Abstract
The response to the COVID-19 pandemic in the U.S prompted abrupt and dramatic changes to social contact patterns. Monitoring changing social behavior is essential to provide reliable input data for mechanistic models of infectious disease, which have been increasingly used to support public health policy to mitigate the impacts of the pandemic. While some studies have reported on changing contact patterns throughout the pandemic, few have reported differences in contact patterns among key demographic groups and none have reported nationally representative estimates. We conducted a national probability survey of US households and collected information on social contact patterns during two time periods: August-December 2020 (before widespread vaccine availability) and March-April 2021 (during national vaccine rollout). Overall, contact rates in Spring 2021 were similar to those in Fall 2020, with most contacts reported at work. Persons identifying as non-White, non-Black, non-Asian, and non-Hispanic reported high numbers of contacts relative to other racial and ethnic groups. Contact rates were highest in those reporting occupations in retail, hospitality and food service, and transportation. Those testing positive for SARS-CoV-2 antibodies reported a higher number of daily contacts than those who were seronegative. Our findings provide evidence for differences in social behavior among demographic groups, highlighting the profound disparities that have become the hallmark of the COVID-19 pandemic.
Keywords: COVID-19, SARS-CoV-2, Transmission, Mathematical models, Social contact, Health disparities
1. Introduction
The response to the COVID-19 pandemic in the U.S., including the closing of schools, workplaces, and businesses, prompted abrupt and dramatic changes to social contact patterns. In the first months of 2020, reducing social contact was the only measure available to ‘flatten the curve’ and blunt the severity of the pandemic. A synchronous, near-universal decline in contact rates occurred across countries in North America, Western Europe, and Asia in the Spring and Summer of 2020, with mean daily contacts dropping from 7 to 26 contacts pre-pandemic to 2–5 contacts per person in the early ‘lockdown’ period (Liu et al., 2021). Through Summer and Fall, as restrictions began to ease, contact patterns slowly rebounded. A key inflection point occurred in November 2020, when the first COVID-19 vaccines became available, signaling the start of a massive national vaccination campaign. Widespread vaccination reduced individuals’ risks of infection and led to declining case rates and hospitalizations, contributing to perceptions of reduced pandemic severity and leading to further relaxation of social distancing policies (Trends in Number of COVID-19 Cases and Deaths in the US Reported to CDC, 2021). Characterizing changing social contact patterns across this time period is critical to better understand behavioral drivers of the trajectory of the pandemic and inform ongoing efforts to estimate the impact of interventions. Systematic data collected in other countries has helped to explain the interplay between contact patterns and transmission dynamics (Zhang et al., 2020, Jarvis et al., 2020), but studies of contact patterns in the U.S. during this period draw primarily from convenience samples which survey unrepresentative segments of the population (Feehan and Mahmud, 2021, Kiti et al., 2021).
While some studies have reported on changing contact patterns throughout the COVID-19 pandemic in the U.S (Liu et al., 2021)., few have reported on differences in contact patterns among key demographic groups. This is particularly important since the burden of the COVID-19 pandemic has disproportionately fallen on low-income and minority populations, with a heavier burden of COVID-19 cases and deaths in low-income and minority populations (Escobar et al., 2021, Macias Gil et al., 2020, Yehia et al., 2020, Jung et al., 2021). To date, research has suggested that such disparities reflect limited capacity for these groups to markedly change their contact patterns due to social and structural factors (Chang et al., 2021, Selden and Berdahl, 2021, Roberts et al., 2020). Indeed, studies on neighborhood-level mobility support the notion that certain demographic groups were more likely to self-isolate than others during the early pandemic (Kissler et al., 2020a, Kishore et al., 2020). Disparities in the impact of COVID-19 persisted as vaccines became available. Inequities in vaccine access and uptake have resulted in suboptimal vaccination rates in many U.S. communities (COVID-19 Vaccination Equity, 2021, M Reitsma, S.A., Goldhaber-Fiebert, J., Joseph, N. , Kates, J. , Levitt, L. , Rouw, A. , Salomon, J. , 2021. Disparities in Reaching COVID-19 Vaccination Benchmarks: Projected Vaccination Rates by Race/Ethnicity as of July 4: Kaiser Family Foundation, 2021., Disparities in COVID-19 Vaccination Rates across Racial and Ethnic Minority Groups in the United States, 2021). A person’s vaccination status as well as the vaccination coverage in their broader community influences the real and perceived risk of infection, shaping differences in social contact patterns across demographic groups as the pandemic continues.
Data are needed on contact patterns over the period of the COVID-19 pandemic in the U.S.; ideally these data should be both nationally representative and investigate differences by demographic groups. Such data are critical to illustrate a national picture of contact patterns driving the changing epidemiology of COVID-19 and provide robust data on the behavioral patterns that may be perpetuating inequities in the impact of the pandemic. Specifically, unbiased estimates of contact will be useful in parameterizing mechanistic models of infectious disease transmission, informing underlying assumptions about the rate at which individuals come into contact with one another which are necessary to accurately simulate spread of infection and the potential impact of interventions. In particular, it is important to capture contact patterns in the period before widespread vaccine availability during which restrictions began to be relaxed, as well as after, in order to guide models aiming to understand the role of vaccines in altering population-level transmission dynamics. With infectious disease models increasingly informing both domestic and global policy, it is essential that the data underlying models maintains fidelity to rapidly changing contact patterns, which represents one of the key sources of uncertainty in COVID-19 transmission models.
We conducted a national probability survey of US households (The COVIDVu Study) with the primary aim to generate nationally representative estimates of the cumulative incidence of SARS-CoV-2 infection (Siegler et al., 2020, Lamba et al., 2021, Sullivan et al., 2021). As a part of this study, we collected information on social contact patterns during two time periods: August-December 2020 (before widespread vaccine availability) and March-April 2021 (during national vaccine rollout). We calculate nationally representative contact rates for the U.S. by demographic group and assess the relationship between contact rates and SARS-CoV-2 serostatus in both periods.
2. Methods
2.1. Sampling
Sampling methods for the COVIDVu study have been previously described (Siegler et al., 2020). Briefly, we used a national address-based household sample, which was chosen to be representative on the basis of age, gender, race/ethnicity, education level, household income, region of residence, and home ownership. To achieve a total sample of at least 4000 responding households, a total of 39,500 addresses were sampled. To ensure adequate participation of key groups, we oversampled households in census tracts with > 50 % Black residents and households with surnames likely to represent Hispanic ethnicity (Lavange et al., 2010). One household member aged ≥ 18 years was randomly selected and offered participation in the study. Baseline surveys were conducted from August-December 2020 and a follow-up survey was conducted in March – April 2021. The baseline period coincided with the relaxation of many pandemic restrictions that had lasted through the Summer of 2020 and the subsequent tightening of restrictions as wintertime case counts began to rise in November and December. The follow-up period coincided with the start of widespread COVID-19 vaccine availability and the fall of case counts after the wintertime surge (Supplemental Fig. 1). Fig. 1.
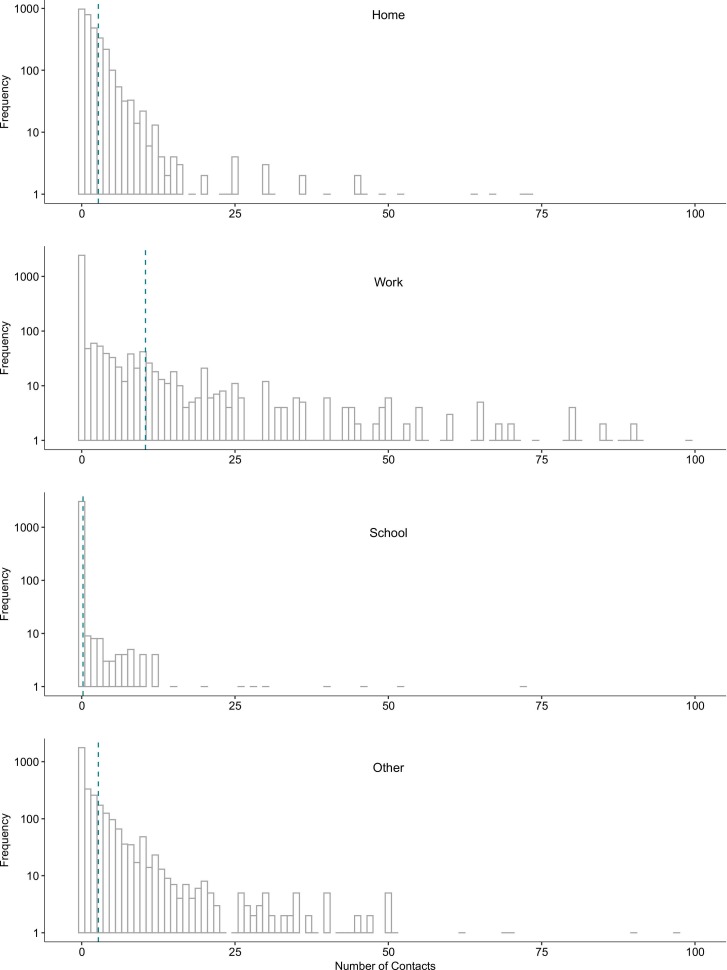
Fig. 1.
Frequency of reported contacts per day by location of contact for a probability sample of 3112 households representing the population aged ≥ 18 years at study baseline, United States, August-December 2020.
To adjust for non-response, we calculated sampling weights. Weights were used to estimate key parameters that represent the non-institutionalized, housed adult population in the U.S. The COVIDVu study was approved by the Emory University Institutional Review Board (STUDY00000695).
2.2. Survey and specimen collection
Consenting participants completed an online survey, which collected the age, sex, race, ethnicity, income, household size, and occupation of participants. We also included questions about person-to-person contacts on the previous day, which we adapted from previous, similar surveys (Kiti et al., 2021, Mossong et al., 2008, Gimma et al., 2021a). We defined a physical contact as any contact involving physical touch, such as a handshake, hug, or kiss and a non-physical contact by an interaction in which the participant was within 6 feet of the other person and exchanged three or more words. For each contact reported in the survey, participants were asked to record the age of the contact and the location where contact occurred (home, work, school, or other). Occupation categories were adapted from those used in the 2018 American Community Survey and based on the North American Industry Classification System (Industry and Occupation Code Lists and Crosswalks, 2021).
Participants self-collected and returned a dried blood spot (DBS) specimen to a central laboratory via prepaid mailer. DBS specimens were tested using the BioRad Platelia Total Antibody test that targets the nucleocapsid protein (i.e., IgA, IgM, IgG; BioRad, Hercules, California) (Sullivan et al., 2020, Guest et al., 2020).
2.3. Statistical analysis
We restricted our analysis to participants who completed the baseline and follow-up surveys and returned DBS specimens from each period with valid total Ig result. Where missing, we imputed responses for all variables involved in calculation of weights, including age, gender, race/ethnicity, and income (Siegler et al., 2020, Andridge and Little, 2010). Using the survey weights, we calculated the mean and median daily number of contacts reported in each time period, overall and by age, sex, location of contact, race/ethnicity, income, occupation, and SARS-CoV-2 serostatus. We performed two-sample t-tests and Kolmogorov-Smirnov tests for the difference in contacts reported at baseline and follow-up, which assess differences in the mean and overall distribution, respectively. We assessed the relationship between number of contacts and serostatus adjusting for age and race/ethnicity, variables which we have previously found to be associated with serostatus (Sullivan et al., 2021). For comparability with analyses of social contact from other countries, we truncated data at 50 contacts per day per contact age group to report total contacts (Jarvis et al., 2020), though we explored other cutoffs to assess how the impact on our findings. All analysis was conducted in R version 3.5.3 and all code is available on GitHub at https://github.com/kbratnelson/covidvu-socialcontact. A dataset including contact variables and key sociodemographic variables, as well as survey weights, is also available on GitHub.
3. Results
39,500 registration materials and kits were mailed to sampled US households. 4654 (12.6 % of sampled households) returned a baseline survey and a DBS specimen with a valid total Ig result during the period August 9-December 8, 2020. Of these, 3112 (66.9 % of participants in the baseline survey) returned a follow-up survey and a DBS specimen with a valid total Ig result during the period March 2-April 18, 2021.
Overall, the mean number of daily contacts was 13.9 (IQR: 2, 10) at baseline and 14.5 (IQR: 2, 11) at follow-up (p of two-sample t-test = 0.552). ( Table 1) Physical contacts increased slightly from 5.9 (IQR: 1, 4) to 7.8 (IQR: 0, 5) contacts (p = 0.322), and non-physical contacts stayed similar (10.2 (IQR: 0, 5) at baseline and 9.6 (IQR: 0, 6) at follow-up, p = 0.722). Most contacts in both survey periods were reported at work (65 % at baseline and 54 % at follow-up), with fewer contacts reported at home (17 % at baseline and 21 % at follow-up) and other locations (17 % at baseline and 22 % at follow-up); the fewest contacts were reported at school (1 % at baseline and 3 % at follow-up). (Fig. 1) (Of note, nearly all participants were adults, so contacts at school were not expected to be common.) While the distribution of contacts in all locations was skewed, this skew was particularly apparent among workplace contacts (90th percentile of the distribution: 16 and 15 for baseline and follow-up, respectively). Contacts reported in each location are truncated at 100 to display the lower end of the distribution for each plot. Vertical dotted line shows the mean for each location (as reported in Table 1).
Table 1.
Mean, median, and quantiles of reported contacts per day by contact location, type, demographic group, and serostatus for a probability sample of 3112 households representing the population aged ≥ 18 years, United States, 2020–2021.
| Baseline |
Follow-up |
Sample |
Weighted Sample |
Statistical tests ** |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Mean contacts | Median contacts | (25th, 75th, 90th percentiles) | Mean contacts | Median contacts | (25th, 75th, 90th percentiles) | N | % (column) | N | % (column) | T-test p-value *** | K-S test p-value *** |
| Overall | 13.9 | 4.0 | 2.0, 10.0, 27.0 | 14.5 | 5.0 | 2.0, 11.0, 26.0 | 3112 | 100 | 252117112 | 100 | 0.552 | 0.180 |
| Nature of contact | ||||||||||||
| Physical | 5.9 | 4.0 | 1.0, 4.0, 10.0 | 7.8 | 2.0 | 0.0, 5.0, 10.0 | – | – | – | – | 0.322 | 0.654 |
| Non-physical | 10.2 | 2.0 | 0.0, 5.0, 16.0 | 9.6 | 2.0 | 0.0, 6.0, 15.0 | – | – | – | – | 0.722 | 0.685 |
| Contact Location | ||||||||||||
| Home | 2.7 | 1.0 | 0.0, 3.0, 5.0 | 3.7 | 2.0 | 1.0, 3.0, 5.0 | – | – | – | – | 0.410 | 0.596 |
| Work | 10.4 | 0.0 | 0.0, 1.0, 16.0 | 9.4 | 0.0 | 0.0, 2.0, 15.0 | – | – | – | – | 0.568 | 0.993 |
| School | 0.2 | 0.0 | 0.0, 0.0, 0.0 | 0.6 | 0.0 | 0.0, 0.0, 0.0 | – | – | – | – | 0.243 | 1.000 |
| Other | 2.7 | 0.0 | 0.0, 2.0, 6.0 | 3.8 | 0.0 | 0.0, 2.8, 7.0 | – | – | – | – | 0.674 | 1.000 |
| Age category | ||||||||||||
| 18–24 | 20.9 | 5.0 | 3, 20, 60 | 17.7 | 7.0 | 3, 16, 62 | 169 | 5.4 | 24823133 | 9.8 | 0.461 | 0.768 |
| 25–34 | 16 | 6.0 | 3, 13, 35 | 23.3 | 7.0 | 3, 14, 40 | 448 | 14.4 | 42037821 | 16.7 | 0.030 | 1.000 |
| 35–44 | 18.3 | 5.0 | 3, 13, 35 | 14.5 | 6.0 | 3, 14, 23 | 503 | 16.2 | 43010914 | 17.1 | 0.097 | 1.000 |
| 45–54 | 17.7 | 5.0 | 2, 11, 32 | 16.8 | 5.0 | 2, 12, 29 | 509 | 16.4 | 41649386 | 16.5 | 0.796 | 1.000 |
| 55–64 | 10.7 | 3.0 | 1, 7, 26 | 12.7 | 4.0 | 1, 10, 20 | 649 | 20.9 | 44192201 | 17.5 | 0.283 | 0.999 |
| 65 + | 5.5 | 2.0 | 1, 5, 10 | 6.0 | 2.0 | 1, 6, 12 | 834 | 26.8 | 56403657 | 22.4 | 0.592 | 1.000 |
| Sex | ||||||||||||
| Female | 12.5 | 4.0 | 2, 9, 26 | 11.3 | 5.0 | 2, 10, 20 | 1907 | 61.3 | 115127131 | 45.7 | 0.158 | 0.576 |
| 15 | 4.0 | 2, 10, 32 | 17.2 | 5.0 | 2, 12, 32 | 1205 | 38.7 | 136989980 | 54.3 | 0.251 | 1.000 | |
| Race/Ethnicity | ||||||||||||
| Hispanic | 11.8 | 4.0 | 2, 12, 34 | 14.8 | 6.0 | 2, 13, 31 | 388 | 12.5 | 41498755 | 16.5 | 0.242 | 0.931 |
| Non-Hispanic White | 14.3 | 4.0 | 2, 9, 26 | 14.0 | 5.0 | 2, 11, 23 | 2118 | 68.1 | 161730015 | 64.1 | 0.796 | 1.000 |
| Non-Hispanic Black | 16.1 | 3.0 | 1, 10, 40 | 15.5 | 3.0 | 1, 9, 42 | 409 | 13.1 | 26371146 | 10.5 | 0.846 | 1.000 |
| Non-Hispanic Asian | 6.8 | 3.0 | 2, 7, 19 | 11.3 | 5.0 | 2, 9, 26 | 145 | 4.7 | 16297586 | 6.5 | 0.019 | 0.626 |
| Non-Hispanic Other | 25.5 | 6.0 | 1, 13, 40 | 28.2 | 6.0 | 2, 21, 208 | 52 | 1.7 | 6219608 | 2.5 | 0.838 | 0.999 |
| Income | ||||||||||||
| $0 to $9,999 | 9.9 | 4.0 | 2, 6, 23 | 14.1 | 5.0 | 1, 10, 54 | 156 | 5.0 | 9175348 | 3.6 | 0.235 | 0.624 |
| $10,000 to $24,999 | 28.9 | 4.0 | 1, 9, 71 | 19.2 | 4.0 | 1, 10, 31 | 282 | 9.1 | 21014175 | 8.3 | 0.102 | 1.000 |
| $25,000 to $49,999 | 16.7 | 4.0 | 1, 12, 39 | 24.5 | 5.0 | 2, 14, 43 | 594 | 19.1 | 42517322 | 16.9 | 0.020 | 0.998 |
| $50,000 to $74,999 | 9.8 | 4.0 | 2, 9, 21 | 12.1 | 5.0 | 2, 11, 20 | 540 | 17.4 | 40975725 | 16.3 | 0.214 | 1.000 |
| $75,000 to $99,999 | 14 | 4.0 | 2, 10, 35 | 12.9 | 4.0 | 2, 9, 24 | 454 | 14.6 | 34753959 | 13.8 | 0.614 | 1.000 |
| $100,000 to $149,999 | 12.9 | 4.0 | 2, 10, 32 | 9.2 | 4.0 | 2, 9, 17 | 534 | 17.2 | 44554817 | 17.7 | 0.009 | 1.000 |
| $150,000 to $199,999 | 12.2 | 4.0 | 2, 9, 26 | 11.8 | 4.0 | 2, 11, 30 | 246 | 7.9 | 25854439 | 10.3 | 0.871 | 1.000 |
| $200,000 or higher | 9.5 | 4.0 | 2, 9, 22 | 12.4 | 6.0 | 2, 12, 22 | 306 | 9.8 | 33271326 | 13.2 | 0.150 | 0.943 |
| Occupation* | ||||||||||||
| Utilities | 1.3 | 0.0 | 0, 0, 4 | 10.1 | 0.0 | 0, 0, 75 | 16 | 0.5 | 1624138 | 0.6 | 0.205 | 1.000 |
| Construction | 8.5 | 5.0 | 0, 16, 26 | 5.9 | 3.0 | 1, 10, 15 | 50 | 1.6 | 7468477 | 3.0 | 0.166 | 0.905 |
| Manufacturing | 4.4 | 2.0 | 0, 8, 15 | 12.3 | 0.0 | 0, 6, 30 | 50 | 1.6 | 8095010 | 3.2 | 0.085 | 0.982 |
| Retail Trade | 92 | 12.0 | 0, 65, 171 | 72.1 | 4.0 | 0, 30, 142 | 84 | 2.7 | 12398395 | 4.9 | 0.638 | 0.690 |
| Transportation and Warehousing | 24.3 | 6.0 | 0, 26, 85 | 3.2 | 0.0 | 0, 4, 10 | 57 | 1.8 | 4028061 | 1.6 | 0.000 | 0.446 |
| Information | 0.8 | 0.0 | 0, 0, 2 | 0.8 | 0.0 | 0, 0, 4 | 55 | 1.8 | 4702720 | 1.9 | 0.926 | 1.000 |
| Finance and Insurance | 1.7 | 0.0 | 0, 0, 10 | 2.2 | 0.0 | 0, 0, 6 | 105 | 3.4 | 9429022 | 3.7 | 0.520 | 1.000 |
| Real Estate and Rental and Leasing | 1.5 | 0.0 | 0, 3, 8 | 1.9 | 0.0 | 0, 5, 6 | 29 | 0.9 | 2407083 | 1.0 | 0.635 | 1.000 |
| Professional, Scientific, and Technical Services | 1.5 | 0.0 | 0, 0, 5 | 2.1 | 0.0 | 0, 0, 6 | 254 | 8.2 | 17627427 | 7.0 | 0.327 | 1.000 |
| Management of Companies and Enterprises | 4 | 0.0 | 0, 0, 26 | 8.0 | 0.0 | 0, 0, 30 | 29 | 0.9 | 2279303 | 0.9 | 0.672 | 1.000 |
| Administrative Services | 4 | 0.0 | 0, 3, 15 | 2.1 | 0.0 | 0, 2, 7 | 25 | 0.8 | 1804659 | 0.7 | 0.380 | 1.000 |
| Educational Services | 8.5 | 0.0 | 0, 10, 20 | 12.2 | 0.0 | 0, 11, 30 | 227 | 7.3 | 11316238 | 4.5 | 0.160 | 1.000 |
| Health Care and Social Assistance | 16.4 | 2.0 | 0, 17, 46 | 10.0 | 2.0 | 0, 12, 29 | 313 | 10.1 | 24401663 | 9.7 | 0.003 | 1.000 |
| Arts, Entertainment, and Recreation | 4.7 | 0.0 | 0, 0, 10 | 3.6 | 0.0 | 0, 0, 5 | 50 | 1.6 | 3116292 | 1.2 | 0.717 | 1.000 |
| Accommodation and Food Services | 29.3 | 0.0 | 0, 10, 49 | 18.1 | 6.0 | 0, 26, 90 | 55 | 1.8 | 7428078 | 2.9 | 0.943 | 1.000 |
| Other Services (except Public Administration) | 10.4 | 0.0 | 0, 5, 10 | 4.2 | 0.0 | 0, 6, 13 | 75 | 2.4 | 6013537 | 2.4 | 0.292 | 1.000 |
| Public Administration | 3.7 | 0.0 | 0, 7, 12 | 2.3 | 0.0 | 0, 2, 9 | 37 | 1.2 | 2938101 | 1.2 | 0.326 | 1.000 |
| Other | 12.7 | 0.0 | 0, 3, 10 | 14.9 | 0.0 | 0, 5, 17 | 250 | 8.0 | 19646552 | 7.8 | 0.731 | 1.000 |
| Household size | ||||||||||||
| 1 | 6.8 | 2.0 | 0, 5, 12 | 8.7 | 2.0 | 0, 8, 19 | 850 | 27.3 | 40909934 | 16.2 | 0.104 | 0.956 |
| 2 | 11.5 | 3.0 | 1, 8, 23 | 12.0 | 3.0 | 1, 8, 21 | 1228 | 39.5 | 104424631 | 41.4 | 0.722 | 1.000 |
| 3 | 14.9 | 5.0 | 2, 12, 32 | 14.4 | 7.0 | 3, 14, 28 | 448 | 14.4 | 42787020 | 17.0 | 0.817 | 0.958 |
| 4 | 20.3 | 6.0 | 3, 14, 42 | 18.2 | 7.0 | 3, 14, 28 | 383 | 12.3 | 39212704 | 15.6 | 0.466 | 0.999 |
| 5 + | 23.6 | 7.0 | 4, 21, 40 | 28.7 | 7.0 | 3, 14, 39 | 203 | 6.5 | 24782822 | 9.8 | 0.464 | 1.000 |
| ` | ||||||||||||
| Serostatus at baseline (August-December 2020) | ||||||||||||
| Positive | 16.6 | 6.0 | 3, 36, 44 | – | – | 138 | 0.0 | 12316038 | 4.9 | – | – | |
| Negative | 13.3 | 4.0 | 2, 9, 26 | – | – | 2974 | 1.0 | 239801073 | 95.1 | – | – | |
| Serostatus at follow-up (March-April 2021) | ||||||||||||
| Positive | – | – | 19.6 | 8.0 | 3, 14, 31 | 350 | 0.1 | 31360651 | 12.4 | – | – | |
| Negative | – | – | 13.7 | 4.0 | 2, 10, 25 | 2762 | 0.9 | 220756460 | 87.6 | – | – | |
* work, not total, contacts
** Statistical tests performed on weighted data
*** The t-test is sensitive to differences in means of the distributions; the K-S test is sensitive to shape of the distribution.
N, total participants. Weighted N: sum of the weights of participants. K-S: Kolmogorov-Smirnov.
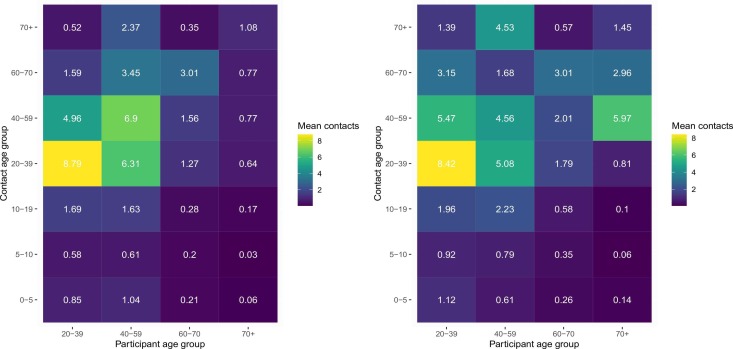
Trends in contact varied by age group ( Fig. 2, Fig. 3). The number of contacts did not substantially change among 18–24 year-olds (p = 0.461), 35–44 year-olds (p = 0.097), and 45–54 year-olds (p = 0.796) from baseline to follow-up; comparatively, contact increased among 25–34 year-olds (mean of 16 contacts at baseline and 23.3 contacts at follow-up, p = 0.030). Contact rates among the elderly (65 +) were the lowest of all age groups and remained similar from baseline to follow-up (mean of 5.5 and 6.0 contacts at baseline and follow-up, respectively, p = 0.592). The number of contacts among men were generally higher than among women and were similar from baseline to follow-up (15.0–17.2, p = 0.251), contacts reported among women were also similar between the two surveys (mean of 12.5 and 11.3 at baseline and follow-up, respectively, p = 0.158).
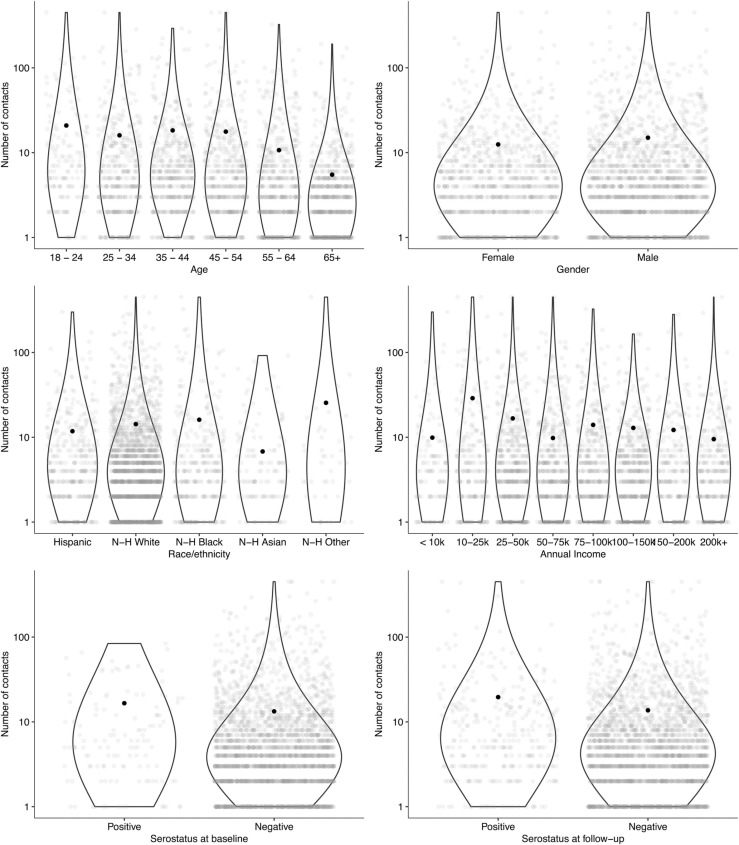
Fig. 2.
Mean reported contacts per day by demographic group and serostatus for a probability sample of 3112 households representing the population aged ≥ 18 years at study baseline, United States, August-December 2020.
Fig. 3.
Mean contact rates per day by age group for a probability sample of 3112 households representing the population aged ≥ 18 years at study baseline (left) and follow-up (right), United States, August 2020-April 2021.
Contact patterns were distinct across socioeconomic and racial groups in both surveys (Fig. 2), but showed little change from baseline to follow-up. Those identifying as Asian reported the lowest contact rates (6.8 and 11.3 mean contacts at baseline and follow-up, respectively, p = 0.019), and those who identified as non-White, non-Black, non-Asian, and non-Hispanic (‘Other’) reported the highest contact rates (baseline mean: 25.5, follow-up mean 28.2, p = 0.838). Number of reported contacts was highest among those reporting annual incomes from $10,000-$49,999. The number of contacts reported at work varied substantially by industry, with jobs in retail (mean 92 contacts), accommodation and food service (mean 29 contacts at baseline), transportation (mean 24 contacts at baseline), healthcare (mean 16 contacts at baseline), manufacturing (mean 12.3 contacts at follow-up), and education (mean 12 contacts at follow-up) accounting for the highest levels of contact.
Participants with a positive serostatus had slightly higher number of contacts than those with a negative serostatus in both periods (16.6 compared to 13.3 contacts at baseline and 19.6 compared to 13.7 contacts at follow-up). (Fig. 2) Those who were seronegative at both surveys reported a lower mean number of contacts than those who were seropositive at either survey (13.4 and 16.8 contacts at baseline and follow-up, respectively). Those who seroconverted from baseline to follow-up reported a higher number of contacts at both surveys (17.0 and 18.2 contacts) than those who did not (13.6 and 14.1 contacts). Number of contacts reported was not associated with serostatus at either survey after adjusting for age group and race/ethnicity.
Violin plots show the distribution of the non-zero numbers of contacts reported by group and grey dots indicate each individual data point. Black dots show the mean for each group (as reported in Table 1).
We chose to truncate contacts at 50 per day per contact age group (9 age groups x 50 contacts = total of 450 contacts) to enable comparison with other pandemic-focused studies of contact. Moreover, we reasoned that the nature of some occupations could lead to several hundred contacts a day. Very few participants reported above the 50 per day per group limit of contacts (5, or 0.2 % of participants, at baseline and 10, or 0.3 % of participants, at follow-up). (Supplemental Table 1) When we used a truncation limit of 29 total contacts per day, consistent with other previous studies of contact (Mossong et al., 2008), we found qualitatively similar results to those reported (mean 7.7 contacts (IQR: 2, 10)) at baseline to 8.1 contacts (IQR: 2, 11) at follow-up. A moderate number of participants reported more than 29 contacts (220 participants (7 %) at baseline and 249 participants (8 %) at follow-up). In general, those reporting a very high number of contacts reported them at work or at ‘other’ locations.
4. Discussion
Overall, national contact rates in Spring 2021 were similar to those in Fall 2020, with most contacts in both surveys reported at work. The number of contacts reported was not uniform across groups, with those identifying as non-White, non-Black, non-Asian, (‘Other’ race) and non-Hispanic reporting high rates of contact relative to other racial and ethnic groups. Contact rates were highest among those with lower incomes and in specific occupational categories, including retail, hospitality and food service, and transportation. While the number of contacts reported were mostly similar from baseline to follow-up, younger adults (aged 25–34) reported higher numbers of contacts at follow-up as compared to baseline. Finally, we found that those testing positive for SARS-CoV-2 antibodies reported a higher number of daily contacts than those who were seronegative. Collectively, these findings provide robust empirical evidence for differences in social behavior among demographic groups, highlighting the profound disparities that have become the hallmark of the COVID-19 pandemic.
The ‘opening up’ of much of the U.S. in mid-2020 saw the lifting of restrictive social distancing measures put into place across much of the country at the beginning of the epidemic. Social contact reached a nadir in Spring 2020, began to rebound by Fall 2020, and according to our findings stayed largely consistent through Spring 2021. The consistency of overall contact rates across these two periods is ostensibly the result of a complex interplay of factors. The expiration of many local mitigation policies in late Summer 2020 (Raifman et al., 2020) likely prompted increases in contact, before the severe wintertime ‘wave’ arrived in November caused contact rates to decline once again. Optimism about the introduction of vaccines in early 2021 gradually began to counterbalance the caution prompted by rising case counts in winter (Trends in Number of COVID-19 Cases and Deaths in the US Reported to CDC, 2021). Together, these factors likely led to largely consistent social contact rates across the two survey periods.
Although pre-pandemic data on social contact is limited, available data suggest that contact rates may have rebounded to closeto pre-pandemic levels, estimated at 12–14 contacts per day for the average adult (Prem et al., 2017, Feehan and Cobb, 2019). Comparisons with pre-pandemic contact data are not straightforward, given that previous studies have use different methods to collect contact data and no previous, nationally representative surveys such as this one have been performed (Hoang et al., 2019). However, we suspect that while overall numbers of contacts may have rebounded, the nature of contact (i.e., physical vs. non-physical, masked vs. unmasked) has changed and, moreover, that these changes occurred unevenly among demographic groups. Of note, this return to pre-pandemic contact levels is in contrast to data from European countries, which has shown that as of early 2021 (UK) and mid-2020 (Belgium), contact rates had yet to return to pre-pandemic levels (Gimma A., Munday J.D., Wong K.L., et al., 2021a . CoMix: Changes in social contacts as measured by the contact survey during the COVID-19 pandemic in England between March 2020 and March 2021. medRxiv 2021:2021b.05.28.21257973., Coletti et al., 2020).
Our finding that social contact rates varied by demographic group in the U.S. has been shown in smaller, less broadly representative studies (Feehan and Mahmud, 2021) We found high contact rates among the youngest age group, 18–24 year-olds, consistent with other data showing that this group typically reported lowest prevalence of mitigation behaviors (Coroiu et al., 2020, Ingram et al., 2021, Hutchins BW et al., 2020). We also found that adults 24–35 increased their contact rates the most from baseline to follow-up, which may reflect the opening of schools and workplaces over this period, and later, increased contacts after becoming partially or fully vaccinated. Minority populations and those with lower incomes reported high mean rates of contact. Together with the fact that the majority of contacts at both baseline and follow-up were reported in the workplace, this is consistent with reports that lower-income and minority groups were more likely to be classified as ‘essential’ workers, a designation which may have required them to continue working in-person or to return to work shortly after the most strict social distancing restrictions were lifted and prior to our baseline survey (Roberts et al., 2020). At least some of this disparity in contact rates is likely due to inflexible and unstable employment situations that are less likely to allow for remote work. Differential contact rates by job type were expected but striking in magnitude. We found contact rates up to ten times the population average in occupations which involve working environments that place employees in close contact with many other people, such as retail, transportation, and food processing. Notably, workplaces in general are an important source of infection and transmission risk for respiratory infections (Edwards et al., 2016, Lan et al., 2020, Murti et al., 2021) and the industries associated with high contact rates in our survey were the settings of several highly publicized outbreaks in 2020 (Waltenburg TV and Rose, 2020, Contreras et al., 2021).
Finally, we found that seropositive individuals were more likely to report higher contact rates at both survey rounds. Many studies have reported on risk factors for infection related to social environment, which have included poverty and crowding (Saloner et al., 2020, Ahmad et al., 2020, Emeruwa et al., 2020). We coupled behavioral information with serological status in a nationally representative sample to show that higher rates of contact are indeed linked with previous infection status. However, we did not find that contact rate was associated with serostatus after adjusting for age and race/ethnicity, suggesting a more central role for demographic factors over purely behavioral metrics, such as contact rate. Linking information on mitigation behaviors and serostatus will be important to understand epidemic dynamics as population-level immunity increases. These dynamics are predicted to be characterized by highly localized outbreaks in space and time driven by ‘pockets’ of susceptible persons (Kissler et al., 2020b, Baker et al., 2020).
The availability of nationally representative data on contact patterns by demographic group fills an important gap in efforts to accurately forecast and explain the trajectory of the COVID-19 pandemic. Traditionally, infectious disease models have rarely incorporated social factors, though they are instrumental in determining disease risk. This is partially because of limited availability of data to properly formulate models that track many population groups, but also because average population models are often favored for their simplicity and greater ease of computation. However, accounting for exposure differences between groups is a critical component of accurately modeling disease dynamics by population segment. To date, most models that have aimed to explain differences in the burden of cases and deaths by demographic group have used proxies of contact, such as mobility measured by mobile phone location data, to represent the frequency of person-person interactions rather than data on direct interactions. However, recent work has shown that during ‘lockdowns’, mobility was likely to be a poor indicator of contact patterns, calling into question the validity of this assumption (Buckee et al., 2021). Data on contact patterns by race, ethnicity, socioeconomic group, and occupation provides the necessary granularity to investigate disease dynamics driving sociodemographic inequities in disease burden. Indeed, modeling studies that have investigated these questions have noted the lack of longitudinal social contact surveys that would provide data to allow proper parameterization of models (Ma et al., 2021). Systematically collected data that is finely stratified by demographic group has to date been a major barrier to COVID-19 research efforts (Krieger et al., 2020). Collecting and making this data available to the modeling community can support the development of more accurate models to inform public health policymaking on continued use of vaccines as well as non-pharmaceutical interventions. As vaccine rollout continues and transmission becomes increasingly concentrated among undervaccinated populations, understanding changing behaviors will be critical to track trends in transmission over time and to understand the potential utility of targeted interventions to mitigate population-level risk.
There were several limitations to this study. First, our response rates were low (<15 %) but standard for address-based surveys. For certain groups at risk of being underrepresented by our initial survey responses, we intentionally oversampled to ensure that such groups were adequately represented, and survey weights were used to help ensure the sample represented the underlying population. Second, contact patterns may have changed over the period spanned by our survey (four months in the baseline survey, one month in the follow-up). In addition, our surveys were conducted before and after the winter holidays, which likely saw transient increases in travel and contact rates; we could not capture these changes. Third, differences in contact patterns on the state and local levels are likely to be significant due to variation in the nature of and adherence to distancing and other mitigation policies as well as differences in the speed of vaccine rollout and eligibility criteria. The national focus of our survey limits the inferences we can make about contact patterns at a finer geographic scale. With respect to the contact survey, we asked participants to record contacts from one day (the day prior to completing the survey) and we do not know to what extent this day might represent general patterns of contact. Moreover, this does not allow us to assess whether reported contacts were unique on that day, or repeated across days. Generally, a higher number of unique contacts would represent higher infection and transmission risk. Fourth, we do not have information on mitigation measures relevant for each contact, which impact the likelihood of transmitting infection during an interaction. Lastly, the age range of the surveyed population limits the generalizability of these results to only adults, as there were not a sufficient number of participants under 18 to make conclusions about contact rates among younger people. This also limits our view into school-based contact, which is potentially an important setting of transmission.
As the pandemic continues, continued collection of data on behavioural indicators by key demographic groups can support the development of detailed COVID-19 models that accurately represent disease dynamics in varied sociodemographic contexts. Continued, systematic collection and analysis of social contact data is critical to understand the past and future trajectory of the epidemic as well as explain differences in disease burden by population segment and through time. Longitudinal social contact data is particularly powerful alongside seropositivity estimates that are finely stratified by demographic group, and can further support efforts to properly fit dynamic models aiming assess interventions to mitigate the continuing impacts of the pandemic.
Funding source
All were funded by the COVIDVu study, National Institutes of Health (NIH) NIAID 3R01AI143875–02S1, the Woodruff Foundation, and the California Department of Public Health. KNN and BAL were funded by GlobalMix NIH NICHD R01 HD097175 and CorporateMix CDC U01CK000572.
CRediT authorship contribution statement
KNN conceptualized the study, conducted data analysis, and write the original draft, EH and NL curated and analyzed data, PH was responsible for project administration, AJS, PSS, HB, TS, and BAL designed the larger COVIDVu study and acquired funding, all authors reviewed and edited the manuscript.
Declarations of interest
No conflicts to report.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.epidem.2022.100605.
Appendix A. Supplementary material
Supplementary material
.
References
- Ahmad K., Erqou S., Shah N., et al. Association of poor housing conditions with COVID-19 incidence and mortality across US counties. PLOS ONE. 2020;15 doi: 10.1371/journal.pone.0241327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andridge R.R., Little R.J.A. A review of hot deck imputation for survey non-response. Int Stat. Rev. 2010;78:40–64. doi: 10.1111/j.1751-5823.2010.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R.E., Park S.W., Yang W., Vecchi G.A., Metcalf C.J.E., Grenfell B.T. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc. Natl. Acad. Sci. 2020;117:30547–30553. doi: 10.1073/pnas.2013182117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckee C., Noor A., Sattenspiel L. Thinking clearly about social aspects of infectious disease transmission. Nature. 2021;595:205–213. doi: 10.1038/s41586-021-03694-x. [DOI] [PubMed] [Google Scholar]
- Chang S., Pierson E., Koh P.W. Mobility network models of COVID-19 explain inequities and inform reopening. Nature. 2021;589:82–87. doi: 10.1038/s41586-020-2923-3. [DOI] [PubMed] [Google Scholar]
- Coletti P., Wambua J., Gimma A. CoMix: comparing mixing patterns in the Belgian population during and after lockdown. Sci. Rep. 2020;10:21885. doi: 10.1038/s41598-020-78540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras Z., Ngo V., Pulido M. Industry sectors highly affected by worksite outbreaks of coronavirus disease, Los Angeles County, California, USA, March 19-September 30, 2020. Emerg. Infect. Dis. 2021;27:1769–1775. doi: 10.3201/eid2707.210425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coroiu A., Moran C., Campbell T., Geller A.C. Barriers and facilitators of adherence to social distancing recommendations during COVID-19 among a large international sample of adults. PLOS ONE. 2020;15 doi: 10.1371/journal.pone.0239795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 Vaccination Equity. Accessed July 29 2021.
- Disparities in COVID-19 Vaccination Rates across Racial and Ethnic Minority Groups in the United States. Washington, D.C.: U.s. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation, Office of Science and Data Policy, 2021.
- Edwards C.H., Tomba G.S., de Blasio B.F. Influenza in workplaces: transmission, workers’ adherence to sick leave advice and European sick leave recommendations. Eur. J. Public Health. 2016;26:478–485. doi: 10.1093/eurpub/ckw031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeruwa U.N., Ona S., Shaman J.L. Associations between built environment, neighborhood socioeconomic status, and SARS-COV-2 infection among pregnant women in New York City. JAMA. 2020;324:390–392. doi: 10.1001/jama.2020.11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar G.J., Adams A.S., Liu V.X., et al. Racial disparities in COVID-19 testing and outcomes: retrospective cohort study in an integrated health system. Ann. Intern Med. 2021;174:786–793. doi: 10.7326/M20-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feehan D.M., Cobb C. Using an online sample to estimate the size of an offline population. Demography. 2019;56:2377–2392. doi: 10.1007/s13524-019-00840-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feehan D.M., Mahmud A.S. Quantifying population contact patterns in the United States during the COVID-19 pandemic. Nat. Commun. 2021;12:893. doi: 10.1038/s41467-021-20990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimma A., Munday J.D., Wong K.L., et al., 2021a . CoMix: Changes in social contacts as measured by the contact survey during the COVID-19 pandemic in England between March 2020 and March 2021. medRxiv 2021:2021b.05.28.21257973. [DOI] [PMC free article] [PubMed]
- Gimma A., Munday J.D., Wong K.L., et al., 2021b . CoMix: Changes in social contacts as measured by the contact survey during the COVID-19 pandemic in England between March 2020 and March 2021. medRxiv 2021a:2021.05.28.21257973. [DOI] [PMC free article] [PubMed]
- Guest J.L., Sullivan P.S., Valentine-Graves M. Suitability and sufficiency of telehealth clinician-observed, participant-collected samples for SARS-CoV-2 Testing: The iCollect cohort pilot study. JMIR Public Health Surveill. 2020;6 doi: 10.2196/19731. e19731-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T., Coletti P., Melegaro A. A systematic review of social contact surveys to inform transmission models of close-contact infections. Epidemiology. 2019;30:723–736. doi: 10.1097/EDE.0000000000001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins BW H.J., Leeb R., Ko J.Y., Odom E., Willey J., Friedman A., Bitsko R. Vol. 69. Centers for Disease Control and Prevention; Atlanta, GA: 2020. COVID-19 Mitigation Behaviors by Age Group - United States, April–June 2020; pp. 1584–1590. (MMWR Morb Mortal Wkly Rep). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Industry and Occupation Code Lists and Crosswalks. Available at: 〈https://www.census.gov/topics/employment/industry-occupation/guidance/code-lists.html〉. 2021.
- Ingram M., Zahabian A., Hur C. Prediction of COVID-19 social distancing adherence (SoDA) on the United States county-level. Humanit. Soc. Sci. Commun. 2021;8:87. [Google Scholar]
- Jarvis C.I., Van Zandvoort K., Gimma A. Quantifying the impact of physical distance measures on the transmission of COVID-19 in the UK. BMC Med. 2020;18:124. doi: 10.1186/s12916-020-01597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J., Manley J., Shrestha V. Coronavirus infections and deaths by poverty status: The effects of social distancing. J. Econ. Behav. Organ. 2021;182:311–330. doi: 10.1016/j.jebo.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore N., Kahn R., Martinez P.P., De Salazar P.M., Mahmud A.S., Buckee C.O. Lockdown related travel behavior undermines the containment of SARS-CoV-2. medRxiv 2020:2020.10.22.20217752.
- Kissler S.M., Kishore N., Prabhu M., et al. Reductions in commuting mobility correlate with geographic differences in SARS-CoV-2 prevalence in New York City. Nat. Commun. 2020;11:4674. doi: 10.1038/s41467-020-18271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiti M.C., Aguolu O.G., Liu C.Y., et al. Social contact patterns among employees in 3 U.S. companies during early phases of the COVID-19 pandemic. Epidemics. 2021;36 doi: 10.1016/j.epidem.2021.100481. April June 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N., Testa C., Hanage W.P., Chen J.T. US racial and ethnic data for COVID-19 cases: still missing in action. Lancet. 2020;396 doi: 10.1016/S0140-6736(20)32220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba K., Bradley H., Shioda K., et al. SARS-CoV-2 cumulative incidence and period seroprevalence: results from a statewide population-based serosurvey in California. Open Forum Infect. Dis. 2021;Vol. 8 doi: 10.1093/ofid/ofab379. ofab379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan F.-Y., Wei C.-F., Hsu Y.-T., Christiani D.C., Kales S.N. Work-related COVID-19 transmission in six Asian countries/areas: a follow-up study. PLOS ONE. 2020;15 doi: 10.1371/journal.pone.0233588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavange L.M., Kalsbeek W.D., Sorlie P.D., et al. Sample design and cohort selection in the hispanic community health study/study of latinos. Ann. Epidemiol. 2010;20:642–649. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.Y., Berlin J., Kiti M.C., et al. Rapid review of social contact patterns during the COVID-19 pandemic. medRxiv 2021:2021.03.12.21253410. [DOI] [PMC free article] [PubMed]
- M Reitsma, S.A., Goldhaber-Fiebert, J., Joseph, N. , Kates, J. , Levitt, L. , Rouw, A. , Salomon, J. , 2021. Disparities in Reaching COVID-19 Vaccination Benchmarks: Projected Vaccination Rates by Race/Ethnicity as of July 4: Kaiser Family Foundation, 2021.
- Ma K.C., Menkir T.F., Kissler S., Grad Y.H., Lipsitch M. Modeling the impact of racial and ethnic disparities on COVID-19 epidemic dynamics. eLife. 2021;10 doi: 10.7554/eLife.66601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias Gil R., Marcelin J.R., Zuniga-Blanco B., Marquez C., Mathew T., Piggott D.A. COVID-19 pandemic: disparate health impact on the hispanic/latinx population in the United States. J. Infect. Dis. 2020;222:1592–1595. doi: 10.1093/infdis/jiaa474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossong J., Hens N., Jit M. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLOS Med. 2008;5 doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murti M., Achonu C., Smith B.T. COVID-19 workplace outbreaks by industry sector and their associated household Transmission, Ontario, Canada, January to June, 2020. J. Occup. Environ. Med. 2021;63:574–580. doi: 10.1097/JOM.0000000000002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prem K., Cook A.R., Jit M. Projecting social contact matrices in 152 countries using contact surveys and demographic data. PLOS Comput. Biol. 2017;13 doi: 10.1371/journal.pcbi.1005697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raifman KN, J. , Jones, D. , Bor, J., Lipson, S., Jay, J., Cole, M., Krawczyk, N., Benfer, E., Chan, P., Galea, S., 2020. COVID-19 US State Policy Database. Ann Arbor, MI: Inter-university Consortium for Political and Social Research.
- Roberts J.D., Dickinson K.L., Koebele E. Clinicians, cooks, and cashiers: examining health equity and the COVID-19 risks to essential workers. Toxicol. Ind. Health. 2020;36:689–702. doi: 10.1177/0748233720970439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner B., Parish K., Ward J.A., DiLaura G., Dolovich S. COVID-19 cases and deaths in federal and state prisons. JAMA. 2020;324:602–603. doi: 10.1001/jama.2020.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden T.M., Berdahl T.A. Risk of severe COVID-19 among workers and their household members. JAMA Intern. Med. 2021;181:120–122. doi: 10.1001/jamainternmed.2020.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler A.J., Sullivan P.S., Sanchez T. Protocol for a national probability survey using home specimen collection methods to assess prevalence and incidence of SARS-CoV-2 infection and antibody response. Ann. Epidemiol. 2020;49:50–60. doi: 10.1016/j.annepidem.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan P.S., Sailey C., Guest J.L. Detection of SARS-CoV-2 RNA and antibodies in diverse samples: protocol to validate the sufficiency of provider-observed, home-collected blood, saliva, and oropharyngeal samples. JMIR Public Health Surveill. 2020;6 doi: 10.2196/19054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan P.S., Siegler A.J., Shioda K. SARS-CoV-2 cumulative incidence, United States, August-December 2020. Clin. Infect. Dis. 2021 [Google Scholar]
- Trends in Number of COVID-19 Cases and Deaths in the US Reported to CDC, by State/Territory. Accessed July 29 2021.
- M.A. Waltenburg TV, C.E. Rose, et al. Update: COVID-19 Among Workers in Meat and Poultry Processing Facilities ― United States, April–May 2020. In: Prevention CfDCa, ed. MMWR Morb Mortal Wkly Rep. Vol. 69. Atlanta, GA, 2020:887–92. [DOI] [PMC free article] [PubMed]
- Yehia B.R., Winegar A., Fogel R., et al. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.18039. e2018039-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Litvinova M., Liang Y. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science. 2020;368:1481–1486. doi: 10.1126/science.abb8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material