Abstract
The field of tissue engineering continues to advance, sometimes in exponential leaps forward, but also sometimes at a rate that does not fulfill the promise that the field imagined a few decades ago. This review is in part a catalog of success in an effort to inform the process of innovation. Tissue engineering has recruited new technologies and developed new methods for engineering tissue constructs that can be used to mitigate or model disease states for study. Key to this antecedent statement is that the scientific effort must be anchored in the needs of a disease state and be working toward a functional product in regenerative medicine. It is this focus on the wildly important ideas coupled with partnered research efforts within both academia and industry that have shown most translational potential. The field continues to thrive and among the most important recent developments are the use of three-dimensional bioprinting, organ-on-a-chip, and induced pluripotent stem cell technologies that warrant special attention. Developments in the aforementioned areas as well as future directions are highlighted in this article. Although several early efforts have not come to fruition, there are good examples of commercial profitability that merit continued investment in tissue engineering.
Impact statement
Tissue engineering led to the development of new methods for regenerative medicine and disease models. Among the most important recent developments in tissue engineering are the use of three-dimensional bioprinting, organ-on-a-chip, and induced pluripotent stem cell technologies. These technologies and an understanding of them will have impact on the success of tissue engineering and its translation to regenerative medicine. Continued investment in tissue engineering will yield products and therapeutics, with both commercial importance and simultaneous disease mitigation.
Keywords: 3D bioprinting, organ-on-a-chip, regenerative medicine, stem cells, tissue engineering
Introduction
Tissue defects and organ loss can result from congenital problems, disease, damage, or surgical removal1,2 and thus, tissue defects need to be regenerated and repaired. In addition, organ function needs to be regained. This is the promise of tissue engineering. Because of the shortages in organ and tissue supply,3 many patients die every day while waiting for a transplant.4,5 Tissue engineering and its popular and governmental support in concept was driven by this organ and tissue deficiency. Therefore, methods to develop autograft-like replacement tissues have been explored and demanded by the funding agencies. The major advance has been the development of the tissue engineering concept in the late 1980s and early 1990s.6
Tissue engineering aims at producing functional tissue constructs for use in reconstruction or regeneration of damaged or lost tissues and organs,7,8 such as skin,9 spinal cord,10 and other organs.11 In addition, secondary gain has been the development of models to study function,12 disease13 and test and develop drugs.14 Tissue engineering can be achieved either ex vivo15 or in situ16 by using various molecules, materials, or cells to stimulate local tissue regenerative capacity. It is important to note that this effort of tissue engineering was a fundamental shift in the approach to the treatment of tissue loss. With the end goal being functional organs with a complex interplay of different cell signals and scaffolds, the effort mandated a “system” approach with engineering principles instead of the traditional reductionist methodology of experimentation. The ultimate end goal was beyond the knowledge of a fundamental mechanism, but rather a product to mitigate or cure a disease state.
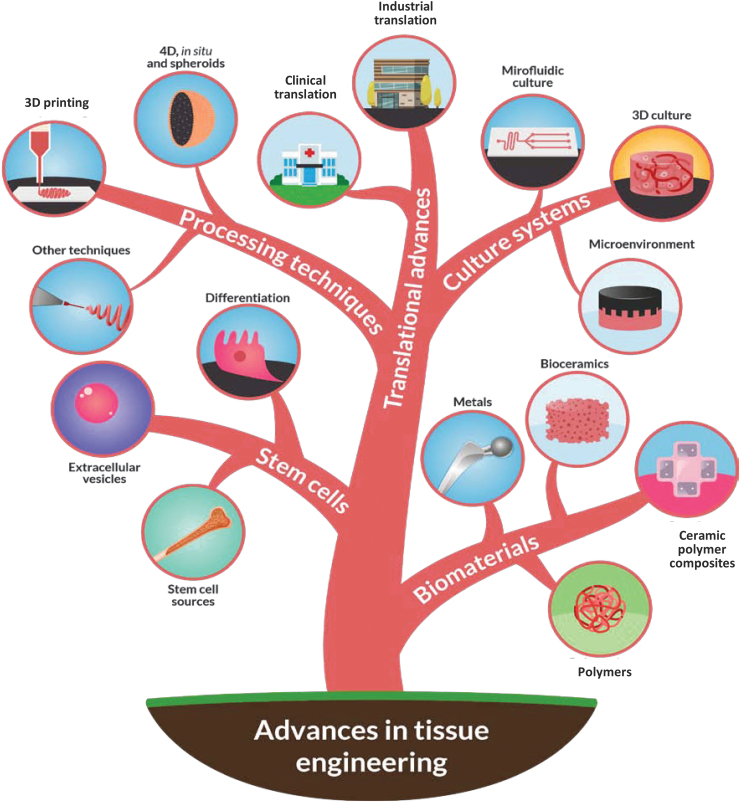
The field of tissue engineering; however, continues to advance, taking advantage of recent developments in areas such as smart biomaterials,16–18 induced pluripotent stem cell (iPSC),19 three-dimensional (3D) bioprinting20,21 technologies, and dynamic culture methods.22,23 It is also renewed by new technologies such as genetic engineering, extracellular vesicles (EVs), and artificial intelligence. Literature in the field is vast, and there are excellent reviews of different aspects of tissue engineering.24–26 Therefore, the purpose of the current article is to highlight only major and recent advances in the field (Fig. 1).
FIG. 1.
Schematic illustration showing overview of major and recent advances in tissue engineering. 3D, three-dimensional; 4D, four-dimensional. Color images are available online.
Advancing Frontiers in Tissue Engineering
Advances in biomaterials and their application in tissue engineering
In tissue engineering, biomaterials are used to provide micro- and nanostructural characteristics, morphology, and surface properties that support cells and can be loaded with appropriate growth factors. Biomaterials used, in the form of matrices or scaffolds for tissue engineering, can be engineered in a way that helps to direct cell growth through specific designs.27 Although biomaterials have been used in tissue engineering since the early 1990s,6,28 how they have been used and the applications they are used for are constantly evolving. Outlined here are recent advances in the use of biomaterials for tissue engineering (Table 1). It is important to note that the field often has an eye on commercialization, such that the Food and Drug Administration (FDA) approval for a new biomaterial may be a hurdle that shapes the evolution of tissue engineering constructs. It is important to note that this barrier can and has in part limited the imagination of the field, because it is often more expeditious to adapt a natural biomaterial or harness one that has an FDA track record instead of designing de novo a material that will require significant vetting before its clinical use.
Table 1.
Advantages, Disadvantages, or Limitations of Different Biomaterials Used for Tissue Engineering
| No. | Biomaterial | Advantages | Disadvantages/limitations | Types of tissue engineering products | Refs. |
|---|---|---|---|---|---|
| A | Polymers | ||||
| 1 | Natural polymers | • Biocompatibility • Cell adhesion motifs • High processability • Elasticity • Degradability |
• Limited mechanical properties | • Various tissues such as heart, bone, liver, and cartilage |
27–31 |
| 2 | ECM | • Mimicking native tissue | • Tissues such as bone, skin, meniscus, and kidney | 32–36 | |
| 3 | Synthetic polymers | • Can be bioresorbable and processed in a controlled way • High mechanical properties |
• Inflammation • No cell adhesion molecules |
• Tissues such as bone, cartilage, nerve, and brain | 37–40 |
| 4 | Hydrogels | • Cells, drugs, and biomolecule delivery • Minimally invasive techniques |
• Mechanical properties • Adhesive strength • Cell adhesion |
• 3D bioprinting • Injectable materials and drug delivery vehicles for regeneration • Minimally invasive regenerative therapeutics • Cartilage regeneration |
41–43 |
| 5 | Smart and functional polymers—composites | • Biological properties • Antibacterial activity • Physical properties, e.g., self-healing, shape-memory, stimuli-responsiveness |
• Controllability of responsiveness may be affected by environment | • Injectable regenerative therapeutics for treating bone defects | 8,44,45 |
| B | Bioceramics | • Bioactive • Biocompatible • High compression strength • 3D printed scaffolds with mechanical characteristics comparable to human cortical bone |
• Low tensile strength • Brittleness • Weak under cyclic or high loads |
• Hard tissue engineering such as bone, cartilage, and tooth | 46–51 |
| C | Ceramic-polymer composites | • Cell incorporation • Enhanced tissue infiltration |
• Brtittleness | • Injectable or 3D-printed composites for dental and cartilage tissue engineering | 52–55 |
| D | Metals | • Biocompatibility • Degradable metal alloys • Improved mechanical properties |
• Uncontrolled corrosion | • Absorbable implants for bone repair • 3D porous scaffolds |
56,57 |
3D, three-dimensional; ECM, extracellular matrixes.
Advances in polymeric biomaterials
Natural polymers
Natural polymers that are most commonly used in tissue engineering are collagen,29 gelatin,30 chitosan,31 alginate,32 hyaluronic acid (HA),33 and polyhydroxyalkanoates (PHAs),34 because of their availability and biocompatibility.35,36 Peptides present on some of them, such as collagen, help cell attachment, migration, and function.29 Silk, a natural polymer,37 has been increasingly popular in many tissue engineering applications due to its high processability, strength, and elasticity.38,39 The PHAs comprise another group of natural polymers with special interest, as they are characterized by degradation through surface erosion that helps to maintain their general structure.40
Guided growth of neuronal cells was observed in vitro following the use of highly aligned electrospun fibers of a blend of the poly(3-hydroxybutyrate) [p(3HB)], and poly(3-hydroxyoctanoate) [p(3HO)].41 In another application, p(3HO) was used to produce cardiac patches, which exhibited favorable mechanical properties closely matching those of native cardiac muscle, and surface topography that enabled efficient cell adhesion and proliferation.42 To produce new constructs with improved properties, polymers can also be used in combination, for example, electrospun fibers made from a combination of chitosan and gelatin were found to enhance bone regeneration capability.43 When PHAs were blended with the synthetic polymer polycaprolactone (PCL) to produce a scaffold that delivers seeded cardiac progenitor cells and implanted in the postmortem murine heart, the implants enabled the adhesion of cardiac progenitor cells, stem cell proliferation, and retention.44
Natural extracellular matrixes
Natural extracellular matrixes (ECM) have been used in a wide range of tissue engineering applications.45,46 The ECM provides a natural structure that maintains some of the biological cues of the native tissues. The ECM chemical cues also help with cell attachment, differentiation, and function. There are various methods that have been used for the preparation of mammalian-tissue-based decellularized matrices, including chemical, biological, and physical methods and their combinations.47 The majority of research, though, has been focused on the decellularization of tissues or organs.48 It was shown that seeding decellularized hearts can result in obtaining contractile hearts by day 4 after keeping them in a bioreactor.49 Using electrical stimulation and physiological load, constructs pump function was achieved by day 8. This represents an interesting area for the application of decellularized ECM in the tissue engineering of various organs.
Kusuma et al. made a major advance by demonstrating that immortalized cell lines can produce high-quality ECM from a single cell source.50 Moreover, processing steps such as homogenization, pepsin digestion, or urea extraction have been used to create solutions that can be used to create surface coatings that retain some of the key properties of the native ECM. The ECM is proposed for numerous applications, due also to its versatile processing characteristics that have already allowed its use in 3D printing51 and electrospinning.52 For example, Kim et al.53 used skin-derived ECM bioink for the 3D printing of skin tissue, with some success. Further, Carvalho et al.54 combined cell-derived ECM with PCL and electrospun the solution to create microfibrous scaffolds for bone tissue engineering. The incorporation of ECM in the fibers enhanced cell proliferation and osteogenic differentiation, maintaining similar mechanical properties to PCL alone. The regulatory requirements of the field allow the strategy for efficient decellularization to appear to be one of the most viable pathways toward a product in short order.49
Despite the many positive attributes of the decellularized matrix for use in the field of tissue engineering, it does also come with limitations. One such limitation is its degradation rate, and this is a property that often needs consideration when using biomaterials for tissue engineering. For optimal regeneration, the degradation rate of decellularized matrix should be closely matched with the regeneration rate of the target tissue, and in many of applications this means that the degradation rate needs to be reduced.55 Current decellularization methods and processes achieve both a thorough removal of all cells and retention of other nonantigenic parts of the original tissue composition that can aid/guide in tissue regeneration.56
Decellularization is also not a “one-size-fits-all” approach, and the protocols must be adapted for different tissue types while integrating factors such as their density and the matrix components. Decellularized matrix produced from tissues, which have specific mechanical properties, must maintain structural matrix components such as collagen fibers and many proteins that are necessary as endoskeleton and thus decellularization protocols need to be tuned to preserving these components. Increased preservation of active factors and structural components would also increase the bioactivity of decellularized matrix, making it an even better natural guidance material for tissue engineering.57
It is important to note why this strategy is imperative and that it is linked to the “systems approach” already mentioned in contrast to precedent scientific reductionist work. In a system approach, the “principle” is that the ECM or the scaffold is imperative to drive and maintain differentiation. One can ask the fundamental question as to whether an osteoblast is an osteoblast when it is not surrounded by its ECM. Many in the stem cell field would argue that the cells and the ECM are intrinsic to one another and that molecular flexibility in differentiation and dedifferentiation occur without the union of the cell and the ECM. With this principle in mind, the strategies of decellularized matrices are rational because we do not have all the cues that are both physical and chronologic to the complex interplay between the cell and its ECM. Certainly, with further study and insight, smart or rational designs will incorporate the natural cues found in the natural ECM and allow synthetic polymers to support cell differentiation with similar efficiency to natural polymers.
Synthetic polymers
Synthetic polymers have been widely used in tissue engineering because they are widely available and inexpensive; can be bioresorbable; and can be processed in a controlled and multitude of ways to make them suitable for different applications. Commonly used synthetic polymers include polylactide (PLA),58–60 polyglycolide (PGA),58 poly(lactide-co-glycolide) (PLGA),59,61,62 PCL,60,63,64 poly(glycerol sebacate) (PGS),64 and polydimethylsiloxane (PDMS). The PDMS has unique applications in tissue engineering among synthetic polymers due to high oxygen (O2) diffusivity, ease of fabrication, biocompatibility, and flexibility.65 It has been explored for engineering of cell sheets,66 and it is widely used for the development of 3D organ-on-a-chip (OoC) cultures67 that helped advancing the field of engineering tissue models tremendously (see the Advances in Microfluidic Culture Systems section). The PDMS is a nonbiodegradable polymer, and it was therefore used more commonly in ex vivo rather than implantable tissue engineering constructs.67
Blends of synthetic polymers were also explored to combine the properties of different materials.68 Synthetic polymers have been also used in combination to build different phases in the resulting structure. For example, Fang et al. used PLA to produce shell and PGA to produce core in electrospun nanofibres.58 The materials were found to accelerate wound healing in vivo. Synthetic polymers can also be combined with natural biomaterials to form semi-synthetic polymers. For example, Jiao et al.63 melt-blended PCL and HA to 3D print scaffolds for bone tissue engineering. Constructs had improved mechanical characteristics as compared with those that were made from PCL alone.
The bioresorbability of many synthetic polymers poses advantages in many tissue engineering applications. However, controlling the rate at which degradation occurs can be a clinical challenge. Xu et al.62 experimented with adding magnesium to PLGA to make composite films with low ranges of magnesium weight percentages, and they found that magnesium extended the duration of degradation and also improved the tensile strength of the films.
Hydrogels
Another advancing recent frontier has been in the area of hydrogels. Hydrogels have been extensively used for 3D bioprinting, which has been a very active area of research in the past few years.69 Hydrogels can be made from various natural or synthetic polymers and have been used for the engineering of different tissues, because of their ability to encapsulate cells,70,71 while having the permeability required for the diffusion of O2 and nutrients across the material. An aspect in which previously they have fallen short is their mechanical properties72 and the lack of adhesiveness.73 Recently, however, these problems were largely addressed. For example, Shirzaei Sani et al.74 produced an adhesive HA/elastin-like polypeptide hybrid hydrogel, which is characterized by remarkable adhesive, antimicrobial activity, and tunable physical properties. This enhances the translation of the hydrogels to the clinical practice as it was limited due to their poor mechanical characteristics, low adhesive strength, and their weakness to inhibit bacterial colonization.
Reinforcement of hydrogels can be achieved through interpenetrating secondary networks.75 The addition of a second network enables conventional hydrogels to be used in many emerging biofabrication techniques toward achieving hierarchical architectures and developing personalized medicine. These interpenetrating hydrogels can find applications in tissue engineering and drug delivery systems as well as in developing in vitro disease models for drug discovery and screening. Hybrid hydrogels were found to have greater adhesive strength to the tissue being engineered, as compared with commercially available tissue adhesives. A great potential of hydrogels is their use as injectable materials to deliver cells, drugs, and biomolecules16 for regenerative purposes that can often be achieved by using minimally invasive techniques.76,77
A recent study78 looking into cartilage repair found that HA hydrogels could be used to encapsulate chondrocytes and support cell survival and the regeneration of cartilaginous tissue. Aside from HA, alginate, and collagen, ECM hydrogels have been used in tissue engineering, and also for cell encapsulation. In addition, microencapsulation of cells to produce microgels was also explored.79–81 Hydrogels such as these can be blended and processed through 3D bioprinting, where cells are encapsulated and printed into designed structures and then crosslinked to provide appropriate mechanical properties. For example, 3D-printed scaffolds of collagen/alginate hydrogel have been used for cartilage tissue engineering82 and ECM hydrogel for cardiac patches.83
The regeneration of damaged tissue can be achieved either via ex vivo or in situ methods. In ex vivo tissue engineering, scaffolds are combined with cells and biomolecules outside the body to obtain cell-laden tissue constructs for implantation (Fig. 2A).16 However, the ex vivo tissue regeneration has limitations, such as tissue morbidity and the lack of reliable cell sources. On the other hand, in situ tissue engineering requires precise control of the biochemical and biophysical cues to stimulate resident host cells and attract cells to the site of injury requiring regeneration (Fig. 2B). On the other hand, in situ tissue regeneration can be achieved by stimulating endogenous cells using either extracellular signals or cell reprogramming. In the first approach, cells are primed via extracellular factors, such as through modulating the biophysical and biochemical characteristics of the biomaterial (Fig. 2B).16 In the second approach, direct manipulation of the cellular gene- expression program is accomplished through cellular reprogramming (Fig. 2B).16 Because of its relation to biomaterials, we review the first approach in this section.
FIG. 2.
Schematic illustration showing (A) Use of traditional ex vivo tissue engineering approach, which is based on the use of cells (1) cultured (2) with growth factors (3) and scaffolds (4) to develop pre-seeded constructs (5) outside the body before their implantation (6). (B) Use of in situ tissue regeneration to harness the innate regenerative capacity in the body either through extracellular signal manipulation by using bioactive (1) or immunomodulatory (2) biomaterials or bioactive molecules (3), or through an intracellular reprograming approach, which employs epigenetic transformation (4), transcription factors (5), gene editing (6), or an RNA-based approach (7). Created with Biorender.com. Color images are available online.
Smart and functional polymers
Smart polymers used in tissue engineering include those with self-healing,84–86 shape memory,76,87–89 or stimuli-responsiveness18,90–92 properties. The ability to change the shape of 3D-printed objects via environmental stimuli, such as heat, moisture, water, pH, or light as a function of time, is known as four-dimensional (4D) printing, and it also has recently gained considerable interest.93 For example, Invernizzi et al. have developed a novel 4D-printable smart material, using PCL and 2-ureido-4[1H]-pyrimidinone (UPy), which is a thermally activated shape memory polymer with self-repairing abilities.94 The incorporation of methacrylates bearing UPy (UPyMA) monomers had provided self-healing properties to the 4D-printed structures, and the possibility to print actuators for soft robotics had been shown for the first time in this work. Synthesizing smart hydrogels that provide both self-healing and shape memory properties at the same time is expected to be further investigated in the following years.95–97
Although there are many clear reasons why smart and functional polymers have gathered attention, they do have drawbacks that should be considered. For example, in the body environment, triggering thresholds of changes, for example, temperature or pH, may affect the responsiveness of these polymers, which, in turn, will affect controllability of the construct and the included cells.69 A shape memory material implanted in the body may lead to injury of the neighboring tissues or loss of function when it returns to its original shape.98 These challenges have to be mitigated before full benefits can be gained from utilizing smart polymers for tissue engineering.
Stimuli-responsive and self-healing hydrogels have also emerged as pharmaceutical carriers for tissue engineering.84,94–105 One such example application of these self-healing hydrogels that is being explored is bone regeneration that can be achieved by providing an optimal microenvironment for new bone formation and for therapeutic drug delivery. Unlike conventional hydrogels, these constructs can resist mechanical stress, while protecting their therapeutic cargos from degradation and maintaining their sustained release for the long-term performance required for bone tissue healing.84,99 To this end, a hydrogel made of chondroitin sulfate (ChS), known for its regenerative capacities, was developed for bone tissue repair with the material being cross-linked to mimic cranial bone characteristics.100
With excellent self-healing, injectability, and in vivo tissue adhesion abilities, ChS-based hydrogel exhibited good cytocompatibility when it was used to encapsulate rat-derived mesenchymal stem cells (MSCs). Most importantly and compared with phosphate-buffered saline-loaded hydrogel, the injection of bone morphogenetic protein (BMP)-4 loaded hydrogel into a murine bone defect model led to defect repair through the formation of new cranial bone tissue, with a significant decrease in the defect size after 12 weeks.
Over the past few years, significant progress in the development of advanced functional polymers with tunable chemical, physical, and biological properties has been achieved.101,102 This resulted in novel applications in 3D and 4D bioprinting103 and drug delivery.104–106 For example, Zhang et al. recently developed a biocompatible hydrogel ink, which contains self-healing precrosslinked hydrogel microparticles of chitosan methacrylate and polyvinyl alcohol hybrid hydrogels. Their results showed advanced structures with a high aspect ratio, and excellent shape accuracy at organ-proper scales could be quickly produced.107
Several drug delivery systems have been based on the use of advanced polymers. Consequently, functional hydrogels that can provide the required dosage in both proper chronicity and location can mitigate complications and enhance success with clinical application.108–110
Advances in bioceramic biomaterials
Ceramics are attractive materials for tissue engineering, because of their highly bioactive and biocompatible characteristics.111 Ceramics, including bioactive glass ceramics, have been used in bone tissue engineering applications for many years due to their well-matched mineral characteristics.112–114 They are strong and osteoconductive, which makes them ideal for application in hard tissue engineering.66–69 They are strong in compression, however, weak in tension, and very brittle. Bioactive ceramic and glass-ceramic scaffolds were also produced by 3D printing processes such as “robocasting”115 and the “freeze extrusion fabrication” that combines extrusion printing with freeze-drying.116 The high strength values of scaffolds fabricated by additive manufacturing result from their ability to maintain highly interconnected channels with high alignment, at a porosity of 50–60%. These scaffolds presented an elastic response under compression, with an average compressive strength of 140 MPa and an elastic modulus of 5–6 GPa, which are comparable to those of the human cortical bone.
Although bioactive ceramic and glass-ceramic scaffolds can effectively mimic porous bone, provide required compressive strength,117 and contain channels in their 3D structure for tissue ingrowth,115 they are brittle and not suitable for applications in locations exposed to cyclic or high loads. Thus, scaffolds made of pure ceramics were not very successful when they were used in load-bearing regions of the body.118 It is also imperative to note that these scaffolds, in particular once seeded with cells and remodeled by biologic ingrowth and calcified ECM production, will change their structural capacity and thus often can be used as a bridge technology. Therefore, the development of advanced scaffolds that can maintain bioactivity properties is required. To achieve this, the obvious engineering solution that has been implemented was the development of composite materials.
Advances in ceramic polymer composite biomaterials
Bioactive glass was used in the form of particles,119 fibers,120 or scaffolds,121 and it was combined with polymers to develop various composites for tissue engineering. For example, bioactive glass nanoparticles have been incorporated into freeze-cast gelatin-chitosan foams with a pore size range between 150 and 300 μm.122 The low strength of the composite was improved by a decrease in its porosity. Bioactive glass–collagen–phosphatidylserine scaffolds (65 wt.% 58S sol–gel bioactive glass) were developed with 75% porosity, a pore size of 300 μm, and a compressive strength of 1.5 MPa.123 However, connectivity between pores was poor, limiting scaffold application in tissue engineering.
In addition, Nikpour et al.124 developed a composite with bioactive glass-ceramic and dextran hydrogel because of its biocompatibility and hydrophilicity, which enable the incorporation of cells and nanoparticles in the structure. Chatzistavrou et al. also looked at the combination of bioactive glass-ceramic particles with appropriate matrixes (e.g., ECM, collagen–fibrin microspheres) and stem cells to enhance odontogenic differentiation and trigger new dentine formation in dental tissue engineering approaches.125,126 Another study also looked at producing nano-bioactive glass-ceramic particles that were incorporated with Calcarea phosphorica aiming at assessing the effect of these nanoparticles on osteoblast differentiation.127 It was found that these particles had osteogenic potential, as they promoted mouse mesenchymal cell proliferation.
Ceramics are also being coated with polymers, which can help in achieving surface functionalization, controlled delivery of growth factors and drugs, and enhanced bioactivity.128 In one example, Luginina et al.64 combined bioactive glass particles with electrospun PGS/PCL for the engineering of cartilage. This combination helped to maintain smaller projected cell areas as well as rounded cell phenotype. Scaffolds made of 13–93 bioactive glass were seeded with rat-bone-marrow-derived MSCs and implanted in the subcutis of rats for 4 weeks, which resulted in tissue infiltration of the scaffolds.121 Moreover, vessels can form inside scaffolds in in vitro cultures and when the construct is implanted in vivo newly formed vessels may connect to the host blood vessels.129 Further, scaffolds made from bioactive glass fibers and PLGA mesh were also developed and investigated for bone tissue engineering using osteoprogenitor cells representing craniosynostotic osteoprogenitors, with the view of using this approach for the reconstruction of the crania of these patients using autologous cells derived from removed tissues.130
Advances in metal biomaterials
Metals are a group of interesting materials that can also be used for developing scaffolds for tissue engineering. At a historic level, gold and other materials with malleable properties have been used before the time of Hippocrates. Because most of metals are not biodegradable, they cannot be replaced by tissues. Therefore, the use of this group of materials for tissue engineering has been very limited except for the recent activity in biodegradable metal alloys, which represents an expanding research frontier. These materials combine both the properties of metals and biodegradability sought in polymers. In addition, their use helps to avoid many problems associated with the use of biodegradable polymers, such as inflammation131,132 and osteolysis.133 In this group of biodegradable metals, magnesium-based alloys have been explored and various implants have been developed especially for application in the treatment of bone tissue.134 A combination of both biodegradable polymers and metals has also been investigated,135 for example, a biodegradable magnesium-reinforced biodegradable PLA membrane was developed for application in guided bone regeneration.136 In future, it is expected to see more studies on the combination of metals with polymers.
Metals, in general, exhibit improved mechanical properties (i.e., yield strength, ultimate tensile strength, hardness, etc.), and they are considered the best alternative for structural support. In addition to mechanical performance, absorbable metals should be compatible and nontoxic with controlled corrosion behavior. A metal that can be considered absorbable should corrode in the body's environment without generating toxic corrosion products. Thus, they should meet an appropriate balance between maintaining the required mechanical performance and corroding within a required period while the native tissue is regenerating.
Iron (Fe), magnesium (Mg), and their alloys have been investigated as absorbable metals, for biomedical applications in cardiovascular and orthopedic surgery. Mg is biocompatible, reduces thrombogenicity, and is critical for several cellular functions, such as intracellular transport, signal transduction, and energy metabolism.137 Absorbable stents138 and bone screws139 made of Mg-based alloys are already commercially available.140,141
However, the uncontrolled and fast corrosion of Mg in biological environments remains a challenge.142 Mg-based alloys are still being optimized toward meeting the expectations of absorbable metallic implants.132 Zinc (Zn) was incorporated as an alternative to Mg, because of its moderate corrosion rate in simulated body fluid.143 In one study, Bowen et al.144 presented an outstanding corrosion behavior and biocompatibility of Zn vascular stents in rat aorta. Current research proposes that Zn alloys could potentially overcome the challenges of Mg alloys used as absorbable implants. Current research work also includes advances in other biomedical applications such as 3D porous Zn scaffolds.145
Through previous research it became clear that each biomaterial brings with it certain advantages and disadvantages, and to create specialized scaffolds and other tissue engineering constructs we must be able to utilize multiple materials in combination, so that their varied advantages can be exploited. In some sense, this requires that the engineering process begins with a clinical problem that dictates the design and needs of the bioresorbable material properties. Early tissue engineering began with a polymer or a construct and looked to find an application. Therefore, as the field has advanced, our approach to design and fabrication should also evolve.
Advances in stem cells and their application in tissue engineering
Although primary cells can be used for tissue engineering,146 the use of stem cells offers the advantage of access to cells that can be directed to differentiate to the desired cell type.147 The use of autologous cells, in particular, can help to avoid the problems associated with allo-transplantation. Therefore, stem cells represent a very important and almost inexhaustible source for tissue engineering148,149 and regenerative medicine.150,151 Stem cells can also be used for engineering tissues either with or without a biomaterial as a matrix152 (Fig. 3). In addition to engineering tissues for regenerative purposes, stem cells were recently used for the engineering of cancer spheroids to develop models for cancer studies.153
FIG. 3.
Engineering of cell sheets composed of cells only using a modified poly(N-isopropylacrylamide) (PNIPAAm) surface.430 Color images are available online.
Advances in stem cell sources
Stem cells are divided according to their differentiation potency into various lineages as totipotent, pluripotent, and multipotent. Totipotent stem cells can give rise to the three primary germ cell layers of the embryo and also give rise to extra-embryonic tissues.154 Pluripotent stem cells can give rise to all tissues in the body, except the placenta and umbilical cord. Embryonic stem cells (ESCs) represent an important type of pluripotent stem cells that were explored for cell therapy and tissue regeneration.155,156 Because of the associated ethical issues and regulatory restrictions, research continued to explore other possibilities.157
In 2006, scientists succeeded in developing pluripotent stem cells from adult somatic cells,19 that is, iPSCs, by using clustered regularly interspaced short palindromic repeats (CRISPR) technology, which can be used for either the activation (CRISPRa) or interference (CRISPRi) with the expression of certain genes.158 The iPSCs are currently being intensively studied because of their pluripotency but without having many of the issues associated with ESCs.159 Today, they represent an advancing frontier in tissue engineering, because of their potential to differentiate into many cell types.158 For example, iPSCs that were generated from human anterior cruciate ligament were used in the repair of ligaments and tendons. Further, iPSCs will be an invaluable tool for the development of personalized therapeutics.160,161
Compared with pluripotent cells, multipotent stem cells such as MSCs can produce only certain cell types.162 MSCs are derived from mesodermal embryonic tissues, have high regenerative ability, and are precursors of different mesenchymal tissues such as bone and cartilage.163 MSCs can be isolated from different tissues such as bone marrow,164 adipose tissue,165–167 amniotic membrane,168 umbilical cord,168–170 placenta,171 dental pulp,172 and other sources that are being continuously explored.173
Recently, MSCs that were isolated from the synovial fluid and synovial membrane were investigated for cartilage tissue engineering.174 Among these, adipose tissue represents an attractive source175 because of its abundance, easiness of accessibility, and the possibility of retrieval of stem cells that were proved to differentiate to different lineages such as fat, bone, and cartilage.176 Generally, MSCs have been the most widely investigated stem cells177,178 for various tissue engineering applications.179 Their versatile behavior in vivo and in vitro made stem cells favorable for research and clinical applications.180,181 Stem cells can also be used for immunomodulation,182,183 which can be explored for application in tissue engineering and regenerative therapy.184
Recent advances in stem cell-derived extracellular vesicles
In addition to iPSCs, advancing frontiers in stem cell technology and its application in tissue engineering include stem cell-derived EVs. EVs are produced by cells in the form of exosomes, microvesicles, or apoptotic bodies; they carry peptides, lipids, or nucleotides such as RNA and DNA, and they have been increasingly recognized as an important means of molecular communication between cells and organs.185 In particular, EVs secreted by MSCs have been investigated for tissue regeneration since they can produce important effects without the need to use cells. They have been investigated for skin, bone, cartilage, and neuronal regeneration.178,186
To increase the efficacy of EVs, MSCs were preconditioned by using hypoxia to produce primed MSCs.187 This paves a new way of devising regenerative strategies based on the use of stem cell-derived EVs, which will help to eliminate many of the problems associated with the use of cell-based products. It is expected that research in this area will expand and extend to clinical translation in the future. It also underscores the importance of closing the knowledge and understanding gap that we still have in relation to the stem cell microenvironment.
Advances in stem cell differentiation
The most important challenge in the development of stem cell-based treatments in tissue engineering applications is the identification of biophysically and biochemically different tissue-specific environments.188 In this process, besides defining differentiation and growth factors that mimic the stem cell environment, it has been reported that determining the physical properties and mechanical forces of stem cell matrix such as morphology and stiffness are also important.189 Studying the effects of ECM biological, physical, and chemical effects on stem cells will help to develop methods that can influence cell differentiation.190 It was also found that making the surface architecture and the stiffness properties of the biomaterials similar to those of certain native tissues favors the differentiation of the stem cells to cells specific to these tissues.191
Stem cell fate control is a crucial issue for stem cell research and applications. In a recent study, magnetic nanoparticles were used to guide stem cell differentiation,192 with the help of an externally applied magnetic field that was used to pull iron oxide particle-laden ESCs together and form spheroids. Then, opposing magnetic fields were used to stretch them and lead to cardiac lineage differentiation. When both physical and chemical factors were combined and applied to ESC, significantly higher myogenic differentiation was observed.193,194
Differentiation of stem cells into desired cell type is possible by identifying factors such as matrix microenvironment and epigenetic mechanisms195 that regulate the fate of stem cells.190,194,196 For instance, an injectable hydrogel was developed by using HA, horseradish peroxidase, galactose oxidase, and tyramine; it was used as a crosslinker. Experiments in mice demonstrated the biocompatibility of the material, which makes it a good candidate for use in biomedical applications such as tissue engineering applications.197
In a recent study, the porosity of hydrogel biomaterial was shown to influence MSCs and their response to insulin-like growth factor-1 (IGF-1).198 Unlike nanoporous alginate hydrogel, microporous ones could sensitize MSCs to the growth factor. Adding cell–cell adhesion mediating molecule (N-cadherin) mimicking peptide to nanoporous alginate added the effect that macroporous had in eliciting MSCs paracrine activity in response to IGF-1. This demonstrated the role of physical properties of the biomaterials further, and also the possibility to influence this by using chemical ways. Combined, these methods will help us to control the behavior of stem cells further in the future and tailoring it toward desired activity and fate, for regenerating desired tissues.
Advances in cell maturation strategies
There are several technologies that have been developed to increase cell maturation by using electrical and physical cues. For influencing cell maturation, physical cues such as surface patterning199 or mechanical stretching200 have been investigated. For example, physical conditioning of cardiomyocytes (both primary myocytes and human pluripotent stem cell-derived cardiomyocytes) embedded in a collagen hydrogel was achieved by using an automated stretch device.200 More recently, the effect of electrical stimulation on cell maturation and the differentiation of human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) was investigated.201 It was found that the application of electrical stimulation during cell differentiation makes hiPSC-CMs behave similar to cardiac cells. It was demonstrated that hiPSC-CMs derived by using electromechanical stimulation can be used to engineer heart tissue.202 In fact, the maturation of early stage cardiomyocytes was achieved by using electrical stimulation for two weeks in the presence of various growth factors. This area represents an advancing frontier in tissue engineering, and it is worth investigating further to also look at the outcome of in vivo applications of cells matured using electrical stimulation.
In another recent study, electrical stimulation of neonatal rat cardiomyocyte-embedded in gelatin hydrogel led to their maturation.203 It was also shown that the organization of the cells within the gelatin hydrogels was improved by employing this strategy. For skeletal muscle tissue engineering, a gelatin-carboxymethyl cellulose biomaterial was combined with carbon nanotubes to increase electrical properties of the biomaterial.204 Electric pulse stimulation was applied and led to enhanced myogenic differentiation and maturation of C2C12 myoblasts to form a skeletal muscle tissue.
Advances in culture systems and their application in tissue engineering
Cell culture is an important and integral part of ex vivo tissue engineering. Because of the rarity of certain cell types in the human body and the potential donor site morbidity associated with the retrieval of cells in large numbers,22,205 cells are propagated outside the body in an environment that can provide nutrients and possible stimulation of cells to proliferate, differentiate, and function.22 This is carried out using static or dynamic culture methods. In recent years, there have been advances in cell culture methods such as 3D, 4D, and microfluidic OoC culture systems. Significant progress has been made in these areas, and therefore, they will be highlighted in this section.
Advances in cell culture microenvironment
The most common drawback of any of cell culture techniques is the need to use media, which may contain animal serum. There is evidence that fetal serum could be the source of endotoxins, mycoplasma, or viral contaminants.206 Also, the serum itself has ECM components that may alter the cell expression of proteins. Human autologous serum can alternatively be used, and it has been shown to be equivalent to fetal animal sera. However, it is often difficult to isolate human autologous serum in sufficient quantities, especially for use in prolonged and large-scale applications.207 Serum-free media tend to substitute individual key components found in serum-containing media, which can be a safer approach.208
Among the key components, are the growth factors specific for the stem cell type and tissue culture application. For instance, Hasegawa and colleagues have created a medium for stem cell culture containing a replacement of wnt with a GSK3β and NFAT inhibitor.209 Despite significant progress made with serum-free media, the use of new approaches for the elimination of protein from the media will make this technology more cost-effective and possible to scale up.
Other parameters of cell culture such as temperature can also be used to influence cells, for example, improving adipogenic differentiation.210 Another area of interest is the co-cultivation induction, where the concomitant culture of stem cells and committed cells is carried out. This technique has been shown to upregulate the properties of the stem cells and to induce a “physiologic” differentiation process without the need for the use of morphogens and other differentiation induction media. In a recent study, cardiomyocytes were cultured together with iPSCs, and it was found that older cardiomyocytes serve as an adequate inducer for stem cell differentiation, recapitulating the environment necessary for cardiac cell differentiation.211
Imprinted micropatterns on the surface of plates allow cell-to-cell adhesion and determine the formation and characteristics of the culture. This approach has seen several potential applications. For example, the use of micropatterned surfaces allowed homogenous stem cell differentiation to chondrocytes.212 In another study, a 3D micropatterned plate was used to culture hepatic endoderm iPSCs.213 The cells quickly reaggregated and formed hundreds of round-shaped spheroids while they efficiently differentiated into hepatocyte-like cells expressing hepatic gene makers. In addition, growth factors can be printed in micropatterned surfaces. For example, a micropattern-immobilized nerve growth factor nanolayer was found to induce neurite growth and regulate neurite formation.214
Advances in 3D culture
Conventional two-dimensional (2D) systems are classically used for stem cell culture. Such culture uses a feeder layer of cells complemented with tissue culture media supplemented with growth factors or cytokines containing cues that support cells and drive them to proliferate or differentiate.215 Two-dimensional cultures have several limitations, including: (1) the deformation of the cells during culture (flattening and elongation), (2) poor differentiation and cell junction formation, (3) unnatural high proliferation rates, and (4) significant differences in gene expression and phenotypes.216 Recent advances in 2D cultures have tried to overcome some of these drawbacks. Adaptations of the tissue culture biomaterial properties have been shown to modify cell fate.
On the other hand, 3D culture systems are better at recapitulating in vivo conditions. Several studies showed the effect of 3D culture systems on improving cell morphology, proliferation, differentiation, and response to stimuli.217 Three-dimensional culture could be divided into either anchorage-dependent (scaffold-based) or anchorage-independent ones using specialized 3D platforms.218 The former can benefit from recent advances in processing techniques mentioned earlier, such as 3D bioprinting20,219,220 and electrospinning,221,222 to create complex structures.223 Such cell culture models should mimic cells' natural environment, providing interactions between the cells and the microenvironment, nutrients, O2, and waste product removal.
Despite several advantages of 3D over 2D culture, 3D culture still have some drawbacks such as uneven distribution of nutrients, growth factors, and O2, which often results in making cells residing far away from the surface of the matrix inactive.224 In addition, many tissue-engineered constructs are looking for regenerative models of culture as opposed to mature quiescent ECM–cell relationships. Increased costs, differences in experiment replication, and data interpretation are additional drawbacks of this type of culture225 that remain to be addressed in future development activities.
Scaffold-based anchorage-dependent culture techniques utilize a scaffold of variable architecture ranging from a simple extracellular-like matrix to complex multilayer structures. Scaffold selection is largely dependent on the target tissue to be engineered, advantaging physical factors providing structural stability and the cellular composition of the target tissue. Three-dimensional bioprinting has revolutionized the construction of such complex structures. However, because the development of functional vasculature in transplantable devices has not been achieved, successful in vivo applications and clinical translation are largely affected.
Special 3D anchorage-independent techniques include the use of a low attachment vessel,226 magnetic levitation,227 or hand-drop technique,228 including the use of magnetic forces.192 The low attachment plate technique uses a culture vessel with an ultra-low attachment coating. Anchorage-independent techniques force cells to aggregate, form spheres, and subsequently create their own ECM. The most common form of these techniques is the spheroid culture, which is used in the engineering of cartilage.229
Magnetic levitation utilizes a magnetic force to levitate cultured cells mixed with magnetic nanoparticles. This technique is shown to have reproducible results and to reduce necrosis in the spheroid core. Stem cells cultured in these conditions maintain their properties and remain quiescent for subsequent clinical use.230 One area of interest in anchorage-independent culture is the development of organoids. Organoid formation involves the utilization of a tissue culture technique that allows self-organizing and self-renewing of 3D cultures. Organoid cultures have been described for several organs, including the kidney, eye, brain, gut, and lungs.231
More recently, Tseng et al. demonstrated the capacity of assembling adipospheres from multiple cell types, including adipose tissue-derived stem cells, endothelial cells, and leukocytes, that recreate tissue organization.232 This technique enabled the formation of vessel-like endothelial structures with lumens and differentiation of unilocular adipocytes. The hand-drop technique utilizes the self-aggregation properties of cells when no attachment wall is found. The cells aggregate to form spheroids, and the control of the volume of the cell suspension enables the control of the spheroid size. The outcome of this type of cell culture is better as compared with that of static cultures.
Recently, investigators explored the conversion of adipocytes to cardiomyocytes for application in cardiovascular tissue engineering.233 In applications for retinal degeneration, the hand-drop technique was utilized to convert adipocytes to retinal precursors and showed improved differentiation yield, with these precursor-like cells responding to glutamate neurotransmitters.234 This technique has been used in many other preclinical studies, including cartilage repair, bone healing, and cardiac tissue regeneration.235,236
Four-dimensional culture platforms utilize a complex 3D-bioprinted or imprinted structure with a predetermined time-dependent dynamic morphological change. This is achieved by the control and manipulation of the behaviors of stem cells responding to cues that aim at replicating the topographical and mechano-biological environment of the target tissue. These systems could find applications in studying tissue biology and pathophysiology, preclinical testing, and tissue biofabrication.237–239 As far as tissue engineering is concerned, the use of 4D culture systems is in its infancy. However, some promising studies were published. For example, Miao et al. have utilized this technique to create neural tissue with a time-dependent self-morphing regulation of neural stem cells that enhances neural differentiation of cells along with significant axonal alignment.238 Further studies will be of interest in this area of research, as it is structurally most replicative of the regenerative process of healing.
Advances in microfluidic culture systems
Microfluidic systems are designed for cultures under perfusion, allowing a continuous supply of O2 and nutrients (Fig. 4). This enables the long-term maintenance of constructs at physiologically relevant nutrient supply rates. The use of a microfluidic-based approach in cartilage regeneration allowed enhanced conjugation of the key growth factor, transforming growth factor-beta 3 and its sustained release.240 In another study, biomimetic neural tissue fibers having hierarchically ordered nerve fibers were created by using a microfluidic system, which contained a coaxial triple-channel chip and a stretching loading device.241 Authors reported good performance of the resulting nerve fibers.
FIG. 4.
(A) Schematic illustration of the design of microfluidic chip that has three parallel gel regions, six gel filling ports, and two medium channels connected to four medium reservoirs. The device also contains a surrounding vacuum channel. Scale bar, 2 mm. (B) The device comprises a microfluidic layer on a polydimethylsiloxane membrane featuring two sets of two capped pillars (inset). The membrane is itself bonded to a coverslip. (C) Schematic illustration showing the final coculture arrangement: embedded in a hydrogel, muscle bundles that are wrapped around and exerted force to the pillars. They are innervated by neurospheres, which are placed in the opposite gel chamber separated by a 1-mm-wide gel region. (D) Schematic illustration showing the differentiation process of the ESCs into motor neurons (MNs). Row 2: Schematic illustration displaying the top and front views of the tissue in the microfluidic device. Row 3: Three-dimensional illustrations showing the version of the device used at the corresponding days. ChR2, and Channelrhodopsin-2; CNTF, ciliary neurotrophic factor; EBs, embryoid bodies; ESCs, embryonic stem cells; GDNF, glia-derived neurotrophic factors; HS, horse serum; RA, retinoic acid; SAG, smoothened agonist. Reproduced from Uzel et al.,431 which is an open-access article distributed under the terms of the Creative Commons Attribution license. Color images are available online.
The microfluidic system was also used for the production of a gene delivery system composed of nanocomplexes of plasmids encoding for BMP-2 and chitosan.242 The results demonstrated the potential of using this system for in situ bone tissue regeneration. Another application of microfluidic systems is the development of OoC platforms, which aim at reproducing the function of organs or tissues.243,244 Applications of OoC are currently limited to the development of basic tissue functions and of certain disease models,245–247 and it points to new avenues for the study of novel tissue engineering strategies.
Advances in processing techniques and their application in tissue engineering
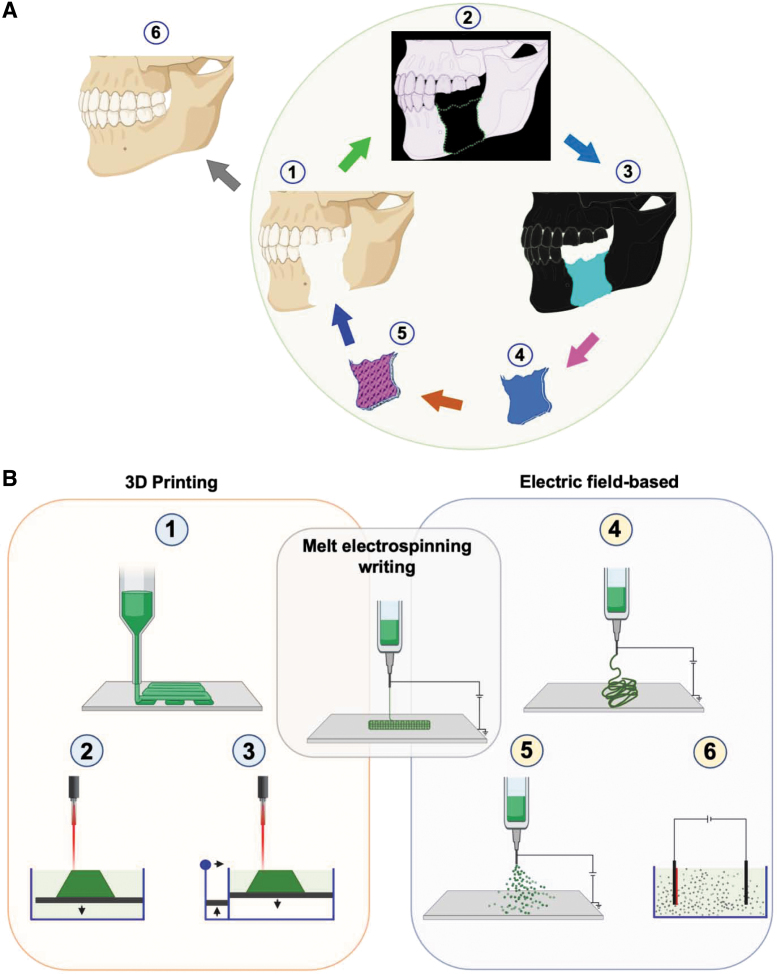
There are several techniques that have been used to develop scaffolds, matrices, or tissue constructs, such as salt leaching, molding, spinning, freeze-drying,248 solvent casting and particulate leaching,249 electrospinning,221,222 selective laser sintering and 3D printing,250 and 4D bioprinting.69 However, we will highlight in this section only the recent developments in the most advancing frontiers of fabrication techniques251 (Fig. 5).
FIG. 5.
Advances in fabrication techniques for tissue engineering. (A) Three-dimensional printing and electric-field-assisted techniques can be very useful for 3D construction of tissue defects (1) based on data-derived from imaging such as computed tomography (2), which is transferred to a design template for 3D bioprinting (4), to produce living constructs (5) that are transplanted to bridge defects and heal damaged tissue (6). (B) In 3D printing (1–3), layer-by-layer deposition of polymeric gel results in the formation of predesigned 3D constructs. In the electric-field-assisted technique (5–6), an electric field is used to control for directing and depositing polymeric fibers. In melt-electrospinning writing (4), both 3D printing and electric-field-assisted methods are combined. Created with Biorender.com. Color images are available online.
Advances in 3D printing
Tissue engineering has adopted the 3D printing technique252 for the fabrication of scaffolds and later to create cell-laden multi-cellular253 and complex254 tissue constructs, and the technique was termed “3D bioprinting.” Three-dimensional bioprinting is gaining increasing popularity, with more companies innovating to produce 3D bioprinters. The method employs most commonly extrusion, inkjet, laser, or stereolithography, with each of these methods having its own advantages and limitations.25,255 Therefore, new approaches include combining 3D bioprinting with conventional manufacturing methods. Different combinations of various fabrication techniques can be used, for example, combining electrospinning with 3D bioprinting256 or 3D printing with 3D bioprinting257,258 to produce advanced scaffolds.
Three-dimensional bioprinting has several advantages over other tissue engineering techniques.259 It allows the creation of well-defined, customized structures that mimic native tissues. These tissues have functional cellular components; therefore, cellular migration from the host is not essential. Further, cellular interaction and key signaling molecules can be incorporated into the design of the printed constructs. The overall cost of 3D bioprinting is lower when compared with currently used graft materials with no donor site morbidity.260 Host tissue regeneration that occurs in pace with the degradation of the implanted 3D-bioprinted construct can hopefully be achieved in future by controlling material properties of the construct bioink. The field is still in its infancy, and therefore we expect to still see shortcomings of current 3D bioprinted constructs.
One problem is the choice of the material that can address both the biology and the anatomy of the tissue to be treated. A lot of our current understanding of these issues is based on our experiments on animals, which may make the design of structures with appropriate properties that fit human tissue structure and function challenging. Further, several aspects of the 3D bioprinting process, such as the isolation of cells, culture conditions, and identification of the signals and growth factors, need to be considered. Our current inability to incorporate vasculature and the potential degradation of the structures limits the success of implanted constructs, and it requires the attention and development of innovative solutions.259,261,262 There are two areas of 3D bioprinting that deserve special discussion, the 4D263 and the in situ264,265 bioprinting.
Despite the numerous challenges, in the past decade, an increasing number of studies were published regarding the creation of biomimetic constructs for future clinical applications. The most important applications so far include skin,266 musculoskeletal267 cardiovascular,268 neural,269 and other tissues.270 The studies present developments made in bioinks that are composed of different biomaterials, cell types, and additives such as growth factors, drugs, or osteoconductive elements, which were tested either in vitro or in vivo.271 In addition, 3D-bioprinted products often have significant contributions from established FDA-approved component parts, including cells, signals, and scaffolds, and thus the fabrication technique can often produce and amalgamate products that incorporate existing technology with a novel effect. Available evidence is encouraging; however, we are far from achieving the full complex organ engineering that tissue engineering has promised.
Advances in 4D, in situ, and spheroid printing
Four-dimensional bioprinting uses smart stimuli-responsive materials,18,90 which are programmed to change their properties and bioactivity over time in response to local or external stimuli.272 An example of the application of 4D bioprinting can be in guided nerve generation, using materials such as graphene hybrid in a 4D construct, which can provide physical guidance, chemical cues, dynamic self-entubulation, and seamless integration.273
In addition, in situ bioprinting265 is of great interest. It employs special hardware and it completely eliminates the need for ex vivo manipulation of the grafts.264 This approach can find applications in cases where the exact dimensions of the tissue are not known preoperatively, such as, for example, after debridement of tissues following trauma, infection, or cancer resection. Several hand-held265 or scanner-controlled 3D printing devices have been developed and the available studies show that such structures retain the high resolution of the 3D bioprinting technique and can match the exact needs of tissues to be constructed.274
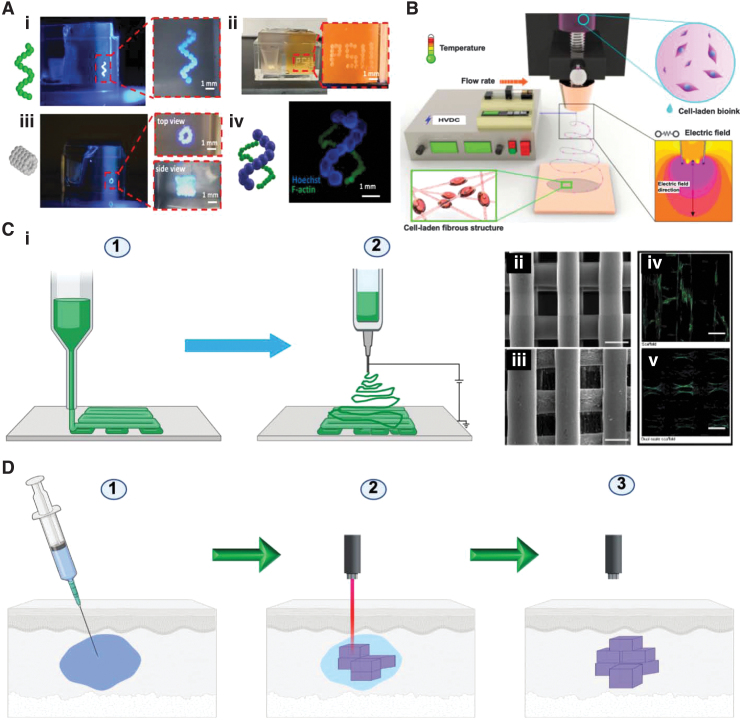
Spheroids were recently used as building blocks of constructs that were produced by 3D bioprinting. In this method, spheroids are sucked in, and then released in a controlled fashion. This approach tries to lend developmental biology approaches. This technique will allow the development of 3D constructs using biomaterial-free bioinks and precise deposition of spheroids into the resulting construct.275 It was also possible to use spheroid-based 3D bioprinting in combination with the freeform 3D bioprinting method that enabled patterning of the printed spheroids into the desired shape of constructs (Fig. 6A).276
FIG. 6.
Various fabrication techniques for tissue engineering constructs. (A) Use of mesenchymal stem cell spheroids to 3D bioprint a helix shape (i), Penn State University initials (ii), 5-layer tubular structures (iii), and double helix-shape constructs (iv). One hundred fifty micrometers (F-actin) and 450 μm (Hoechst) in radius in 1.2% Carbopol yield-stress gel. Magnified zone is indicated by dashed red line. Reproduced from Ayan et al.,276 which is licensed under a Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). (B) Cell-electrospinning process with the processing parameters. Reproduced from Hong et al.,432 which is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/). (C) Combined electrospinning and 3D printing. (i) Schematic illustration of the composite scaffold with electrospinning and 3D printing. Step (1) polymer polycaprolactone was used to 3D print the constructs and electrospinning to produce nanofibers, resulting in the formation of dual-scale scaffolds. Created with Biorender.com. Scanning electron microscope images of the scaffolds that were produced by using 3D printing (ii) and dual-scale scaffolds that were produced by using electrospinning and 3D printing (iii) (scale bar = 300 μm). Confocal laser scanning microscopy images of the scaffolds that were produced by using 3D printing (iv) and dual-scale scaffolds that were produced by using electrospinning and 3D printing (v) (scale bar = 300 μm). Reproduced from Vyas et al.,433 which is an open access article distributed under the terms of the Creative Commons CC BY license (ii–v). (D) Intravital 3D bioprinting, which is carried out by injecting a solution of the polymer into a certain tissue site to be treated in a living body. In this example, a two-photon excitation is used for the construction of a 3D object by gelating the polymer solution, and object intravital imaging is used for identification. Created with Biorender.com. Color images are available online.
Advances in other processing techniques
Electrospinning is a versatile technique relying on the use of an electric field to produce thin micro- and nanofibers277 that can also be combined with drugs.222,278,279 This technique has also been used for some time to produce nanofiber-based scaffolds that mimic ECM in several aspects and explored for the engineering of various tissue constructs277,280 such as bone,281 cartilage,282 nerve conduits,283 blood vessels,284 skin,24 and other tissues. In addition to its use for ex vivo tissue engineering, electrospinning was also experimented for in situ applications, for example, for the treatment of skin wounds24 and for ocular drug delivery.285
Different materials have been utilized for electrospinning, and various modifications of the technique of the spinning process have been developed to allow for combining the benefits and properties of more than one material.286 For example, in coaxial electrospinning, it is possible to use a material in the core and a different one in the shell that can have different degradation profiles and can be loaded with different molecules or drugs.287 Examples of the successful use of co-axial287 and triaxial288 electrospinning techniques include the engineering of osteochondral tissues.289 Among the interesting recent advances in electrospinning is cell-laden electrospinning (Fig. 6B), in which muscle cells were encapsulated in fibrin.290 It has been found that incorporating these cells and modifying electrospinning conditions significantly enhanced cell viability under a 4.5 kV electric field.
Another advancing frontier involves the use of electrospinning in combination with 3D printing and bioprinting to bring in various properties, such as reinforcement, to improve the mechanical properties of the resulting 3D-bioprinted constructs.291 In addition, combining electrospinning with 3D printing provides a microporous structure that can enhance cell proliferation and infiltration of the structure292 (Fig. 6C). Further, more control over the process of fiber laying of electrospinning, which classically randomly falls on the collecting surface, enabled the use of the techniques in a similar way as 3D printing.293 Once this is well controlled, it can be one form of 3D printing and bioprinting in future, used on its own.
In addition, melt-spinning has been used for tissue engineering.294 Melt electrospinning-based printing is an emerging printing technique that can print fibers with diameter in the range of nanometers, providing a high degree of resolution, porosity, and pore interconnectivity.295–297 For example, Brown et al. combined melt electrospinning with a digitally controlled collector and developed a new class of 3D printer called melt-electrospinning writing (MEW), which enabled the deposition of well-defined filaments.298 The MEW has the advantage of avoiding problems related to solvents that are used in conventional electrospinning.299
Recently, and for the first time it was possible to have automated coupled melt-electrospinning and melt-electrowriting, by using a modified elongated nozzle to direct-write melt-electrospun polymeric thin fibers onto a collection surface.300 In one interesting development, multilayers were developed by electrospinning, and layers have complementary moieties that lead to the formation of covalent bonds (such as hydrazide and aldehyde groups) between electrospun fibers when they are brought together under mechanical loading.301 The technique can be useful in tissue engineering of advanced structures in future, which can become stronger on exposure to stress, for example, blood vessel engineering,284 guided nerve regeneration,283 or tendon282 and ligament engineering.302
All these advances open new avenues and application territories of the techniques and provide us with more options and versatility toward mimicking the complexity and heterogeneity of the native tissues to be engineered, by combining various processing techniques. With these novel approaches, we come one step closer toward developing successful engineered constructs, ex vivo or in situ.
Translational advances in tissue engineering
Successful transfer of technology from bench to industrial production of engineered tissue products has been progressive, but slow, in part because the clinicians often do not have embedded design and architecture input to the early stage tissue engineered constructs. Although there was a bolus of products, primarily focused on engineered skin tissue, that was approved in the late 1990s and early 2000s,303 only a few products have subsequently emerged.303 Although a few individuals have gained expertise in translating basic tissue engineering research to commercial products, researchers in the field of tissue engineering and regenerative medicine (TERM), overall, lack experience in translational sciences. One factor that may highlight the impact and enable faster commercial translation is academic–industry partnerships among tissue engineers, clinical investigators, clinicians, and industry partners.
Advances in clinical translation
Influencing factors
Clinical translation is affected by several factors that are related to the technology, approval, and acceptance by doctors304,305 and patients.305 Although there have been advances in the field, clinical translation has been limited, not because of science or technology, but largely due to factors including scalability, cost, regulatory issues, and uptake.305 There are engineered tissue products that are in clinical use or are moving toward clinical translation such as skin, cartilage, bone, vascular grafts, cardiac tissues, and bladder.305,306 More complex structures such as heart, lung, liver, and kidney have been recreated and are still in preclinical animal studies. Clinical translation of complex structures and whole organs face a completely different set of challenges.305
Acellular products
Successful clinical applications of engineered tissue products have flourished in the past couple of decades,304 and they were approved by the United States FDA, for example, Integra for skin and INFUSE for bone regeneration.76,307,308 The former is composed of collagen, glycosaminoglycans, and polysiloxane, and it was approved by FDA in 2002 for use in the treatment of burns307 and then for the treatment of diabetic foot ulcers in 2016.309 INFUSE is a BMP2-containing collagen sponge that was also approved by the FDA in 2002 for use in lumbar fusion.310 In addition to these biomaterials, the FDA has also approved native-tissue derived ECM for application in the treatment of complex wounds311 and nerve regeneration.312
Currently, there are several clinical trials exploring the use of materials in achieving in situ regeneration of nervous,10,313 cardiac,314 and musculoskeletal315 tissues. The Humacyte acellular vascular graft, which is made by laying smooth ECM on PGA with cultured smooth muscle cells that are subsequently removed from the graft,316 is now in an open-label, nonrandomized, phase II clinical trial317 for patients with life-threatening limb or torso vascular trauma. The primary outcome measures include primary graft patency along with frequency and severity of adverse outcomes.
For cartilage and osteochondral repair, TruFit and MaioRegen acellular devices have been developed. TruFit is composed of a PLGA, 10% calcium sulfate, PGA fibers, and surfactant and is used for cartilage repair.318 Unfortunately, a 2-year clinical study showed no significant improvement in knee scores.319 Compared with autologous osteochondral transplantation, knee scores were worse in the group that received TruFit.320 Other clinical studies with TruFit showed improvement in symptoms and radiologic outcomes but lack direct comparison with conventional surgical cartilage repair techniques.321,322 Although there was excitement with MaioRegen (a three-layer scaffold composed of collagen I and hydroxyapatite) for the treatment of osteochondral lesions when medium-term results showed significant improvement of knee scores,323 the 5-year results showed failure of repair.324
Cellular products
Several notable cellular products have been approved by the FDA. In 1997, TransCyte, which is composed of fibroblasts and nylon mesh,325 was approved for the treatment of burns.326 In 1998, Apligraf, which is composed of fibroblasts, keratinocytes, and collagen matrix,327 and another product for the treatment of skin venous ulcers328 were approved. For the treatment of nonhealing diabetic foot ulcers,329 in 2001, the FDA approved Dermagraft, a construct made from a synthetic polymer, PGA with fibroblasts. OrCel is a collagen sponge-based scaffold seeded with keratinocytes and fibroblasts, which was used in the treatment of burns.330 Laserskin and Hyalograft are hyaluronan-based matrices seeded with fibroblasts and keratinocytes, which were used in the treatment of diabetic foot ulcers331 and chronic wounds of the lower extremity.332 Matriderm® is an acellular matrix composed of coupled collagen and elastin,333 which can be seeded with fibroblasts and keratinocytes and used for the treatment of full-thickness skin loss.333
Most of the identified cellularized products are for skin regeneration.334 Although these products are very valuable, it is also important to develop and test cellular products for the treatment of tissues with much less intrinsic regenerative capacity such as the heart.335 Even though many of these products have not yet been approved or are not widely utilized in the field, it is important to mention that an iPSC-derived cell sheet based product (TERUMO BCT) for the treatment of heart failure was developed, but it has not yet been approved by the FDA.336 This cellular therapy may have a major impact on the treatment of heart failure, which constitutes one of the major causes of mortality in the world.337
Autologous cellular products
The FDA-approved autologous cell-based products include matrix-assisted autologous chondrocyte implantation (MACI), which is composed of matrix and chondrocytes and used for the treatment of full-thickness cartilage defects, and they were approved in 2001.76,307 Since 2001, several MACI products have been commercialized,318 including BioSeed®-C338 and Hyalograft® C.339 Fibrin glue is used in BioSeed as a cell carrier, and polyglactin 910/poly-p-dioxanone fleece is used as a scaffold. Significant benefits were demonstrated in clinical studies.338,340 Hyalograft C, which uses HA as a matrix, has been investigated in 28 trials. Relative to microfracture therapy, Hyalograft C showed improved patient scores.318 However, Hyalograft C did not undergo a phase III clinical trial and was withdrawn from the market318 due to problems with manufacturing practices and comparative studies.341
Other MACI products available outside of the United States include CaReS®, which uses collagen type I hydrogel seeded with autologous chondrocytes. The results of a prospective multicenter clinical trial in 116 patients (49 women and 67 men; mean age, 32.5 ± 8.9 years) demonstrated significant improvement in the knee scores at 12–60 months after treatment with CaReS; there was a significant reduction in global pain scores and an improvement in the health-related quality of life (SF-36) scores.342
In 2017, phase III clinical trial of another MACI product, NeoCart®, was completed. NeoCart relies on the use of type I collagen scaffold.343 At the 5-year follow-up, although magnetic resonance imaging showed significant improvements from earlier follow-up time points, subchondral bone lesions were seen in 80% of patients. NOVOCART® 3D, a third-generation ACI, employs type I/III collagen biphasic scaffolds344; clinical trials have been performed.345 and phase III clinical trials are in progress.346–349 One report showed significantly improved knee scores from the preoperative state.350 NOVOCART 3D may serve as the treatment of choice for children and adolescents.351
Several other engineered tissues reached the clinical testing stage but faced several challenges. In 2010, tissue-engineered trachea that employed a decellularized allograft352 along with autologous MSCs was implanted into a 12-year-old boy.353 Though successful, full restoration of the biomechanical properties of the trachea took a long time (18 months). Bladder tissue reconstruction was another success.
In 2005, Atala et al. reported implantation of engineered bladder in patients needing cystoplasty for end-stage bladder disease.354 Collagen or PGA-collagen scaffolds were seeded with the patient's own bladder cells. Unfortunately, a phase II study consisting of children with neurogenic bladder resulting from spina bifida showed no functional improvement in bladder compliance or capacity and the prevalence of serious adverse events prevented further development.355 The performance of the engineered bladder is still far from replacing that conventional use of gastrointestinal tissue for augmentation cystoplasty.306 As a result, a combination of techniques with neurovascular muscle transfer and tissue engineering was proposed.356 The common thread is that neovascularization had to occur in vivo on the construct, because the vascular component of tissue engineering is not optimized.
However, some clinical success was seen in large vessel tissue engineering related to congenital heart disease. In one patient, tissue-engineered pulmonary artery using PCL–PLA copolymer (weight ratio, 1:1) reinforced with woven PGA tubular scaffolds and seeded with autologous peripheral vein-derived cells was found to be successful, and follow-up at seven months showed no evidence of graft occlusion or aneurysms.357
Later, the same group showed both safety and absence of adverse events at four years in a cohort of 25 patients with congenital heart disease who had extracardiac total cavopulmonary surgeries with tissue-engineered vascular grafts serving as conduits.358 One major challenge hindering clinical application of engineered vessel grafts is related to standardizing the engineered parameters such as scaffold structure and materials, for which 3D printing may offer solutions in terms of reproducibility.306 Another interesting product is Holoclar®, which employs autologous stem cells that are cultured on a fibrin matrix and transplanted to treat damaged outer layer of the cornea; Holoclar was approved by the European Commission in 2015.306,359,360
Biomaterial-free cellular products
Biomaterial-free cellular grafts have also been tested in patients. In one case report, iPSC-derived retinal pigment epithelial (RPE) sheets were used in the treatment of age-related macular degeneration (AMD) of a 70-year-old woman.361 Two phase I/II clinical studies have been conducted to assess the safety and tolerability of ESC-derived RPEs in the treatment of Stargardt's disease (n = 9 patients) and patients with AMD (n = 9) and provided first evidence of medium- to long-term safety, graft survival, and possible biological activity of these cells. No evidence of adverse proliferation or rejection was seen in these patients who were followed up for a median duration of 22 months.362
Three-dimensional bioprinted products
There are opportunities for translating 3D bioprinting technologies into the clinic. However, there remain significant challenges and limitations that need to be addressed. Some challenges include the production of tissue constructs that have a clinically relevant size, function, and vascularization.363 Tissues such as cartilage, bone, and skin are more feasible than complex tissues such as the myocardium. The physiologic components and functional requirements necessary for mimicking native tissues are significantly more challenging to engineer. Successful engineering of complex tissues requires time, development of multicomponent bioinks,271 and improvements in materials, cell sourcing, and fabrication techniques.363 Scalability and costs are added barriers. To the best of our knowledge, there are no 3D-bioprinted products currently undergoing clinical testing. To advance translational aspects of 3D printing and bioprinting, we have organized sessions in the Annual meeting of the Society for Biomaterials, 2019 and World Biomaterials Congress 2020.
Industrial translational advances
Product concepts in the TERM field are challenging. Despite promising clinical outcomes,364 many therapeutics have limited insight into the target mechanism of action. This, in turn, leads to a poor understanding of the critical quality attributes that function, in part, to gauge acceptable levels of variability, either inherent to the biology or due to the process.365 Further, a critical eye needs to be kept on the efficacy and approach to developing therapies given the cost of resources for development and translation to the clinic.364
Many initial product concepts emerge from basic science research, largely supported by federal grants from the National Institutes of Health (NIH) and National Science Foundation (NSF). These funding mechanisms explicitly favor innovation, which may come at the cost of advancing simpler effective approaches. In addition, for some areas, the patent landscape is crowded and complicated requiring some product concepts to needlessly contort to remain unconflicted.366 The ultimate goal, however, is to develop a therapeutic that has a clear increase in efficacy over the standard of care,367 but if not careful the long path of translation may induce drift away from that goal.
As these products move from concept to realization, there are a host of business-related challenges that emerge.368 Even an efficacious approach needs to have a tenable business model to be ubiquitously and consistently available to patients. If the process to manufacture the product is not scalable, only a limited number of patients can benefit from the product. Further, if the financial models for generating the product, looking at both cost of goods and reimbursement levels, are not favorable, then eventually no corporate entity can support production. These considerations are often taken too late, leading to false starts as we try to develop therapies for aiding patients.
Limitations in supporting production infrastructure also comprise a concern for the nascent TERM industry. Although some larger corporations will develop full manufacturing and testing facilities internally, widespread translation will require contract organizations to support smaller businesses. However, there is a paucity of Contract Manufacturing Organizations (CMOs) and Contract Testing Organizations (CTOs) that are experienced in the technical aspects unique to the TERM field. In particular, the robust workforce for manufacturing and testing for this field is lacking. Fortunately, efforts by academic programs have recently emerged to address the specialized workforce required production.369
Another production concern is the availability of proper raw materials. For example, the lack of specialized cytokines and biomaterials that are cGMP grade will remain an issue until the demand for enough is established.370 Even for raw materials shared with other more established industries, such as Pharma, the TERM industry does not currently require the scale of material to provide the leverage needed to implement common supply chain strategies, in turn affecting the cost of goods. Unfortunately, only as more TERM products are translated toward the commercial scale will these resource issues be more fully addressed.
The challenges for the translation of TERM products include issues from tenable product concepts to manufacturing issues to regulatory hurdles.371 One of the major hurdles in getting TERM products to the clinic has been the time for regulatory review and approval in the United States.304 The FDA has developed programs to accelerate the process, including the Regenerative Medicine Advanced Therapy (RMAT) designation, which was enacted in the 21st Century Cures Act in December 2016.372 The RMAT designation applies to those regenerative medicine therapies that target serious or life-threatening conditions and has the potential to address unmet medical needs. Although this relatively new regulatory pathway will help ease one obstacle, many practical challenges still exist in commercializing TERM products. Only as the field continues to forge forward will some of these issues be overcome. Continued discussions among the community, such as ones at the TERMIS meetings, are critical for identifying the problems and sharing the solutions.
Challenges and Future Directions
There are already more than 100,000 publications and 9000 patents in the field of tissue engineering,371 but many bottlenecks still exist at the translational interface. At this time, the pipeline for academic–industry collaborations with active participation by clinical investigators and clinicians is underdeveloped. Additional efforts in tissue engineering need to address both the scientific challenges and translational potential to achieve synergistic success that will have an impactful benefit on patients in the clinic.
One of the most important challenges in tissue engineering has been the death of cells in the scaffolds after their implantation in the body.365 Cells can survive on diffusion only at a distance of ∼100–200 μm away from the source of nutrient supply.373 Because angiogenesis takes time,374 various strategies have been explored to provide cells in the engineered tissue with essential nutrients and O2 while awaiting new vessel formation.375–377 One strategy that has been developed recently is to deliver O2 into the engineered tissues by using O2-generating biomaterials,378 which have been shown to be also effective when they are used as a part of 3D-bioprinted tissue constructs.379
To enhance angiogenesis, accurate cell positioning in printed constructs380 and angiogenic growth factors381 can play important roles to avoid failure of engineered tissues382 or implanted constructs.383,384 To ensure continued blood supply to engineered constructs, strategies for vascularization,375,385 or prevascularization of scaffolds through the use of microsurgery were investigated.377 The need for a functional vascular network increases with the complexity and size of the target tissue or organ. These vascular networks could be used to support the grafts during the immediate postfabrication period.
Although many of the early tissue engineering experiments were proof-of-concept and demonstrated function,386 they were mostly carried out in immune-deficient animals387 where normal immune reactions are not functional. After implantation in immunocompetent animals, immune response to the construct is a challenging problem.388 This response includes nonspecific inflammatory reactions to matrix materials and possible reactions to allogeneic cells.389
Various strategies to address these issues have been developed, such as the use of autologous cells and the use of biocompatible materials. In addition, the exploration of autologous native ECM derived materials has been pursued.390 The use of immune reaction modulating agents such as anti-inflammatory agents391–393 embedded or integrated with the biomaterial has been explored for optimizing reactions toward implanted biodegradable materials. Further, cellular constructs that have no foreign materials added represent an interesting approach.394 Recent developments in the use of in situ tissue regeneration can be used as an alternative to ex vivo engineering, and it will help to avoid many of the current problems associated with the ex vivo engineering approach.395
Successful results of tissue engineering were demonstrated in early short- to medium-term animal experiments. Later, it was shown that function cannot be always sustained, such as it was seen in experiments with pancreatic endocrine396 and liver tissue engineering.397 Therefore, strategies to enhance the survival and function of implanted engineered tissue constructs were investigated.398 Durability of the implanted engineered tissue constructs is an important aspect, and long-term studies are required to demonstrate this. In vivo imaging399 and cell tracking400 have recently evolved and they enable better evaluation and monitoring during the postimplantation period. Sensor technology is an emerging area that can be taken as an enabling tool to advance our capabilities in monitoring our implants further and pursue timely intervention if needed.401,402
Many of the engineered tissues are small in size because of limitation, partly imposed by difficulty in providing nourishment to deeper parts of the engineered tissue constructs and required vascularization for larger-sized constructs. Even with the most recent developments in the use of 3D bioprinting for engineering tissues, the production of clinically relevant sizes of constructs remains a challenge.258 Some strategies have been developed to address this, such as the printing of supportive structures258 or printing into a supportive sacrificial material.403 The latter is hoped to help produce larger constructs. It remains, however, to get mechanically stable constructs that can preserve their physical characteristics and mechanical properties for a time enough to support tissues while they are in the healing stage. Most challenges in this sense are related to constructs intended for use in hard tissue such as the bone. Important strategies can be sought by combining acellular frames and scaffolds with cellular constructs, as has been suggested earlier.20
The use of engineered tissue products may be associated with safety issues related to cell, material, and molecule sources, during retrieval, processing, storage, transport, and application phases.255,404 Ethical issues are especially related to the source of cells, for example, xenogeneic grafts, chimeric constructs, or ESCs.405 Also, aspects related to the use of stem cell therapies that are risky, untested, and unproven scientifically by unregulated clinics need to be addressed.406 Further, ethical aspects related to applications and making the therapy available when needed and for patients who need it are important, given the lack of availability of sufficient organs and tissues needed to provide vital functions and reduce the death of patients on the waiting list.407 Ethical aspects related to clinical trials should be properly analyzed and addressed. In clinical trials, it is sometimes difficult to design appropriate control groups because of ethical reasons, and therefore, results should be evaluated accordingly, and so also when applications are submitted for approval by regulatory bodies.
Because of financial reasons or the availability of other therapeutic alternatives, health service providers and insurance companies may not provide or approve engineered tissue products, which imposes another challenge facing wider application of engineered tissues. In addition, acceptance by doctors408 and patients is also an important factor that will influence the future of the use of engineered tissue therapeutics. Wider clinical application will, thus, be influenced by patient education, marketing, safety, and efficacy proof. Influencing factors will also include safety, efficacy, and price as well as coverage of the products by insurance companies for defined indications, where alternatives are not available, inefficient, or more expensive. It is clear that there is a niche for engineered tissue products in certain clinical indications, for example, skin for the treatment of face lesions, younger patients, and large burn wounds.409
In the future, we expect to see more of in situ tissue engineering that can be accomplished by creating an environment in vivo that stimulates an individual's own resident cells to achieve regeneration. This can be achieved via various approaches that may employ bioresponsive materials to influence immune cells, progenitors, or stem cells, or utilize transcription factors and RNA-based strategies to reprogram cells.16 Further, the use of in situ264,265 and intravital410 (Fig. 6D) 3D bioprinting will advance our capabilities further toward achieving less or minimally invasive delivery of regenerative therapeutics.76
Using iPSCs411 and stem cell-derived EVs,412 as well as customized implants using 3D printing413,414 allows for the development of more customized and personalized treatment modalities in the future. There is a significant need for the development of autologous endocrine tissues, for example, for the treatment of diabetes,396 and for musculoskeletal tissues that are important for craniomaxillofacial reconstruction,397 which can be addressed by using these new tissue and cellular engineering approaches.
One advancing frontier is related to the use of electroconductive materials for tissue engineering, which can be useful in many applications such as neural tissue engineering.415,416 Care of the patients will be advanced by integrating capabilities of sensors and actuators; communication and remote control, which will enable real-time monitoring of implanted constructs and timely intervention by providing the right treatment at the right time.417 Diagnosis, design of treatment, installation of implants, follow-up, and optimization will benefit from advances made through the Internet of Things.418
Advances in microfluidic OoC systems245 have led to the publication of several experiments on studying tissues, developing disease models, and testing drugs.419 However, their use for advancing tissue engineering for the purpose of regenerative medicine needs still to be harnessed and there are many untapped opportunities to be explored. In addition, OoC systems will enhance our ability to perform cell culture studies in a 3D dynamic environment, which makes it possible to mimic the in vivo microenvironment.243 Growth and spread of cells can be monitored in a controlled manner on microstructures created with 3D printers, and it can be possible to mimic the real-time events.420 Further, the use of such advanced in vitro systems will allow us to also test and decide on the ideal cell type for use in the engineering of certain tissues and defined clinical applications.
Next-generation studies in tissue engineering applications will be especially focused on the use of smart biomaterials,421–423 stem cell studies,424 development of nanotechnology, new biofabrication techniques, and the integration of advances made in synthetic biology.425,426 Especially with increasing studies and published results on stem cells, it will become easier to imitate target tissues and organs.427 Further studies in the area would allow overcoming the safety and efficacy concerns encountered with many stem cell types, such as the iPSCs and MSCs. Methodologies to isolate, ex vivo manipulate, and culture these cells need further evaluation.428
In tissue engineering applications, the selection and design of biomaterials that are suitable for target tissue and organ is one of the most important issues. In addition, the harmony and integrity of the cells with biomaterials have made the use of biological materials as scaffolds useful for the integration of organs with 3D systems.429 In this context, the most important issue will be to increase the use of technological applications such as computer modeling, artificial intelligence, OoC platforms, and 3D printing for understanding the interactions of cells and tissues with biomaterials in vivo. Thus, it will be possible to use the new-generation biocompatible smart materials in tissue engineering applications and to meet patient requirements in real time.305
Conclusions
Overall, it can be concluded that our current armamentarium in tissue engineering has made different advances at different levels, including biomaterials, stem cell technologies, fabrication techniques, industrial production innovation, and clinical applications. Through the integration of these facets by multidisciplinary teams with sustained funding, future developments should lead to optimized tissue constructs, successful products, and wider adoption for clinical application.
Acknowledgments
Thanks are due to Dr. Mohammed Xohdy and to Mr. Adam Marsh for drawing Figure 1.
Disclosure Statement
No competing financial interests exist.
Funding Information
The authors acknowledge funding from the National Institutes of Health (1UG3TR003148-01) and the American Heart Association (18TPA34230036 and 442611-NU-80922). Dr. Nguyen receives grant funding from the American Heart Association (18TPA34170049), the National Institutes of Health (R01HL148182), and the Veterans Health Administration (I01-CX001901).
References
- 1. Mulzer, J., Schutte, B., Lindner, J., Okpanyi, S.N., and Eurich, R.. Studies on DNA content of tissues and organs. 1. Changes in cell content of some mesenchymal and parenchymatous rat organs during development, maturation, and in part aging (determinations of DNA). Arzneimittelforschung 29, 207, 1979. [PubMed] [Google Scholar]
- 2. Chong, A.S., and Alegre, M.L.. The impact of infection and tissue damage in solid-organ transplantation. Nat Rev Immunol 12, 459, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wagener, G. Multiple listings: good for a few, but no solution for the organ shortage. Transplantation 104, 671, 2020. [DOI] [PubMed] [Google Scholar]
- 4. Colvin, M., Smith, J.M., Hadley, N., et al. OPTN/SRTR 2016 annual data report: heart. Am J Transplant 18(S1), 291, 2018. [DOI] [PubMed] [Google Scholar]
- 5. Giwa, S., Lewis, J.K., Alvarez, L., et al. The promise of organ and tissue preservation to transform medicine. Nat Biotechnol 35, 530, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Langer, R., and Vacanti, J.P.. Tissue engineering. Science 260, 920, 1993. [DOI] [PubMed] [Google Scholar]
- 7. Vacanti, J.P., and Langer, R.. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 354 Suppl 1, Si32, 1999. [DOI] [PubMed] [Google Scholar]
- 8. Langer, R. Tissue engineering: perspectives, challenges, and future directions. Tissue Eng 13, 1, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Natan, B., Hanjun, K., Marcus, J.G., et al. Biofabrication of endothelial cell, dermal fibroblast, and multilayered keratinocyte layers for skin tissue engineering. Biofabrication 2020 [Epub ahead of print]; DOI: 10.1088/1758-5090/aba503. [DOI] [PubMed] [Google Scholar]
- 10. Ashammakhi, N., Kim, H.-J., Ehsanipour, A., et al. Regenerative therapies for spinal cord injury. Tissue Eng Part B Rev 25, 471, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saveleva, M.S., Ivanov, A.N., Prikhozhdenko, E.S., et al. Hybrid functional materials for tissue engineering: synthesis, in vivo drug release and SERS effect. J Phys Conf Ser 1461, 012150, 2020. [Google Scholar]
- 12. Gonzalez-Andrades, M., Garzon, I., Alaminos, M., et al. Advances in the field of tissue engineering and regenerative medicine: state of the art and regulatory issues. J Biomater Tiss Eng 3, 245, 2013. [Google Scholar]
- 13. Mohammadinejad, R., Kumar, A., Ranjbar-Mohammadi, M., et al. Recent advances in natural gum-based biomaterials for tissue engineering and regenerative medicine: a review. Polymers (Basel) 12, 176, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jensen, G., Morrill, C., and Huang, Y.. 3D tissue engineering, an emerging technique for pharmaceutical research. Acta Pharm Sin B 8, 756, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh, A. Biomaterials innovation for next generation ex vivo immune tissue engineering. Biomaterials 130, 104, 2017. [DOI] [PubMed] [Google Scholar]
- 16. Gaharwar, A.K., Singh, I., and Khademhosseini, A.. Engineered biomaterials for in situ tissue regeneration. Nat Rev Mater 5, 686, 2020. [Google Scholar]
- 17. Koons, G.L., Diba, M., and Mikos, A.G.. Materials design for bone-tissue engineering. Nat Rev Mater 5, 584, 2020. [Google Scholar]
- 18. Ashammakhi, N., and Kaarela, O.. Stimuli-responsive biomaterials: next wave. J Craniofac Surg 28, 1647, 2017. [DOI] [PubMed] [Google Scholar]
- 19. Takahashi, K., and Yamanaka, S.. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663, 2006. [DOI] [PubMed] [Google Scholar]
- 20. Ashammakhi, N., Hasan, A., Kaarela, O., et al. Advancing frontiers in bone bioprinting. Adv Healthc Mater 8, 1801048, 2019. [DOI] [PubMed] [Google Scholar]
- 21. Jorgensen, A.M., Yoo, J.J., and Atala, A.. Solid organ bioprinting: strategies to achieve organ function. Chem Rev 120, 11093, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hacking, S.A., Ashammakhi, N., and Khademhosseini, A. 2.1.3—Cells and surfaces in vitro. In: Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., and Yaszemski, M.J., eds. Biomaterials Science (Fourth Edition). Academic Press, 2020, pp. 661–681. [Google Scholar]
- 23. Nguyen, L., Bang, S., and Noh, I.. Tissue regeneration of human mesenchymal stem cells on porous gelatin micro-carriers by long-term dynamic in vitro culture. Tissue Eng Regen Med 16, 19, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kamel, R.A., Ong, J.F., Eriksson, E., Junker, J.P.E., and Caterson, E.J.. Tissue engineering of skin. J Am Coll Surg 217, 533, 2013. [DOI] [PubMed] [Google Scholar]
- 25. Murphy, S.V., and Atala, A.. 3D bioprinting of tissues and organs. Nat Biotechnol 32, 773, 2014. [DOI] [PubMed] [Google Scholar]
- 26. Ashammakhi, N., Ndreu, A., Nikkola, L., Wimpenny, I., and Yang, Y.. Advancing tissue engineering by using electrospun nanofibers. Regen Med 3, 547, 2008. [DOI] [PubMed] [Google Scholar]
- 27. Chan, B.P., and Leong, K.W.. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J 17(S4), 467, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dolcimascolo, A., Calabrese, G., Conoci, S., and Parenti, R. Innovative Biomaterials for Tissue Engineering. IntechOpen, 2019. [Google Scholar]
- 29. Sell, S.A., Wolfe, P.S., Garg, K., McCool, J.M., Rodriguez, I.A., and Bowlin, G.L.. The use of natural polymers in tissue engineering: a focus on electrospun extracellular matrix analogues. Polymers (Basel) 2, 522, 2010. [Google Scholar]
- 30. Massoumi, B., Abbasian, M., Khalilzadeh, B., et al. Gelatin-based nanofibrous electrically conductive scaffolds for tissue engineering applications. Int J Polym Mater Polym Biomater 70, 693, 2020. [Google Scholar]
- 31. Nezhad-Mokhtari, P., Akrami-Hasan-Kohal, M., and Ghorbani, M.. An injectable chitosan-based hydrogel scaffold containing gold nanoparticles for tissue engineering applications. Int J Biol Macromol 154, 198, 2020. [DOI] [PubMed] [Google Scholar]
- 32. Chawla, D., Kaur, T., Joshi, A., and Singh, N.. 3D bioprinted alginate-gelatin based scaffolds for soft tissue engineering. Int J Biol Macromol 144, 560, 2020. [DOI] [PubMed] [Google Scholar]
- 33. Nazir, R., Bruyneel, A., Carr, C., and Czernuszka, J.. Collagen type I and hyaluronic acid based hybrid scaffolds for heart valve tissue engineering. Biopolymers 110, e23278, 2019. [DOI] [PubMed] [Google Scholar]
- 34. Pramanik, N., Dutta, K., Basu, R.K., and Kundu, P.P.. Aromatic pi-conjugated curcumin on surface modified polyaniline/polyhydroxyalkanoate based 3D porous scaffolds for tissue engineering applications. ACS Biomater Sci Eng 2, 2365, 2016. [DOI] [PubMed] [Google Scholar]
- 35. Wang, X.Y., Wang, G., Liu, L., and Zhang, D.Y.. The mechanism of a chitosan-collagen composite film used as biomaterial support for MC3T3-E1 cell differentiation. Sci Rep 6, 39322, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O'Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater Today 14, 88, 2011. [Google Scholar]
- 37. Kundu, B., Kurland, N.E., Bano, S., et al. Silk proteins for biomedical applications: bioengineering perspectives. Prog Polym Sci 39, 251, 2014. [Google Scholar]
- 38. Najberg, M., Haji Mansor, M., et al. Aerogel sponges of silk fibroin, hyaluronic acid and heparin for soft tissue engineering: composition-properties relationship. Carbohydr Polym 237, 116107, 2020. [DOI] [PubMed] [Google Scholar]
- 39. Brito-Pereira, R., Correia, D.M., Ribeiro, C., et al. Silk fibroin-magnetic hybrid composite electrospun fibers for tissue engineering applications. Compos Part B Eng 141, 70, 2018. [Google Scholar]
- 40. Jendrossek, D., and Handrick, R.. Microbial degradation of polyhydroxyalkanoates. Annu Rev Microbiol 56, 403, 2002. [DOI] [PubMed] [Google Scholar]
- 41. Lizarraga-Valderrama, L.R., Taylor, C.S., Claeyssens, F., Haycock, J.W., Knowles, J.C., and Roy, I.. Unidirectional neuronal cell growth and differentiation on aligned polyhydroxyalkanoate blend microfibres with varying diameters. J Tissue Eng Regen Med 13, 1581, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bagdadi, A.V., Safari, M., Dubey, P., et al. Poly(3-hydroxyoctanoate), a promising new material for cardiac tissue engineering. J Tissue Eng Regen Med 12, e495, 2018. [DOI] [PubMed] [Google Scholar]
- 43. Ranganathan, S., Balagangadharan, K., and Selvamurugan, N.. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int J Biol Macromol 133, 354, 2019. [DOI] [PubMed] [Google Scholar]
- 44. Constantinides, C., Basnett, P., Lukasiewicz, B., et al. In vivo tracking and 1H/19F magnetic resonance imaging of biodegradable polyhydroxyalkanoate/polycaprolactone blend scaffolds seeded with labeled cardiac stem cells. ACS Appl Mater Interfaces 10, 25056, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dhandayuthapani, B., Yoshida, Y., Maekawa, T., and Kumar, D.S.. Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci 2011, 1, 2011. [Google Scholar]
- 46. Ashammakhi, A., Apu, E.H., and Caterson, E.J. Self-healing biomaterials to heal tissues. J Craniofac Surg 2020 [Epub ahead of print]; DOI: 10.1097/SCS.0000000000007267. [DOI] [PubMed] [Google Scholar]
- 47. Ge, F., Lu, Y., Li, Q., and Zhang, X.. Decellularized extracellular matrices for tissue engineering and regeneration. Adv Exp Med Biol 1250, 15, 2020. [DOI] [PubMed] [Google Scholar]
- 48. Fernandez-Perez, J., and Ahearne, M.. The impact of decellularization methods on extracellular matrix derived hydrogels. Sci Rep 9, 14933, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ott, H.C., Matthiesen, T.S., Goh, S.K., et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 14, 213, 2008. [DOI] [PubMed] [Google Scholar]
- 50. Kusuma, G.D., Brennecke, S.P., O'Connor, A.J., et al. Decellularized extracellular matrices produced from immortal cell lines derived from different parts of the placenta support primary mesenchymal stem cell expansion. PLoS ONE 12, e0171488. DOI: 10.1371/journal.pone.0171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dzobo, K., Motaung, K.S.C.M., and Adesida, A.. Recent trends in decellularized extracellular matrix bioinks for 3D printing: an updated review. Int J Mol Sci 20, 4628, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schoen, B., Avrahami, R., Baruch, L., et al. Electrospun extracellular matrix: paving the way to tailor-made natural scaffolds for cardiac tissue regeneration. Adv Funct Mater 27, 1700427, 2017. [Google Scholar]
- 53. Kim, B.S., Kwon, Y.W., Kong, J.-S., et al. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials 168, 38, 2018. [DOI] [PubMed] [Google Scholar]
- 54. Carvalho, M.S., Silva, J.C., Udangawa, R.N., et al. Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue engineering. Mater Sci Eng C 99, 479, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wassenaar, J.W., Braden, R.L., Osborn, K.G., and Christman, K.L.. Modulating in vivo degradation rate of injectable extracellular matrix hydrogels. J Mater Chem B 4, 2794, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Obata, T., Tsuchiya, T., Akita, S., et al. Utilization of natural detergent potassium laurate for decellularization in lung bioengineering. Tissue Eng Part C Methods 25, 459, 2019. [DOI] [PubMed] [Google Scholar]
- 57. Liao, J., Xu, B., Zhang, R.H., Fan, Y.B., Xie, H.Q., and Li, X.M.. Applications of decellularized materials in tissue engineering: advantages, drawbacks and current improvements, and future perspectives. J Mater Chem B 8, 10023, 2020. [DOI] [PubMed] [Google Scholar]
- 58. Fang, Y., Zhu, X., Wang, N., et al. Biodegradable core-shell electrospun nanofibers based on PLA and γ-PGA for wound healing. Eur Polym J 116, 30, 2019. [Google Scholar]
- 59. Qi, F., Wu, J., Li, H., and Ma, G.. Recent research and development of PLGA/PLA microspheres/nanoparticles: a review in scientific and industrial aspects. Front Chem Sci Eng 13, 14, 2019. [Google Scholar]
- 60. Sartore, L., Inverardi, N., Pandini, S., Bignotti, F., and Chiellini, F.. PLA/PCL-based foams as scaffolds for tissue engineering applications. Mater Today 7, 410, 2019. [Google Scholar]
- 61. Sun, X., Wang, J., Wang, Y., and Zhang, Q.. Collagen-based porous scaffolds containing PLGA microspheres for controlled kartogenin release in cartilage tissue engineering. Artif Cells Nanomed Biotechnol 46, 1957, 2018. [DOI] [PubMed] [Google Scholar]
- 62. Xu, T.O., Kim, H.S., Stahl, T., and Nukavarapu, S.P.. Self-neutralizing PLGA/magnesium composites as novel biomaterials for tissue engineering. Biomed Mater 13, 035013, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jiao, Z., Luo, B., Xiang, S., Ma, H., Yu, Y., and Yang, W.. 3D printing of HA/PCL composite tissue engineering scaffolds. Adv Ind Eng Polym Res 2, 196, 2019. [Google Scholar]
- 64. Luginina, M., Schuhladen, K., Orru, R., Cao, G., Boccaccini, A.R., and Liverani, L.. Electrospun PCL/PGS composite fibers incorporating bioactive glass particles for soft tissueengineering applications. Nanomaterials 10, 978, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu, J., Zheng, H.Y., Dai, X.Y., Poh, P.S.P., Machens, H.G., and Schilling, A.F.. Transparent PDMS bioreactors for the fabrication and analysis of multi-layer pre-vascularized hydrogels under continuous perfusion. Front Bioeng Biotechnol 8, 568934, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Qian, Z.C., Ross, D., Jia, W.K., Xing, Q., and Zhao, F.. Bioactive polydimethylsiloxane surface for optimal human mesenchymal stem cell sheet culture. Bioact Mater 3, 167, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wu, Q.R., Liu, J.F., Wang, X.H., et al. Organ-on-a-chip: recent breakthroughs and future prospects. Biomed Eng Online 19, 9, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. de Cassan, D., Becker, A., Glasmacher, B., et al. Blending chitosan-g-poly(caprolactone) with poly(caprolactone) by electrospinning to produce functional fiber mats for tissue engineering applications. J Appl Polym Sci 137, 48650, 2020. [Google Scholar]
- 69. GhavamiNejad, A., Ashammakhi, N., Wu, X.Y., and Khademhosseini, A.. Crosslinking strategies for 3D bioprinting of polymeric hydrogels. Small 16, e2002931, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bjørge, I.M., Costa, A.M.S., Silva, A.S., Vidal, J.P.O., Nóbrega, J.M., and Mano, J.F.. Tuneable spheroidal hydrogel particles for cell and drug encapsulation. Soft Matter 14, 5622, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Huang, Q., Zou, Y., Arno, M.C., et al. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem Soc Rev 46, 6255, 2017. [DOI] [PubMed] [Google Scholar]
- 72. Al-Sabanh, A., Burnell, S.E.A., Simoes, I.N., et al. Structural and mechanical characterization of crosslinked and sterilised nanocellulose-based hydrogels for cartilage tissue engineering. Carbohyd Polym 212, 242, 2019. [DOI] [PubMed] [Google Scholar]
- 73. Roy, C.K., Guo, H.L., Sun, T.L., et al. Self-adjustable adhesion of polyampholyte hydrogels. Adv Mater 27, 7344, 2015. [DOI] [PubMed] [Google Scholar]
- 74. Shirzaei Sani, E., Portillo-Lara, R., Spencer, A., et al. Engineering adhesive and antimicrobial hyaluronic acid/elastin-like polypeptide hybrid hydrogels for tissue engineering applications. ACS Biomater Sci Eng 4, 2528, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dhand, A.P., Galarraga, J.H., and Burdick, J.A.. Enhancing biopolymer hydrogel functionality through interpenetrating networks. Trends Biotechnol 39, 519, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ashammakhi, N., Ahadian, S., Darabi, M.A., et al. Minimally invasive and regenerative therapeutics. Adv Mater 31, e1804041, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. GhavamiNejad, A., Lu, B., and Wu, X.Y.. Transdermal drug delivery via microneedle patches. In: Unnithan, A.R., Sasikala, A.R.K., Park, C.H., and Kim, C.S., eds. Biomimetic Nanoengineered Materials for Advanced Drug Delivery. Netherlands: Elsevier, 2019, pp. 37–52. [Google Scholar]
- 78. Han, S.-S., Yoon, H.Y., Yhee, J.Y., et al. In situ cross-linkable hyaluronic acid hydrogels using copper free click chemistry for cartilage tissue engineering. Polym Chem 9, 20, 2018. [Google Scholar]
- 79. Kumar Meena, L., Rather, H., Kedaria, D., and Vasita, R.. Polymeric microgels for bone tissue engineering applications—a review. Int J Polym Mater Polym Biomater 69, 381, 2020. [Google Scholar]
- 80. Jiang, W., Li, M., Chen, Z., and Leong, K.W.. Cell-laden microfluidic microgels for tissue regeneration. Lab Chip 16, 4482, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Newsom, J.P., Payne, K.A., and Krebs, M.D.. Microgels: modular, tunable constructs for tissue regeneration. Acta Biomater 88, 32, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yang, X., Lu, Z., Wu, H., Li, W., Zheng, L., and Zhao, J.. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater Sci Eng C 83, 195, 2018. [DOI] [PubMed] [Google Scholar]
- 83. Noor, N., Shapira, A., Edri, R., Gal, I., Wertheim, L., and Dvir, T.. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv Sci (Weinh) 2019, 1900344. DOI: 10.1002/advs.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Talebian, S., Mehrali, M., Taebnia, N., et al. Self-healing hydrogels: the next paradigm shift in tissue engineering? Adv Sci (Weinh) 6, 1801664, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Saunders, L., and Ma, P.X.. Self-healing supramolecular hydrogels for tissue engineering applications. Macromol Biosci 19, e1800313, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rammal H GA, Erdem, A., Mbeleck, R., et al. Advances in biomedical applications of self-healing hydrogels. Mater Chem Front 5, 4368, 2021. [Google Scholar]
- 87. Montgomery, M., Ahadian, S., Davenport Huyer, L., et al. Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat Mater 16, 1038, 2017. [DOI] [PubMed] [Google Scholar]
- 88. Xia, Y., He, Y., Zhang, F., Liu, Y., and Leng, J.. A review of shape memory polymers and composites: mechanisms, materials, and applications. Adv Mater 33, e2000713, 2020. [DOI] [PubMed] [Google Scholar]
- 89. Darabi, M.A., Khosrozadeh, A., Wang, Y., et al. An alkaline based method for generating crystalline, strong, and shape memory polyvinyl alcohol biomaterials. Adv Sci 7, 1902740, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lu, Y., Aimetti, A.A., Langer, R., and Gu, Z.. Bioresponsive materials. Nat Rev Mater 2, 16075, 2016. [Google Scholar]
- 91. Tang, J.D., Mura, C., and Lampe, K.J.. Stimuli-responsive, pentapeptide, nanofiber hydrogel for tissue engineering. J Am Chem Soc 141, 4886, 2019. [DOI] [PubMed] [Google Scholar]
- 92. Sood, N., Bhardwaj, A., Mehta, S., and Mehta, A.. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv 23, 758, 2016. [DOI] [PubMed] [Google Scholar]
- 93. Zhou, W.X., Qiao, Z., Zare, E.N., et al. 4D-printed dynamic materials in biomedical applications: chemistry, challenges, and their future perspectives in the clinical sector. J Med Chem 63, 8003, 2020. [DOI] [PubMed] [Google Scholar]
- 94. Invernizzi, M., Turri, S., Levi, M., and Suriano, R.. 4D printed thermally activated self-healing and shape memory polycaprolactone-based polymers. Eur Polym J 101, 169, 2018. [Google Scholar]
- 95. Gyarmati, B., Szilágyi, B.Á., and Szilágyi, A.. Reversible interactions in self-healing and shape memory hydrogels. Eur Polym J 93, 642, 2017. [Google Scholar]
- 96. Obiweluozor, F.O., GhavamiNejad, A., Park, C.H., and Kim, C.S.. Mussel inspired locomotive: the moisture induced actuation in a poly(vinyl alcohol) film containing melanin-like dopamine nano spheres. RSC Adv 6, 65089, 2016. [Google Scholar]
- 97. Obiweluozor, F.O., GhavamiNejad, A., Maharjan, B., Kim, J., Park, C.H., and Kim, C.S.. A mussel inspired self-expandable tubular hydrogel with shape memory under NIR for potential biomedical applications. J Mater Chem B 5, 5373, 2017. [DOI] [PubMed] [Google Scholar]
- 98. Sun, J., Liu, Y.Y., and Leng, J.S.. Mechanical properties of shape memory polymer composites enhanced by elastic fibers and their application in variable stiffness morphing skins. J Intell Mater Syst Struct 26, 2020, 2015. [Google Scholar]
- 99. Liu, Y., andHsu S-h., Synthesis and biomedical applications of self-healing hydrogels. Front Chem 6, 449, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lü, S., Bai, X., Liu, H., et al. An injectable and self-healing hydrogel with covalent cross-linking in vivo for cranial bone repair. J Mater Chem B 5, 3739, 2017. [DOI] [PubMed] [Google Scholar]
- 101. Drury, J.L., and Mooney, D.J.. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24, 4337, 2003. [DOI] [PubMed] [Google Scholar]
- 102. Zou, W., Dong, J., Luo, Y., Zhao, Q., and Xie, T.. Dynamic covalent polymer networks: from old chemistry to modern day innovations. Adv Mater 29, 1606100, 2017. [DOI] [PubMed] [Google Scholar]
- 103. Unagolla, J.M., and Jayasuriya, A.C.. Hydrogel-based 3D bioprinting: a comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl Mater Today 18, 100479, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li, J., and Mooney, D.J.. Designing hydrogels for controlled drug delivery. Nat Rev Mater 1, 1, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Vermonden, T., Censi, R., and Hennink, W.E.. Hydrogels for protein delivery. Chem Rev 112, 2853, 2012. [DOI] [PubMed] [Google Scholar]
- 106. Fletcher, N.A., Babcock, L.R., Murray, E.A., and Krebs, M.D.. Controlled delivery of antibodies from injectable hydrogels. Mater Sci Eng C 59, 801, 2016. [DOI] [PubMed] [Google Scholar]
- 107. Zhang, H., Cong, Y., Osi, A.R., et al. Direct 3D printed biomimetic scaffolds based on hydrogel microparticles for cell spheroid growth. Adv Funct Mater 30, 1910573, 2020. [Google Scholar]
- 108. Ashammakhi, N. Drug release: proper control to help clinical application discussion. J Craniofac Surg 29, 124, 2018. [DOI] [PubMed] [Google Scholar]
- 109. GhavamiNejad, A., Park, C.H., and Kim, C.S.. In situ synthesis of antimicrobial silver nanoparticles within antifouling zwitterionic hydrogels by catecholic redox chemistry for wound healing application. Biomacromolecules 17, 1213, 2016. [DOI] [PubMed] [Google Scholar]
- 110. GhavamiNejad, A., SamariKhalaj, M., Aguilar, L.E., Park, C.H., and Kim, C.S.. pH/NIR light-controlled multidrug release via a mussel-inspired nanocomposite hydrogel for chemo-photothermal cancer therapy. Sci Rep 6, 33594, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Baino, F., Novajra, G., and Vitale-Brovarone, C.. Bioceramics and scaffolds: a winning combination for tissue engineering. Front Bioeng Biotechnol 3, 202, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tellisi, N., and Ashammakhi, N.. Comparison of meshes, gels and ceramic for cartilage tissue engineering in vitro. Eur J Plast Surg 35, 159, 2012. [Google Scholar]
- 113. Rahaman, M.N., Day, D.E., Bal, B.S., et al. Bioactive glass in tissue engineering. Acta Biomater 7, 2355, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. El-Rashidy, A.A., Roether, J.A., Harhaus, L., Kneser, U., and Boccaccini, A.R.. Regenerating bone with bioactive glass scaffolds: a review of in vivo studies in bone defect models. Acta Biomater 62, 1, 2017. [DOI] [PubMed] [Google Scholar]
- 115. Fu, Q., Saiz, E., and Tomsia, A.P.. Direct ink writing of highly porous and strong glass scaffolds for load-bearing bone defects repair and regeneration. Acta Biomater 7, 3547, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Huang, T.S., Rahaman, M.N., Doiphode, N.D., et al. Porous and strong bioactive glass (13–93) scaffolds fabricated by freeze extrusion technique. Mater Sci Eng C Mater 31, 1482, 2011. [Google Scholar]
- 117. Wu, Z.Y., Hill, R.G., Yue, S., Nightingale, D., Lee, P.D., and Jones, J.R.. Melt-derived bioactive glass scaffolds produced by a gel-cast foaming technique. Acta Biomater 7, 1807, 2011. [DOI] [PubMed] [Google Scholar]
- 118. Gao, C.D., Deng, Y.W., Feng, P., et al. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int J Mol Sci 15, 4714, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Du, X.Y., Fu, S.Y., and Zhu, Y.F.. 3D printing of ceramic-based scaffolds for bone tissue engineering: an overview. J Mater Chem B 6, 4397, 2018. [DOI] [PubMed] [Google Scholar]
- 120. Esfahani, H., Jose, R., and Ramakrishna, S.. Electrospun ceramic nanofiber mats today: synthesis, properties, and applications. Materials (Basle) 10, 1238, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Fu, Q.A., Rahaman, M.N., Bal, B.S., Kuroki, K., and Brown, R.F.. In vivo evaluation of 13–93 bioactive glass scaffolds with trabecular and oriented microstructures in a subcutaneous rat implantation model. J Biomed Mater Res A 95a, 235, 2010. [DOI] [PubMed] [Google Scholar]
- 122. Peter, M., Binulal, N.S., Nair, S.V., Selvamurugan, N., Tamura, H., and Jayakumar, R.. Novel biodegradable chitosan-gelatin/nano-bioactive glass ceramic composite scaffolds for alveolar bone tissue engineering. Chem Eng J 158, 353, 2010. [Google Scholar]
- 123. Xu, C.X., Su, P.Q., Chen, X.F., et al. Biocompatibility and osteogenesis of biomimetic bioglass-collagen-phosphatidylserine composite scaffolds for bone tissue engineering. Biomaterials 32, 1051, 2011. [DOI] [PubMed] [Google Scholar]
- 124. Nikpour, P., Salimi-Kenari, H., Fahimipour, F., et al. Dextran hydrogels incorporated with bioactive glass-ceramic: nanocomposite scaffolds for bone tissue engineering. Carbohydr Polym 190, 281, 2018. [DOI] [PubMed] [Google Scholar]
- 125. Chatzistavrou, X., Fenno, J.C., Faulk, D., et al. Fabrication and characterization of bioactive and antibacterial composites for dental applications. Acta Biomater 10, 3723, 2014. [DOI] [PubMed] [Google Scholar]
- 126. Chatzistavrou, X., Rao, R.R., Caldwell, D.J., et al. Collagen/fibrin microbeads as a delivery system for Ag-doped bioactive glass and DPSCs for potential applications in dentistry. J Non Cryst Solids 432, 143, 2016. [Google Scholar]
- 127. Dinesh Kumar, S., Mohamed Abudhahir, K., Selvamurugan, N., et al. Formulation and biological actions of nano-bioglass ceramic particles doped with Calcarea phosphorica for bone tissue engineering. Mater Sci Eng C 83, 202, 2018. [DOI] [PubMed] [Google Scholar]
- 128. Subhapradha, N., Abudhahir, M., Aathira, A., Srinivasan, N., and Moorthi, A.. Polymer coated mesoporous ceramic for drug delivery in bone tissue engineering. Int J Biol Macromol 110, 65, 2018. [DOI] [PubMed] [Google Scholar]
- 129. Tsigkou, O., Pomerantseva, I., Spencer, J.A., et al. Engineered vascularized bone grafts. Proc Natl Acad Sci U S A 107, 3311, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Santos-Ruiz, L., Mowatt, D.J., Marguerie, A., et al. Potential use of craniosynostotic osteoprogenitors and bioactive scaffolds for bone engineering. J Tissue Eng Regen M 1, 199, 2007. [DOI] [PubMed] [Google Scholar]
- 131. Crupi, A., Costa, A., Tarnok, A., Melzer, S., and Teodori, L.. Inflammation in tissue engineering: the Janus between engraftment and rejection. Eur J Immunol 45, 3222, 2015. [DOI] [PubMed] [Google Scholar]
- 132. Rezk, A.I., Sasikala, A.R.K., Nejad, A.G., et al. Strategic design of a mussel-inspired in situ reduced Ag/Au-nanoparticle coated magnesium alloy for enhanced viability, antibacterial property and decelerated corrosion rates for degradable implant Applications. Sci Rep 9, 117, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yusop, A.H., Bakir, A.A., Shaharom, N.A., Kadir, M.R.A., and Hermawan, H.. Porous biodegradable metals for hard tissue scaffolds: a review. Int J Biomater 2012, 641430, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sheikh, Z., Najeeb, S., Khurshid, Z., Verma, V., Rashid, H., and Glogauer, M.. Biodegradable materials for bone repair and tissue engineering applications. Materials 8, 5744, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ferrández-Montero, A., Lieblich, M., González-Carrasco, J.L., et al. Development of biocompatible and fully bioabsorbable PLA/Mg films for tissue regeneration applications. Acta Biomater 98, 114, 2019. [DOI] [PubMed] [Google Scholar]
- 136. Zhang, H.Y., Jiang, H.B., Kim, J.E., Zhang, S., Kim, K.M., and Kwon, J.S.. Bioresorbable magnesium-reinforced PLA membrane for guided bone/tissue regeneration. J Mech Behav Biomed Mater 112, 104061, 2020. [DOI] [PubMed] [Google Scholar]
- 137. Saris, N.E.L., Mervaala, E., Karppanen, H., Khawaja, J.A., and Lewenstam, A.. Magnesium—an update on physiological, clinical and analytical aspects. Clin Chim Acta 294, 1, 2000. [DOI] [PubMed] [Google Scholar]
- 138. Bosiers, M., Peeters, P., D'Archambeau, O., et al. ; AMS INSIGHT Investigators.. AMS INSIGHT-absorbable metal stent implantation for treatment of below-the-knee critical limb ischemia: 6-month analysis. Cardiovasc Intervent Radiol 32, 424, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Windhagen, H., Radtke, K., Weizbauer, A., et al. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: short term results of the first prospective, randomized, controlled clinical pilot study. Biomed Eng Online 12, 62, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Seitz, J.M., Lucas, A., and Kirschner, M.. Magnesium-based compression screws: a novelty in the clinical use of implants. JOM 68, 1177, 2016. [Google Scholar]
- 141. Cerrato, E., Barbero, U., Romero, J.A.G., et al. Magmaris (TM) resorbable magnesium scaffold: state-of-art review. Future Cardiol 15, 267, 2019. [DOI] [PubMed] [Google Scholar]
- 142. Gu, X.N., Zheng, Y.F., Cheng, Y., Zhong, S.P., and Xi, T.F.. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 30, 484, 2009. [DOI] [PubMed] [Google Scholar]
- 143. Vojtech, D., Kubasek, J., Serak, J., and Novak, P.. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater 7, 3515, 2011. [DOI] [PubMed] [Google Scholar]
- 144. Kornev, V.A., Grebenik, E.A., Solovieva, A.B., Dmitriev, R.I., and Timashev, P.S.. Hydrogel-assisted neuroregeneration approaches towards brain injury therapy: a state-of-the-art review. Comput Struct Biotecnol 16, 488, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Capek, J., Jablonska, E., Lipov, J., Kubatik, T.F., and Vojtech, D.. Preparation and characterization of porous zinc prepared by spark plasma sintering as a material for biodegradable scaffolds. Mater Chem Phys 203, 249, 2018. [Google Scholar]
- 146. Howard, D., Buttery, L.D., Shakesheff, K.M., and Roberts, S.J.. Tissue engineering: strategies, stem cells and scaffolds. J Anat 213, 66, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Uz, U., Gunhan, K., Vatansever, S., Kivanc, M., and Yuceturk, A.V.. Novel simple strategy for cartilage tissue engineering using stem cells and synthetic polymer scaffold. J Craniofac Surg 30, 940, 2019. [DOI] [PubMed] [Google Scholar]
- 148. Toma, J.G., Akhavan, M., Fernandes, K.J., et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol 3, 778, 2001. [DOI] [PubMed] [Google Scholar]
- 149. Blau, H.M., Brazelton, T.R., and Weimann, J.M.. The evolving concept of a stem cell: entity or function? Cell 105, 829, 2001. [DOI] [PubMed] [Google Scholar]
- 150. Bajada, S., Mazakova, I., Richardson, J.B., and Ashammakhi, N.. Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Regen Med 2, 169, 2008. [DOI] [PubMed] [Google Scholar]
- 151. Bajada, S., Mazakova, I., Ashton, B.A., Richardson, J., and Ashammakhi, N. Stem cells in regenerative medicine. In: Topics in Tissue Engineering. Oulu, Finland: Oulu, FinlandTopics in Tissue Engineering, 2008. Accessed September 29, 2020. Available from: https://www.oulu.fi/spareparts/ebook_topics_in_t_e_vol4/abstracts/bajada.pdf [DOI] [PubMed]
- 152. Choi, A., Yoon, H., Han, S.J., Lee, J.H., Rhyou, I.H., and Kim, D.S.. Rapid harvesting of stem cell sheets by thermoresponsive bulk poly(N-isopropylacrylamide) (PNIPAAm) nanotopography. Biomater Sci 8, 5260, 2020. [DOI] [PubMed] [Google Scholar]
- 153. Kundu, B., Bastos, A.R.F., Brancato, V., et al. Mechanical property of hydrogels and the presence of adipose stem cells in tumor stroma affect spheroid formation in the 3D osteosarcoma model. ACS Appl Mater Interfaces 11, 14548, 2019. [DOI] [PubMed] [Google Scholar]
- 154. Umehara, H., Kimura, T., Ohtsuka, S., et al. Efficient derivation of embryonic stem cells by inhibition of glycogen synthase kinase-3. Stem Cells 25, 2705, 2007. [DOI] [PubMed] [Google Scholar]
- 155. Xu, R.H., Chen, X., Li, D.S., et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol 20, 1261, 2002. [DOI] [PubMed] [Google Scholar]
- 156. Khademhosseini, A., Ashammakhi, N., Karp, J.M., et al. Chapter 27—Embryonic stem cells as a cell source for tissue engineering. In: Lanza, R., Langer, R., Vacanti, J.P., and Atala, A., eds. Principles of Tissue Engineering (Fifth Edition). London, UK: Academic Press, 2020, pp. 467–490. [Google Scholar]
- 157. Zacharias, D.G., Nelson, T.J., Mueller, P.S., and Hook, C.C.. The science and ethics of induced pluripotency: what will become of embryonic stem cells? Mayo Clin Proc 86, 634, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhang, K., Chooi, W.H., Liu, S., et al. Localized delivery of CRISPR/dCas9 via layer-by-layer self-assembling peptide coating on nanofibers for neural tissue engineering. Biomaterials 256, 120225, 2020. [DOI] [PubMed] [Google Scholar]
- 159. Somoza, R.A., and Rubio, F.J.. Cell therapy using induced pluripotent stem cells or somatic stem cells: this is the question. Curr Stem Cell Res Ther 7, 191, 2012. [DOI] [PubMed] [Google Scholar]
- 160. Kiskinis, E., and Eggan, K.. Progress toward the clinical application of patient-specific pluripotent stem cells. J Clin Invest 120, 51, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Paik, D.T., Chandy, M., and Wu, J.C.. Patient and disease–specific induced pluripotent stem cells for discovery of personalized cardiovascular drugs and therapeutics. Pharmacol Rev 72, 320, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Rosenbaum, A.J., Grande, D.A., and Dines, J.S.. The use of mesenchymal stem cells in tissue engineering. Organogenesis 4, 23, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ding, D.C., Shyu, W.C., and Lin, S.Z.. Mesenchymal stem cells. Cell Transplant 20, 5, 2011. [DOI] [PubMed] [Google Scholar]
- 164. Oryan, A., Kamali, A., Moshiri, A., and Baghaban Eslaminejad, M.. Role of mesenchymal stem cells in bone regenerative medicine: what is the evidence? Cells Tissues Organs 204, 59, 2017. [DOI] [PubMed] [Google Scholar]
- 165. Strioga, M., Viswanathan, S., Darinskas, A., Slaby, O., and Michalek, J.. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 21, 2724, 2012. [DOI] [PubMed] [Google Scholar]
- 166. Niemelä, S., Miettinen, S., Sarkanen, J.R., and Ashammakhi, N. Adipose tissue and adipocyte differentiation: molecular and cellular aspects and tissue engineering applications. 2008. In: Topics in Tissue Engineering. Oulu, Finland: Oulu UniversityTopics in Tissue Engineering. Accessed September 29, 2020. Available from: https://www.oulu.fi/spareparts/ebook_topics_in_t_e_vol4/abstracts/niemela.pdf
- 167. Niemelä, S.M., Miettinen, S., Konttinen, Y., et al. Fat tissue: views on reconstruction and exploitation. J Craniofac Surg 18, 325, 2007. [DOI] [PubMed] [Google Scholar]
- 168. Hendrijantini, N., and Hartono, P.. Phenotype characteristics and osteogenic differentiation potential of human mesenchymal stem cells derived from amnion membrane (HAMSCs) and umbilical cord (HUC-MSCs). Acta Inform Med 27, 72, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Yaghoubi, Y., Movassaghpour, A., Zamani, M., Talebi, M., Mehdizadeh, A., and Yousefi, M.. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci 233, 116733, 2019. [DOI] [PubMed] [Google Scholar]
- 170. Rajabzadeh, N., Fathi, E., and Farahzadi, R.. Stem cell-based regenerative medicine. Stem Cell Investig 6, 19, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Abumaree, M.H., Abomaray, F.M., Alshabibi, M.A., AlAskar, A.S., and Kalionis, B.. Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta 59, 87, 2017. [DOI] [PubMed] [Google Scholar]
- 172. Amiryaghoubi, N., Pesyan, N.N., Fathi, M., and Omidi, Y.. Injectable thermosensitive hybrid hydrogel containing graphene oxide and chitosan as dental pulp stem cells scaffold for bone tissue engineering. Int J Biol Macromol 162, 1338, 2020. [DOI] [PubMed] [Google Scholar]
- 173. Gargett, C.E., Schwab, K.E., and Deane, J.A.. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update 22, 137, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Amemiya, M., Tsuji, K., Katagiri, H., et al. Synovial fluid-derived mesenchymal cells have non-inferior chondrogenic potential and can be utilized for regenerative therapy as substitute for synovium-derived cells. Biochem Biophys Res Commun 523, 465, 2020. [DOI] [PubMed] [Google Scholar]
- 175. Dai, R., Wang, Z., Samanipour, R., Koo, K.-I., and Kim, K.. Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int 2016, 6737345, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zuk, P.A., Zhu, M., Mizuno, H., et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7, 211, 2001. [DOI] [PubMed] [Google Scholar]
- 177. Yong, K.W., Choi, J.R., Choi, J.Y., and Cowie, A.C.. Recent advances in mechanically loaded human mesenchymal stem cells for bone tissue engineering. Int J Mol Sci 21, 5816, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Maqsood, M., Kang, M., Wu, X., Chen, J., Teng, L., and Qiu, L.. Adult mesenchymal stem cells and their exosomes: sources, characteristics, and application in regenerative medicine. Life Sci 256, 118002, 2020. [DOI] [PubMed] [Google Scholar]
- 179. Al-Moraissi, E.A., Oginni, F.O., Holkom, M.A.M., Mohamed, A.A.S., and Al-Sharani, H.M.. Tissue-engineered bone using mesenchymal stem cells versus conventional bone grafts in the regeneration of maxillary alveolar bone: a systematic review and meta-analysis. Int J Oral Maxillofac Implants 35, 79, 2020. [DOI] [PubMed] [Google Scholar]
- 180. Docheva, D., Popov, C., Mutschler, W., and Schieker, M.. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. J Cell Mol Med 11, 21, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Galanis, E., Atherton, P.J., Viker, K.B., et al. Phase I/II trial of adipose tissue derived mesenchymal stem cell delivery of a measles virus strain engineered to express the sodium iodide symporter in ovarian cancer patients. Mol Ther 28, 589, 2020. [Google Scholar]
- 182. Aurora, A.B., and Olson, E.N.. Immune modulation of stem cells and regeneration. Cell Stem Cell 15, 15, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Nasef, A., Ashammakhi, N., and Fouillard, L.. Immunomodulatory effect of mesenchymal stromal cells: possible mechanisms. Regen Med 3, 531, 2008. [DOI] [PubMed] [Google Scholar]
- 184. Li, H., Shen, S., Fu, H., et al. Immunomodulatory functions of mesenchymal stem cells in tissue engineering. Stem Cells Int 2019, 9671206, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Bavisotto, C.C., Scalia, F., Gammazza, A.M., et al. Extracellular vesicle-mediated cell-cell communication in the nervous system: focus on neurological diseases. Int J Mol Sci 20, 434, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Jiang, T., Wang, Z., and Sun, J.. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-β/Smad signaling pathway. Stem Cell Res Ther 11, 198, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Pendse, S., Kale, V., and Vaidya, A.. Extracellular vesicles isolated from mesenchymal stromal cells primed with hypoxia: novel strategy in regenerative medicine. Curr Stem Cell Res Ther 16, 243, 2021. [DOI] [PubMed] [Google Scholar]
- 188. Higuchi, A., Ling, Q.-D., Chang, Y., Hsu, S.-T., and Umezawa, A.. Physical cues of biomaterials guide stem cell differentiation fate. Chem Rev 113, 3297, 2013. [DOI] [PubMed] [Google Scholar]
- 189. Engler, A.J., Sen, S., Sweeney, H.L., and Discher, D.E.. Matrix elasticity directs stem cell lineage specification. Cell 126, 677, 2006. [DOI] [PubMed] [Google Scholar]
- 190. Padhi, A., and Nain, A.S.. ECM in differentiation: a review of matrix structure, composition and mechanical properties. Ann Biomed Eng 48, 1071, 2020. [DOI] [PubMed] [Google Scholar]
- 191. Sung, T.C., Li, H.F., Higuchi, A., et al. Human pluripotent stem cell culture on polyvinyl alcohol-co-itaconic acid hydrogels with varying stiffness under xeno-free conditions. J Vis Exp 57314, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Du, V., Luciani, N., Richard, S., et al. A 3D magnetic tissue stretcher for remote mechanical control of embryonic stem cell differentiation. Nat Commun 8, 400, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Tannaz, N.A., Ali, S.M., Nooshin, H., et al. Comparing the effect of uniaxial cyclic mechanical stimulation and chemical factors on myogenin and Myh2 expression in mouse embryonic and bone marrow derived mesenchymal stem cells. Mol Cell Biomech 11, 19, 2014. [PubMed] [Google Scholar]
- 194. Even-Ram, S., Artym, V., and Yamada, K.M.. Matrix control of stem cell fate. Cell 126, 645, 2006. [DOI] [PubMed] [Google Scholar]
- 195. Hsieh, J., and Gage, F.H.. Epigenetic control of neural stem cell fate. Curr Opin Genet Dev 14, 461, 2004. [DOI] [PubMed] [Google Scholar]
- 196. Zhao, X., Cui, K., and Li, Z.. The role of biomaterials in stem cell-based regenerative medicine. Future Med Chem 11, 1777, 2019. [DOI] [PubMed] [Google Scholar]
- 197. Wang, L., Li, J., Zhang, D., et al. Dual-enzymatically crosslinked and injectable hyaluronic acid hydrogels for potential application in tissue engineering. RSC Adv 10, 2870, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Qazi, T.H., Mooney, D.J., Duda, G.N., and Geissler, S.. Niche-mimicking interactions in peptide-functionalized 3D hydrogels amplify mesenchymal stromal cell paracrine effects. Biomaterials 230, 119639, 2020. [DOI] [PubMed] [Google Scholar]
- 199. Abdellatef, S.A., Ohi, A., Nabatame, T., and Taniguchi, A.. The effect of physical and chemical cues on hepatocellular function and morphology. Int J Mol Sci 15, 4299, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Zimmermann, W.H., Tiburcy, M., and Eschenhagen, T.. Cardiac tissue engineering: a clinical perspective. Future Cardiol 3, 435, 2007. [DOI] [PubMed] [Google Scholar]
- 201. Crestani, T., Steichen, C., Neri, E., et al. Electrical stimulation applied during differentiation drives the hiPSC-CMs towards a mature cardiac conduction-like cells. Biochem Biophys Res Commun 533, 376, 2020. [DOI] [PubMed] [Google Scholar]
- 202. Dhahri, W., Romagnuolo, R., and Laflamme, M.A.. Training heart tissue to mature. Nat Biomed Eng 2, 351, 2018. [DOI] [PubMed] [Google Scholar]
- 203. Zhang, F., Qu, K.Y., Li, X.P., et al. Gelatin-based hydrogels combined with electrical stimulation to modulate neonatal rat cardiomyocyte beating and promote maturation. Bio-Des Manuf 4, 100, 2021. [Google Scholar]
- 204. Das, A., Ojha, M., Subramanyam, P., and Deepa, M.. New volumetric CNT-doped gelatin-cellulose scaffolds for skeletal muscle tissue engineering. Nanoscale Adv 2, 2885, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Giannoudis, P.V., and Pountos, I.. Tissue regeneration. The past, the present and the future. Injury 36 Suppl 4, S2, 2005. [DOI] [PubMed] [Google Scholar]
- 206. Nims, R.W., and Price, P.J.. Best practices for detecting and mitigating the risk of cell culture contaminants. In Vitro Cell Dev Biol Anim 53, 872, 2017. [DOI] [PubMed] [Google Scholar]
- 207. Pountos, I., Georgouli, T., and Giannoudis, P.V.. The effect of autologous serum obtained after fracture on the proliferation and osteogenic differentiation of mesenchymal stem cells. Cell Mol Biol (Noisy-le-grand) 54, 33, 2008. [PubMed] [Google Scholar]
- 208. Zhang, X., Wang, L., Zhang, X., et al. The use of KnockOut serum replacement (KSR) in three dimensional rat testicular cells co-culture model: an improved male reproductive toxicity testing system. Food Chem Toxicol 106, 487, 2017. [DOI] [PubMed] [Google Scholar]
- 209. Yasuda, S.Y., Ikeda, T., Shahsavarani, H., et al. Chemically defined and growth-factor-free culture system for the expansion and derivation of human pluripotent stem cells. Nat Biomed Eng 2, 173, 2018. [DOI] [PubMed] [Google Scholar]
- 210. Tirza, G., Solodeev, I., Sela, M., et al. Reduced culture temperature attenuates oxidative stress and inflammatory response facilitating expansion and differentiation of adipose-derived stem cells. Stem Cell Res Ther 11, 35, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Chu, A.J., Zhao, E.J., Chiao, M., and Lim, C.J.. Co-culture of induced pluripotent stem cells with cardiomyocytes is sufficient to promote their differentiation into cardiomyocytes. PLoS One 15, e0230966, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Li, B., Gao, Y., Guo, L., et al. Synthesis of photo-reactive poly (vinyl alcohol) and construction of scaffold-free cartilage like pellets in vitro. Regen Biomater 5, 159, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Zhang, R.R., Takebe, T., Miyazaki, L., et al. Efficient hepatic differentiation of human induced pluripotent stem cells in a three-dimensional microscale culture. Methods Mol Biol 1210, 131, 2014. [DOI] [PubMed] [Google Scholar]
- 214. Kim, S.M., Ueki, M., Ren, X., Akimoto, J., Sakai, Y., and Ito, Y.. Micropatterned nanolayers immobilized with nerve growth factor for neurite formation of PC12 cells. Int J Nanomed 14, 7683, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Pountos, I., Corscadden, D., Emery, P., and Giannoudis, P.V.. Mesenchymal stem cell tissue engineering: techniques for isolation, expansion and application. Injury 38 Suppl 4, S23, 2007. [DOI] [PubMed] [Google Scholar]
- 216. Costa, E.C., Moreira, A.F., de Melo-Diogo, D., Gaspar, V.M., Carvalho, M.P., and Correia, I.J.. 3D tumor spheroids: an overview on the tools and techniques used for their analysis. Biotechnol Adv 34, 1427, 2016. [DOI] [PubMed] [Google Scholar]
- 217. Antoni, D., Burckel, H., Josset, E., and Noel, G.. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci 16, 5517, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Ho, W.J., Pham, E.A., Kim, J.W., et al. Incorporation of multicellular spheroids into 3-D polymeric scaffolds provides an improved tumor model for screening anticancer drugs. Cancer Sci 101, 2637, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Davoodi, E., Sarikhani, E., Montazerian, H., et al. Extrusion and microfluidic-based bioprinting to fabricate biomimetic tissues and organs. Adv Mater Technol 5, 1901044, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Atala, A., and Forgacs, G.. Three-dimensional bioprinting in regenerative medicine: reality, hype, and future. Stem Cells Transl Med 8, 744, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Gao, X., Han, S., Zhang, R., Liu, G., and Wu, J.. Progress in electrospun composite nanofibers: composition, performance and applications for tissue engineering. J Mater Chem B 7, 7075, 2019. [DOI] [PubMed] [Google Scholar]
- 222. Nikkola, L., Morton, T., Balmayor, E.R., et al. Fabrication of electrospun poly(d,l lactide-co-glycolide)80/20 scaffolds loaded with diclofenac sodium for tissue engineering. Eur J Med Res 20, 54, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Jensen, C., and Teng, Y.. Is it time to start transitioning from 2D to 3D cell culture? Front Mol Biosci 7, 33, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front Pharmacol 9, 6, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Kapałczyńska, M., Kolenda, T., Przybyła, W., et al. 2D and 3D cell cultures—a comparison of different types of cancer cell cultures. Arch Med Sci 14, 910, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Merten, O.W. Advances in cell culture: anchorage dependence. Philos Trans R Soc B Lond B Biol Sci 370, 20140040, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Souza, G.R., Molina, J.R., Raphael, R.M., et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol 5, 291, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Abe-Fukasawa, N., Otsuka, K., Aihara, A., Itasaki, N., and Nishino, T.. Novel 3D liquid cell culture method for anchorage-independent cell growth, cell imaging and automated drug screening. Sci Rep 8, 3627, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Kronemberger, G.S., Dalmônico, G.M.L., Rossi, A.L., et al. Scaffold- and serum-free hypertrophic cartilage tissue engineering as an alternative approach for bone repair. Artif Organs 44, E288, 2020. [DOI] [PubMed] [Google Scholar]
- 230. Anil-Inevi, M., Yilmaz, E., Sarigil, O., Tekin, H.C., and Ozcivici, E.. Single cell densitometry and weightlessness culture of mesenchymal stem cells using magnetic levitation. Methods Mol Biol 2125, 15, 2020. [DOI] [PubMed] [Google Scholar]
- 231. Dutta, D., Heo, I., and Clevers, H.. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med 23, 393, 2017. [DOI] [PubMed] [Google Scholar]
- 232. Tseng, H., Daquinag, A.C., Souza, G.R., and Kolonin, M.G.. Three-dimensional magnetic levitation culture system simulating white adipose tissue. Methods Mol Biol 1773, 147, 2018. [DOI] [PubMed] [Google Scholar]
- 233. Bagheri-Hosseinabadi, Z., Seyedi, F., Mollaei, H.R., Moshrefi, M., and Seifalian, A.. Combination of 5-azaytidine and hanging drop culture convert fat cell into cardiac cell. Biotechnol Appl Biochem 68, 92, 2021. [DOI] [PubMed] [Google Scholar]
- 234. Salehi, H., Razavi, S., Esfandiari, E., Kazemi, M., Amini, S., and Amirpour, N.. Application of hanging drop culture for retinal precursor-like cells differentiation of human adipose-derived stem cells using small molecules. J Mol Neurosci 69, 597, 2019. [DOI] [PubMed] [Google Scholar]
- 235. Lawrence, L.M., Cottrill, A., Valluri, A., et al. Minimally manipulative method for the expansion of human bone marrow mesenchymal stem cells to treat osseous defects. Int J Mol Sci 20, 612, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Kondo, S., Nakagawa, Y., Mizuno, M., et al. Transplantation of aggregates of autologous synovial mesenchymal stem cells for treatment of cartilage defects in the femoral condyle and the femoral groove in microminipigs. Am J Sports Med 47, 2338, 2019. [DOI] [PubMed] [Google Scholar]
- 237. Ji, K., Sameni, M., Osuala, K., Moin, K., Mattingly, R.R., and Sloane, B.F.. Spatio-temporal modeling and live-cell imaging of proteolysis in the 4D microenvironment of breast cancer. Cancer Metastasis Rev 38, 445, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Miao, S., Cui, H., Esworthy, T., et al. 4D self-morphing culture substrate for modulating cell differentiation. Adv Sci (Weinh) 7, 1902403, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Nies, C., Rubner, T., Lorig, H., et al. A microcavity array-based 4D cell culture platform. Bioengineering (Basel) 6, 50, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Qasim, M., Le, N.X.T., Nguyen, T.P.T., Chae, D.S., Park, S.J., and Lee, N.Y.. Nanohybrid biodegradable scaffolds for TGF-β3 release for the chondrogenic differentiation of human mesenchymal stem cells. Int J Pharm 581, 119248, 2020. [DOI] [PubMed] [Google Scholar]
- 241. Chen, S., Wu, C., Liu, A., et al. Biofabrication of nerve fibers with mimetic myelin sheath-like structure and aligned fibrous niche. Biofabrication 3, 035013, 2020. [DOI] [PubMed] [Google Scholar]
- 242. Malek-Khatabi, A., Javar, H.A., Dashtimoghadam, E., Ansari, S., Hasani-Sadrabadi, M.M., and Moshaverinia, A.. In situ bone tissue engineering using gene delivery nanocomplexes. Acta Biomater 108, 326, 2020. [DOI] [PubMed] [Google Scholar]
- 243. Ashammakhi, N., Darabi, M.A., Çelebi-Saltik, B., et al. Microphysiological systems: next generation systems for assessing toxicity and therapeutic effects of nanomaterials. Small Methods 4, 1900589, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Bhatia, S.N., and Ingber, D.E.. Microfluidic organs-on-chips. Nat Biotechnol 32, 760, 2014. [DOI] [PubMed] [Google Scholar]
- 245. Ashammakhi, N., Elkhammas, E., and Hasan, A.. Translating advances in organ-on-a-chip technology for supporting organs. J Biomed Mater Res B Appl Biomater 107, 2006, 2019. [DOI] [PubMed] [Google Scholar]
- 246. Sontheimer-Phelps, A., Hassell, B.A., and Ingber, D.E.. Modelling cancer in microfluidic human organs-on-chips. Nat Rev Cancer 19, 65, 2019. [DOI] [PubMed] [Google Scholar]
- 247. Zheng, F., Fu, F., Cheng, Y., Wang, C., Zhao, Y., and Gu, Z.. Organ-on-a-chip systems: microengineering to biomimic living systems. Small 12, 2253, 2016. [DOI] [PubMed] [Google Scholar]
- 248. Brougham, C.M., Levingstone, T.J., Shen, N.A., et al. Freeze-drying as a novel biofabrication method for achieving a controlled microarchitecture within large, complex natural biomaterial scaffolds. Adv Healthc Mater 6, 2017. DOI: 10.1002/adhm.201700598. [DOI] [PubMed] [Google Scholar]
- 249. Prasad, A., Sankar, M.R., and Katiyar, V.. State of art on solvent casting particulate leaching method for orthopedic scaffolds fabrication. Mater Today 4, 898, 2017. [Google Scholar]
- 250. Eltom, A., Zhong, G.Y., and Muhammad, A.. Scaffold techniques and designs in tissue engineering functions and purposes: a review. Adv Mater Sci Eng 2019, 3429527, 2019. [Google Scholar]
- 251. Dutta, R.C., Dey, M., Dutta, A.K., and Basu, B.. Competent processing techniques for scaffolds in tissue engineering. Biotechnol Adv 35, 240, 2017. [DOI] [PubMed] [Google Scholar]
- 252. Hull, C.W., inventor; UVP, Inc., assignee. Apparatus for production of three-dimensional objects by stereolithography. USA Patent 4,575,330 1986. [Google Scholar]
- 253. Vijayavenkataraman, S., Yan, W.C., Lu, W.F., Wang, C.H., and Fuh, J.Y.H.. 3D bioprinting of tissues and organs for regenerative medicine. Adv Drug Deliv Rev 132, 296, 2018. [DOI] [PubMed] [Google Scholar]
- 254. Mandrycky, C., Wang, Z.J., Kim, K., and Kim, D.H.. 3D bioprinting for engineering complex tissues. Biotechnol Adv 34, 422, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Belk L TN, MacDonald, H., Erdem, A., Ashammakhi, N., and Pountos, I.. Safety considerations in 3D bioprinting using Mesenchymal Stem Cells. Front Bioeng Biotechnol 8, 924, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Paul, K., Darzi, S., McPhee, G., et al. 3D bioprinted endometrial stem cells on melt electrospun poly ɛ-caprolactone mesh for pelvic floor application promote anti-inflammatory responses in mice. Acta Biomater 97, 162, 2019. [DOI] [PubMed] [Google Scholar]
- 257. Merceron, T.K., Burt, M., Seol, Y.J., et al. A 3D bioprinted complex structure for engineering the muscle-tendon unit. Biofabrication 7, 035003, 2015. [DOI] [PubMed] [Google Scholar]
- 258. Kang, H.W., Lee, S.J., Ko, I.K., Kengla, C., Yoo, J.J., and Atala, A.. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 34, 312, 2016. [DOI] [PubMed] [Google Scholar]
- 259. Jessop, Z.M., Al-Sabah, A., Gardiner, M.D., Combellack, E., Hawkins, K., and Whitaker, I.S.. 3D bioprinting for reconstructive surgery: principles, applications and challenges. J Plast Reconstr Aesthet Surg 70, 1155, 2017. [DOI] [PubMed] [Google Scholar]
- 260. Kahl, M., Gertig, M., Hoyer, P., Friedrich, O., and Gilbert, D.F.. Ultra-low-cost 3D bioprinting: modification and application of an off-the-shelf desktop 3D-printer for biofabrication. Front Bioeng Biotechnol 7, 184, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Fulco, I., Miot, S., Haug, M.D., et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet 384, 337, 2014. [DOI] [PubMed] [Google Scholar]
- 262. Nerem, R.M., and Seliktar, D.. Vascular tissue engineering. Annu Rev Biomed Eng 3, 225, 2001. [DOI] [PubMed] [Google Scholar]
- 263. Ashammakhi, N., Ahadian, S., Fan, Z.J., et al. Advances and future perspectives in 4D bioprinting. Biotechnol J 13, e1800148, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Ashammakhi, N., Ahadian, S., Pountos, I., et al. In situ three-dimensional printing for reparative and regenerative therapy. Biomed Microdevices 21, 42, 2019. [DOI] [PubMed] [Google Scholar]
- 265. Ying, G., Manríquez, J., Wu, D., et al. An open-source handheld extruder loaded with pore-forming bioink for in situ wound dressing. Mater Today Bio 8, 100074, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Lei, Z.Y., and Wu, P.Y.. A supramolecular biomimetic skin combining a wide spectrum of mechanical properties and multiple sensory capabilities. Nat Commun 9, 1134, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Potyondy, T.U.J., Tebon, P.J., Byambaa, B., et al. Recent advances in 3D bioprinting of musculoskeletal tissues. Biofabrication 2020 [Epub ahead of print]; DOI: 10.1088/1758-5090/abc8de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Liang, C.Y., Hu, Y.C., Wang, H.S., et al. Biomimetic cardiovascular stents for in vivo re-endothelialization. Biomaterials 103, 170, 2016. [DOI] [PubMed] [Google Scholar]
- 269. Khoyratee, F., Grassia, F., Saighi, S., and Levi, T.. Optimized real-time biomimetic neural network on FPGA for bio-hybridization. Front Neurosci 13, 377, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Jain, A., and Bansal, R.. Applications of regenerative medicine in organ transplantation. J Pharm Bioallied Sci 7, 188, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Ashammakhi, N., Ahadian, S., Xu, C., et al. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater Today Bio 1, 100008, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Tamay, D.G., Dursun Usal, T., Alagoz, A.S., Yucel, D., Hasirci, N., and Hasirci, V.. 3D and 4D printing of polymers for tissue engineering applications. Front Bioeng Biotechnol 7, 164, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Miao, S., Cui, H., Nowicki, M., et al. Stereolithographic 4D bioprinting of multiresponsive architectures for neural engineering. Adv Biosyst 2, 1800101, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Cui, H.T., Nowicki, M., Fisher, J.P., and Zhang, L.G.. 3D bioprinting for organ regeneration. Adv Healthc Mater 6, 10.1002/adhm.201601118, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Dong Nyoung, H., Bugra, A., Madhuri, D., et al. Aspiration-assisted bioprinting of co-cultured osteogenic spheroids for bone tissue engineering. Biofabrication 2020 [Epub ahead of print]; DOI: 10.1088/1758-5090/abc1bf. [DOI] [PubMed] [Google Scholar]
- 276. Ayan, B., Celik, N., Zhang, Z., et al. Aspiration-assisted freeform bioprinting of pre-fabricated tissue spheroids in a yield-stress gel. Commun Phys 3, 183, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Yang, Y., Nikkola, L., Ylikauppila, H., and Ashammakhi N, eds. Investigation of cell attachment on the scaffolds manufactured by electrospun PCL-hyaluronan blend. TNT2006 “Trends in Nanotechnology” Conference 2006; Grenoble, France, 2006. [Google Scholar]
- 278. Will, O.M., Purcz, N., Chalaris, A., et al. Increased survival rate by local release of diclofenac in a murine model of recurrent oral carcinoma. Int J Nanomedicine 11, 5311, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. GhavamiNejad, A., Unnithan, A.R., Ramachandra, A., et al. Mussel-inspired electrospun nanofibers functionalized with size-controlled silver nanoparticles for wound dressing application. ACS Appl Mater Interfaces 7, 12176, 2015. [DOI] [PubMed] [Google Scholar]
- 280. Bhardwaj, N., and Kundu, S.C.. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv 28, 325, 2010. [DOI] [PubMed] [Google Scholar]
- 281. Chee, B.S., de Lima, G.G., Devine, D., and Nugent, M.J.D. Electrospun hydrogels composites for bone tissue engineering. Applications of Nanocomposite Materials in Orthopedics. 2019, pp. 39–70. DOI: 10.1016/B978-0-12-813740-6.00003-X. [DOI] [Google Scholar]
- 282. Chen, W.M., Xu, Y., Liu, Y.Q., et al. Three-dimensional printed electrospun fiber-based scaffold for cartilage regeneration. Mater Des 179, 107886, 2019. [Google Scholar]
- 283. Frost, H.K., Andersson, T., Johansson, S., et al. Electrospun nerve guide conduits have the potential to bridge peripheral nerve injuries in vivo (vol 8, 16716, 2018). Sci Rep 9, 10017, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Karkan, S.F., Davaran, S., Rahbarghazi, R., Salehi, R., and Akbarzadeh, A.. Electrospun nanofibers for the fabrication of engineered vascular grafts. J Biol Eng 13, 83, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Göttel, B., de Souza e Silva, J.M., Santos de Oliveira, C., et al. Electrospun nanofibers—a promising solid in-situ gelling alternative for ocular drug delivery. Eur J Pharm Biopharm 146, 125, 2020. [DOI] [PubMed] [Google Scholar]
- 286. GhavamiNejad, A., Sasikala, A.R.K., Unnithan, A.R., et al. Mussel-inspired electrospun smart magnetic nanofibers for hyperthermic chemotherapy. Adv Funct Mater 25, 2867, 2015. [Google Scholar]
- 287. Aguilar, L.E., GhavamiNejad, A., Park, C.H., and Kim, C.S.. On-demand drug release and hyperthermia therapy applications of thermoresponsive poly-(NIPAAm-co-HMAAm)/polyurethane core-shell nanofiber mat on non-vascular nitinol stents. Nanomed Nanotechnol 13, 527, 2017. [DOI] [PubMed] [Google Scholar]
- 288. Nagiah, N., Murdock, C.J., Bhattacharjee, M., Nair, L., and Laurencin, C.T.. Development of tripolymeric triaxial electrospun fibrous matrices for dual drug delivery applications. Sci Rep 10, 609, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Casanova, M.R., Reis, R.L., Martins, A., and Neves, N.M.. The use of electrospinning technique on osteochondral tissue engineering. In: Oliveira, J.M., Reis, S.P.R.L., and Roman, J.S., eds. Osteochondral Tissue Engineering: nanotechnology, Scaffolding-Related Developments and Translation. vol. 1058, 2018. New York City: Springer International Publishing, pp. 247–263. [Google Scholar]
- 290. Guo, Y., Gilbert-Honick, J., Somers, S.M., Mao, H.-Q., and Grayson, W.L.. Modified cell-electrospinning for 3D myogenesis of C2C12s in aligned fibrin microfiber bundles. Biochem Biophys Res Commun 516, 558, 2019. [DOI] [PubMed] [Google Scholar]
- 291. Maver, T., Smrke, D.M., Kurecic, M., Gradisnik, L., Maver, U., and Kleinschek, K.S.. Combining 3D printing and electrospinning for preparation of pain-relieving wound-dressing materials. J Solgel Sci Technol 88, 33, 2018. [Google Scholar]
- 292. Yu, Y.X., Hua, S., Yang, M.K., et al. Fabrication and characterization of electrospinning/3D printing bone tissue engineering scaffold. RSC Adv 6, 110557, 2016. [Google Scholar]
- 293. Chen, Y.J., Shafiq, M., Liu, M.Y., Morsi, Y., and Mo, X.M.. Advanced fabrication for electrospun three-dimensional nanofiber aerogels and scaffolds. Bioact Mater 5, 963, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Vogel, R., Tandler, B., Haussler, L., Jehnichen, D., and Brunig, H.. Melt spinning of poly(3-hydroxybutyrate) fibers for tissue engineering using alpha-cyclodextrin/polymer inclusion complexes as the nucleation agent. Macromol Biosci 6, 730, 2006. [DOI] [PubMed] [Google Scholar]
- 295. Park, J.-S. Electrospinning and its applications. Adv Nat Sci Nanosci Nanotechnol 1, 043002, 2011. [Google Scholar]
- 296. Bisht, G.S., Canton, G., Mirsepassi, A., et al. Controlled continuous patterning of polymeric nanofibers on three-dimensional substrates using low-voltage near-field electrospinning. Nano Lett 11, 1831, 2011. [DOI] [PubMed] [Google Scholar]
- 297. Hochleitner, G., Jungst, T., Brown, T.D., et al. Additive manufacturing of scaffolds with sub-micron filaments via melt electrospinning writing. Biofabrication 7, 035002, 2015. [DOI] [PubMed] [Google Scholar]
- 298. Brown, T.D., Dalton, P.D., and Hutmacher, D.W.. direct writing by way of melt electrospinning. Adv Mater 23, 5651, 2011. [DOI] [PubMed] [Google Scholar]
- 299. Costa, P.F., Vaquette, C., Zhang, Q., Reis, R.L., Ivanovski, S., and Hutmacher, D.W.. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J Clin Periodontol 41, 283, 2014. [DOI] [PubMed] [Google Scholar]
- 300. Großhaus, C., Bakirci, E., Berthel, M., et al. Melt electrospinning of nanofibers from medical-grade poly(ɛ-caprolactone) with a modified nozzle. Small 16, 2003471, 2020. [DOI] [PubMed] [Google Scholar]
- 301. Davidson, M.D., Ban, E., Schoonen, A.C.M., et al. Mechanochemical adhesion and plasticity in multifiber hydrogel networks. Adv Mater 32, 1905719, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Saveh-Shemshaki, N., S.Nair, L., and Laurencin, C.T.. Nanofiber-based matrices for rotator cuff regenerative engineering. Acta Biomater 94, 64, 2019. [DOI] [PubMed] [Google Scholar]
- 303. Yu, J.R., Navarro, J., Coburn, J.C., et al. Current and future perspectives on skin tissue engineering: key features of biomedical research, translational assessment, and clinical application. Adv Healthc Mater 8, e1801471, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. O'Donnell, B.T., Ives, C.J., Mohiuddin, O.A., and Bunnell, B.A.. Beyond the present constraints that prevent a wide spread of tissue engineering and regenerative medicine approaches. Front Bioeng Biotechnol 7, 95, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Chandra, P.K., Soker, S., and Atala, A. Chapter 1—Tissue engineering: current status and future perspectives. In: Lanza, R., Langer, R., Vacanti, J.P., and Atala, A., eds. Principles of Tissue Engineering (Fifth Edition). London, UK: Academic Press, 2020, pp. 1–35. [Google Scholar]
- 306. Colombo, F., Sampogna, G., Cocozza, G., Guraya, S., and Forgione, A.. Regenerative medicine: clinical applications and future perspectives. J Microsc Ultrastruct 5, 1, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Yano, K., Speidel, A.T., and Yamato, M.. Four Food and Drug Administration draft guidance documents and the REGROW Act: a litmus test for future changes in human cell- and tissue-based products regulatory policy in the United States? J Tissue Eng Regen Med 12, 1579, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Mao, A.S., and Mooney, D.J.. Regenerative medicine: current therapies and future directions. Proc Natl Acad Sci U S A 112, 14452, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Pahon, E. FDA Approves Integra Omnigraft Dermal Regeneration Matrix to Treat Diabetic Foot Ulcers. US Food and Drug Administration, 2016. Updated January 7, 2016; accessed November 1, 2020. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-integra-omnigraft-dermal-regeneration-matrix-treat-diabetic-foot-ulcers
- 310. Anonymous. Premarket Approval: INFUSE Bone Graft/LT-CAGE Lumbar Tapered Fusion Device: Food and Drud Adimistration; 2002. Accessed November 1, 2020. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P000058
- 311. Kimmel, H., and Gittleman, H.. Retrospective observational analysis of the use of an architecturally unique dermal regeneration template (Derma Pure®) for the treatment of hard-to-heal wounds. Int Wound J 14, 666, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Means, K.R.Jr., Rinker, B.D., Higgins, J.P., Payne, S.H.Jr., Merrell, G.A., and Wilgis, E.F.. A multicenter, prospective, randomized, pilot study of outcomes for digital nerve repair in the hand using hollow conduit compared with processed allograft nerve. Hand (N Y) 11, 144, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Anonymous. The INSPIRE Study: Probable Benefit of the Neuro-Spinal Scaffold for Treatment of AIS A Thoracic Acute Spinal Cord Injury. US National Library of Medicine, 2014. Updated December 18, 2019; accessed November 1, 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT02138110
- 314. Anonymous. A Study of VentriGel in Post-MI Patients. US National Library of Medicine, 2014. Updated October 25, 2019; accessed November 1, 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT02305602
- 315. Anonymous. Musculotendinous Tissue Repair Unit and Reinforcement (MTURR). US National Library of Medicine, 2011. Updated August 14, 2017; accessed November 1, 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT01292876
- 316. Dahl, S.L., Kypson, A.P., Lawson, J.H., et al. Readily available tissue-engineered vascular grafts. Sci Transl Med 3, 68ra9, 2011. [DOI] [PubMed] [Google Scholar]
- 317. Anonymous. Humacyte Human Acellular Vessel (HAV) in Patients with Vascular Trauma. ClinicalTrials.gov, 2016. Updated October 12, 2020; accessed November 8, 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT03005418
- 318. Jiang, S., Guo, W., Tian, G., et al. Clinical application status of articular cartilage regeneration techniques: tissue-engineered cartilage brings new hope. Stem Cells Int 2020, 5690252, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Azam, A., Forster, M., and Robertson, A.. Clinical and radiological outcome for Trufit Plug in the treatment of chondral and osteochondral lesions at a minimum of 2years. J Orthop 15, 47, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Hindle, P., Hendry, J.L., Keating, J.F., and Biant, L.C.. Autologous osteochondral mosaicplasty or TruFit plugs for cartilage repair. Knee Surg Sports Traumatol Arthrosc 22, 1235, 2014. [DOI] [PubMed] [Google Scholar]
- 321. Bugelli, G., Ascione, F., Dell'Osso, G., Zampa, V., and Giannotti, S.. Biphasic bioresorbable scaffold (TruFit®) in knee osteochondral defects: 3-T MRI evaluation of osteointegration in patients with a 5-year minimum follow-up. Musculoskelet Surg 102, 191, 2018. [DOI] [PubMed] [Google Scholar]
- 322. Di Cave, E., Versari, P., Sciarretta, F., Luzon, D., and Marcellini, L.. Biphasic bioresorbable scaffold (TruFit Plug®) for the treatment of osteochondral lesions of talus: 6- to 8-year follow-up. Foot 33, 48, 2017. [DOI] [PubMed] [Google Scholar]
- 323. Berruto, M., Delcogliano, M., de Caro, F., et al. Treatment of large knee osteochondral lesions with a biomimetic scaffold: results of a multicenter study of 49 patients at 2-year follow-up. Am J Sports Med 42, 1607, 2014. [DOI] [PubMed] [Google Scholar]
- 324. D'Ambrosi, R., Valli, F., De Luca, P., Ursino, N., and Usuelli, F.G.. MaioRegen osteochondral substitute for the treatment of knee defects: a systematic review of the literature. J Clin Med 8, 783, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Anonymous. Premarket approval (PMA): transcyte human fibroblast-derived temporary skin substitute. Food and Drug Administration, 1998. Accessed November 1, 2020. Available from: www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P960007
- 326. Noordenbos, J., Doré, C., and Hansbrough, J.F.. Safety and efficacy of TransCyte for the treatment of partial-thickness burns. J Burn Care Rehabil 20, 275, 1999. [DOI] [PubMed] [Google Scholar]
- 327. Wilkins, L.M., Watson, S.R., Prosky, S.J., Meunier, S.F., and Parenteau, N.L.. Development of a bilayered living skin construct for clinical applications. Biotechnol Bioeng 43, 747, 1994. [DOI] [PubMed] [Google Scholar]
- 328. Zaulyanov, L., and Kirsner, R.S.. A review of a bi-layered living cell treatment (Apligraf) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin Interv Aging 2, 93, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Marston, W.A. Dermagraft, a bioengineered human dermal equivalent for the treatment of chronic nonhealing diabetic foot ulcer. Expert Rev Med Devices 1, 21, 2004. [DOI] [PubMed] [Google Scholar]
- 330. Still, J., Glat, P., Silverstein, P., Griswold, J., and Mozingo, D.. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns 29, 837, 2003. [DOI] [PubMed] [Google Scholar]
- 331. Caravaggi, C., De Giglio, R., Pritelli, C., et al. HYAFF 11-based autologous dermal and epidermal grafts in the treatment of noninfected diabetic plantar and dorsal foot ulcers. A prospective, multicenter, controlled, randomized clinical trial. Diabetes Care 26, 2853, 2003. [DOI] [PubMed] [Google Scholar]
- 332. Uccioli, L. A clinical investigation on the characteristics and outcomes of treating chronic lower extremity wounds using the tissuetech autograft system. Int J Low Extrem Wounds 2, 140, 2003. [DOI] [PubMed] [Google Scholar]
- 333. Min, J.H., Yun, I.S., Lew, D.H., Roh, T.S., and Lee, W.J.. The use of matriderm and autologous skin graft in the treatment of full thickness skin defects. Arch Plast Surg 41, 330, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Vig, K., Chaudhari, A., Tripathi, S., et al. Advances in skin regeneration using tissue engineering. Int J Mol Sci 18, 789, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Ghiroldi, A., Piccoli, M., Cirillo, F., et al. Cell-based therapies for cardiac regeneration: a comprehensive review of past and ongoing strategies. Int J Mol Sci 19, 3194, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Therapeutic Apheresis. https://www.terumobct.com/Pages/Therapeutic%20Apheresis/Spectra%20Optia/Not-Approved-for-US.aspx (accessed August 4, 2021).
- 337. Davidson, P.M., Newton, P.J., Tankumpuan, T., Paull, G., and Dennison-Himmelfarb, C.. Multidisciplinary management of chronic heart failure: principles and future trends. Clin Ther 37, 2225, 2015. [DOI] [PubMed] [Google Scholar]
- 338. Ossendorf, C., Kaps, C., Kreuz, P.C., Burmester, G.R., Sittinger, M., and Erggelet, C.. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res Ther 9, R41, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Brix, M.O., Stelzeneder, D., Trattnig, S., Windhager, R., and Domayer, S.E.. Cartilage repair of the knee with Hyalograft C:(R) Magnetic Resonance Imaging assessment of the glycosaminoglycan content at midterm. Int Orthop 37, 39, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Kreuz, P.C., Müller, S., Freymann, U., et al. Repair of focal cartilage defects with scaffold-assisted autologous chondrocyte grafts: clinical and biomechanical results 48months after transplantation. Am J Sports Med 39, 1697, 2011. [DOI] [PubMed] [Google Scholar]
- 341. Wylie, J.D., Hartley, M.K., Kapron, A.L., Aoki, S.K., and Maak, T.G.. Failures and reoperations after matrix-assisted cartilage repair of the knee: a systematic review. Arthroscopy 32, 386, 2016. [DOI] [PubMed] [Google Scholar]
- 342. Schneider, U., Rackwitz, L., Andereya, S., et al. A prospective multicenter study on the outcome of type I collagen hydrogel-based autologous chondrocyte implantation (CaReS) for the repair of articular cartilage defects in the knee. Am J Sports Med 39, 2558, 2011. [DOI] [PubMed] [Google Scholar]
- 343. Crawford, D.C., DeBerardino, T.M., and Williams, R.J., 3rd. NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: an FDA phase-II prospective, randomized clinical trial after two years. J Bone Joint Surg Am 94, 979, 2012. [DOI] [PubMed] [Google Scholar]
- 344. Gaissmaier, C.F.J., Schewe, B., Albrecht, D., et al. Development of NOVOCART® 3D, a novel system of scaffold augmented transplantation of autologous chondrocytes. In: Zanasi, S., Brittberg, M., and Marcacci, M., eds. Basic Science, Clinical Repair and Reconstruction of Articular Cartilage Defects: Current Status and Prospects. Bologna, Italy: Timeo Editore s.r.l., 2006, pp. 573–585. [Google Scholar]
- 345. Anonymous. Non-Interventional Study to Evaluate Safety and Efficacy of NOVOCART 3D in Patients with Cartilage Defects (NISANIK). ClinicalTrials.gov, 2015. Updated December 3, 2019; accessed November 8, 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT02348697
- 346. Anonymous. Non-Interventional Study with NOVOCART® 3D for the Treatment of Cartilage Defects of the Knee in Pediatric Patients (JUNOVO). ClinicalTrials.gov, 2017. Updated March 31, 2020; accessed November 8, 2020. Available from: https://clinicaltrials.gov/ct2/history/NCT04186208?V_2=View#StudyPageTop
- 347. Anonymous. Phase III Study to Evaluate Safety and Effectiveness of NOVOCART 3D Plus vs. Microfracture in Knee Cartilage Defects (N3D). ClinicalTrials.gov, 2012. Updated August 18, 2020; accessed November 8, 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT01656902
- 348. Anonymous. NOVOCART 3D Treatment Following Microfracture Failure. ClinicalTrials.gov, 2017. Updated January 31, 2019; accessed November 8, 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT03219307
- 349. Anonymous. NOVOCART®3D for Treatment of Articular Cartilage of the Knee (N3D). ClinicaTtrials.gov, 2013. Updated October 24, 2019; accessed November 8, 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT01957722
- 350. Müller, P.E., Gallik, D., Hammerschmid, F., et al. Third-generation autologous chondrocyte implantation after failed bone marrow stimulation leads to inferior clinical results. Knee Surg Sports Traumatol Arthrosc 28, 470, 2020. [DOI] [PubMed] [Google Scholar]
- 351. Niethammer, T.R., Holzgruber, M., Gülecyüz, M.F., Weber, P., Pietschmann, M.F., and Müller, P.E.. Matrix based autologous chondrocyte implantation in children and adolescents: a match paired analysis in a follow-up over three years post-operation. Int Orthop 41, 343, 2017. [DOI] [PubMed] [Google Scholar]
- 352. Baiguera, S., Jungebluth, P., Burns, A., et al. Tissue engineered human tracheas for in vivo implantation. Biomaterials 31, 8931, 2010. [DOI] [PubMed] [Google Scholar]
- 353. Elliott, M.J., De Coppi, P., Speggiorin, S., et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet 380, 994, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Atala, A., Bauer, S.B., Soker, S., Yoo, J.J., and Retik, A.B.. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 367, 1241, 2006. [DOI] [PubMed] [Google Scholar]
- 355. Joseph, D.B., Borer, J.G., De Filippo, R.E., Hodges, S.J., and McLorie, G.A.. Autologous cell seeded biodegradable scaffold for augmentation cystoplasty: phase II study in children and adolescents with spina bifida. J Urol 191, 1389, 2014. [DOI] [PubMed] [Google Scholar]
- 356. Stenzl, A., Ninkovic, M., Ashammakhi, N., Eder, I.E., and Bartsch, G.. [Reconstruction of the lower urinary tract. Developments at the beginning of a new century]. Urologe A 40, 368, 2001. [DOI] [PubMed] [Google Scholar]
- 357. Shin'oka T, Imai, Y., and Ikada, Y.. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med 344, 532, 2001. [DOI] [PubMed] [Google Scholar]
- 358. Kurobe, H., Maxfield, M.W., Breuer, C.K., and Shinoka, T.. Concise review: tissue-engineered vascular grafts for cardiac surgery: past, present, and future. Stem Cells Transl Med 1, 566, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Pellegrini, G., Ardigò, D., Milazzo, G., et al. Navigating market authorization: the path holoclar took to become the first stem cell product approved in the european union. Stem Cells Transl Med 7, 146, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Anonymous. Europe approves Holoclar®, the first stem cell-based medicinal product. EuroStemCell. Accessed November 1, 2020. Available from: https://www.eurostemcell.org/story/europe-approves-holoclar-first-stem-cell-based-medicinal-product
- 361. Anonymous. Transplantation of iPSC-derived RPE sheet into first AMD patient. Riken Center for Developmental Biology, 2014. Accessed November 1, 2020. Available from: http://www.cdb.riken.jp/en/news/2014/researches/0915_3047.html
- 362. Schwartz, S.D., Regillo, C.D., Lam, B.L., Eliott, D., Rosenfeld, P.J., Gregori, N.Z., Hubschman, J.P., Davis, J.L., Heilwell, G., Spirn, M., Maguire, J., Gay, R., Bateman, J., Ostrick, R.M., Morris, D., Vincent, M., Anglade, E., Del Priore, L.V., Lanza, R.. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385, 509, 2015. [DOI] [PubMed] [Google Scholar]
- 363. Murphy, S.V., De Coppi, P., and Atala, A.. Opportunities and challenges of translational 3D bioprinting. Nat Biomed Eng 4, 370, 2020. [DOI] [PubMed] [Google Scholar]
- 364. Hunsberger, J., Harrysson, O., Shirwaiker, R., et al. Manufacturing road map for tissue engineering and regenerative medicine technologies. Stem Cell Transl Med 4, 130, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Evans, C.H. Barriers to the clinical translation of orthopedic tissue engineering. Tissue Eng Part B Rev 17, 437, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Lu, L.C., Arbit, H.M., Herrick, J.L., Segovis, S.G., Maran, A., and Yaszemski, M.J.. Tissue engineered constructs: perspectives on clinical translation. Ann Biomed Eng 43, 796, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Webber, M.J., Khan, O.F., Sydlik, S.A., Tang, B.C., and Langer, R.. A perspective on the clinical translation of scaffolds for tissue engineering. Ann Biomed Eng 43, 641, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Chapekar, M.S. Tissue engineering: challenges and opportunities. J Biomed Mater Res 53, 617, 2000. [DOI] [PubMed] [Google Scholar]
- 369. Nerem, R.M. Cellular engineering. Ann Biomed Eng 19, 529, 1991. [DOI] [PubMed] [Google Scholar]
- 370. Chen, F.M., and Liu, X.H.. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci 53, 86, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Ghaemi, R.V., Siang, L.C., and Yadav, V.G.. Improving the rate of translation of tissue engineering products. Adv Healthc Mater 8, 1900538, 2019. [DOI] [PubMed] [Google Scholar]
- 372. Barlas, S. The 21st Century Cures Act: FDA implementation one year later: some action, some results, some questions. P T 43, 149, 2018. [PMC free article] [PubMed] [Google Scholar]
- 373. Jain, R.K., Au, P., Tam, J., Duda, D.G., and Fukumura, D.. Engineering vascularized tissue. Nat Biotechnol 23, 821, 2005. [DOI] [PubMed] [Google Scholar]
- 374. Schneider, M.K., Ioanas, H.-I., Xandry, J., and Rudin, M.. An in vivo wound healing model for the characterization of the angiogenic process and its modulation by pharmacological interventions. Sci Rep 9, 6004, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Shahabipour, F., Ashammakhi, N., Oskuee, R.K., et al. Key components of engineering vascularized 3-dimensional bioprinted bone constructs. Transl Res 216, 57, 2020. [DOI] [PubMed] [Google Scholar]
- 376. Rath, S.N., Pryymachuk, G., Bleiziffer, O.A., et al. Hyaluronan-based heparin-incorporated hydrogels for generation of axially vascularized bioartificial bone tissues: in vitro and in vivo evaluation in a PLDLLA-TCP-PCL-composite system. J Mater Sci Mater Med 22, 1279, 2011. [DOI] [PubMed] [Google Scholar]
- 377. Penttilä, H.T.R-M., Waris, T., Ellä, V., Kellomäki, M., Törmälä, P., and Ashammakhi, N., eds. Combining prefabricated microvascularied perichondrial flaps and bioabsorbable polylactide nonwoven scaffolds to tissue engineered cartilage. Joint Meeting of the Tissue Engineering Society International (TESI) and the European Tissue Engineering Society (ETES); 10–13.10.2004; Lausanne, Switzerland. [Google Scholar]
- 378. Ashammakhi, N., Darabi, M.A., Kehr, N.S., et al. Advances in controlled oxygen generating biomaterials for tissue engineering and regenerative therapy. Biomacromolecules 21, 56, 2020. [DOI] [PubMed] [Google Scholar]
- 379. Erdem, A., Darabi, M.A., Nasiri, R., et al. 3D bioprinting of oxygenated cell-laden gelatin methacryloyl constructs. Adv Healthc Mater 9, 1901794, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Wust, S., Muller, R., and Hofmann, S.. Controlled positioning of cells in biomaterials-approaches towards 3D tissue printing. J Funct Biomater 2, 119, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Lee, J.W., Choi, Y.J., Yong, W.J., et al. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication 8, 015007, 2016. [DOI] [PubMed] [Google Scholar]
- 382. Puumanen, K.A., Ruuskanen, M.M., Ashammakhi, N., et al. Tissue engineering of bone in muscle by using free periosteal grafts with a self-reinforced polyglycolide membrane scaffold. An experimental study in growing rabbits. Eur J Plast Surg 23, 39, 2000. [Google Scholar]
- 383. Kawecki, F., Clafshenkel, W.P., Auger, F.A., et al. Self-assembled human osseous cell sheets as living biopapers for the laser-assisted bioprinting of human endothelial cells. Biofabrication 10, 035006, 2018. [DOI] [PubMed] [Google Scholar]
- 384. Cui, X., and Boland, T.. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 30, 6221, 2009. [DOI] [PubMed] [Google Scholar]
- 385. Tresoldi, C., Bianchi, E., Pellegata, A.F., Dubini, G., and Mantero, S.. Estimation of the physiological mechanical conditioning in vascular tissue engineering by a predictive fluid-structure interaction approach. Comput Methods Biomech Biomed Engin 20, 1077, 2017. [DOI] [PubMed] [Google Scholar]
- 386. Guilak, F., Butler, D.L., Goldstein, S.A., and Baaijens, F.P.T.. Biomechanics and mechanobiology in functional tissue engineering. J Biomech 47, 1933, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. McGovern, J.A., Griffin, M., and Hutmacher, D.W.. Animal models for bone tissue engineering and modelling disease. Dis Model Mech 11, dmm033084, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Boehler, R.M., Graham, J.G., and Shea, L.D.. Tissue engineering tools for modulation of the immune response. Biotechniques 51, 239, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Yuan, T., Li, K.F., Guo, L.K., Fan, H.S., and Zhang, X.D.. Modulation of immunological properties of allogeneic mesenchymal stem cells by collagen scaffolds in cartilage tissue engineering. J Biomed Mater Res A 98a, 332, 2011. [DOI] [PubMed] [Google Scholar]
- 390. Sittinger, M., Bujia, J., Rotter, N., Reitzel, D., Minuth, W.W., and Burmester, G.R.. Tissue engineering and autologous transplant formation: practical approaches with resorbable biomaterials and new cell culture techniques. Biomaterials 17, 237, 1996. [DOI] [PubMed] [Google Scholar]
- 391. Nikkola, L., Vapalahti, K., Huolman, R., Seppala, J., Harlin, A., and Ashammakhi, N.. Multilayer implant with triple drug releasing properties. J Biomed Nanotechnol 4, 331, 2008. [Google Scholar]
- 392. Piras AM CF, Chiellini, E., Nikkola, L., and Ashammakhi, N.. New multicomponent bioerodible electrospun nanofibers for dual-controlled drug release. J Bioact Compat Polym 23, 423, 2008. [Google Scholar]
- 393. Farah, S., Doloff, J.C., Müller, P., et al. Long-term implant fibrosis prevention in rodents and non-human primates using crystallized drug formulations. Nat Mater 18, 892, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Washington, K.S., and Bashur, C.A.. Delivery of antioxidant and anti-inflammatory agents for tissue engineered vascular grafts. Front Pharmacol 8, 659, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Dias, J.R., Ribeiro, N., Baptista-Silva, S., et al. In situ enabling approaches for tissue regeneration: current challenges and new developments. Front Bioeng Biotechnol 8, 85, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Sokolowska, P., Janikiewicz, J., Jastrzebska, E., Brzozka, Z., and Dobrzyn, A.. Combinations of regenerative medicine and Lab-on-a-chip systems: new hope to restoring the proper function of pancreatic islets in diabetes. Biosens Bioelectron 167, 112451, 2020. [DOI] [PubMed] [Google Scholar]
- 397. Langer, R., and Vacanti, J.. Advances in tissue engineering. J Pediatr Surg 51, 8, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Luo, J.S., Qin, L.F., Zhao, L.P., et al. Tissue-engineered vascular grafts with advanced mechanical strength from human iPSCs. Cell Stem Cell 26, 251, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Fragogeorgi, E.A., Rouchota, M., Georgiou, M., Velez, M., Bouziotis, P., and Loudos, G.. In vivo imaging techniques for bone tissue engineering. J Tissue Eng 10, 2041731419854586, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Kim, S.H., Park, J.H., Kwon, J.S., et al. NIR fluorescence for monitoring in vivo scaffold degradation along with stem cell tracking in bone tissue engineering. Biomaterials 258, 120267, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Xu, Y.Y., Wu, X.Y., Guo, X., et al. The boom in 3D-printed sensor technology. Sensors (Basel) 17, 1166, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Ashammakhi, N.H.A., Unluturk, B.D., Quintero, S.A., et al. Biodegradable implantable sensors: materials design, fabrication, and applications. Adv Funct Mater. 2021. DOI: 10.1002/adfm.202104149. [DOI] [Google Scholar]
- 403. Lee, A., Hudson, A.R., Shiwarski, D.J., et al. 3D bioprinting of collagen to rebuild components of the human heart. Science 365, 482, 2019. [DOI] [PubMed] [Google Scholar]
- 404. Pountos, I., Tellisi, N., and Ashammakhi, N.. Three-dimensional bioprinting: safety, ethical, and regulatory considerations. In: Guvendiren, M., ed. 3D Bioprinting in Medicine: Technologies, Bioinks, and Applications. Cham: Springer International Publishing, 2019, pp. 191–203. [Google Scholar]
- 405. Afshar, L. Ethical issues in perinatal tissue derivation and regenerative medicine. Stem Cells Biol Reg 229, 2016. [Google Scholar]
- 406. Mohammadi, D. The dangers of unregulated stem-cell marketing. Lancet 390, 1823, 2017. [DOI] [PubMed] [Google Scholar]
- 407. Seetapun, D., and Ross, J.J.. Eliminating the organ transplant waiting list: the future with perfusion decellularized organs. Surgery 161, 1474, 2017. [DOI] [PubMed] [Google Scholar]
- 408. Hollister, S.J., and Murphy, W.L.. Scaffold translation: barriers between concept and clinic. Tissue Eng Part B Rev 17, 459, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Boyce, S.T., and Lalley, A.L.. Tissue engineering of skin and regenerative medicine for wound care. Burns Trauma 6, 4, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Urciuolo, A., Poli, I., Brandolino, L., et al. Intravital three-dimensional bioprinting. Nat Biomed Eng 4, 901, 2020. [DOI] [PubMed] [Google Scholar]
- 411. Maffioletti, S.M., Sarcar, S., Henderson, A.B.H., et al. Three-dimensional human iPSC-derived artificial skeletal muscles model muscular dystrophies and enable multilineage tissue engineering. Cell Rep 23, 899, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Burrello, J., Monticone, S., Gai, C., Gomez, Y., Kholia, S., and Camussi, G.. Stem cell-derived extracellular vesicles and immune-modulation. Front Cell Dev Biol 4, 83, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Tellisi, N., Ashammakhi, N.A., Billi, F., and Kaarela, O.. Three dimensional printed bone implants in the clinic. J Craniofac Surg 29, 2363, 2018. [DOI] [PubMed] [Google Scholar]
- 414. Abelseth, E., Abelseth, L., De la Vega, L., Beyer, S.T., Wadsworth, S.J., and Willerth, S.M.. 3D printing of neural tissues derived from human induced pluripotent stem cells using a fibrin-based bioink. ACS Biomater Sci Eng 5, 234, 2019. [DOI] [PubMed] [Google Scholar]
- 415. Xu, C., Guan, S., Wang, S.P., et al. Biodegradable and electroconductive poly(3,4-ethylenedioxythiophene)/carboxymethyl chitosan hydrogels for neural tissue engineering. Mat Sci Eng C Mater 84, 32, 2018. [DOI] [PubMed] [Google Scholar]
- 416. Alizadeh, P.S.M., Tutar, R., Apu, E.H., Unluturk, B., Contag, C.H., and Ashammakhi, N.. Use of electroconductive biomaterials for engineering tissues by 3D printing and 3D bioprinting. Essays Biochem 65, 441, 2021. [DOI] [PubMed] [Google Scholar]
- 417. Ashammakhi, N., Darabi, M.A., and Pountos, I.. The dynamic cycle of future personalized and regenerative therapy. J Craniofac Surg 30, 623, 2019. [DOI] [PubMed] [Google Scholar]
- 418. Akyildiz, I.F., Chen, J., Ghovanloo, M., et al. Microbiome-gut-brain axis as a biomolecular communication network for the internet of bio-nanothings. IEEE Access 7, 136161, 2019. [Google Scholar]
- 419. Tian, C., Tu, Q., Liu, W.M., and Wang, J.Y.. Recent advances in microfluidic technologies for organ-on-a-chip. Trac-Trend Anal Chem 117, 146, 2019. [Google Scholar]
- 420. Ashammakhi, N., Darabi, M.A., Çelebi-Saltik, B., et al. Therapeutic nanomaterials: microphysiological systems: next generation systems for assessing toxicity and therapeutic effects of nanomaterials (small methods 1/2020). Small Methods 4, 2070001, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Zhang, K., Wang, S.P., Zhou, C.C., et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res 6, 31, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. GhavamiNejad, A., Li, J., Lu, B., et al. Glucose-responsive composite microneedle patch for hypoglycemia-triggered delivery of native glucagon. Adv Mater 31, e1901051, 2019. [DOI] [PubMed] [Google Scholar]
- 423. GhavamiNejad, A., Lu, B., Giacca, A., and Wu, X.Y.. Glucose regulation by modified boronic acid-sulfobetaine zwitterionic nanogels—a non-hormonal strategy for the potential treatment of hyperglycemia. Nanoscale 11, 10167, 2019. [DOI] [PubMed] [Google Scholar]
- 424. Nostro, M.C., and Keller, G.. Generation of beta cells from human pluripotent stem cells: potential for regenerative medicine. Semin Cell Dev Biol 23, 701, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425. Hoffman, T., Antovski, P., Tebon, P., et al. Synthetic biology and tissue engineering: toward fabrication of complex and smart cellular constructs. Adv Funct Mater 30, 1909882, 2020. [Google Scholar]
- 426. Diltemiz, S.E.T.M., de Barros, N.R., Heinamaki, J., Seidlits, S., and Ashammakhi, N. Use of artificial cells as drug carriers. Mater Chem Front 2021, DOI: 10.1039/d1qm00717c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427. Bradley, J.A., Bolton, E.M., and Pedersen, R.A.. Stem cell medicine encounters the immune system. Nat Rev Immunol 2, 859, 2002. [DOI] [PubMed] [Google Scholar]
- 428. Ibarretxe, G., Alvarez, A., Cañavate, M.L., Hilario, E., Aurrekoetxea, M., and Unda, F.. Cell reprogramming, IPS limitations, and overcoming strategies in dental bioengineering. Stem Cells Int 2012, 365932, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429. Khademhosseini, A., Langer, R., Borenstein, J., and Vacanti, J.P.. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A 103, 2480, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Tang, Z.L., Akiyama, Y., and Okano, T.. Temperature-responsive polymer modified surface for cell sheet engineering. Polymers (Basel) 4, 1478, 2012. [Google Scholar]
- 431. Uzel, S.G.M., Platt, R.J., Subramanian, V., et al. Microfluidic device for the formation of optically excitable, three-dimensional, compartmentalized motor units. Sci Adv 2, e1501429, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Hong, J., Yeo, M., Yang, G.H., and Kim, G.. Cell-electrospinning and its application for tissue engineering. Int J Mol Sci 20, 6208, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Vyas, C., Ates, G., Aslan, E., Hart, J., Huang, B., and Barto, P.. Three-dimensional printing and electrospinning dual-scale polycaprolactone scaffolds with low-density and oriented fibers to promote cell alignment. 3D Print Addit Manuf 7, 105, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]