Abstract
T regulatory cells (Tregs) have a key role in the maintenance of immune homeostasis and the regulation of immune tolerance by preventing the inflammation and suppressing the autoimmune responses. Numerical and functional deficits of these cells have been reported in systemic lupus erythematosus (SLE) patients and mouse models of SLE, where their imbalance and dysregulated activities have been reported to significantly influence the disease pathogenesis, progression and outcomes. Most studies in SLE have focused on CD4+ Tregs and it has become clear that a critical role in the control of immune tolerance after the breakdown of self-tolerance is provided by CD8+ Tregs. Here we review the role, cellular and molecular phenotypes, and mechanisms of action of CD8+ Tregs in SLE, including ways to induce these cells for immunotherapeutic modulation in SLE.
Keywords: immune tolerance, CD8+ Tregs, lupus, immune homeostasis, anti-DNA Ab
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by widespread inflammation, autoantibody production, and immune complex deposition. SLE affects major organ systems in the body, with lupus nephritis as a leading cause of death.[1, 2, 3]
In SLE, immune homeostasis is impaired. Many investigations have attempted to modulate the abnormal immune regulation in SLE, having as a therapeutic goal the restoration of immune self-tolerance and the suppression of the activity and number of pathogenic cells and the production of auto-antibodies by inducing T regulatory cells (Tregs).[4, 5, 6, 7, 8, 9, 10] Many bio-technology and pharmaceutical companies are also currently working to translate the knowledge on the biology of Tregs and/or to bioengineer Tregs into transformational medicines that could benefit patients with various inflammatory and autoimmune diseases including SLE.
While a decrease in the number and/or function of CD4+ Tregs has been extensively studied in SLE,[11, 12, 13, 14, 15, 16, 17, 18, 19] the role and characterization of CD8+ Tregs in the disease is less clear. Our group identified and characterized a CD8+ T cell subset that prevented the generation of pathogenic autoantibody production and maintained immune self-tolerance in murine lupus.[6, 8]
The investigation of the regulatory networks, genes, and signaling pathways involved in the regulation of the functional activity and survival of CD8+ Tregs can be important for the development of therapies of restoration of immune homeostasis in SLE and other autoimmune diseases. The critical questions toward a clinical translational use of the findings are: (1) What is/are the precise surface phenotype(s) of the CD8+ Tregs which suppress autoantibody production? (2) What are the critical molecular elements in the CD8+ Tregs that are required for their survival, expansion, and suppression of helper T cell activity and suppression of autoantibody production by B cells? (3) What are the roles of transforming growth factor (TGF)-β, Bcl2, regulator of G-protein signaling (RGS) proteins, and interferons (IFNs) expression in the suppressive mechanisms of the CD8+ Tregs? (4) Can peptides that target Major Histocompatibility Complex (MHC) I/II T-cell domains augment the CD8+ Treg activity in SLE patients?
This review will discuss the aspects of Treg-mediated immune regulation, current knowledge in the field and approaches of Treg-based immunotherapy for improved management of SLE.
Cellular and molecular phenotypes of CD8+ T regulatory Cells (Tregs)
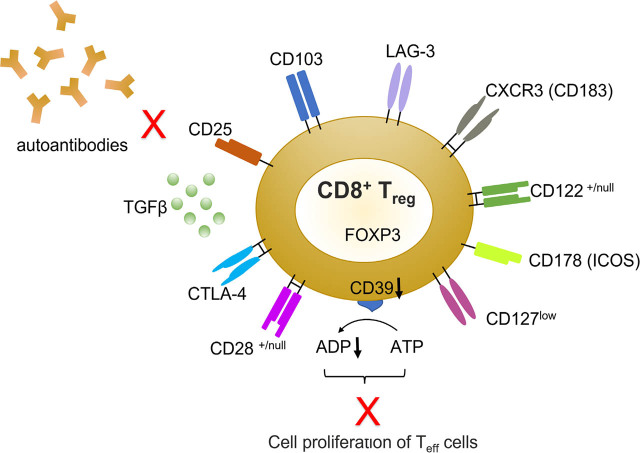
Although several cellular and molecular markers have been described for the identification of CD8+ Tregs (see Table 1, Figure 1, 2 and [20]), there is no single surface marker that is specific for CD8+ Tregs.
Table 1.
CD8+ Treg markers and mechanisms of action.
| Subset | Natural/induced | Markers | Mechanism of action | Ref. |
|---|---|---|---|---|
| CD8+FoxP3+ (mice) | Induced | PD-1low, CD62Lhigh, CCR7low | Secretion of TGF-β | [6, 8, 21] |
| CD8, CD8α, CD25high, CD28low/high, FoxP3, CTLA-4, CD103, CD122, CXCR3, LAG-3, CD127low (mice, humans) | Natural/induced | CD25high, CD28low/high, FoxP3, CTLA-4, CD103, CD122, CXCR3, LAG-3, CD127low | Secretion of IL-10, Reduction of IFN-γ, Cell-to-cell contact dependent | [20, 22, 23, 24, 25] |
| CD8, Qa-1, NKG2A (CD94) (mice) | Natural | Qa-1 (mice), HLA-E (humans), Ly49 | Suppress T effector cells, use perforin | [23, 26, 27, 28, 29] |
| CD8, CD25, FoxP3 (humans) | Natural | CD8, CD25, FoxP3, CD127low (mice and humans) | Suppress T effector cells | [22] |
| CD8 (mice) | Natural | CD28+CD28−, CD103, CD122, ICOS+ in mice | Suppress T effector cells | [22, 30, 31, 32, 33, 34, 35, 36] |
| CD8, ILT3/ILT4 (mice) | Natural | ILT3, ILT4 | Make APCs tolerogenic | [37, 38] |
| CD8, CD103 (mice) | Induced | CD103 | CD39, attenuate glomerular endothelial cell damage | [20, 22, 23, 24, 25, 35, 39] |
| CD8, CD25, CXCR3 (CD183) CD178 (ICOS) (humans) | Natural | CD8+CD25hi, CD183+ CD178+FoxP3+ | Suppress B cells proliferation and IgG production | [40] |
| CD8, CD28 (humans) | Natural | CD8+CD28− | Inhibit T cell proliferation and cytotoxic functions | [21, 33] |
APC, antigen presenting cells; ILT, Ig-like transcript; IgG, immunoglobulin; LAG-3, lymphocyte activation gene 3; PD-1, programed death-1; ICOS, Inducible co-stimulator.
Figure 1.
CD8+ Tregs SLE. In SLE, subsets of CD8+CD25+FoxP3+ Tregs—whose additional phenotypic markers are schematically depicted here—can suppress the activity of T effector (Teff) cells and APCs, also suppressing autoantibody production through the secretion of TGF-β and other cytokines/chemokines. APC, antigen presenting cells; LAG-3, lymphocyte activation gene 3; SLE, systemic lupus erythematosus; Tregs, T regulatory cells; and Teff, T effector. Modified from Martha R. Vieyra-Lobato, Jorge Vela-Ojeda, Laura Montiel-Cervantes, Rubén López-Santiago, Martha C. Moreno-Lafont, “Description of CD8+ Regulatory T Lymphocytes and Their Specific Intervention in Graft-versus-Host and Infectious Diseases, Autoimmunity, and Cancer”, Journal of Immunology Research, vol. 2018, Article ID 3758713, 16 pages, 2018. https://doi.org/10.1155/2018/3758713
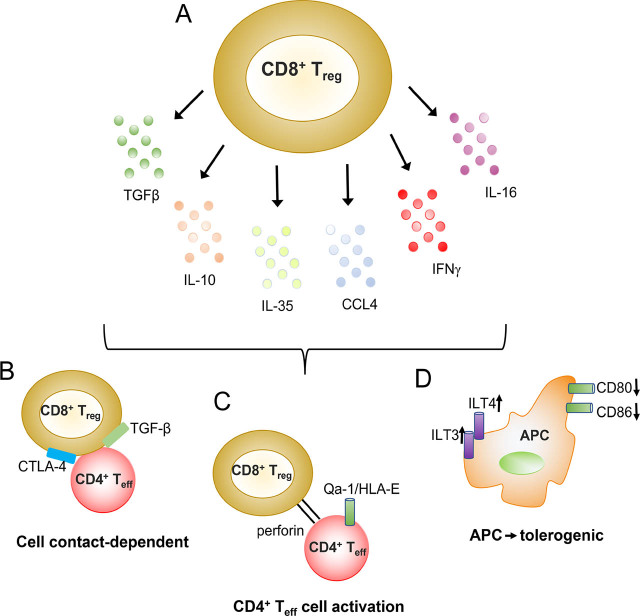
Figure 2.
Schematic representation of the mechanisms of immune suppression of CD8+ Tregs in SLE. A. CD8+ Tregs secrete cytokines/chemokines such as TGFβ, IL-10, and CCL4 that suppress immune responses. B. CD8+ Tregs can also suppress in a cell contact-dependent fashion that may depend on the surface expression of membrane-bound TGFβ (and/or CTLA-4). C. MHC class I-restricted CD8+ Tregs are capable to kill activated CD4+ T effector (Teff) cells that express Qa-1/HLA-E. D. CD8+ Tregs can render APCs tolerogenic by downregulating co-stimulatory molecules such as CD80 and CD86, and upregulating inhibitory receptors such as ILT3 and ILT4. APC, antigen presenting cells; ILT, Ig-like transcript; SLE, systemic lupus erythematosus; and Tregs, T regulatory cells. Modified with permission from Ref # 20, Dinesh RK et al, Autoimmun Rev. 2010 Jun;9(8):560-8. Copyright, 2010, Elsevier.
Isolated CD8+ Tregs frequently express several genes that include CD8α, FoxP3, CD25high, CD28low, CTLA-4, CD122, CD103, CD38, CD45RA, CD45RO, CD56, CXCR3, lymphocyte activation gene 3 (LAG-3), and CD127low.[20, 22, 23, 24, 25]
Analogous to the CD8+CD122+ T cells found in mice, Shi et al. showed that in humans CD8+CXCR3 (CD183+) T cells were regulatory in nature and mediated suppressive functions through IL-10.[41] In mice, CD8+CD122+ T cells contained populations which were both positive and negative for the expression of programed death-1 (PD-1); however, the suppressive activity was only present in the PD-1+ subset and depended on production of IL-10.[42] Also in mice, Deng et al. reported that CD8+CD103+ Tregs inhibited the progression of lupus nephritis by attenuating glomerular endothelial cell injury,[43] and the adoptive transfer of CD8+CD103+ inducible Tregs (iTregs) to Murphy Roths Large (MRL)/lpr mice associated with decreased levels of autoantibodies, reduced renal pathological lesions, lowered renal deposition of IgG/C3, and less proteinuria.[43]
CD8+CD28− and CD8+CD28low Tregs were reported in mice and in human,[44] while CD8+CD183+CD25highCD278+ Tregs that inhibited B-cell proliferation and immunoglobulin (IgG), IgM. IgA production were identified by Gupta and colleagues in humans.[45]
Our group showed that the treatment of (New Zealand Black X New Zealand White)F1 (BWF1) lupus-prone mice with the anti-DNA-based peptide pCons induced distinct populations of CD8+ Tregs.[6, 8, 30, 31] Those CD8+ Tregs included both CD8+CD28− and CD8+CD28+ cells but the expression of FoxP3 and TGF-β mRNAs was higher and longer-lasting in the Tregs of the CD28− subset.[6] Other pCons-induced molecular markers[6, 8] included are RGS2low, RGS16, RGS17, Bcl-2 Associated X-protein (BAXlow), glutamic pyruvate transaminase (GPT-2low), and growth arrest and DNA damage inducible 45β protein (GADD45β). The phenotype of the pCons-induced CD8+ Tregs that protected lupus mice and reduced anti-DNA autoantibodies and proteinuria[6, 8, 21, 39] also included programed cell death-1 (PD1low), CD62Lhigh, and CCR7low (Singh et al., in press, Front Immunol (2021) doi: 10.3389/fimmu.2021.718359).
Cellular and molecular markers of CD4+ Tregs
There are similarities and differences between CD8+ Tregs and CD4+ Tregs. Compared to CD8+ Tregs, CD4+ Tregs have been better characterized (Table 2). Markers for human and murine CD4+ Tregs include CD25, FOXP3, CD127low, GITR, CTLA-4, CD28, GARP, HLA-DR, CD45RA, CD45RO, ICOS, Bcl-6, CCR6, CD39, CD73, CD49d, and Helios.[40, 46, 47] Nocentini et al. showed that CD4+CD25low and GITR+ T cells had a regulatory phenotype and suppressed the proliferation of T effector cells, were expanded in inactive lupus patients.[48] Others found that human Tregs preferentially expressed tumor necrosis factor receptor 2 (TNFR2), in addition to CD25, FoxP3, and CD45RO+ markers,[49, 50] and Okubo et al. demonstrated that tumor necrosis factor-alpha (TNF-α) or a TNFR2 agonist promoted the expansion in vitro of TNFR2+ Tregs with a strong suppressive function.[51]
Table 2.
CD4+ Tregs markers and mechanisms of action.
| Subset | Natural/induced | Markers | Mechanisms of Action | Ref. |
|---|---|---|---|---|
| CD4+ Tregs (mice, humans) | Induced/natural | CD4, CD25, FoxP3, IL-10, IL-35, GITR, CD127low | Suppress T effector cells, cell-to-cell contact, downregulation of CD80/CD86, metabolic disruption | [18, 40, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75] |
| CD4+ Tregs (humans) | Natural | CD4, CD25, GARP, CD45RA/RO, CCR6 Helios, CD127low | Suppress T effector cells | [35, 40, 46, 47, 63, 76, 77, 78] |
| Tr1 | Natural | CD4, CD25, IL-10, IL-35 | Suppress T effector cells, induction of B7-H4 on APCs through IL-10, TGF-β, IL-35 | [40, 46, 47, 79] |
| Th1, Th2, Th3 | Natural | CD4, CD25, CXCR CXCR3+ T cells that can produce IFN-γ, IL-4 | Suppress T effector cells through IL-10, TGF-β, IFN-γ, IL-4 | [63, 64, 65, 66, 67] |
| IL-17+ FoxP3+ Tregs (mice, humans) | Natural | CD4, FOXP3, CCR6, RORγt | Suppress CD4+ T cell proliferation | [40, 46, 47] |
| CD45RA+ FoxP3low Tregs (mice, humans) | Natural | CD4, CD45RA, FOXP3 | Resting Tregs | [40, 46, 47] |
| Follicular Tregs (mice) | Natural | CD4, Foxp3, CXCR5, Bcl6 | Germinal centers | [40, 46, 47] |
| CD4+CD25low/−GITR+ (humans) | Natural | CD4+ CD25low/−GITR+ | Suppress T effector cells | [48] |
| CD4+CD25+ CXCR2+FoxP3+ CD45RO+ (humans) | Natural/induced | CD4+CD25+ CXCR2+FoxP3+ CD45RO+ | Suppress T effector cells | [51] |
| CD4+CD161+ FoxP3+ (humans) | Natural | CD127low, IL-2, IFNγ, IL-17 | Suppress T effector cells | [57, 58] |
| CD4+CXCR5+ FoxP3+ (mice, humans) | Natural | CD4+CXCR5+ | Suppress B-cell antibody production | [59] |
| Follicular CD4+Bcl6−FoxP3+ Tregs (mice, humans) | Natural | CD4+Bcl6−FoxP3+ | Suppress germinal center reactions | [60] |
APC, antigen presenting cells; Tregs, T regulatory cells; Tr1, type-1 regulatory.
Also CD4+FoxP3− type-1 regulatory (Tr1) cells that express IL-10 are involved in the maintenance of tolerance and display strong immunosuppressive functions.[52, 53, 54] Duhen et al. identified CD4+ Tregs subsets based on the expression of chemokine receptors, with differentially expressed lineage-specific transcription factors that responded differently to Th1, Th2, and Th17.[55, 56] Pesenacker et al. and Afzali et al. defined a new subset of Tregs in human cord blood with a CD4+CD161+ phenotype that, although proinflammatory in nature, had a similar suppressive potential as conventional Tregs,[57, 58] while Chung et al., and Linterman et al. identified a subset of CD4+ Tregs expressing CXCR5 and Bcl6 that localized in the germinal centers of both mice and humans.[59, 60] Other tissue-resident Tregs can be mostly activated cells with memory suppression.[61, 62]
Induction of CD8+ and CD4+ Tregs in SLE
Homeostatic balance in the controlled regulation of the immune response is impaired in lupus patients,[80] and decreased numbers of CD4+ and CD8+ Tregs associate with accelerated and deteriorating pathology in animal models and in humans with SLE,[4, 12, 13, 14, 15, 16, 21] indicating that Tregs play an important role in the protection from SLE.[6, 8, 21, 23, 26, 32, 33, 34, 35, 81, 82]
We reported that both CD4+ and CD8+ Tregs are functionally deficient in both BWF1 mice and patients with SLE (they are as well reduced in other autoimmune conditions).[4, 5, 6, 7, 8, 9, 10, 76, 77, 78] While CD4+ Tregs have been intensively studied,[63, 64, 65, 66, 67] less is known about the CD8+ Tregs in the suppression of autoimmunity.
Functional properties of peptide-induced CD8+ and CD4+ Tregs in SLE
The functional properties of CD8+ Tregs can be modulated by the administration of anti-DNA-based peptides to alter disease progression.[6, 8, 21, 83, 84, 85, 86, 87, 88, 89] We showed that BWF1 lupus mice were protected from autoimmune disease after i.v. injection of high doses of pCons, an artificial peptide based on the VH sequence of murine anti-dsDNA antibodies that is presented by both MHC class I and II molecules.[83] Immune tolerance induced by the pCons peptide associated with an expansion of both CD8+ and CD4+ Tregs that independently suppressed the proliferation of naïve CD4+ T cells and B cells.[6, 8, 18, 21, 39] pCons induced CD4+ Tregs with high FoxP3 expression and suppressed anti-DNA autoantibody production both in vitro and in vivo but also induced an expansion of CD8+[6, 21, 90] that suppressed autoimmune responses in a FoxP3-dependent manner.[6, 8, 21] After pCons administration, CD8+ Tregs developed a unique genetic/molecular profile consisting of the upregulation of genes including FoxP3, Trp53, Bcl2, CCR7, IFNAR1, and Ifi202b (Table 3). Downregulated genes included RGS2, GPT2, BAX, PD1, CTLA4, CD122, GADD45, and phosphodiesterase 3b (PDE3b).[91] In all, their suppressive capacity depended on the expression of FoxP3, PD1, and IFI202b.[8, 39]
Table 3.
Gene changes in CD8+ Tregs induced by anti-DNA antibody-based peptide in lupus mice.
| Upregulated genes | Downregulated genes |
|---|---|
| Foxp3, IL-2, TGF-β, CD25, CD28, Trp53, CD122, Bcl2, CCR7, IFNAR1, Ifi202b | RGS2, GPT2, BAX, PD1, CTLA-4, GADD45β, PDE3b |
PDE3b, phosphodiesterase 3b; RGS, regulator of G-protein signaling; Tregs, T regulatory cells.
While extensive studies have evaluated the role of CD4+ Tregs as suppressor of autoimmune responses, the mode of action of CD8+ Tregs have been explored less[6, 8, 16, 18, 21, 92, 93, 94, 95, 96] but shown to prevent lupus-like disease in murine graft versus host disease (GVHD).[97, 98, 99]
The induction of CD8+ Cytotoxic T lymphocytes (CTLs) is responsible for the killing of autoantibody-producing B cells and the inhibition of murine lupus.[100]
A nucleosomal histone peptide in (SWR × NZB)F1 (SNF1) mice delays lupus nephritis and B-cell activation by inducing (CD4+ and CD8+) TGF-β+ Tregs in mice[85, 101, 102] and also blocks pathogenic autoimmune responses in human SLE.[103]
Interestingly, SLE patients treated with methylprednisolone have CD8+ Tregs associated with decreased disease activity,[104] and CD8+ Tregs are induced by all-trans retinoic acid.[105]
The MHC class 1b molecule Qa-1 restricted CD8+ α/α+ TCR α/β+ T cells has been shown to regulate immunity in mice,[27, 106, 107] and a population of Qa-1-restricted CD8+ T cells can inhibit murine lupus-like disease by targeting autoreactive CD4+ T follicular helper cells (TFH).[23, 28] Peptide-specific CD8+ Tregs that suppress partly through perforin have also been described,[23, 26, 28, 29]; other tolerogenic peptides based on the light chain complementarity-determining region 1 (hCDR1) of human anti-dsDNA antibodies that induce CD4+CD25+ and CD8+CD28− Tregs, which suppressed lymphocyte proliferation and autoantibody production in BWF1 lupus mice have also been described.[1, 19, 20, 87, 108]
Transcription factors and mechanisms of action of Tregs
FoxP3 is a critical transcription factor in the regulatory activity of both CD4+ and CD8+ Tregs.[109] A decreased expression of FoxP3 results in loss of tolerance to self-antigens in SLE patients,[110] and SLE patients have a decreased expression of FoxP3 as compared to healthy matched controls.[77]
Recent studies have shown that both CD4+ and CD8+ Tregs express another transcription factor, Helios, which appears as essential for the maintenance of a stable phenotype and suppressive activity during inflammation and autoimmunity.[111] Helios is a member of the Ikaros gene transcription factor family expressed by FoxP3+ Tregs (both in mice and humans). It is thought that Helios+ cell subsets arise from thymus while Helios− subsets are induced from FoxP3− T cells. Helios+ T cells are highly suppressive and express more highly demethylated Treg-specific demethylated region (TSDR) that facilitate FoxP3 transcription and therefore expression.[112] Helios+ human memory Tregs appear to co-express (T cell immunoreceptor with Ig and ITIM domains (TIGIT) and Fc receptor-like protein 3 (FCRL3),[113] and suppressive Helios+ FoxP3+ Tregs with migratory potential are expanded in inflamed tissues of SLE patients with active disease.[114]
It seems that transcription factor, BTB Domain And CNC Homolog 2 (Bach2), is also important for Tregs, since a loss of it results in Th2-mediated inflammatory lung disease while its expression is required for TGF-β-induced FoxP3 expression and the suppression of T effector cells.[115, 116, 117]
CD4+CD25+LAG+ Tregs are instead regulated by Early growth response 2 (Egr2), a zinc-finger transcription factor required for the induction of T cell anergy, and produce TGF-β3 in an Egr2- and Fas-dependent manner.[118]
Another transcription factor, nuclear factor erythroid 2-related factor 2 (NRF2), is a transcriptional activator which regulates oxidative stress.[119] Although specific functions of NRF2 in Tregs are not fully understood, a recent study has shown that NRF2 is a negative regulator of Treg function and that FoxP3 specific activation of NRF2 results in the loss of immune tolerance and the accumulation of IFN-γ-producing T effector cells and inflammation.[120] In SLE, several lines of evidence suggest that NRF2 plays a central role in the pathogenesis of the disease by exerting anti-inflammatory effects—although others show pro-inflammatory effects. One study showed that aged female NRF2-deficient mice were prone to develop a condition closely resembling human SLE,[121] and another study in B6/lpr mice associated NRF2 deficiency with lupus nephritis and Th17 cells.[122] Mechanistically, NRF2 binds together with small Maf proteins to the antioxidant response element (ARE) in the regulatory regions of target genes and with KEAP1 (Kelch ECH associating protein 1), a repressor protein that binds to NRF2 and promotes its degradation by the ubiquitin-proteasome pathway. Genetic deletion of Keap1 resulted in higher percentages of Tregs,[123] and the absence of NRF2 in donor T cells enhanced the persistence of Tregs and reduced systemic inflammation in murine GVHD.[124]
Notwithstanding the above consideration, the general mechanisms of actions of the Tregs include: (1) suppression of T and B cells through inhibitory cytokines; (2) induction of cytolysis in target cells; (3) targeting antigen presenting cells (APC) such as dendritic cells, and (4) metabolic disruption in target cells.
Tregs secrete inhibitory cytokines such as IL-10, TGF-β, and IL-35 that can suppress target cells including APCs and CD4+CD25− T effector cells.[68, 69, 125, 126] For example, pCons-induced Tregs secreted TGF-β and IL-10,[6, 8, 18, 21, 76] is also observed in other studies.[70, 71, 72]
The cytolysis of target cells by Tregs involved perforin and granzyme B.[18, 90]
Tregs can also target directly APCs to suppress their function or render them tolerogenic through an upregulation of inhibitory receptors such as Ig-like transcript (ILT)-3 and ILT-4.[37, 38] Bezie et al. showed that CD8+FoxP3+ Tregs depend on the expression of CTLA-4 to suppress T effector cells in vitro,[73] and other studies found that Tregs can downregulate costimulatory molecules such as CD80 and CD86 on the APCs.[36, 74, 79]
Finally, the “metabolic disruption” in target cells by Tregs causes suppression of T effector cells by utilizing/sequestering IL-2 and/or IL-15, thus depriving the target cells of critical growth factors.[75, 127]
Concluding remarks
Studies and findings on Tregs are ready to be translated into approaches for the restoration of immune tolerance in SLE and advancement toward clinical settings. In particular, the bioengineering of Tregs and the use of polyclonal and antigen-specific Treg cell therapies based on CD4+ and CD8+ chimeric-antigen-receptor (CAR) Tregs in ongoing investigations by many biotechnology and pharmaceutical companies are providing encouraging results that appear to rapidly translate into the clinical practices.[128, 129, 130, 131, 132, 133, 134] More research will allow to fine-tuning and avoid off-target effects in different Tregs-based immunotherapies, optimizing the immunotherapeutic benefits for SLE patients.
Footnotes
Funding
This work was supported by the NIH grants AR54034, AI 083894, AI65645 to RPS; UCLA Senate Core Grant to BHH and RPS; UCLA Oppenheimer Clinical Seed Grant and American Autoimmune Related Disease Association grant to RPS.
Conflict of Interest
Dr. Hahn has accepted funds for advisory work from Aurinia, GSL, and UCB in the last 12 months. The authors declare that there is no additional financial or commercial conflict of interest.
References
- [1].Crispin JC, Kyttaris VC, Terhorst C. et al. T Cells as Therapeutic Targets in SLE. Nat Rev Rheumatol. 2010;6:317–125. doi: 10.1038/nrrheum.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Crow MK. Collaboration, Genetic Associations, and Lupus Erythematosus. N Engl J Med. 2008;358:956–961. doi: 10.1056/NEJMe0800096. [DOI] [PubMed] [Google Scholar]
- [3].Hahn BH. Lessons in Lupus: The Mighty Mouse. Lupus. 2001;10:589–593. doi: 10.1191/096120301682430140. [DOI] [PubMed] [Google Scholar]
- [4].Filaci G, Bacilieri S, Fravega M. et al. Impairment of CD8+ T Suppressor Cell Function in Patients with Active Systemic Lupus Erythematosus. J Immunol. 2001;166:6452–6457. doi: 10.4049/jimmunol.166.10.6452. [DOI] [PubMed] [Google Scholar]
- [5].Karpouzas GA, La Cava A, Ebling FM. et al. Differences Between CD8+ T Cells in Lupus-Prone (NZB x NZW)F1 Mice and Healthy (BALB/c x NZW)F1 Mice May Influence Autoimmunity in the Lupus Model. Eur J Immunol. 2004;34:2489–2499. doi: 10.1002/eji.200424978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Singh RP, La Cava A, Wong M. et al. CD8+ T Cell-Mediated Suppression of Autoimmunity in A Murine Lupus Model of Peptide-Induced Immune Tolerance Depends on Foxp3 Expression. J Immunol. 2007;178:7649–7657. doi: 10.4049/jimmunol.178.12.7649. [DOI] [PubMed] [Google Scholar]
- [7].Singh RP, Hahn BH, La Cava A. Tuning Immune Suppression in Systemic Autoimmunity with Self-Derived Peptides. Inflamm Allergy Drug Targets. 2008;7:253–259. doi: 10.2174/187152808786848423. [DOI] [PubMed] [Google Scholar]
- [8].Singh RP, La Cava A, Hahn BH. pConsensus Peptide Induces Tolerogenic CD8+ T Cells in Lupus-Prone (NZB x NZW)F1 Mice by Differentially Regulating Foxp3 and PD1 Molecules. J Immunol. 2008;180:2069–2080. doi: 10.4049/jimmunol.180.4.2069. [DOI] [PubMed] [Google Scholar]
- [9].Skaggs BJ, Singh RP, Hahn BH. Induction of Immune Tolerance by Activation of CD8+ T Suppressor/Regulatory Cells in Lupus-Prone Mice. Hum Immunol. 2008;69:790–796. doi: 10.1016/j.humimm.2008.08.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Suzuki M, Konya C, Goronzy JJ. et al. Inhibitory CD8+ T Cells in Autoimmune Disease. Hum Immunol. 2008;69:781–789. doi: 10.1016/j.humimm.2008.08.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Crispin JC, Alcocer-Varela J, de Pablo P. et al. Immunoregulatory Defects in Patients with Systemic Lupus Erythematosus in Clinical Remission. Lupus. 2003;12:386–393. doi: 10.1191/0961203303lu368oa. [DOI] [PubMed] [Google Scholar]
- [12].Crispin JC, Martinez A, Alcocer-Varela J. Quantification of Regulatory T Cells in Patients with Systemic Lupus Erythematosus. J Autoimmun. 2003;21:273–276. doi: 10.1016/s0896-8411(03)00121-5. [DOI] [PubMed] [Google Scholar]
- [13].Liu MF, Wang CR, Fung LL. et al. Decreased CD4+CD25+ T Cells in Peripheral Blood of Patients with Systemic Lupus Erythematosus. Scand J Immunol. 2004;59:198–202. doi: 10.1111/j.0300-9475.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- [14].Miyara M, Amoura Z, Parizot C. et al. Global Natural Regulatory T Cell Depletion in Active Systemic Lupus Erythematosus. J Immunol. 2005;175:8392–8400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- [15].Valencia X, Yarboro C, Illei G. et al. Deficient CD4+CD25high T Regulatory Cell Function in Patients with Active Systemic Lupus Erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- [16].Scalapino KJ, Tang Q, Bluestone JA. et al. Suppression of Disease in New Zealand Black/New Zealand White Lupus-Prone Mice by Adoptive Transfer of Ex Vivo Expanded Regulatory T Cells. J Immunol. 2006;177:1451–1459. doi: 10.4049/jimmunol.177.3.1451. [DOI] [PubMed] [Google Scholar]
- [17].Horwitz DA, Gray JD, Zheng SG. The Potential of Human Regulatory T Cells Generated Ex Vivo as A Treatment for Lupus and Other Chronic Inflammatory Diseases. Arthritis Res. 2002;4:241–246. doi: 10.1186/ar414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].La Cava A, Ebling FM, Hahn BH. Ig-Reactive CD4+CD25+ T Cells from Tolerized (New Zealand Black x New Zealand White)F1 Mice Suppress In Vitro Production of Antibodies to DNA. J Immunol. 2004;173:3542–3548. doi: 10.4049/jimmunol.173.5.3542. [DOI] [PubMed] [Google Scholar]
- [19].Horwitz DA, Zheng SG, Gray JD. et al. Regulatory T Cells Generated Ex Vivo as an Approach for the Therapy of Autoimmune Disease. Semin Immunol. 2004;16:135–143. doi: 10.1016/j.smim.2003.12.009. [DOI] [PubMed] [Google Scholar]
- [20].Dinesh RK, Skaggs BJ, La Cava A. et al. CD8+ Tregs in Lupus, Autoimmunity, and Beyond. Autoimmun Rev. 2010;9:560–568. doi: 10.1016/j.autrev.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hahn BH, Singh RP, La Cava A. et al. Tolerogenic Treatment of Lupus Mice with Consensus Peptide Induces Foxp3-Expressing, Apoptosis-Resistant, TGFβ-Secreting CD8+ T Cell Suppressors. J Immunol. 2005;175:7728–7737. doi: 10.4049/jimmunol.175.11.7728. [DOI] [PubMed] [Google Scholar]
- [22].Churlaud G, Pitoiset F, Jebbawi F. et al. Human and Mouse CD8+CD25+FOXP3+ Regulatory T Cells at Steady State and During Interleukin-2 Therapy. Front Immunol. 2015;6:171. doi: 10.3389/fimmu.2015.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim HJ, Verbinnen B, Tang X. et al. Inhibition of Follicular T-Helper Cells by CD8+ Regulatory T Cells is Essential for Self Tolerance. Nature. 2010;467:328–332. doi: 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pomie C, Menager-Marcq I, van Meerwijk JP. Murine CD8+ Regulatory T Lymphocytes: The New Era. Hum Immunol. 2008;69:708–714. doi: 10.1016/j.humimm.2008.08.288. [DOI] [PubMed] [Google Scholar]
- [25].Tang XL, Smith TR, Kumar V. Specific Control of Immunity by Regulatory CD8 T Cells. Cell Mol Immunol. 2005;2:11–19. [PubMed] [Google Scholar]
- [26].Kim HJ, Wang X, Radfar S. et al. CD8+ T Regulatory Cells Express the Ly49 Class I MHC Receptor and are Defective in Autoimmune Prone B6-Yaa Mice. Proc Natl Acad Sci USA. 2011;108:2010–2015. doi: 10.1073/pnas.1018974108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Smith TR, Kumar V. Revival of CD8+ Treg-Mediated Suppression. Trends Immunol. 2008;29:337–342. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- [28].Lu L, Cantor H. Generation and Regulation of CD8+ Regulatory T Cells. Cell Mol Immunol. 2008;5:401–406. doi: 10.1038/cmi.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Leavenworth JW, Tang X, Kim HJ. et al. Amelioration of Arthritis through Mobilization of Peptide-Specific CD8+ Regulatory T Cells. J Clin Invest. 2013;123:1382–1389. doi: 10.1172/JCI66938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rifa’i M, Kawamoto Y, Nakashima I. et al. Essential Roles of CD8+CD122+ Regulatory T Cells in the Maintenance of T Cell Homeostasis. J Exp Med. 2004;200:1123–1134. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rifa’i M, Shi Z, Zhang SY. et al. CD8+CD122+ Regulatory T Cells Recognize Activated T Cells Via Conventional MHC Class I-AlphabetaTCR Interaction and Become IL-10-Producing Active Regulatory Cells. Int Immunol. 2008;20:937–947. doi: 10.1093/intimm/dxn052. [DOI] [PubMed] [Google Scholar]
- [32].Colovai AI, Mirza M, Vlad G. et al. Regulatory CD8+CD28− T Cells in Heart Transplant Recipients. Hum Immunol. 2003;64:31–37. doi: 10.1016/s0198-8859(02)00742-5. [DOI] [PubMed] [Google Scholar]
- [33].Filaci G, Fenoglio D, Fravega M. et al. CD8+CD28− T Regulatory Lymphocytes Inhibiting T Cell Proliferative and Cytotoxic Functions Infiltrate Human Cancers. J Immunol. 2007;179:4323–4334. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- [34].Najafian N, Chitnis T, Salama AD. et al. Regulatory Functions of CD8+CD28− T Cells in an Autoimmune Disease Model. J Clin Invest. 2003;112:1037–1048. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhang L, Bertucci AM, Ramsey-Goldman R. et al. Regulatory T Cell (Treg) Subsets Return in Patients with Refractory Lupus Following Stem Cell Transplantation, and TGF-Beta-Producing CD8+ Treg Cells are Associated with Immunological Remission of Lupus. J Immunol. 2009;183:6346–6358. doi: 10.4049/jimmunol.0901773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cederbom L, Hall H, Ivars F. CD4+CD25+ Regulatory T Cells Down-Regulate Co-Stimulatory Molecules on Antigen-Presenting Cells. Eur J Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- [37].Chang CC, Ciubotariu R, Manavalan JS. et al. Tolerization of Dendritic Cells by T(S) Cells: The Crucial Role of Inhibitory Receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- [38].Manavalan JS, Kim-Schulze S, Scotto L. et al. Alloantigen Specific CD8+CD28− FOXP3+ T Suppressor Cells Induce ILT3+ ILT4+ Tolerogenic Endothelial Cells, Inhibiting Alloreactivity. Int Immunol. 2004;16:1055–1068. doi: 10.1093/intimm/dxh107. [DOI] [PubMed] [Google Scholar]
- [39].Dinesh R, Hahn BH, La Cava A. et al. Interferon-Inducible Gene 202b Controls CD8+ T Cell-Mediated Suppression in Anti-DNA Ig Peptide-Treated (NZB x NZW)F1 Lupus Mice. Genes Immun. 2011;12:360–369. doi: 10.1038/gene.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ballke C, Gran E, Baekkevold ES. et al. Characterization of Regulatory T-Cell Markers in CD4+ T Cells of the Upper Airway Mucosa. PloS ONE. 2016;11:e0148826. doi: 10.1371/journal.pone.0148826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shi Z, Okuno Y, Rifa’i M. et al. Human CD8+CXCR3+ T Cells Have the Same Function as Murine CD8+CD122+ Treg. Eur J Immunol. 2009;39:2106–2119. doi: 10.1002/eji.200939314. [DOI] [PubMed] [Google Scholar]
- [42].Dai H, Wan N, Zhang S. et al. Cutting Edge: Programmed Death-1 Defines CD8+CD122+ T Cells as Regulatory Versus Memory T Cells. J Immunol. 2010;185:803–807. doi: 10.4049/jimmunol.1000661. [DOI] [PubMed] [Google Scholar]
- [43].Deng W, Xu M, Meng Q. et al. CD8+CD103+ iTregs Inhibit the Progression of Lupus Nephritis by Attenuating Glomerular Endothelial Cell Injury. Rheumatology (Oxford) 58:2039–2050. doi: 10.1093/rheumatology/kez112. [DOI] [PubMed] [Google Scholar]
- [44].Vuddamalay Y, van Meerwijk JP. CD28− and CD28lowCD8+ Regulatory T Cells: Of Mice and Men. Front Immunol. 2017;8:31. doi: 10.3389/fimmu.2017.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gupta S, Su H, Agrawal S. CD8 Treg Cells Inhibit B-Cell Proliferation and Immunoglobulin Production. Int Arch Allergy Immunol. 2020;181:947–955. doi: 10.1159/000509607. [DOI] [PubMed] [Google Scholar]
- [46].Miyara M, Gorochov G, Ehrenstein M. et al. Human FoxP3+ Regulatory T Cells in Systemic Autoimmune Diseases. Autoimmun Rev. 2011;10:744–755. doi: 10.1016/j.autrev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- [47].Chen X, Oppenheim JJ. Resolving the Identity Myth: Key Markers of Functional CD4+FoxP3+ Regulatory T Cells. Int Immunopharmacol. 2011;11:1489–1496. doi: 10.1016/j.intimp.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nocentini G, Alunno A, Petrillo MG. et al. Expansion of Regulatory GITR+CD2low/−CD4+ T Cells in Systemic Lupus Erythematosus Patients. Arthritis Res Ther. 2014;16:444. doi: 10.1186/s13075-014-0444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Annunziato F, Cosmi L, Liotta F. et al. Phenotype, Localization, and Mechanism of Suppression of CD4+CD25+ Human Thymocytes. J Exp Med. 2002;196:379–387. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chen X, Subleski JJ, Hamano R. et al. Co-Expression of TNFR2 and CD25 Identifies More of the Functional CD4+FOXP3+ Regulatory T Cells in Human Peripheral Blood. Eur J Immunol. 2010;40:1099–1106. doi: 10.1002/eji.200940022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Okubo Y, Mera T, Wang L. et al. Homogeneous Expansion of Human T-Regulatory Cells Via Tumor Necrosis Factor Receptor 2. Sci Rep. 2013;3:3153. doi: 10.1038/srep03153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gagliani N, Magnani CF, Huber S. et al. Coexpression of CD49b and LAG-3 Identifies Human and Mouse T Regulatory Type 1 Cells. Nat Med. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- [53].Geem D, Harusato A, Flannigan K. et al. Harnessing Regulatory T Cells for the Treatment of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:1409–1418. doi: 10.1097/MIB.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mascanfroni ID, Takenaka MC, Yeste A. et al. Metabolic Control of Type 1 Regulatory T Cell Differentiation by AHR and HIF1-β. Nat Med. 2015;21:638–646. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Duhen T, Duhen R, Lanzavecchia A. et al. Functionally Distinct Subsets of Human FOXP3+ Treg Cells that Phenotypically Mirror Effector Th Cells. Blood. 2012;119:4430–4440. doi: 10.1182/blood-2011-11-392324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Duhen T, Geiger R, Jarrossay D. et al. Production of Interleukin 22 but not Interleukin 17 by A Subset of Human Skin-Homing Memory T Cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- [57].Pesenacker AM, Bending D, Ursu S. et al. CD161 Defines the Subset of FoxP3+ T Cells Capable of Producing Proinflammatory Cytokines. Blood. 2013;121:2647–2658. doi: 10.1182/blood-2012-08-443473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Afzali B, Mitchell PJ, Edozie FC. et al. CD161 Expression Characterizes A Subpopulation of Human Regulatory T Cells that Produces IL-17 in a STAT3-Dependent Manner. Eur J Immunol. 2013;43:2043–2054. doi: 10.1002/eji.201243296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Linterman MA, Pierson W, Lee SK. et al. Foxp3+ Follicular Regulatory T Cells Control the Germinal Center Response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chung Y, Tanaka S, Chu F. et al. Follicular Regulatory T Cells Expressing Foxp3 and Bcl-6 Suppress Germinal Center Reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kalekar LA, Rosenblum MD. Regulatory T Cells in Inflammatory Skin Disease: from Mice to Humans. Int Immunol. 2019;31:457–463. doi: 10.1093/intimm/dxz020. [DOI] [PubMed] [Google Scholar]
- [62].Mizui M, Tsokos GC. Targeting Regulatory T Cells to Treat Patients with Systemic Lupus Erythematosus. Front Immunol. 2018;9:786. doi: 10.3389/fimmu.2018.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chatila TA. Role of Regulatory T Cells in Human Diseases. J Allergy Clin Immunol. 2005;116:949–959. doi: 10.1016/j.jaci.2005.08.047. quiz 960. [DOI] [PubMed] [Google Scholar]
- [64].Chatila TA. Regulatory T Cells: Key Players in Tolerance and Autoimmunity. Endocrinol Metab Clin North Am. 2009;38:265–272. vii. doi: 10.1016/j.ecl.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Chatila TA, Blaeser F, Ho N. et al. JM2 Encoding a Fork Head-Related Protein, is Mutated in X-Linked Autoimmunity-Allergic Dis-regulation Syndrome. J Clin Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chatila TA, Williams CB. Foxp3: Shades of Tolerance. Immunity. 2012;36:693–694. doi: 10.1016/j.immuni.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chatila TA, Williams CB. Regulatory T Cells: Exosomes Deliver Tolerance. Immunity. 2014;41:3–5. doi: 10.1016/j.immuni.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nakamura K, Kitani A, Strober W. Cell Contact-Dependent Immunosuppression by CD4+CD25+ Regulatory T Cells is Mediated by Cell Surface-Bound Transforming Growth Factor β. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Collison LW, Workman CJ, Kuo TT. et al. The Inhibitory Cytokine IL-35 Contributes to Regulatory T-Cell Function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- [70].Hawrylowicz CM, O’Garra A. Potential Role of Interleukin-10-Secreting Regulatory T Cells in Allergy and Asthma. Nat Rev Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- [71].Annacker O, Asseman C, Read S. et al. Interleukin-10 in the Regulation of T Cell-Induced Colitis. J Autoimmun. 2003;20:277–279. doi: 10.1016/s0896-8411(03)00045-3. [DOI] [PubMed] [Google Scholar]
- [72].Joetham A, Takeda K, Taube C. et al. Naturally Occurring Lung CD4+CD25+ T Cell Regulation of Airway Allergic Responses Depends on IL-10 Induction of TGF-β. J Immunol. 2007;178:1433–1442. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- [73].Bezie S, Anegon I, Guillonneau C. Advances on CD8+ Treg Cells and their Potential in Transplantation. Transplantation. 2018;102:1467–1478. doi: 10.1097/TP.0000000000002258. [DOI] [PubMed] [Google Scholar]
- [74].Kryczek I, Wei S, Zou L. et al. Cutting Edge: Induction of B7-H4 on APCs through IL-10: Novel Suppressive Mode for Regulatory T Cells. J Immunol. 2006;177:40–44. doi: 10.4049/jimmunol.177.1.40. [DOI] [PubMed] [Google Scholar]
- [75].Pandiyan P, Zheng L, Ishihara S. et al. CD4+CD25+Foxp3+ Regulatory T Cells Induce Cytokine Deprivation-Mediated Apoptosis of Effector CD4+ T Cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- [76].Singh RP, Hahn BH, Bischoff DS. Effects of Peptide-Induced Immune Tolerance on Murine Lupus. Front Immunol. 2021;12:662901. doi: 10.3389/fimmu.2021.662901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Singh RP, Bischoff DS. Sex Hormones and Gender Influence the Expression of Markers of Regulatory T Cells in SLE Patients. Front Immunol. 2021;12:619268. doi: 10.3389/fimmu.2021.619268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Giang S, Horwitz DA, Bickerton S. et al. Nanoparticles Engineered as Artificial Antigen-Presenting Cells Induce Human CD4+ and CD8+ Tregs that are Functional in Humanized Mice. Front Immunol. 2021;12:628059. doi: 10.3389/fimmu.2021.628059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Davila E, Kang YM, Park YW. et al. Cell-Based Immunotherapy with Suppressor CD8+ T Cells in Rheumatoid Arthritis. J Immunol. 2005;174:7292–7301. doi: 10.4049/jimmunol.174.11.7292. [DOI] [PubMed] [Google Scholar]
- [80].Crispin JC, Oukka M, Bayliss G. et al. Expanded Double Negative T Cells in Patients with Systemic Lupus Erythematosus Produce IL-17 and Infiltrate the Kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ben-David H, Sharabi A, Dayan M. et al. The Role of CD8+CD28− Regulatory Cells in Suppressing Myasthenia Gravis-Associated Responses by A Dual Altered Peptide Ligand. Proc Natl Acad Sci USA. 2007;104:17459–17464. doi: 10.1073/pnas.0708577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Sharabi A, Mozes E. The Suppression of Murine Lupus by A Tolerogenic Peptide Involves foxp3-expressing CD8 Cells that are Required for the Optimal Induction and Function of foxp3-expressing CD4 Cells. J Immunol. 2008;181:3243–3251. doi: 10.4049/jimmunol.181.5.3243. [DOI] [PubMed] [Google Scholar]
- [83].Hahn BH, Singh RR, Wong WK. et al. Treatment with A Consensus Peptide based on Amino Acid Sequences in Autoantibodies Prevents T Cell Activation by Autoantigens and Delays Disease Onset in Murine Lupus. Arthritis Rheum. 2001;44:432–441. doi: 10.1002/1529-0131(200102)44:2<432::AID-ANR62>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- [84].Kang HK, Liu M, Datta SK. Low-dose Peptide Tolerance Therapy of Lupus Generates Plasmacytoid Dendritic Cells that Cause Expansion of Autoantigen-Specific Regulatory T Cells and Contraction of Inflammatory th17 Cells. J Immunol. 2007;178:7849–7858. doi: 10.4049/jimmunol.178.12.7849. [DOI] [PubMed] [Google Scholar]
- [85].Kang HK, Michaels MA, Berner BR. et al. Very Low-Dose Tolerance with Nucleosomal Peptides Controls Lupus and Induces Potent Regulatory T Cell Subsets. J Immunol. 2005;174:3247–3255. doi: 10.4049/jimmunol.174.6.3247. [DOI] [PubMed] [Google Scholar]
- [86].Riemekasten G, Langnickel D, Enghard P. et al. Intravenous Injection of a D1 Protein of the Smith Proteins Postpones Murine Lupus and Induces Type 1 Regulatory T Cells. J Immunol. 2004;173:5835–5842. doi: 10.4049/jimmunol.173.9.5835. [DOI] [PubMed] [Google Scholar]
- [87].Sharabi A, Haviv A, Zinger H. et al. Amelioration of Murine Lupus by A Peptide, Based on the Complementarity Determining Region-1 of an Autoantibody as Compared to Dexamethasone: Different Effects on Cytokines and Apoptosis. Clin Immunol. 2006;119:146–155. doi: 10.1016/j.clim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- [88].Singh RR, Ebling FM, Albuquerque DA. et al. Induction of Auto-antibody Production is Limited in Nonautoimmune Mice. J Immunol. 2002;169:587–594. doi: 10.4049/jimmunol.169.1.587. [DOI] [PubMed] [Google Scholar]
- [89].Boldin MP, Taganov KD, Rao DS. et al. miR-146a is A Significant Brake on Autoimmunity, Myeloproliferation, and Cancer in Mice. J Exp Med. 2011;208:1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hahn BH, Anderson M, Le E. et al. Anti-DNA Ig Peptides Promote Treg Cell Activity in Systemic Lupus Erythematosus Patients. Arthritis Rheum. 2008;58:2488–2497. doi: 10.1002/art.23609. [DOI] [PubMed] [Google Scholar]
- [91].Dinesh R, Hahn BH, Singh RP. Gender and Sex Hormones Influence CD4+ Regulatory T Cells and their Expression of FoxP3 in Healthy People and in SLE. Arthritis Rheum. 2010;62:1257. [Google Scholar]
- [92].Kohm AP, Carpentier PA, Anger HA. et al. Cutting Edge: CD4+CD25+ Regulatory T Cells Suppress Antigen-Specific Autore-active Immune Responses and Central Nervous System Inflammation During Active Experimental Autoimmune Encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- [93].Hoffmann P, Ermann J, Edinger M. et al. Donor-Type CD4+CD25+ Regulatory T Cells Suppress Lethal Acute Graft-versus-host Disease After Allogeneic Bone Marrow Transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Malek TR, Yu TR, Vincek V. et al. CD4 Regulatory T Cells Prevent Lethal Autoimmunity in IL-2Rβ-Deficient Mice. Implications for the Nonredundant Function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- [95].Kumar V, Sercarz E. An Integrative Model of Regulation Centered on Recognition of TCR Peptide/MHC Complexes. Immunol Rev. 2001;182:113–121. doi: 10.1034/j.1600-065x.2001.1820109.x. [DOI] [PubMed] [Google Scholar]
- [96].Zheng SG, Wang JH, Koss MN. et al. CD4+ and CD8+ Regulatory T Cells Generated Ex Vivo with IL-2 and TGF-β Suppress a Stimulatory Graft-Versus-Host Disease with A Lupus-Like Syndrome. J Immunol. 2004;172:1531–1539. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- [97].Puliaev R, Puliaeva I, Welniak LA. et al. CTL-Promoting Effects of CD40 Stimulation Outweigh B Cell-Stimulatory Effects Resulting in B Cell Elimination and Disease Improvement in A Murine Model of Lupus. J Immunol. 2008;181:47–61. doi: 10.4049/jimmunol.181.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Puliaeva I, Puliaev R, Via CS. Therapeutic Potential of CD8+ Cytotoxic T Lymphocytes in SLE. Autoimmun Rev. 2009;8:219–223. doi: 10.1016/j.autrev.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Via CS, Sharrow SO, Shearer GM. Role of Cytotoxic T Lymphocytes in the Prevention of Lupus-Like Disease Occurring in A Murine Model of Graft-vs-Host Disease. J Immunol. 1987;139:1840–1849. [PubMed] [Google Scholar]
- [100].Fan GC, Singh RR. Vaccination with Minigenes Encoding V(H)-Derived Major Histocompatibility Complex Class I-Binding Epitopes Activates Cytotoxic T Cells that Ablate Autoantibody-Producing B Cells and Inhibit Lupus. Journal Exp Med. 2002;196:731–741. doi: 10.1084/jem.20020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kang SM, Tang Q, Bluestone JA. CD4+CD25+ Regulatory T Cells in Transplantation: Progress, Challenges and Prospects. Am J Transplant. 2007;7:1457–1463. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- [102].Kang HK, Chiang MY, Liu M. et al. The Histone Peptide H4 71-94 Alone is More Effective than A Cocktail of Peptide Epitopes in Controlling Lupus: Immunoregulatory Mechanisms. J Clin Immunol. 2011;31:379–394. doi: 10.1007/s10875-010-9504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Zhang L, Bertucci AM, Ramsey-Goldman R. et al. Major Pathogenic Steps in Human Lupus can be Effectively Suppressed by Nucleosomal Histone Peptide Epitope-Induced Regulatory Immunity. Clin Immunol. 2013;149:365–378. doi: 10.1016/j.clim.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Tsai YG, Lee CY, Lin TY. et al. CD8+ Treg Cells Associated with Decreasing Disease Activity after Intravenous Methylprednisolone Pulse Therapy in Lupus Nephritis with Heavy Proteinuria. PloS One. 2014;9:e81344. doi: 10.1371/journal.pone.0081344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ma J, Liu Y, Li Y. et al. Differential Role of All-Trans Retinoic Acid in Promoting the Development of CD4+ and CD8+ Regulatory T Cells. J Leuk Biol. 2014;95:275–283. doi: 10.1189/jlb.0513297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Tang X, Maricic I, Kumar V. Anti-TCR Antibody Treatment Activates A Novel Population of Nonintestinal CD8α/α+ TCR α/β+ Regulatory T Cells and Prevents Experimental Autoimmune Encephalomyelitis. J Immunol. 2007;178:6043–6050. doi: 10.4049/jimmunol.178.10.6043. [DOI] [PubMed] [Google Scholar]
- [107].Tang X, Maricic I, Purohit N. et al. Regulation of Immunity by A Novel Population of Qa-1-Restricted CD8α/α+TCRα/β+ T Cells. J Immunol. 2006;177:7645–7655. doi: 10.4049/jimmunol.177.11.7645. [DOI] [PubMed] [Google Scholar]
- [108].Eilat E, Dayan M, Zinger H. et al. The Mechanism by Which A Peptide Based on Complementarity-Determining Region-1 of A Pathogenic Anti-DNA Auto-Ab Ameliorates Experimental Systemic Lupus Erythematosus. Proc Natl Acad Sci USA. 2001;98:1148–1153. doi: 10.1073/pnas.98.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Hori S, Nomura T, Sakaguchi S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- [110].Atfy M, Amr GE, Elnaggar AM. et al. Impact of CD4+CD25high Regulatory T-Cells and FoxP3 Expression in the Peripheral Blood of Patients with Systemic Lupus Erythematosus. Egypt J Immunol. 2009;16:117–126. [PubMed] [Google Scholar]
- [111].Nakagawa H, Wang L, Cantor H. et al. New Insights into the Biology of CD8 Regulatory T Cells. Adv Immunol. 2018;140:1–20. doi: 10.1016/bs.ai.2018.09.001. [DOI] [PubMed] [Google Scholar]
- [112].Thornton AM, Lu J, Korty PE. et al. Helios+ and Helios− Treg Subpopulations are Phenotypically and Functionally Distinct and Express Dissimilar TCR Repertoires. Eur J Immunol. 2019;49:398–412. doi: 10.1002/eji.201847935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Bin Dhuban K, d’Hennezel E, Nashi E. et al. Coexpression of TIGIT and FCRL3 Identifies Helios+ Human Memory Regulatory T Cells. J Immunol. 2015;194:3687–3696. doi: 10.4049/jimmunol.1401803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Alexander T, Sattler A, Templin L. et al. Foxp3+ Helios+ Regulatory T Cells are Expanded in Active Systemic Lupus Erythematosus. Ann Rheum Dis. 2013;72:1549–1558. doi: 10.1136/annrheumdis-2012-202216. [DOI] [PubMed] [Google Scholar]
- [115].Roychoudhuri R, Hirahara K, Mousavi K. et al. BACH2 Represses Effector Programs to Stabilize T(reg)-Mediated Immune Homeostasis. Nature. 2013;498:506–510. doi: 10.1038/nature12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kim EH, Gasper DJ, Lee SH. et al. Bach2 Regulates Homeostasis of Foxp3+ Regulatory T Cells and Protects Against Fatal Lung Disease in Mice. J Immunol. 2014;192:985–995. doi: 10.4049/jimmunol.1302378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Muto A, Tashiro S, Nakajima O. et al. The Transcriptional Programme of Antibody Class Switching Involves the Repressor Bach2. Nature. 2004;429:566–571. doi: 10.1038/nature02596. [DOI] [PubMed] [Google Scholar]
- [118].Okamura T, Sumitomo S, Morita K. et al. TGF-β3-Expressing CD4+CD25+LAG3+ Regulatory T Cells Control Humoral Immune Responses. Nat Commun. 2015;6:6329. doi: 10.1038/ncomms7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Ohl K, Tenbrock K. Oxidative Stress in SLE T Cells, is NRF2 Really the Target to Treat? Front Immunol. 2021;12:633845. doi: 10.3389/fimmu.2021.633845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Klemm P, Rajendiran A, Fragoulis A. et al. Nrf2 Expression Driven by Foxp3 Specific Deletion of Keap1 Results in Loss of Immune Tolerance in Mice. Eur J Immunol. 2020;50:515–524. doi: 10.1002/eji.201948285. [DOI] [PubMed] [Google Scholar]
- [121].Ma Q, Battelli L, Hubbs AF. Multiorgan Autoimmune Inflammation, Enhanced Lymphoproliferation, and Impaired Homeostasis of Reactive Oxygen Species in Mice Lacking the Antioxidant-Activated Transcription Factor Nrf2. Am J Pathol. 2006;168:1960–1974. doi: 10.2353/ajpath.2006.051113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhao M, Chen H, Ding Q. et al. Nuclear Factor Erythroid 2-Related Factor 2 Deficiency Exacerbates Lupus Nephritis in B6/lpr Mice by Regulating Th17 Cell Function. Sci Rep. 2016;6:38619. doi: 10.1038/srep38619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Noel S, Martina MN, Bandapalle S. et al. T Lymphocyte-Specific Activation of Nrf2 Protects from AKI. J Am Soc Nephrol. 2015;26:2989–3000. doi: 10.1681/ASN.2014100978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Tsai JJ, Velardi E, Shono Y. et al. Nrf2 Regulates CD4+ T Cell-Induced Acute Graft-Versus-Host Disease in Mice. Blood. 2018;132:2763–2774. doi: 10.1182/blood-2017-10-812941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Green EA, Gorelik L, McGregor CM. et al. CD4+CD25+ T Regulatory Cells Control Anti-Islet CD8+ T Cells through TGF-β-TGF-β Receptor Interactions in Type 1 Diabetes. Proc Natl Acad Sci USA. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Nakamura K, Kitani A, Fuss I. et al. TGF-β 1 Plays an Important Role in the Mechanism of CD4+CD25+ Regulatory T Cell Activity in Both Humans and Mice. J Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- [127].Thornton AM, Shevach EM. CD4+CD25+ Immunoregulatory T Cells Suppress Polyclonal T Cell Activation In Vitro by Inhibiting Interleukin 2 Production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].MacDonald KG, Hoeppli RE, Huang Q. et al. Alloantigen-Specific Regulatory T Cells Generated with A Chimeric Antigen Receptor. J Clin Invest. 2016;126:1413–1424. doi: 10.1172/JCI82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Boardman DA, Philippeos C, Fruhwirth GO. et al. Expression of A Chimeric Antigen Receptor Specific for Donor HLA Class I Enhances the Potency of Human Regulatory T Cells in Preventing Human Skin Transplant Rejection. Am J Transplant. 2017;17:931–943. doi: 10.1111/ajt.14185. [DOI] [PubMed] [Google Scholar]
- [130].Noyan F, Zimmermann K, Hardtke-Wolenski M. et al. Prevention of Allograft Rejection by Use of Regulatory T Cells with an MHC-Specific Chimeric Antigen Receptor. Am J Transplant. 2017;17:917–930. doi: 10.1111/ajt.14175. [DOI] [PubMed] [Google Scholar]
- [131].De Paula Pohl A, Schmidt A, Zhang AH. et al. Engineered Regulatory T Cells Expressing Myelin-Specific Chimeric Antigen Receptors Suppress EAE Progression. Cell Immunol. 2020;358:104222. doi: 10.1016/j.cellimm.2020.104222. [DOI] [PubMed] [Google Scholar]
- [132].Yu Y, Ma X, Gong R. et al. Recent Advances in CD8+ Regulatory T Cell Research. Oncol Lett. 2018;15:8187–8194. doi: 10.3892/ol.2018.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Flippe L, Bezie S, Anegon I. et al. Future Prospects for CD8+ Regulatory T Cells in Immune Tolerance. Immunol Rev. 2019;292:209–224. doi: 10.1111/imr.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Bezie S, Charreau B, Vimond N. et al. Human CD8+ Tregs Expressing a MHC-Specific CAR Display Enhanced Suppression of Human Skin Rejection and GVHD in NSG Mice. Blood Adv. 2019;3:3522–3538. doi: 10.1182/bloodadvances.2019000411. [DOI] [PMC free article] [PubMed] [Google Scholar]