement strategies.
Historically, the 6MWT has been indicated as a surrogate test for the evaluation of functional capacity and a significant determinant of prognosis in patients with HF.4–6 In the current study, patients with shorter 6MWD were independently associated with worse outcomes and the 6MWD showed incremental prognostic values over conventional HF risk factors. Our results confirmed that the evaluation of functional capacity is important for the management of elderly patients with HF. However, 6MWT is sometimes difficult to perform in elderly patients with concomitant orthopaedic and/or neurological disorders. The 6MWT could not be performed in 115 patients, and among them, 91 patients were successfully evaluated using the SPPB. The SPPB has emerged as a comprehensive objective measure of physical performance including a slow gait, weakness, and balance impairment,25 which has better concurrent validity when compared to other measures of frailty.26,27 The strength of the SPPB in clinical practice is its simplicity and reproducibility, which only requires 5–10 min to complete; hence, it can be integrated into patient management without excessive time consumption. As an objective measure of physical performance, the SPPB was adopted in multiple observational studies that consistently found an association among incident disability, hospital admission, and mortality.14,17,25,28–31 A systematic review and meta-analysis of 17 observational studies encompassing 16 534 participants has revealed a significant association between low SPPB scores and all-cause mortality.32 Another meta-analysis including 22 598 patients with HF has reported that patients with poor physical performance in the 6MWT, SPPB, and gait speed test showed a higher risk of hospitalization or mortality.33 In the current analysis including elderly patients with HF, the SPPB score was low at a median of 8. As SPPB can be used to classify physical limitations and/or physical frailty, identifying frail or prefrail patients using the SPPB score may be important for further clinical treatment such as cardiac rehabilitation. Rinaldo et al. have reported that a lower SPPB score was associated with a higher complication rate in the post-acute phase in elderly (≥75 years) patients with cardiac surgery, HF, or acute coronary syndrome.34 In the current analysis, we found that the SPPB score provides additive prognostic information to conventional risk factors, but it is inferior to that of 6MWD. As there is no fixed cut-off SPPB score, we have added sensitivity analyses using continuous SPPB score and 6MWD. The results support our primary results that 6MWD is superior to SPPB in terms of outcomes prediction. Nevertheless, given its ease of use, we believe that the SPPB might be a useful alternative, especially in patients who are incapable of performing the 6MWT. Further studies are required to test this hypothesis.
Strengths and limitations
The strength of the present study lies in the fact that this is a real-world large-scale registry focusing on elderly patients with HF, and provides entirely novel insights regarding the prognostic significance of the SPPB score compared to 6MWD in hospitalized elderly patients with AHF. However, the current study has several limitations. First, this was a post hoc analysis of a prospective observational cohort study with inherent associated limitations. Despite covariate adjustments, we could not exclude the influence of other measured and unmeasured confounding factors. Second, in this analysis, we used the median value of the 6MWD as the cut-off value for grouping. This is because we thought that the value of 300 m, which is often used as a marker of decreased physical function, may be significantly high for our elderly HF cohort. Therefore, we added the sensitivity analysis using the cut-off value of 300 m and 6MWD as a continuous value, and we found that the results remained significant. Third, it is possible that our data cannot be generalized to all patients with AHF. Particularly, the current cohort excluded patients who were unable to walk independently. Although we believe that the SPPB is better suited for more vulnerable and frail patients, further studies are needed to test this hypothesis. However, as SPPB measures different domains of physical performance compared to 6WMT, both instruments should be assessed if possible.
CONCLUSIONS
Reduced functional capacity assessed by either the SPPB or 6MWT was associated with worse outcomes in hospitalized elderly patients with AHF. The incremental prognostic value over the conventional risk factors was superior in 6MWT than that in SPPB.
Lead author biography

Takeshi Kitai, MD, PhD, has a staff physician in the department of Cardiovascular Medicine, at the National Cerebral and Cardiovascular Center in Japan. He received medical degree from Osaka City University in 2004 and PhD from Kyoto University Graduate School of Medicine in 2013. He completed his post-doc clinical research fellowship at the Cleveland Clinic in 2017. As a clinician-scientist and practicing heart failure/transplant cardiologist, Dr. Kitai's research focuses on understanding pathophysiological mechanisms that contribute to disease progression in heart failure and other organ dysfunctions.
Funding
FRAGILE-HF was supported by Novartis Pharma Research Grants and Japan Heart Foundation Research Grant.
Conflict of interest: Dr. Kamiya has received research funding from Eiken Chemical Co. Ltd. Dr. Takatoshi Kasai is affiliated with a department endowed by Philips Respironics, ResMed, Teijin Home Healthcare, and Fukuda Denshi. Dr. Nobuyuki Kagiyama is affiliated with a department funded by Philips Healthcare; Asahi KASEI Corporation; Inter Reha Co., Ltd; and Toho Holdings Co., Ltd based on collaborative research agreements. Dr. Yuya Matsue is affiliated with a department endowed by Philips Respironics, ResMed, Teijin Home Healthcare, and Fukuda Denshi, and received an honorarium from Otsuka Pharmaceutical Co. The remaining authors have nothing to disclose.
Data availability: The data underlying this article will be shared on reasonable request to the corresponding author
Contributor Information
Takeshi Kitai, Department of Cardiovascular Medicine, Kobe City Medical Center General Hospital, Kobe, Japan; Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center, 6-1 Kishibe Shin-machi, Osaka 564-8565, Japan.
Takayuki Shimogai, Department of Rehabilitation, Kobe City Medical Center General Hospital, Kobe, Japan.
W H Wilson Tang, Department of Cardiovascular Medicine, Kaufman Center for Heart Failure, Heart and Vascular Institute, Cleveland Clinic, Cleveland, OH, USA.
Kentaro Iwata, Department of Rehabilitation, Kobe City Medical Center General Hospital, Kobe, Japan.
Andrew Xanthopoulos, Department of Cardiology, University General Hospital of Larissa, Larissa, Greece.
Shuto Otsuka, Department of Rehabilitation, Kobe City Medical Center General Hospital, Kobe, Japan.
Fumika Nakada, Department of Rehabilitation, Kobe City Medical Center General Hospital, Kobe, Japan.
Rina Yokoyama, Department of Rehabilitation, Kobe City Medical Center General Hospital, Kobe, Japan.
Kentaro Kamiya, Department of Rehabilitation, School of Allied Health Science, Kitasato University, Sagamihara, Japan.
Hiroshi Saito, Department of Rehabilitation, Kameda Medical Center, Kamogawa, Japan; Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan.
Kazuya Saito, Department of Rehabilitation, The Sakakibara Heart Institute of Okayama, Okayama, Japan.
Emi Maekawa, Department of Cardiovascular Medicine, Kitasato University School of Medicine, Sagamihara, Japan.
Masaaki Konishi, Division of Cardiology, Yokohama City University Medical Center, Yokohama, Japan.
Yuki Ogasahara, Department of Nursing, The Sakakibara Heart Institute of Okayama, Okayama, Japan.
Kentaro Jujo, Department of Cardiology, Nishiarai Heart Center Hospital, Tokyo, Japan.
Hiroshi Wada, Department of Cardiovascular Medicine, Saitama Medical Center, Jichi Medical University, Saitama, Japan.
Takatoshi Kasai, Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan; Cardiovascular Respiratory Sleep Medicine, Juntendo Univeristy Graduate School of Medicine, Tokyo, Japan.
Shinichi Momomura, Saitama Citizens Medical Center, Saitama, Japan.
Chayakrit Krittanawong, Section of Cardiology, Baylor School of Medicine, Houston, TX, USA.
John Skoularigis, Department of Cardiology, University General Hospital of Larissa, Larissa, Greece.
Filippos Triposkiadis, Department of Cardiology, University General Hospital of Larissa, Larissa, Greece.
Nobuyuki Kagiyama, Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan; Department of Digital Health and Telemedicine R&D, Juntendo University, Tokyo, Japan.
Yutaka Furukawa, Department of Cardiovascular Medicine, Kobe City Medical Center General Hospital, Kobe, Japan.
Yuya Matsue, Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan; Cardiovascular Respiratory Sleep Medicine, Juntendo Univeristy Graduate School of Medicine, Tokyo, Japan.
REFERENCES
- 1. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: a report from the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 2. Coats AJS, Forman DE, Haykowsky M, Kitzman DW, McNeil A, Campbell TS, Arena R. Physical function and exercise training in older patients with heart failure. Nat Rev Cardiol 2017;14:550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piepoli MF, Spoletini I, Rosano G. Monitoring functional capacity in heart failure. Eur Heart J Suppl 2019;21:M9–M12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, Coke LA, Fleg JL, Forman DE, Gerber TC, Gulati M, Madan K, Rhodes J, Thompson PD, Williams MA; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 2013;128:873–934. [DOI] [PubMed] [Google Scholar]
- 5. Bittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, Kronenberg MW, Kostis JB, Kohn RM, Guillote M. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA 1993;270:1702–1707. [PubMed] [Google Scholar]
- 6. Forman DE, Arena R, Boxer R, Dolansky MA, Eng JJ, Fleg JL, Haykowsky M, Jahangir A, Kaminsky LA, Kitzman DW, Lewis EF, Myers J, Reeves GR, Shen WK; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017;135:e894–e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 8. Reflection paper on physical frailty: instruments for baseline characterisation of older populations in clinical trials. EMA/CHMP/77809/2015. https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-physical-frailty-instruments-baseline-characterisation-older-populations-clinical_en.pdf.
- 9. Saji M, Higuchi R, Tobaru T, Iguchi N, Takanashi S, Takayama M, Isobe M. Impact of frailty markers for unplanned hospital readmission following transcatheter aortic valve implantation. Circ J 2018;82:2191–2198. [DOI] [PubMed] [Google Scholar]
- 10. Matsushita K, Ballew SH, Sang Y, Kalbaugh C, Loehr LR, Hirsch AT, Tanaka H, Heiss G, Windham BG, Selvin E, Coresh J. Ankle-brachial index and physical function in older individuals: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis 2017;257:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsue Y, Kamiya K, Saito H, Saito K, Ogasahara Y, Maekawa E, Konishi M, Kitai T, Iwata K, Jujo K, Wada H, Kasai T, Nagamatsu H, Ozawa T, Izawa K, Yamamoto S, Aizawa N, Yonezawa R, Oka K, Momomura S, Kagiyama N. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: The FRAGILE-HF cohort study. Eur J Heart Fail 2020;22:2112–2119. [DOI] [PubMed] [Google Scholar]
- 12. Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993;88:107–115. [DOI] [PubMed] [Google Scholar]
- 13. Subra J, Gillette-Guyonnet S, Cesari M, Oustric S, Vellas B, Platform T; Platform Team. The integration of frailty into clinical practice: preliminary results from the Gerontopole. J Nutr Health Aging 2012;16:714–720. [DOI] [PubMed] [Google Scholar]
- 14. Volpato S, Cavalieri M, Sioulis F, Guerra G, Maraldi C, Zuliani G, Fellin R, Guralnik JM. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci 2011;66A:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 16. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, Limacher MC, Mahaffey KW, Mehran R, Nissen SE, Smith EE, Targum SL. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (writing committee to develop cardiovascular endpoints data standards). J Am Coll Cardiol 2015;66:403–469. [DOI] [PubMed] [Google Scholar]
- 17. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, Limacher MC, Mahaffey KW, Mehran R, Nissen SE, Smith EE, Targum SL. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (writing committee to develop cardiovascular endpoints data standards). J Am Coll Cardiol 2015;66:403–469. [DOI] [PubMed] [Google Scholar]
- 17. Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, Espeland MA, Fielding RA, Gill TM, Groessl EJ, King AC, Kritchevsky SB, Manini TM, McDermott MM, Miller ME, Newman AB, Rejeski WJ, Sink KM, Williamson JD; LIFE study investigators. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. Jama 2014;311:2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA 2011;305:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cooper R, Kuh D, Hardy R; Mortality Review Group; FALCon and HALCyon study Teams. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ 2010;341:c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 21. Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, Walston J. Frailty consensus: a call to action. J Am Med Direc Assoc 2013;14:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc 2010;58:681–687. [DOI] [PubMed] [Google Scholar]
- 24. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. da Camara SM, Alvarado BE, Guralnik JM, Guerra RO, Maciel AC. Using the Short Physical Performance Battery to screen for frailty in young-old adults with distinct socioeconomic conditions. Geriatr Gerontol Int 2013;13:421–428. [DOI] [PubMed] [Google Scholar]
- 27. Ostir GV, Volpato S, Fried LP, Chaves P, Guralnik JM, Women's Health and Aging Study. Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women’s Health and Aging Study. J Clin Epidemiol 2002;55:916–921. [DOI] [PubMed] [Google Scholar]
- 28. Chiarantini D, Volpato S, Sioulis F, Bartalucci F, Del Bianco L, Mangani I, Pepe G, Tarantini F, Berni A, Marchionni N, Di Bari M. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Card Fail 2010;16:390–395. [DOI] [PubMed] [Google Scholar]
- 29. Khan H, Kalogeropoulos AP, Georgiopoulou VV, Newman AB, Harris TB, Rodondi N, Bauer DC, Kritchevsky SB, Butler J. Frailty and risk for heart failure in older adults: the health, aging, and body composition study. Am Heart J 2013;166:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Warraich HJ, Kitzman DW, Whellan DJ, Duncan PW, Mentz RJ, Pastva AM, Nelson MB, Upadhya B, Reeves GR. Physical function, frailty, cognition, depression, and quality of life in hospitalized adults >/=60 years with acute decompensated heart failure with preserved versus reduced ejection fraction. Circ Heart Fail 2018;11:e005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Panas LJ, Siordia C, Angel RJ, Eschbach K, Markides KS. Physical performance and short-term mortality in very old Mexican Americans. Exp Aging Res 2013;39:481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pavasini R, Guralnik J, Brown JC, di Bari M, Cesari M, Landi F, Vaes B, Legrand D, Verghese J, Wang C, Stenholm S, Ferrucci L, Lai JC, Bartes AA, Espaulella J, Ferrer M, Lim JY, Ensrud KE, Cawthon P, Turusheva A, Frolova E, Rolland Y, Lauwers V, Corsonello A, Kirk GD, Ferrari R, Volpato S, Campo G. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med 2016;14:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fuentes-Abolafio IJ, Stubbs B, Pérez-Belmonte LM, Bernal-López MR, Gómez-Huelgas R, Cuesta-Vargas AI. Physical functional performance and prognosis in patient with heart failure: a systemic review and meta-analysis. BMC Cardiovasc Disorders 2020;20:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rinaldo L, Caligari M, Acquati C, Nicolazzi S, Paracchini G, Sardano D, Giordano A, Marcassa C, Corrà U. Functional capacity assessment and minimal clinically important difference in post-acute cardiac patients: the role of Short Physical Performance Battery. Eur J Prev Cardiol 2021; doi: 10.1093/eurjpc/zwab044. [DOI] [PubMed] [Google Scholar]