Abstract
Coronavirus disease 2019 (COVID-19) infection evokes severe proinflammatory storm and pulmonary infection with the number of confirmed cases (more than 200 million) and mortality (5 million) continue to surge globally. A number of vaccines (e.g., Moderna, Pfizer, Johnson/Janssen and AstraZeneca vaccines) have been developed over the past two years to restrain the rapid spread of COVID-19. However, without much of effective drug therapies, COVID-19 continues to cause multiple irreversible organ injuries and is drawing intensive attention for cell therapy in the management of organ damage in this devastating COVID-19 pandemic. For example, mesenchymal stem cells (MSCs) have exhibited promising results in COVID-19 patients. Preclinical and clinical findings have favored the utility of stem cells in the management of COVID-19-induced adverse outcomes via inhibition of cytokine storm and hyperinflammatory syndrome with coinstantaneous tissue regeneration capacity. In this review, we will discuss the existing data with regards to application of stem cells for COVID-19.
Keywords: COVID-19, Stem cells, Organ damage, Cytokine storm, MicroRNA
Graphical Abstract
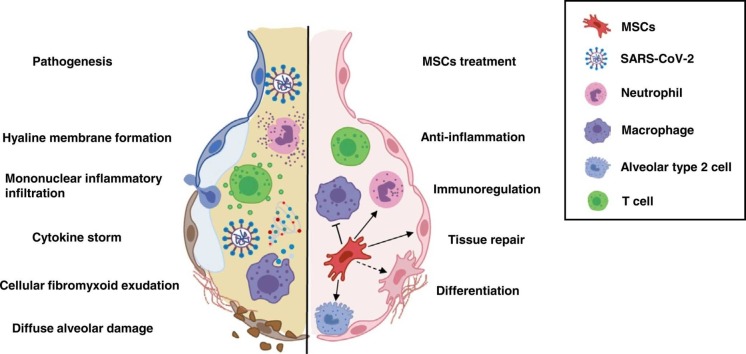
Proposed mechanisms for MSCs function in severe COVID-19 patients.
1. Introduction
Novel coronavirus infectious disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is rapidly spreading, afflicting 200 million world populations with > 5 million mortalities over the past two years. Compared with SARS-CoV, SARS-CoV-2 displays a much stronger contingency. COVID-19 patients present fever, fatigue, cough, pneumonia, and acute respiratory distress syndrome (ARDS) at the advanced stages [1], [2]. Other than respiratory symptoms, COVID-19 evokes cardiovascular symptoms such as cardiac anomalies, chest pain, palpitation, and acute cardiovascular injury, particularly those with pre-existing cardiovascular issues [3], [4], [5]. Reminiscent of influenza and human immunodeficiency virus (HIV), SARS-CoV-2 viruses carry RNAs as their genome and possess a high rate of mutation. Newly identified SARS-CoV-2 variant has greatly challenged the development of vaccines and therapies. For instance, antibody-based treatment developed against one variant must be redesigned following viral mutation. Meanwhile, viral variants develop resistance against antiviral drugs and mild viral variants may spontaneously become virulent [6]. In this context, therapeutic and preventive measures are imminently needed to deal with viral mutations. Nevertheless, COVID-19 infected patients are currently left with only symptomatic management, given limited available information for COVID-19 treatment despite intense efforts towards vaccines and therapeutics [7]. Antiviral drugs used for COVID-19 such as remdesivir, hydroxychloroquine and lopinavir/ritonavir are often without high-quality proof validating their effectiveness [8], [9], [10]. Besides, adverse side effects including diarrhea, nausea, vomiting, fatigue, hepatotoxicity, arrhythmias, cognitive deficit and delirium have been reported following these drug administrations [11], [12]. Apart from direct antiviral treatment for COVID-19, immunomodulatory therapeutic regimens have been suggested to potentially retard disease progression and save life. Many immunotherapeutic approaches were used in COVID-19 patients, including convalescent plasma therapy, glucocorticoid therapy, and anti-interleukin (IL-6) receptor antibody therapy [13], [14], [15]. However, glucocorticoid can dampen the ability to fight against infection and impose severe sequelae such as osteonecrosis of the femoral head, in spite of alleviation in toxic symptoms associated with pulmonary infection [16], [17]. Consequently, unfavorable side effects and uncertain treatment efficacy warrant further in-depth studies to fully elucidate the safety and effectiveness of immunomodulatory strategies. Specific treatment options are still lacking for severe and critical cases. At this point, blood purification and extracorporeal membrane oxygenation (ECMO) device are the predominant lifesaving therapies for COVID-19-induced ARDS and refractory respiratory failure. However, underlying compound immune damages originated from extracorporeal circuit initiation during ECMO therapy should be considered [18]. When classical medicine treatment, mechanical ventilation and ECMO cannot reconcile lung function in critically ill COVID-19 patients, lung transplantation is currently the only effective strategy although not a routine for end-stage pulmonary disease [19]. Notably, cell-based therapies utilizing stem cells, especially mesenchymal stem cells (MSCs), represent a spotlight of therapeutic research for human diseases. Due to the feasibility, high proliferative rate, multi-linage differentiation ability and free of ethical problems, MSCs may provide an emerging avenue for ameliorating adverse effects of COVID-19 [20]. Here we cover recent contemporary viewpoints with regards to the benefit and challenges of stem cell-based COVID-19 therapy.
2. Methods
2.1. Search strategy
A comprehensive search was conducted in PubMed for relevant published articles through February 2022. The National Institutes of Health (NIH) guidelines were referenced for treatment recommendations. Clinicaltrials.gov and clinicaltrialsregister.eu were referenced for registered ongoing clinical trials. The following keywords were used: “SARS-CoV-2”, “COVID-19”, “stem cells”, “mesenchymal stem cells”, “cell therapy”, “tissue repair”, “organ damage”, “cytokine storm”, “microRNA”, “extracellular vesicles”, “human pluripotent stem cell”, and “organoid” with the Boolean operators “OR” and “AND”. To ensure capture of all relevant articles, the reference lists of selected articles were searched.
2.2. Inclusion criteria
In vitro, pre-clinical in vivo and clinical interventional studies were included where stem cells or MSCs-derived extracellular vesicles were used as therapies to manage COVID-19-induced multiple irreversible organ injuries. Journal articles, clinical trials, case reports, meta-analyses, and systematic reviews were included only if reported data were pertinent to the application of stem cells for COVID-19 treatment.
2.3. Exclusion criteria
We excluded studies involving other respiratory infected diseases such as SARS and MERS, or studies in a language other than English. Publications prior COVID-19 were excluded except for highly regarded and widely referenced ones.
2.4. Study quality assessment
Title and abstract screening were conducted independently by two reviewers (YEL and AA) using pre-defined inclusion and exclusion criteria. Publications which did not fulfill the eligibility criteria were excluded. Subsequent full-text review was performed by the same reviewers. MD and JR were consulted for resolving the differences in opinion. Study quality evaluation were based on the PRISMA checklist.
2.5. Data extraction
The following descriptive information was extracted from study texts, tables, and figures: study characteristics (publication year, study design, and country of study origin); clinical characteristics (symptoms, complications, and population); characteristics of intervention (types, dosage, and frequency), and main findings (outcomes such as adverse reactions, prognosis, clinical laboratory and radiological parameters).
3. Results
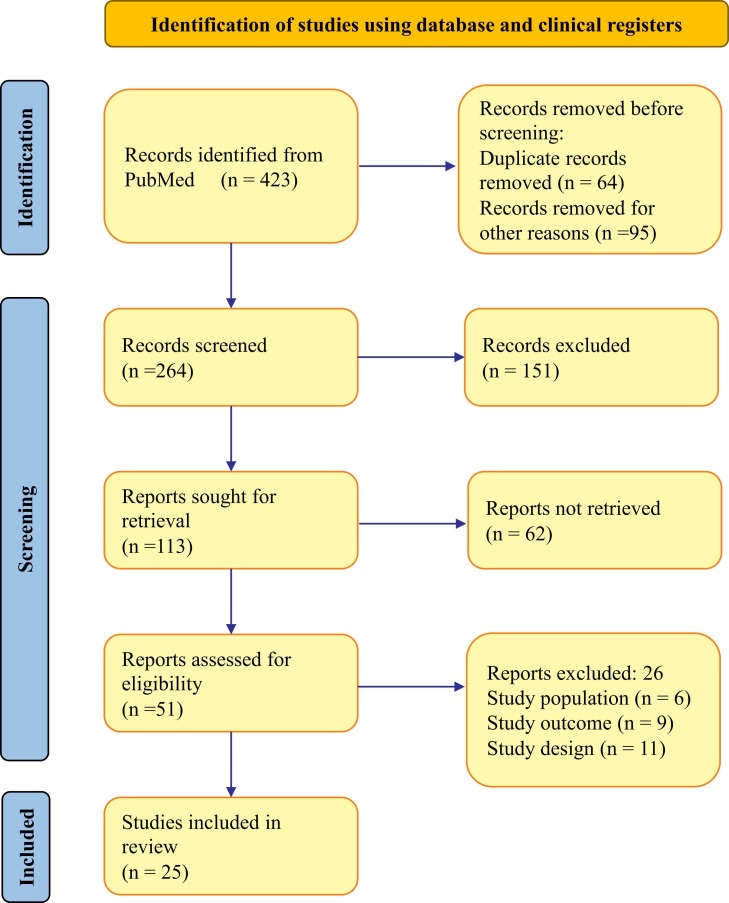
Our systemic search of PubMed database yielded 423 studies, among which 159 reports were excluded due to duplication or other reasons. Following the subsequent screening of the titles and abstracts, 51 articles met the inclusion criteria and underwent for full evaluation. Of these, 26 publications were removed according to the exclusion criteria, resulting in inclusion of 25 studies here in this review to gain insight on MSCs treatment for patients with COVID-19. Fig. 1 summarizes the PRISMA flowchart of the search and selection procedure.
Fig. 1.
PRISMA diagram showing the flow chart of report search and screening.
Despite ample undergoing clinical trials, few results have been released regarding the clinical efficacy of MSCs in pneumonia treatment in COVID-19 patients ( Table 1). In a case of an elderly female COVID-19 patient, intravenous delivery of human umbilical cord-MSCs (three doses every 3 days) were found to safely and sufficiently modulate immune responses favoring tissue repair [21]. Clinical improvement was evident after the second administration. Moreover, inflammatory cells/neutrophils declined, whereas, lymphocyte count was elevated [21]. Besides, a single-center open-labeled study including 7 COVID-19 patients with distinct severity reveals benefit of MSCs infusion. In particular, MSCs treatment elevated peripheral lymphocyte counts, reduced C‐reactive protein (CRP) and diminished hyperactivated cytokine producing immune cells (e.g., CXCR3 + natural killer cells, CXCR3 +CD4 + and CXCR3 +CD8 + T cells), leading to suppression of inflammation. Furthermore, TNF‐α levels declined simultaneously with an increase in IL-10 following MSCs treatment. Moreover, MSCs conferred protection and rejuvenation of alveolar epithelial cells, and modulated lung milieu to improve pulmonary function and retard pulmonary fibrosis. Besides, RNA-Seq analysis revealed that transplanted MSCs were immune against COVID-19 infection due to suppression of TMPRSS2 and ACE2, and release of paracrine and anti-inflammatory factors including brain derived neurotrophic factor (BDNF), nerve growth factor (NGF), VEGF, nitric oxide associated 1 (NOA1), leukemia inhibitory factor (LIF), galanin and gmap prepropeptide, a neuroendocrine peptide (GAL), TGF-β, epidermal growth factor (EGF), fibroblast growth factor (FGF), and HGF. All patients receiving stem cells survived. These results have pinpointed potential safety and efficacy of stem cell administration in COVID-19 patients [22]. Moreover, multiple clinical trials found similar outcomes with suppressed pro-inflammatory cytokines, solid lung lesion and improved reserve capacity following umbilical cord MSCs treatment in COVID-19 patients, along with prolonged survival time and shortened recovery duration [23], [24], [25], [26], [27]. In another clinical study, allogeneic adipose tissue-MSCs were delivered in 13 mechanical ventilated COVID-19 patients. Injection of two intravenous doses of adipose tissue-MSCs was found to abate inflammatory factors including IL-6, lactate dehydrogenase (LDH), ferritin, and CRP, as well as enhancement of CD4 + /CD8 + T cells and B-lymphocytes [28]. Furthermore, fibrinogen and D-dimer protein (a product of fibrin degradation) were remarkably declined 5 days following the first dose, thus, preventing thromboembolism in these COVID-19 patients [28]. Overall, outcomes from registered clinical trials are largely in favor of the efficacy and safety of stem cells in COVID-19 therapy.
Table 1.
Clinical studies of MSCs treatment for patients with COVID-19.
| Clinical trial ID | Study design | Phase | MSCs’ source | Indications | Dose and route of administration | N | General outcomes |
|---|---|---|---|---|---|---|---|
| ChiCTR2000029990[22] | Open label, single center, case-control | Phase 1 | MSCs | Moderate/Severe/Critical | 1 round of 1 × 106 cells/kg, IV | 7 | Markedly ameliorated symptoms and pulmonary dysfunction. |
| NCT04355728[23] | Double‐blind, randomized trial | Phase 1/2a | Umbilical cord | ARDS | 2 rounds of 10 ± 2 × 107 cells/round on days 0 and 3, IV | 24 | Safety for UC-MSCs, significantly reduced proinflammatory cytokines and improved patient prognosis. |
| NCT04288102[24] | Randomized, double-blind, placebo-controlled trial | Phase 2 | Umbilical cord | Severe | 3 rounds of 4 × 107 cells/dose on days 0, 3 and 6, IV | 100 | Safety for UC-MSCs, improved overall lung lesion volume, solid-component lesion. |
| ChiCTR2000031494[25] | Open-label, randomized, standard treatment-controlled trial | Phase 1 | Umbilical cord | Severe/Critical | 1 round of 2 × 106 cells/kg, IV | 41 | Clinical improvement following MSCs delivery |
| NCT04252118[26] | Open label, single center, case-control | Phase 1 | Umbilical cord | Moderate/Severe | 3 rounds of 3 × 107 cells/dose on days 0, 3 and 6, IV | 18 | Safe and well-tolerated intravenous administration of UC-MSCs to patients with moderate and severe COVID-19. |
| IRCT20200217046526N2 [27] |
Phase 1 | Placental and umbilical cord | ARDS | 3 rounds of 2 × 108 cells/round on days 0, 2 and 4, IV | 11 | Relieved respiratory distress and decreased inflammatory biomarkers by MSCs. | |
| NCT04348461[28] | Prospective nonrandomized open-label cohort | Phase 1 | Adipose-derived | Severe/Critical | 1, 2 or 3 rounds of 1 × 106 cells/kg, IV | 13 | No adverse events, amelioration of biological, radiological and ventilatory parameters correlated to clinical response. |
| National Medical Products Administration of China[90] | Case report | Allogenic, UC-MSCs |
Critical | 3 rounds of 5 × 107 cells/dose, IV | 1 | Remission of inflammation symptom, good tolerance, improved patient prognosis. | |
| ChiCTR2000029606[91] | Case report | Allogeneic, menstrual blood-derived | ARDS | 3 rounds of 1 × 106 cells/kg on days 0, 1 and 3, IV | 2 | Improved and well-tolerated partial pressure of oxygen (PO2) and fraction of inspired O2 (FiO2). | |
| National Medical Products Administration of China[92] | Prospective nonrandomized open-label cohort | Phase 1 | hESC-IMRCs | COVID-19 patients with lung fibrosis | 1–3 rounds of 3 × 106 cells/kg, IV | 27 | Reduced pulmonary fibrotic lesions. |
ARDS acute respiratory distress syndrome; hESC-IMRCs human embryonic stem cell-derived immunity and matrix-regulatory cells; IV intravenous injection; MSC mesenchymal stem cell; UC-MSCs umbilical cord mesenchymal stem cells; 6MWD 6-minute walking distance
4. Discussion
4.1. Pathogenic characteristics of COVID-19
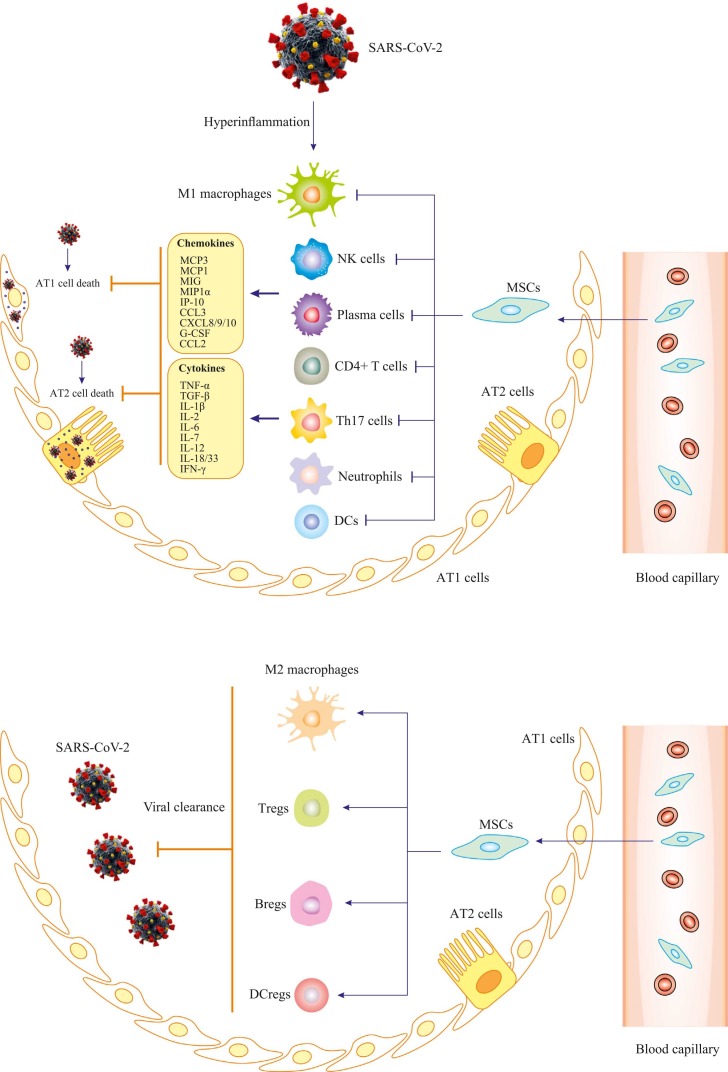
SARS-CoV-2 belongs to a single-strand RNA Coronaviridae family. Genomic analysis of SARS-CoV-2 revealed that spike protein (S-protein) in viral structure binds to angiotensin converting enzyme 2 (ACE2) in host cells [29]. Host proteases including transmembrane protease serine protease 2 (TMPRSS2), cathepsin L, and furin then cleave S-protein to facilitate endocytosis of SARS-CoV-2. Single-cell RNA sequencing revealed that TMPRSS2 is co-expressed with ACE2 in lungs, bronchial branches and nasal epithelial cells, indicating tropism of SARS-CoV-2 infection [30], [31], [32]. Besides, the cluster of differentiation 147 (CD147) transmembrane protein provides a novel route for SARS-CoV-2 invasion, albeit with low affinity for S-protein compared with ACE2. This receptor provides a reasonable explication for lower risk of COVID-19 infection in females, higher susceptibility in the elderly and patients with co-morbidities such as diabetes mellitus, obesity and asthma [5], [33]. Following cell entrance, virus replicates to elicit a series of cell damaging effects, triggering immune response for clearance of the virus [34]. COVID-19 patients often display high levels of pro-inflammatory cytokines including IL-1β, IL-6, interferon-γ (IFN-γ) and chemokines (CCL2 and CXCL10), associated with massive lung involvement [3]. Postmortem examination revealed bilateral diffuse injury of alveolus, accompanied by fibrous mucinous exudation and interstitial mononuclear inflammatory infiltration in COVID-19 patients ( Fig. 2, Fig. 3) [35]. Therefore, it is vital to manage infection timely to avoid development of critical conditions.
Fig. 2.
Proposed mechanisms for MSCs function in severe COVID-19 patients. Firstly, SARS-CoV-2 infects respiratory tract; infiltration of immune cells (monocytes/macrophages, neutrophils, natural killer cells, T cells and B cells) increases; then cytokine storm (such as TNF‐α, IL-2, IL-6, MCP1 and IP10) occurs. Hyaline membrane formation, cellular fibrous mucinous exudation and diffuse alveolar damage develop. After stem cells administration, the number of infiltrated immune cells significantly reduces, and damaged lung tissue is repaired. MSCs have properties of anti-inflammation, immunoregulation and tissue regeneration, although detailed mechanisms underlying these roles remain to be further elucidated. MCP3, monocyte-chemotactic protein 3; MCP1, monocyte-chemotactic protein 1; MIG, monokine induced by gamma; MIP1α, macrophage inflammatory protein 1α; IP-10, interferon gamma-induced protein 10; CCL3, C-C motif chemokine ligand 3; CXCL8/9/10, C-X-C motif chemokine ligand 8/9/10; G-CSF, granulocyte colony-stimulating factor; CCL2, C-C motif chemokine ligand 2; IL-1β, IL-1 beta protein; IFN-γ, interferon gamma; NK cells, natural killer cells; DCs, dendritic cells; Th17, T helper 17 cells; Tregs, T regulatory cells; Bregs, B regulatory cells.
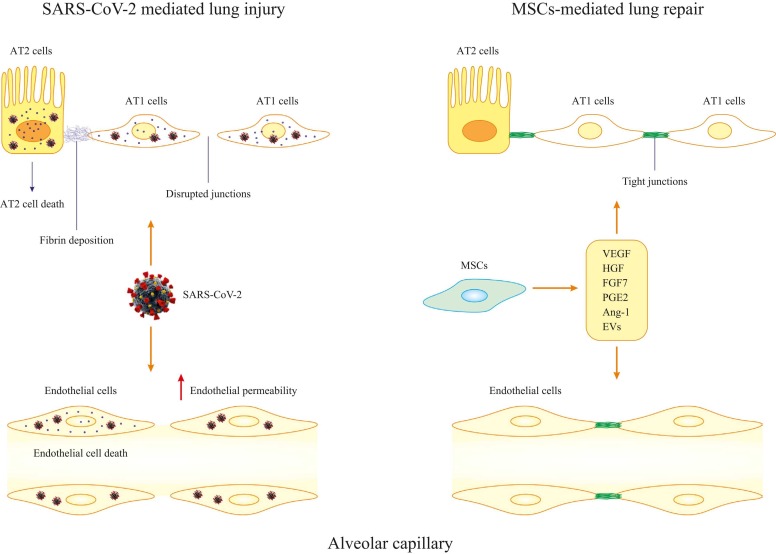
Fig. 3.
MSCs-mediated lung repair. During invasion of SARS-CoV-2 to lung alveolar capillary, both endothelial cells and epithelial cells are damaged. Cell death and disruption of cell junctions are vividly observed during the infection. However, application of MSCs leads to reformation of tight junctions and suppression of cell death in both endothelial cells and epithelial cells.
Despite major advances in the understanding of SARS-CoV2 and COVID-19 since late 2019, the pathobiology of COVID-19 remains poorly elucidated. Different cell types are infected at various stages of disease progression [36]. Upon inhalation, the nasal cavity hosts SARS-CoV-2, where it binds epithelial cells through ACE2, initiating viral replication [37]. Besides, in vitro evidence suggested that SARS-CoV mainly infects ciliated cells in airways, although low expression of ACE2 in airway conducting cells makes this doubtful [38]. Hence, it is perceived that virus is locally propagated while the response level of innate immune system might be limited. At this low burden stage of CoV-2 infection, nasal swabs help to identify viral infection [39]. Later on, SARS-CoV descends the respiratory tract, resulting in ignition of more immune responses. For advanced stages, CXCL10 levels are indicative of clinical outcomes [40]. Of note, virus-harboring epithelial cells produce interferon-λ (IFN-λ) and interferon-β (IFN-β) [41]. In addition, CXCL10 could be utilized as a possible SARS-CoV marker. Eighty percent of COVID-19 patients exhibit mild symptoms with infection mainly within the upper airways. Eventually, 20% of these patients manifest pulmonary infiltrates, which subsequently develops into severe anomaly [36]. In more advanced stage, SARS-CoV infects pulmonary gas exchange compartments and granular pneumocytes (type II cells), particularly, subpleural and peripheral cells [42]. Consequently, SARS-CoV disseminate within type II cells, and ample viral particles are liberated, causing apoptosis [43]. The endpoints would be manifestations of dispersed alveolar impairment, pulmonary fibrosis, and elimination of majority of alveolar type II cells, culminating in abnormal wound healing and severe fibrosis and scaring.
4.2. MSCs: A promising treatment
Cell-induced and particularly stem cell treatment has become a potential therapeutic method for incurable diseases. For stem cell-based therapy, MSCs have attracted much attention [44], [45]. MSCs are pluripotent stem cells originating from placenta, umbilical blood and bone marrows in autologous and allogenic sources, widely used for viral diseases including acute lung injury (ALI) in influenza, chronic hepatitis in hepatitis B virus (HBV) and HIV [46], [47]. MSCs inhibit cytokine storm and hyperinflammatory syndrome in COVID-19 patients with coinstantaneous innate immunity strengthening, in addition to preservation of lung alveolar endothelial and epithelial cells and facilitation of clearance ability [48], [49]. This is consistent with the property of MSCs in migrating to the site of injury to secrete paracrine factors and modulate epi- and endo-thelial cell permeability and inflammation. In addition to inflammation and infection, MSCs are capable of repairing and regenerating epithelial and endothelial tissues within pulmonary alveoli through restoration of cellular element in the niche. After receiving diverse signals from lung damage, MSCs secrete various growth factors [e.g., transforming growth factor β1 (TGF-β1), platelet-derived growth factor (PDGF) and neurotrophin] in extracellular matrix (ECM). MSCs also release ECM components (such as laminin 5 and decorin) to mimic the effect of growth factor [50], [51]. Apart from paracrine factors, MSCs also mediate tissue regeneration by transporting organelles such as mitochondria through tunnel nanotube or vesicular transfer. Mitochondrial transfer from damaged cells promotes recipients to restore functionality and avoid the emergence of excessive inflammation [52]. MSCs also serves as precursors for specialized compositions to replenish niche cells. Based on engrafted microenvironment, MSCs will change their immune phenotypes. For example, toll-like receptors 4 (TLR4) activation switches MSCs to the pro-inflammatory phenotype MSC1, whereas an anti-inflammatory phenotype of MSC2 will be endowed via Poly(I:C) activation of TLR3 [50], [53]. In immunoregulation of host cells, MSC2 will differentiate into new lung epithelial tissues to repair lung scarring in COVID-19 patients, thus attenuating response elicited by viral injury, and fostering macrophages pro-angiogenic phenotype to stimulate lung tissue regeneration in COVID-19 patients (Fig. 2, Fig. 3) [54].
Notably, MSCs offer protection against idiopathic pulmonary fibrosis and ALI, indicating the promises of MSCs in COVID-19 pulmonary fibrosis [55]. MSCs are expected to benefit cardiovascular damage in COVID-19. MSCs promote angiogenesis in a paracrine manner, for example, secretion of angiogenic factors [56]. Besides, MSCs may promote cardiac injury recovery through facilitation of resident myocardial cell proliferation, related to enhanced DNA synthesis and signal gene [e.g., vascular endothelial growth factor (VEGF) and cyclin A2] in cardiomyocytes, while suppressing apoptosis [57]. Application of MSCs in complications evoke by COVID-19 is supported by the FDA approval of Emergency Use Authorization (EUA). A number of clinical trials are underway using MSCs (Table 1) thus it is pertinent to examine the number, source and patient groups for therapy. Here we cover recent contemporary viewpoints with regards to the benefit and challenges of stem cell-based therapy.
4.3. MSCs-derived extracellular vesicles in COVID-19 therapy
Although MSCs treatment has become a potential therapeutic modality for COVID-19 patients, limitation and challenge exist for its application such as variations in safety and cell viability. Intriguingly, MSCs-originated extracellular vesicles (EVs) may dodge or minimize these shortcomings to fully elaborate immunoregulatory, anti-inflammatory and anti-fibrotic properties of MSCs. EVs usually refer to microvesicles (MVs) and exosomes. Exosomes are membraned compartments around 200 nm in diameter or less generated by the majority of eukaryotic cells. However, MVs ranges around 1000 nm in diameter [58]. There is a growing notion that MSCs-derived EVs may be implemented for cell-free therapeutic purposes owing to their endocrine/paracrine effects and safety compared to cell-based therapies such as low excessive cell proliferation and contamination with malignant cells [59], [60]. Characteristics of EVs encompass reduced risk of immune responses, less proliferative thus low risk of tumorigenesis, and small size to easily pass through capillaries [61]. Mechanistically, EVs bind targeted membrane receptors and transfer their contents to cytosol via endocytosis [62]. MSCs-derived EVs carry inside their lipid bilayer, immune regulators, cytokines, messenger RNAs (mRNAs) and small non-coding RNAs (ncRNAs) from parental cells which can modulate behavior of target cells [63], [64], [65]. EVs are suggested to exhibit great clinical potential for the delivery of microRNAs (miRNAs), as negative regulators of mRNA [66]. miRNAs are endogenous small ncRNAs of 18–25 nucleotides which can be found in MSCs-originated EVs, and are linked to post-transcriptional gene degradation or repression [67]. They are unstable and small in structures and have a short half-life in the circulation, thus techniques capable of ameliorating the delivery to specific sites and prolonging the half-life are essential. As such, EVs may be advantageous in serving as transfer cargo carriers. After internalizing of EVs by target cells, miRNAs are released into cytoplastic matrix and with the help of enzyme complexes, they bind to target mRNA via base-pairing in the 3’ untranslated region (UTR) or 5’ UTR gene regions. Given that complementary miRNAs potentially target virus and play an antiviral role by binding to conserved 3’ UTR, targeting 3’ UTR within viral genome might be a critical strategy for antiviral efficacy [68]. Indeed, Chauhan and colleagues demonstrated that miRNAs function in host cells through decreasing receptors expression, avoiding viral invasion and replication, and minimizing the spread of infection [69].
Ample evidence has revealed that the MSCs-EVs-miRNAs combination mainly pinpoints proteins such as BCL2 apoptosis regulator (BCL2), NLR family pyrin domain containing 3 (NLRP3), prolyl 4-hydroxylase subunit alpha 1(P4HA1), insulin like growth factor 1 receptor (IGF1R), Cyclin G1 (CCNG1), signal transducer and activator of transcription 3 (STAT3), semaphorin 3 A (SEMA3A), phosphatase and tensin homolog (PTEN), methyl-CpG binding protein 2 (MECP2), dynamin-related protein 1 (DRP1), and transforming growth factor beta receptor I (TGFBR1) [70], [71]. Besides, bioinformatic analysis predicted that miRNA carried by MSCs-originated EVs suppresses proinflammatory cytokines, relieves coagulation and cell death [28]. Specifically, miR-125a-3p attaches to 3’ UTR of IFN, tumor necrosis factor (TNF) and Factor XIII genes. This multi-targeted method helps to greatly reduce cell death, coagulation disorder and systemic inflammation in patients with severe COVID-19, resulting in improved clinical prognosis. Two other miRNAs, miR-202–3p and miR-769–3p might mitigate cell death preventing tissue injury via synergistically binding to the 3’ UTR of IFN and TNF genes inhibiting their protein translation. Moreover, let-7e-5p targets the 3’ UTR of CASP8, RIPK1 and TNF genes involved in cell death signaling pathway, it also attaches to the 3’ UTR of Factor VIII gene of the coagulation cascade [6], [72]. Based on this analysis, multi-target feature of MSCs-originated EVs miRNAs might be advantageous in comparison with strategies gearing towards a single pathway, for example, Anakira or Tocilizumab. On the other hand, a prospective nonrandomized open-label cohort study examined exosomes (ExoFlo™) originated from allogeneic bone marrow MSCs in the treatment for COVID-19. ExoFlo™ was found to effectively restore oxygenation capacity, downregulate cytokine storm, reconstitute immunity and improve prognosis in COVID-19 patients [73]. Overall, MSCs-derived EVs seem to be a promising tool to be implemented in clinical settings.
For ALI and ARDS in COVID-19, MSCs are believed to execute their efficacy by targeting endothelial, inflammatory and infectious factors. For instance, MSCs release IL-13, IL-6, granulocyte macrophage-colony stimulating factor (GM-CSF), prostaglandin E2 (PGE2), and keratinocyte growth factor-2 (KGF2), which boost phagocytosis and confer anti-fibrotic, anti-apoptotic, and anti-inflammatory effects [74], [75], [76] (Fig. 2, Fig. 3). As a result of PGE2 secretion, pro-inflammatory M1 macrophages are polarized to the anti-inflammatory M2 macrophages [77]. Also, released growth factors including hepatocyte growth factor (HGF), VEGF, and KGF2 confer protection of alveolar cells by restoring ATP and capillary barrier [78], [79]. Moreover, MSCs suppress chemotactic characteristics, differentiation, and propagation of B cells [80] (Fig. 3). Besides, multiple preclinical examinations demonstrated the therapeutic potential of MSCs-EVs in ARDS, ALI, and other pulmonary conditions mainly through reduction of inflammation and epithelial damage and increased clearance of edema [81], [82]. Intratracheal MSCs administration was evinced to enhance MSCs accessibility in pulmonary endothelium and alveolar epithelium [83].
MSCs-derived exosomes suppress cytokine secretion and reinitiate anti-viral defenses of the host [84]. EVs obtained from bone marrow-MSCs have been applied in the treatment of ARDS. EVs were found to suppress edema and inflammation in the lung reminiscent of MSCs [85]. It was revealed that MSCs-induced manipulation of macrophages in a murine model of ARDS was mainly attributed to EVs [86]. In addition, MSC-EVs exerted protective impacts on lipopolysaccharide-mediated lung injury via modulation of alveolar macrophages in murine models [86]. Also, EVs-mediated transfer of healthy mitochondria culminated in macrophage polarization from M1 to M2 owing to the higher oxidative phosphorylation [86]. Furthermore, MSC-EVs-transferred mitochondria boosted lung injury repair due to increased mitochondrial activity in human alveolar cells [87].
4.4. Ideal COVID-19 patient candidates for stem cell therapy
Most patients do not display noticeable symptoms in early stage of COVID-19 infection. Only a small fraction (2%) of patients experienced sore throat, cough, fever and muscle pain. As the disease progresses, patients suffer shortness of breath, leading to suddenly deteriorated health. Severe immune deficiency appears to be the primary mortality cause, as attested by excessive storm of cytokines such as TNF, IL-2, IL-6, MCP1 and IP10 [3]. In advanced COVID-19 infection, immune system is overactivated and produces excess cytokines, creating an unpleasant environment. As such, cytokine storm prompts respiratory dysfunction, secondary infection, organ (including cardiac) damage and ARDS, leaving patients with difficulty of breath and ultimately death [88]. It is advised that stem cells be given to critically ill COVID-19 patients needing ventilation (respiration rate > 30 times/min, resting pulse oxygen saturation < 93%, partial pressure of PaO2/FuO2 < 300 mmHg) as choices for better prognosis [89]. Stem cells promote respiratory function and ameliorate self-defensive machinery including antibody production. Besides, elderly COVID-19 patients with comorbidities including asthma, diabetes and cardiovascular diseases are least likely to recovery from COVID-19 infection. With disease progression, the regenerative ability and immune system of these populations are further compromised, making them severely prone to SARS-CoV-2. Given the impaired immune system, it takes much longer to produce antibody against the virus, resulting in development of severe pneumonia and ARDS in these patients. Stem cell treatment seems to impose hope for these individuals not only for COVID-19 infection but also for comorbidities [22], [90], [91], [92].
4.5. Future modalities to enhance MSCs efficacy in COVID-19 therapy
Although MSCs are capable of migrating to sites of injury, the number of implantations is relatively small. In order for MSCs to migrate to injured tissues, the tissue must chemoattract MSCs from bloodstream first, which relies on markers on MSC surface [such as vascular cell adhesion molecule (VCAM)− 1, CD18, CD24, CD44], as well as their interaction with particular markers in target tissues [93]. Modification of MSCs coating has revealed promises for strengthening MSCs treatment potency in COVID-19 in preclinical models [94]. Coating MSCs with sialyl Lewis X (SLeX), an important mediator identified on white blood cell surface, participates in migration of leucocyte to inflammatory tissues, the first step in migration process. Interestingly, migration of SLeX-modified MSCs to the inflammatory endothelium is reinforced in vivo compared to original MSCs [95]. Another approach of MSCs engineering is to target antigens expressed in diseased sites using antibodies. For example, an in vivo study employed anti-VCAM-1 to modify MSCs prior to infusion into an experimental colitis model. Increased migration of anti-VCAM-1-modified MSCs to damaged sited was observed [96]. Moreover, biocompatible/biodegradable MSC coating is advantageous in MSC retention in cardiac tissues and can be engineered to reinforce retention in lung tissues after intravenous administration [97], [98].
Similarly, nanotechnology is explored to reinforce MSCs delivery and increase treatment potency in COVID-19. Nanocarriers act as a delivery platform for cells, drugs and vaccines due to their specificity, selectivity and continued release. Chitosan is a safe, biocompatible and biodegradable polymer for drug deliver to lungs in infectious diseases. Due to its permeability and mucoadhesive capacity, as well as its ability to attach to specific site, it serves as a particulate conveyor for drugs in the lung [99]. Thus, integrating MSCs with chitosan hydrogel can improve their treatment potency by enhancing targeting, adhesion and permeation. Mehta and colleagues inferred favorable outcomes of nano-carriers including nanotheranostics, polysaccharide nanoparticles and mesoporous silica nanoparticles in COVID-19 treatment. Hence, integration with MSCs represents a novel COVID-19 therapy [100]. Besides, several nanomaterials were shown to exhibit anti-viral potential (e.g., heparan sulfate proteoglycan, gold nanoparticles), and their integration with MSCs may duplicate their treatment efficacy [101]. It is noteworthy that nano-synthetic stem cells (LIFNano) can inhibit cytokine storm in COVID-19 patients [102]. LIFNano carries LIF approximately 1000 times more efficiently compared with the MSCs-releasing soluble LIF, thus offering protection against ALI [103]. Therefore, LIFNano can be regarded as an substitute for MSC treatment due to its high volume and capacity to suppress cytokine storm as well as repair injured lung tissue [102].
Furthermore, MSCs can be preconditioned with other compounds in order to synergize their effect and improve prognosis in COVID-19 patients. For example, MSCs can be pretreated with vitamin D that serves as a valid immunoregulator [104]. Because MSCs may undergo through apoptosis following infusion, a process that can be alleviated by preconditioning with antioxidants. Astaxanthin, a strong antioxidant, has a protective effect for MSCs via surmounting oxidative stress; reducing hydrogen peroxide which can evoke apoptosis; and increasing the production of natural cell antioxidants, for example, heme oxygenase-1 (HO-1). Such procedures can help protect MSCs and improve their survival under hostile circumstances, thereby improving their treatment potency in COVID-19 patients [105]. Intriguingly, selenium is regarded as a low-toxic antioxidant with antiviral characteristic. It can be applied in the original form or rather in nanoform (nanoSe), integrating with MSCs to relive COVID-19 symptoms [106].
4.6. Novel stem cell- and organoid-based modeling for SARS-CoV-2 research
As a substitute for immortalized cell lines or model organisms, human pluripotent stem cells (hPSCs) can be leveraged as versatile extracorporal models providing human-based data for COVID-19 study. hPSCs comprise human embryonic stem cells (hESCs) and human induced PSCs (hiPSCs) produced by somatic cell reprogramming [107]. Given the short life of SARS-CoV-2, its multi-organ targeting/damage, difficulty of acquisition of target organs, classical models cannot fully satisfy the research purpose. Nonetheless, hPSC-derived models can expedite evaluation of preclinical toxicity and safety as well as the effective planning of clinical trials compared with regular modeling systems. Specifically, organoids are stem cell-originated self-structured three-dimensional cultures in vitro and imitate the physiological environment of natural organs [108], [109], [110]. Lung organoid constructed by pneumocytes expressing ACE2 mimicked interferon-mediated inflammatory response in SARS-CoV-2 infection with less surface surfactants and apoptosis in alveolar cells [111]. Moreover, Pei and coworker employed hESCs to establish lung organoids including alveolar and airway organoids and identified that club, ciliated, and alveolar type 2 cells (AT2s) were mostly prone to SARS-CoV-2 infection. RNA sequencing disclosed early cellular responses to virus infection including an unanticipated downregulation of metabolism, particularly lipid metabolism, as well as the well-known upregulation of immune response [112]. Besides, hiPSCs-derived AT2s adapted to air-liquid interface culture exhibited upregulation of proinflammation signaling cascade including NF-κB following COVID-19 infection in conjunction with abnormal alveolar structure [113]. With regards to the orientation of SARS-CoV-2 infection, human pancreatic β cells and hepatic organoids were deemed highly susceptible to SARS-CoV-2 infection, consolidating the potential utility of adult primary islets and cholangiocyte and hepatocyte organoids [114]. Indeed, hPSC-derived cells/organoids provide valued models for comprehending cellular/organ responses to SARS-CoV-2 infection.
Regarding to cardiotoxicity of SARS-CoV-2, several studies have employed myocardial cells originated from hiPSCs and noted elevated levels of viral RNA in the culture medium, along with compromised contractility and beating rhythm as well as apparent apoptosis. Molecular analysis revealed that SARS-CoV-2 of hPSC-derived cardiomyocytes (hPSC-CMs) was relied on cathepsin-dependent endosomal pathway for viral entry. Furthermore, viral infection elicited innate immunoreaction in the hPSC-CM model via upregulating interleukin immunomodulator and anti-viral pathway genes [115], [116]. Moreover, hPSC-CMs also displayed great potentiality as model for investigating cardiac safety of novel anti-SARS-CoV-2 drugs from clinical perspectives. In order to evaluate the anti-viral effects of a group of protein kinase inhibitors, Garcia and colleagues analyzed these compounds and noted that berzosertib, an ataxia telangiectasia and Rad3-related (ATR) kinase inhibitor, exhibited a profound effect on SARS-CoV-2 infection of hPSC-CMs [117]. Similarly, Mills and associates utilized hPSC-originated cardiac organoids to examine the impact of bromodomain protein (BRD) inhibitors on BRD4, an epigenetic regulator of cardiac malfunction evoked by SARS-CoV-2 infection. These authors found that the inhibitor INCB054329 was able to prevent cardiac cytokine storm-evoked diastolic dysfunction [118]. Future work utilizing hPSC-derived cardiovascular cells should be advantageous to pave the way for the management of cardiovascular complications in COVID-19 patients.
5. Limitations
The present study suffers from several limitations. First, due to the methodology employed, the sample size was modest, and information discussed is primarily for critically ill COVID-19 patients. Nonetheless, MSCs seem to be useful for COVID-19 treatment without severe adverse effects. Besides, majority of studies included here lacked the proper control group, and might have a moderate to high risk of bias due to short follow-up period, inappropriate study designs, therapeutic regimens and dosage of drugs. It would be difficult to draw confirmative conclusion on cell-based therapy due to the absence of reliable data from controlled, randomized, multicenter trials.
6. Perspectives and conclusions
With the escalating COVID-19 pandemic, effective interventions are pertinent to alleviate the burden on health system. Stem cell-based cell therapy has shown promises in COVID-19 management. In particular, MSCs might probably be a good choice for COVID-19 patients, given their accessibility from various tissues, MSCs can be cryopreserved until utilized, with their feature and potency characterized in pre-clinical and clinical research. Nevertheless, some apprehensions must be considered with regards to the use of stem cells for COVID‐19 infection. For example, yielding for sufficient stem cells in a short time period is a challenge for emergency care in critical COVID-19 cases. In vitro, amplification of MSCs can be time-consuming and might decrease the potency of amplified cells. Therefore, a better understanding of stem cell treatment is necessary to satisfy the clinical application of COVID-19. Further work should focus on issues involving generation of genetically modified MSCs and large abundance of EVs capable of safely transporting therapeutic components or factors. Ultimately, optimizing clinical-grade MSCs and consensus on registered clinical trials dependent on cellular product characteristics and delivery mode will facilitate laying foundation for safe and efficacious MSCs treatment of COVID-19.
Author contributions
YEL and AA were involved in acquisition, analysis, interpretation of data and drafting of the manuscript. MD and JR were involved in study concept and design, and medical writing assistance. All authors read and approved the final manuscript.
Conflict of Interest Statement
None of the authors have any potential conflict of interest to declare.
Acknowledgments
None.
References
- 1.Su M., Xu S., Weng J. A bibliometric study of COVID-19 research in Web of Science. Pharm. Res. 2021;169 doi: 10.1016/j.phrs.2021.105664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaco R., Leoni O., Cukaj G., Ercolanoni M., Carnovale C. A new stratification model for a population health risk assessment, based on a large cohort of patients infected by COVID-19. Pharm. Res. 2021;168 doi: 10.1016/j.phrs.2021.105598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lionetti V., Bollini S., Coppini R., Gerbino A., Ghigo A., Iaccarino G., Madonna R., Mangiacapra F., Miragoli M., Moccia F., Munaron L., Pagliaro P., Parenti A., Pasqua T., Penna C., Quaini F., Rocca C., Samaja M., Sartiani L., Soda T., Tocchetti C.G., Angelone T. Understanding the heart-brain axis response in COVID-19 patients: A suggestive perspective for therapeutic development. Pharm. Res. 2021;168 doi: 10.1016/j.phrs.2021.105581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren J., Wu N.N., Wang S., Sowers J.R., Zhang Y. Obesity cardiomyopathy: evidence, mechanisms, and therapeutic implications. Physiol. Rev. 2021;101(4):1745–1807. doi: 10.1152/physrev.00030.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J.H., Choi Y., Lim C.W., Park J.M., Yu S.H., Kim Y., Han H.J., Kim C.H., Song Y.S., Kim C., Yu S.R., Oh E.Y., Lee S.M., Moon J. Potential Therapeutic Effect of Micrornas in Extracellular Vesicles from Mesenchymal Stem Cells against SARS-CoV-2. Cells. 2021;10:9. doi: 10.3390/cells10092393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho W.S., Zhang R., Tan Y.L., Chai C.L.L. COVID-19 and the promise of small molecule therapeutics: Are there lessons to be learnt? Pharm. Res. 2022;179 doi: 10.1016/j.phrs.2022.106201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen M.S. Hydroxychloroquine for the Prevention of Covid-19 - Searching for Evidence. N. Engl. J. Med. 2020;383(6):585–586. doi: 10.1056/NEJMe2020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siemieniuk R.A., Bartoszko J.J., Ge L., Zeraatkar D., Izcovich A., Kum E., Pardo-Hernandez H., Qasim A., Martinez J.P.D., Rochwerg B., Lamontagne F., Han M.A., Liu Q., Agarwal A., Agoritsas T., Chu D.K., Couban R., Cusano E., Darzi A., Devji T., Fang B., Fang C., Flottorp S.A., Foroutan F., Ghadimi M., Heels-Ansdell D., Honarmand K., Hou L., Hou X., Ibrahim Q., Khamis A., Lam B., Loeb M., Marcucci M., McLeod S.L., Motaghi S., Murthy S., Mustafa R.A., Neary J.D., Rada G., Riaz I.B., Sadeghirad B., Sekercioglu N., Sheng L., Sreekanta A., Switzer C., Tendal B., Thabane L., Tomlinson G., Turner T., Vandvik P.O., Vernooij R.W., Viteri-García A., Wang Y., Yao L., Ye Z., Guyatt G.H., Brignardello-Petersen R. Drug treatments for covid-19: living systematic review and network meta-analysis. Bmj. 2020;370:m2980. doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao Y.C., Deng Q.X., Dai S.X. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: An evaluation of the evidence. Travel Med Infect. Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izcovich A., Siemieniuk R.A., Bartoszko J.J., Ge L., Zeraatkar D., Kum E., Qasim A., Khamis A.M., Rochwerg B., Agoritsas T., Chu D.K., McLeod S.L., Mustafa R.A., Vandvik P., Brignardello-Petersen R. Adverse effects of remdesivir, hydroxychloroquine and lopinavir/ritonavir when used for COVID-19: systematic review and meta-analysis of randomised trials. BMJ Open. 2022;12(3) doi: 10.1136/bmjopen-2020-048502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yildirim F.S., Sayan M., Sanlidag T., Uzun B., Ozsahin D.U., Ozsahin I. Comparative evaluation of the treatment of COVID-19 with multicriteria decision-making techniques. J. Health Eng. 2021;2021:8864522. doi: 10.1155/2021/8864522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., Kong Y., Ren L., Wei Q., Mei H., Hu C., Tao C., Yang R., Wang J., Yu Y., Guo Y., Wu X., Xu Z., Zeng L., Xiong N., Chen L., Wang J., Man N., Liu Y., Xu H., Deng E., Zhang X., Li C., Wang C., Su S., Zhang L., Wang J., Wu Y., Liu Z. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. Jama. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D., Criner G.J., Kaplan-Lewis E., Baden R., Pandit L., Cameron M.L., Garcia-Diaz J., Chávez V., Mekebeb-Reuter M., Lima de Menezes F., Shah R., González-Lara M.F., Assman B., Freedman J., Mohan S.V. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N. Engl. J. Med. 2021;384(1):20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres A., Sibila O., Ferrer M., Polverino E., Menendez R., Mensa J., Gabarrús A., Sellarés J., Restrepo M.I., Anzueto A., Niederman M.S., Agustí C. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. Jama. 2015;313(7):677–686. doi: 10.1001/jama.2015.88. [DOI] [PubMed] [Google Scholar]
- 17.Zhao J.P., Hu Y., Du R.H., Chen Z.S., Jin Y., Zhou M., Zhang J., Qu J.M., Cao B. [Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):183–184. doi: 10.3760/cma.j.issn.1001-0939.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Henry B.M. COVID-19, ECMO, and lymphopenia: a word of caution. Lancet Respir. Med. 2020;8(4) doi: 10.1016/S2213-2600(20)30119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han W., Zhu M., Chen J., Zhang J., Zhu S., Li T., Cai H., Fang Q., Wei G., Liang T. Lung Transplantation for Elderly Patients With End-Stage COVID-19 Pneumonia. Ann. Surg. 2020;272(1):e33–e34. doi: 10.1097/SLA.0000000000003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh T.U., Parida S., Lingaraju M.C., Kesavan M., Kumar D., Singh R.K. Drug repurposing approach to fight COVID-19. Pharm. Rep. 2020;72(6):1479–1508. doi: 10.1007/s43440-020-00155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang B., Chen J., Li T., Wu H., Yang W., Li Y., Li J., Yu C., Nie F., Ma Z. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: s case report. Medicine. 2020;99:31. doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., Fan J., Wang W., Deng L., Shi H., Li H., Hu Z., Zhang F., Gao J., Liu H., Li X., Zhao Y., Yin K., He X., Gao Z., Wang Y., Yang B., Jin R., Stambler I., Lim L.W., Su H., Moskalev A., Cano A., Chakrabarti S., Min K.J., Ellison-Hughes G., Caruso C., Jin K., Zhao R.C. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanzoni G., Linetsky E., Correa D., Messinger Cayetano S., Alvarez R.A., Kouroupis D., Alvarez Gil A., Poggioli R., Ruiz P., Marttos A.C., Hirani K., Bell C.A., Kusack H., Rafkin L., Baidal D., Pastewski A., Gawri K., Leñero C., Mantero A.M.A., Metalonis S.W., Wang X., Roque L., Masters B., Kenyon N.S., Ginzburg E., Xu X., Tan J., Caplan A.I., Glassberg M.K., Alejandro R., Ricordi C. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021;10(5):660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi L., Huang H., Lu X., Yan X., Jiang X., Xu R., Wang S., Zhang C., Yuan X., Xu Z., Huang L., Fu J.L., Li Y., Zhang Y., Yao W.Q., Liu T., Song J., Sun L., Yang F., Zhang X., Zhang B., Shi M., Meng F., Song Y., Yu Y., Wen J., Li Q., Mao Q., Maeurer M., Zumla A., Yao C., Xie W.F., Wang F.S. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target Ther. 2021;6(1):58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shu L., Niu C., Li R., Huang T., Wang Y., Huang M., Ji N., Zheng Y., Chen X., Shi L., Wu M., Deng K., Wei J., Wang X., Cao Y., Yan J., Feng G. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):361. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng F., Xu R., Wang S., Xu Z., Zhang C., Li Y., Yang T., Shi L., Fu J., Jiang T., Huang L., Zhao P., Yuan X., Fan X., Zhang J.Y., Song J., Zhang D., Jiao Y., Liu L., Zhou C., Maeurer M., Zumla A., Shi M., Wang F.S. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct. Target Ther. 2020;5(1):172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashemian S.R., Aliannejad R., Zarrabi M., Soleimani M., Vosough M., Hosseini S.E., Hossieni H., Keshel S.H., Naderpour Z., Hajizadeh-Saffar E., Shajareh E., Jamaati H., Soufi-Zomorrod M., Khavandgar N., Alemi H., Karimi A., Pak N., Rouzbahani N.H., Nouri M., Sorouri M., Kashani L., Madani H., Aghdami N., Vasei M., Baharvand H. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 2021;12(1):91. doi: 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sánchez-Guijo F., García-Arranz M., López-Parra M., Monedero P., Mata-Martínez C., Santos A., Sagredo V., Álvarez-Avello J.-M., Guerrero J.E., Pérez-Calvo C. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cascella M., Rajnik M., Aleem A., Dulebohn S.C., Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. [PubMed]
- 30.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T., Winter H., Meister M., Veith C., Boots A.W., Hennig B.P., Kreuter M., Conrad C., Eils R. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. Embo J. 2020;39(10) doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang K., Chen W., Zhang Z., Deng Y., Lian J.Q., Du P., Wei D., Zhang Y., Sun X.X., Gong L., Yang X., He L., Zhang L., Yang Z., Geng J.J., Chen R., Zhang H., Wang B., Zhu Y.M., Nan G., Jiang J.L., Li L., Wu J., Lin P., Huang W., Xie L., Zheng Z.H., Zhang K., Miao J.L., Cui H.Y., Huang M., Zhang J., Fu L., Yang X.M., Zhao Z., Sun S., Gu H., Wang Z., Wang C.F., Lu Y., Liu Y.Y., Wang Q.Y., Bian H., Zhu P., Chen Z.N. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target Ther. 2020;5(1):283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Z., McGoogan J.M. Characteristics of and important lessons From the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. Jama. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reyfman P.A., Walter J.M., Joshi N., Anekalla K.R., McQuattie-Pimentel A.C., Chiu S., Fernandez R., Akbarpour M., Chen C.-I., Ren Z. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am. J. Respir. Crit. care Med. 2019;199(12):1517–1536. doi: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. The. Eur. Respir. J. 2020;55:4. doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang N.L., Chan P.K., Wong C.K., To K.F., Sung Wu.A.K., Hui Y.M., Sung D.S., Lam J.J. CW. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin. Chem. 2005;51(12):2333–2340. doi: 10.1373/clinchem.2005.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hancock A.S., Stairiker C.J., Boesteanu A.C., Monzón-Casanova E., Lukasiak S., Mueller Y.M., Stubbs A.P., García-Sastre A., Turner M., Katsikis P.D. Transcriptome analysis of infected and bystander type 2 alveolar epithelial cells during influenza A virus infection reveals in vivo wnt pathway downregulation. J. Virol. 2018;92:21. doi: 10.1128/JVI.01325-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J., Wu X., Zeng W., Guo D., Fang Z., Chen L., Huang H., Li C. Chest CT findings in patients with coronavirus disease 2019 and its relationship with clinical features. Invest. Radiol. 2020;55(5):257–261. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian Z., Travanty E.A., Oko L., Edeen K., Berglund A., Wang J., Ito Y., Holmes K.V., Mason R.J. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am. J. Respir. Cell Mol. Biol. 2013;48(6):742–748. doi: 10.1165/rcmb.2012-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golchin A., Farahany T.Z., Khojasteh A., Soleimanifar F., Ardeshirylajimi A. The clinical trials of mesenchymal stem cell therapy in skin diseases: an update and concise review. Curr. Stem Cell Res Ther. 2019;14(1):22–33. doi: 10.2174/1574888X13666180913123424. [DOI] [PubMed] [Google Scholar]
- 45.Benavides-Castellanos M.P., Garzon-Orjuela N., Linero I. Effectiveness of mesenchymal stem cell-conditioned medium in bone regeneration in animal and human models: a systematic review and meta-analysis. Cell Regen. 2020;9(1):5. doi: 10.1186/s13619-020-00047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Behnke J., Kremer S., Shahzad T., Chao C.M., Böttcher-Friebertshäuser E., Morty R.E., Bellusci S., Ehrhardt H. MSC based therapies-new perspectives for the injured lung. J. Clin. Med. 2020;9:3. doi: 10.3390/jcm9030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kallmeyer K., Ryder M.A., Pepper M.S. Mesenchymal stromal cells: a possible reservoir for HIV-1? Stem Cell Rev. Rep. 2022 doi: 10.1007/s12015-021-10298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arabpour E., Khoshdel S., Tabatabaie N., Akhgarzad A., Zangiabadian M., Nasiri M.J. Stem cells therapy for COVID-19: a systematic review and meta-analysis. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.737590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y., Wei Z., Ma X., Xu J., Zhao X., Cao Q., Di G. Efficacy and safety of mesenchymal stromal cells therapy for COVID-19 infection: a systematic review and meta-analysis. Curr. Stem Cell Res Ther. 2021 doi: 10.2174/1574888X16666211206145839. [DOI] [PubMed] [Google Scholar]
- 50.Sagaradze G.D., Basalova N.A., Efimenko A.Y., Tkachuk V.A. Mesenchymal Stromal Cells as Critical Contributors to Tissue Regeneration. Front Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.576176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Purcaru O.S., Artene S.A., Barcan E., Silosi C.A., Stanciu I., Danoiu S., Tudorache S., Tataranu L.G., Dricu A. The Interference between SARS-CoV-2 and Tyrosine Kinase Receptor Signaling in Cancer. Int J. Mol. Sci. 2021;22:9. doi: 10.3390/ijms22094830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahrouf-Yorgov M., Augeul L., Da Silva C.C., Jourdan M., Rigolet M., Manin S., Ferrera R., Ovize M., Henry A., Guguin A., Meningaud J.P., Dubois-Randé J.L., Motterlini R., Foresti R., Rodriguez A.M. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 2017;24(7):1224–1238. doi: 10.1038/cdd.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurte M., Vega-Letter A.M., Luz-Crawford P., Djouad F., Noël D., Khoury M., Carrión F. Time-dependent LPS exposure commands MSC immunoplasticity through TLR4 activation leading to opposite therapeutic outcome in EAE. Stem Cell Res Ther. 2020;11(1):416. doi: 10.1186/s13287-020-01840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shammaa R., El-Kadiry A.E., Abusarah J., Rafei M. Mesenchymal stem cells beyond regenerative medicine. Front Cell Dev. Biol. 2020;8:72. doi: 10.3389/fcell.2020.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gad E.S., Salama A.A.A., El-Shafie M.F., Arafa H.M.M., Abdelsalam R.M., Khattab M. The Anti-fibrotic and Anti-inflammatory Potential of Bone Marrow-Derived Mesenchymal Stem Cells and Nintedanib in Bleomycin-Induced Lung Fibrosis in Rats. Inflammation. 2020;43(1):123–134. doi: 10.1007/s10753-019-01101-2. [DOI] [PubMed] [Google Scholar]
- 56.Zhu M., Chu Y., Shang Q., Zheng Z., Li Y., Cao L., Chen Y., Cao J., Lee O.K., Wang Y., Melino G., Lv G., Shao C., Shi Y. Mesenchymal stromal cells pretreated with pro-inflammatory cytokines promote skin wound healing through VEGFC-mediated angiogenesis. Stem Cells Transl. Med. 2020;9(10):1218–1232. doi: 10.1002/sctm.19-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ellison-Hughes G.M., Colley L., O'Brien K.A., Roberts K.A., Agbaedeng T.A., Ross M.D. The Role of MSC Therapy in Attenuating the Damaging Effects of the Cytokine Storm Induced by COVID-19 on the Heart and Cardiovascular System. Front Cardiovasc Med. 2020;7 doi: 10.3389/fcvm.2020.602183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawson C., Vicencio J.M., Yellon D.M., Davidson S.M. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J. Endocrinol. 2016;228(2):R57–R71. doi: 10.1530/JOE-15-0201. [DOI] [PubMed] [Google Scholar]
- 59.de Almeida D.C., Donizetti-Oliveira C., Barbosa-Costa P., Origassa C.S., Câmara N.O. In search of mechanisms associated with mesenchymal stem cell-based therapies for acute kidney injury. Clin. Biochem. Rev. 2013;34(3):131. [PMC free article] [PubMed] [Google Scholar]
- 60.Togel F., Weiss K., Yang Y., Hu Z., Zhang P., Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am. J. Physiol. -Ren. Physiol. 2007;292(5):F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 61.Abraham A., Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl. Med. 2020;9(1):28–38. doi: 10.1002/sctm.19-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cocucci E., Racchetti G., Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19(2):43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 63.Harrell C.R., Jovicic N., Djonov V., Arsenijevic N., Volarevic V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells. 2019;8:12. doi: 10.3390/cells8121605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bulut Ö., GÜrsel İ. Mesenchymal stem cell derived extracellular vesicles: promising immunomodulators against autoimmune, autoinflammatory disorders and SARS-CoV-2 infection. Turk. J. Biol. 2020;44(3):273–282. doi: 10.3906/biy-2002-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Brien K., Breyne K., Ughetto S., Laurent L.C., Breakefield X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020;21(10):585–606. doi: 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ji F., Li L., Li Z., Jin Y., Liu W. Mesenchymal stem cells as a potential treatment for critically ill patients with coronavirus disease 2019. Stem Cells Transl. Med. 2020;9(7):813–814. doi: 10.1002/sctm.20-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu J., Clark A.G. Impact of microRNA regulation on variation in human gene expression. Genome Res. 2012;22(7):1243–1254. doi: 10.1101/gr.132514.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shah V., Shah J. Recent trends in targeting miRNAs for cancer therapy. J. Pharm. Pharm. 2020;72(12):1732–1749. doi: 10.1111/jphp.13351. [DOI] [PubMed] [Google Scholar]
- 69.Chauhan N., Jaggi M., Chauhan S.C., Yallapu M.M. COVID-19: fighting the invisible enemy with microRNAs. Expert Rev. Anti Infect. Ther. 2021;19(2):137–145. doi: 10.1080/14787210.2020.1812385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen L., F-b Lu, D-z Chen, J-l Wu, L-m Xu, M-h Zheng, Li H., Huang Y., Jin X.-Y., Gong Y.-W. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol. Immunol. 2018;93:38–46. doi: 10.1016/j.molimm.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 71.Lou G., Yang Y., Liu F., Ye B., Chen Z., Zheng M., Liu Y. MiR‐122 modification enhances the therapeutic efficacy of adipose tissue‐derived mesenchymal stem cells against liver fibrosis. J. Cell. Mol. Med. 2017;21(11):2963–2973. doi: 10.1111/jcmm.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schultz I.C., Bertoni A.P.S., Wink M.R. Mesenchymal stem cell-derived extracellular vesicles carrying mirna as a potential multi target therapy to COVID-19: an in silico analysis. Stem Cell Rev. Rep. 2021;17(2):341–356. doi: 10.1007/s12015-021-10122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020;29(12):747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang Y.S., Ahn S.Y., Yoo H.S., Sung S.I., Choi S.J., Oh W.I., Park W.S. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J. Pediatr. 2014;164(5):966–972. doi: 10.1016/j.jpeds.2013.12.011. e966. [DOI] [PubMed] [Google Scholar]
- 75.Simonson O.E., Mougiakakos D., Heldring N., Bassi G., Johansson H.J., Dalén M., Jitschin R., Rodin S., Corbascio M., El, Andaloussi S. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl. Med. 2015;4(10):1199–1213. doi: 10.5966/sctm.2015-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson J.G., Liu K.D., Zhuo H., Caballero L., McMillan M., Fang X., Cosgrove K., Vojnik R., Calfee C.S., Lee J.-W. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir. Med. 2015;3(1):24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin L., Deng Z., Zhang J., Yang C., Liu J., Han W., Ye P., Si Y., Chen G. Mesenchymal stem cells promote type 2 macrophage polarization to ameliorate the myocardial injury caused by diabetic cardiomyopathy. J. Transl. Med. 2019;17(1):1–14. doi: 10.1186/s12967-019-1999-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goolaerts A., Pellan-Randrianarison N., Larghero J., Vanneaux V., Uzunhan Y., Gille T., Dard N., Planès C., Matthay M.A., Clerici C. Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2014;306(11):L975–L985. doi: 10.1152/ajplung.00242.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Islam M.N., Das S.R., Emin M.T., Wei M., Sun L., Westphalen K., Rowlands D.J., Quadri S.K., Bhattacharya S., Bhattacharya J. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tabera S., Pérez-Simón J.A., Díez-Campelo M., Sánchez-Abarca L.I., Blanco B., López A., Benito A., Ocio E., Sánchez-Guijo F.M., Cañizo C. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. haematologica. 2008;93(9):1301–1309. doi: 10.3324/haematol.12857. [DOI] [PubMed] [Google Scholar]
- 81.Rani S., Ryan A.E., Griffin M.D., Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol. Ther. 2015;23(5):812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang X.-D., Shi L., Monsel A., Li X.-Y., Zhu H.-L., Zhu Y.-G., Qu J.-M. Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by Ang-1 mRNA. Stem Cells. 2017;35(7):1849–1859. doi: 10.1002/stem.2619. [DOI] [PubMed] [Google Scholar]
- 83.MacLoughlin R.J., Higgins B.D., Laffey J.G., O’Brien T. Optimized aerosol delivery to a mechanically ventilated rodent. J. Aerosol Med. Pulm. Drug Deliv. 2009;22(4):323–332. doi: 10.1089/jamp.2008.0717. [DOI] [PubMed] [Google Scholar]
- 84.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29(12):747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu Yg Feng Xm, Abbott J., Fang Xh Hao, Monsel Q., Qu Jm A., Matthay M.A., Lee J.W. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin‐induced acute lung injury in mice. Stem Cells. 2014;32(1):116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morrison T.J., Jackson M.V., Cunningham E.K., Kissenpfennig A., McAuley D.F., O’Kane C.M., Krasnodembskaya A.D. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am. J. Respir. Crit. care Med. 2017;196(10):1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khatri M., Richardson L.A., Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res. Ther. 2018;9(1):1–13. doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choudhery M.S., Harris D.T. Stem cell therapy for COVID-19: Possibilities and challenges. Cell Biol. Int. 2020;44(11):2182–2191. doi: 10.1002/cbin.11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu S., Peng D., Qiu H., Yang K., Fu Z., Zou L. Mesenchymal stem cells as a potential therapy for COVID-19. Stem Cell Res Ther. 2020;11(1):169. doi: 10.1186/s13287-020-01678-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liang B., Chen J., Li T., Wu H., Yang W., Li Y., Li J., Yu C., Nie F., Ma Z., Yang M., Xiao M., Nie P., Gao Y., Qian C., Hu M. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Med. (Baltim. ) 2020;99(31) doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang L., Jiang Y., Zhu M., Chen L., Zhou X., Zhou C., Ye P., Chen X., Wang B., Xu Z., Zhang Q., Xu X., Gao H., Wu X., Li D., Jiang W., Qu J., Xiang C., Li L. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front Med. 2020;14(5):664–673. doi: 10.1007/s11684-020-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu J., Zhou X., Tan Y., Wang L., Li T., Li Z., Gao T., Fan J., Guo B., Li W., Hao J., Wang X., Hu B. Phase 1 trial for treatment of COVID-19 patients with pulmonary fibrosis using hESC-IMRCs. Cell Prolif. 2020;53(12) doi: 10.1111/cpr.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kavanagh D.P., Robinson J., Kalia N. Mesenchymal stem cell priming: fine-tuning adhesion and function. Stem Cell Rev. Rep. 2014;10(4):587–599. doi: 10.1007/s12015-014-9510-7. [DOI] [PubMed] [Google Scholar]
- 94.Wu P.J., Peng H., Li C., Abdel-Latif A., Berron B.J. Adhesive stem cell coatings for enhanced retention in the heart tissue. ACS Appl. Bio Mater. 2020;3(5):2930–2939. doi: 10.1021/acsabm.9b01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sarkar D., Spencer J.A., Phillips J.A., Zhao W., Schafer S., Spelke D.P., Mortensen L.J., Ruiz J.P., Vemula P.K., Sridharan R., Kumar S., Karnik R., Lin C.P., Karp J.M. Engineered cell homing. Blood. 2011;118(25):e184–e191. doi: 10.1182/blood-2010-10-311464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen Q., Li Y., Chen Z., Du H., Wan J. Anti-VCAM 1 antibody-coated mesenchymal stromal cells attenuate experimental colitis via immunomodulation. Med Sci. Monit. 2019;25:4457–4468. doi: 10.12659/MSM.914238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gottipati A., Chelvarajan L., Peng H., Kong R., Cahall C.F., Li C., Tripathi H., Al-Darraji A., Ye S., Elsawalhy E., Abdel-Latif A., Berron B.J. Gelatin based polymer cell coating improves bone marrow-derived cell retention in the heart after myocardial infarction. Stem Cell Rev. Rep. 2019;15(3):404–414. doi: 10.1007/s12015-018-9870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lim S.K., Giebel B., Weiss D.J., Witwer K.W., Rohde E. Vol. 29. 2020. Re: "Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19" by Sengupta et al. pp. 877–878. (Stem Cells Dev). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rasul R.M., Tamilarasi Muniandy M., Zakaria Z., Shah K., Chee C.F., Dabbagh A., Rahman N.A., Wong T.W. A review on chitosan and its development as pulmonary particulate anti-infective and anti-cancer drug carriers. Carbohydr. Polym. 2020;250 doi: 10.1016/j.carbpol.2020.116800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mehta M., Prasher P., Sharma M., Shastri M.D., Khurana N., Vyas M., Dureja H., Gupta G., Anand K., Satija S., Chellappan D.K., Dua K. Advanced drug delivery systems can assist in targeting coronavirus disease (COVID-19): A hypothesis. Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang Z., Zhang X., Shu Y., Guo M., Zhang H., Tao W. Insights from nanotechnology in COVID-19 treatment. Nano Today. 2021;36 doi: 10.1016/j.nantod.2020.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Metcalfe S.M. Mesenchymal stem cells and management of COVID-19 pneumonia. Med Drug Disco. 2020;5 doi: 10.1016/j.medidd.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Quinton L.J., Mizgerd J.P., Hilliard K.L., Jones M.R., Kwon C.Y., Allen E. Leukemia inhibitory factor signaling is required for lung protection during pneumonia. J. Immunol. 2012;188(12):6300–6308. doi: 10.4049/jimmunol.1200256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rastogi A., Bhansali A., Khare N., Suri V., Yaddanapudi N., Sachdeva N., Puri G.D., Malhotra P. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study) Post. Med J. 2022;98(1156):87–90. doi: 10.1136/postgradmedj-2020-139065. [DOI] [PubMed] [Google Scholar]
- 105.Mohammadi S., Barzegari A., Dehnad A., Barar J., Omidi Y. Astaxanthin protects mesenchymal stem cells from oxidative stress by direct scavenging of free radicals and modulation of cell signaling. Chem. Biol. Inter. 2021;333 doi: 10.1016/j.cbi.2020.109324. [DOI] [PubMed] [Google Scholar]
- 106.He L., Zhao J., Wang L., Liu Q., Fan Y., Li B., Yu Y.L., Chen C., Li Y.F. Using nano-selenium to combat coronavirus disease 2019 (COVID-19)? Nano Today. 2021;36 doi: 10.1016/j.nantod.2020.101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Simoneau C.R., Ott M. Modeling multi-organ infection by SARS-CoV-2 using stem cell technology. Cell Stem Cell. 2020;27(6):859–868. doi: 10.1016/j.stem.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sandilya S., Singh S. Development of islet organoids from human induced pluripotent stem cells in a cross-linked collagen scaffold. Cell Regen. 2021;10(1):38. doi: 10.1186/s13619-021-00099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hou J., Zhou Q., Zhu X., Peng J., Xiong J.W. Diverse biological and engineering strategies towards organ regeneration. Cell Regen. 2021;10(1):34. doi: 10.1186/s13619-021-00098-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li H., Wang Y., Zhang M., Wang H., Cui A., Zhao J., Ji W., Chen Y.G. Establishment of porcine and monkey colonic organoids for drug toxicity study. Cell Regen. 2021;10(1):32. doi: 10.1186/s13619-021-00094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Katsura H., Sontake V., Tata A., Kobayashi Y., Edwards C.E., Heaton B.E., Konkimalla A., Asakura T., Mikami Y., Fritch E.J., Lee P.J., Heaton N.S., Boucher R.C., Randell S.H., Baric R.S., Tata P.R. Human lung stem cell-based alveolospheres provide insights into SARS-CoV-2-mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell. 2020;27(6):890–904. doi: 10.1016/j.stem.2020.10.005. e898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pei R., Feng J., Zhang Y., Sun H., Li L., Yang X., He J., Xiao S., Xiong J., Lin Y., Wen K., Zhou H., Chen J., Rong Z., Chen X. Host metabolism dysregulation and cell tropism identification in human airway and alveolar organoids upon SARS-CoV-2 infection. Protein Cell. 2021;12(9):717–733. doi: 10.1007/s13238-020-00811-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang J., Hume A.J., Abo K.M., Werder R.B., Villacorta-Martin C., Alysandratos K.D., Beermann M.L., Simone-Roach C., Lindstrom-Vautrin J., Olejnik J., Suder E.L., Bullitt E., Hinds A., Sharma A., Bosmann M., Wang R., Hawkins F., Burks E.J., Saeed M., Wilson A.A., Mühlberger E., Kotton D.N. SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell Stem Cell. 2020;27(6):962–973. doi: 10.1016/j.stem.2020.09.013. e967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang L., Han Y., Nilsson-Payant B.E., Gupta V., Wang P., Duan X., Tang X., Zhu J., Zhao Z., Jaffré F., Zhang T., Kim T.W., Harschnitz O., Redmond D., Houghton S., Liu C., Naji A., Ciceri G., Guttikonda S., Bram Y., Nguyen D.T., Cioffi M., Chandar V., Hoagland D.A., Huang Y., Xiang J., Wang H., Lyden D., Borczuk A., Chen H.J., Studer L., Pan F.C., Ho D.D., tenOever B.R., Evans T., Schwartz R.E., Chen S. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27(1):125–136. doi: 10.1016/j.stem.2020.06.015. e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bojkova D., Wagner J.U.G., Shumliakivska M., Aslan G.S., Saleem U., Hansen A., Luxán G., Günther S., Pham M.D., Krishnan J., Harter P.N., Ermel U.H., Frangakis A.S., Milting H., Zeiher A.M., Klingel K., Cinatl J., Dendorfer A., Eschenhagen T., Tschöpe C., Ciesek S., Dimmeler S. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc Res. 2020;116(14):2207–2215. doi: 10.1093/cvr/cvaa267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sharma A., Garcia G., Arumugaswami V., Svendsen C.N. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. bioRxiv. 2020 doi: 10.1016/j.xcrm.2020.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Garcia G., Jr., Sharma A., Ramaiah A., Sen C., Purkayastha A., Kohn D.B., Parcells M.S., Beck S., Kim H., Bakowski M.A., Kirkpatrick M.G., Riva L., Wolff K.C., Han B., Yuen C., Ulmert D., Purbey P.K., Scumpia P., Beutler N., Rogers T.F., Chatterjee A.K., Gabriel G., Bartenschlager R., Gomperts B., Svendsen C.N., Betz U.A.K., Damoiseaux R.D., Arumugaswami V. Antiviral drug screen identifies DNA-damage response inhibitor as potent blocker of SARS-CoV-2 replication. Cell Rep. 2021;35(1) doi: 10.1016/j.celrep.2021.108940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mills R.J., Humphrey S.J., Fortuna P.R.J., Lor M., Foster S.R., Quaife-Ryan G.A., Johnston R.L., Dumenil T., Bishop C., Rudraraju R., Rawle D.J., Le T., Zhao W., Lee L., Mackenzie-Kludas C., Mehdiabadi N.R., Halliday C., Gilham D., Fu L., Nicholls S.J., Johansson J., Sweeney M., Wong N.C.W., Kulikowski E., Sokolowski K.A., Tse B.W.C., Devilée L., Voges H.K., Reynolds L.T., Krumeich S., Mathieson E., Abu-Bonsrah D., Karavendzas K., Griffen B., Titmarsh D., Elliott D.A., McMahon J., Suhrbier A., Subbarao K., Porrello E.R., Smyth M.J., Engwerda C.R., MacDonald K.P.A., Bald T., James D.E., Hudson J.E. BET inhibition blocks inflammation-induced cardiac dysfunction and SARS-CoV-2 infection. Cell. 2021;184(8):2167–2182. doi: 10.1016/j.cell.2021.03.026. e2122. [DOI] [PMC free article] [PubMed] [Google Scholar]