Abstract
PURPOSE
Fatigue is recognized as one of the most burdensome and long-lasting adverse effects of cancer and cancer treatment. We aimed to characterize long-term fatigue trajectories among breast cancer survivors.
METHODS
We performed a detailed longitudinal analysis of fatigue using a large ongoing national prospective clinical study (CANcer TOxicity, ClinicalTrials.gov identifier: NCT01993498) of patients with stage I-III breast cancer treated from 2012 to 2015. Fatigue was assessed at diagnosis and year 1, 2, and 4 postdiagnosis. Baseline clinical, sociodemographic, behavioral, tumor-related, and treatment-related characteristics were available. Trajectories of fatigue and risk factors of trajectory-group membership were identified by iterative estimates of group-based trajectory models.
RESULTS
Three trajectory groups were identified for severe global fatigue (n = 4,173). Twenty-one percent of patients were in the high-risk group, having risk estimates of severe global fatigue of 94.8% (95% CI, 86.6 to 100.0) at diagnosis and 64.6% (95% CI, 59.2 to 70.1) at year 4; 19% of patients clustered in the deteriorating group with risk estimates of severe global fatigue of 13.8% (95% CI, 6.7 to 20.9) at diagnosis and 64.5% (95% CI, 57.3 to 71.8) at year 4; 60% were in the low-risk group with risk estimates of 3.6% (95% CI, 2.5 to 4.7) at diagnosis and 9.6% (95% CI, 7.5 to 11.7) at year 4. The distinct dimensions of fatigue clustered in different trajectory groups than those identified by severe global fatigue, being differentially affected by sociodemographic, clinical, and treatment-related factors.
CONCLUSION
Our findings highlight the multidimensional nature of cancer-related fatigue and the complexity of its risk factors. This study helps to identify patients with increased risk of severe fatigue and to inform personalized interventions to ameliorate this problem.
INTRODUCTION
Cancer-related fatigue is one of the most burdensome and potentially long-lasting effects of breast cancer (BC) treatment.1-3 A substantial number of previous studies demonstrated that most patients experience fatigue during active treatment and resume usual energy after the end of treatment; however, 20%-30% of patients experience persistent severe fatigue for many years.4-9 Previous studies also suggested that cancer-related fatigue is multifactorial. Medically, psychologically, socially, and economically fragile patients at the time of diagnosis have a higher risk of enduring persistent fatigue after cancer.5 There is also an increasing recognition that fatigue is multidimensional and includes physical, emotional, and cognitive components.10
CONTEXT
Key Objective
There is considerable interindividual variability in long-term trajectories of patient-reported fatigue after early breast cancer primary therapy. We aimed to identify latent clusters of patients at risk for severe cancer-related fatigue and to assess the relationship of host factors with trajectory-group membership.
Knowledge Generated
Most patients fared well over time; however, a cluster reported significantly worsened global cancer-related fatigue after primary treatment and never recovered to pretreatment values for up to 4 years after diagnosis. The distinct fatigue dimensions were differentially affected by multiple factors, including sociodemographic and clinical factors and treatment-related factors. Common determinants of worse trajectory membership included emotional distress, particularly depression, and receipt of hormonal therapy.
Relevance
This study will help identify clusters of patients with early breast cancer who are at risk of persistent cancer-related fatigue and facilitate personalized interventions.
Classic analytic approaches that describe the average population level may not capture the interindividual variability of the longitudinal trajectory of cancer-related fatigue. However, this variability can be more extensively and granularly explored by clustering techniques such as growth mixture models or latent class analyses, which can identify subgroups of patients with similar longitudinal trajectories over time.11,12 Only a few studies have attempted to identify distinct groups of patients who experience distinct fatigue trajectories after BC diagnosis using this methodological approach.7,13-15 These previous studies have been limited by small samples and a lack of comprehensive longitudinal examination spanning from diagnosis to long-term survivorship. Furthermore, there are no reports that have evaluated the trajectories of the distinct dimensions of fatigue.
In this study, we used CANTO (ClinicalTrials.gov identifier: NCT01993498) data16 to describe patterns of global fatigue over 4 years after BC diagnosis and to assess risk factors of distinct fatigue trajectory groups over time. We also examined the trajectories of the distinct dimensions of fatigue, including physical, emotional, and cognitive fatigue.
METHODS
Data Source
CANTO cohort data were used.16 Briefly, CANTO enrolled female patients with stage I-II-III BC. Data used in the present study had been collected at diagnosis (baseline, before treatment onset) and then at year 1 (3-6 months after completion of primary surgery, chemotherapy, and/or radiation therapy), 2, and 4 follow-up visits after diagnosis. Adjuvant endocrine and/or antihuman epidermal growth factor receptor 2 (HER2) treatment was allowed during follow-up. All participants signed informed consent. CANTO is a study of chronic toxicities among disease-free survivors. Recurrence of disease, including BC nodal or distant recurrence (ie, other than local BC recurrence), second primary cancers, or death are reasons for study termination. Patients may also terminate their participation to CANTO at any time because of withdrawal of consent to study participation/personal choice and lost to follow-up. The national regulatory and ethics committee approved the study (ID-RCB:2011-A01095-36,11-039).
Study Cohort
Six thousand six hundred nineteen women diagnosed with BC from 2012 to 2015, with a follow-up of at least 4 years after diagnosis were initially included. Patients who did not respond to the baseline (diagnosis, before the treatment onset) fatigue assessment and did not have ≥ 1 follow-up visit were excluded. The potential for bias in selection was evaluated by comparing respondents and nonrespondents. The latter were older, with more comorbidities, lower income, and higher-stage BC. However, no significant differences were present (data are not shown). The final analytic cohort included 5,692 patients (the flowchart of inclusion is available in the Data Supplement, online only).
Variables of Interest
Outcome variable.
The primary outcome of interest was global fatigue, assessed by the European Organisation for Research and Treatment of Cancer [EORTC] Quality of Life Questionnaire [QLQ]-C30. Secondarily, we evaluated the physical, emotional, and cognitive dimensions of fatigue using the EORTC QLQ-FA12. Both instruments provide a four-point Likert scale response for each item: not at all, a little, quite a bit, and very much.17-19 All items are converted to a 0-100 scale using a standard scoring algorithm, where higher scores reflect greater fatigue severity. All outcomes were defined as a binary variable, with scores ≥ 40 of 100 defining severe fatigue (threshold defined per Abrahams et al5 for global fatigue and retained for the distinct dimensions on the basis of correlation analyses showing good concordance [data not shown]).
Exposure variables.
Data collected by medical record review at diagnosis included age; socioeconomic status including education level and income; marital status; Charlson comorbidity index; tobacco use; body mass index (BMI); physical activity; tumor stage; breast surgery; axillary management; and receipt of radiotherapy, chemotherapy, endocrine therapy, and antihuman HER2 therapy. Patient-reported outcomes were collected at diagnosis including pain and insomnia assessed as per EORTC-QLQC30, physical activity as per Global Physical Activity Questionnaire-16, and depression and anxiety as per the Hospital Anxiety and Depression Scale. In addition, hot flashes at diagnosis were assessed by in-person nurse evaluations (Common terminology criteria for adverse events v4.0).
Statistical Analysis
Patient characteristics were descriptively summarized for the whole cohort.
Definition of Trajectory Groups
Multivariable latent class models using Group-Based Trajectory Modeling (GBTM) assessed longitudinal variations in repeated measures of fatigue.11,12,20-22 The procedure is extensively presented in the Data Supplement. Each identified trajectory group was assigned a label name that briefly describes the associated fatigue outcome pattern. Characteristics in each group were then descriptively summarized.
Predictors of Trajectory-Group Membership
Risk factors for group membership with trajectory groups were identified after the definition of the best-fitting model, exponentiating model-based estimates to obtain odds ratios and respective 95% CIs. Membership to the trajectory group with the lowest risk levels of severe fatigue over time was chosen as a reference to assess risk factors for clustering into groups with worse fatigue patterns.
Sensitivity Analyses
We used an extension of GBTM to address potential nonrandom participant dropout (eg, truncation because of BC recurrence, second cancer, or death events) that may vary across groups. In addition, subgroup analyses by systemic treatment received were performed to assess membership risk factors.
Statistical analysis was performed using SAS statistical software version 9.4 (SAS Institute Inc) and the PROC Traj Procedure developed for SAS. Statistical significance was defined with a two-sided P value < .05.
RESULTS
Cohort Characteristics
Among the whole cohort (N = 5,692), the mean age at diagnosis was 56.2 years (standard deviation = 11.3); 56.3% had a household income < 3,000 Euros/month. The majority of the cohort did not have relevant comorbidities (79.8%). Altogether, 50% of patients had stage I BC. Sixty-one percent of patients had anxiety symptoms, and 18.2% depressive symptoms at diagnosis. Of note, the proportion of borderline or case anxiety was highest at diagnosis (61% of patients), reduced at year 1 (44.6%), and plateaued over time at year 2 (44.4%) and year 4 (43.5%) after diagnosis. Overall, 73.2% had breast-conserving surgery, 90.9% received radiotherapy, 53.2% received chemotherapy, 81.4% received hormonal therapy, and 11.8% received anti-HER2 therapy. The prevalence of severe fatigue over 4 years reached 35.6% for global fatigue, 35.0% for physical, 25.4% for emotional, and 13.3% for cognitive fatigue. Cohort characterization and fatigue metrics over time are provided in the Data Supplement.
Definition of Trajectory Groups
Metrics used for model selection are available in the Data Supplement. On the basis of an average posterior probability of group membership close to or > .75, there was a relatively high degree of certainty in group assignment.11,12,21 The Maximum Likelihood Estimates parameters of the models are provided in the Data Supplement.
Severe Global Fatigue
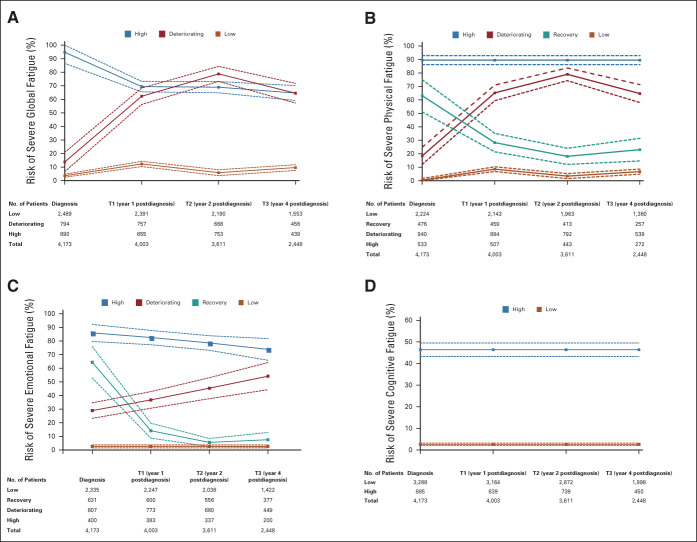
Our final model (n = 4,173) identified three trajectory groups of severe global fatigue, graphically represented in Figure 1A. The first group (21% of patients; high) had a persistently high risk of severe global fatigue over time (estimated risk of severe fatigue 94.8% [95% CI, 86.6 to 100.0] at diagnosis and 64.6% [95% CI, 59.2 to 70.1] at year 4) with corresponding mean fatigue scores of 62.3 of 100 (95% CI for the mean, 61.2 to 63.5) at diagnosis and 52.1 of 100 (49.7 to 54.5) at year 4. In the second trajectory group (19%; deteriorating), the risk of severe fatigue was low at diagnosis and increased substantially over time (estimated risk of severe fatigue 13.8% [95% CI, 6.7 to 20.9] at diagnosis and 64.5% [95% CI, 57.3 to 71.8] at year 4), where fatigue started off with an excellent mean score at diagnosis (24.5 of 100, 95% CI for the mean, 23.7 to 25.4), but then increased significantly at year 1 not recovering by year 4 (51.3 of 100; 95% CI for the mean, 51.3 to 53.2). The best-performing trajectory group in terms of fatigue symptoms included the majority of patients in this analysis (60%; low), reporting a persistently low risk of severe global fatigue (estimated risk of severe fatigue 3.6% [95% CI, 2.5 to 4.7] at diagnosis and 9.6% [95% CI, 7.5 to 11.7] at year 4), with the highest mean score of 23.1 of 100 (95% CI for the mean, 22.3 to 23.8).
FIG 1.
Trajectory groups according to the best-fitting prediction model of (A) severe global fatigue, (B) severe physical fatigue, (C) severe emotional fatigue, and (D) severe cognitive fatigue. Solid lines represent the predicted trajectories of risk estimate, and dashed lines represent the respective 95% CIs of risk estimate. T, time.
The Data Supplement displays the mean scores of fatigue over time of the identified groups and the observed and predicted risk of severe fatigue. The distribution of patient characteristics at diagnosis by trajectory group is presented in Table 1. Significant risk multivariable factors for membership to worse trajectory groups (high and deteriorating group) included age (younger age) and contextual (being single), behavioral (tobacco use), and clinical characteristics (comorbidities, high BMI, and symptoms at diagnosis). Particularly, among treatment-related factors, receipt of hormonal therapy was significantly associated with increased likelihood of membership to the deteriorating group compared with the low group, with an adjusted odds ratio (95% CI) of 1.38 (1.02 to 1.86).
TABLE 1.
Distribution of Patient Characteristics at Diagnosis and Breast Cancer Primary Treatment by Severe Global Trajectory Group and Multinomial Logistic Regression of Factors Associated With Severe Global Fatigue Trajectory-Group Membership (v group C: low, n = 2,489 [60%])
Domains of Fatigue
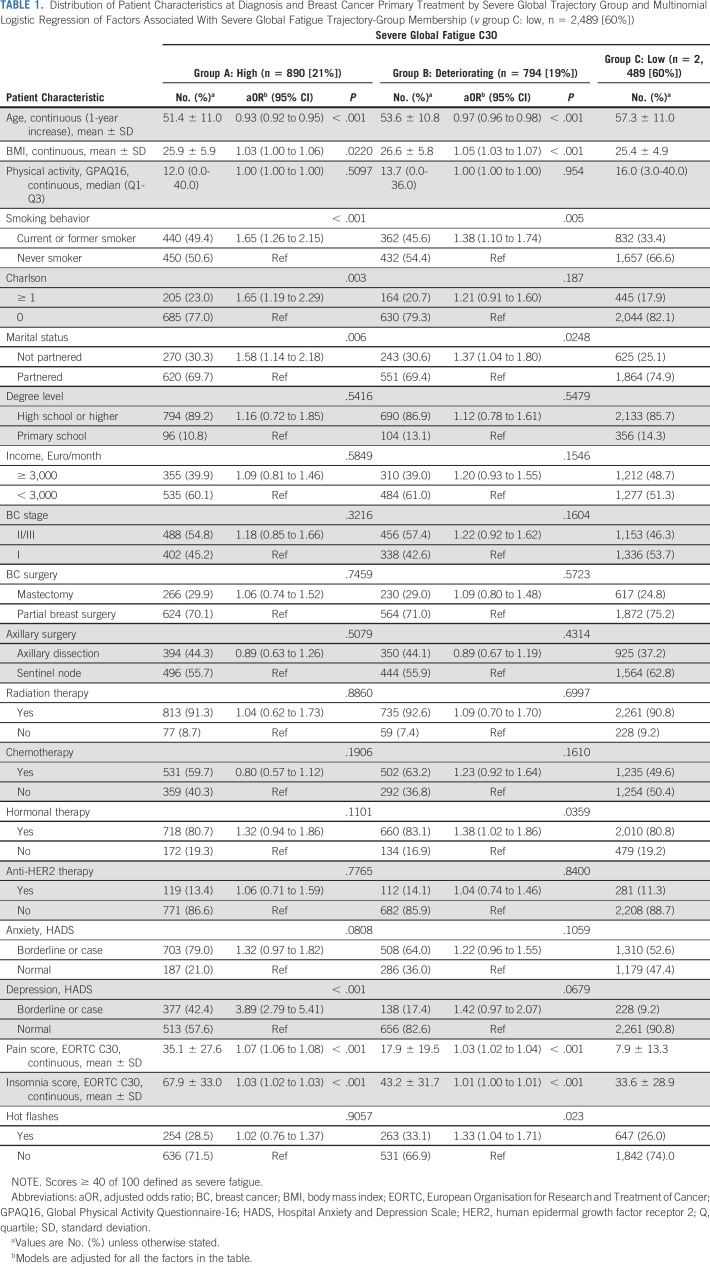
The distinct dimensions of fatigue were clustered in different trajectory groups than those identified by severe global fatigue. For physical fatigue, the final model identified four trajectory groups of severe physical fatigue, graphically represented in Figure 1B. One group had high-risk estimates of severe physical fatigue over time (13% of patients; high). The risk of severe physical fatigue in the second trajectory group (23%; deteriorating) was low at diagnosis but increased over time. The third group represented patients with high-risk estimates of severe physical fatigue at diagnosis. By year 4, the risk estimate decreased being low (11%; recovery). Finally, the best trajectory group included most patients in this analysis (53%; low), reporting a persistently low risk estimate of severe physical fatigue over time. The distribution of patient characteristics at diagnosis by trajectory group is presented in Table 2. Significant multivariable risk factors for membership were similar to the ones reported for severe global fatigue. Consistent with models of global fatigue, hormonal therapy was associated with higher odds of belonging to the deteriorating group compared with low.
TABLE 2.
Distribution of Patient Characteristics at Diagnosis and Breast Cancer Primary Treatment by Severe Physical Trajectory Group and Multinomial Logistic Regression (v reference group D: low, n = 2,224 [53%])
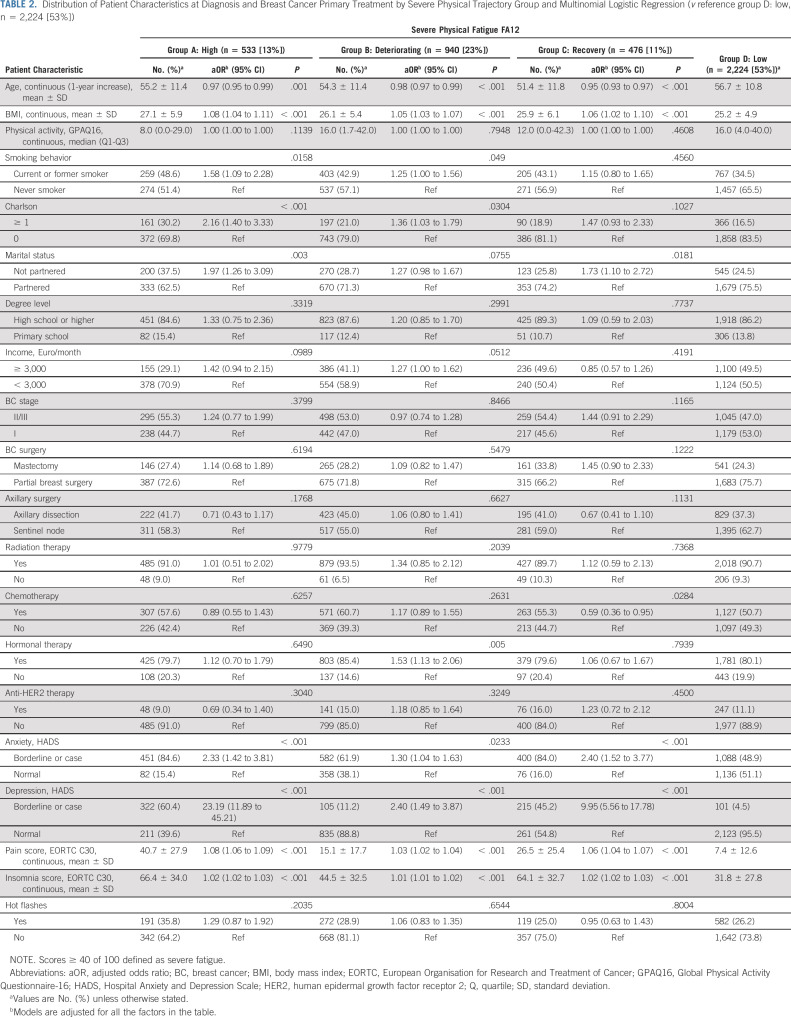
Similarly, the final model for severe emotional fatigue included four trajectory groups (Fig 1C). Ten percent of patients reported a high-risk estimate of severe emotional fatigue over time (high group), 19% belonged to the deteriorating group, 15% of patients belonged to the recovery group, and the best trajectory group included the majority of patients in this analysis (56%; low) having a low-risk estimate of severe emotional fatigue over time. The distribution of patient characteristics at diagnosis by trajectory group is presented in Table 3. The most consistent associations were seen between emotional distress–related variables and emotional fatigue.
TABLE 3.
Distribution of Patient Characteristics at Diagnosis and Breast Cancer Primary Treatment by Severe Emotional Trajectory Group (v reference group C: recovery, n = 631 [15%])
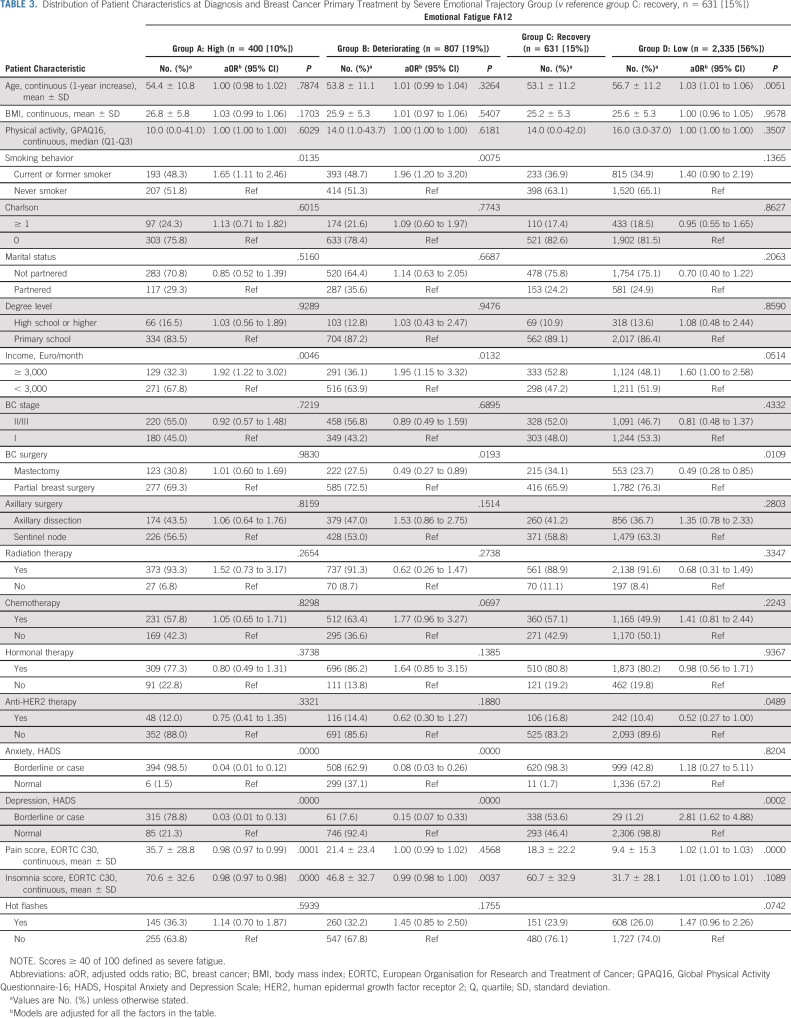
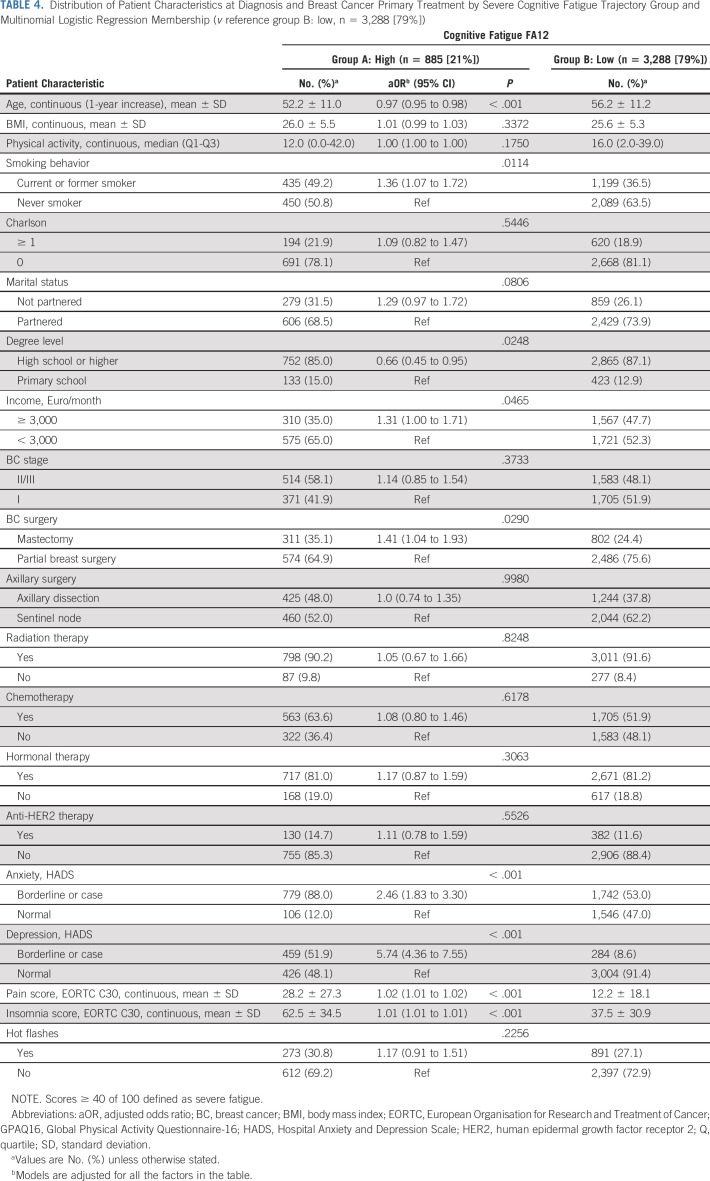
Finally, the model for severe cognitive fatigue included two trajectory groups (Fig 1D). The majority (79%) of patients clustered in the low trajectory group, and only 21% reported an overall high risk of severe cognitive fatigue (high group) over time. The distribution of patient characteristics at diagnosis by trajectory group and results for group membership are presented in Table 4, with age, smoking, having a mastectomy, and physical and emotional symptomatology at diagnosis increasing the risk of belonging to the high group.
TABLE 4.
Distribution of Patient Characteristics at Diagnosis and Breast Cancer Primary Treatment by Severe Cognitive Fatigue Trajectory Group and Multinomial Logistic Regression Membership (v reference group B: low, n = 3,288 [79%])
Results of sensitivity analyses also showed great consistency regarding the number of trajectory groups identified and risk factors for long-term high or deteriorating fatigue trajectories. Particularly, the analyses yielded similar results regardless of subgrouping by systemic adjuvant treatment received (Data Supplement).
DISCUSSION
This study highlights the multidimensional nature of cancer-related fatigue and the complexity of its risk factors that include both common and dimension-specific determinants. The distinct dimensions of fatigue that we examined presented different longitudinal patterns and were differentially affected by multiple factors, including classical sociodemographic and clinical factors and treatment-related factors. Common determinants of trajectory membership were found, including emotional distress—particularly depression—that emerged as the most potent driver of fatigue trajectory, independent of the explored dimension. Receipt of systemic treatments—particularly hormonal therapy—was a consistent risk factor for developing severe fatigue and clearly isolated groups of patients with deteriorating fatigue symptoms compared with those at low risk of fatigue, across different dimensions of the symptom.
More than 50% of cancer survivors suffer from at least one severe post-treatment symptom, calling for an improved survivorship care model that focuses on managing long-term and late effects of treatment.23 Substantial evidence shows that long and late effects of cancer treatment are often underaddressed.24 Therefore, in recent years, the concept of personalized survivorship care has emerged. This concept implies patient stratification on the basis of understanding longitudinal determinants of long‐term and late effects and the development of appropriate care pathways to allow tailored care.25 In this setting, our study focused on performing an extensive characterization of the patterns of one of the most prevalent and distressing symptoms after a BC diagnosis and cancer-related fatigue and on informing risk stratification that is clinically actionable.
Previous literature has suggested that the patient population that is at higher risk of cancer-related fatigue includes (1) those who are more fragile at the time of diagnosis, including being young, belonging to lower socioeconomic class, not partnered, having higher BMI, comorbidities, being a smoker, presenting heavier psychologic distress (depressive and anxiety symptoms), and concomitant symptom burden and (2) those who receive specific classes of treatment such as chemotherapy and endocrine therapy.4-9,26 Some previous studies have suggested that there are clusters of patients who experience distinct trajectories of fatigue.27 For example, Bower et al examined post-treatment fatigue trajectories in 191 women with early-stage BC. This cohort15 had a follow-up time of 4.3 years after treatment and identified four distinct patterns of fatigue after BC: (1) low fatigue group (44%), where patients presented low fatigue levels throughout the duration of the observation; (2) recovery group (28%), where patients were fatigued at the initial post-treatment assessment but then recovered their energy levels; (3) late group (17%) where patients presented with low post-treatment levels of fatigue but then gradually started to report increased fatigue levels over time; and (4) high fatigue group (11%), where fatigue was persistently elevated across the study period. In this study, psychologic factors, such as depressive symptoms and childhood adversity, were the strongest predictors of membership in high and recovery groups. Furthermore, there was a suggestion that treatment factors played a role in fatigue trajectories, with chemotherapy being associated with membership to the recovery group and endocrine therapy contributing to the fatigue observed in the high group.15 Recently, the same authors identified consistent trajectories among 270 BC survivors, defining five fatigue groups: stable low (66%), stable high (13%), decreasing (4%), increasing (9%), and reactive (8%).28 Our study confirms and expands on these previous studies. Particularly, our study calls for the implementation of several actions in the clinical care of patients with BC.
First, by describing consistent and consolidated variables associated with worse trajectories of cancer-related fatigue, our data can assist clinicians to focus on relevant patient characteristics that are consistently associated with long-term patterns of severe fatigue. These variables include age, BMI, tobacco use, receipt of hormonal therapy, and concomitant symptom burden (pain, insomnia, and depression). An accurate and systematic screening of cancer-related fatigue and associated risk factors—at diagnosis and over the survivorship period—can therefore help in the identification of the most vulnerable.
Second, by stressing that several of the cancer-related fatigue risk factors are modifiable, this study advocates for the implementation of symptom management and health promotion strategies since diagnosis. Strategies addressing modifiable risk factors include healthy weight management, smoking cessation, and cognitive-behavioral therapy for concomitant symptoms such as sleep disturbances and emotional distress, or specific treatment of chronic pain, which should be considered early in the patient journey. In addition, there are patients whose fatigue trajectory is characterized by high levels of pretreatment fatigue, and therefore, they deserve the upfront implementation of strategies to mitigate this symptom since the moment of diagnosis. This is particularly relevant considering that fatigue is probably only one of the outcomes of cancer-related accelerating aging that can be affected by these modifiable host-specific factors.29
Third, our study findings are provocative in that not all the dimensions of cancer-related fatigue seem to have the same longitudinal evolution and determinants. Depending on the dimension, an interaction between treatment exposures and host vulnerabilities such as psychologic factors, sociodemographic factors such as age and loneliness, and clinical features such as comorbidities can promote and render fatigue more persistent in cancer survivors. This calls for a comprehensive evaluation at diagnosis and with attention to examination of risk factors in light of their dimension-specific effect.
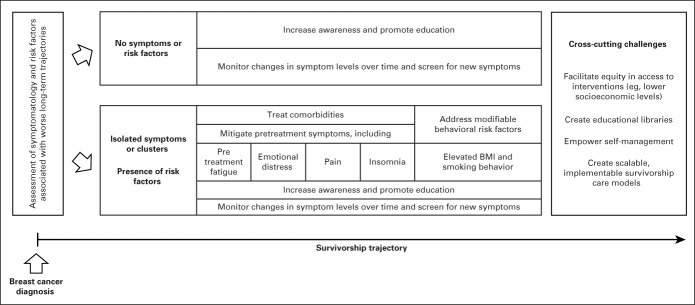
Our study advocates for a systematic pretreatment screening for fatigue and risk factors among patients newly diagnosed with BC and for the implementation of a proactive management of cancer-related fatigue and risk factors over the course of the survivorship period. In addition, efforts should be made to truly transform the survivorship journey in a fully patient-centered model, by promoting patient participation in the process of care, through increased awareness and education and interdisciplinary referral and access to interventions to better manage survivorship-related issues including long-term symptom management. Particularly, patients should be educated to self-monitor changes in fatigue levels over time, encouraged to be attentive to symptoms that can herald the onset of persistent fatigue, and advised to seek medical help if a persistent deterioration of energy levels exists (Fig 2).30-35
FIG 2.
A comprehensive patient-centered survivorship care model building on predicted longitudinal symptom patterns to avoid long-term deterioration. BMI, body mass index.
Finally, by highlighting the complexity of post-treatment symptom patterns, this study also offers insight into multiple challenges that are ahead of the implementation of a survivorship model aiming to deliver comprehensive, patient-centered survivorship care. One of these challenges is the need of scalable models, which is a particularly relevant topic in an era of oncology workforce shortage and professional burnout.36,37 Relying on digital solutions may facilitate the uptake of personalized care models in diverse settings. The use of electronic monitoring to understand post-treatment trajectories and promote patient care holds the promise to lead to greater awareness and better management of treatment-related symptoms.38,39 Telemedicine self-management programs showed promising results on prevalent symptoms of cancer survivors.40-47
We acknowledge several limitations of this study. First, CANTO is a longitudinal study with the common limitation of increasing response attrition the further away from study entry. Therefore, we acknowledge potential for selection and attrition bias over time. However, GBTM can accommodate missing outcome data, and sensitivity analyses trying to address these points confirmed the robustness of our findings.20 Second, there are some well-known determinants of fatigue that could not be explored, including psychologic measures such as childhood adversity and biologic markers such as inflammatory cytokines.9,15 Third, our population only included French survivors, and results may not be fully generalizable, but still included patients from across the country. As lower-income/less-educated and older patients, other subpopulations might be under-represented in this study, warranting a dedicated approach in future studies. Fourth, we included a population diagnosed between 2012 and 2015, and treatment practices have slightly changed since. Fifth, because of the observational design, we cannot exclude unmeasured confounding and no formal adjustment for multiplicity has been performed given the exploratory modeling. Finally, the risk models for belonging to particular trajectory groups may underestimate the uncertainty obtained from the trajectory modeling in the first stage.
In conclusion, this study provides insight into the long-term evolution and fluctuation of fatigue symptomatology that may arise from chronic illnesses other than malignancies and offers an actionable clinical perspective. Identifying key risk factors and underlying mechanisms is critical for developing and deploying targeted interventions to reduce the burden of long-term effects from cancer and its treatment, including fatigue.
Acknowledgments
ACKNOWLEDGMENT
The authors would like to thank Editage for editing and reviewing this manuscript for English language.
Ines Vaz-Luis
Honoraria: AstraZeneca (Inst), Amgen (Inst), Pfizer (Inst)
Barbara Pistilli
Consulting or Advisory Role: Puma Biotechnology, Pierre Fabre, Novartis, Myriad Genetics, AstraZeneca, Daiichi Sankyo/UCB Japan
Research Funding: Pfizer (Inst), Puma Biotechnology (Inst), Merus (Inst), Daiichi-Sankyo (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, MSD Oncology, Novartis, Pierre Fabre
Paul H. Cottu
Honoraria: Pfizer, Novartis (Inst), Roche, NanoString Technologies (Inst), Lilly
Consulting or Advisory Role: Pfizer, Lilly
Research Funding: Pfizer (Inst)
Travel, Accommodations, Expenses: Roche, Pfizer
Florence Lerebours
Consulting or Advisory Role: AstraZeneca, Eisai, Lilly, Pierre Fabre, Roche, Pfizer
Travel, Accommodations, Expenses: Lilly, Novartis, Pfizer, Roche, Pierre Fabre
Sarah Dauchy
Honoraria: Servier, Novartis, MSD Oncology, BMS, Nutricia
Travel, Accommodations, Expenses: Servier
Suzette Delaloge
Consulting or Advisory Role: AstraZeneca (Inst), Sanofi (Inst), Besins Healthcare (Inst), Rappta Therapeutics (Inst)
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Roche/Genentech (Inst), Puma Biotechnology (Inst), Lilly (Inst), Novartis (Inst), Sanofi (Inst), Exact Sciences (Inst), Bristol Myers Squibb (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, Novartis (Inst)
Nancy U. Lin
Consulting or Advisory Role: Seattle Genetics, Puma Biotechnology, Daiichi Sankyo, Denali Therapeutics, AstraZeneca, Prelude Therapeutics, Voyager Therapeutics, Affinia Therapeutics, Pfizer
Research Funding: Genentech (Inst), Pfizer (Inst), Seattle Genetics (Inst), Merck (Inst), Zion (Inst)
Patents, Royalties, Other Intellectual Property: Royalties for chapter in Up-to-Date regarding management of breast cancer brain metastases, Royalties, Jones & Bartlett
Patricia A. Ganz
Leadership: Intrinsic LifeSciences
Stock and Other Ownership Interests: Xenon Pharma, Intrinsic LifeSciences, Silarus Therapeutics, Teva, Novartis, Merck, Johnson & Johnson, Pfizer, GlaxoSmithKline, Abbott Laboratories
Consulting or Advisory Role: InformedDNA, Vifor Pharma, Ambys Medicines, Global Blood Therapeutics, GlaxoSmithKline, Ionis Pharmaceuticals, Akebia Therapeutics, Protagonist Therapeutics, Regeneron, Sierra Oncology, Rockwell Medical Technologies Inc, Astellas Pharma, Gossamer Bio, American Regent, Disc Medicine, Blue Note Therapeutics, Grail
Research Funding: Blue Note Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Related to iron metabolism and the anemia of chronic disease, Up-to-Date royalties for section editor on survivorship
Travel, Accommodations, Expenses: Intrinsic LifeSciences
Ann H. Partridge
Patents, Royalties, Other Intellectual Property: I receive small royalty payments for coauthoring the breast cancer survivorship section of UpToDate
Open Payments Link: https://openpaymentsdata.cms.gov/physician/835197
Fabrice André
Stock and Other Ownership Interests: Pegacsy
Research Funding: AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), Lilly (Inst), Roche (Inst), Daiichi (Inst)
Travel, Accommodations, Expenses: Novartis, Roche, GlaxoSmithKline, AstraZeneca
Stefan Michiels
Consulting or Advisory Role: IDDI, Sensorion, Biophytis, Servier, Yuhan, Amaris Consulting, Roche
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the European Society for Medical Oncology (ESMO) Congress, Munich, Germany, October 20, 2018 (abstract 4614).
SUPPORT
Supported by a Career Catalyst Research grant from Susan G. Komen (CCR17483507) and grants from Odyssea, the French Foundation for Cancer Research (ARC), and Foundation Gustave Roussy to I.V.-L and by a Career Pathway Grant in Symptom Management from Conquer Cancer, the ASCO Foundation and Rising Tide Foundation for Clinical Cancer Research to A.D.M. Supported by CANTO under the Investment for the Future program managed by the National Research Agency (ANR), Grant No. ANR-10-COHO-0004 and by the PRISM project funded by the Agence Nationale de la Recherche under Grant No. ANR-18-IBHU-0002. Supported by Susan G. Komen, Conquer Cancer Foundation of ASCO and Rising Tide Foundation for Clinical Cancer Research, French Foundation for Cancer Research, Foundation Gustave Roussy, and French Government (Agence Nationale de la recherché [ANR]).
DATA SHARING STATEMENT
Data will be available upon request to Unicancer R&D.
AUTHOR CONTRIBUTIONS
Conception and design: Ines Vaz-Luis, Antonio Di Meglio, Patricia A. Ganz, Ann H. Partridge, Stefan Michiels
Administrative support: Anne-Laure Martin
Provision of study materials or patients: Barbara Pistilli, Sibille Everhard, Anne-Laure Martin, Paul H. Cottu, Florence Lerebours, Charles Coutant
Collection and assembly of data: Sibille Everhard, Anne-Laure Martin
Data analysis and interpretation: Ines Vaz-Luis, Antonio Di Meglio, Julie Havas, Mayssam El-Mouhebb, Pietro Lapidari, Daniele Presti, Davide Soldato, Barbara Pistilli, Agnes Dumas, Gwenn Menvielle, Cecile Charles, Florence Lerebours, Charles Coutant, Suzette Delaloge, Nancy U. Lin, Ann H. Partridge, Fabrice André, Stefan Michiels
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Long-Term Longitudinal Patterns of Patient-Reported Fatigue After Breast Cancer: A Group-Based Trajectory Analysis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ines Vaz-Luis
Honoraria: AstraZeneca (Inst), Amgen (Inst), Pfizer (Inst)
Barbara Pistilli
Consulting or Advisory Role: Puma Biotechnology, Pierre Fabre, Novartis, Myriad Genetics, AstraZeneca, Daiichi Sankyo/UCB Japan
Research Funding: Pfizer (Inst), Puma Biotechnology (Inst), Merus (Inst), Daiichi-Sankyo (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, MSD Oncology, Novartis, Pierre Fabre
Paul H. Cottu
Honoraria: Pfizer, Novartis (Inst), Roche, NanoString Technologies (Inst), Lilly
Consulting or Advisory Role: Pfizer, Lilly
Research Funding: Pfizer (Inst)
Travel, Accommodations, Expenses: Roche, Pfizer
Florence Lerebours
Consulting or Advisory Role: AstraZeneca, Eisai, Lilly, Pierre Fabre, Roche, Pfizer
Travel, Accommodations, Expenses: Lilly, Novartis, Pfizer, Roche, Pierre Fabre
Sarah Dauchy
Honoraria: Servier, Novartis, MSD Oncology, BMS, Nutricia
Travel, Accommodations, Expenses: Servier
Suzette Delaloge
Consulting or Advisory Role: AstraZeneca (Inst), Sanofi (Inst), Besins Healthcare (Inst), Rappta Therapeutics (Inst)
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Roche/Genentech (Inst), Puma Biotechnology (Inst), Lilly (Inst), Novartis (Inst), Sanofi (Inst), Exact Sciences (Inst), Bristol Myers Squibb (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, Novartis (Inst)
Nancy U. Lin
Consulting or Advisory Role: Seattle Genetics, Puma Biotechnology, Daiichi Sankyo, Denali Therapeutics, AstraZeneca, Prelude Therapeutics, Voyager Therapeutics, Affinia Therapeutics, Pfizer
Research Funding: Genentech (Inst), Pfizer (Inst), Seattle Genetics (Inst), Merck (Inst), Zion (Inst)
Patents, Royalties, Other Intellectual Property: Royalties for chapter in Up-to-Date regarding management of breast cancer brain metastases, Royalties, Jones & Bartlett
Patricia A. Ganz
Leadership: Intrinsic LifeSciences
Stock and Other Ownership Interests: Xenon Pharma, Intrinsic LifeSciences, Silarus Therapeutics, Teva, Novartis, Merck, Johnson & Johnson, Pfizer, GlaxoSmithKline, Abbott Laboratories
Consulting or Advisory Role: InformedDNA, Vifor Pharma, Ambys Medicines, Global Blood Therapeutics, GlaxoSmithKline, Ionis Pharmaceuticals, Akebia Therapeutics, Protagonist Therapeutics, Regeneron, Sierra Oncology, Rockwell Medical Technologies Inc, Astellas Pharma, Gossamer Bio, American Regent, Disc Medicine, Blue Note Therapeutics, Grail
Research Funding: Blue Note Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Related to iron metabolism and the anemia of chronic disease, Up-to-Date royalties for section editor on survivorship
Travel, Accommodations, Expenses: Intrinsic LifeSciences
Ann H. Partridge
Patents, Royalties, Other Intellectual Property: I receive small royalty payments for coauthoring the breast cancer survivorship section of UpToDate
Open Payments Link: https://openpaymentsdata.cms.gov/physician/835197
Fabrice André
Stock and Other Ownership Interests: Pegacsy
Research Funding: AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), Lilly (Inst), Roche (Inst), Daiichi (Inst)
Travel, Accommodations, Expenses: Novartis, Roche, GlaxoSmithKline, AstraZeneca
Stefan Michiels
Consulting or Advisory Role: IDDI, Sensorion, Biophytis, Servier, Yuhan, Amaris Consulting, Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bower JE: Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 11:597-609, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell SA: Cancer-related fatigue: State of the science. PM R 2:364-383, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Bower JE, Ganz PA, Desmond KA, et al. : Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol 18:743-753, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Andrykowski MA, Donovan KA, Laronga C, et al. : Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer 116:5740-5748, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrahams HJG, Gielissen MFM, Schmits IC, et al. : Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: A meta-analysis involving 12 327 breast cancer survivors. Ann Oncol 27:965-974, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Dhruva A, Dodd M, Paul SM, et al. : Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nurs 33:201-212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donovan KA, Jacobsen PB, Andrykowski MA, et al. : Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manage 28:373-380, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieboer P, Buijs C, Rodenhuis S, et al. : Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: A longitudinal study. J Clin Oncol 23:8296-8304, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Bower JE, Asher A, Garet D, et al. : Testing a biobehavioral model of fatigue before adjuvant therapy in women with breast cancer. Cancer 125:633-641, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weis J, Tomaszewski KA, Hammerlid E, et al. : International psychometric validation of an EORTC quality of life module measuring cancer related fatigue (EORTC QLQ-FA12). J Natl Cancer Inst 109, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Nagin DS, Odgers CL: Group-based trajectory modeling (nearly) two decades later. J Quant Criminol 26:445-453, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagin DS, Odgers CL: Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 6:109-138, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Donovan KA, Small BJ, Andrykowski MA, et al. : Utility of a cognitive-behavioral model to predict fatigue following breast cancer treatment. Health Psychol 26:464-472, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Identification of distinct fatigue trajectories in patients with breast cancer undergoing adjuvant chemotherapy. Profiles RNS. https://profiles.uchicago.edu/profiles/display/17340461 [DOI] [PMC free article] [PubMed]
- 15.Bower JE, Wiley J, Petersen L, et al. : Fatigue after breast cancer treatment: Biobehavioral predictors of fatigue trajectories. Health Psychol 37:1025-1034, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaz-Luis I, Cottu P, Mesleard C, et al. : UNICANCER: French prospective cohort study of treatment-related chronic toxicity in women with localised breast cancer (CANTO). ESMO Open 4:e000562, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husson O, Rooij BH, Kieffer J, et al. : The EORTC QLQ‐C30 summary score as prognostic factor for survival of patients with cancer in the “real‐world”: Results from the population‐based PROFILES registry. Oncologist 25:e722-e732, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aaronson NK, Ahmedzai S, Bergman B, et al. : The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365-376, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Giesinger JM, Kieffer JM, Fayers PM, et al. : Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol 69:79-88, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Nagin DS, Jones BL, Passos VL, et al. : Group-based multi-trajectory modeling. Stat Methods Med Res 27:2015-2023, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Group-Based Modeling of Development—Daniel S. Nagin. Harvard University Press. https://www.hup.harvard.edu/catalog.php?isbn=9780674016866 [Google Scholar]
- 22.Choi CWJ, Stone RA, Kim KH, et al. : Group-based trajectory modeling of caregiver psychological distress over time. Ann Behav Med 44:73-84, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira ARR, Di Meglio A, Pistilli B, et al. : Differential impact of endocrine therapy and chemotherapy on quality of life of breast cancer survivors: A prospective patient-reported outcomes analysis. Ann Oncol 30:1784-1795, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Di Meglio A, Charles C, Martin E, et al. : Uptake of recommendations for posttreatment cancer-related fatigue among breast cancer survivors. J Natl Compr Canc Netw 10.6004/jnccn.2021.7051 [Epub ahead of print on February 7, 2022] [DOI] [PubMed]
- 25.Alfano CM, Mayer DK, Bhatia S, et al. : Implementing personalized pathways for cancer follow‐up care in the United States: Proceedings from an American Cancer Society–American Society of Clinical Oncology summit. CA Cancer J Clin 69:234-247, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Meglio A, Havas J, Soldato D, et al. : Development and validation of a predictive model of severe fatigue after breast cancer diagnosis: Toward a personalized framework in survivorship care. J Clin Oncol 40:1111-1123, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bean HR, Diggens J, Ftanou M, et al. : Insomnia and fatigue symptom trajectories in breast cancer: A longitudinal cohort study. Behav Sleep Med 19:814-827, 2021. [DOI] [PubMed] [Google Scholar]
- 28.Bower JE, Ganz PA, Irwin MR, et al. : Do all patients with cancer experience fatigue? A longitudinal study of fatigue trajectories in women with breast cancer. Cancer 127:1334-1344, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carroll JE, Bower JE, Ganz PA: Cancer-related accelerated ageing and biobehavioural modifiers: A framework for research and clinical care. Nat Rev Clin Oncol 2021 1-15, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Comprehensive Cancer Network: Cancer-related fatigue (version 2.2022). https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bower JE, Bak K, Berger A, et al. : Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical Oncology clinical practice guideline adaptation. J Clin Oncol 32:1840-1850, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mary Dwyer N, Rashmi Kumar M, Swarm R, et al. : Continue NCCN Guidelines Panel Disclosures NCCN GuidelinesTM Version 2.2011 Panel Members Adult Cancer Pain. 2011 [Google Scholar]
- 33.National Comprehensive Cancer Network: Distress management (version 2.2022). https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf [DOI] [PubMed] [Google Scholar]
- 34.Franzoi MA, Agostinetto E, Perachino M, et al. : Evidence-based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer. Lancet Oncol 22:e303-e313, 2021 [DOI] [PubMed] [Google Scholar]
- 35.Demark-Wahnefried W, Schmitz KH, Alfano CM, et al. : Weight management and physical activity throughout the cancer care continuum. CA Cancer J Clin 68:64-89, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer DK, Alfano CM: Personalized risk-stratified cancer follow-up care: Its potential for healthier survivors, happier clinicians, and lower costs. J Natl Cancer Inst 111:442-448, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takvorian SU, Balogh E, Nass S, et al. : Developing and sustaining an effective and resilient oncology careforce: Opportunities for action. J Natl Cancer Inst 112:663-670, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basch E, Deal AM, Dueck AC, et al. : Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 318:197-198, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Absolom K, Warrington L, Hudson E, et al. : Phase III randomized controlled trial of eRAPID: eHealth intervention during chemotherapy. J Clin Oncol 39:734-747, 2021 [DOI] [PubMed] [Google Scholar]
- 40.Abrahams HJG, Gielissen MFM, Donders RRT, et al. : The efficacy of Internet-based cognitive behavioral therapy for severely fatigued survivors of breast cancer compared with care as usual: A randomized controlled trial. Cancer 123:3825-3834, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Yun YH, Lee KS, Kim YW, et al. : Web-based tailored education program for disease-free cancer survivors with cancer-related fatigue: A randomized controlled trial. J Clin Oncol 30:1296-1303, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Zachariae R, Amidi A, Damholdt MF, et al. : Internet-delivered cognitive-behavioral therapy for insomnia in breast cancer survivors: A randomized controlled trial. J Natl Cancer Inst 110:880-887, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hummel SB, Van Lankveld JJDM, Oldenburg HSA, et al. : Efficacy of Internet-based cognitive behavioral therapy in improving sexual functioning of breast cancer survivors: Results of a randomized controlled trial. J Clin Oncol 35:1328-1340, 2017 [DOI] [PubMed] [Google Scholar]
- 44.Atema V, Van Leeuwen M, Kieffer JM, et al. : Efficacy of internet-based cognitive behavioral therapy for treatment-induced menopausal symptoms in breast cancer survivors: Results of a randomized controlled trial. J Clin Oncol 37:809-822, 2019 [DOI] [PubMed] [Google Scholar]
- 45.Compen F, Bisseling E, Schellekens M, et al. : Face-to-face and internet-based mindfulness-based cognitive therapy compared with treatment as usual in reducing psychological distress in patients with cancer: A multicenter randomized controlled trial. J Clin Oncol 36:2413-2421, 2018 [DOI] [PubMed] [Google Scholar]
- 46.Conklin HM, Ogg RJ, Ashford JM, et al. : Computerized cognitive training for amelioration of cognitive late effects among childhood cancer survivors: A randomized controlled trial. J Clin Oncol 33:3894-3902, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bray VJ, Dhillon HM, Bell ML, et al. : Evaluation of a web-based cognitive rehabilitation program in cancer survivors reporting cognitive symptoms after chemotherapy. J Clin Oncol 35:217-225, 2017 [DOI] [PubMed] [Google Scholar]