Abstract
Aims
Long-chain polyunsaturated fatty acids (PUFAs) generate diverse bioactive lipid mediators, which tightly regulate vascular inflammation. The effects of omega-3 PUFA supplementation in cardiovascular prevention however remain controversial. In addition to direct dietary intake, fatty acid desaturases (FADS) determine PUFA levels. Increased arterial stiffness represents an independent predictor of mortality and cardiovascular events. The aim of the present study was to determine the association of PUFA intake, FADS1 genotype, and FADS expression with arterial stiffness.
Methods and results
A cross-sectional population-based cohort study of 1464 participants without overt cardiovascular disease was conducted. Dietary intake was assessed using a food frequency questionnaire. Arterial stiffness was assessed by carotid–femoral pulse wave velocity (cfPWV), and the FADS1 locus variant was determined. Blood cell transcriptomics was performed in a subset of 410 individuals. Pulse wave velocity was significantly associated with the FADS1 locus variant. Differential associations between PWV and omega-3 PUFA intake were observed depending on the FADS1 genotype. High omega-3 PUFA intake attenuated the FADS1 genotype-dependent associations. Carriers of the minor FADS1 locus variant exhibited increased expression of FADS2, which is associated with PWV.
Conclusion
Taken together, these findings point to FADS1 genotype-dependent associations of omega-3 PUFA intake on subclinical cardiovascular disease. These findings may have implications for identifying responders and non-responders to omega-3 PUFA supplementation and open up for personalized dietary counselling in cardiovascular prevention.
Keywords: Arterial stiffness, Dietary, Inflammation, Omega-3 fatty acids, Prevention, STANISLAS cohort
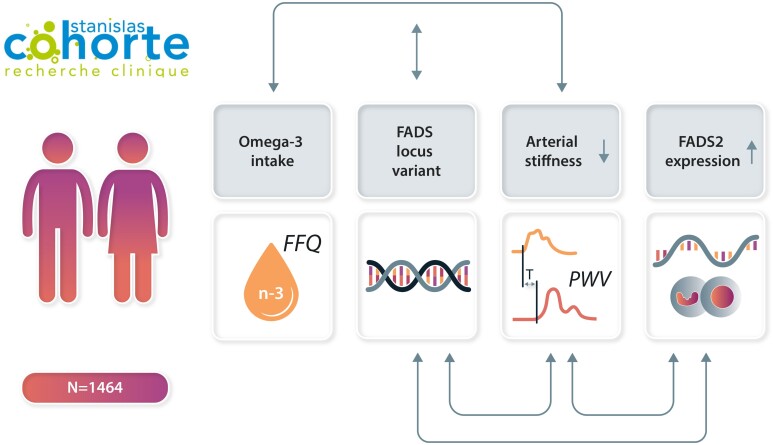
Graphical Abstract
Graphical Abstract.
Interactions between omega-3 intake, FADS genotype, and RNA expression for association with arterial stiffness.
Introduction
Long-chain polyunsaturated fatty acids (PUFAs) generate diverse bioactive lipid mediators, which tightly regulate inflammatory processes. Omega-6 PUFA arachidonic acid (AA) is the precursor for proinflammatory lipid mediators, e.g. 4-series leukotrienes and 2-series prostaglandins, formed through lipoxygenase and cyclooxygenase activities, respectively. When using the omega-3 PUFA eicosapentaenoic acid (EPA) as the substrate, the latter enzymatic pathways generate 5-series leukotrienes and 3-series prostaglandins, which are less biologically active and hence act as anti-inflammatory mediators by competition with the AA pathway. Furthermore, EPA and docosahexaenoic acid (DHA) are metabolized into specific lipid mediators involved in the resolution of inflammation.1 These include resolvins, maresins, and protectins, collectively referred to specialized proresolving mediators (SPMs),2 which act as ‘stop signals’ in vascular inflammation3 with beneficial effects in models of, for example, atherosclerosis, intimal hyperplasia, and vascular calcification.4
In the randomized controlled trial (RCT) REDUCE-IT, a high dose of EPA (4 g) provided a 25% risk reduction for cardiovascular events.5 Other recent RCTs of omega-3 PUFA supplementation have however been neutral for the primary cardiovascular outcomes,6,7 raising the notion that omega-3 PUFA formulations, doses, and the relative EPA and DHA contents may be decisive cardiovascular prevention effects of omega-3 PUFAs,8 which is supported by a recent meta-analysis.9 In addition, cardiovascular risk reduction by omega-3 PUFA supplementation is more pronounced in subjects with low basal dietary omega-3 PUFA intake,6 supporting that the overall omega-3 PUFA exposure is of importance for cardiovascular health.
Increased arterial stiffness, measured as carotid–femoral pulse wave velocity (cfPWV), represents an independent predictor of mortality and cardiovascular events.10 An increased PWV occurs as a result of ageing and/or subclinical pathophysiological process in the vascular wall, in which chronic non-resolving inflammation may play a key role.3,10 Whereas higher circulating omega-3 PUFAs are associated with lower PWV,11,12 results from studies examining omega-3 PUFA intake and arterial stiffness are conflicting.13,14 Data from large population-based cohorts are hence needed for determining if the dietary contribution of omega-3 PUFAs is sufficient to induce beneficial effects on arterial stiffness.
In addition to direct dietary intake, fatty acid desaturases (FADS) 1 and 2 catalyse an endogenous synthesis of AA and EPA from dihomo-γ-linolenic acid and eicosatetraenoic acid, respectively. Genome-wide association studies have identified single-nucleotide polymorphisms (SNPs) in the FADS1 gene that are associated with FADS expression and that determines the fatty acid composition of phospholipids.15 A Mendelian randomization study recently showed that the associations of fatty acids with risk of 15 cardiovascular diseases were driven by the intronic SNP rs174547 in FADS1, with the minor allele predicting a lower cardiovascular risk.16 The association between the FADS1 locus variant and PWV has been reported in a limited cohort of obese individuals,14 but no previous study has evaluated the association between FADS1 genotype, PUFA intake, and PWV in a large population nor as a measure of subclinical vascular alterations in the absence of overt cardiovascular disease. Whereas several studies have shown that variations within the FADS1 gene influence PUFA levels following fish oil supplementation or dietary interventions,17–19 little is known about a possible gene–diet interaction for FADS1 with habitual dietary omega-3 PUFA intake.
Based on the above, the aim of the present study was to determine the association of PUFA intake, FADS1 rs174547 genotype, and FADS gene expression with PWV.
Methods
Study population
Participants attending the fourth visit of the longitudinal familial cohort STANISLAS (Suivi Temporaire Annuel Non-Invasif de la Santé des Lorrains Assurés Sociaux)20 were included in this study. The STANISLAS cohort is a population-based study of 1006 families that each comprise two parents and at least two children (4295 participants) from the Lorraine region (eastern France) recruited during 1993–95 at the Center for Preventive Medicine. The participants were of French origin and free of acute or chronic disease. A total of 1705 participants underwent the fourth examination from 2011 to 2016. After exclusion of participants with missing information on food intake (n = 10), without subclinical organ damage assessment data (n = 56), cardiovascular disease history (n = 26), and genetic data (n = 86) as well as those with a daily energy intake either below 1000 or above 5000 kcal (n = 63), the cross-sectional study population who underwent at the fourth examination consisted of 1464 participants. A total of 410 individuals had available transcriptomic data and were included in the gene expression analysis (see Supplementary material online, Figure S1). The research protocol was conducted according to the Declaration of Helsinki and was approved by the local ethics committee (Comité de Protection des Personnes Est III, Nancy, France). All study participants gave written informed consent to participate.
Polyunsaturated fatty acid and dietary intake assessment
Dietary intake was assessed using a validated food frequency questionnaire (FFQ) as previously described.21,22 In brief, the participants reported their consumption frequency and portion size of 133 food items over the previous 3 months. The consumption frequency was reported using six levels in the questionnaire, ranging from ‘never or rarely’ to ‘twice or more a day’. The portion size of each food item was estimated using standard serving sizes and food models. Polyunsaturated fatty acids intake, i.e. DHA, EPA, docosapentaenoic acid (DPA), linoleic acid (LA), alpha-linolenic acid (ALA), and AA, was estimated from all the items in a scale of grams per day by multiplying the consumption frequency of each item by the nutrient content of selected portions. Nutritional data were extracted from the French food composition database established by the French Data Centre on Food Quality (Ciqual, last updated in 2013). The ratio omega 6/omega 3 is calculated as follows: (AA + LA)/(EPA + DHA + DPA + ALA).
Carotid–femoral pulse wave velocity
The cfPWV was measured using a Complior device (Alam Medical, France) in a quiet room after at least 10 mins of rest in a supine position according to the recommendations of the European Network for the Noninvasive Investigation of Large Arteries.20,23 Two sensors were placed simultaneously on the carotid artery and the femoral artery. Two measurements were made, with cfPWV calculated as their mean. If the two measurements differed by > 0.5 m/s, a third measurement was made, and the cfPWV was then calculated as the median of the three measurements. The onboard foot-to-foot algorithm based on the second-derivative waveforms was used to determine the transit time. The carotid-to-femoral, carotid-to-sternal-notch, and sternal-notch-to-carotid distances were measured with a measuring tape. The distance used for cfPWV calculation was 0.8 times the direct carotid–femoral distance. The cfPWV was calculated as distance divided by transit time.
Genotyping
Genotyping was performed as previously described.24 In brief, whole blood DNA was extracted using Gentra Puregene Blood Kit (Qiagen, Hilden, Germany) and stored at −20°C. Genotyping was conducted at the Centre National de Recherche en Génomique Humaine (Evry, France) using two DNA chips from Illumina (Exome Array and Global Screening Array), and data for the FADS1 SNP rs174547 were extracted for 1611 individuals after quality control steps (call rate 100% and HWeq = 0.06).
Fatty acid desaturases gene expression
Whole blood RNA was extracted from PAXgene Blood RNA Tubes (Qiagen, Hilden, Germany) using MagMAX for the Stabilized Blood Tubes RNA Isolation Kit (Life Technologies, Villebon sur Yvette, France) on a King Fisher Duo Prime automate (Thermo Fisher Scientific, Dardilly, France). Extracted RNAs were quantified using a Nanodrop spectrophotometer, and the quality was assessed using an RNA ScreenTape system (Agilent, les Ulis, France). Transcriptome analysis was conducted at IMoPA (Vandoeuvre-les-Nancy, France) using Clariom D® assays (Affymetrix, Thermo Fisher Scientific, Dardilly, France). The differential expression of the FADS1 and FADS2 genes was assessed in a subgroup of 410 individuals. Briefly, the analysis of differential gene expression according to rs174547 genotype was carried out with Transcription Analysis Console Software 4.0.2, including SST-RMA intensity normalization followed by statistical gene expression analysis with the Limma R package (e-Bayes method), with a statistical threshold [false discovery rate (FDR) < 0.05]. Gene expression values refer to normalized intensities values (in log2) obtained from Clariom D® microarrays for each individual. Quantitative gene expression value from normalized intensities is expressed and plotted as normalized intensity units (NIUs). The Mann–Whitney–Wilcoxon test was applied to compare means of normalized intensities between the appropriated rs174547 genotype groups.
Covariates
A self-reported questionnaire was used to collect demographic and socioeconomic information, such as age, sex, education level, smoking status, and physical activity, as well as information about disease history and treatment.25 Anthropometric measurements such as weight, height, and waist circumference (WC) were recorded during clinical examination. Blood samples were collected, and the serum concentrations of the following biomarkers were measured: fasting glucose, high-density lipoprotein cholesterol (HDL-C), and triglycerides. Office blood pressure20 and 24 h ambulatory blood pressure26,27 were measured. In brief, participants underwent a 24 h recording of the ambulatory blood pressure using a Spacelabs 90207 ambulatory monitor (Spacelabs Medical), with the monitoring cuff placed around the non-dominant arm. The blood pressure system was programmed to make measurements every 15 mins from 6 a.m. to 10 p.m. and every 30 mins from 10 p.m. to 6 a.m. Metabolic syndrome was defined according to National Cholesterol Education Program ATP328 as the presence of ≥3 of the following components: elevated WC (>102 cm for men, >88 cm for women); elevated triglycerides (≥1.5 g/L) or treated by lipid-lowering drugs; reduced HDL-C (<0.4 g/L for men, <0.5 g/L for women); elevated office systolic blood pressure (SBP, ≥130 mm Hg), elevated diastolic blood pressure (DBP, ≥85 mm Hg), or treated by anti-hypertensive drugs; or elevated glucose (≥1.10 g/L) or treated by anti-diabetic drugs. The condition of elevated triglycerides was defined as ≥1.5 g/L or treated by lipid-lowering drugs.
Statistics
Participant characteristics were stratified by sex and compared using a χ2 test for categorical variables and either Student’s t-test or Wilcoxon non-parametric test for continuous variables. Polyunsaturated fatty acid and energy intake by the FADS1 rs174547 genotype were compared using ANOVA. Mixed models were performed to assess the associations between EPA or DHA intake and PWV. A random effect on family was added in the models to take into account the familial link between some individuals. Adjustment variables were selected after testing the associations between the covariates and PWV (see Supplementary material online, Table S1). Only variables with P < 0.05 were retained: age, sex, body mass index (BMI), SBP. As the PUFAs were highly correlated (r = 0.99, P < 0.0001), adjustment for other PUFAs than the ones tested in the analyses was not performed. Third, mixed models were used as described above to assess the associations between the rs174547 FADS1 locus variant and PWV. Additive and recessive models were performed as follows: for the additive model, homozygotes for minor allele (CC) were noted 0, heterozygotes (CT) were noted 1, and homozygotes for the major allele (TT) were noted 2. For the recessive model, CT and TT genotypes were grouped together and noted 1 as previously described.29 Genotypes were also used for stratification, according to additive and recessive models. Sensitivity analyses were performed after using stratification by sex and PUFA intake. The associations of FADS1 and FADS2 expression levels with PUFA intake and/or PWV were assessed using a mixed model, as described above. Statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Fatty acid intake
The characteristics of the study population according to sex are shown in Table 1. The overall PUFA intake was higher in men compared with that in women and represented a higher proportion of total energy intake in women than that in men (Table 1). The median overall omega-6 to omega-3 intake ratio was almost two-fold above the recommended 4:1, with a higher ratio in men than that in women. The FADS1 locus variant had the expected distribution16 and was not significantly different across sexes (Table 1). There were no significant differences in PUFA intake by the FADS1 locus variant (see Supplementary material online, Table S2).
Table 1.
Study population characteristics
| All | Men | Women | P-value | |
|---|---|---|---|---|
| N | 1464 | 711 | 753 | |
| Socio-demographics | ||||
| Age (years) | 55 (34–60) | 57 (34–61) | 54 (34–59) | <0.001 |
| Smokers | 296 (20%) | 152 (21%) | 144 (19%) | 0.28 |
| Education level | 0.58 | |||
| Low | 540 (37%) | 272 (38%) | 268 (36%) | |
| Intermediate | 224 (15%) | 107 (15%) | 117 (16%) | |
| High | 699 (48%) | 332 (47%) | 367 (49%) | |
| BMI (kg/m²) | 25 (22.5–28.3) | 25.8 (23.7–28.7) | 24.0 (21.7–27.8) | <0.001 |
| Energy expenditure (MET-min/week) | 1782 (656–4158) | 2322 (792–5976) | 1440 (554–3192) | <0.001 |
| Food intake | ||||
| Energy intake (kcal/day) | 2242 (1764–2865) | 2545 (2061–3077) | 1951 (1538–2498) | <0.001 |
| Carbohydrates (g/day) | 254.9 ± 99.9 | 286.1 ± 102.5 | 225.5 ± 87.7 | <0.001 |
| Lipids (g/day) | 97.8 ± 40.5 | 106.0 ± 39.5 | 90.0 ± 39.9 | <0.001 |
| Proteins (g/day) | 99.5 ± 36.7 | 109.8 ± 37.0 | 89.7 ± 33.6 | <0.001 |
| Saturated fat (g/day) | 37.6 ± 16.2 | 40.9 ± 16.0 | 34.3 ± 15.8 | <0.001 |
| MUFA (g/day) | 38.8 ± 17.3 | 42.1 ± 17.1 | 35.6 ± 16.9 | <0.001 |
| PUFA (g/day) | 14.7 ± 7.7 | 15.6 ± 7.3 | 13.9 ± 8.0 | <0.001 |
| EPA (mg/day) | 158.1 ± 125.1 | 165.6 ± 119.2 | 151.0 ± 130.1 | 0.03 |
| DHA (mg/day) | 239.2 ± 187.7 | 250.5 ± 177.7 | 228.5 ± 192.5 | 0.02 |
| w6/w3 ratio | 7.3 ± 2.6 | 7.5 ± 2.7 | 7.1 ± 2.6 | 0.002 |
| Carbohydrates (mg/kcal/day) | 107.7 ± 20.5 | 108.0 ± 20.4 | 107.5 ± 20.5 | 0.63 |
| Lipids (mg/kcal/day) | 41.1 ± 8.0 | 39.9 ± 7.7 | 42.2 ± 8.0 | 0.001 |
| Proteins (mg/kcal/day) | 42.4 ± 8.1 | 41.8 ± 7.7 | 42.9 ± 8.5 | 0.008 |
| Saturated fat (mg/kcal/day) | 15.8 ± 3.6 | 15.4 ± 3.5 | 16.1 ± 3.6 | 0.001 |
| MUFA (mg/kcal/day) | 16.3 ± 4.1 | 15.9 ± 3.9 | 16.7 ± 4.2 | 0.001 |
| PUFA (mg/kcal/day) | 6.2 ± 2.2 | 5.9 ± 1.9 | 6.5 ± 2.4 | 0.001 |
| EPA (µg/kcal/day) | 69.6 ± 53.5 | 65.2 ± 47.5 | 73.8 ± 58.4 | 0.002 |
| DHA (µg/kcal/day) | 105.4 ± 79.1 | 98.6 ± 70.7 | 111.8 ± 85.9 | 0.001 |
| Clinics | ||||
| Metabolic syndrome | 332 (27%) | 180 (31%) | 152 (24%) | <0.001 |
| Diabetes | 76 (5%) | 49 (7%) | 27 (4%) | 0.004 |
| Fasting glucose (g/L) | 0.9(0.8–1) | 0.9 (0.9–1.0) | 0.9 (0.8–0.9) | <0.001 |
| HbA1C (%) | 5.6(5.3–5.8) | 5.6 (5.4–5.8) | 5.5 (5.3–5.8) | 0.10 |
| Use of anti-diabetic drugs | 58 (4%) | 39 (6%) | 19 (3%) | 0.004 |
| Elevated triglycerides | 385 (26%) | 234 (33%) | 151 (20%) | <0.001 |
| Triglycerides (g/L) | 0.9(0.7–1.3) | 1.0 (0.7–1.4) | 0.9 (0.8–0.9) | <0.001 |
| Total cholesterol (g/L) | 2.1(1.9–2.4) | 2.1 (1.8–2.4) | 2.1 (1.9–2.5) | <0.001 |
| HDL-C (g/L) | 0.6(0.5–0.7) | 0.5 (0.4–0.6) | 0.6 (0.5–0.7) | <0.001 |
| LDL-C (g/L) | 1.3(1.1–1.6) | 1.3 (1.1–1.6) | 1.3 (1.1–1.6) | 0.21 |
| Use of lipid-lowering drugs | 208 (14%) | 127 (18%) | 81 (11%) | <0.001 |
| Hypertension | 565 (39%) | 326 (49%) | 239 (34%) | <0.001 |
| 24 h SBP (mmHg) | 120.1 ± 10.2 | 123.6 ± 9.3 | 117.0 ± 10.0 | <0.001 |
| 24 h DBP (mmHg) | 74.3 ± 7.2 | 76.2 ± 6.9 | 72.6 ± 7.0 | <0.001 |
| Use of anti-hypertensive drugs | 277 (19%) | 151 (21%) | 126 (17%) | 0.03 |
| FADS1 rs174547 genotype | CC: 134 (9%) | CC: 62 (9%) | CC: 72 (10%) | 0.71 |
| CT: 663 (45%) | CT: 329 (46%) | CT: 334 (44%) | ||
| TT: 667 (46%) | TT: 320 (45%) | TT: 347 (46%) |
BMI, body mass index; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid; DHA, docosahexaenoic acid; HbA1C, glycated haemoglobin; HDL-C, plasma high-density cholesterol; LDL-C, plasma low-density cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure. Hypertension is defined as elevated blood pressure (130/80) and/or declared hypertension and/or use of at least one anti-hypertensive drug; diabetes is defined by high fasting glucose (>1.26 g/L) and/or declared diabetes and/or use of at least one anti-diabetic drug. Metabolic syndrome definition is specified in the Methods section.
Association of eicosapentaenoic acid and docosahexaenoic acid intake with pulse wave velocity
In age- and sex-adjusted analyses, DHA and EPA were negatively associated with PWV at the limit of significance {see Supplementary material online, Table S3, β [95% confidence interval (CI): −0.39 (−0.78; −0.007); −0.56 (−1.13; 0.01), respectively, P = 0.05 for both]}. Further adjustment for SBP, BMI, and energy intake attenuated the observed associations to not reaching statistical significance [β (95% CI): −0.46 (−1.07; 0.15), P = 0.14 for EPA, and −0.32 (−0.73; 0.09), P = 0.13 for DHA]. Given the differences in PUFA intake and PWV between men and women in the present cohort (Table 1), further analyses were performed by sex. The inverse association of PWV with DHA and EPA was significant in males [β: −0.70 (−1.36; −0.04), P = 0.04 for DHA, and −1.01 (−1.99; −0.03), P = 0.04 for EPA] but not in females [β: −0.16 (−0.60; 0.29), P = 0.49 for DHA, and −0.23 (−0.89; 0.43), P = 0.49 for EPA] in the age-adjusted analysis, but these associations were non-significant in both sexes after further adjustment for BMI, SBP, and energy intake [males: −0.64 (−1.34; 0.06), P = 0.07; females: −0.09 (−0.56; 0.37), P = 0.69 for DHA; males: −0.92 (−1.97; 0.13), P = 0.08, females: −0.15 (−0.84; 0.54), P = 0.67 for EPA].
FADS1 locus variant confers lower pulse wave velocity
Pulse wave velocity was significantly associated with the FADS1 locus variant [β (95% CI): 0.15 (0.04; 0.27), P = 0.007], with the lowest PWV in carriers of the minor C-allele. The association remained significant after adjustments for BMI and SBP (Table 2). Adjusting on energy intake or each PUFA did not change the association. The significant association between the FADS1 locus variant and PWV observed in the age- and sex-adjusted analysis (Table 2) was reproduced only in men in a sex-stratified analysis [β (95% CI): 0.20 (0.01; 0.38), P = 0.03]. In contrast, no significant association was observed in females [β (95% CI): 0.10 (−0.03; 0.23), P = 0.13]. These sex-dependent differences were retained in models adjusting for age, BMI, SBP, and PUFA intake (see Supplementary material online, Table S4).
Table 2.
Multivariate association between the FADS1 rs174547 genotype (risk per T allele) and pulse wave velocity (N = 1464)
| rs174547 | β (95% CI) | P-value |
|---|---|---|
| M0 | 0.15 (0.04; 0.27) | 0.007 |
| M1 | 0.15 (0.04; 0.27) | 0.01 |
| M1 + EPA | 0.15 (0.04; 0.26) | 0.01 |
| M1 + DHA | 0.15 (0.03; 0.26) | 0.01 |
| M1 + total energy intake | 0.15 (0.04; 0.27) | 0.01 |
Mixed model with random effect on family.
M0: adjusted for age and sex.
M1: M0 + adjusted for BMI and SBP.
Association of fatty acid intake with pulse wave velocity according to the FADS1 locus variant
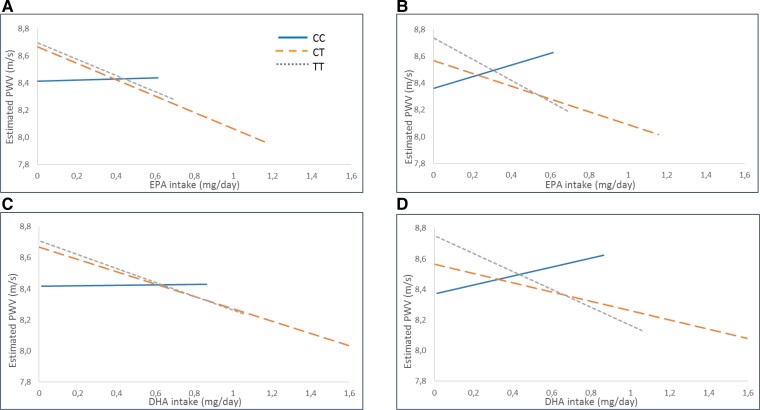
The association of fatty acid intake with PWV after stratification according to the genotype is shown in Figure 1 (EPA Figure 1A and B and DHA Figure 1C and D). The projection of the estimation after linear regression analysis was adjusted on either sex and age (Model 0; Figure 1A and C) or on sex, age, BMI, and SBP (Model 1; Figure 1B and D). The results indicated a recessive pattern for the minor allele with similar associations in the CT and TT genotypes, whereas the associations between intake of either EPA or DHA with PWV were largely attenuated in homozygous C-allele carriers (Figure 1). Grouping the CT and TT genotypes revealed a significant negative association for DHA [β −0.42 (−0.83; −0.01); P = 0.04] and a borderline negative association for EPA [β −0.61 (−1.22; 0); P = 0.05] with PWV in the age- and sex-adjusted analysis. These associations were somewhat attenuated after further adjustments for BMI and SBP [β −0.41 (−0.82; 0), P = 0.05 for DHA, and β −0.60(−1.23; −0.01), P = 0.06 for EPA]. In contrast, no significant associations for either DHA or EPA intake were observed in the CC genotype in crude [β 0.02 (−1.23; 1.27), P = 0.98 for DHA; β 0.04(−1.81; 1.89), P = 0.96 for EPA] or adjusted models [β 0.30(−1.01; 1.61), P = 0.66 for DHA; β 0.44 (−0.87; 1.75), P = 0.66 for EPA]. There were, however, no significant interactions between genotype and fatty acid intake in any of the models.
Figure 1.
Association of polyunsaturated fatty acid intake from either eicosapentaenoic acid (A and B) or docosahexaenoic acid (C and D) with pulse wave velocity stratified by different genotypes of FADS1 rs174547 after estimation with linear regression adjusted for sex and age (Model 0) and sex, age, body mass index, and systolic blood pressure (Model 1). Solid blue lines represent homozygous for the minor allele (CC), and dotted orange lines represent heterozygous (CT) and scattered grey lines represent homozygous for the major allele (TT) (all interactions are non-significant—N = 1464).
Association of FADS1 locus variant with pulse wave velocity according to fatty acid intake
Stratifying the population based on the median into high and low dietary PUFA intake revealed a significant association between FADS1 rs174547 genotypes and PWV in individuals with low intake of EPA and DHA and a low w6/w3 ratio (Table 3). These associations remained significant after further adjustment for BMI and SBP. In contrast, for subjects with a dietary intake of EPA and DHA and w6/w3 ratio above median, no significant associations for the genotype were observed.
Table 3.
Association between the FADS1 rs174547 genotype (risk per T allele) and PWV stratified by polyunsaturated fatty acid’ median (</≥)
| < Median | ≥ Median | |||
|---|---|---|---|---|
| β (95% CI) | P-value | β (95% CI) | P-value | |
| EPA | ||||
| M0 | 0.17 (0.02; 0.33) | 0.03 | 0.13 (−0.03; 0.29) | 0.11 |
| M1 | 0.21 (0.05; 0.37) | 0.01 | 0.09 (−0.07; 0.26) | 0.26 |
| DHA | ||||
| M0 | 0.20 (0.04; 0.36) | 0.01 | 0.11 (−0.05; 0.27) | 0.18 |
| M1 | 0.23 (0.07; 0.39) | 0.006 | 0.07 (−0.09; 0.24) | 0.37 |
| Ratio w6/w3 | ||||
| M0 | 0.20 (0.04; 0.36) | 0.02 | 0.09 (−0.06; 0.25) | 0.24 |
| M1 | 0.18 (0.02; 0.34) | 0.02 | 0.10 (−0.07; 0.26) | 0.25 |
Mixed model with random effect on family.
M0: adjusted for age and sex.
M1: M0 + adjusted for BMI and SBP.
The medians were 0.13 g/day for EPA, 0.19 g/day for DHA, and 6.87 for the w6/w3 ratio.
FADS2 gene expression according to FADS1 locus variant and pulse wave velocity
Since PWV is significantly associated with the FADS1 rs174547 genotype [β 0.15 (0.04; 0.27), P = 0.007] and the FADS1 locus variant is only associated with FADS2 expression, associations of FADS1 and FADS2 expression levels with PVW were subsequently assessed.
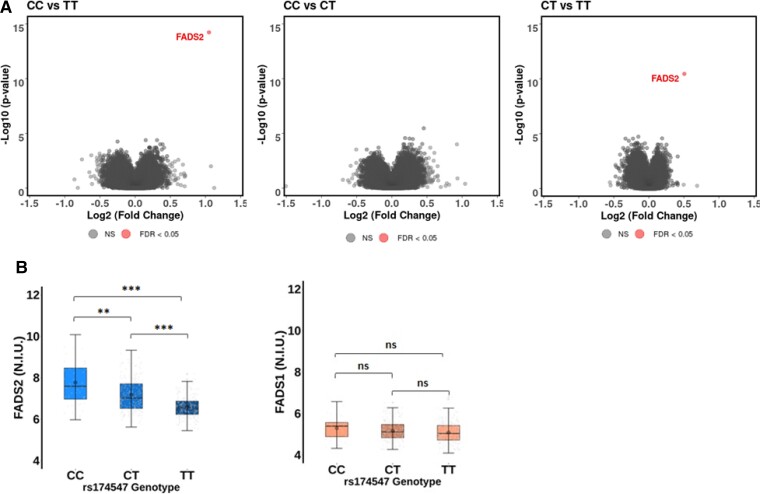
The age, sex, and FADS1 rs174547 genotype distribution in the subgroup of 410 individuals included in the transcriptomic analysis are shown in Supplementary material online, Table S5. In the analysis of the whole transcriptome after application of statistical threshold filter for FDR (q-value) ≤0.05, only transcripts of the FADS2 gene were differentially expressed between the FADS1 rs174547 genotypes (Figure 2A). The overall FADS2 expression was higher than FADS1 expression (Figure 2B) and significantly different between the FADS1 rs174547 genotypes, with the TT genotype being associated with the lowest FADS2 expression levels (Figure 2B). FADS2 expression levels were significantly higher in CC vs. TT (FC = 2.07; P = 5.86e−15; q = 7.96e−10) and CT vs. TT (FC = 1.42; P = 3.48e−11; q = 4.73e−06) FADS1 rs174547 genotypes (Figure 2B). In contrast, none of the genotype comparisons (CC vs.TT, CT vs. TT, and CC vs. CT) identified any significant differences in FADS1 gene expression (Figure 2B).
Figure 2.
Association of fatty acid desaturases gene expression with the FADS1 rs174547 genotype in a subgroup of 410 individuals. (A) Volcano plots representing the significance [−log10(P-value)] vs. the fold change [Log2(Fold Change)] for differentially expressed genes in CC vs. TT (left), CC vs. CT (middle), and CT vs. TT (right) comparisons. Each gene is represented by a dot with grey dot corresponding to false discovery rate > 0.05 and red dot to false discovery rate < 0.05. (B) Normalized intensities of FADS2 (left panel) and FADS1 (right panel) according to FADS1 rs174547 genotype. Normalized intensities have been extracted from cluster IDs TC1100011050.hg.1 and TC1100013022.hg.1, representing FADS1 and FADS2 gene expression, respectively. Mann–Whitney–Wilcoxon significancce at: *P < 0.05, **P < 0.01, ***P < 0001. ns, non-significant; P > 0.05.
In age- and sex-adjusted analyses, FADS2 expression was negatively associated with PWV [β −0.16 (−0.3; −0.02), P = 0.03]. The significance was slightly increased after adjustments for BMI and SBP [β −0.19 (−0.32; −0.05), P = 0.01; Table 4]. No significant association was found for FADS1 expression with PWV (Table 4). Sex-stratified analyses revealed a stronger negative association between FADS 2 mRNA and PWV in males than females (Table 4).
Table 4.
Associations between FADS1 and FADS2 gene expression and pulse wave velocity in the subgroup of the STANISLAS cohort with transcriptomic data (n = 410)
| β (95% CI) | P-value | |
|---|---|---|
| All (n = 410) | ||
| FADS1 | ||
| M0 | −0.04 (−0.26; 0.18) | 0.73 |
| M1 | −0.10 (−0.34; 0.14) | 0.40 |
| FADS2 | ||
| M0 | −0.16 (−0.30; −0.02) | 0.03 |
| M1 | −0.19 (−0.33; −0.05) | 0.01 |
| Men (n = 205) | ||
| FADS1 | ||
| M0 | −0.09 (−0.4; 0.22) | 0.57 |
| M1 | −0.07 (−0.42; 0.28) | 0.71 |
| FADS2 | ||
| M0 | −0.27 (−0.48; −0.05) | 0.01 |
| M1 | −0.30 (−0.70; −0.08) | 0.02 |
| Women (n = 205) | ||
| FADS1 | ||
| M0 | −0.01 (−0.16; 0.28) | 0.97 |
| M1 | −0.16 (−0.47; 0.15) | 0.32 |
| FADS2 | ||
| M0 | −0.07 (−0.26; 0.13) | 0.48 |
M0, adjusted for sex and age.
M1, M0 + adjusted for BMI and SBP.
Discussion
Three major findings supporting the role of omega-3 PUFA, FADS1 locus variant, and FADS2 expression in arterial stiffness emerge from the present study. First, the FADS1 locus variant was significantly associated with PWV. Second, differential associations between the FADS1 locus variant and PWV were observed depending on the omega-3 PUFA intake, with high omega-3 PUFA intake attenuating the FADS1 locus variant-dependent associations. Third, carriers of the minor FADS1 locus variant exhibited increased expression of FADS2 without alterations of FADS1 expression. Taken together, these findings point to an association of FADS with PWV contingent on omega-3 PUFA intake.
The association of higher EPA and DHA intake with lower PWV lost statistical significance after adjustments in the present study. In a subgroup analysis of the Age, Gene/Environment Susceptibility-Reykjavik (AGES-Reykjavik) Study, circulating levels of EPA and DHA, but not FFQ-evaluated fish oil consumption, were associated with lower cfPWV.12 Likewise, in 351 subjects from the HealthTrack Study, omega-3 PUFA intake was not significantly associated with brachial-ankle PWV.13 Beneficial effects of omega-3 PUFA on arterial stiffness have received support from interventional studies showing lowered PWV after fish oil supplementation.30 Cardiovascular risk factors, PUFA levels and doses, as well as differential effects of EPA and DHA are some possible factors behind the conflicting results from both observational studies of PUFA intake and/or levels and clinical trials of omega-3 PUFA supplementation.9
In addition to dietary intake, endogenous biosynthesis from plant-derived shorter-chain omega-3 PUFA contribute to the systemic and tissue levels of EPA and DHA.31 This conversion of PUFA involves the desaturase activities of the FADS enzymes, which in turn are partially determined by genetic variations.15,32 In the present study, the minor C-allele of the FADS1 rs174547 genotype was associated with lower cfPWV. These findings are in line with the lower risk recently reported to be associated with this genotype for a range of cardiovascular diseases,16,29,33 and extend the observation to subclinical arterial disease in otherwise healthy individuals.
Importantly, although the interactions between genotype and fatty acid intake were not significant, we observed that unlike in CC carriers, in CT and TT carriers, there was a significant association between higher EPA/DHA intake and lower PWV. Moreover, we observed only in the group with low PUFA intake that there was an association between the FADS1 locus variant and PWV (compared with CC, increasing PWV for CT and TT). This suggests that high omega-3 PUFA intake may compensate for an unfavourable FADS1 locus genotype.
Desaturase-dependent biosynthetic pathways may represent a less important source of total DHA and EPA at high fish consumption.31 The consequences of this for the cardiovascular phenotype, as suggested by the present results for cfPWV, have however not previously been reported. Taken together, these findings raise the notion that an unfavourable FADS1 locus variant effect on PWV could be neutralized by high omega-3 PUFA intake and that less pronounced beneficial effects of omega-3 PUFA intake could be anticipated in carriers of the protective FADS1 locus variant.
An increase in the dietary omega-6 to omega-3 ratio has previously been associated with an increased risk of acute coronary syndromes.34 Likewise, the ratio of the downstream lipid mediators from these PUFAs, e.g. resolvin D1 to leukotriene B4 ratio, has been associated with preclinical atherosclerosis.35 However, results are conflicting on how alterations in PUFA status as a result of FADS1 polymorphisms are modified by dietary intake of EPA and DHA. The large observational CHARGE consortium reported that the association of FADS1 variants with circulating EPA and DHA were independent of fish consumption,15 but that this association may vary depending on the blood fraction used for PUFA measurements, e.g. plasma vs. erythrocyte membranes.36
In addition to reducing Δ5-desaturase activity and lowering AA levels,15 the rs174547 CC genotype increases FADS2 (but not FADS1) mRNA expression and Δ6-desaturase activity, resulting in increased levels of DHA in aortic valves.29 Although the FADS1 locus variant has been associated with altered FADS1 expression in other tissues, the results of the transcriptomic analysis in the present study support FADS2 (but not FADS1) expression being increased in the blood from carriers of the minor FADS1 locus variant. Importantly, FADS2 expression was inversely associated with PWV, reinforcing a connection between the FADS1 locus variant and arterial stiffness through FADS2 expression.
Several differential observations were noted between males and females in the present study. Consistent with other studies,37 males had higher total PUFA intake, whereas the PUFA proportion of total energy intake was higher in females. In addition, men had a higher omega-6 to omega-3 ratio than the intake in females. Also, the negative association between EPA and DHA intake and PWV was significant for males but not for females in the age-adjusted analysis, albeit non-significant in both sexes for the fully adjusted models. Furthermore, the FADS genotype and FADS2 expression predicted PWV significantly only in males but not in females, which is similar to the previously reported association between the consumed and circulating proportions of omega-3 PUFAs being less pronounced in men who are homozygous for the major allele of another FADS1 SNP (rs174550 in linkage disequilibrium with rs174547).38 Other possible factors underlying the sex-specific associations between PUFA intake and cardiovascular phenotypes in the present and previous39 studies include differences in risk factors, comorbidities, and metabolic differences across the sexes. The latter has received support from the observation that females have a significantly greater increase in the EPA content of plasma phospholipids compared with males over the same period on the same diet.40
The possible mechanisms involved in the role of EPA and DHA intake with reduced arterial stiffness may involve changes in the downstream lipid mediators. Indeed, whereas omega-6-derived leukotriene B4 has been associated with increased PWV,41 proresolving mediators derived from DHA and EPA exert beneficial effects on the vascular wall.3,4
The present study is the first to link the FADS1 rs174547 genotype with dietary omega-3 PUFA intake to a beneficial arterial phenotype in the absence of known cardiovascular disease. The strengths include analyses based on a large general and initially healthy population-based cohort with the availability of detailed information on diet, genetics, and extensive cardiovascular phenotyping, including PWV. Certain limitations should however be acknowledged. We did not measure the levels of EPA and DHA in this study. Ideally, these measures should be performed in erythrocyte membranes, which are not available in the STANISLAS family cohort. Even if the diet was assessed through declarative data over the past 3 months and can underestimate the real consumption, FFQ is well detailed for PUFA intake and a reliable tool to evaluate the habits of food consumption regarding PUFAs.22 Nonetheless, we did not collect information on dietary supplements rich in n-3 PUFA, which is used by 13.8% of French adults.42 In addition, we did not measure downstream PUFA-derived lipid mediators. The observational and cross-sectional design of the study also means that no causal relationship can be concluded for the observed associations with arterial stiffness.
In summary, the present study identified significant associations between omega-3 PUFA intake, FADS1 locus variant, FADS2 expression, and cfPWV. Importantly, the unfavourable genotype effect on arterial stiffness was blunted by a high omega-3 PUFA intake. In conclusion, the FADS1 locus variant may affect the impact of omega-3 PUFA intake on subclinical cardiovascular disease. These findings may have implications for identifying responders and non-responders to omega-3 supplementation and open up for precision medicine through personalized dietary counselling in cardiovascular prevention.
Lead author biography

Patrick Rossignol, MD, PhD, is a Nephrologist and Vascular medicine specialist, ESC/ESH certified hypertension specialist, Professor of Therapeutics at the University of Lorraine, France. He is heading the Nancy University Hospital Inserm Plurithematic Clinical Investigation Centre. He is coordinating the French multidisciplinary network F-CRIN (French Clinical research Infrastructure Network): Cardiovascular and Renal Clinical Trialists (INI-CRCT) www.inicrct.org. He is mainly involved in clinical trials, in heart failure, hypertension, and chronic kidney disease. Hyperkalaemia is one of his main areas of interest (https://expertscape.com/ex/hyperkalemia). He has published more than 400 peer review publications and is the co-founder of the SME CardioRenal.
Supplementary Material
Acknowledgements
The authors are highly grateful to the Vandoeuvre-Lès Nancy Centre de Médecine Préventive staff and Dr Sophie Visvikis-Siest (INSERM U1122) who managed the STANISLAS Cohort for the first three visits. The authors deeply thank the Staff of the Clinical Investigation Center and other personnel involved in the Stanislas Cohort management. Biostatisticians: R. Fay, Z. Lamiral, J.L. Machu. Computer scientists: N. Boucenna, C. Gallina-Müller, P.L. Maclot, T. Sas. Co-investigators: K. Chau, P. Di Patrizio, D. Dobre, D. Gonthier, O. Huttin, L. Malingrey, V. Mauffrey, A. Olivier, T. Poyeton, E. Steyer, G. Watfa. Data managers: P. Cimon, E. Eby, L. Merckle. Data entry operators: M. Batsh, O. Blanger, C. Bottelin, N. Haskour, V. Jacquet, M.C. Przybylski, Y. Saribekyan, H. Thomas, M. Vallée. Echocardiographists, echographists: M. Ben Sassi, S. Cario, Y. Camara, S. Coiro, Z. Frikha, A. Kearney-Schwartz, C. Selton-Suty, G. Watfa. Imaging engineer: E. Bozec. Laboratory engineer: J. Nuée-Capiaumont. Technicians: J. Fruminet, M. Kuntz, J. Ravey, E. Rousseau, C. Tachet. Project managers: S. Bouali, C. Hertz. Quality engineer: X. Lepage. Registered nurses: M. Giansily, L. Poinsignon, N. Robin, M. Schmartz, M. Senn, E. Micor-Patrignani, M. Toutlemonde. Hospital technician: M.T. Fleurot. Resident physicians: R. Alvarez-Vasquez, M. Amiot, M. Angotti, E. Babel, M. Balland, A. Bannay, P. Basselin, P. Benoit, J. Bercand, M. Bouazzi, E. Boubel, N. Boucherab-Brik, F. Boyer, C. Champagne, S.A. Chenna, J. Clochey, D. Czolnowski, J. Dal-Pozzolo, L. Desse, B. Donetti, G. Dugelay, C. Friang, M. Galante, M. Garel, A. Gellenoncourt, A. Guillin, M.L. Hariton, M. Hinsiger, E. Haudiquet, J.M. Hubert, A. Hurtaud, J. Jabbour, S. Jeckel, A. Kecha, G. Kelche, C. Kieffert, E. Laurière, M. Legay, A. Mansuy, O. Millet-Muresan, N. Meyer, E. Mourton, A.L. Naudé, A.C. Pikus, M. Poucher, M. Prot, A. Quartino, M. Saintot, A. Schiavi, R. Schumman, M. Serot, C. Sert, R. Siboescu, S. Terrier-de-la-Chaise, A. Thiesse, L. Thietry, M. Vanesson, M. Viellard. Secretaries: E. De Amorin, C. Villemain, N. Ziegler. Study coordinators: E. Dauchy, S. Laurent, and all persons not listed above who helped in the funding, initiation, accrual, management, and analysis of the fourth visit of the STANISLAS cohort. Steering committee: Pierre Mutzenhardt, Mehdy Siaghy, Patrick Lacolley, Marie-Ange Luc, Pierre Yves Marie, Jean-Michel Vignaud. Advisory members: Sophie Visvikis-Siest, Faiez Zannad. Technical committee: Christiane Branlant, Isabelle Behm-Ansmant, Jean-Michel Vignaud, Christophe Philippe, Jacques Magdalou, Faiez Zannad, Patrick Rossignol. Scientific committee: Laurence Tiret, Denis Wahl, Athanase Benetos, Javier Diez, Maurizio Ferrari, Jean Louis Gueant, Georges Dedoussis, François Alla, François Gueyffier, Pierre-Yves Scarabin, Claire Bonithon Kopp, Xavier Jouven, Jean-Claude Voegel, Jan Staessen. The authors also thank CRB Lorrain of Nancy CHRU for biobank handling. The authors are also grateful to the Department of Public Health of the University of Liège for allowing them to use their food frequency questionnaire (https://www.dssp-uliege.be/FFQ).
Data availability
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
M.B. is an awardee of a Gutenberg Chair of Excellence from the Région Grand Est and the Eurométropole de Strasbourg (France) and supported by the Swedish Research Council (2019-01486). The STANISLAS study is sponsored by Nancy CHRU. This work is supported by the French Ministry of Health ‘Programme Hospitalier de Recherche Clinique Inter regional 2013’, by the Contrat de Plan Etat-Lorraine and FEDER Lorraine, and a public grant overseen by the French National Research Agency (ANR) as part of the second ‘Investissements d’Avenir’ program FIGHT-HF (reference: ANR-15-RHU-0004) and by the French PIA project ‘Lorraine Université d’Excellence’, reference ANR-15-IDEX-04-LUE, by FEDER Lorraine and CPER IT2MP. It is also supported by FOCUS-MR (reference: ANR-15-CE14-0032-01), ERA-CVD EXPERT (reference: ANR-16-ECVD-0002-02), and the Fondation de Recherche en Hypertension Artérielle.
Conflict of interest: none declared.
References
- 1. Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014;510:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest 2018;128:2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bäck M, Yurdagul A Jr, Tabas I, Oorni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol 2019;16:389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carracedo M, Artiach G, Arnardottir H, Bäck M. The resolution of inflammation through omega-3 fatty acids in atherosclerosis, intimal hyperplasia, and vascular calcification. Semin Immunopathol 2019;41:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM, REDUCE-IT Investigators . Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 6. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, D’Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE, VITAL Research Group . Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 2019;380:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, Mozaffarian D, Ridker PM, Ray KK, Katona BG, Himmelmann A, Loss LE, Rensfeldt M, Lundström T, Agrawal R, Menon V, Wolski K, Nissen SE. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA 2020;324:2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bäck M, Hansson GK. Omega-3 fatty acids, cardiovascular risk, and the resolution of inflammation. FASEB J 2019;33:1536–1539. [DOI] [PubMed] [Google Scholar]
- 9. Sarajlic P, Artiach G, Larsson SC, Bäck M. Dose-dependent risk reduction for myocardial infarction with eicosapentaenoic acid: a meta-analysis and meta-regression including the STRENGTH trial. Cardiovasc Drugs Ther 2021;35:1079–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Safar ME, Asmar R, Benetos A, Blacher J, Boutouyrie P, Lacolley P, Laurent S, London G, Pannier B, Protogerou A, Regnault V. French Study Group on Arterial Stiffness . Interaction between hypertension and arterial stiffness. Hypertension 2018;72:796–805. [DOI] [PubMed] [Google Scholar]
- 11. Sekikawa A, Shin C, Masaki KH, Barinas-Mitchell EJ, Hirooka N, Willcox BJ, Choo J, White J, Evans RW, Fujiyoshi A, Okamura T, Miura K, Muldoon MF, Ueshima H, Kuller LH, Sutton-Tyrrell K, Group EJS. Association of total marine fatty acids, eicosapentaenoic and docosahexaenoic acids, with aortic stiffness in Koreans, whites, and Japanese Americans. Am J Hypertens 2013;26:1321–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reinders I, Murphy RA, Song X, Mitchell GF, Visser M, Cotch MF, Garcia ME, Launer LJ, Eiriksdottir G, Gudnason V, Harris TB, Brouwer IA. Higher plasma phospholipid n-3 PUFAs, but Lower n-6 PUFAs, are associated with lower pulse wave velocity among older adults. J Nutr 2015;145:2317–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Senevirathne A, Neale E, Peoples G, Tapsell L. Relationship between long-chain omega-3 polyunsaturated fatty acid intake and ankle brachial index, pulse wave velocity and resting heart rate in a sample of overweight adults: a secondary analysis of baseline data in the HealthTrack study. Nutr Diet 2019;76:95–103. [DOI] [PubMed] [Google Scholar]
- 14. Kim H, Park S, Yang H, Choi YJ, Huh KB, Chang N. Association between fish and shellfish, and omega-3 PUFAs intake and CVD risk factors in middle-aged female patients with type 2 diabetes. Nutr Res Pract 2015;9:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lemaitre RN, Tanaka T, Tang W, Manichaikul A, Foy M, Kabagambe EK, Nettleton JA, King IB, Weng LC, Bhattacharya S, Bandinelli S, Bis JC, Rich SS, Jacobs DR Jr, Cherubini A, McKnight B, Liang S, Gu X, Rice K, Laurie CC, Lumley T, Browning BL, Psaty BM, Chen YD, Friedlander Y, Djousse L, Wu JH, Siscovick DS, Uitterlinden AG, Arnett DK, Ferrucci L, Fornage M, Tsai MY, Mozaffarian D, Steffen LM, McCarthy MI. Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genet 2011;7:e1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yuan S, Bäck M, Bruzelius M, Mason AM, Burgess S, Larsson S. Plasma phospholipid fatty acids, FADS1 and Risk of 15 cardiovascular diseases: a mendelian randomisation study. Nutrients 2019;11:3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al-Hilal M, Alsaleh A, Maniou Z, Lewis FJ, Hall WL, Sanders TA, O’Dell SD, Team Ms . Genetic variation at the FADS1-FADS2 gene locus influences delta-5 desaturase activity and LC-PUFA proportions after fish oil supplement. J Lipid Res 2013;54:542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Porenta SR, Ko YA, Raskin L, Gruber SB, Mukherjee B, Baylin A, Ren J, Djuric Z. Interaction of fatty acid genotype and diet on changes in colonic fatty acids in a Mediterranean diet intervention study. Cancer Prev Res (Phila) 2013;6:1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cormier H, Rudkowska I, Lemieux S, Couture P, Julien P, Vohl MC. Effects of FADS and ELOVL polymorphisms on indexes of desaturase and elongase activities: results from a pre-post fish oil supplementation. Genes Nutr 2014;9:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferreira JP, Girerd N, Bozec E, Mercklé L, Pizard A, Bouali S, Eby E, Leroy C, Machu JL, Boivin JM, Lamiral Z, Rossignol P, Zannad F. Cohort profile: rationale and design of the fourth visit of the STANISLAS cohort: a familial longitudinal population-based cohort from the Nancy region of France. Int J Epidemiol 2018;47:395–395j. [DOI] [PubMed] [Google Scholar]
- 21. Wagner S, Lioret S, Girerd N, Duarte K, Lamiral Z, Bozec E, Van den Berghe L, Hoge A, Donneau AF, Boivin JM, Mercklé L, Zannad F, Laville M, Rossignol P, Nazare JA. Association of dietary patterns derived using reduced-rank regression with subclinical cardiovascular damage according to generation and sex in the STANISLAS cohort. J Am Heart Assoc 2020;9:e013836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sauvageot N, Alkerwi A, Albert A, Guillaume M. Validation of the food frequency questionnaire used to assess the association between dietary habits and cardiovascular risk factors in the NESCAV study. J Nutr Food Sci 2013;3:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chau K, Girerd N, Bozec E, Ferreira JP, Duarte K, Nazare JA, Laville M, Benetos A, Zannad F, Boivin JM, Rossignol P. Association between abdominal adiposity and 20-year subsequent aortic stiffness in an initially healthy population-based cohort. J Hypertens 2018;36:2077–2084. [DOI] [PubMed] [Google Scholar]
- 24. Xhaard C, Dandine-Roulland C, Villemereuil P, Floch EL, Bacq-Daian D, Machu JL, Ferreira JP, Deleuze JF, Zannad F, Rossignol P, Girerd N. Heritability of a resting heart rate in a 20-year follow-up family cohort with GWAS data: Insights from the STANISLAS cohort. Eur J Prev Cardiol 2021;28:1334–1341. [DOI] [PubMed] [Google Scholar]
- 25. Gillingham LG, Harding SV, Rideout TC, Yurkova N, Cunnane SC, Eck PK, Jones PJ. Dietary oils and FADS1-FADS2 genetic variants modulate [13C]alpha-linolenic acid metabolism and plasma fatty acid composition. Am J Clin Nutr 2013;97:195–207. [DOI] [PubMed] [Google Scholar]
- 26. Ferreira JP, Girerd N, Bozec E, Machu JL, Boivin JM, London GM, Zannad F, Rossignol P. Intima-media thickness is linearly and continuously associated with systolic blood pressure in a population-based cohort (STANISLAS Cohort Study). J Am Heart Assoc 2016;5:e003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lopez-Sublet M, Girerd N, Bozec E, Machu JL, Ferreira JP, Zannad F, Mourad JJ, Rossignol P. Nondipping pattern and cardiovascular and renal damage in a population-based study (the STANISLAS cohort study). Am J Hypertens 2019;32:620–628. [DOI] [PubMed] [Google Scholar]
- 28. Expert Panel on Detection Evaluation, Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 29. Plunde O, Larsson SC, Artiach G, Thanassoulis G, Carracedo M, Franco-Cereceda A, Eriksson P, Bäck M. FADS1 (Fatty Acid Desaturase 1) Genotype associates with aortic valve FADS mRNA expression, fatty acid content and calcification. Circ Genom Precis Med 2020;13:e002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tousoulis D, Plastiras A, Siasos G, Oikonomou E, Verveniotis A, Kokkou E, Maniatis K, Gouliopoulos N, Miliou A, Paraskevopoulos T, Stefanadis C. Omega-3 PUFAs improved endothelial function and arterial stiffness with a parallel antiinflammatory effect in adults with metabolic syndrome. Atherosclerosis 2014;232:10–16. [DOI] [PubMed] [Google Scholar]
- 31. Minihane AM. Impact of genotype on EPA and DHA status and responsiveness to increased intakes. Nutrients 2016;8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Plunde O, Larsson S, Artiach G, Thanassoulis G, Carracedo M, Franco-Cereceda A, Eriksson P, Bäck M. FADS1 genotype associates with aortic valve FADS mRNA expression, fatty acid content and calcification. Circ Genom Precis Med 2020;13: e002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen HY, Cairns BJ, Small AM, Burr HA, Ambikkumar A, Martinsson A, Theriault S, Munter HM, Steffen B, Zhang R, Levinson RT, Shaffer CM, Rong J, Sonestedt E, Dufresne L, Ljungberg J, Naslund U, Johansson B, Ranatunga DK, Whitmer RA, Budoff MJ, Nguyen A, Vasan RS, Larson MG, Harris WS, Damrauer SM, Stark KD, Boekholdt SM, Wareham NJ, Pibarot P, Arsenault BJ, Mathieu P, Gudnason V, O’Donnell CJ, Rotter JI, Tsai MY, Post WS, Clarke R, Soderberg S, Bosse Y, Wells QS, Smith JG, Rader DJ, Lathrop M, Engert JC, Thanassoulis G. Association of FADS1/2 locus variants and polyunsaturated fatty acids with aortic stenosis. JAMA Cardiol 2020;5:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Serikawa T, Miura S, Okabe M, Hongo H, Tokutome M, Yoshikawa T, Takesue K, Adachi S, Osaka K, Matsukawa R, Yanagi D, Nozoe M, Kozai T, Hironaga K, Saku K, Yamamoto Y. Ratio of eicosapentaenoic acid to arachidonic acid is a critical risk factor for acute coronary syndrome in middle-aged older patients as well as younger adult patients. J Cardiol 2014;63:35–40. [DOI] [PubMed] [Google Scholar]
- 35. Thul S, Labat C, Temmar M, Benetos A, Bäck M. Low salivary resolvin D1 to leukotriene B4 ratio predicts carotid intima media thickness: a novel biomarker of non-resolving vascular inflammation. Eur J Prev Cardiol 2017;24:903–906. [DOI] [PubMed] [Google Scholar]
- 36. Smith CE, Follis JL, Nettleton JA, Foy M, Wu JH, Ma Y, Tanaka T, Manichakul AW, Wu H, Chu AY, Steffen LM, Fornage M, Mozaffarian D, Kabagambe EK, Ferruci L, Chen YD, Rich SS, Djousse L, Ridker PM, Tang W, McKnight B, Tsai MY, Bandinelli S, Rotter JI, Hu FB, Chasman DI, Psaty BM, Arnett DK, King IB, Sun Q, Wang L, Lumley T, Chiuve SE, Siscovick DS, Ordovas JM, Lemaitre RN. Dietary fatty acids modulate associations between genetic variants and circulating fatty acids in plasma and erythrocyte membranes: meta-analysis of nine studies in the CHARGE consortium. Mol Nutr Food Res 2015;59:1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Agence nationale de sécurité sanitaire alimentation e, travail (ANSES) . Étude individuelle nationale des consommations alimentaires 3 (INCA 3). In. June 2017 ed; 2017.
- 38. Takkunen MJ, de Mello VD, Schwab US, Kuusisto J, Vaittinen M, Agren JJ, Laakso M, Pihlajamaki J, Uusitupa MI. Gene-diet interaction of a common FADS1 variant with marine polyunsaturated fatty acids for fatty acid composition in plasma and erythrocytes among men. Mol Nutr Food Res 2016;60:381–389. [DOI] [PubMed] [Google Scholar]
- 39. Joensen AM, Overvad K, Dethlefsen C, Johnsen SP, Tjønneland A, Rasmussen LH, Schmidt EB. Marine n-3 polyunsaturated fatty acids in adipose tissue and the risk of acute coronary syndrome. Circulation 2011;124:1232–1238. [DOI] [PubMed] [Google Scholar]
- 40. Childs CE, Kew S, Finnegan YE, Minihane AM, Leigh-Firbank EC, Williams CM, Calder PC. Increased dietary α-linolenic acid has sex-specific effects upon eicosapentaenoic acid status in humans: re-examination of data from a randomised, placebo-controlled, parallel study. Nutr J 2014;13:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Labat C, Temmar M, Nagy E, Bean K, Brink C, Benetos A, Bäck M. Inflammatory mediators in saliva associated with arterial stiffness and subclinical atherosclerosis. J Hypertens 2013;31:2251–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pouchieu C, Andreeva VA, Péneau S, Kesse-Guyot E, Lassale C, Hercberg S, Touvier M. Sociodemographic, lifestyle and dietary correlates of dietary supplement use in a large sample of French adults: results from the NutriNet-Santé cohort study. Br J Nutr 2013;110:1480–1491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author.