An unexpected global outbreak of monkeypox virus challenges our knowledge of this long-known pathogen and raises questions about the reasons for its re-emergence. Here, we discuss the unusual aspects of this outbreak and the urgency for a coordinated public-health strategy and present knowledge gaps on epidemiology and molecular epidemiology, clinical presentation, transmission, treatment, and prevention. Similar to other re-emerging viruses of the past decades, the current monkeypox outbreak highlights the fact that threats of emerging pathogens can only be addressed on a global scale by improving preparedness and control strategies in endemic countries before viruses re-emerge globally. “Any new disease that is emerging faster than our understanding is never under control" (Margret Chan, World Health Assembly, 2013).
More than 2 years into the coronavirus 2019 (COVID-19) pandemic, monkeypox arises in a population tired of emerging viruses and public health measures. This must not lead to inaction, and severe acute respiratory syndrome 2 (SARS-CoV-2) demonstrates the importance of timely targeted interventions to arising public-health threats.
SARS-CoV-2 and monkeypox are very dissimilar viruses—one is a never-seen-before RNA virus, and the other is a DNA virus known for decades with zoonotic transmission endemic to Sub-Saharan Africa. Yet, there are more unknowns than knowns during the early days of world-wide outbreaks.
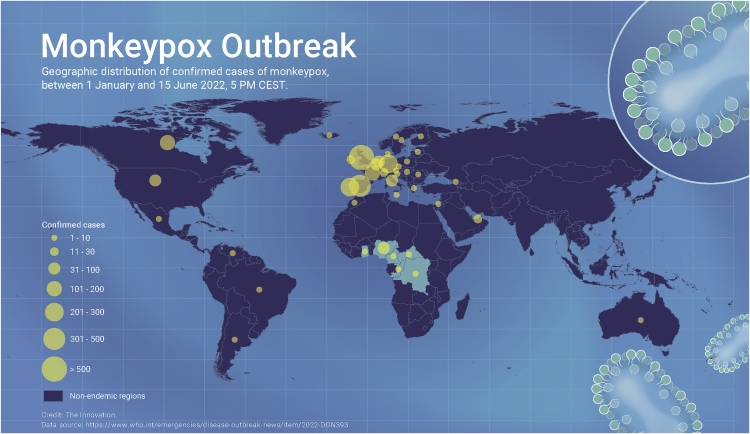
The current monkeypox outbreak comprises a multitude of cases with more than >2000 worldwide as of June 15th (Figure 1), in a novel population in previously non-endemic regions, spanning 41 countries across four WHO regions (the region of the Americas as well as the European, Eastern Mediterranean, and Western Pacific regions). Full genome sequences were immediately available from multiple countries, thanks to the massively increased sequencing capacity during the SARS-CoV-2 pandemic. Molecular epidemiology surprisingly suggests a much longer period of unrecognized, ongoing human-to-human spread, potentially even in the range of years.
Figure 1.
Monkeypox outbreak 2022 global map. Credit: The Innovation.
As with any re-emerging virus, a piece of the puzzle is missing: why is a long-known virus suddenly emerging like this? Thus, monkeypox should not be considered a mild or easily containable disease until transmission dynamics, viral kinetics, clinical presentation, and outcomes are better understood. Few data are available regarding poxviruses genomic evolution in humans, supporting the need to hit quick and hard, especially without widely available treatments and vaccines.
Monkeypox was first identified in 1958 in imported macaques as the cause of a mild fever associated with a pustular rash.1 Like humans, monkeys are only incidental hosts; small mammals are the most likely reservoir in Sub-Saharan Africa, where the disease is endemic. The exact reservoir is still unknown, with squirrels seemingly the best candidates. Close contacts with rodents, such as hunting for bushmeat, are therefore risk factors in endemic countries. For decades, cases have been regularly confirmed in Central and/or West Africa, caused by two different clades, termed the Congolese and West African clades, with the latter being less virulent and less transmissible. Before 2022, few patients were diagnosed outside Africa. This included an epidemic in the United States caused by the importation of infected rodents and some returning travelers since 2018.2 In all instances, secondary cases were rare. In humans, monkeypox virus transmits via direct contact with body fluid or a skin/mucosal lesions, contaminated fomites such as bedding or clothing, or large respiratory droplets. The role of aerosol transmission is less clear and currently under debate. Usually, transmission requires close and prolonged contact between humans. Currently, the increasingly frequent cases diagnosed out of Africa, without clear transmission chains, are mostly observed in those who had multiple sex partners and/or identify as men who have sex with men. Sexual transmission of orthopoxviruses has already been suggested for monkeypox3 and vaccinia viruses; however, close contact during intimate relationships is consistent with the known transmission modes.
Any (re-)emerging virus shakes our certitudes: for Ebola and Zika viruses, not only was sexual transmission documented but so were many other aspects of these long-known viruses that only became visible during large outbreaks. Monkeypox virus was discovered more than 60 years ago, yet viral kinetics in blood, saliva, the throat, and sores are poorly known, especially during the incubation period and after apparent recovery. To what extent poxviruses are present or can persist in semen and lead to sexual transmission while asymptomatic is unknown. We have to balance staying humble and acknowledging these uncertainties with not letting knowledge gaps delay necessary action.
The peculiar epidemiological situation of the current monkeypox outbreak, mostly driven—as is the rule for infectious disease epidemics—by human behaviors presents several challenges. While the affected community should not be stigmatized—monkeypox is not primarily transmitted sexually and can infect anyone in close contact with an infected case—communication has to be directed toward the primary risk group. Those at greater risk of exposure must be urgently informed to prevent risky behaviors. We must quickly identify and care for infected patients and apply strict infection control measures to halt transmission before it enters the general population and groups known to be at higher risk of severe disease (pregnant women, the immunocompromised, and children).
The classical clinical description of monkeypox disease, after 6 to 21 days of incubation, consists of a flu-like illness with fever and lymphadenopathies, followed by a vesico-pustular rash (first on the mucosa and the face, spreading to the body up to palms and soles), which likely represents only a fraction of symptomatic infections.4 Currently, many cases start with very mild illness and atypical rash such as small, isolated, painless ulcerated lesions located on the genitals and/or the peri-anal region. Thus, case definitions must be swiftly adapted with new data and must be promptly and effectively communicated to healthcare providers, at-risk communities, and the general population; clinicians don’t look for diseases and patients do not seek care if they are unaware of a risk. The size of the outbreak requires a simultaneous, unanimous international message—a challenging yet crucial task for health authorities.
That no casualties have yet been reported during the present outbreak is reassuring for the currently affected group but cannot be extrapolated to others. This clade, with an estimated lethality rate between 1% and 3.3%,3 cannot be considered a mild disease. In endemic regions, the lethality rate has been even up to 14% in children <4 years old, and monkeypox is known to cause miscarriage in pregnant women. Although monkeypox is probably underdiagnosed in most endemic countries, and poor access to healthcare as well as underlying conditions influence disease outcomes, these numbers are concerning, especially with restricted access to drugs and vaccines. As clinicians, public-health specialists, and virologists, we cannot allow monkeypox to extend geographically, establish new reservoirs, nor maintain continuous transmission chains amongst humans.
Monkeypox and its close relative variola belong to the Poxviridae family. Variola is the etiologic agent of smallpox, the first and only human virus to be eradicated. In contrast to monkeypox, which infects a range of mammalian species, variola was restricted to humans. This allowed eradication through vaccination—but it took about 200 years and a coordinated, expensive, international effort to finally declare the world free from smallpox in 1980. Afterward, vaccination was stopped, resulting in no cross-protective smallpox-vaccine-derived immunity in those under 40–50 years old. Furthermore, immunity from smallpox vaccination, now decades old, is waning in the vaccinated. A long-standing hypothesis postulates that eventually another virus will fill this empty ecological niche, and monkeypox is not a candidate for eradication. Monkeypox has already been described in many small mammals and primates. While no case has yet been reported in common pets, the full host range, especially in a novel geographic context, is unknown. Best public-health practices must include precautions against human-to-animal spread. As poxviruses are rather stable, any role of environmental contamination, such as wastewater or household or hospital waste should be carefully examined.
Although poxviruses, as DNA viruses, have a lower evolutionary rate than RNA viruses such as SARS- CoV-2, they have strategies that allow for remarkable genomic plasticity—the prerequisite for quickly adapting to new conditions. For monkeypox virus, a mutation rate of around 105–106 mutations per site per year was described in previous literature, representing 1–2 mutations per genome per year. Preliminary results of available sequences from the current 2022 monkeypox epidemic show that sequences fall into the same clade but have a difference by about 40 mutations from the closest 2018 sequences.5 Previously, few sequences were available from Africa, most certainly underrepresenting the genomic diversity of monkeypox virus. The clinical or public-health significance of these mutations remains yet to be elucidated.
While the drug Tecovirimat is suggested as a possible treatment against monkeypox by some health authorities, existing efficacy evidence is scarce, mostly from in vitro experiments or animal experiments. International efforts are already ongoing to organize clinical trials aiming to confirm their safety and efficacy against monkeypox disease: another beneficial side effect of the COVID-19 pandemic, which created the infrastructure for swift implementation of international studies. Currently it is most important to note that although vaccines and treatments exist, their availability on a national or even global scale is very limited. Therefore, the existence of vaccines and treatments should not lead to laziness from authorities nor be an excuse to delay the response.
We must act quickly while there is still a chance to control the outbreak. “Once burned, twice shy” should be the guiding leitmotiv for international health authorities.
Finally, the current outbreak sheds light on a neglected disease endemic to many African countries, responsible for disease and death since many decades. As efforts are put in place to control the spread of the disease in Europe and North America, one should not forget that this is an opportunity and a responsibility to respond at a larger scale by addressing the burden of monkeypox in Africa. The next important step would be to focus on epidemic preparedness instead of response. Let us not forget that Ebola is striking yet again and that polio recently resurfaced in continents where it was previously eradicated. It is time to look up.
Acknowledgments
Declaration of interests
The authors declare no competing interests.
Published: June 23, 2022
References
- 1.Magnus P. von., Andersen E.K., Petersen K.B., et al. A pox-like disease in cynomolgus monkeys. Acta Pathol. Microbiol. Scand. 1959;46:156–176. [Google Scholar]
- 2.Adler H., Gould S., Hine P., et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect. Dis. 2022;24 doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogoina D., Izibewule J.H., Ogunleye A., et al. The 2017 human monkeypox outbreak in Nigeria—Report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS One. 2019;14:e0214229. doi: 10.1371/journal.pone.0214229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCollum A.M., Damon I.K. Human monkeypox. Clin. Infect. Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- 5.Nextstrain.org Genomic Epidemiology of Monkeypox Virus. 2022. https://nextstrain.org/monkeypox/hmpxv1