Abstract
Growing evidence has established that a subset of dopamine (DA) neurons co-release glutamate and express vesicular glutamate transporter 2 (VGLUT2). VGLUT2 expression in DA neurons plays a key role in selective vulnerability to DA neurodegeneration in Parkinson’s Disease (PD). In this review, we summarize recent findings on impacts of VGLUT2 expression and glutamate co-release from DA neurons on selective DA neuron vulnerability. We present evidence that DA neuron VGLUT2 expression may be neuroprotective, boosting DA neuron resilience in the context of ongoing neurodegenerative processes in PD. We highlight genetic and pesticide models of PD that have provided mechanistic insights into selective DA neuron vulnerability. Finally, we discuss potential neuroprotective mechanisms, focusing on roles of VGLUT2 and glutamate in promoting mitochondrial health, and diminishing oxidative stress and excitotoxicity. Elucidating these mechanisms may ultimately lead to more effective treatments to boost DA neuron resilience that can slow or even prevent DA neurodegeneration.
Keywords: Dopamine, glutamate, co-release, VGLUT2, Parkinson’s Disease, neurodegeneration
Graphical Abstract
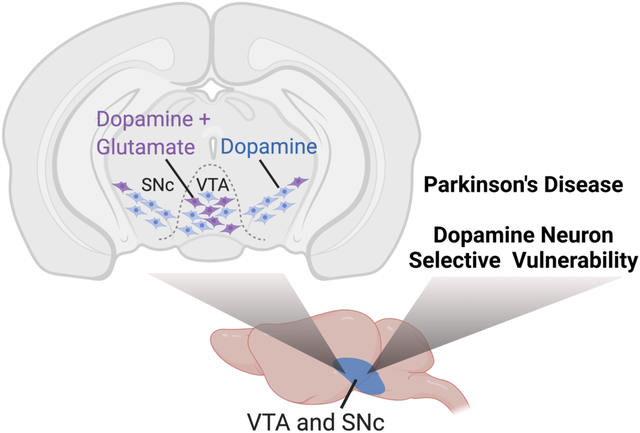
Overview
Following early evidence of glutamate transmission in cultured dopamine (DA) neurons1, 2, subsequent studies verified that a minority of DA neurons express vesicular glutamate transporter 2 (VGLUT2) and co-release glutamate. This phenomenon is conserved across evolution, having been observed in flies, rodents, non-human primates, and humans3–5. The majority of midbrain DA/glutamate neurons localize to the medial ventral tegmental area (VTA) and project to the medial shell of the nucleus accumbens (NAc), while a smaller DA/glutamate neuron population in the lateral substantia nigra pars compacta (SNc) projects to the tail of the striatum6, 7. Remarkably, almost all DA neurons express VGLUT2 during development, only to repress VGLUT2 in adulthood8, 9. However, VGLUT2 expression is increased in DA neurons, and associated with DA neuron survival, after exposure to insults10–12. This raises the question of what roles VGLUT2 expression and glutamate co-transmission may play in selective DA neuron vulnerability in neurodegenerative diseases including Parkinson’s Disease (PD). Here, we summarize findings on DA neuron VGLUT2 and its impact on selective DA neuron vulnerability, with a focus on pesticide and genetic models of PD. We also explore potential mechanisms of DA neuroprotection conferred by VGLUT2 expression.
VGLUT2 and Selective DA Neuron Vulnerability in PD
Even before the discovery of DA/glutamate neurons, changes in glutamatergic neurotransmission within DAergic brain areas were observed in response to DA neuron loss where treatment with DA neurotoxin 6-hydroxydopamine (6OHDA) increases striatal glutamate release13. Importantly, the proportion of VGLUT2-expressing DA neurons in the VTA and their projections to the NAc increases after single exposures to DA neurotoxins (e.g., 6OHDA, MPTP) at early postnatal periods and in adulthood2, 11, 14, 15 or to chronic α-synuclein exposures in mouse PD models12. This is accompanied by upregulated VGLUT2 expression in surviving DA neurons9, 12. Conversely, VGLUT2 cKO in DA neurons exacerbates DA neurodegeneration in response to 6OHDA and MPTP9, 11, 16, and leads to fewer striatal connections after a 6OHDA-induced lesion16; VGLUT2 cKO mice also show impairments in DA-dependent basal and psychostimulant-induced locomotion after MPTP3, 11, 17. In humans, elevated striatal VGLUT2 expression was discovered in brains of PD patients18. Consistent with this, VGLUT2-expressing DA neurons are enriched in the SNc of PD patients versus controls12, further suggesting that VGLUT2 boosts DA neuron resilience in PD. Overall, these findings suggest roles for VGLUT2 in selective DA neuron vulnerability.
Males are likelier to develop PD versus females19 and age remains the greatest risk factor in PD20. In Drosophila, the Drosophila ortholog of VGLUT, dVGLUT, mediates sex differences in vulnerability to DA neurodegeneration5. These studies showed that DA neuron dVGLUT expression increases with age, and RNA interference (RNAi)-mediated knockdown of dVGLUT in DA neurons increases age-related DA neurodegeneration5. Further, there are evolutionarily conserved sex differences in DA neuron VGLUT expression with females exhibiting greater DA neuron VGLUT expression than males in Drosophila, rats, and humans5. Consequently, DA neuron VGLUT is implicated in age- and sex-related differences in DA neurodegeneration. Nevertheless, questions remain concerning the translatability of these age and sex differences from animal models to clinical PD. Recent work showing greater resilience of VGLUT2-expressing SNc DA neurons was only conducted on male subjects12. Therefore, investigating DA neuron VGLUT2 expression in female PD patients is a critical next step and may provide a novel mechanism by which females are more protected from DA neurodegeneration in PD.
Though regulation of endogenous VGLUT2 expression appears neuroprotective for DA neurons, heterologous VGLUT2 overexpression in DA neurons offers conflicting results. In mice, DA neuron-specific VGLUT2 overexpression was selectively neuroprotective in one study and neurotoxic in another9, 11. Recent work demonstrated that the lowest levels of heterologous VGLUT2 overexpression are closest to physiological levels of upregulation which boost DA neuron resilience to MPTP5. In contrast, higher levels of VGLUT2 overexpression increase selective vulnerability of midbrain DA neurons5. Since most DA neurons only transiently express VGLUT2 during development before losing VGLUT2 expression by adulthood9, these mature DA neurons may not be equipped for sustained VGLUT2 overexpression. This suggests DA neuron VGLUT2 expression must be finely tuned through endogenous regulatory mechanisms to enhance DA neuron resilience. Circumventing such mechanisms by changing VGLUT2 expression at either extreme impacts resilience5. Thus, insufficient VGLUT upregulation may not boost neuronal resilience enough to withstand cell stress, whereas too much VGLUT2 expression diminishes resilience.
DA Neuron VGLUT2 and Selectivity Vulnerability in Pesticide Models of PD
Pesticide exposure can lead to DA neurodegeneration across several animal models and to PD in humans21–23. Pesticide models induce progressive DA neuron loss and reproduce key features of PD neuropathology that are not observed with neurotoxicants like 6OHDA or MPTP, including accumulation of intracellular α-synuclein and polyubiquitin aggregates in DA neurons22–27. We will focus on paraquat and rotenone, pesticides that cause selective DA neurodegeneration and differ mechanistically.
Paraquat, one of the most used pesticides globally, is associated with increased PD risk22. Occupational exposure to paraquat doubles PD risk and even indirect exposures boost PD risk28. In mice, paraquat causes SNc DA neurodegeneration, analogous to the pattern of cell loss in early PD29. Paraquat achieves these effects via generation of reactive oxygen species (ROS) that impair mitochondrial function and ultimately lead to DA neurodegeneration30 (Figure 1A).
Figure 1. Mechanisms of pesticide-induced SNc dopamine neurodegeneration.
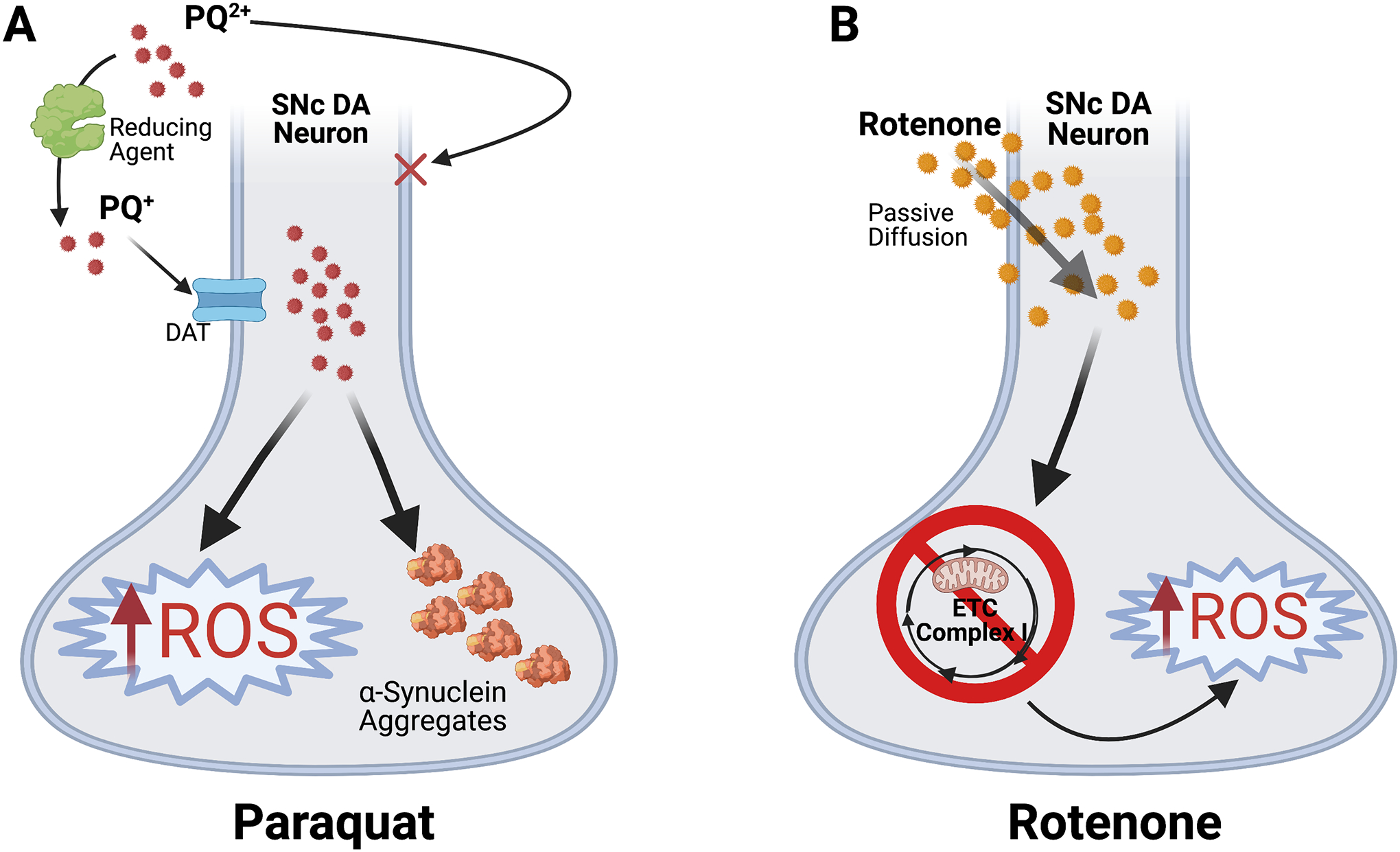
(A) Paraquat (PQ) enters the organism in its native divalent cation state, PQ2+, which cannot cross the plasma membrane. However, conversion to PQ+ by a reducing agent enables PQ+ to selectively enter DA neurons via the dopamine transporter (DAT)34. PQ+ accumulates in neurons where it induces increased ROS generation and α-synuclein aggregation35. Resulting oxidative stress contributes to SNc DA neurodegeneration. (B) Rotenone is lipophilic and freely crosses the membrane via passive diffusion36. Rotenone then induces ROS buildup and neurotoxic oxidative stress via direct inhibition of Complex I of the mitochondrial electron transport chain (ETC)25.
Rotenone is associated with sporadic PD and/or amplifying pre-existing PD risk25, 31. Rotenone exposure causes selective degeneration of nigral DA neurons through oxidative stress via direct inhibition of Complex I of the mitochondrial electron transport chain25 (Figure 1B). Chronic, systemic rotenone administration in rats recapitulates hallmark PD pathology, causing progressive deficits in neuroinflammatory and autophagy-lysosomal pathways, as well as accumulation of α-synuclein and polyubiquitin aggregates in DA neurons23, 25. Moreover, as in PD, VTA DA neurons are relatively spared after rotenone versus the SNc25.
Though the mechanisms underlying selective vulnerability of midbrain DA neurons remain unclear, we can glean insights using pesticide models. Vesicular transporters may offer mechanistic clues. Vesicular monoamine transporter 2 (VMAT2) facilitates DA loading into vesicles. In this process, VMAT2 sequesters DA into synaptic vesicles, diminishing cytoplasmic DA available for degradation into products that raise toxic ROS to damage mitochondria and injure DA neurons via oxidative stress32, 33. Besides VMAT2, VGLUT2 expression in DA neurons is also protective against rotenone10, consistent with VGLUT2’s ability to boost resilience in DA neurons across different PD models.
Mechanisms of Protection of VGLUT2-Expressing DA Neurons
Since PD is a pleiotropic, multifactorial illness based on complex gene-environment interactions, familial PD cases caused by mutations in specific genes have provided vital insights into PD pathogenesis and mechanisms of selective DA neuron vulnerability. In addition to α-synuclein, there is substantial evidence of PD associations with PINK1 and Parkin genes which encode a serine/threonine ubiquitin kinase and an E3 ubiquitin ligase, respectively37, 38. PINK1 and Parkin proteins have major functions in mitophagy, a form of autophagy involving the selective trafficking of mitochondria for degradation37, 39 (Figure 2A). Impairment or disruption of PINK1/Parkin functions contribute to PD pathogenesis39, 40. Consistent with this, cells with decreased PINK1 display reduced mitochondrial membrane potential, respiratory impairments, mitochondrial calcium overload, and heightened ROS production. PINK1 mutants that are no longer trafficked to mitochondria cannot form functional Parkin/PINK1 complexes, causing accumulation of damaged, dysfunctional mitochondria41, 42. This boosts mitochondrial ROS production as well as upregulates TH to increase cytoplasmic DA levels available for oxidation into DA quinones. These effects collectively contribute to ROS generation and oxidative stress43, ultimately leading to DA cell death in PD (Figure 2B).
Figure 2. Roles of Parkin/PINK1 in mitochondrial quality control and effects of PINK1 mutants in DA neurons.
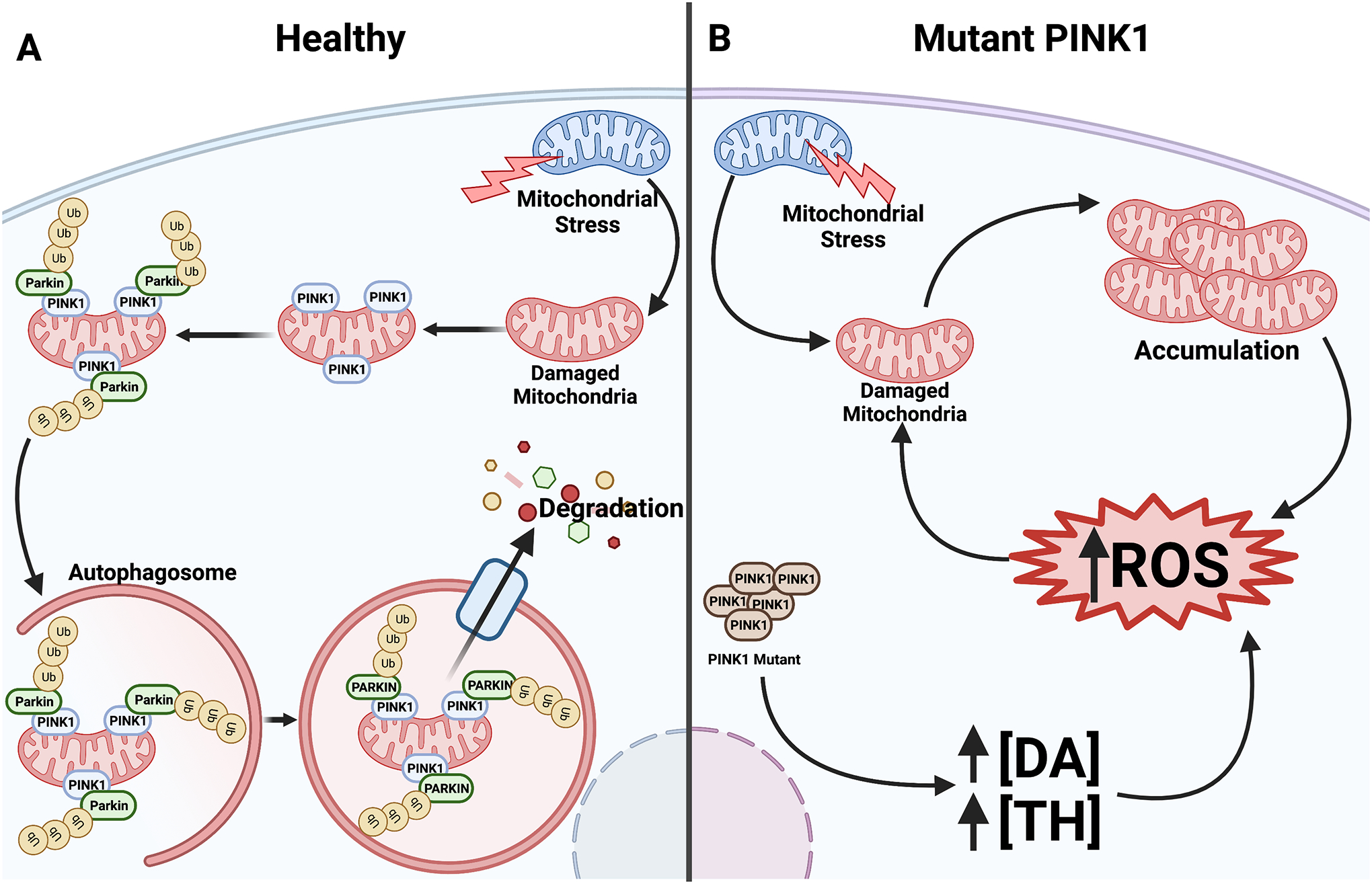
(A) PINK1 accumulates on damaged mitochondria, which signals the E3 ubiquitin ligase PARKIN to bind44. PARKIN requires PINK1 to activate it45, resulting in ubiquitination which targets the mitochondria for degradation by autophagosome-mediated mitophagy. (B) PINK1 mutants have difficulty localizing to and binding damaged mitochondria. In the absence of functional Parkin/PINK1 complexes to signal mitophagy, damaged mitochondria accumulate which leads to ROS buildup. Mutant PINK1 also upregulates TH expression to increase cytoplasmic DA. Resulting elevation in ROS generation further boosts oxidative stress to cause DA neurodegeneration.
An imbalance between ROS production and antioxidant activity leads to mitochondrial dysfunction and has been cited as potential cause of PD. PD-linked DJ-1 (encoded by PARK7) has a neuroprotective function by maintaining or restoring the balance between mitochondrial ROS generation and antioxidant activity. DJ-1 achieves this as an antioxidant scavenger or redox sensor, as well as a redox-sensitive chaperone that inhibits α-synuclein accumulation46, 47. Indeed, mice deficient in DJ-1 exhibit a fragmented mitochondrial phenotype which causes oxidative stress in DA neurons and can be rescued by PINK1 and Parkin46.
Studies in flies, rodents, and human brain suggest VGLUT (VGLUT2 in mammals and dVGLUT in Drosophila) expression in DA neurons mediates conserved neuroadaptive responses to insults which may play a role in selective vulnerability in PD5, 10. We posit that VGLUT’s ability to diminish cytotoxic intracellular ROS and maintain mitochondrial health is integral to its neuroprotective properties (Figure 3A). Several lines of evidence link DA neuron VGLUT to mitochondrial function: 1) Recent electron microscopy studies revealed that VGLUT2+ synaptic vesicles in nerve terminals projecting to the NAc are in closer proximity to mitochondria versus other vesicle populations48. 2) Mitochondrial availability is linked to synaptic dVGLUT levels49. 3) VGLUT2 enhances DA sequestration into synaptic vesicles during periods of increased neuronal activity3, 50. This diminishes cytoplasmic DA available for degradation into products that raise toxic ROS and produce mitochondrial dysfunction. Indeed, VGLUT2-expressing DA neurons are less vulnerable to rotenone-induced cell loss10. Finally, VGLUT2 may be an important modulator of axon arborization in DA neurons, suggesting that VGLUT2 may help DA neurons re-form axonal branching in response to stressors. VGLUT2 cKO in DA neurons impairs axonal reinnervation in the striatum in response to 6OHDA16. This relationship between VGLUT2 expression and axonal arborization may be regulated by glial-derived neurotrophic factor (GDNF)16 and brain-derived neurotrophic factor and tropomyosin-receptor kinase B (BDNF/TrkB) signaling11.
Figure 3. Potential mechanisms of VGLUT2-mediated protection from neurotoxicity.
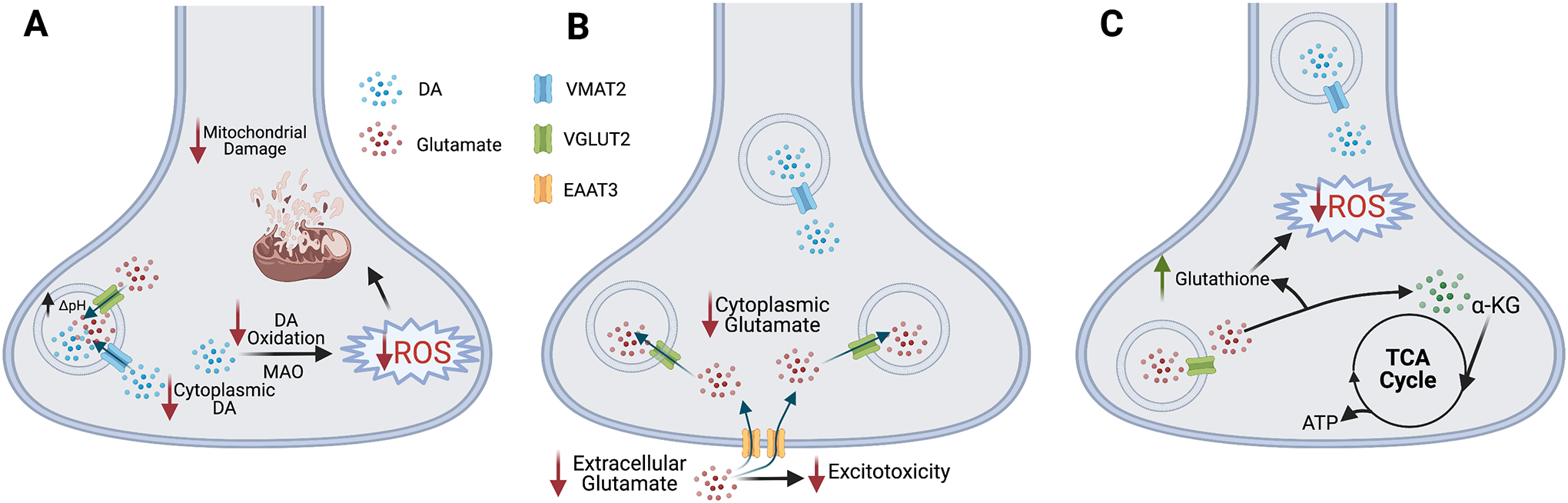
(A) VGLUT2 enhances DA loading into synaptic vesicles via VMAT2 by increasing the vesicular pH gradient (ΔpH)50. This decreases cytoplasmic DA levels, making less free DA available to generate neurotoxic ROS that damage mitochondria. (B) VGLUT2-mediated glutamate sequestration lowers cytoplasmic glutamate to diminish glutamatergic excitotoxicity. VGLUT2-expressing DA neurons also express EAAT3, which removes glutamate from the extracellular space, preventing glutamate-mediated excitotoxicity. (C) Glutamate can be converted to α-ketoglutarate (α-KG) to feed into the TCA cycle and maintain mitochondrial ATP production. Glutamate also replenishes glutathione to reduce ROS accumulation and protect DA neurons.
The greater resilience of VGLUT2-expressing DA neurons may also stem from glutamate-dependent mechanisms (Figures 3B, 3C). VGLUT2 limits glutamate availability by vesicular glutamate sequestration after insults to protect neurons from glutamate excitotoxicity51 (Figure 3B). Additionally, midbrain DA neurons express the glutamate transporter EAAT3. EAAT3 transports glutamate into the cell from the extracellular space and is dynamically trafficked at the plasma membrane to modulate synaptic glutamate signaling52. This suggests EAAT3 function in DA/glutamate neurons may boost resilience by increasing reuptake of synaptic glutamate as protection against glutamate-mediated excitotoxicity (Figure 3B). Moreover, glutamate serves as an anaplerotic energy source as well as a source of the antioxidant glutathione. Glutamate can be converted to α-ketoglutarate either directly in neurons or in nearby astrocytes to continue fueling the TCA cycle to maintain ATP synthesis during stress53, 54. Also, as glutathione synthesis depends on glutamate55, glutamate availability helps replenish glutathione to protect DA neurons from oxidative stress (Figure 3C). The combination of these mechanisms may explain the greater resiliency of DA neurons that co-release glutamate.
Finally, while the neuroprotective mechanisms proposed above are cell autonomous, VGLUT2-expressing DA neurons may also be protected through non-cell autonomous mechanisms. Recent work has begun to investigate this by focusing on DA/glutamate neuron projections to cholinergic interneurons56, 57. Glutamate co-release from VGLUT2-expressing DA neurons drives cholinergic interneuron burst-firing, causing acetylcholine-mediated stimulation of nicotinic acetylcholine receptors on the terminals of the DA/glutamate neurons to cause further DA/glutamate co-release7. Such a positive feedback loop may enable VGLUT2-expressing DA neurons to maintain their function and ultimately enable survival during cell stress.
Concluding Remarks and Future Perspectives
Many questions remain concerning the physiological relevance of DA/glutamate co-transmission and its relevance to healthy and disease states. Indeed, though almost all DA neurons express VGLUT2 as they develop, the regulatory mechanisms by which most DA neurons switch off VGLUT2 expression post-development or how these cells re-activate VGLUT2 expression in response to insults remain open questions. Increasing evidence shows that DA neuron VGLUT2 may act as a determinant of selective DA neuron vulnerability in PD. While we propose potential mechanisms by which VGLUT2 and glutamate modulate protection of DA neurons, future work is required to clarify these mechanisms. Elucidating these mechanisms may ultimately lead to new, more effective treatments for neuropsychiatric disorders including PD.
Acknowledgements
All figures were created with BioRender.com.
Funding
This work is supported by the National Institutes of Health F31NS118811 (SAB), R21AG068607 (ZF), R21DA052419 (ZF), and R21AA028800 (ZF).
Abbreviations
- α-KG
α-ketoglutarate
- cKO
conditional knockout
- DA
dopamine
- DAT
dopamine transporter
- EAAT3
excitatory amino acid transporter 3
- ETC
electron transport chain
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NAc
nucleus accumbens
- PD
Parkinson’s disease
- PQ
paraquat
- RNAi
RNA interference
- SNc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- VMAT2
vesicular monoamine transporter 2
- VTA
ventral tegmental area
- 6OHDA
6-hydroxydopamine
- VGLUT2
vesicular glutamate transporter 2
Footnotes
The authors declare no competing financial interest.
References
- 1.Sulzer D; Joyce MP; Lin L; Geldwert D; Haber SN; Hattori T; Rayport S, Dopamine neurons make glutamatergic synapses in vitro. J Neurosci 1998, 18 (12), 4588–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dal Bo G; St-Gelais F; Danik M; Williams S; Cotton M; Trudeau LE, Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. J Neurochem 2004, 88 (6), 1398–405. [DOI] [PubMed] [Google Scholar]
- 3.Hnasko TS; Chuhma N; Zhang H; Goh GY; Sulzer D; Palmiter RD; Rayport S; Edwards RH, Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron 2010, 65 (5), 643–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Root DH; Wang HL; Liu B; Barker DJ; Mod L; Szocsics P; Silva AC; Magloczky Z; Morales M, Glutamate neurons are intermixed with midbrain dopamine neurons in nonhuman primates and humans. Scientific reports 2016, 6, 30615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buck SA; Steinkellner T; Aslanoglou D; Villeneuve M; Bhatte SH; Childers VC; Rubin SA; De Miranda BR; O’Leary EI; Neureiter EG; Fogle KJ; Palladino MJ; Logan RW; Glausier JR; Fish KN; Lewis DA; Greenamyre JT; McCabe BD; Cheetham CEJ; Hnasko TS; Freyberg Z, Vesicular glutamate transporter modulates sex differences in dopamine neuron vulnerability to age-related neurodegeneration. Aging Cell 2021, e13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poulin JF; Caronia G; Hofer C; Cui Q; Helm B; Ramakrishnan C; Chan CS; Dombeck DA; Deisseroth K; Awatramani R, Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat Neurosci 2018, 21 (9), 1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mingote S; Amsellem A; Kempf A; Rayport S; Chuhma N, Dopamine-glutamate neuron projections to the nucleus accumbens medial shell and behavioral switching. Neurochem Int 2019, 129, 104482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumas S; Wallen-Mackenzie A, Developmental Co-expression of Vglut2 and Nurr1 in a Mes-Di-Encephalic Continuum Preceeds Dopamine and Glutamate Neuron Specification. Front Cell Dev Biol 2019, 7, 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinkellner T; Zell V; Farino ZJ; Sonders MS; Villeneuve M; Freyberg RJ; Przedborski S; Lu W; Freyberg Z; Hnasko TS, Role for VGLUT2 in selective vulnerability of midbrain dopamine neurons. J Clin Invest 2018, 128 (2), 774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buck SA; Miranda BR; Logan RW; Fish KN; Greenamyre JT; Freyberg Z, VGLUT2 is a determinant of dopamine neuron resilience in a rotenone model of dopamine neurodegeneration. J Neurosci 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen H; Marino RAM; McDevitt RA; Bi GH; Chen K; Madeo G; Lee PT; Liang Y; De Biase LM; Su TP; Xi ZX; Bonci A, Genetic deletion of vesicular glutamate transporter in dopamine neurons increases vulnerability to MPTP-induced neurotoxicity in mice. Proc Natl Acad Sci U S A 2018, 115 (49), E11532–E11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinkellner T; Conrad SW; Kovacs I; Rissman RA; Lee EB; Trojanowski JQ; Freyberg Z; Roy S; Luk KC; Lee VM; Hnasko TS, Dopamine neurons exhibit emergent glutamatergic identitiy in Parkinson’s disease. Brain 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindefors N; Ungerstedt U, Bilateral regulation of glutamate tissue and extracellular levels in caudate-putamen by midbrain dopamine neurons. Neurosci Lett 1990, 115 (2–3), 248–52. [DOI] [PubMed] [Google Scholar]
- 14.Berube-Carriere N; Riad M; Dal Bo G; Levesque D; Trudeau LE; Descarries L, The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. The Journal of comparative neurology 2009, 517 (6), 873–91. [DOI] [PubMed] [Google Scholar]
- 15.Dal Bo G; Berube-Carriere N; Mendez JA; Leo D; Riad M; Descarries L; Levesque D; Trudeau LE, Enhanced glutamatergic phenotype of mesencephalic dopamine neurons after neonatal 6-hydroxydopamine lesion. Neuroscience 2008, 156 (1), 59–70. [DOI] [PubMed] [Google Scholar]
- 16.Kouwenhoven WM; Fortin G; Penttinen AM; Florence C; Delignat-Lavaud B; Bourque MJ; Trimbuch T; Luppi MP; Salvail-Lacoste A; Legault P; Poulin JF; Rosenmund C; Awatramani R; Trudeau LE, VGluT2 Expression in Dopamine Neurons Contributes to Postlesional Striatal Reinnervation. J Neurosci 2020, 40 (43), 8262–8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birgner C; Nordenankar K; Lundblad M; Mendez JA; Smith C; le Greves M; Galter D; Olson L; Fredriksson A; Trudeau LE; Kullander K; Wallen-Mackenzie A, VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc Natl Acad Sci U S A 2010, 107 (1), 389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashani A; Betancur C; Giros B; Hirsch E; El Mestikawy S, Altered expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in Parkinson disease. Neurobiol Aging 2007, 28 (4), 568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jurado-Coronel JC; Cabezas R; Avila Rodriguez MF; Echeverria V; Garcia-Segura LM; Barreto GE, Sex differences in Parkinson’s disease: Features on clinical symptoms, treatment outcome, sexual hormones and genetics. Front Neuroendocrinol 2018, 50, 18–30. [DOI] [PubMed] [Google Scholar]
- 20.Reeve A; Simcox E; Turnbull D, Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res Rev 2014, 14, 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang A; Costello S; Cockburn M; Zhang X; Bronstein J; Ritz B, Parkinson’s disease risk from ambient exposure to pesticides. Eur J Epidemiol 2011, 26 (7), 547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldman SM, Environmental toxins and Parkinson’s disease. Annu Rev Pharmacol Toxicol 2014, 54, 141–64. [DOI] [PubMed] [Google Scholar]
- 23.Cannon JR; Tapias V; Na HM; Honick AS; Drolet RE; Greenamyre JT, A highly reproducible rotenone model of Parkinson’s disease. Neurobiol Dis 2009, 34 (2), 279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Miranda BR; Fazzari M; Rocha EM; Castro S; Greenamyre JT, Sex Differences in Rotenone Sensitivity Reflect the Male-to-Female Ratio in Human Parkinson’s Disease Incidence. Toxicological sciences : an official journal of the Society of Toxicology 2019, 170 (1), 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betarbet R; Sherer TB; MacKenzie G; Garcia-Osuna M; Panov AV; Greenamyre JT, Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 2000, 3 (12), 1301–6. [DOI] [PubMed] [Google Scholar]
- 26.Drolet RE; Cannon JR; Montero L; Greenamyre JT, Chronic rotenone exposure reproduces Parkinson’s disease gastrointestinal neuropathology. Neurobiology of disease 2009, 36 (1), 96–102. [DOI] [PubMed] [Google Scholar]
- 27.Tanner CM; Goldman SM; Ross GW; Grate SJ, The disease intersection of susceptibility and exposure: chemical exposures and neurodegenerative disease risk. Alzheimers Dement 2014, 10 (3 Suppl), S213–25. [DOI] [PubMed] [Google Scholar]
- 28.Tanner CM; Ross GW; Jewell SA; Hauser RA; Jankovic J; Factor SA; Bressman S; Deligtisch A; Marras C; Lyons KE; Bhudhikanok GS; Roucoux DF; Meng C; Abbott RD; Langston JW, Occupation and risk of parkinsonism: a multicenter case-control study. Archives of neurology 2009, 66 (9), 1106–13. [DOI] [PubMed] [Google Scholar]
- 29.Dwyer Z; Rudyk C; Farmer K; Beauchamp S; Shail P; Derksen A; Fortin T; Ventura K; Torres C; Ayoub K; Hayley S, Characterizing the protracted neurobiological and neuroanatomical effects of paraquat in a murine model of Parkinson’s disease. Neurobiol Aging 2021, 100, 11–21. [DOI] [PubMed] [Google Scholar]
- 30.Kuter K; Nowak P; Golembiowska K; Ossowska K, Increased reactive oxygen species production in the brain after repeated low-dose pesticide paraquat exposure in rats. A comparison with peripheral tissues. Neurochem Res 2010, 35 (8), 1121–30. [DOI] [PubMed] [Google Scholar]
- 31.Tanner CM; Kamel F; Ross GW; Hoppin JA; Goldman SM; Korell M; Marras C; Bhudhikanok GS; Kasten M; Chade AR; Comyns K; Richards MB; Meng C; Priestley B; Fernandez HH; Cambi F; Umbach DM; Blair A; Sandler DP; Langston JW, Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect 2011, 119 (6), 866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lohr KM; Bernstein AI; Stout KA; Dunn AR; Lazo CR; Alter SP; Wang M; Li Y; Fan X; Hess EJ; Yi H; Vecchio LM; Goldstein DS; Guillot TS; Salahpour A; Miller GW, Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo. Proc Natl Acad Sci U S A 2014, 111 (27), 9977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hastings TG; Lewis DA; Zigmond MJ, Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci U S A 1996, 93 (5), 1956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rappold PM; Cui M; Chesser AS; Tibbett J; Grima JC; Duan L; Sen N; Javitch JA; Tieu K, Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc Natl Acad Sci U S A 2011, 108 (51), 20766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manning-Bog AB; McCormack AL; Li J; Uversky VN; Fink AL; Di Monte DA, The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem 2002, 277 (3), 1641–4. [DOI] [PubMed] [Google Scholar]
- 36.Heinz S; Freyberger A; Lawrenz B; Schladt L; Schmuck G; Ellinger-Ziegelbauer H, Mechanistic Investigations of the Mitochondrial Complex I Inhibitor Rotenone in the Context of Pharmacological and Safety Evaluation. Scientific reports 2017, 7, 45465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mani S; Sevanan M; Krishnamoorthy A; Sekar S, A systematic review of molecular approaches that link mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurol Sci 2021, 42 (11), 4459–4469. [DOI] [PubMed] [Google Scholar]
- 38.Tan EK; Skipper LM, Pathogenic mutations in Parkinson disease. Hum Mutat 2007, 28 (7), 641–53. [DOI] [PubMed] [Google Scholar]
- 39.Park GH; Park JH; Chung KC, Precise control of mitophagy through ubiquitin proteasome system and deubiquitin proteases and their dysfunction in Parkinson’s disease. BMB Rep 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H; Chan DC, Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet 2009, 18 (R2), R169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okatsu K; Oka T; Iguchi M; Imamura K; Kosako H; Tani N; Kimura M; Go E; Koyano F; Funayama M; Shiba-Fukushima K; Sato S; Shimizu H; Fukunaga Y; Taniguchi H; Komatsu M; Hattori N; Mihara K; Tanaka K; Matsuda N, PINK1 autophosphorylation upon membrane potential dissipation is essential for Parkin recruitment to damaged mitochondria. Nat Commun 2012, 3, 1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ando M; Fiesel FC; Hudec R; Caulfield TR; Ogaki K; Gorka-Skoczylas P; Koziorowski D; Friedman A; Chen L; Dawson VL; Dawson TM; Bu G; Ross OA; Wszolek ZK; Springer W, The PINK1 p.I368N mutation affects protein stability and ubiquitin kinase activity. Mol Neurodegener 2017, 12 (1), 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou ZD; Refai FS; Xie SP; Ng SH; Chan CH; Ho PG; Zhang XD; Lim TM; Tan EK, Mutant PINK1 upregulates tyrosine hydroxylase and dopamine levels, leading to vulnerability of dopaminergic neurons. Free Radic Biol Med 2014, 68, 220–33. [DOI] [PubMed] [Google Scholar]
- 44.Ge P; Dawson VL; Dawson TM, PINK1 and Parkin mitochondrial quality control: a source of regional vulnerability in Parkinson’s disease. Mol Neurodegener 2020, 15 (1), 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gladkova C; Maslen SL; Skehel JM; Komander D, Mechanism of parkin activation by PINK1. Nature 2018, 559 (7714), 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irrcher I; Aleyasin H; Seifert EL; Hewitt SJ; Chhabra S; Phillips M; Lutz AK; Rousseaux MW; Bevilacqua L; Jahani-Asl A; Callaghan S; MacLaurin JG; Winklhofer KF; Rizzu P; Rippstein P; Kim RH; Chen CX; Fon EA; Slack RS; Harper ME; McBride HM; Mak TW; Park DS, Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet 2010, 19 (19), 3734–46. [DOI] [PubMed] [Google Scholar]
- 47.Tanudjojo B; Shaikh SS; Fenyi A; Bousset L; Agarwal D; Marsh J; Zois C; Heman-Ackah S; Fischer R; Sims D; Melki R; Tofaris GK, Phenotypic manifestation of α-synuclein strains derived from Parkinson’s disease and multiple system atrophy in human dopaminergic neurons. Nature communications 2021, 12 (1), 3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinkellner T; Madany M; Haberl MG; Zell V; Li C; Hu J; Mackey M; Ramachandra R; Adams S; Ellisman MH; Hnasko TS; Boassa D, Genetic Probe for Visualizing Glutamatergic Synapses and Vesicles by 3D Electron Microscopy. ACS Chem Neurosci 2021, 12 (4), 626–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu JY; Hannan SB; Drager NM; Vereshchagina N; Krahl AC; Fu Y; Elliott CJH; Han Z; Jahn TR; Rasse TM, Autophagy inhibition rescues structural and functional defects caused by the loss of mitochondrial chaperone Hsc70–5 in Drosophila. Autophagy 2021, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aguilar JI; Dunn M; Mingote S; Karam CS; Farino ZJ; Sonders MS; Choi SJ; Grygoruk A; Zhang Y; Cela C; Choi BJ; Flores J; Freyberg RJ; McCabe BD; Mosharov EV; Krantz DE; Javitch JA; Sulzer D; Sames D; Rayport S; Freyberg Z, Neuronal Depolarization Drives Increased Dopamine Synaptic Vesicle Loading via VGLUT. Neuron 2017, 95 (5), 1074–1088 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blandini F; Porter RH; Greenamyre JT, Glutamate and Parkinson’s disease. Mol Neurobiol 1996, 12 (1), 73–94. [DOI] [PubMed] [Google Scholar]
- 52.Underhill SM; Wheeler DS; Li M; Watts SD; Ingram SL; Amara SG, Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons. Neuron 2014, 83 (2), 404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Divakaruni AS; Wallace M; Buren C; Martyniuk K; Andreyev AY; Li E; Fields JA; Cordes T; Reynolds IJ; Bloodgood BL; Raymond LA; Metallo CM; Murphy AN, Inhibition of the mitochondrial pyruvate carrier protects from excitotoxic neuronal death. J Cell Biol 2017, 216 (4), 1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plaitakis A; Shashidharan P, Glutamate transport and metabolism in dopaminergic neurons of substantia nigra: implications for the pathogenesis of Parkinson’s disease. Journal of neurology 2000, 247 Suppl 2, Ii25–35. [DOI] [PubMed] [Google Scholar]
- 55.Franco R; Cidlowski JA, Apoptosis and glutathione: beyond an antioxidant. Cell death and differentiation 2009, 16 (10), 1303–14. [DOI] [PubMed] [Google Scholar]
- 56.Chuhma N; Mingote S; Yetnikoff L; Kalmbach A; Ma T; Ztaou S; Sienna AC; Tepler S; Poulin JF; Ansorge M; Awatramani R; Kang UJ; Rayport S, Dopamine neuron glutamate cotransmission evokes a delayed excitation in lateral dorsal striatal cholinergic interneurons. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai Y; Ford CP, Dopamine Cells Differentially Regulate Striatal Cholinergic Transmission across Regions through Corelease of Dopamine and Glutamate. Cell Rep 2018, 25 (11), 3148–3157 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]


