Abstract
Introduction
The necessity of a booster dose is a matter that has not been as yet illuminated, although it is noted that neutralizing antibody titers decrease over time. We aimed therefore to evaluate antibody titers and seroconversion rates after a booster mRNA vaccine and a booster inactivated vaccine.
Methods
A total of 322 participants were divided into three main groups, with two subgroups each, based on their vaccinations and previous infection history. The levels of anti-SARS-CoV-2 Ig-G were analyzed with the Elecsys® Anti-SARS-CoV-2 S assay.
Results
The antibody titers showed a linear and significant increase from one vaccine group to the other, displaying progressive changes from group 2IV to group 3IV, and then to group 2IV/mRNA. All of the seronegative participants were in the 2IV(-) subgroup; 93.3% of the participants whose antibody titers were above the upper limit were in the 2IV/mRNA group. Doctors were much more inclined to have a booster dose and mRNA vaccines than nurses. The status of being a doctor increases the rate of having a booster dose 7.8 times; likewise, each annual increase in age increases the rate 1.05 times.
Conclusion
Anti-SARS-CoV-2 IgG levels decrease over time. The antibody response rate to only two doses of the inactivated vaccine was meager, so a booster dose is necessary to maintain the effectiveness of inactivated vaccines. The third dose of the vaccine, especially that of the mRNA vaccine, which was found to be much more superior to the inactivated vaccine, should be strongly recommended.
Key Words: Booster dose, COVID‐19, Inactivated vaccine, mRNA vaccine, Anti-SARS-CoV-2
1. Introduction
Many vaccine and drug development studies were initiated after the Coronavirus 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) broke out in December 2019. While an effective antiviral agent has not been developed yet, inactivated vaccines, messenger RNA (mRNA) vaccines, and adenovirus vaccines were developed not long after the identification of SARS-CoV-2, and emergency use approvals were obtained for these vaccines [1], [2], [3]. Vaccination campaigns were launched rapidly and widely around the world. Shortly after the inactivated vaccine (CoronaVac) was given emergency use approval by the Republic of Turkey Ministry of Health, vaccinations were first given to health care workers (HCW), beginning in January 2021. The HCWs received two doses of the CoronaVac vaccine with a recommended dosing interval of 28 days between the first and second doses. In addition to the inactivated vaccine, the BNT162b2mRNA vaccine (Pfizer and BioNTech) began to be implemented in Turkey at the beginning of April 2021. The Turkish Ministry of Health recommended administering the third booster dose with an optional inactivated or mRNA vaccine as of the beginning of July 2021 for healthcare workers (HCWs) and the elderly.
CoronaVac is an inactivated and aluminum-adjuvant vaccine developed by Sinovac Life Science Company, Beijing, China [4]. The BNT162b2mRNA vaccine produced by Pfizer and BioNTech was the first vaccine approved for emergency use by the United States Food and Drug Administration [5].
In this study, we aimed to evaluate antibody titers and seroconversion rates after a booster mRNA vaccine and a booster inactivated vaccine, administered after two doses of inactivated vaccines, by examining the anti-SARS-CoV-2 IgG response in HCWs with and without a previous history of SARS-CoV-2 infection, and to determine the inclination toward vaccination of nurses and doctors.
2. Materials and methods
2.1. Study design and population
The study group consists of 322 participants (232 (72%) women, 90 (28%) men, 116 (36%) doctors, 206 (64%) nurses) who worked at the Children's Hospital of Ankara City Hospital. The mean age was 31.4 ± 8.01 years old, and the median age was 28 years old. Our study includes three groups: those who had only two doses of inactivated vaccine [2IV group, 83 paticipants (25.8%)], those who had two doses of inactivated vaccine and a booster dose of inactivated vaccine [3IV group, 23 participants (7.1%)], and those who had two doses of inactivated vaccine and a booster dose of mRNA vaccine [2IV/mRNA group, 216 participants (67.1%)]. Among the participants, 93 (28.9%) had a previous history of COVID-19 infection (diagnosed by naso- pharyngeal swab polymerase chain reaction (PCR) result), 229 (71.1%) did not. Participants in each group who had a previous history of COVID-19 infection were indicated as (+), and those who did not were indicated as (-).The study did not include those with immunodeficiency, those receiving immunosuppressive therapy, and those with a history of multiple COVID-19 infections. In order to determine the anti-SARS-CoV-2 immunoglobulin G (IgG) antibody levels, blood samples from 322 HCWs who were volunteers were collected. The duration between the last vaccination and sampling was 6 to 8 months for those who had only two doses of inactivated vaccine, and 2 to 4 months for those who had inactivated or mRNA booster doses.
2.2. Detection of anti-SARS-CoV-2 antibodies
The samples were analyzed at the Ankara Microbiology Reference Laboratories of the Public Health General Directorate of Turkey. Analyses were performed in a macroELISA device (Roche Cobas 8000-e801analyzer) with an electrochemiluminescent (ECLIA) technique using a commercial kit (Elecsys Anti-SARS-CoV-2 S, Roche). The Elecsys Anti‑SARS‑CoV‑2 S assay utilizes a recombinant protein representing the RBD of the virus's S antigen in a double antigen sandwich test format which favors the quantitative determination of high-affinity antibodies to SARS‑CoV‑2. The tests were run and evaluated according to the manufacturer's instructions. Values below the measurement limit resulted in < 0.40 U/mL. Values above the measuring range resulted in >250 U/mL, and these samples were reanalysed at 1/50 automatic dilution. Values above the measurement limit resulted in >12,500 U/mL. A cut-off index (COI) < 0.80 U/mL was considered negative for anti-SARS-CoV-2 antibodies, while a COI ≥ ≥ 0.80 U/mL was considered positive. In order to correlate the units (U) of the Elecsys® Anti-SARS-CoV-2 S assay with the “binding antibody units” (BAU) of the first WHO International Standard for anti-SARS-CoV-2 immunoglobulin, a 0.972 coefficient was used in line with the manufacturer's recommendations [6]. A mathematical transposition of Roche specific U to BAU of the WHO International Standard followed the equation: Roche U = 0.972*BAU.
2.3. Statistical analysis
The Statistical Package for the Social Sciences (SPSS) version 23.0 (IBM Corp., Armonk, New York, USA) was used to analyze the data. Descriptive statistics (including frequencies and means) for all variables were calculated. The results were expressed as mean ± standard deviation, median, range (minimum-maximum), and number (%). The difference between groups in categorical variables was analyzed using the chi-square test. The Kolmogorov Smirnov test was used to examine whether the numerical variables showed normal distribution. The Mann-Whitney U test investigated differences between the two independent groups in terms of binary variables. The Kruskal-Wallis test was used for comparisons of more than one independent group. The level of statistical significance was established as p < 0.05. A logistic regression model was created to reveal the factors affecting having a booster dose and to estimate the odds ratio (OR) and confidence interval (CI).
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
This study was conducted in conformity with the principles of the Declaration of Helsinki and approved by the Republic of Turkey Ministry of Health, the Ethics Committee of Ankara City Hospital Ethics Committee, and the Institutional Review Board of the Children's Hospital of Ankara City Hospital.
3. Results
Descriptive features of the participants were demonstrated in Table 1 . While 239 (74.2%) of the participants had a booster dose with an inactivated or mRNA vaccine, 83 (25.8%) did not. No relationship was found between gender or previous history of COVID-19 infection and having a booster dose (p > 0.05 each). A significant relationship was found between occupation and having a booster dose; the rate of having a booster dose was 93% for doctors and 63% for nurses (p< 0.001) (Table 2 ). When vaccine preferences were examined according to professions, 90.5% of the doctors preferred an mRNA booster, 2.6% preferred an inactive vaccine booster, while among nurses, 53.9% preferred an mRNA booster, and 9.7% preferred an inactive vaccine booster (p< 0.001). Additionally, there was a significant relationship between age and having a booster dose; the older the age of the HCWs, the higher was the rate of having a booster dose (p = 0.002). In the present study, the multivariate logistic regression analysis was used to examine associations, and it was revealed that status as a doctor increases the rate of having a booster dose 7.8 times (OR = 7.81, 95% CI 3.59–16.95, p < 0.001); likewise, each annual increase in age increases the rate 1.05 times (OR = 1.05, 95% CI 1.009–1.085, p = 0.014) (Table 2).
Table 1.
Descriptive features of the participants.
| Variables | n = 322 |
|---|---|
| Age (year) (mean±SD) (median) | 31.4 ± 8.01 (median: 28) |
| Gender Female Male |
232 (72%) 90 (28%) |
| Occupation Pediatrician Pediatric nurse |
116 (36%) 206 (64%) |
| Previous history of COVID-19 infection Yes No |
93 (28.9%) 229 (71.1%) |
| Vaccine preferences 2IV 3IV 2IV/mRNA |
83 (25.8%) 23 (7.1%) 216 (67.1%) |
Table 2.
Descriptive features of the participants based on vaccination preferences.
| Variables |
Booster (-) (n = 83) |
Booster (+) (n = 239) |
p value |
Adjusted OR (95% CI) for having a booster dose |
|---|---|---|---|---|
| Age (year) (mean±SD) (median) |
29 ± 6.88 (median: 26) |
32.22 ± 8.22 (median: 29) |
p = 0.002 |
1.05 (1.009–1.085) (p = 0.014) |
| Gender Female Male |
62 (25.3%) 21 (74.7%) |
69 (28.9%) 170 (71.1%) |
p > 0.05 |
0.91 (0.48–1.65) (p = 0.72) |
| Occupation Pediatrician Pediatric nurse |
8 (9.6%) 75 (90.4%) |
108 (45.2%) 131 (54.8%) |
p< 0.001 |
7.81 (3.59–16.95) (p < 0.001) |
| Previous history of COVID-19 infection Yes No |
29 (34.9%) 54 (65.1%) |
64 (26.8%) 175 (73.2%) |
p > 0.05 |
0.69 (0.39–1.23) (p = 0.21) |
| Anti-SARS-CoV-2 IgG (BAU) (mean±SD),(median, IQR) |
2000.43 ± 4050.51 186.21 (57.61–777.77) |
9659.02 ± 4293.32 12,376.54 (7155.34–12,860) |
p< 0.001 |
OR: Odds ratio, C.I.: Confidence interval.
No relationship was found between age, gender, or previous history of COVID-19 infection and antibody levels (p > 0.05 each). When the antibody levels of the HCWs were examined, 3.41% (n = 11) of the samples were seronegative (< 20 BAU), and 36.95% (n = 119) were above the upper limit of 12,860 BAU that the test could detect. All of the seronegative participants were in the 2IV(-) subgroup; 93.3% of the participants whose antibody titers were above the upper limit were in the 2IV/mRNA group.
When the antibody levels of the participants who had and did not have a booster dose were compared, it was seen that the antibody levels of those who had a booster dose were much higher than the others (p< 0.001) (Table 2). Anti-SARS-CoV-2 IgG levels based on vaccines showed statistically significant differences between groups. Antibody titers were the highest in the 2IV/mRNA group (p < 0.001). The antibody levels of the 3IV group were lower than the 2IV/mRNA group but higher than the 2IV group (p < 0.001). The lowest antibody levels were observed in the 2IV group.
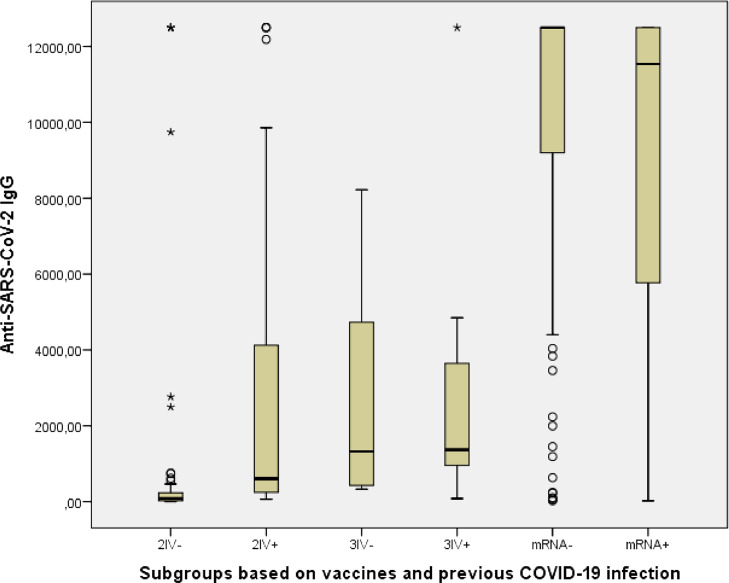
The antibody levels of the subgroups were compared with each other based on their past COVID-19 infection history. Measured anti-SARS-CoV-2 IgG levels for the 2IV(-) and 2IV(+) subgroups showed statistically significant differences, it was higher in the 2IV+ subgroup (p < 0.001). But the 3IV(-) and 3IV(+) subgroups and the 2IV/mRNA(-) and 2IV/mRNA(+) subgroups did not showed difference (p > 0.05 each) (Table 3 and Fig. 1 ). Although there was no significant difference in antibody levels between the 2IV/mRNA(-) and 2IV/mRNA(+) subgroups, the antibody levels of each of these two subgroups were significantly much higher than all other subgroups (p < 0.001 each). While the antibody levels of both the 3IV(+) and 3IV(-) subgroups were significantly higher than the 2IV(-) subgroup (p < 0.001 each), surprisingly, no difference was found between the 2IV(+) subgroup and the 3IV(-) subgroup (p > 0.05), or likewise between the 2IV(+) and the 3IV(+) subgroups (p > 0.05).
Table 3.
Anti-SARS-CoV-2 IgG levels based on vaccines, doses and COVID-19 infection history.
| Groups | Number (%) | Median (IQR) Anti-SARS-CoV-2 IgG levels (BAU) | p | Seronegativity rate,% (n) | Rate of participants with a maximum titer,% (n) |
|---|---|---|---|---|---|
| 2IV(-) | 54 (16.8%) | 88.27 (25.64–247.17) |
p < 0.001 | 20.37% (n = 11) | 7.4%, (n = 4) |
| 2IV(+) | 29 (9.0%) | 626.54 (238.68–4782.92) |
0% | 10.34%, (n = 3) | |
| 3IV(-) | 13 (4.0%) | 1361.11 (441.36–5128.09) |
p > 0.05 | 0% | 0% |
| 3IV(+) | 10 (3.1%) | 1409.47 (969.39–4060.70) |
0% | 10%, (n = 1) | |
| 2IV/mRNA(-) | 162 (50.3%) | 12,860 (9437.76–12,860) |
p > 0.05 | 0% | 53.9%, (n = 86) |
| 2IV/mRNA(+) | 54 (16.8%) | 11,871.91 (5912.55–12,860) |
0% | 46.3%, (n = 25) |
Fig. 1.
Distribution of Anti-SARS-CoV-2 IgG levels of the study groups.
4. Discussion
In this study, the seroconversion rates and anti-SARS-CoV-2 IgG antibody levels of HCWs who had only two doses of inactivated vaccine, two doses of inactivated vaccine with a booster dose of inactivated vaccine, and two doses of inactivated vaccine with a booster dose of mRNA vaccine were evaluated. To the best of the authors’ knowledge, this is one of the first studies to show the difference between the antibody levels induced by a single inactivated vaccine booster dose and a single mRNA vaccine booster dose, each after two doses of inactivated COVID-19 vaccine.
In several studies conducted in the prevaccination period, it was found that nurses were less inclined to get vaccinated against COVID-19 than physicians, meaning that vaccine reluctance was higher in nurses [7], [8], [9], [10]. In addition to the low vaccine acceptance rate noted among nurses for COVID-19 vaccines, a low vaccine acceptance rate for seasonal influenza vaccine was also displayed by this group as compared to physicians in the literature [11,12]. In terms of our study, nurses were less likely to accept a third booster dose against COVID-19 than physicians, which is consistent with previous vaccine hesitancy findings. Furthermore, nurses showed a remarkable reluctance compared to doctors with regard to receiving mRNA vaccines. Having more confidence in vaccines, having a higher degree of medical training, a higher level of risk perception, and fear of COVID-19 may contribute to an increased uptake of vaccines among doctors [13].
In previous studies, the antibody response induced by COVID-19 infection or vaccination was found to be sufficient [3,[14], [15], [16]]. In later studies, however, it was observed that antibody titers decreased in patient follow-ups [17,18]. In a study with an extended period of time, it was shown that antibody levels increased at a peak after 30 days of BNT162b2 vaccination and, subsequently, reduced until they reached a plateau at 60 days after the second dose, with similar values observed after 90 days [19]. It was also shown that mRNA vaccines elicit rapid immune responses in seropositive individuals with post-vaccine antibody titres that are comparable to or exceed titres in naive individuals vaccinated with two doses [19,20]. In recent publications, therefore, a booster dose has been recommended for individuals who have been vaccinated with two doses of inactivated vaccine and for special groups such as individuals with a kidney transplant or solid organ transplant who had received the mRNA vaccine but have low seroconversion rates [18,[21], [22], [23]]. The antibody response in our study was found at the lowest titer in the group that received two doses of inactivated vaccine, which supports this recommendation. Similarly, all seronegative participants in our study were in the two-doses inactivated vaccine group. No seronegative participants were found in the mRNA or inactivated booster groups.
Kesin et al. found in their study that an inactivated vaccine booster dose after two doses of inactivated vaccine yielded 1.7 times an increase in median values of anti-spike antibody titers; BNT162b2 administration as the third vaccine dose boosted anti-spike antibody median titers by a factor of 46.6 [24]. In a study of the Ministry of Health of Chile led by Araos et al., 4.7 million participants initially immunized with CoronaVac were analyzed, of whom nearly two million had a booster dose. In the case of CoronaVac, effectiveness increased from 56% to 80.2%, while Pfizer-BioNTech rose from 56% to 90%, and AstraZeneca climbed from 56% to 93%. Regarding the prevention of hospitalization, the effectiveness of the CoronaVac booster vaccine increased from 84% to 88%, Pfizer-BioNTech from 84% to 87%, and AstraZeneca from 84% to 96.3% [25]. Similar to these studies, in our study, it was found that the mRNA booster dose induced the highest antibody level. In addition, most of the participants (93.3%) with the highest antibody levels were in the mRNA booster group. Interestingly, it was also found in the current study that there was no difference between the antibody levels of patients with a previous history of COVID-19 infection who had not had a booster dose, and the antibody levels of patients with or without a previous history of COVID-19 infection who had received a third inactivated vaccine.
The present study had several limitations. First, this was a single-center study. Secondly, the prevaccination levels of SARS-CoV-2 antibodies in the participants were unknown. Moreover, since it was a cross-sectional study, the time between vaccination and sampling could not be standardized. Additionally, the antibody level that predicts protection is unknown.
In conclusion, Anti-SARS-CoV-2 IgG levels decrease over time. The antibody response rate to only two doses of the inactivated vaccine was meager, so the results suggest that a booster dose is necessary to maintain the effectiveness of inactivated vaccines. The third dose of the vaccine, especially that of the mRNA vaccine, which was found to be much more superior to the inactivated vaccine, should be strongly recommended. We consider that doing so will ensure the continuity of herd immunity until multicenter and prospective clinical efficacy studies are conducted.
5. Funding
This manuscript is not under simultaneous consideration by any other publication. An honorarium, grant, or other form of payment was not given to any author to produce the manuscript. All authors significantly contributed the work, have approved the final manuscript and takes full responsibility for the manuscript.
CRediT authorship contribution statement
Metin Yigit: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Visualization, Supervision. Aslinur Ozkaya-Parlakay: Conceptualization, Methodology, Supervision. Yasemin Cosgun: Conceptualization, Methodology, Resources, Data curation. Yunus Emre Ince: Writing – original draft, Investigation, Visualization. Furkan Kalayci: Investigation, Resources, Data curation. Naci Yilmaz: Investigation, Resources, Data curation. Emrah Senel: Conceptualization, Writing – review & editing, Supervision.
Conflicts of interest
The authors have indicated they have no conflicts of interest relevant to this article to disclose.
Funding/Support
None.
References
- 1.Zhang Y., Zeng G., Pan H., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramasamy M.N., Minassian A.M., Ewer K.J., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma O., Sultan A.A., Ding H., Triggle C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020;11 doi: 10.3389/FIMMU.2020.585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ledford H. US authorization of first COVID vaccine marks new phase in safety monitoring. NatureNature. 2020;588:377–378. doi: 10.1038/d41586-020-03542-4. [DOI] [PubMed] [Google Scholar]
- 6.WHO/BS.2020.2403 . 2021. Establishment of the WHO International Standard and Reference Panel For anti-SARS-CoV-2 Antibody.https://www.who.int/publications/m/item/WHO-BS-2020.2403 accessed Dec 3. [Google Scholar]
- 7.Dror A.A., Eisenbach N., Taiber S., et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020;35:775–779. doi: 10.1007/s10654-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gagneux-Brunon A., Detoc M., Bruel S., et al. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: a cross-sectional survey. J. Hosp. Infect. 2021;108:168–173. doi: 10.1016/j.jhin.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang K., Wong E.L.Y., Ho K.F., et al. Intention of nurses to accept coronavirus disease 2019 vaccination and change of intention to accept seasonal influenza vaccination during the coronavirus disease 2019 pandemic: a cross-sectional survey. Vaccine. 2020;38:7049–7056. doi: 10.1016/j.vaccine.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yigit M., Ozkaya-Parlakay A., Senel E. Evaluation of COVID-19 vaccine acceptance of healthcare providers in a tertiary Pediatric hospital. Hum. Vaccin. Immunother. 2021;17:2946–2950. doi: 10.1080/21645515.2021.1918523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguilar-Díaz F del C., Jiménez-Corona M.E., Ponce-de-León-Rosales S. Influenza vaccine and healthcare workers. Arch. Med. Res. 2011;42:652–657. doi: 10.1016/j.arcmed.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Dror A.A., Eisenbach N., Taiber S., et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020;35:775–779. doi: 10.1007/s10654-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M., Luo Y., Watson R., et al. Healthcare workers’ (HCWs) attitudes and related factors towards COVID-19 vaccination: a rapid systematic review. Postgrad. Med. J. 2021;0:1–7. doi: 10.1136/postgradmedj-2021-140195. [DOI] [PubMed] [Google Scholar]
- 14.Bayram A., Demirbakan H., Günel Karadeniz P., Erdoğan M., Koçer I. Quantitation of antibodies against SARS-CoV-2 spike protein after two doses of CoronaVac in healthcare workers. J. Med. Virol. 2021;93:5560–5567. doi: 10.1002/jmv.27098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson L.A., Anderson E.J., Rouphael N.G., et al. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., Zeng G., Pan H., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anand S.P., Prévost J., Nayrac M., et al. Longitudinal analysis of humoral immunity against SARS-CoV-2 Spike in convalescent individuals up to 8 months post-symptom onset. Cell Rep. Med. 2021;2 doi: 10.1016/J.XCRM.2021.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yigit M., Ozkaya-Parlakay A., Cosgun Y., Ince Y.E., Bulut Y.E., Senel E. Should a third booster dose be scheduled after two doses of CoronaVac? A single-center experience. J. Med. Virol. 2022;94:287–290. doi: 10.1002/jmv.27318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vietri M.T., Albanese L., Passariello L., et al. Evaluation of neutralizing antibodies after vaccine BNT162b2: preliminary data. J. Clin. Virol. 2022;146 doi: 10.1016/j.jcv.2021.105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padoan A., Dall'Olmo L., della Rocca F, et al. Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin. Chim. Acta. 2021;519:60–63. doi: 10.1016/j.cca.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Werbel W.A., Boyarsky B.J., Ou M.T., et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: A Case Series. 174 (2021) 1330–1332, doi: 10.7326/L21-0282. [DOI] [PMC free article] [PubMed]
- 22.Yue L., Xie T., Yang T., et al. A third booster dose may be necessary to mitigate neutralizing antibody fading after inoculation with two doses of an inactivated SARS-CoV-2 vaccine. J. Med. Virol. 2022;94:35–38. doi: 10.1002/jmv.27334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benotmane I., Gautier G., Perrin P., et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 Doses. JAMAJAMA. 2021;326:1063–1065. doi: 10.1001/jama.2021.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keskin A.U., Bolukcu S., Ciragil P., Topkaya A.E. SARS-CoV-2 specific antibody responses after third CoronaVac or BNT162b2 vaccine following two-dose CoronaVac vaccine regimen. J. Med. Virol. 2022;94:39–41. doi: 10.1002/jmv.27350. [DOI] [PubMed] [Google Scholar]
- 25.Gob.cl - Article President Piñera presents results of first study on Covid-19 booster dose. Revealing Increased Effectiveness. 2021 https://www.gob.cl/en/news/president-pinera-presents-results-first-study-covid-19-booster-dose-revealing-increased-effectiveness/ (accessed Dec 3) [Google Scholar]