Abstract
Background
Acute respiratory distress syndrome (ARDS) represents the most severe course of COVID‐19 (caused by the SARS‐CoV‐2 virus), usually resulting in a prolonged stay in an intensive care unit (ICU) and high mortality rates. Despite the fact that most affected individuals need invasive mechanical ventilation (IMV), evidence on specific ventilation strategies for ARDS caused by COVID‐19 is scarce. Spontaneous breathing during IMV is part of a therapeutic concept comprising light levels of sedation and the avoidance of neuromuscular blocking agents (NMBA). This approach is potentially associated with both advantages (e.g. a preserved diaphragmatic motility and an optimised ventilation‐perfusion ratio of the ventilated lung), as well as risks (e.g. a higher rate of ventilator‐induced lung injury or a worsening of pulmonary oedema due to increases in transpulmonary pressure). As a consequence, spontaneous breathing in people with COVID‐19‐ARDS who are receiving IMV is subject to an ongoing debate amongst intensivists.
Objectives
To assess the benefits and harms of early spontaneous breathing activity in invasively ventilated people with COVID‐19 with ARDS compared to ventilation strategies that avoid spontaneous breathing.
Search methods
We searched the Cochrane COVID‐19 Study Register (which includes CENTRAL, PubMed, Embase, Clinical Trials.gov WHO ICTRP, and medRxiv) and the WHO COVID‐19 Global literature on coronavirus disease to identify completed and ongoing studies from their inception to 2 March 2022.
Selection criteria
Eligible study designs comprised randomised controlled trials (RCTs) that evaluated spontaneous breathing in participants with COVID‐19‐related ARDS compared to ventilation strategies that avoided spontaneous breathing (e.g. using NMBA or deep sedation levels). Additionally, we considered controlled before‐after studies, interrupted time series with comparison group, prospective cohort studies and retrospective cohort studies. For these non‐RCT studies, we considered a minimum total number of 50 participants to be compared as necessary for inclusion. Prioritised outcomes were all‐cause mortality, clinical improvement or worsening, quality of life, rate of (serious) adverse events and rate of pneumothorax. Additional outcomes were need for tracheostomy, duration of ICU length of stay and duration of hospitalisation.
Data collection and analysis
We followed the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions.
Two review authors independently screened all studies at the title/abstract and full‐text screening stage. We also planned to conduct data extraction and risk of bias assessment in duplicate. We planned to conduct meta‐analysis for each prioritised outcome, as well as subgroup analyses of mortality regarding severity of oxygenation impairment and duration of ARDS. In addition, we planned to perform sensitivity analyses for studies at high risk of bias, studies using NMBA in addition to deep sedation level to avoid spontaneous breathing and a comparison of preprints versus peer‐reviewed articles. We planned to assess the certainty of evidence using the GRADE approach.
Main results
We identified no eligible studies for this review.
Authors' conclusions
We found no direct evidence on whether early spontaneous breathing in SARS‐CoV‐2‐induced ARDS is beneficial or detrimental to this particular group of patients. RCTs comparing early spontaneous breathing with ventilatory strategies not allowing for spontaneous breathing in SARS‐CoV‐2‐induced ARDS are necessary to determine its value within the treatment of severely ill people with COVID‐19. Additionally, studies should aim to clarify whether treatment effects differ between people with SARS‐CoV‐2‐induced ARDS and people with non‐SARS‐CoV‐2‐induced ARDS.
Plain language summary
Spontaneous breathing activity in COVID‐19‐related lung failure
Is early spontaneous breathing beneficial in the treatment of lung failure in individuals with COVID‐19?
People with severe COVID‐19 can present with lung failure, which is called acute respiratory distress syndrome (ARDS). This requires invasive mechanical ventilation through a breathing tube. It is possible to allow breathing, triggered by the patient (called spontaneous breathing), whilst being on a ventilator. However, it is unclear whether this is beneficial for such individuals, especially in the early phase of ventilation.
Key messages
We found no evidence if spontaneous breathing is beneficial in the treatment of lung failure due to COVID‐19.
What are the advantages of early spontaneous breathing in ARDS?
The advantage of spontaneous breathing during mechanical ventilation is the preserved movement of the diaphragm (the major muscle for breathing located under the lungs). It leads to better distribution of the inhaled air, especially in the pulmonary alveoli (small air sacs within the lungs) close to the diaphragm. In general, ventilation procedures with possible spontaneous breathing require lower doses of sedatives (which slow down brain activity). Since these can cause low blood pressure, it can additionally reduce the administration of cardiovascular medicines.
Can early spontaneous breathing be harmful in the treatment of ARDS?
During spontaneous breathing under mechanical ventilation, increased pressure fluctuations in the lungs may occur. Increased pressure difference within the lung is the main cause of ventilator‐associated lung injury.
What is the alternative to using early spontaneous breathing?
Spontaneous breathing may be suppressed by increased sedation or blockade of the nerves innervating muscles by medicines that allow for breathing (called neuromuscular blockade). The advantage of complete ventilator‐based breathing is a lower oxygen consumption of the muscles and the reduced risk of self‐inflicted lung injury.
What did we want to find out?
We wanted to evaluate the benefits and harms of early spontaneous breathing activity in ventilated people with COVID‐19 with ARDS compared to ventilation strategies that avoid spontaneous breathing.
What did we do?
We searched for studies that compared early spontaneous breathing during invasive mechanical ventilation with mandatory invasive ventilation and neuromuscular blockade in people with ARDS related to COVID‐19. People could have been any age, sex or ethnicity.
What did we find?
After systematic search, we found no records that met the inclusion criteria.
Main results
We identified no eligible studies for this review.
What are the limitations of the evidence?
To date, there are no studies that have compared early spontaneous breathing during invasive mechanical ventilation to mandatory invasive ventilation without spontaneous breathing in people with ARDS related to COVID‐19.
How up‐to‐date is this evidence?
Our evidence is up‐to‐date to 2 March 2022.
Background
This work is part of a series of Cochrane Reviews investigating treatments and therapies for coronavirus disease 2019 (COVID‐19). Reviews of this series share information in the background section and methodology based on published reviews about remdesivir (Ansems 2021), monoclonal antibodies (Kreuzberger 2021), and convalescent plasma (Chai 2020), and are part of the German research project "CEOsys" (COVID‐19 Evidence‐Ecosystem; CEOsys 2021).
Description of the condition
COVID‐19 is a rapidly spreading infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). On 11 March 2020, the World Health Organization (WHO) declared the current COVID‐19 outbreak a pandemic (WHO 2020a). COVID‐19 is unprecedented in comparison to previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) with 774 deaths and Middle East respiratory syndrome (MERS) with 858 deaths (WHO 2015; WHO 2019). Despite intensive international efforts to contain its spread, as of April 2022, there have been over 494 million confirmed cases, including over 6.1 million deaths (WHO 2022a). In the meantime, the appearance of SARS‐CoV‐2 variants (such as B.1.1.7 (Alpha), first identified in the UK in late 2020, B.1.617.2 lineage (Delta), first identified in India in December 2020 and B.1.1.529 lineage (Omicron), first identified in South Africa in November 2021) with higher transmissibility further increases infection rates (ECDC 2020; WHO 2020b; PHE 2021; WHO 2022b).
The median incubation time is estimated to be five to six days, and 97.5% of symptomatic cases develop symptoms within 11.5 days of exposure (Lauer 2020). Sore throat, cough, fever, headache, fatigue, and myalgia or arthralgia are the most commonly reported symptoms (Struyf 2021). Other symptoms include dyspnoea, chills, nausea or vomiting, diarrhoea, nasal congestion or loss of smell. Most infected people have mild symptoms (approximately 80%, Wu 2020), or remain asymptomatic (Buitrago‐Garcia 2020). A smaller proportion is affected by severe (approximately 11% to 20%) or critical (approximately 1% to 5%) disease with hospitalisation and intensive care unit (ICU) admittance due to respiratory failure, septic shock or multiple organ dysfunction syndrome (Wu 2020; Funk 2021).
In one case series from 12 hospitals in New York (USA), 14% of people hospitalised due to COVID‐19 were treated in ICU (Richardson 2020). Evaluations of people during the first COVID‐19 wave in Germany showed an estimate of 14% to 37% of this proportion (Schilling 2020; Tolksdorf 2020). In one observational study of 10,021 hospitalised adults in Germany with a confirmed COVID‐19 diagnosis, 17% received mechanical ventilation (non‐invasive and invasive; Karagiannidis 2020). One large meta‐analysis including 45 studies with 16,561 patients from 17 countries showed that approximately 76.1% of all patients admitted to the ICU were diagnosed with acute respiratory distress syndrome (ARDS), and 67.7% required invasive mechanical ventilation (IMV) (Tan 2021). Mortality rates are high in those most seriously ill people with COVID‐19. In two systematic reviews and meta‐analyses of international studies, the proportion of patients who died among those treated in ICU was estimated at 28% to 34% and among of those receiving IMV at 83% (Potere 2020; Tan 2021).
ARDS is an acute deterioration of lung function caused by a variety of pathological states. Most often, bacterial infection triggers ARDS, but also viral (e.g. influenza A virus) or fungal infection, trauma, autoimmune disease (e.g. granulomatosis with polyangiitis), or incorrect IMV may cause ARDS. The clinical picture of ARDS is relatively uniform regardless of which pathological entity triggered the deterioration of lung function and is generally defined by the Berlin definition of ARDS from 2012 (The ARDS Definition Task Force 2012): ARDS is a syndrome with acute onset (less than one week from clinical insult to new or worsened respiratory function), bilateral radiological opacities, non‐cardiac pulmonary oedema and decreased oxygenation.
Description of the intervention
Enabling early spontaneous breathing (within 48 hours after onset of ARDS) in people who are invasively ventilated, combining appropriate ventilator settings with avoidance of neuromuscular blockade or deep sedation, or both.
Basic ventilatory strategies for people with ARDS are relatively standardised: IMV with sufficiently high positive end‐expiratory pressure (PEEP) levels, sufficiently low peak inspiratory pressure (PIP) levels, low driving pressure levels, low tidal volumes, and fraction of inspiratory oxygen (FiO2) as low as possible with a target arterial oxygen saturation (SaO2) of 92% to 96% (Fichtner 2018; Fichtner 2019; Gottlieb 2022).
For other aspects of IMV it is less clear how to treat people with severe ARDS. The optimal amount and proportion of time of spontaneous breathing activity whilst being mechanically ventilated is particularly unclear, and results from randomised controlled trials (RCTs) show conflicting data. While increased spontaneous breathing may reduce ventilation‐perfusion mismatch and reduce diaphragm atrophy, it may also increase oxygen consumption and promote self‐inflicted lung injury (Meyer 2021). The use of neuromuscular blocking agents (NMBA) serves as a surrogate for ventilatory strategies that avoid spontaneous breathing during IMV. Treatment with NMBA also requires deep sedation due to total muscle relaxation in an otherwise awake patient. Contradictory data from RCTs on people with early severe non‐COVID‐19‐ARDS led to inconsistent guideline recommendations and heterogeneity in clinical routine treatment concepts related to spontaneous breathing of people receiving IMV (Papazian 2010; Moss 2019). Another ongoing Cochrane Review addresses the use of NMBA in non‐SARS‐CoV‐2‐induced ARDS separately (Kuriyama 2021).
How the intervention might work
While spontaneous breathing during IMV is enabled by reduced sedation and avoidance of neuromuscular blocking, spontaneous breathing is suppressed by increased sedation or pharmacological neuromuscular blocking.
During spontaneous breathing while connected to a ventilator, diaphragm innervation and contraction are preserved, putatively reducing diaphragm atrophy. Furthermore, airflow into the diaphragm adjacent alveoli may be facilitated by active diaphragm contractions, reducing ventilation‐perfusion mismatch. Since suppression of spontaneous breathing needs deeper sedation or neuromuscular blocking, enabling spontaneous breathing reduces need for sedation and therefore shortens the duration of IMV due to accelerated weaning.
In contrast, spontaneous breathing is equivalent to active muscle contraction and consequently may increase consumption and subsequently demand of oxygen, which may already be compromised due to ARDS conditions. Furthermore, it may be hypothesised that active contractions of the diaphragm decrease intrathoracic pressure with consecutive pulmonary oedema and increased biotrauma of the alveoli inducing self‐inflicted lung injury.
In people with SARS‐CoV‐2 infection experiencing severe‐to‐critical COVID‐19, moderate‐to‐severe ARDS is the hallmark of the clinical picture. Therefore, all controversies regarding ventilatory modes and settings emerge for SARS‐CoV‐2‐induced ARDS. As a consequence, since ARDS represents a syndrome, not a single pathological entity, the amount of heterogeneity of ARDS may be high, and it has been hypothesised that ARDS caused by SARS‐CoV‐2 differs substantially from non‐SARS‐CoV‐2‐induced ARDS (Meyer 2021). In part, this resulted in experience‐based treatment recommendations for SARS‐CoV‐2‐induced ARDS, which are openly contradictory to clinical guidelines and evidence (Marini 2020).
Therefore, for people with SARS‐CoV‐2‐induced ARDS, it is unclear whether it is different from non‐SARS‐CoV‐2‐induced ARDS to an extent that either allows or requires major deviation from evidence‐based guidelines or if these guidelines, which provide treatment strategies for ARDS due to all known triggers besides SARS‐CoV‐2, should be adhered to. For SARS‐CoV‐2‐triggered ARDS, some experts recommend spontaneous breathing not before the "very end of the weaning process" (Marini 2020). In contrast, according to the German evidence‐based guidelines for people without severe ARDS early enabling and supporting spontaneous breathing is suggested regardless of the trigger of the respiratory insufficiency (Fichtner 2018; Fichtner 2019).
For people with SARS‐CoV‐2‐induced ARDS, the frequency of early NMBA use as a surrogate for strategies suppressing spontaneous breathing varies, ranging from 25% of all people with SARS‐CoV‐2‐induced ARDS in one observational study from New York City, USA to 88% in a cohort from France, Belgium and Switzerland (Cummings 2020; Schmidt 2021). To date, observational data on rates of spontaneously breathing people undergoing IMV as well as data on ventilator settings is scarce and can, therefore, only be roughly estimated by the number of people not exposed to NMBA or deep sedation.
Why it is important to do this review
IMV is the cornerstone of ARDS treatment, but can also aggravate ARDS. Therefore, ventilatory therapy should be cautiously applied.
The amount and proportion of time during IMV devoted to spontaneous breathing is unclear: while there is evidence for all patients with respiratory insufficiency, mild and moderate ARDS to support spontaneous breathing, for severe ARDS no such recommendation can be given due to lack of direct evidence and conflicting indirect evidence from studies on NMBA use in early severe ARDS. For the new SARS‐CoV‐2‐induced ARDS, some experts recommend following the guidelines for ARDS triggered by all non‐SARS‐CoV‐2 causes, while other experts recommend avoiding all spontaneous breathing efforts during IMV (e.g. using NMBA).
Therefore, this systematic review aimed to assess the benefits and harms of early spontaneous breathing in SARS‐CoV‐2‐induced ARDS compared to deep sedation and NMBA usage during ventilatory therapy.
Objectives
To assess the benefits and harms of early spontaneous breathing activity in invasively ventilated people with COVID‐19 with ARDS compared to ventilation strategies that avoid spontaneous breathing.
Methods
Criteria for considering studies for this review
Types of studies
The main description of methods is based on the standard template of the Cochrane Haematology review group and is in line with a series of Cochrane Reviews investigating treatments and therapies against COVID‐19 as part of German Evidence Ecosystem CEOsys. Specific adaptions related to the research question were made if necessary. We adhered to the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021a). The protocol for this review was registered with PROSPERO on 31 March 2021 (Frank 2021).
Given that RCTs, if conducted appropriately, provide the best evidence for experimental therapies in highly controlled therapeutic settings, we considered them for inclusion. We had planned to include non‐standard RCT designs, such as cluster‐randomised studies (Higgins 2021b) and cross‐over studies. In cross‐over studies, we would have only considered results from the first period before cross‐over because COVID‐19 is not a chronic condition, and its exact course and long‐term effects are yet to be defined. Based on a pilot search for literature, we expected to identify few RCTs. Therefore, we chose to additionally include quasi‐RCTs, controlled before‐after studies, interrupted time series with comparison group, prospective cohort studies and retrospective cohort studies as eligible study types. For non‐RCTs, we considered a minimum total number of 50 participants to be compared as necessary for inclusion.
We would have included the following formats, if sufficient information was available on study design, characteristics of participants, interventions and outcomes:
full‐text publications;
preprint articles;
abstract publications;
results published in trials registries.
Types of participants
We considered studies with adults on IMV with SARS‐CoV‐2‐induced ARDS, and we did not exclude any studies based on gender, ethnicity, disease severity or setting.
If studies enrolled only a subset of relevant participants, we planned to include the relevant study only if data specific to this subgroup were available.
Types of interventions
We considered the following interventions:
light sedation without usage of neuromuscular blockade combined with modes of mechanical ventilation, enabling or supporting spontaneous breathing activity of the participant (minute volume support (e.g. biphasic positive airway pressure), tidal volume support (e.g. pressure support ventilation) and adaptive support (e.g. adaptive support ventilation)) early in the course of ARDS.
We considered the following comparisons:
mandatory ventilation mode combined with deep sedation level or neuromuscular blockade.
Types of outcome measures
Primary outcomes
We evaluated core outcomes in accordance with the Core Outcome Measures in Effectiveness Trials Initiative for people with COVID‐19 (COMET 2021; WHO 2020c), and additional outcomes that were prioritised by consumer representatives and the German guideline panel for SARS‐CoV‐2 inpatient therapy. Prioritised outcomes are underlined.
Effectiveness of spontaneous breathing activity
All‐cause mortality (at up to day 28, day 60, in ICU, in hospital and longest follow‐up (time‐to‐event estimate)).
-
Clinical improvement or worsening.
Ventilator‐free days within 28 and 60 days.
Time to liberation from IMV.
New need for extracorporeal membrane oxygenation (ECMO) therapy.
Need for tracheostomy.
Duration of ICU stay or time to discharge from ICU.
Duration of hospitalisation or time to discharge from hospital.
Quality of life, including fatigue and functional independence; assessed with standardised scales (e.g. WHOQOL‐100) at longest follow‐up available.
Safety of spontaneous breathing activity
Adverse events, any grade (defined as number of participants with event of any grade).
Serious adverse events (defined as number of participants with event).
Incidence of pneumothorax.
If we had found studies with other outcomes, we would have considered them for narrative review only.
Timing of outcome measurement
In case of time‐to‐event analysis (e.g. for time to discharge from hospital and time to mortality), we included the outcome measure based on the longest follow‐up time. We also collected information on outcomes from all other time points reported in the publications. We considered adverse events occurring during active treatment as well as long‐term adverse events occurring after active treatment.
Secondary outcomes
We considered no other outcomes.
Search methods for identification of studies
Electronic searches
Our information specialists (MIM, AV) searched the following COVID‐19‐specific electronic databases without restrictions from their inception to 2 March 2022 (date of last search for both databases):
-
Cochrane COVID‐19 Study Register (CCSR) (www.covid-19.cochrane.org), comprising:
PubMed, weekly updates;
Embase.com, weekly updates;
ClinicalTrials.gov (www.clinicaltrials.gov), daily updates;
World Health Organization International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch), weekly updates;
medRxiv (www.medrxiv.org), weekly updates;
Cochrane Central Register of Controlled Trials (CENTRAL), monthly updates;
WHO COVID‐19 Global literature on coronavirus disease (search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/).
In addition, systematic reviews were identified via the US Department for Veterans Affairs Evidence Synthesis Program (www.covid19reviews.org/), Epistemonikos (www.epistemonikos.org/), and by using PubMed's similar article algorithm and exporting the first 10 similar articles of eight known relevant systematic reviews.
For the full search strategies of all databases, see Appendix 1.
Searching other resources
We searched for other potentially eligible studies by searching the reference lists of included studies and relevant systematic reviews.
Data collection and analysis
Selection of studies
Teams of two review authors (FH, CH, JF, NO, CG, SD, AK) independently screened the titles and abstracts of all retrieved studies using Covidence. In the case of disagreement or if the relevance was unclear, we progressed the study to full‐text screening. The teams of two review authors then assessed the full‐text articles of those studies deemed potentially relevant. If the two review authors were unable to reach a consensus, they consulted the review authors FF and SL to reach a final decision.
We documented the study selection process in a flow chart, as recommended in the PRISMA statement (Moher 2009), outlining the total numbers of references retrieved and the numbers of included and excluded studies. We listed all studies that we excluded during full‐text screening and the reasons for their exclusion in the Characteristics of excluded studies table.
Data extraction and management
We planned to extract data according to the guidelines proposed by Cochrane (Li 2021). Two review authors planned to independently extract data from each study and in duplicate, using a customised data extraction form developed in Microsoft Excel (Microsoft 2018). Any disagreements were to be resolved by discussion or by consulting a third review author if necessary.
We planned extract the following information, where reported.
General information: author, title, source, publication date, country, language, duplicate publications.
Study characteristics: trial design, setting, and dates; source of participants; inclusion/exclusion criteria; comparability of groups; treatment cross‐overs; compliance with assigned treatment; length of follow‐up.
Participant characteristics: age, gender, ethnicity, number of participants recruited/allocated/evaluated, additional diagnoses, severity of disease, previous treatments, concurrent treatments, comorbidities (e.g. diabetes, respiratory disease, hypertension, immunosuppression, obesity, heart failure).
Interventions: ventilation mode and parameter setting, clinically assessed spontaneous breathing activity, documented low sedation level.
Control interventions: ventilation mode and parameter setting, dosage and duration of NMBA, type of NMBA.
Outcomes: as specified in Types of outcome measures section.
Risk of bias assessment: randomisation process, deviations from the intended interventions, missing outcome data, measurement of the outcome, selection of the reported result.
Assessment of risk of bias in included studies
Two review authors planned to independently assess the included studies for methodological quality and risk of bias. If the review authors were unable to reach a consensus, a third review author was to be consulted.
We planned to use the RoB 2 tool to assess the risk of bias of included RCTs (Sterne 2019). The tool allows for the assessment of the following types of bias, as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021b):
bias arising from the randomisation process;
bias due to deviations from the intended interventions;
bias due to missing outcome data;
bias in measurement of the outcome;
bias in selection of the reported result.
For cross‐over studies, we planned to use the RoB 2 tool as described above, as we would have only considered results from the first period before cross‐over, making this akin to a parallel RCT. For cluster‐RCTs, we planned to add a domain to assess bias arising from the timing of identification and recruitment of participants in relation to the timing of randomisation (Eldridge 2021; Higgins 2021b).
For all signalling questions, the following judgement options were available.
Yes: if there was firm evidence that the question was fulfilled in the study (i.e. the study was at low or high risk of bias for the given the direction of the question).
Probably yes: a judgement was made that the question was fulfilled in the study (i.e. the study was at low or high risk of bias given the direction of the question).
No: if there was firm evidence that the question was unfilled in the study (i.e. the study was at low or high risk of bias for the given the direction of the question).
Probably no: a judgement was made that the question was unfilled in the study (i.e. the study was at low or high risk of bias given the direction of the question).
No information: if the study report did not provide sufficient information to allow any judgement.
The algorithms within RoB 2 allow for assigning each domain one of the following levels of bias.
Low risk of bias.
Some concerns.
High risk of bias.
Subsequently, the tool comprises an overall risk of bias judgement for each prespecified outcome in each study in accordance with the following suggestions.
Low risk of bias: we judged the trial at low risk of bias for all domains for this result.
Some concerns: we judged the trial to raise some concerns in at least one domain for this result, but not at high risk of bias for any domain.
High risk of bias: we judged the trial at high risk of bias in at least one domain for the result, or we judged the trial to have some concerns for multiple domains in a way that substantially lowered confidence in the results.
For this review, we considered the effect of the assignment to the intervention (the intention‐to‐treat (ITT) effect), thus, we planned to perform all assessments with RoB 2 on this effect. The outcomes relevant for assessment were those featured in the summary of findings table. We planned to use the RoB 2 Excel tool to implement RoB 2 (available on the riskofbias.info website); however, we identified no relevant studies.
For non‐RCTs, we planned to use the Risk of Bias in Non‐randomised Studies of Interventions (ROBINS‐I) tool (Sterne 2016; Sterne 2020). ROBINS‐I allows for the assessment of the following domains.
Bias due to confounding.
Bias in selection of participants into the study.
Bias in classification of interventions.
Bias due to deviations from intended interventions.
Bias due to missing data.
Bias in measurement of outcomes.
Bias in selection of the reported result.
For each signalling question, the following options are available.
Yes.
Probably yes.
Probably no.
No.
No information.
The tool also comprises an overall risk of bias judgement for each prespecified outcome.
Low risk.
Moderate risk.
Serious risk.
Critical risk.
Measures of treatment effect
For continuous outcomes, we were interested in the mean, standard deviation and total number of participants in both treatment and control groups, which could then have been used to calculate either the mean difference (MD; had studies used the same scale) or the standardised mean difference (SMD; had studies used different scales).
For dichotomous outcomes, we were interested in the number of events and total number of participants in both treatment and control groups, which could then have been used to calculate the risk ratio (RR).
If available, we planned to extract and report hazard ratios (HRs) for time‐to‐event outcomes (e.g. time to death) and the corresponding 95% confidence intervals.
Unit of analysis issues
The aim of this review was to summarise trials that analysed data at the level of the individual. However, had we identified relevant studies at the cluster‐level, we would have ensured that these accounted for clustering in their design or analysis or both. If studies comparing more than two relevant arms had been identified, we would have assessed whether to combine multiple treatment groups, if they were sufficiently homogeneous, or to compare each treatment group with the comparator separately. The latter would have required splitting the comparator group, so as to avoid unit of analysis issues (Higgins 2021b).
Dealing with missing data
Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions suggests several potential sources for missing data: at study level, at outcome level and at summary data level (Deeks 2021). At all levels, it is important to differentiate between data 'missing at random', which may often be unbiased, and 'not missing at random', which may bias study and thus review results.
In case of missing data which precluded the assessment of eligibility, the assessment of risk of bias or the incorporation of a study into the data synthesis, we planned to contact the authors via email or telephone. With regard to the data synthesis, we planned to analyse only reported data (i.e. we did not plan to impute missing outcome data).
Assessment of heterogeneity
We planned to assess the heterogeneity of treatment effects between trials using a Chi² test (P < 0.05 considered significant), the I² statistic (Higgins 2003) (I² statistic > 30% to signify moderate heterogeneity, I² statistic > 75% to signify considerable heterogeneity; Deeks 2021) and visual examination.
Assessment of reporting biases
We searched trials registries to identify completed trials that have not been published elsewhere, to minimise or determine publication bias. We intended to explore potential publication bias by generating a funnel plot and statistically testing this by conducting a linear regression test for meta‐analyses involving at least 10 trials (Sterne 2019). We would have considered P < 0.1 as significant for this test. Where we suspected publication bias due to small‐study effects, we planned to conduct sensitivity analyses to assess whether these small studies were disproportionately driving results.
Data synthesis
If the clinical and methodological characteristics of individual studies were sufficiently homogeneous, we planned to pool the data in a meta‐analysis, according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021). We planned to use Review Manager Web (RevMan Web) software for analyses (Review Manager Web 2021). One review author would have entered the data into the software, and a second review author planned to check the data for accuracy. When meta‐analysis was feasible, we had planned to use the random‐effects model as we assumed that the intervention effects were related but were not the same for the included studies. For dichotomous outcomes, we would have performed meta‐analyses using the Mantel‐Haenszel method under a random‐effects model to calculate the summary (combined) intervention effect estimate as a weighted average of the intervention effects estimated in the individual studies. For continuous outcomes, we would have used the inverse‐variance method. We planned to present descriptive statistics only, if we deemed meta‐analysis inappropriate for a certain outcome because of heterogeneity or because of serious study limitations leading to considerably high risk of bias.
Subgroup analysis and investigation of heterogeneity
Because of clinical relevance, we planned to perform subgroup analyses of mortality for the following characteristics.
Severity of oxygenation impairment (as oxygen pressure in arterial blood (PaO2)/FiO2 ratio) at baseline (< 100 mmHg; 100 mmHg to 200 mmHg, 201 mmHg to 300 mmHg).
Duration of ARDS (spontaneous breathing activity within 48 hours versus after 48 hours of ARDS onset).
For comparisons with heterogeneity above 80%, where the two planned subgroup analyses did not explain heterogeneity, we had planned to conduct exploratory subgroup analyses to identify potential causes. However, any findings based on these additional subgroup analyses would have been clearly described as exploratory, to avoid any risk of selective reporting of findings.
Sensitivity analysis
We planned to perform sensitivity analyses for the following potential confounders.
Risk of bias domains (studies with a low risk of bias or some concerns versus studies with a high risk of bias).
Studies using NMBA in addition to deep sedation level alone to avoid spontaneous breathing.
Comparison of preprints versus peer‐reviewed articles.
Comparison of premature termination of studies with completed studies.
Additionally, if we suspected that due to publication bias, small‐study effects were biasing the effects estimated in meta‐analysis, we planned to conducted sensitivity analyses comparing the results of random‐effects versus fixed‐effect meta‐analysis (Page 2022).
Summary of findings and assessment of the certainty of the evidence
We (FH, FG, FF, SL, LW, JB) planned to use the GRADE approach for interventions evaluated in RCTs (GRADEpro GDT), as recommended in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021). We planned to resolve disagreements by discussion; if this was unsuccessful, another review author decided.
The GRADE approach uses five domains (risk of bias, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty in the body of evidence for each prioritised outcome.
We planned to potentially downgrade our certainty of evidence for:
serious (–1) or very serious (–2) risk of bias;
serious (–1) or very serious (–2) inconsistency;
serious (–1) or very serious (–2) uncertainty about directness;
serious (–1) or very serious (–2) imprecise or sparse data;
serious (–1) or very serious (–2) probability of reporting bias.
As a result of applying these criteria, the GRADE system allows assignment of the following levels of certainty to a body of evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
According to Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions, the "most critical and/or important health outcomes, both desirable and undesirable, limited to seven or fewer outcomes" should be included in the summary of findings table(s) (Schünemann 2021). We planned to assess the certainty of the outcomes prioritised according to the Core Outcome Set for intervention studies (COMET 2021) and patient‐relevance (critical outcomes visualised by underlining).
Effectiveness of spontaneous breathing activity
All‐cause mortality (at up to day 28, day 60, in ICU, in hospital, and longest follow‐up (time‐to‐event estimate)).
-
Clinical improvement or worsening.
Ventilator‐free days within 28 and 60 days.
Time to liberation from IMV.
New need for ECMO therapy.
Need for tracheostomy.
Duration of ICU stay or time to discharge from ICU.
Duration of hospitalisation or time to discharge from hospital.
Quality of life, including fatigue and functional independence; assessed with standardised scales (e.g. WHOQOL‐100) at longest follow‐up available.
Safety of spontaneous breathing activity
Adverse events, any grade (defined as number of participants with event of any grade).
Serious adverse events (defined as number of participants with event).
Incidence of pneumothorax.
Results
Description of studies
Results of the search
We performed the database searches on 2 March 2022 and identified 1716 records. After removing duplicates, we screened titles and abstracts of 1562 records. We excluded 1502 records that did not meet the inclusion criteria. All of the remaining 60 records were excluded at the full‐text screening stage. Thus, no studies satisfied the eligibility criteria. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1).
1.
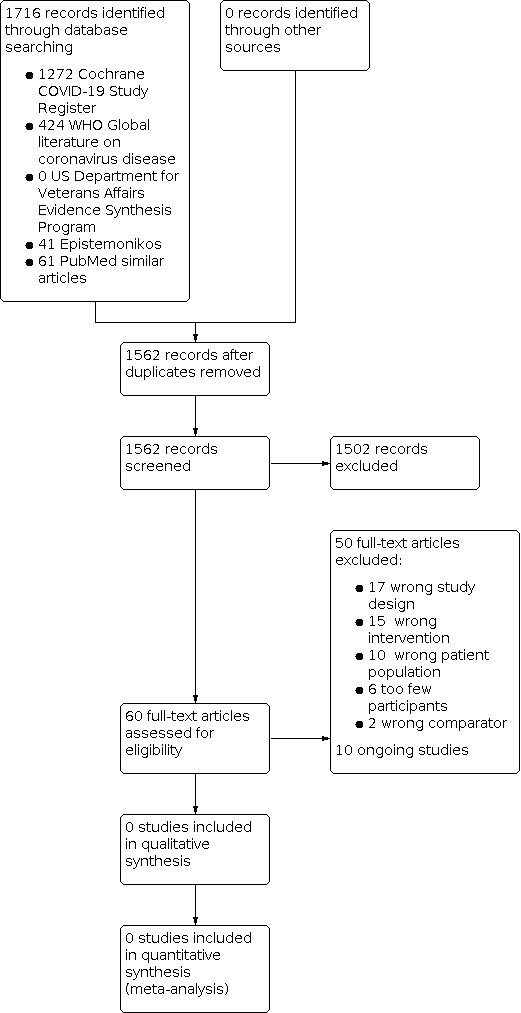
Study flow diagram.
Included studies
We identified no eligible studies for this review.
Excluded studies
We excluded 50 references (50 studies) that did not match our inclusion criteria (more details are provided in the Characteristics of excluded studies table); specifically, they were excluded for the following reasons:
17 studies applied a non‐eligible study design;
15 did not investigate spontaneous breathing;
10 studies did not include people with SARS‐CoV‐2‐induced‐ARDS;
six studies had a small study population (fewer than 50 participants).
two studies did not compare spontaneous breathing modes to controlled ventilation modes.
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
We identified 10 ongoing studies (see Characteristics of ongoing studies table).
Risk of bias in included studies
We identified no eligible studies for this review.
Allocation
We identified no eligible studies for this review.
Blinding
We identified no eligible studies for this review.
Incomplete outcome data
We identified no eligible studies for this review.
Selective reporting
We identified no eligible studies for this review.
Other potential sources of bias
We identified no eligible studies for this review.
Effects of interventions
We identified no eligible studies for this review.
Discussion
The aim of this review was to assess the benefits and harms of early spontaneous breathing during IMV compared to ventilation strategies that avoid spontaneous breathing. In this first version of the review, we identified no eligible studies for inclusion. Therefore, we cannot provide direct evidence on the value of early spontaneous breathing during IMV in SARS‐CoV‐2‐induced ARDS to date.
Clinicians worldwide face being challenged with an unprecedented emergence of a new pathogen causing respiratory failure. Naturally, a debate arises about whether ARDS caused by SARS‐CoV‐2 parallels non‐SARS‐CoV‐2‐induced ARDS in terms of treatment recommendations, amongst these recommendations for or against the use of spontaneous breathing in invasively ventilated people.
ARDS is a syndrome induced by a variety of pathological states, including bacterial, viral and fungal infections, as well as trauma, autoimmune disease or even mechanical strain delivered by invasive ventilation. While treatment of the underlying cause of ARDS can widely differ between the different pathological states, ventilatory strategies for people with ARDS are relatively standardised and more or less uniquely applied in clinical settings. For example, for all forms of ARDS not induced by SARS‐CoV‐2, mechanical ventilation with sufficiently high PEEP levels, sufficiently low PIP levels, low driving pressure levels, low tidal volumes and FiO2 as low as possible with a target SaO2 of 92% to 96% is recommended (Fichtner 2018; Fichtner 2019; Gottlieb 2022).
The amount and proportion of spontaneous breathing during mechanical ventilation is less clear. In one key study from 2010 usage of NMBA in early ARDS resulted in a reduction in mortality (Papazian 2010). This result was not reproduced in further studies. In contrast, some studies could not show any drawbacks of enabling early spontaneous breathing for non‐SARS‐CoV‐2‐induced ARDS (Zhou 2017; Hirshberg 2018; Moss 2019). Further large RCTs investigating spontaneous breathing in people with non‐SARS‐CoV‐2‐induced ARDS are awaiting publication (BiRDS trial: NCT01862016) or ongoing (PReSPON trial: NCT04228471). Evidence‐based guidelines suggest early spontaneous breathing for all patients mechanically ventilated without severe ARDS (Fichtner 2018; Fichtner 2019). For people with severe ARDS, there are conflicting recommendations from evidence‐based guidelines ranging from not recommending for or against early spontaneous breathing to considering different forms of short‐term NMBA usage (Fichtner 2018; Fichtner 2019; Papazain 2019; Alhazzani 2020a). The latter is also suggested by the recent surviving sepsis campaign guidelines on the management of critically ill adults with COVID‐19 based on some guidelines (Alhazzani 2020b). Two trials on early spontaneous breathing in ARDS analysed the subgroup of participants with more severe ARDS (Zhou 2017: PaO2/FiO2 less than 100 mmHg; Moss 2019: oxygenation ratio (PaO2/FiO2) less than 120 mmHg) and found no disadvantages of early spontaneous breathing. Therefore, for non‐SARS‐CoV‐2‐induced ARDS, enabling early spontaneous breathing is either weakly recommended or at least considered not to be harmful by Aslam and colleagues in one recently published narrative review (Aslam 2021).
Early in the SARS‐CoV‐2 pandemic experts hypothesised on two distinct SARS‐CoV‐2‐induced ARDS subtypes based on retrospective and observational data: H‐type and L‐type (Marini 2020). While the H‐type resembled the typical clinical features of ARDS, the L‐type is postulated to be a distinct pathophysiological entity. According to the authors, these entities should be treated differently according to their pulmonary mechanical properties: while the H‐type should be treated (more or less) according to recommendations for "typical" ARDS, the L‐type should be treated with low PEEP levels and more liberal tidal volume (Marini 2020). Furthermore, they suggest that spontaneous breathing should be enabled "only at the very end of the weaning process" (Marini 2020, p. 2330).
More recently, the unique lung injury induced by SARS‐CoV‐2 has been questioned. In one prospective observational pilot study on 27 people with ARDS, SARS‐CoV‐2‐induced ARDS differed from viral but not SARS‐CoV‐2‐induced ARDS in some inflammatory features, but oxygenation ratio, minute ventilation, lung compliance or overall survival were not different between groups, which led the authors to conclude that deviation from evidence‐based recommendations for treatment of ARDS is not justified by these results (Bain 2021).
Grasselli and colleagues compared functional and morphological characteristics in a cohort of 301 people with COVID‐19 to people from the LUNG‐SAFE study (Grasselli 2020). The LUNG‐SAFE study is an observational multicentre, international, prospective cohort study of people with non‐SARS‐CoV‐2 on invasive or non‐invasive ventilation in 459 ICUs from 50 countries (Bellani 2016). The comparison suggests that SARS‐CoV‐2‐induced ARDS and non‐SARS‐CoV‐2‐induced ARDS are similar in many aspects (Grasselli 2020). In one multicentre prospective observational study of 742 invasively ventilated patients, Ferrando 2020 found that SARS‐CoV‐2‐induced ARDS predominantly exhibited the same characteristics as non‐SARS‐CoV‐2‐induced ARDS, including similar mortality. The PRoVENT‐COVID trial from the Netherlands showed that the application of ventilatory strategies for non‐SARS‐CoV‐2‐induced ARDS was feasible, and that clinical characteristics from the included participants did not suggest a unique SARS‐CoV‐2‐induced ARDS phenotype (Botta 2021).
The similarity of SARS‐CoV‐2‐induced ARDS and non‐SARS‐CoV‐2 induced ARDS was affirmed by a Delphi‐based expert consensus statement, which resulted in 86.5% agreement to the statement, "The pathophysiology of C‐ARF [COVID‐19‐related acute respiratory failure] is similar to that of ARDS" and in 100% agreement to the statement "lung protective ventilation should be used for patients with C‐ARF on IMV", which is the hallmark of evidence‐based guidelines on ARDS (Nasa 2021). While the same expert consensus statement contended that NMBAs "may be considered" during the early phase of invasive ventilation, early spontaneous breathing was not referenced.
The German living guideline "recommendations for the therapy of hospitalised patients with COVID‐19" stated – given these trials – that due to lack of randomised trials on ventilatory therapy specifically in COVID‐19, the recommendations for ventilatory strategies are based on the most recently published guidelines for invasive ventilation in acute respiratory failure (Kluge 2021).
Since ARDS is a syndrome induced by a variety of pathological states and SARS‐CoV‐2‐induced ARDS mimics non‐SARS‐CoV‐2‐induced ARDS in many aspects including outcome if treated according to the evidence‐based guidelines for non‐SARS‐CoV‐2‐induced ARDS, it seems reasonable that SARS‐CoV‐2‐induced ARDS represents a subset of ARDS, not a unique, standalone pathological entity, albeit this classification is still subject to an ongoing debate.
Nevertheless, facing the lack of distinct trials that investigate specific ventilatory strategies including spontaneous breathing and the use of NMBAs in SARS‐CoV‐2‐induced ARDS it is paramount that trials in SARS‐CoV‐2‐induced ARDS be conducted so that these remaining uncertainties and debates can be clarified, and so that patients can receive the most effective treatment.
Summary of main results
We identified no eligible studies for this review. Therefore, we found no direct evidence on whether early spontaneous breathing in SARS‐CoV‐2‐induced ARDS is beneficial or detrimental to this particular group of patients. For this reason, we can make no recommendations for or against the use of early spontaneous breathing in SARS‐CoV‐2‐induced ARDS until further studies are published.
Overall completeness and applicability of evidence
We identified no eligible studies for this review in a complete search for evidence.
Quality of the evidence
We identified no eligible studies for this review.
Potential biases in the review process
We identified no eligible studies for this review.
Agreements and disagreements with other studies or reviews
We identified no eligible studies for this review.
Authors' conclusions
Implications for practice.
We found no direct evidence on whether early spontaneous breathing in SARS‐CoV‐2‐induced acute respiratory distress syndrome (ARDS) is beneficial or detrimental to this particular group of patients.
Implications for research.
There is no direct evidence of the impact of early spontaneous breathing in SARS‐CoV‐2‐induced ARDS on clinical outcomes. Because ARDS is a syndrome and not a unique pathological entity, it is possible that early spontaneous breathing in SARS‐CoV‐2‐induced ARDS may have different beneficial or adverse effects compared with non‐SARS‐CoV‐2‐induced ARDS. Furthermore, it is also possible that SARS‐CoV‐2‐induced ARDS disaggregates into different disease entities or disease stages, which might show different effects induced by early spontaneous breathing. Therefore, randomised controlled trials (RCT) comparing early spontaneous breathing with ventilatory strategies not allowing for spontaneous breathing in people with SARS‐CoV‐2‐induced ARDS are necessary to answer these questions. Such RCTs could also assess whether the specific SARS‐CoV‐2 disease state influences the treatment effect; if people with non‐SARS‐CoV‐2‐induced ARDS were also included, these could additionally aim to clarify whether treatment effects differ between people with SARS‐CoV‐2‐induced ARDS and people with non‐SARS‐CoV‐2‐induced ARDS.
Acknowledgements
This work is part of a series of reviews investigating treatments and therapies for COVID‐19 as part of the project CEOsys. Text passages in the Background section (e.g. Description of the intervention and Why it is important to do this review) are shared between reviews of this series. We thank the authors of the first published reviews of this series for providing and sharing this information. Moreover, we thank the Cochrane Haematology working group for use of the template for the description of methods.
The research was part of a project supported by the German Federal Ministry of Education and Research (NaFoUniMedCovid19, funding number: 01KX2021; part of the project CEOsys). The contents of this document reflect only the review authors' views, and the German Ministry is not responsible for any use that may be made of the information it contains.
FH, FG, CH, NO, JF, MG, AK, VT, FF, and SL are thankful for the support of their colleagues within the Department of Anaesthesia and Intensive Care at the University of Leipzig Medical Centre whilst working on the manuscript of this review during the COVID‐19 pandemic.
Editorial and peer‐reviewer contributions
The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Harald Herkner, Co‐ordinating Editor of the Cochrane Emergency and Critical Care Group.
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Anne‐Marie Stephani, Cochrane Central Editorial Service.
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service.
Copy Editor (copy editing and production): Anne Lawson, Copy Edit Support, Cochrane.
Peer‐reviewers (provided comments and recommended an editorial decision): Takeshi Yoshida, MD, PhD Associate Professor, Department of Anesthesiology and Intensive Care Medicine, Osaka University Graduate School of Medicine (clinical review); Sharon Einav, MD, MCE, Shaare Zedek Medical Center Intensive Care Unit and Hebrew University Faculty of Medicine, Jerusalem Israel (clinical review); Ruowen Jiang, Xi'an fifth Hospital (consumer review); Rachel Richardson, Cochrane Evidence Production and Methods Directorate (methods review); Robin Featherstone, Cochrane Central Editorial Service (search review).
Appendices
Appendix 1. Search strategies
Primary literature
Cochrane COVID‐19 Study Register (CCSR)
"neuromuscular blockade" OR "neuromuscular blockades" OR "neuromuscular blockage" OR "neuromuscular blocking" OR "neuromuscular blocked" OR "neuromuscular blocker" OR "neuromuscular blockers" OR "neuromuscular block" OR "neuromuscular nondepolarizing agent" OR "neuromuscular nondepolarizing agents" OR "muscle relaxant" OR "muscle relaxation" OR "muscle relaxants" OR "neuromuscular blocking agent" OR "neuromuscular blocking agents" OR succinylcholin OR suxamethonium OR rocuronium OR atracurium OR cisatracurium OR "assisted ventilation" OR "supported ventilation" OR "pressure support ventilation" OR "PSV" OR "assisted spontaneous breathing" OR "ASB" OR "synchronized intermittent mandatory ventilation" OR "SIMV" OR "synchronised intermittent mandatory ventilation" OR "A/C ventilation" OR "AC ventilation" OR "Assist Control Ventilation" OR "intermittent positive pressure ventilation" OR "mandatory ventilation" OR "controlled ventilation" OR "VCV" OR "PCV" OR "IPPV" OR "positive pressure respiration" OR "controlled mechanical ventilation" OR "mandatory mechanical ventilation" OR "controlled positive pressure ventilation" OR "mandatory positive pressure ventilation" OR "controlled PPV" OR "mandatory PPV" OR "controlled invasive ventilation" OR "mandatory invasive ventilation" OR "pressure regulated volume control" OR "PRVC" OR "airway pressure release ventilation" OR "APRV" OR "biphasic positive airway pressure" OR "bipap" OR "Bi Level" OR "bi vent" OR "duoPAP" OR "biphase" OR "bilevel" OR "bi pap" OR "bi phasic positive airway pressure" OR "smartcare" OR "smartcare/ps" OR "automated weaning" OR "closed loop" OR "adaptive support ventilation" OR "ASV" OR "intellivent" OR "Proportional Assist Ventilation" OR "Proportional Assisted Ventilation" Or "Proportional Assist Ventilator" OR "pav" OR "neurally adjusted ventilatory assist" OR "neurally adjusted ventilator" OR "nava" OR "automatic tube compensation" OR "atc" = 1272 records
World Health Organization COVID‐19 Global literature on coronavirus disease Title, abstract, subject: "neuromuscular blockade" OR "neuromuscular blockades" OR "neuromuscular blockage" OR "neuromuscular blocking" OR "neuromuscular blocked" OR "neuromuscular blocker" OR "neuromuscular blockers" OR "neuromuscular block" OR "neuromuscular nondepolarizing agent" OR "neuromuscular nondepolarizing agents" OR "muscle relaxant" OR "muscle relaxation" OR "muscle relaxants" OR "neuromuscular blocking agent" OR "neuromuscular blocking agents" OR succinylcholin OR suxamethonium OR rocuronium OR atracurium OR cisatracurium OR "assisted ventilation" OR "supported ventilation" OR "pressure support ventilation" OR "PSV" OR "assisted spontaneous breathing" OR "ASB" OR "synchronized intermittent mandatory ventilation" OR "SIMV" OR "synchronised intermittent mandatory ventilation" OR "A/C ventilation" OR "AC ventilation" OR "Assist Control Ventilation" OR "intermittent positive pressure ventilation" OR "mandatory ventilation" OR "controlled ventilation" OR "VCV" OR "PCV" OR "IPPV" OR "positive pressure respiration" OR "controlled mechanical ventilation" OR "mandatory mechanical ventilation" OR "controlled positive pressure ventilation" OR "mandatory positive pressure ventilation" OR "controlled PPV" OR "mandatory PPV" OR "controlled invasive ventilation" OR "mandatory invasive ventilation" OR "pressure regulated volume control" OR "PRVC" OR "airway pressure release ventilation" OR "APRV" OR "biphasic positive airway pressure" OR "bipap" OR "Bi Level" OR "bi vent" OR "duoPAP" OR "biphase" OR "bilevel" OR "bi pap" OR "bi phasic positive airway pressure" OR "smartcare" OR "smartcare/ps" OR "automated weaning" OR "closed loop" OR "adaptive support ventilation" OR "ASV" OR "intellivent" OR "Proportional Assist Ventilation" OR "Proportional Assisted Ventilation" Or "Proportional Assist Ventilator" OR "pav" OR "neurally adjusted ventilatory assist" OR "neurally adjusted ventilator" OR "nava" OR "automatic tube compensation" OR "atc" = 424 records
Evidence syntheses
PubMed Similar Articles Search
10 first records for PMIDs: 30379668, 33095344, 30949778, 28936695, 28013329, 31112383, 32066488, 33444180 = 66 records
US VA Evidence Synthesis Program Covid‐19 Reviews
searched each term separately: "breathing"; "neuromuscular" = 0 relevant records
Epistemonikos
title, abstract:
("neuromuscular block*" OR "muscle relax*") AND ("acute respiratory" OR ARDS) ("neuromuscular block*" OR "muscle relax*") AND (COVID OR COVID19) (spontan* AND breathing) AND (COVID OR COVID19) (spontan* AND breathing) AND ("acute respiratory" OR ARDS) = 35 records
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agarwal 2013 | Wrong patient population |
| Angeles 2020 | Wrong intervention |
| Ball 2021 | Wrong intervention |
| Becher 2019 | Wrong patient population |
| Bonny 2020 | Wrong intervention |
| Buiteman‐Kruizinga 2020 | Wrong study design |
| Buiteman‐Kruizinga 2021 | Wrong study design |
| Caley 2021 | Wrong study design |
| Chelly 2020 | Wrong comparator |
| Cotton 2021 | Wrong intervention |
| Courcelle 2020 | Wrong intervention |
| Devlin 2022 | Wrong study design |
| Diniz‐Silva 2020 | Wrong comparator |
| Ego 2021 | Wrong study design |
| Esnault 2020 | Wrong study design |
| Gainnier 2004 | Wrong patient population |
| Gao 2022 | Wrong patient population |
| Groetzinger 2016 | Wrong intervention |
| Guervilly 2017 | Too few participants |
| Ingebrigtson 2021 | Wrong intervention |
| Jain 2021 | Too few participants |
| Kallet 2018 | Wrong intervention |
| Karayiannis 2021 | Wrong study design |
| Knafelj 2021 | Too few participants |
| Kressin 2021 | Too few participants |
| Lee 2022 | Wrong study design |
| Li 2017 | Wrong patient population |
| Li Bassi 2021 | Wrong intervention |
| Lyu 2014 | Wrong patient population |
| Mauri 2020 | Wrong intervention |
| McCue 2020 | Wrong study design |
| Needham 2012 | Wrong patient population |
| Papazian 2010 | Wrong patient population |
| Perinkulam Sathyanarayanan 2021 | Too few participants |
| Renes 2020 | Wrong study design |
| Rizvi 2021 | Wrong study design |
| Rodrigo Castroviejo 2021 | Wrong study design |
| Ruan 2021 | Wrong study design |
| Sella 2020 | Wrong intervention |
| Serrano 2020 | Wrong study design |
| Song 2016 | Wrong patient population |
| Tsolaki 2020 | Wrong intervention |
| van der Zee 2020 | Wrong intervention |
| Villar 2006 | Wrong intervention |
| Vine 2021 | Wrong study design |
| Wongtangman 2021 | Wrong intervention |
| Wu 2021 | Wrong study design |
| Zhou 2017 | Wrong patient population |
| Ziehr 2020 | Wrong study design |
| Zorbas 2021 | Too few participants |
Characteristics of ongoing studies [ordered by study ID]
ictrp‐RBR‐2z3f7k.
| Study name | Artificial ventilation setting in patients infected with COVID‐19 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
IRCT20150724023314N4.
| Study name | The effect of APRV‐LTV mechanical ventilation mode on arterial blood gases, ventilation indices and vital signs in patients with COVID‐19 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
NCT04386369.
| Study name | Evaluation of airway pressure release ventilation in COVID‐19 ARDS (APRV‐COVID19) |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
NCT04399317.
| Study name | Flow controlled ventilation in ARDS associated with COVID‐19 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
NCT04411459.
| Study name | Risk factors for prolonged invasive mechanical ventilation in COVID‐19 acute respiratory distress syndrome |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
NCT04459533.
| Study name | Sparing in neuromuscular blockade in COVID 19 ICU (TOF‐COVID) |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
NCT04486729.
| Study name | Respiratory mechanics and gas exchange characteristics in patient with SARS‐CoV‐2 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
NCT04497454.
| Study name | Mechanical ventilation strategy for coronavirus disease 2019 (COVID‐19) |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
NCT04922814.
| Study name | Comparison for the effect of neuromuscular blocking agents versus sedation alone on severe ARDS patients due to COVID‐19 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
NCT05248243.
| Study name | Recruitment assessment in patients with acute respiratory distress syndrome and Covid‐19 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Differences between protocol and review
We made the following changes to the published protocol (Frank 2021).
Title
We further specified the title of the review by adding 'early' for spontaneous breathing.
Types of interventions
We clarified our types of intervention. We defined our intervention to be spontaneous breathing during invasive ventilation identified by ventilator settings enabling or supporting spontaneous breathing. On the contrary, ventilation strategies that encompass neuromuscular blockading agents (NMBA), deep sedation or ventilator modes not allowing for spontaneous breathing served as control. This was because most clinicians tend to use deep sedation or NMBA in COVID‐19‐ARDS (Schmidt 2021).
Types of outcome measures
We specified outcomes regarding effectiveness and safety of spontaneous breathing in invasively ventilated individuals with SARS‐CoV‐2‐induced ARDS after a guideline consortium (CEOsys) that occurred after protocol registration. This approach was implemented in all reviews of CEOsys. We created outcome categories and added/specified the following outcomes for invasively ventilated participants with SARS‐CoV‐2‐induced ARDS as follows.
Effectiveness of spontaneous breathing activity
All‐cause mortality (at up to day 28, day 60, in ICU, in hospital, and longest follow‐up (time‐to‐event estimate)).
-
Clinical improvement or worsening.
Ventilator‐free days within 28 and 60 days.
Time to liberation from IMV.
New need for ECMO therapy.
Need for tracheostomy.
Duration of ICU stay or time to discharge from ICU.
Duration of hospitalisation or time to discharge from hospital.
Quality of life, including fatigue and functional independence; assessed with standardised scales (e.g. WHOQOL‐100) at longest follow‐up available.
Safety of spontaneous breathing activity
Adverse events, any grade (defined as number of participants with event of any grade).
Serious adverse events (defined as number of participants with event).
Rate of pneumothorax.
The predefined outcome measures hospital‐acquired infection and need for renal replacement therapy were removed in the review for compaction. Adverse events should include aforementioned outcomes and serve as the leading parameter on safety.
Subgroup analysis and investigation of heterogeneity
We specified and expanded subgroup analyses of mortality as follows.
Severity of oxygenation impairment (as PaO2/FiO2 ratio) at baseline (< 100 mmHg; 100 mmHg to 200 mmHg, 201 mmHg to 300 mmHg).
Duration of ARDS (spontaneous breathing activity within 48 hours versus after 48 hours of ARDS onset).
Sensitivity analysis
We decided to additionally conduct sensitivity analyses for the following potential confounders.
Risk of bias domains (studies with a low risk of bias or some concerns versus studies with a high risk of bias).
Studies using NMBA in addition to deep sedation level alone to avoid spontaneous breathing.
Comparison of preprints versus peer‐reviewed articles.
Considering studies using NMBA in addition to deep sedation level alone to avoid spontaneous breathing, there is evidence that deep sedation itself has negative effects on outcomes in invasively ventilated people. Therefore, performing this sensitivity analysis we aim to distinguish between specific effects of NMBAs and effects of deep sedation levels.
Contributions of authors
FH: clinical expertise, study selection, data extraction and assessment, conception and writing of the manuscript.
LW: statistical expertise; data management, extraction, analysis and assessment; writing of the manuscript.
FG: clinical expertise, study selection, data extraction and assessment, conception and writing of the manuscript.
DS: clinical expertise, study selection, data extraction and assessment, writing of the manuscript.
JF: clinical expertise, study selection, writing of the manuscript.
MGo: clinical expertise and advice, study selection, proofreading of the manuscript.
MGr: protocol and concept, proofreading of the manuscript.
CG: clinical expertise, study selection, data extraction and assessment, writing of the manuscript.
CH: clinical expertise, study selection, writing of the manuscript.
AK: clinical expertise, study selection, proofreading of the manuscript.
MIM: search strategy design and conduct of the search, drafting of manuscript.
OM: clinical expertise and advice, proofreading of the manuscript.
NO: clinical expertise, study selection, writing of the manuscript.
VT: clinical expertise, study selection, data extraction and assessment, proofreading of the manuscript.
AV: search strategy design and conduct of the search, drafting of manuscript.
FF: clinical expertise and advice, data extraction and assessment, conception, writing and proofreading of the manuscript.
JB: methodological expertise and advice, data extraction and assessment, conception, writing and proofreading of the manuscript.
SL: clinical expertise and advice, data extraction and assessment, conception, writing and proofreading of the manuscript.
Sources of support
Internal sources
-
University Hospital Leipzig, Germany
Department of Anesthesiology and Intensive Care Medicine
-
University Hospital Goettingen, Germany
Department of Intensive Care Medicine
External sources
-
Federal Ministry of Education and Research, Germany
This review is part of the CEOsys project funded by the Network of University Medicine (Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF)), grant number 01KX2021.
Declarations of interest
FH: works as an Intensive Care Medicine physician and is member of the CEOsys project (no direct funding).
LW: is member of the CEOsys project funded by the Network of University Medicine (Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF)), grant number 01KX2021, paid to the institution.
FG: works as an Intensive Care Medicine physician and is a member of the CEOsys project funded by the Network of University Medicine (NUM) by the Federal Ministry of Education and Research of Germany (BMBF), grant number 01KX2021, paid to the institution.
DS: works as an Intensive Care Medicine physician and is member of the CEOsys project (no direct funding).
JF: works as an Intensive Care Medicine physician and is member of the CEOsys project funded by the Network of University Medicine (NUM) by the Federal Ministry of Education and Research of Germany (BMBF), grant number 01KX2021, paid to the institution.
MGo: works as an Intensive Care Medicine Consultant and is a member of the CEOsys project (no direct funding).
MGr: works as an Intensive Care Medicine physician and is a member of the CEOsys project funded by the Network of University Medicine (NUM) by the Federal Ministry of Education and Research of Germany (BMBF), grant number 01KX2021, paid to the institution.
CG: works as an Intensive Care Medicine physician and is a member of the CEOsys project (no direct funding).
CH: works as an Intensive Care Medicine physician and is a member of the CEOsys project funded by the Network of University Medicine (NUM) by the Federal Ministry of Education and Research of Germany (BMBF), grant number 01KX2021, paid to the institution.
AK: works as an Intensive Care Medicine physician and is a member of the CEOsys project funded by the Network of University Medicine (NUM) by the Federal Ministry of Education and Research of Germany (BMBF), grant number 01KX2021, paid to the institution.
MIM: is member of the CEOsys project funded by the Network of University Medicine (NUM) by the Federal Ministry of Education and Research of Germany (BMBF), grant number 01KX2021, paid to the institution.
OM: works as an Intensive Care Medicine Consultant and is a member of the CEOsys project (no direct funding).
NO: works as an Intensive Care Medicine physician and is member of the CEOsys project funded by the Network of University Medicine (NUM) by the Federal Ministry of Education and Research of Germany (BMBF), grant number 01KX2021, paid to the institution.
VT: works as an Intensive Care Medicine Consultant and is a member of the CEOsys project (no direct funding).
AV: works as Information Scientist and Librarian of the University of Leipzig Library (no direct funding).
FF: works as an Intensive Care Medicine Consultant and is a member of the CEOsys project (no direct funding).
JB: The Chair for Public Health and Health Services Research at the Institute for Medical Information Processing, Biometry and Epidemiology is part of the CEOsys project funded by the Network of University Medicine (NUM) by the Federal Ministry of Education and Research of Germany (BMBF), grant number 01KX2021, paid to the institution.
SL: works as an Intensive Care Medicine Consultant and is a member of the CEOsys project (no direct funding).
contributed equally (first author)
contributed equally (first author)
contributed equally (last author)
contributed equally (last author)
New
References
References to studies excluded from this review
Agarwal 2013 {published data only}
- Agarwal R, Srinivasan A, Aggarwal A, Gupta D. Adaptive support ventilation for complete ventilatory support in acute respiratory distress syndrome: a pilot, randomized controlled trial. Respirology 2013;18(7):1108-15. [DOI: ] [DOI] [PubMed] [Google Scholar]
Angeles 2020 {published data only}
- Angeles G, Zattera L, Sole C, Fernandez A, De Peray C, Benet P, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 does not differ from other causes of ARDS. Intensive Care Medicine Experimental 2020;8:139. [DOI: 10.1186/s40635-020-00354-8] [DOI] [Google Scholar]
Ball 2021 {published data only}
- Ball L, Robba C, Maiello L, Herrmann J, Gerard SE, Xin Y, et al. Computed tomography assessment of PEEP-induced alveolar recruitment in patients with severe COVID-19 pneumonia. Critical Care 2021;25(1):81. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Becher 2019 {published data only}
- Becher T, Adelmeier A, Frerichs I, Weiler N, Schädler D. Adaptive mechanical ventilation with automated minimization of mechanical power – a pilot randomized cross-over study. Critical Care 2019;23:338. [DOI: 10.1186/s13054-019-2610-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonny 2020 {published data only}
- Bonny V, Janiak V, Spadaro S, Pinna A, Demoule A, Dres M. Correction to: effect of PEEP decremental on respiratory mechanics, gas exchange, pulmonary regional ventilation, and hemodynamics in patients with SARS-Cov-2-associated acute respiratory distress syndrome. Critical Care 2020;24(1):675. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buiteman‐Kruizinga 2020 {published data only}
- Buiteman-Kruizinga L, Mkadami H, Schultz M, Heiden P. Effect of fully-automated versus non-automated ventilation on mechanical power of ventilation in COVID-19 – an observational crossover study. Intensive Care Medicine Experimental 2020;8:150. [DOI: ] [Google Scholar]
Buiteman‐Kruizinga 2021 {published data only}
- Buiteman-Kruizinga L, Mkdami H, Neto A, Kruizinga M, Botta M, Schultz M et al. Effect of INTELLiVENT-ASV versus conventional ventilation on ventilation intensity in patients with COVID-19 ARDS – an observational study. Journal of Clinical Medicine 2021;10:5409. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caley 2021 {published data only}
- Caley L, Dinis A, Oliveira T, Neto Real A, Narciso S, Pereira T, et al. Neuromuscular blocking agents in COVID 19 patients. Intensive Care Medicine Experimental 2021;9:138-9. [DOI: 10.1186/s40635-021-00413-8] [DOI] [Google Scholar]
Chelly 2020 {published data only}
- Chelly J, Mazerand S, Jochmans S, Weyer CM, Pourcine F, Ellrodt O, et al. Automated vs. conventional ventilation in the ICU: a randomized controlled crossover trial comparing blood oxygen saturation during daily nursing procedures (I-NURSING). Critical Care 2020;24(1):453. [DOI: 10.1186/s13054-020-03155-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cotton 2021 {published data only}
- Cotton S, Husain A, Meehan M, Zawaydeh Q, Malhotra A, Sweeney D. The influence of paralytics on the safety and efficacy of prone positioning in COVID19 ARDS. American Journal of Respiratory and Critical Care Medicine 2021;203:TP48. [DOI: 10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts.A2508] [DOI] [Google Scholar]
Courcelle 2020 {published data only}
- Courcelle R, Gaudry S, Serck N, Blonz G, Lascarrou JB, Grimaldi D. Neuromuscular blocking agents (NMBA) for COVID-19 acute respiratory distress syndrome: a multicenter observational study. Critical Care 2020;24(1):446. [DOI: 10.1186/s13054-020-03164-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Devlin 2022 {published data only}
- Devlin J, Train S, Burns K, Massaro A, Vasseur J, Selvan K, et al. Critical care pharmacist attitudes and perceptions of neuromuscular blocker infusions in ARDS. Critical Care Medicine 2022;50(1):472. [DOI: 10.1097/01.ccm.0000810124.49603.b5] [DOI] [Google Scholar]
Diniz‐Silva 2020 {published data only}
- Diniz-Silva F, Moriya HT, Alencar AM, Amato MB, Carvalho CR, Ferreira JC. Neurally adjusted ventilatory assist vs. pressure support to deliver protective mechanical ventilation in patients with acute respiratory distress syndrome: a randomized crossover trial. Annals of Intensive Care 2020;10(1):18. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ego 2021 {published data only}
- Ego A, Peluso L, Gorham J, Diosdado A, Restuccia G, Creteur J, et al. Use of sedatives and neuromuscular-blocking agents in mechanically ventilated patients with COVID-19 ARDS. Microorganisms 2021;9(11):2393. [DOI: 10.3390/microorganisms9112393] [DOI] [PMC free article] [PubMed] [Google Scholar]
Esnault 2020 {published data only}
- Esnault P, Cardinale M, Hraiech S, Goutorbe P, Baumstrack K, Prud'homme E, et al. High respiratory drive and excessive respiratory efforts predict relapse of respiratory failure in critically ill patients with COVID-19. American Journal of Respiratory and Critical Care Medicine 2020;202(8):1173-8. [DOI: 10.1164/rccm.202005-1582LE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gainnier 2004 {published data only}
- Gainnier M, Roch A, Forel JM, Thirion X, Arnal JM, Donati S, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Critical Care Medicine 2004;32(1):113-9. [DOI: 10.1097/01.CCM.0000104114.72614.BC] [DOI] [PubMed] [Google Scholar]
Gao 2022 {published data only}
- Gao Z, Li C, Chen H, Xie J, Liu L, Yang Y. A meta-analysis of the effect of neuromuscular blocking agents on oxygenation in patients with acute respiratory distress syndrome [肌松剂对急性呼吸窘迫综合征患者氧合影响的Meta分析]. Zhonghua Nei Ke Za Zhi 2022;61(1):86-94. [DOI: 10.3760/cma.j.cn112138-20210114-00036] [DOI] [PubMed] [Google Scholar]
Groetzinger 2016 {published data only}
- Groetzinger LM, Rivosecchi RM, Kane-Gill SL, Donahoe MP. Association between train-of-four values and gas exchange indices in moderate to severe acute respiratory distress syndrome. Annals of Pharmacotherapy 2016;50(12):1009-15. [DOI: ] [DOI] [PubMed] [Google Scholar]
Guervilly 2017 {published data only}
- Guervilly C, Bisbal M, Forel JM, Mechati M, Lehingue S, Bourenne J, et al. Effects of neuromuscular blockers on transpulmonary pressures in moderate to severe acute respiratory distress syndrome. Intensive Care Medicine 2017;43(3):408-18. [DOI: 10.1007/s00134-016-4653-4] [DOI] [PubMed] [Google Scholar]
Ingebrigtson 2021 {published data only}
- Ingebrigtson M, Farina N, Miller J, Renius K, McSparron J, Kenes M. Cisatracurium dosing requirements in COVID-19 compared to non-COVID-19 patients. Journal of Pharmacy Practice 2021 Oct 21 [Epub ahead of print]. [DOI: 10.1177/08971900211052835] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jain 2021 {published data only}covidwho‐1193852
- Jain S, Londono C, Uribe-Marquez S, Nowak K. Conventional protective LTV versus APRV for patients with severe COVID-19 disease. Critical Care Medicine 2021;49(1):68. [DOI: 10.1097/01.ccm.0000726548.79729.74] [EMBASE: covidwho-1193852] [DOI] [Google Scholar]
Kallet 2018 {published data only}
- Kallet RH, Zhuo H, Yip V, Gomez A, Lipnick MS. Spontaneous breathing trials and conservative sedation practices reduce mechanical ventilation duration in subjects with ARDS. Respiratory Care 2018;63(1):1-10. [DOI: 10.4187/respcare.05270] [DOI] [PubMed] [Google Scholar]
Karayiannis 2021 {published data only}
- Karayiannis D, Maragkouli A, Mikropoulos T, Sarri A, Kanavou A, Katsagoni C, et al. Neuromuscular blockade administration is associated with altered energy expenditure in critically ill intubated patients with COVID-19. Clinical Nutrition 25 May 2021 [Epub ahead of print]. [DOI: 10.1016/j.clnu.2021.05.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knafelj 2021 {published data only}
- Knafelj R, Noc M. Can we use adaptive support ventilation (ASV) in COVID-19? Intensive Care Medicine Experimental 2021:154-5. [DOI: 10.1186/s40635-021-00413-8] [DOI] [Google Scholar]
Kressin 2021 {published data only}
- Kressin C, Thompson Bastin M, Ather A, Gopinath A, Srour H, Schadler A, et al. Cisatracurium continuous infusion versus no neuromuscular blockade for acute respiratory distress syndrome on venovenous extracorporeal membrane oxygenation. Journal of Clinical Pharmacology 2021;61(11):1415-20. [DOI: 10.1002/jcph.1933] [DOI] [PubMed] [Google Scholar]
Lee 2022 {published data only}
- Lee B, Lee S, Baek M, Baek A, Na Y, Kim J, et al. Lower driving pressure and neuromuscular blocker use are associated with decreased mortality in patients with COVID-19 ARDS. Respiratory Care 2022;67(2):216-26. [DOI: 10.4187/respcare.09577] [DOI] [PubMed] [Google Scholar]
Li 2017 {published data only}
- Li J, Luo Z, Li X, Huang Z, Han J, Li Z, et al. Effect of different transpulmonary pressures guided mechanical ventilation on respiratory and hemodynamics of patients with ARDS: a prospective randomized controlled trial. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2017;29(1):39-44. [DOI: 10.3760/cma.j.issn.2095-4352.2017.01.009] [DOI] [PubMed] [Google Scholar]
Li Bassi 2021 {published data only}
- Li Bassi G, Gibbons K, Suen J, Dalton H, White N, Corley A et al. Use of neuromuscular blocking agents in mechanically ventilated patients with COVID‑19: a propensity score analysis. Intensive Care Medicine Experimental 29 Sep 2021 [Epub ahead of print]. [DOI: 10.1186/s40635-021-00413-8] [DOI] [Google Scholar]
Lyu 2014 {published data only}
- Lyu G, Wang X, Jiang W, Cai T, Zhang Y. Clinical study of early use of neuromuscular blocking agents in patients with severe sepsis and acute respiratory distress syndrome. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014;26(5):325-9. [DOI: 10.3760/cma.j.issn.2095-4352.2014.05.008] [DOI] [PubMed] [Google Scholar]
Mauri 2020 {published data only}
- Mauri T, Spinelli E, Scotti E, Colussi G, Basile M, Crotti S et al. Potential for lung recruitment and ventilation-perfusion mismatch in patients with the acute respiratory distress syndrome from coronavirus disease 2019. Critical Care Medicine 2020;48(8):1129-34. [DOI: 10.1097/CCM.0000000000004386] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCue 2020 {published data only}
- McCue C, Norman S, Macdonald C, Cowan R. Inflammatory response trajectory is associated with increased failure to wean neuromuscular blockade in ventilated COVID-19 patients. Intensive Care Medicine Experimental 2020:463. [DOI: 10.1186/s40635-020-00354-8] [DOI] [Google Scholar]
Needham 2012 {published data only}
- Needham CJ, Brindley PG. Best evidence in critical care medicine: the role of neuromuscular blocking drugs in early severe acute respiratory distress syndrome. Canadian Journal of Anesthesia 2012;59(1):105-8. [DOI: 10.1007/s12630-011-9615-2] [DOI] [PubMed] [Google Scholar]
Papazian 2010 {published data only}
- Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. New England Journal of Medicine 2010;363(12):1107-16. [DOI: 10.1056/NEJMoa1005372] [DOI] [PubMed] [Google Scholar]
Perinkulam Sathyanarayanan 2021 {published data only}
- Perinkulam Sathyanarayanan S, Hamid K, Hamza M, Jamous F. Airway pressure release ventilation use in COVID-19 ARDS: a single-center observational study. Chest 2021;160(4):1089-90. [DOI: 10.1016/j.chest.2021.07.1005] [DOI] [Google Scholar]
Renes 2020 {published data only}
- Renes M, Nijsten M. Muscle relaxation does not reduce CO2 production in mechanically ventilated COVID patients. Intensive Care Medicine Experimental 2020:572. [PMID: 10.1186/s40635-020-00354-8] [DOI] [Google Scholar]
Rizvi 2021 {published data only}
- Rizvi G, Yamane D, Davidson D, Williams J, Heinz E. Sedation, narcotic and neuromuscular blockade in mechanically ventilated patients with COVID-19. Trends in Anaesthesia and Critical Care 2021;39:19-20. [DOI: 10.1016/j.tacc.2021.06.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodrigo Castroviejo 2021 {published data only}
- Rodrigo Castroviejo N, Ferrigno G, Madrid Romero V, Bektran Bernaldez R, Sanmartino Gonzales C, Berenguer Rodriguez M, et al. ARDS in SARS‑COV2 pneumonia: adjuvant measures of mechanical ventilation. Intensive Care Medicine Experimental 2021:131. [DOI: 10.1186/s40635-021-00413-8] [DOI] [Google Scholar]
Ruan 2021 {published data only}
- Ruan E, Gong M, Chen J. Spontaneous awakening trials in mechanically ventilated patients with COVID-19 and how increased compliance impacts clinical outcomes. In: American Thoracic Society International Conference Abstracts. 2021:TP048. [DOI: 10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts.A2522] [DOI]
Sella 2020 {published data only}
- Sella N, Zarantonello F, Andreatta G, Gagliardi V, Boscolo A, Navalesi P. Positive end-expiratory pressure titration in COVID-19 acute respiratory failure: electrical impedance tomography vs. PEEP/FiO2 tables. Critical Care 2020;24(1):540. [DOI: 10.1186/s13054-020-03242-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Serrano 2020 {published data only}
- Serrano C, Ruiz Garcia A, Higuera Lucas J, Tato J, Vaduva C, Arteaga J, et al. Factors associated to mortality in COVID 19 patients on mechanical ventilation. Intensive Care Medicine Experimental 2020:142-3. [PMID: 10.1186/s40635-020-00354-8] [DOI] [Google Scholar]
Song 2016 {published data only}
- Song S, Tian H, Yang X, Hu Z. The clinical effect of airway pressure release ventilation for acute lung injury/acute respiratory distress syndrome. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2016;28(1):15-21. [DOI: 10.3760/cma.j.issn.2095-4352.2016.01.004] [DOI] [PubMed] [Google Scholar]
Tsolaki 2020 {published data only}
- Tsolaki V, Siempos I, Magira E, Kokkoris S, Zakynthinos GE, Zakynthinos S. PEEP levels in COVID-19 pneumonia. Critical Care 2020;24(1):303. [DOI: 10.1186/s13054-020-03049-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Zee 2020 {published data only}
- Zee P, Somhorst P, Endeman H, Gommers D. Electrical impedance tomography for positive end-expiratory pressure titration in COVID-19-related acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 2020;202(2):280-4. [DOI: 10.1164/rccm.202003-0816LE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Villar 2006 {published data only}
- Villar J, Kacmarek RM, Pérez-Méndez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Critical Care Medicine 2006;34(5):1311-8. [DOI: 10.1097/01.CCM.0000215598.84885.01] [DOI] [PubMed] [Google Scholar]
Vine 2021 {published data only}
- Vine J, Soloveichik V, Vinepinsky E, Nini A, Nevo A, Borohovitz A, et al. Airway pressure release ventilation vs low tidal volume ventilation in COVID-19 associated ARDS. Intensive Care Medicine Experimental 2021:189-90. [DOI: 10.1186/s40635-021-00413-8] [DOI] [Google Scholar]
Wongtangman 2021 {published data only}
- Wongtangman K, Santer P, Wachtendorf L, Azimaraghi O, Baedorf Kassis E, Teja B. Association of sedation, coma, and in-hospital mortality in mechanically ventilated patients with coronavirus disease 2019-related acute respiratory distress syndrome: a retrospective cohort study. Critical Care Medicine 2021;49(9):1524-34. [DOI: 10.1097/CCM.0000000000005053] [DOI] [PubMed] [Google Scholar]
Wu 2021 {published data only}
- Wu F, Li M, Zhang Z, Shang J, Guo Y, Li Y. Sedation, analgesia, and muscle relaxation during VV-ECMO therapy in patients with severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2): a single-center, retrospective, observational study. Frontiers in Medicine 2021;8:762740. [DOI: 10.3389/fmed.2021.762740] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2017 {published data only}
- Zhou Y, Jin X, Lv Y, Wang P, Yang Y, Liang G, et al. Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome. Intensive Care Medicine 2017;43:1648-59. [DOI: 10.1007/s00134-017-4912-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziehr 2020 {published data only}
- Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. American Journal of Respiratory and Critical Care Medicine 2020;201(12):1560-4. [DOI: 10.1164/rccm.202004-1163LE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zorbas 2021 {published data only (unpublished sought but not used)}
- Airway pressure release ventilation for mechanically ventilated patients with COVID-19 in Western Australian intensive care units: an observational study. pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/ictrp-ACTRN12620000474954 (accessed prior to 18 May 2022). [ANZCTR: ictrp-ACTRN12620000474954]
- Zorbas J, Ho K, Litton E, Wibrow B, Fysh E, Anstey M. Airway pressure release ventilation in mechanically ventilated patients with COVID-19: a multicenter observational study. Acute and Critical Care 2021;36(2):143-50. [DOI: 10.4266/acc.2021.00017] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ictrp‐RBR‐2z3f7k {published data only}
- ictrp-RBR-2z3f7k. Artificial ventilation setting in patients infected with COVID-19. pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/ictrp-RBR-2z3f7k (accessed prior to 18 May 2022). [INTERNATIONAL CLINICAL TRIALS REGISTRY PLATFORM: ictrp-RBR-2z3f7k]
IRCT20150724023314N4 {published data only}
- IRCT20150724023314N4. The effect of APRV-LTV mechanical ventilation mode on arterial blood gases, ventilation indices and vital signs in patients with COVID-19. www.irct.ir/trial/57913 (first received 4 September 2021). [IRANIAN REGISTRY OF CLINICAL TRIALS: IRCT20150724023314N4]
NCT04386369 {published data only}
- NCT04386369. Evaluation of airway pressure release ventilation in COVID-19 ARDS (APRV-COVID19). clinicaltrials.gov/ct2/show/NCT04386369 (first received 13 May 2020). [CLINICALTRIALS.GOV: NCT04386369]
NCT04399317 {published data only}
- NCT04399317. Flow controlled ventilation in ARDS associated with COVID-19. clinicaltrials.gov/ct2/show/NCT04399317 (first received 22 May 2020). [CLINICALTRIALS.GOV: NCT04399317]
NCT04411459 {published data only}
- NCT04411459. Risk factors for prolonged invasive mechanical ventilation in COVID-19 acute respiratory distress syndrome. clinicaltrials.gov/ct2/show/NCT04411459 (first received 2 June 2020). [CLINICALTRIALS.GOV: NCT04411459]
NCT04459533 {published data only}
- NCT04459533. Sparing in neuromuscular blockade in COVID 19 ICU (TOF-COVID). clinicaltrials.gov/ct2/show/NCT04459533 (first received 7 July 2020). [CLINICALTRIALS.GOV: NCT04459533]
NCT04486729 {published data only}
- NCT04486729. Respiratory mechanics and gas exchange characteristics in patient with SARS-CoV-2. clinicaltrials.gov/ct2/show/NCT04486729 (first received 27 July 2020). [CLINICALTRIALS.GOV: NCT04486729]
NCT04497454 {published data only}
- NCT04497454. Mechanical ventilation strategy for coronavirus disease 2019 (COVID-19). clinicaltrials.gov/ct2/show/NCT04497454 (first received 4 August 2020). [CLINICALTRIALS.GOV: NCT04497454]
NCT04922814 {unpublished data only}
- NCT04922814. Comparison for the effect of neuromuscular blocking agents versus sedation alone on severe ARDS patients due to COVID-19. clinicaltrials.gov/ct2/show/NCT04922814 (first received 11 June 2021). [CLINICALTRIALS.GOV: NCT04922814]
NCT05248243 {published data only}
- NCT05248243. Recruitment assessment in patients with acute respiratory distress syndrome and Covid-19. clinicaltrials.gov/ct2/show/NCT05248243 (first received 21 February 2022). [CLINICALTRIALS.GOV: NCT05248243]
Additional references
Alhazzani 2020a
- Alhazzani W, Belley-Cote E, Møller M, Angus D, Papazain L, Arabi Y, et al. Neuromuscular blockade in patients with ARDS: a rapid practice guideline. Intensive Care Medicine 2020;46:1977-86. [DOI: 10.1007/s00134-020-06227-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alhazzani 2020b
- Alhazzani W, Møller M, Arabi Y, Loeb M, Ng Gong M, Fan E, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Critical Care Medicine 2020;48(6):e440-69. [DOI: 10.1097/CCM.0000000000004363] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ansems 2021
- Ansems K, Grundeis F, Dahms K, Mikolajewska A, Thieme V, Piechotta V, et al. Remdesivir for the treatment of COVID-19. Cochrane Database of Systematic Reviews 2021, Issue 8. Art. No: CD014962. [DOI: 10.1002/14651858.CD014962] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aslam 2021
- Aslam TN, Klitgaard TL, Hofsø K, Rasmussen BS, Laake JH. Spontaneous versus controlled mechanical ventilation in patients with acute respiratory distress syndrome. Current Anesthesiology Reports 2021;11:85-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bain 2021
- Bain W, Yang H, Shah F, Suber T, Drohan C, Al-Yousif N, et al. COVID-19 versus non-COVID-19 acute respiratory distress syndrome: comparison of demographics, physiologic parameters, inflammatory biomarkers, and clinical outcomes. Annals of the American Thoracic Society 2021;18(7):1202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bellani 2016
- Bellani G, Laffey J, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. Journal of the American Medical Association 2016;315:788-800. [DOI] [PubMed] [Google Scholar]
Botta 2021
- Botta M, Tsonas A, Pillay J, Boers L, Algera A, Bos L, et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respiratory Medicine 2021;9:139-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buitrago‐Garcia 2020
- Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci Aziz M, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Medicine 2020;17(9):e1003346. [DOI: 10.1371/journal.pmed.1003346] [DOI] [PMC free article] [PubMed] [Google Scholar]
CEOsys 2021
- CEOsys. German COVID-19 evidence-ecosystem. www.covid-evidenz.de (accessed 31 August 2021).
Chai 2020
- Chai KL, Valk SJ, Piechotta V, Kimber C, Monsef I, Doree C, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013600. [DOI: 10.1002/14651858.CD013600.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
COMET 2021
- COMET. Core outcome set developers’ response to COVID-19 (April 2021). www.comet-initiative.org/Studies/Details/1538 (accessed 7 September 2021).
Cummings 2020
- Cummings M, Baldwin M, Abrams D, Jacobson S, Meyer B, Balough E, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020;395(10239):1763-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
ECDC 2020
- European Centre for Disease Prevention and Control. Rapid increase of a SARS-CoV-2 variant with multiple spike protein mutations observed in the United Kingdom, 2020. www.ecdc.europa.eu/sites/default/files/documents/SARS-CoV-2-variant-multiple-spike-protein-mutations-United-Kingdom.pdf (accessed prior to 18 May 2022). [URL: www.ecdc.europa.eu/sites/default/files/documents/SARS-CoV-2-variant-multiple-spike-protein-mutations-United-Kingdom.pdf]
Ferrando 2020
- Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, Hernández M, Gea A, Arruti E, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Medicine 2020;46:2200-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fichtner 2018
- Fichtner F, Mörer O, Laudi S, Weber-Carstens S, Nothacker M, Kaisers U. Clinical practice guideline: mechanical ventilation and extracorporeal membrane oxygenation in acute respiratory insufficiency. Deutsches Ärzteblatt International 2018;115(50):840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fichtner 2019
- Fichtner F, Mörer O, Weber-Carstens S, Nothacker M, Kaisers U, Laudi S. Clinical guideline for treating acute respiratory insufficiency with invasive ventilation and extracorporeal membrane oxygenation: evidence based recommendations for choosing modes and setting parameters of mechanical ventilation. Respiration 2019;98:357-72. [DOI: 10.1159/000502157] [DOI] [PubMed] [Google Scholar]
Funk 2021
- Funk T, Pharris A, Spiteri G, Bundle N, Melidou A, Carr M, et al. Characteristics of SARS-CoV-2 variants of concern B.1.1.7, B.1.351 or P.1: data from seven EU/EEA countries, weeks 38/2020 to 10/2021. Eurosurveillance 2021;26(16):pii=2100348. [DOI: 10.2807/1560-7917.ES.2021.26.16.2100348] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gottlieb 2022
- Gottlieb J, Capetian P, Hamsen U, Janssens W, Karagiannidis C, Kluge S, et al. Oxygen in the acute care of adults: short version of the German S3 guideline. Medizinische Klinik, Intensivmedizin und Notfallmedizin 2022;117(1):4-15. [DOI: 10.1007/s00063-021-00884-3] [DOI] [PMC free article] [PubMed]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed after 21 December 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Grasselli 2020
- Grasselli G, Tonetti T, Protti A, Langer T, Girardis M, Bellani G, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respiratory Medicine 2020;8(12):1201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI: 10.1136/bmj.327.7414.557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Higgins 2021b
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Hirshberg 2018
- Hirshberg E, Lanspa M, Peterson J, Carpenter L, Wilson E, Brown S, et al. Randomized feasibility trial of a low tidal volume-airway pressure release ventilation protocol compared with traditional airway pressure release ventilation and volume control ventilation protocols. Critical Care Medicine 2018;46(12):1943-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karagiannidis 2020
- Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respiratory Medicine 2020;8:853-62. [DOI: 10.1016/ S2213-2600(20)30316-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kluge 2021
- Kluge S, Janssens U, Welte T, Weber-Carstens S, Schälte G, Spinner C, et al. Recommendations for the therapy of hospitalised patients with COVID-19 [Empfehlungen zur stationären Therapie von Patienten mit COVID-19]. www.awmf.org/leitlinien/detail/ll/113-001LG.html (accessed prior to 18 May 2022).
Kreuzberger 2021
- Kreuzberger N, Hirsch C, Chai KL, Piechotta V, Valk SJ, Estcourt LJ, et al. SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013825. [DOI: 10.1002/14651858.CD013825] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuriyama 2021
- Kuriyama A, Jackson J. Neuromuscular blocking agents for acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2021, Issue 6. Art. No: CD014693. [DOI: 10.1002/14651858.CD014693] [DOI] [Google Scholar]
Lauer 2020
- Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Annals of Internal Medicine 2020;172(9):577-82. [DOI: 10.7326/M20-0504] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2021
- Li T, Higgins JP, Deeks JJ. Chapter 5: Collecting data. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Marini 2020
- Marini J, Gattinoni L. Management of COVID-19 respiratory distress. Journal of the American Medical Association 2020;323(22):2329-30. [DOI] [PubMed] [Google Scholar]
Meyer 2021
- Meyer N, Gattinoni L, Calfee C. Acute respiratory distress syndrome. Lancet 2021;398(10300):622-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Microsoft 2018 [Computer program]
- Microsoft Excel. Microsoft Corporation, 2018. Available at office.microsoft.com/excel.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moss 2019
- Moss M, Huang D, Brower R, Ferguson N, Ginde A, Gong M, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. New England Journal of Medicine 2019;380(21):1997-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nasa 2021
- Nasa P, Azoulay E, Khanna A, Jain R, Gupta S, Javeri Y, et al. Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. Critical Care 2021;25:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01862016
- NCT01862016. Early spontaneous breathing in acute respiratory distress syndrome (BiRDS). clinicaltrials.gov/ct2/show/NCT01862016 (first received 24 May 2013). [CLINICALTRIALS.GOV: NCT01862016]
NCT04228471
- NCT04228471. Early PReserved SPONtaneous breathing activity in mechanically ventilated patients with ARDS (PReSPON). clinicaltrials.gov/ct2/show/NCT04228471 (first received 14 January 2020). [CLINICALTRIALS.GOV: NCT04228471]
Page 2022
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Papazain 2019
- Papazain L, Aubron C, Brochard L, Chiche JD, Combes A, Dreyfuss D, et al. Formal guidelines: management of acute respiratory distress syndrome. Annals of Intensive Care 2019;9:69. [DOI: 10.1186/s13613-019-0540-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
PHE 2021
- Public Health England. SARS-CoV-2 variants of concern and variants under investigation in England – Technical briefing 14, 2021. www.gov.uk/government/publications/investigation-of-novel-sars-cov-2-variant-variant-of-concern-20201201 (accessed prior to 18 May 2022). [URL: www.gov.uk/government/publications/investigation-of-novel-sars-cov-2-variant-variant-of-concern-20201201]
Potere 2020
- Potere N, Valeriani E, Candeloro M, Tana M, Porreca E, Abbate A, et al. A higher mortality rate and long ventilation times differentiate COVID-19 from severe respiratory infections in flu waves [Eine höhere Letalität und lange Beatmungsdauer unterscheiden COVID-19 von schwer verlaufenden Atemwegsinfektionen in Grippewellen]. Critical Care 2020;24(1):389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager Web 2021 [Computer program]
- Review Manager Web (RevMan Web). Version 3.4.0. The Cochrane Collaboration, 2021. Available at revman.cochrane.org.
Richardson 2020
- Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. Journal of the American Medical Association 2020;323(20):2052-9. [DOI: 10.1001/jama.2020.6775] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schilling 2020
- Schilling J, Lehfeld AS, Schumacher D, Ullrich A, Diercke M, Buda S, et al. Disease severity of the first COVID-19 wave in Germany using reporting data from the national notification system. Journal of Health Monitoring 2020;5(S11):2-20. [DOI: 10.25646/7170] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2021
- Schmidt M, Hajage D, Demoule A, Pham T, Combes A, Dres M, et al. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Medicine 2021;47(1):60-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savovic J, Page MJ, Elbers RG, Blencowe N, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI: 10.1136/bmj.l4898] [DOI] [PubMed] [Google Scholar]
Sterne 2020
- Sterne JA, Hernán MA, McAleenan A, Reeves BC, Higgins JP. Chapter 25: Assessing risk of bias in a non-randomized study. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Struyf 2021
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2021
- Tan E, Song J, Deane A, Plummer M. Global impact of coronavirus disease 2019 infection requiring admission to the ICU. Chest Journal 2021;159(2):524-36. [DOI: 10.1016/j.chest.2020.10.014] [DOI] [PMC free article] [PubMed] [Google Scholar]
The ARDS Definition Task Force 2012
- The ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. Journal of the American Medical Association 2012;307(23):2526-33. [DOI] [PubMed] [Google Scholar]
Tolksdorf 2020
- Tolksdorf K, Buda S, Schuler E, Wieler LH, Haas W. Higher lethality and long duration of ventilation distinguish COVID-19 from severe respiratory tract infections in influenza waves. [Eine höhere Letalität und lange Beatmungsdauer unterscheiden COVID-19 von schwer verlaufenden Atemwegsinfektionen in Grippewellen]. Epidemiologisches Bulletin 2020;41:3-10. [DOI: 10.25646/7111] [DOI] [Google Scholar]
WHO 2015
- World Health Organization. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. www.who.int/publications/m/item/summary-of-probable-sars-cases-with-onset-of-illness-from-1-november-2002-to-31-july-2003 (accessed 22 July 2021). [URL: www.who.int/publications/m/item/summary-of-probable-sars-cases-with-onset-of-illness-from-1-november-2002-to-31-july-2003]
WHO 2019
- World Health Organization. Middle East respiratory syndrome coronavirus (MERSCoV). www.who.int/emergencies/mers-cov/en (accessed 22 July 2021). [URL: www.who.int/emergencies/mers-cov/en]
WHO 2020a
- World Health Organization. Timeline: WHO's COVID-19 response. www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline (accessed 22 July 2021). [URL: www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline]
WHO 2020b
- World Health Organization. SARS-CoV-2 variants, 2020. www.who.int/emergencies/disease-outbreak-news/item/2020-DON305 (accessed prior to 18 May 2022). [URL: www.who.int/emergencies/disease-outbreak-news/item/2020-DON305]
WHO 2020c
- WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infectious Diseases 2020;20(8):e192-7. [DOI: 10.1016/S1473-3099(20)30483-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2022a
- World Health Organization. WHO coronavirus disease (COVID-19) dashboard. covid19.who.int (accessed 11 April 2022). [URL: covid19.who.int]
WHO 2022b
- World Health Organization. Update on Omicron, 2021. www.who.int/news/item/28-11-2021-update-on-omicron (accessed prior to 18 May 2022). [URL: www.who.int/news/item/28-11-2021-update-on-omicron]
Wu 2020
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239-42. [DOI: 10.1001/jama.2020.2648] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Frank 2021
- Frank J, Herchenhahn C, Wedekind L, Olbrich N, Hohmann F, Fichtner F, et al. Spontaneous versus mandatory modes in invasively ventilated people with respiratory failure in COVID-19 (part of German Ecosystem CEO-sys), 2021. www.crd.york.ac.uk/prospero/display_record.php?RecordID=245089 (accessed 17 May 2022).


