Abstract
A mucoidal strain of Rhodococcus rhodochrous was resistant to 10% (vol/vol) n-hexadecane, while its rough derivatives were sensitive. When the extracellular polysaccharide (EPS) produced by the mucoidal strain was added to cultures of the rough strains, the rough strains gained resistance to n-hexadecane. Thus, EPS confer tolerance to n-hexadecane in members of the genus Rhodococcus.
The genus Rhodococcus is a group of bacteria that exhibit a diverse range of metabolic activities. Some rhodococci have the ability to degrade a variety of organic compounds, including man-made xenobiotic compounds such as polychlorinated biphenyls, while others are capable of degrading numerous aliphatic or aromatic hydrocarbons (4, 6, 17, 18). We prepared the aromatic fraction (AF) of Arabian light crude oil by silica gel chromatography as indicated in Table 1 and screened 75 Rhodococcus strains for growth on the AF. The growth medium used was SWY (5.0 g of NH4NO3, 0.1 g of FeC6H5O7 · nH2O, 0.1 g of K2HPO4, and 0.25 g of yeast extract in 1 liter of filtered seawater, pH 7.8) supplemented with 1% (vol/vol) AF (SWYAF). None of the strains tested grow in SWY, but six strains exhibited significant growth in SWYAF. All six of these strains were mucoidal in colony morphotype (Table 1).
TABLE 1.
Growth of rhodococci on seawater-based medium containing the AF of Arabian light crude oila
| Species | Strain | Growth | Colony morphology |
|---|---|---|---|
| Rhodococcus australis | ATCC 35215 | − | R |
| Rhodococcus coprophilus | ATCC 29080 | − | R |
| Rhodococcus equi | IAM 3223 | − | R |
| Rhodococcus equi | IFO 14956 | − | S/M |
| Rhodococcus erythropolis | ATCC 19369 | + | R |
| Rhodococcus erythropolis | ATCC 27854 | − | S/R |
| Rhodococcus erythropolis | ATCC 47072 | − | S/R |
| Rhodococcus erythropolis | DSM 1069 | − | S/R |
| Rhodococcus erythropolis | IFO 15567 | − | S |
| Rhodococcus erythropolis | JCM 3201 | − | R |
| Rhodococcus fascians | IFO 15528 | − | R |
| Rhodococcus globerulus | ATCC 14346 | + | R |
| Rhodococcus globerulus | ATCC 14898 | − | S |
| Rhodococcus globerulus | ATCC 15076 | + | R |
| Rhodococcus globerulus | ATCC 15903 | − | S/R |
| Rhodococcus globerulus | ATCC 19370 | − | S |
| Rhodococcus globerulus | ATCC 21022 | ++ | S-M |
| Rhodococcus globerulus | ATCC 21292 | − | R |
| Rhodococcus globerulus | ATCC 21505 | − | S/R |
| Rhodococcus globerulus | ATCC 21506 | + | S/R |
| Rhodococcus globerulus | ATCC 21602 | − | S/R |
| Rhodococcus globerulus | ATCC 25669 | − | R |
| Rhodococcus globerulus | ATCC 25688 | − | R |
| Rhodococcus globerulus | ATCC 31130 | − | R |
| Rhodococcus globerulus | IFO 14531 | − | S/R |
| Rhodococcus opacus | ATCC 17039 | − | R |
| Rhodococcus opacus | ATCC 51881 | − | R |
| Rhodococcus opacus | ATCC 51882 | − | R |
| Rhodococcus percolatus | JCM 10087 | − | S/R |
| Rhodococcus rhodnii | ATCC 35071 | − | S |
| Rhodococcus rhodochrous | ATCC 12483 | − | S/R |
| Rhodococcus rhodochrous | ATCC 12674 | − | S/R |
| Rhodococcus rhodochrous | ATCC 13808 | − | R |
| Rhodococcus rhodochrous | ATCC 14341 | − | R |
| Rhodococcus rhodochrous | ATCC 14347 | − | S/R |
| Rhodococcus rhodochrous | ATCC 14348 | − | R |
| Rhodococcus rhodochrous | ATCC 14349 | − | R |
| Rhodococcus rhodochrous | ATCC 14350 | − | R |
| Rhodococcus rhodochrous | ATCC 15610 | − | R |
| Rhodococcus rhodochrous | ATCC 15905 | − | R |
| Rhodococcus rhodochrous | ATCC 15906 | − | R |
| Rhodococcus rhodochrous | ATCC 15998 | − | R |
| Rhodococcus rhodochrous | ATCC 17041 | + | M/R |
| Rhodococcus rhodochrous | ATCC 17043 | − | S |
| Rhodococcus rhodochrous | ATCC 17895 | − | R |
| Rhodococcus rhodochrous | ATCC 184 | − | S |
| Rhodococcus rhodochrous | ATCC 19067 | − | S/R |
| Rhodococcus rhodochrous | ATCC 19140 | − | M/R |
| Rhodococcus rhodochrous | ATCC 19149 | − | R |
| Rhodococcus rhodochrous | ATCC 19150 | − | S,M/R |
| Rhodococcus rhodochrous | ATCC 21197 | − | R |
| Rhodococcus rhodochrous | ATCC 21198 | − | R |
| Rhodococcus rhodochrous | ATCC 21199 | − | R |
| Rhodococcus rhodochrous | ATCC 21243 | + | R |
| Rhodococcus rhodochrous | ATCC 21291 | − | R |
| Rhodococcus rhodochrous | ATCC 21766 | + | R |
| Rhodococcus rhodochrous | ATCC 21785 | − | R |
| Rhodococcus rhodochrous | ATCC 21924 | − | R |
| Rhodococcus rhodochrous | ATCC 271 | − | R |
| Rhodococcus rhodochrous | ATCC 29670 | − | R |
| Rhodococcus rhodochrous | ATCC 29675 | − | R |
| Rhodococcus rhodochrous | ATCC 33025 | − | R |
| Rhodococcus rhodochrous | ATCC 33258 | ++ | M |
| Rhodococcus rhodochrous | ATCC 33278 | − | R |
| Rhodococcus rhodochrous | ATCC 4001 | − | R |
| Rhodococcus rhodochrous | ATCC 4004 | − | R |
| Rhodococcus rhodochrous | ATCC 4276 | − | R |
| Rhodococcus rhodochrous | ATCC 53968 | ++ | M |
| Rhodococcus rhodochrous | ATCC 9356 | − | S/R |
| Rhodococcus rhodochrous | ATCC 999 | − | R |
| Rhodococcus ruber | IFO 15591 | − | R |
| Rhodococcus zopfii | ATCC 51349 | − | R |
| Rhodococcus sp. | KL6 | ++ | M |
| Rhodococcus sp. | PR4 | ++ | M |
| Rhodococcus sp. | PG7-2 | ++ | M |
The AF was prepared by column chromatography by using Silica Gel C-200 (Wako Pure Chemicals) activated at 180°C for 20 h. Arabian crude oil dissolved in n-hexane at a concentration of 100 mg/ml was applied to the column, and the column was serially eluted with 3 bed volumes of n-hexane, benzene–n-hexane (1:1), and chloroform. The eluate obtained with benzene–n-hexane (1:1) was kept and used as the AF. The growth of rhodococci in SWYAF was examined at 30°C. M, mucoid; S, smooth; R, rough; R/M, a few rough colonies appeared among mucoidal colonies; R-M, rough sectors appeared in mucoidal colonies; ++, cultures became turbid; +, cultures became less turbid; cultures remained transparent and almost all of the oil adhered to the inner surfaces of tubes.
Nineteen of the 75 strains tested showed spontaneous rough-smooth colony morphotype changes at high frequencies. Three of these strains, Rhodococcus rhodochrous ATCC 17041, ATCC 19140, and ATCC 19150, were selected, and mucoidal derivatives were obtained from the original strains. Subsequently, rough derivatives were obtained from the mucoidal variants. The mucoidal clones of R. rhodochrous ATCC 17041, ATCC 19140, and ATCC 19150 showed good growth on SWYAF, whereas the parental strains and the rough derivatives of the mucoidal variants showed no or poor growth on SWYAF.
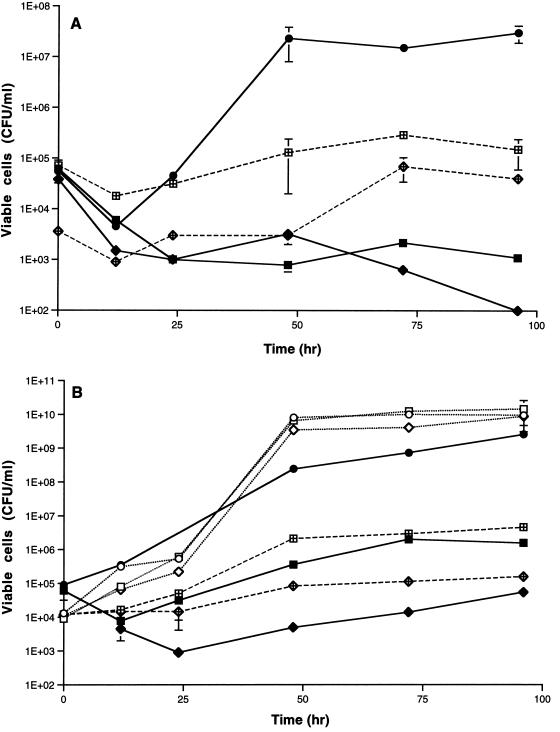
These data suggested that there was an association between mucoidal morphology and the ability to grow on the AF of the crude oil. To investigate further, we employed three colony morphology mutants, S-2, R-1, and R-2, derived from R. rhodochrous CF222 (11, 16). Mucoidal strain S-2 grew well on SWYAF, whereas rough strains R-1 and R-2 did not (Fig. 1A). Plasmid pK4I-7 transformed S-2 from mucoidal to rough colony morphology, and production of extracellular polysaccharide (EPS) was suppressed in the resulting rough transformants (7). Growth of these transformants was inhibited greatly in SWYAF, supporting the hypothesis that there is an association between mucoidal morphology and the ability to grow on the AF.
FIG. 1.
Growth of the colony morphology mutants derived from R. rhodochrous CF222 in SWYAF (A) or in YGAF (B) in the presence or absence of 100 μg of S-2 EPS per ml. The growth temperature was 30°C. (A) Symbols: ●, growth of S-2 in SWYAF; ■, growth of R-1 in SWYAF; ⧫, growth of R-2 in SWYAF; ⊞, growth of R-1 in SWYAF in the presence of S-2 EPS; •, growth of R-2 in SWYAF in the presence of S-2 EPS. (B) Symbols: ○, growth of S-2 in YG; □, growth of R-1 in YG; ▵, growth of R-2 in YG; ●, growth of S-2 in YGAF; ■, growth of R-1 in YGAF; ⧫, growth of R-2 in YGAF; ⊞, growth of R-1 in YGAF in the presence of EPS; •, growth of R-2 in YGAF in the presence of EPS.
Strains S-2, R-1, and R-2 grew on YG (1% [wt/vol] glucose and 1% [wt/vol] yeast extract dissolved in distilled water, pH 7.2). Mucoidal strain S-2 showed good growth on YG containing 1% (vol/vol) AF (YGAF), while growth of rough strain R-2 was greatly inhibited by the AF. Growth of rough strain R-1 was also inhibited by the AF but to a lesser extent (Fig. 1B). From these observations, we concluded that the rough strains could not grow on the AF because they are sensitive to it.
To characterize the tolerance of Rhodococcus strains to various hydrocarbons, an organic solvent tolerance test was performed as described by Aono et al. (2), and the results are shown in Table 2. Mucoidal strain S-2 showed good growth on plates overlaid with n-dodecane, n-pentadecane, n-tetradecane, and n-hexadecane, whereas two rough strains did not. No or little difference among the three strains was detected in growth on plates overlaid with low-molecular-weight volatile compounds. The three strains were all resistant to short-chain n-alkanes and cylohexane, while they were all sensitive to alkylbenzenes. The results suggest that the rough morphotype renders cells specifically sensitive to medium-chain-length n-alkanes.
TABLE 2.
Organic solvent tolerance testa
| Organic solvent | Tolerance of:
|
||
|---|---|---|---|
| Strain S-2 | Strain R-1 | Strain R-2 | |
| n-Hexadecane | ++ | − | − |
| n-Tetradecane | ++ | ± | − |
| n-Pentadecane | ++ | ± | − |
| n-Dodecane | ++ | ± | − |
| n-Hexane | ++ | ++ | ++ |
| Cyclohexane | ++ | ++ | ++ |
| n-Pentane | ++ | ++ | ++ |
| p-Xylene | − | − | − |
| Toluene | − | − | − |
| Benzene | − | − | − |
This experiment was done as described by Aono et al. (2), and the levels of growth of the strains were determined optically. ++, confluent growth; ±, poor growth; −, no growth.
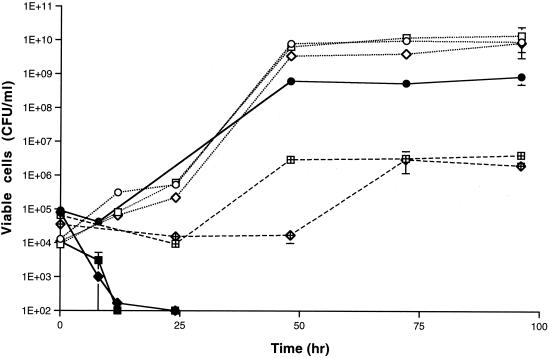
When 10% (vol/vol) n-hexadecane was added to Rhodococcus cultures growing on YG, the numbers of viable cells of the rough strains decreased below the detection limit on day 2, whereas mucoidal strain S-2 was less affected (Fig. 2). When aliquots of n-hexadecane layers from the cultures were spread on YG agar plates and the plates were incubated at 30°C for 96 h, no colonies were formed from cultures of the smooth and rough strains, indicating that viable cells were not present in n-hexadecane layers of the samples. These results showed that the cells of the rough strains but not the cells of the smooth strain were killed in the presence of n-hexadecane under the conditions used.
FIG. 2.
Growth of colony morphology mutants in YG containing 10% (vol/vol) n-hexadecane in the presence or absence of 100 μg of S-2 EPS per ml. The growth temperature was at 30°C. Symbols: ○, growth of S-2 in YG; □, growth of R-1 in YG; ◊, growth of R-2 in YG; ●, growth of S-2 in YG containing n-hexadecane; ■, growth of R-1 in YG containing n-hexadecane; ⧫, growth of R-2 in YG containing n-hexadecane; ⊞, growth of R-1 in YG containing n-hexadecane in the presence of EPS; •, growth of R-2 in YG containing n-hexadecane in the presence of EPS.
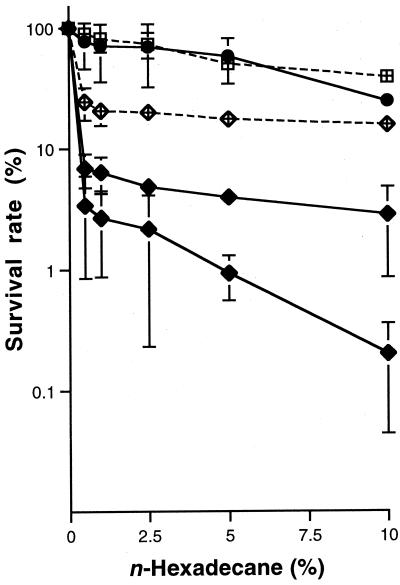
Next, we examined the sensitivity of resting cells to n-hexadecane. Late-logarithmic-phase cells of the three strains grown on YG were suspended in saline and treated with n-hexadecane as described in the legend to Fig. 3. The numbers of viable cells of all of the strains were decreased by treatment with n-hexadecane in a dose-dependent manner, and the level of tolerance to n-hexadecane was determined to be S-2 ≫ R-1 > R-2. No colonies formed when aliquots of n-hexadecane layers were spread on YG agar plates.
FIG. 3.
Survival rates of colony morphology mutants treated with 10% (vol/vol) n-hexadecane in the presence or absence of 100 μg of S-2 EPS per ml. Symbols: ●, S-2 in the absence of EPS; ■, R-1 in the absence of EPS; ⧫, R-2 in the absence of EPS; ⊞, R-1 in the presence of EPS; •, R-2 in the presence of EPS. Cells grown to the late logarithmic phase in YG were harvested by centrifugation at 4,000 × g for 10 min, washed twice with 5 ml of saline, and resuspended in 5 ml of saline. The cell suspensions were diluted 104-fold, and 5 ml aliquots of the diluted cell suspensions were dispensed into sterile tubes containing 0, 2.5, 5, 12.5, 25, or 50 mg of n-hexadecane. Each sample was vortexed twice for 30 s with a 5-s interval between treatments and then left until complete separation of the aqueous and n-hexadecane layers occurred. An aliquot (1 ml) of the aqueous layer was transferred to a new tube and plated onto YG agar plates after appropriate dilution with saline. The plates were incubated at 30°C for 48 h.
Since mucoidal strain S-2 produced much more EPS than the rough strains (15) and rough transformants with pK4I-7 produced (7), we examined the effect of EPS produced by S-2 (S-2 EPS) on the tolerance of the rough strains to the AF or to n-hexadecane. Cells (10 g, wet weight) of S-2 grown on YG agar plates were harvested by scraping and suspended in water. The cell suspension was shaken at 120 rpm for 10 min at 25°C and centrifuged at 14,000 × g for 10 min, and then the supernatant was transferred to new tubes. DNase and RNase were each added to the supernatant to a final concentration of 1 μg/ml, and the supernatant was incubated at 37°C for 16 h. Subsequently, the solution was treated with proteinase K (10 μg/ml) at 37°C for 2 h, and the sample was purified by phenol-chloroform treatment. After five dialysis treatments, each against 5 liters of water for more than 3 h at 4°C, the sample was lyophilized. This preparation was designated S-2 EPS. Preliminary characterization showed that S-2 EPS consisted of acidic polysaccharides containing d-glucose, d-galactose, d-mannose, d-glucuronic acid, and lipids.
Addition of 0.1 mg of S-2 EPS per ml either to SWY or to YG containing no hydrocarbon did not enhance growth of the strains tested compared to growth in the absence of S-2 EPS. However, the responses of the rough strains to S-2 EPS in the presence of the AF or n-hexadecane were different. Growth inhibition by the AF was attenuated in the rough strains by the addition of 0.1 mg of S-2 EPS per ml both in SWYAF (Fig. 1A) and in YGAF (Fig. 1B). When S-2 EPS was added, the numbers of viable R-1 and R-2 cells at all sampling times increased about 10- to 100-fold compared to those in the absence of EPS.
Growth of the R-1 and R-2 strains in YG containing 10% (vol/vol) n-hexadecane in the presence and in the absence of S-2 EPS was also examined (Fig. 2). When S-2 EPS was added, growth of the R-1 and R-2 strains was rescued from inhibition by n-hexadecane.
The effect of S-2 EPS on survival of the rough strains in the presence of n-hexadecane under resting conditions was also investigated (Fig. 3). When S-2 EPS was added, survival of the R-1 and R-2 cells in the presence of 10% (vol/vol) n-hexadecane increased approximately 10- to 100-fold. The results suggested that EPS produced by S-2 protect the rough strains from the toxicity of the hydrocarbon.
Finally, we examined the effect of S-2 EPS on the survival of other rough strains of the genus Rhodococcus. R. coprophilus ATCC 29080, R. erythropolis IFO 15567 and JCM 3201, R. globerulus IFO 14531, R. opacus ATCC 51881, R. rhodochrous ATCC 13808, and R. zopfii ATCC 51349 were used in these experiments. The results are shown in Table 3. The number of viable cells was decreased by treatment with 10% (vol/vol) n-hexadecane for all of the strains tested, and the killing effect was attenuated by addition of S-2 EPS. The results supported and generalized our notion that S-2 EPS protects rough Rhodococcus strains from the toxicity of n-hexadecane.
TABLE 3.
Effect of S-2 EPS on the survival of resting cells of Rhodococcus strains treated with 10% (vol/vol) n-hexadecanea
| Strain | % of surviving cells
|
|
|---|---|---|
| Without EPS | With EPS | |
| R. coprophilus ATCC 29080 | 0.1 (±0.2) | 1.8 (±0.78) |
| R. erythropolis IFO 15567 | 0.9 (±1.2) | 75.3 (±81.6) |
| R. erythropolis JCM 3201 | 0.1 (±0.1) | 48.6 (±25.4) |
| R. globerulus IFO 14531 | 0.01 (±0.01) | 8.6 (±0.1) |
| R. opacus ATCC 51881 | 1.1 (±1.0) | 18.2 (±1.1) |
| R. rhodochrous ATCC 13808 | 0 (±0) | 72.6 (±6.7) |
| R. zopfii ATCC 51349 | 0.5 (±0.5) | 46.7 (±23.6) |
The EPS concentration used in this test was 100 μg/ml. The values are averages based on at least two independent experiments; the values in parentheses are standard errors.
The mechanism of tolerance to oils and n-hexadecane associated with S-2 EPS is not known at present. There are several reports demonstrating that addition of a biosurfactant or chemically synthesized surfactant enhances the biodegradation of organic solvents by bacteria (3, 5). It has also been shown that the level of tolerance to hydrocarbons is elevated by the production of various surface-active compounds (8, 13). Emulsification was observed when S-2 EPS was mixed with various oils. Therefore, one of the possible mechanisms for tolerance is that the surfactant activity of S-2 EPS renders cells resistant to the AF and n-hexadecane. Some Rhodococcus strains produce biosurfactant molecules in response to n-alkanes. These molecules are predominantly glycolipids (10), but other types have also been reported (9, 12, 14). S-2 EPS is a high-molecular-weight complex of acidic polysaccharides and lipids (our preliminary results) and, therefore, different from previously reported Rhodococcus biosurfactants.
The rough strains of Rhodococcus have strongly hydrophobic surfaces, while the surfaces of the smooth strains are hydrophilic. S-2 EPS has been shown to lower the cell surface hydrophobicity of rough strains of Rhodococcus, indicating that S-2 EPS functions as a hydrophilin (15). Aono and Kobayashi (1) reported that low cell surface hydrophobicity serves as a defense mechanism against organic solvents. It is thus possible that S-2 EPS lowers the cell surface hydrophobicity and establishes tolerance to oils and n-hexadecane.
Acknowledgments
We thank H. Kasai and H. Miyashita for valuable discussions and encouragement. We also acknowledge M. Nozawa and T. Aizawa for technical assistance.
This study was performed as part of the Industrial Science and Technology Frontier Program supported by the New Energy and Industrial Technology Development Organization. This study was also supported in part by a grant from Nihon University to M.S. and M.N. and by a “High-Tech Research Center Project” of the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Aono R, Kobayashi H. Cell surface properties of organic solvent-tolerant mutants of Escherichia coli K-12. Appl Environ Microbiol. 1997;63:3637–3642. doi: 10.1128/aem.63.9.3637-3642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aono R, Kobayashi M, Nakajima H, Kobayashi H. A close correlation between improvement of organic solvent tolerance levels and alteration of resistance toward low levels of multiple antibiotics in Escherichia coli. Biosci Biotechnol Biochem. 1995;59:213–218. doi: 10.1271/bbb.59.213. [DOI] [PubMed] [Google Scholar]
- 3.Barkay T, Navon-Venezia S, Ron E Z, Rosenberg E. Enhancement of solubilization and biodegradation of polyaromatic hydrocarbons by the bioemulsifier alasan. Appl Environ Microbiol. 1999;65:2697–2702. doi: 10.1128/aem.65.6.2697-2702.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell K S, Philip J C, Aw D W J, Christofi N. The genus Rhodococcus. J Appl Microbiol. 1998;85:195–210. doi: 10.1046/j.1365-2672.1998.00525.x. [DOI] [PubMed] [Google Scholar]
- 5.Bruheim P, Bredholt H, Eimhjellen K. Bacterial degradation of emulsified crude oil and the effect of various surfactants. Can J Microbiol. 1997;43:17–22. doi: 10.1139/m97-003. [DOI] [PubMed] [Google Scholar]
- 6.Finnerty W R. The biology and genetics of the genus Rhodococcus. Annu Rev Microbiol. 1992;46:193–218. doi: 10.1146/annurev.mi.46.100192.001205. [DOI] [PubMed] [Google Scholar]
- 7.Iwabuchi N, Sunairi M, Nakajima M. Cloning a DNA fragment from Rhodococcus rhodochrous, which suppresses its mucoidal morphology. Actinomycetology. 1997;11:59–63. [Google Scholar]
- 8.Kobayashi H, Takami H, Hirayama H, Kobata K, Usami R, Horikoshi K. Outer membrane changes in a toluene-sensitive mutant of toluene-tolerant Pseudomonas putida IH-2000. J Bacteriol. 1999;181:4493–4998. doi: 10.1128/jb.181.15.4493-4498.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurane R, Matsuyama H. Production of a bioflocculant by mixed culture. Biosci Biotechnol Biochem. 1994;58:1589–1594. doi: 10.1271/bbb.58.1589. [DOI] [PubMed] [Google Scholar]
- 10.Lang S, Philp J C. Surface-active lipids in rhodococci. Antonie Leeuwenhoek. 1998;74:59–70. doi: 10.1023/a:1001799711799. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima M, Wakayama Y, Murooka H. Scanning electron microscopic studies on cell arrangement within colonies of S and R strains of Nocardia sp. Bull Coll Agric Vet Med Nihon Univ. 1980;37:107–121. [Google Scholar]
- 12.Neu T R, Dengler T, Jann B, Poralla K. Structural studies of an emulsion-stabilizing exopolysaccharide produced by an adhesive, hydrophobic Rhodococcus strain. J Gen Microbiol. 1992;138:2531–2537. doi: 10.1099/00221287-138-12-2531. [DOI] [PubMed] [Google Scholar]
- 13.Pinkart H C, White D C. Phospholipid biosynthesis and solvent tolerance in Pseudomonas putida strains. J Bacteriol. 1997;179:4219–4226. doi: 10.1128/jb.179.13.4219-4226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Severn W B, Richards J C. The structure of the specific capsular polysaccharide of Rhodococcus equi serotype 4. Carbohydr Res. 1999;320:209–222. doi: 10.1016/s0008-6215(99)00138-x. [DOI] [PubMed] [Google Scholar]
- 15.Sunairi M, Iwabuchi N, Yoshizawa Y, Murooka H, Nakajima M. Cell-surface hydrophobicity and scum formation of Rhodococcus rhodochrous strains with different colonial morphologies. J Appl Microbiol. 1997;82:204–210. doi: 10.1111/j.1365-2672.1997.tb02852.x. [DOI] [PubMed] [Google Scholar]
- 16.Wakayama Y, Nakajima M, Murooka H. Isolation and characterization of S and R strains of Nocardia sp. CF222. Bull Coll Agric Vet Med Nihon Univ. 1980;37:99–105. [Google Scholar]
- 17.Warhurst A M, Fewson C A. Biotransformations catalyzed by the genus Rhodococcus. Crit Rev Biotechnol. 1994;14:29–73. doi: 10.3109/07388559409079833. [DOI] [PubMed] [Google Scholar]
- 18.Whyte L G, Hawari J, Zhou E, Bourbonniere L, Inniss W E, Greer C W. Biodegradation of variable-chain-length alkanes at low temperatures by a psychrotrophic Rhodococcus sp. Appl Environ Microbiol. 1998;64:2578–2584. doi: 10.1128/aem.64.7.2578-2584.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]