Abstract
Resveratrol is a polyphenol that has been well studied and has demonstrated anti-viral and anti-inflammatory properties that might mitigate the effects of COVID-19. Outpatients (N = 105) were recruited from central Ohio in late 2020. Participants were randomly assigned to receive placebo or resveratrol. Both groups received a single dose of Vitamin D3 which was used as an adjunct. The primary outcome measure was hospitalization within 21 days of symptom onset; secondary measures were ER visits, incidence of pneumonia, and incidence of pulmonary embolism. Five patients chose not to participate after randomization. Twenty-one-day outcome was determined of all one hundred participants (mean [SD] age 55.6 [8.8] years; 61% female). There were no clinically significant adverse events attributed to resveratrol. Outpatients in this phase 2 study treated with resveratrol had a lower incidence compared to placebo of: hospitalization (2% vs. 6%, RR 0.33, 95% CI 0.04–3.10), COVID-19 related ER visits (8% vs. 14%, RR 0.57, 95% CI 0.18–1.83), and pneumonia (8% vs. 16%, RR 0.5, 95% CI 0.16–1.55). One patient (2%) in each group developed pulmonary embolism (RR 1.00, 95% CI: 0.06–15.55). This underpowered study was limited by small sample size and low incidence of primary adverse events consequently the results are statistically similar between treatment arms. A larger trial could determine efficacy.
Trial Registrations: ClinicalTrials.gov NCT04400890 26/05/2020; FDA IND #150033 05/05/2020.
Subject terms: Medical research, Outcomes research, Translational research, Infectious diseases, Viral infection, Health care, Nutrition, Therapeutics
Introduction
Study rationale
Resveratrol (RV) is a polyphenolic phytoalexin produced by certain plants in response to injury or infection. RV has been associated with a variety of positive health effects in areas of inflammation, cardiovascular diseases, cognitive disease, cancer, diabetes, and infectious disease (including viral diseases)1,2. RV is readily available commercially as a dietary supplement produced from plant extracts or by genetically engineered yeast3. COVID-19 is the disease caused by a novel coronavirus (SARS-CoV-2) that can result in life threatening complications, including lung injury. Approved outpatient treatment options for COVID-19 were limited to symptomatic treatment at the time this study. Vaccines were notably not yet available. Multiple lines of preclinical data suggest that RV could be effective against coronavirus disease 2019 (Fig. 1).
Figure 1.
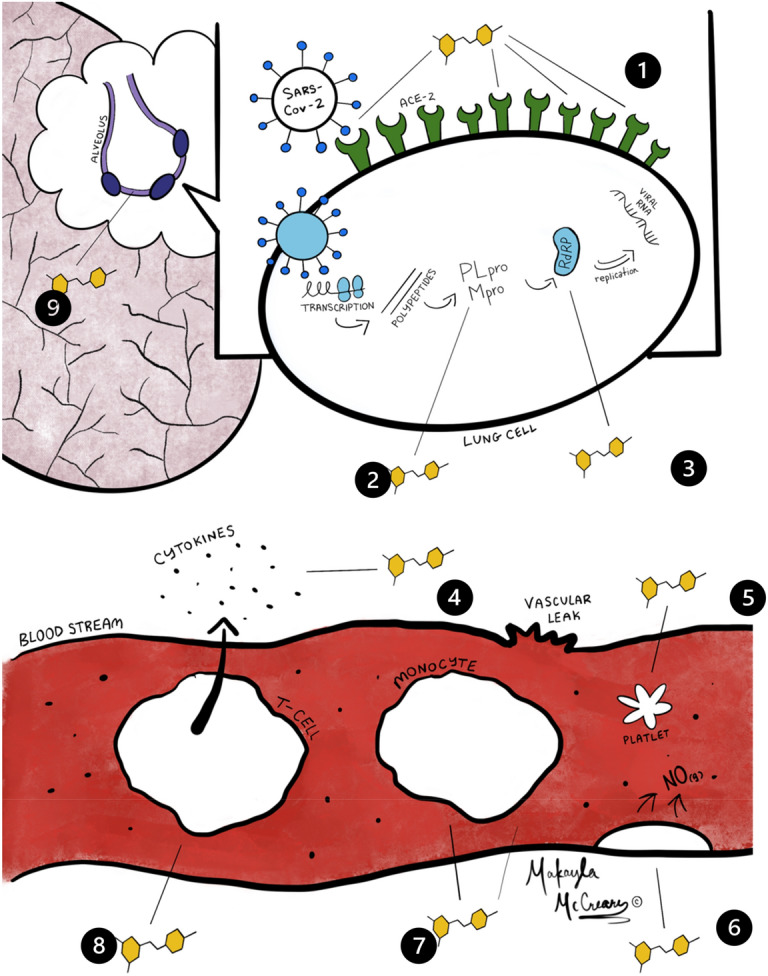
Summary of potential resveratrol effects on virus and host. 1. Inhibits Spike protein to ACE2 binding4,5. 2. Inhibits transcription of viral proteases (Mpro and PLpro)6–9. 3. Inhibits RNA-dependent RNA polymerase10. 4. Inhibits proinflammatory cytokines11–13. 5. Inhibits platelet aggregation14,15. 6. Activates endothelial Nitric Oxide (antiviral and vasoprotective)16–18. 7. Inhibits proinflammatory NF-kB19. 8. Inhibits proinflammatory Th-17 T-cells20. 9. Stimulates the production of glutathione in lung epithelium cells21–23.
Background
SARS-CoV-2 is characterized by surface spike proteins that bind to the Angiotensin-Converting Enzyme 2 (ACE2) of the respiratory tract. After entry into the cell, a variety of processes occur, including the down regulation of ACE2, subsequent destruction of the pneumocyte, the release of inflammatory mediators, and the subsequent release of cytokines (IL1, IL6, TNF-α) and reactive oxygen species24,25. A “cytokine storm” results in further damage to the alveoli and the development of Acute Respiratory Distress Syndrome (ARDS)25. Resveratrol’s multimodal antiviral, anti-inflammatory, and antioxidant properties as well as its ability to upregulate ACE2 receptors could be helpful in reducing the clinical effects of COVID-19.
ACE2 upregulation
In addition to ACE2 being a binding site for coronavirus (CoV), it is also associated with protective effects in SARS induced lung injury26,27. ACE2 may attenuate vascular permeability, inflammatory cell infiltration, pulmonary edema, hyaline membrane formation, and prevent acute lung injury28. Resveratrol has been shown to upregulate ACE229. A deficiency of ACE2 caused by SARS is associated with lung injury28. The upregulation of ACE2 by resveratrol might provide protective effects in COVID-1928,30–32.
Anti-viral effects
RV has demonstrated antiviral effects in a variety of animal and human diseases2. Specific to CoV, in vitro studies demonstrate that RV inhibits MERS-CoV infection by decreasing nucleocapsid protein expression resulting in reduced viral production and increased cell survival33. Starting at the first steps in the infection, in silico modeling suggests that RV would interfere with the binding of CoV spike protein to the ACE2 receptor (Fig. 1)4,5. In silico analysis also suggests that RV would inhibit COVID-19 RNA Dependent Polymerase and Papain-like Protease (PLpro) (Fig. 1) which could explain the inhibition of nucleocapsid protein described by Lin et al.6,10,33. In silico analysis also demonstrates potential inhibition of the coronavirus main proteinase (Mpro) which would be an additional mechanism of inhibiting viral replication7.
Anti-inflammatory effects
COVID-19 is associated with the potential for excessive inflammation. Coronavirus has been shown to activate Toll-Like Receptor 4, increase pro-inflammatory cytokines IL-1, IL-6, CCL5 (RANTES) and TNF-α leading to an unbalanced inflammatory response and damaging inflammation34–37. In contrast, RV has been shown to reduce inflammation via a variety of mechanisms (Fig. 1)11–13,38. RV has been demonstrated to inhibit TLR4 activation, decreasing the release of inflammatory cytokines in the macrophages of patients with COPD, and inhibit the proinflammatory transcription factor NF-κB14,19,39. RV has also demonstrated inhibition of pro-inflammatory Th17 helper T-cells (Fig. 1)20. Inhibition of NF-κB has been shown to increase survival in a mouse model of SARS-COV140.
The anti-inflammatory effects of RV might be beneficial in mitigating the cytokine storm that is associated with ARDS and the high mortality of COVID-1925. A mouse model of cytokine storm showed a 100% mortality reduced to 0% in RV treated mice with minimal lung injury in the treated group41. RV has demonstrated protective effects in lipopolysaccharide induced lung injury, a mouse model of ARDS42,43. The proposed mechanism is RV’s inhibition of NLRP3 inflammasomes42. Inhibition of NLRP3 inflammasomes in another proposed therapeutic target in COVID-1944.
Antioxidant effects
Depletion of the endogenous antioxidant glutathione has been attributed to poor outcomes and death in patient with COVID-19 (Fig. 1)21. The use of antioxidants has been proposed in the treatment of COVID-1945. RV’s antioxidant properties as well as its ability to induce glutathione synthesis might provide additional outcome benefits22.
Animal models of viral infections
As the above discussion regarding RV’s effects are largely based on in vitro models of disease, there is always a concern regarding whether in vitro models will translate into in vivo efficacy. Multiple animal studies have shown that RV does improve outcomes in animal models of viral infections. A porcine model of pseudorabies virus, a respiratory illness, shows that piglets inoculated with the virus had no mortality compared to a 40% mortality in the untreated group. Specifically, that study showed alveolar destruction in the untreated group vs mild lung injury in the RV treated group. The proposed mechanism is inhibition of IκB kinase by RV46. It is notable that a drug prediction analysis of SARS-CoV-2 suggests that IκB kinase inhibition is a potential target for COVID-1947. Similarly, a murine model of H1N1 influenza showed a 60% survival rate in RV treated mice compared to 20% in placebo treated mice48. In Respiratory Syncytial Virus (RSV) infected mice, RV treated mice showed significantly less lung damage compared to untreated mice49.
Vitamin D3
Vitamin D3 was included in the treatment protocol as an adjunct to RV based upon prior research showing that it has synergistic anti-inflammatory effects, inhibiting IL-6 and TNF-α11. Both treatment arms received a single 100,000 IU dose of D3 to quickly assure adequate serum concentrations of D3, as well as to potentially remove vitamin D deficiency as a confounding variable, noting that multiple publications raised concerns that vitamin D deficiency might be associated with worse outcomes in COVID-1950–52. The empiric use of vitamin D3 could lower the overall incidence of adverse outcomes in both groups in this study.
Materials and methods
Study design
Overview
This study was a phase 2, double-blind, randomized, placebo-controlled trial to evaluate the safety and explore the efficacy of RV plus vitamin D3 based on the hypothesis that RV with the adjunct vitamin D3 can reduce hospitalization and morbidity in patients with COVID-19. The study was approved by the U.S. Food and Drug Administration as an investigational new drug trial (FDA IND #150033 05/05/2020; ClinicalTrials.gov NCT04400890 26/05/2020), and the institutional review board of Mount Carmel Health Systems in Columbus, Ohio, USA. All patients were provided informed consent and screening remotely via phone interview, educated via online animated presentation, and e-consented via REDCap electronic data capture tools hosted at the Ohio State University Medical Center that incorporated questions from the REDCap Shared Library53,54.
Patients were recruited primarily from the Mount Carmel Health System testing centers by way of “cold calls” to patients 45 and older who tested positive for COVID-19. A few patients were recruited in response to research advertisements in the central Ohio area (social media, radio, and yard sign advertising), as well as physician referrals. Due to pandemic related safety concerns, the patients remained in quarantine within their home with all trial contact via phone, email, and web (REDCap), with contactless delivery of study packets via courier/mail. Packets were delivered within 7 days from the onset of symptoms, typically < 24-h after consent was signed.
Due to reports of patients self-medicating with investigational drugs (e.g., hydroxychloroquine) in the setting of COVID-19, the specific nature of the trial substance was concealed from subjects until after the study was complete. Patients were informed that they were receiving a “commercially available dietary supplement”, but the use of RV was not disclosed. The use of Vitamin D3 was open-label for both groups.
Patients were provided with a study packet containing identically prepared capsules containing a 15-day supply of either resveratrol or placebo, a one-time dose of vitamin D3, a thermometer, a pulse oximeter, and an instruction booklet with dosing log.
Data was collected via REDCap surveys on days 1–15, 21, 30, and day 60 with adverse symptoms assessed using selected PRO-CTCAE questions55. All patients were given daily online reminders of when to seek medical care based upon CDC recommendations. Primary and secondary outcome measures (including hospitalization, ER visits, history of chest imaging, and pneumonia) were assessed by phone interviews after 21 days from randomization. All radiology reports were reviewed by the principal investigator.
Sample size determination
The maximum total number of randomized subjects was capped at 200 by FDA request. Power analyses were conducted for the primary outcome measure (hospitalization) assuming multiple placebo arm hospitalization rates and effect sizes, as well as for secondary outcome measures.
At the time the protocol was developed, the rate of hospitalization among confirmed cases of COVID-19 ranged between 21% in the 45–54 age bracket, up to 31% for patient’s > 8556. (We now know hospitalization rates are much lower than initially described). A planned sample size of 190 subjects with complete observations yielded 80% power at the 5% two-sided significance level to detect a difference in the primary endpoint (hospitalization) rate of 10% in the resveratrol arm versus 25% in the placebo arm.
An interim analysis was completed by an independent data and safety monitoring board. The analysis used the Hwang-Shih-De Cani alpha spending function with parameter gamma = − 4 (O’Brien-Fleming–like) for the upper (superiority) bound under the null hypothesis with total one-sided Type I error 2.5%, and for the lower (safety or futility) bound under the alternative hypothesis with total Type II error 20% (80% power). Under the assumption of a binding futility bound and a placebo arm hospitalization rate of 25%, the probability of declaring futility at the interim analysis is 3% if the resveratrol arm hospitalization rate is 10% (alternative hypothesis), 55% if the resveratrol arm hospitalization rate is 25% (null hypothesis), and 75% if the resveratrol arm hospitalization rate is 30%. The R package gsDesign was used to determine stopping boundaries.
Participants
Due to low risk of hospitalization (the primary outcome measure), patients younger than 45 were excluded56. Patients were eligible for enrollment if they tested positive for SARS-CoV-2 and would have symptoms for less than 7 days by the expected delivery date of study packet. Exclusion criteria included cognitive impairment that would prevent the patient from cooperating with study procedures; asymptomatic patients; known history of cirrhosis, hepatic impairment, or Hepatitis C; known of history of renal impairment as measured by an eGFR of < 60 mL/min; patients receiving chemotherapy or who are on chronic immunosuppressants; allergy to grapes or rice; co-morbidities with a high likelihood of hospitalization within 30 days; currently pregnant; hospitalization; patients taking immunosuppressants and drug interactions in medications with a narrow therapeutic index. Patients on “statins” and PDE-5 inhibitors were instructed to withhold while on the study treatment. Potential confounding medication use (or intent to use) such as hydroxychloroquine and later bamlanivimab were exclusionary. No patients reported taking ivermectin. One patient had been prescribed an antibiotic (azithromycin) at the time of their initial testing. Patients were asked not to take other dietary supplements. The use of over-the-counter symptom treatment was permitted. Vaccines were not yet available during the enrollment period. Enrollment occurred prior to the detection of alpha, delta, and subsequent variants in the United States.
It is notable that the renal disfunction exclusion was an FDA requirement. Prior research has explored possible benefits of RV for patients with chronic kidney disease57. Furthermore, increased plasma levels of RV that might be attained in the setting of kidney disease might be beneficial.
Randomization
The random allocation list was blocked and stratified by a third-party group. Randomization used balanced blocks of size 2 or 4, selected randomly for each block. Randomization schedules were generated and rejected until the randomization schedule was balanced at 100, 200, and 210 subjects to align with the planned interim and final analyses, and in case of a 10-subject overrun. During the trial, only the third-party group and Data Safety Monitoring Board (DSMB) had access to the randomization list. The study personnel created identical-looking packets with identical-appearing study agents containing a 15-day dosing regimen according to the random allocation list. Study personnel were blinded to the contents of the distributed packets, with bottles only differentiated by a tamper-resistant serial number label applied by the third-party group which corresponded to the randomization list.
Blinding
A disinterested third party (Capital University, Department of Mathematics, Columbus, Ohio) was hired to assign tamper resistant serial number stickers as either RV or placebo based upon the output of randomization script from the R statistical software58. The third party, using a two-person team to provided validation, assigned serial numbers to appropriate manufacturer sealed RV or placebo bottles. The prepared bottles were returned to the research team such that the bottle could only be differentiated by the serial numbers. The randomization table of the serial number labels was kept only by the third party and the Data Safety Monitoring Board until the completion of the study.
Intervention
Patients received identically appearing bottles containing 60 identically appearing capsules of either > 98% pure trans-resveratrol (from Japanese Knotweed Root, Polygonum cuspidatum extract) (500 mg per capsule) or placebo (brown rice flour) (both prepared and bottled by Vita-Age, Vancouver, BC) with instructions to take 2 capsules 4 times per day for at least 7 days, and up to 15 days if COVID-19 symptoms persisted. Dosing was determined based upon published IC50 of resveratrol against MERS-COV and previously published pharmacokinetic literature of resveratrol plus its metabolites. (See the study protocol PDF at www.clinicaltrials.gov/ct2/show/NCT04400890 for detailed dose justification and products certificates of analysis.)
Participant monitoring and follow-up
Starting on day 1, and continuing daily for 15 days, subjects were contacted via automated e-mail. Messages included a reminder to take their study medication as scheduled and complete the daily surveys. Subjects were asked to complete a short questionnaire covering: (1) symptoms that could be related to COVID-19 (e.g., fever, cough, dyspnea), their frequency and severity; (2) report any other related or non-related medical events; (3) any medications they have taken to relieve symptoms, or other new medications they have not previously reported to study personnel; and (4) any visits they have made to healthcare providers, outpatient centers or hospitals, and details regarding those visits. Subjects received reminders when to seek care if they experience symptoms that are worsening or that are concerning to them.
Participants were sent a PRO-CTCAE questions on days 1, 8, 15, 21, 30, and 60 to monitor adverse events.
All subjects provided a surrogate/secondary contact (spouse/family member/friend) in order to determine the subject’s status if the subject could not be reached. All patients or their secondary contact were interviewed for follow up after 21 days from randomization (no participants were lost to follow up for their post-21 day follow up brief interview).
Endpoints
Hospitalizations were determined based on query of subject or the subject’s secondary contact, and the patient’s medical records. Additional outcomes include assessing number of days with fever, and to assess symptoms, including dyspnea and fatigue. Questionnaires to assess symptoms and adverse events were based on the PRO-CTCAE (Supplemental Tables S-2b, and S-3b)55.
Statistical analysis
Data management
Anonymized data were extracted from REDCap and processed into a dataset with one row per participant. Self-reported symptom and adverse event data were retained for every patient contact over the 21 days following randomization. Data were analyzed using Stata version 1759.
Primary analysis (including missing data)
The primary analysis is a comparison of the proportion of persons in the two groups who were hospitalized within 21 days of symptom onset. The comparison was evaluated using Fisher’s exact test, considering the difference to be statistically significant if the two-sided p-value is smaller than 0.05. The analysis uses the intent-to-treat method where all participants are analyzed as part of their randomization group, regardless of whether and when they withdrew from the study and regardless of whether or how well they complied with the study protocol. Missing outcome data were subject to tipping point sensitivity analysis to understand what distribution of missingness, if any, would change the conclusion reached using complete case analysis60.
Secondary analyses (including missing data)
Secondary outcomes were analyzed using Fisher’s exact test, also, with no adjustment for multiple comparisons. Those outcomes were also subject to tipping point analysis of missing outcome data.
Sub-group analyses
The primary and secondary endpoints were analyzed among planned sizable sub-groups using Fisher’s exact test with no adjustment for multiple comparisons.
Adverse events
PRO-CTCAE questions vary in format to either recording the presence or absence of symptoms, or to grading the frequency, severity, and interference in activities of daily living of symptoms. Severity is graded as 0 = None, 1 = Mild, 2 = Moderate, 3 = Severe, 4 = Very Severe. Frequency is graded as 0 = Never, 1 = Rarely, 2 = Occasionally, 3 = Frequently, 4 = Almost constantly. Interference is graded as 1 = Not at all, 2 = A little bit, 3 = Somewhat, 4 = Quite a bit, 5 = Very much. Presence is graded as 0 = No, 1 = Yes55.
Participants were asked about symptoms a) at enrollment (current symptoms), b) in a daily diary during 15 days of treatment (symptoms today), and c) on days 1, 8, 15, and 21 of the study (over the past 7 days). For questions about presence of a symptom, prevalence was compared using Fisher’s exact test. For questions about severity, frequency, or interference with activities of daily living (ADL), the proportion who answered 1+ and the proportion who answered 3+ were compared using Fisher’s exact test. Responses at enrollment or on day 1 of the study were used to characterize differences between study groups at baseline. Responses on days 2–21 were used to characterize differences in effects of placebo vs. resveratrol.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the US Food & Drug Administration (Investigation New Drug application #150033) on June 29, 2020 and subsequently by the Mount Carmel Health Systems IRB (Study # 200412-4) on August 10, 2020.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Results
Study participants
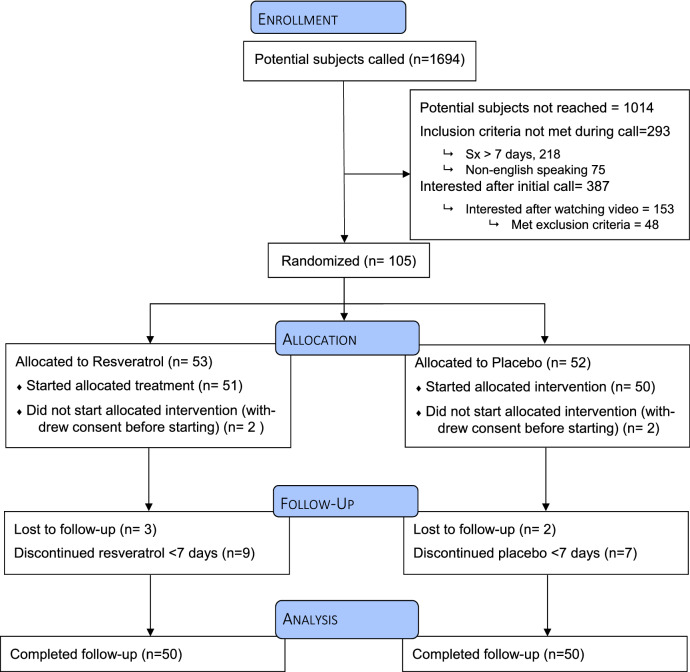
Between September 13, 2020 and December 11, 2020, 1,694 patients were telephoned within 24-h of testing positive for COVID-19 to be recruited into the clinical trial (Fig. 2). One-hundred-five were enrolled and randomized (Table 1). Five withdrew after receiving treatment packets (four withdrew before starting treatment and one withdrew after one treatment day citing “too many pills” as reason for withdrawal).
Figure 2.
CONSORT Diagram.
Table 1.
Baseline characteristics of participants.
| Placebo (N = 52) | Resveratrol (N = 53) | Overall (N = 105) | p value | |
|---|---|---|---|---|
| Age | t test p = 0.7139 | |||
| Mean (SD) | 55.7 (8.55) | 56.3 (9.46) | 56.0 (8.98) | |
| Median [Min, Max] | 54.0 [45.0, 75.0] | 55.0 [45.0, 84.0] | 55.0 [45.0, 84.0] | |
| Sex | chi-square p = 0.3623 | |||
| Male | 19 (36.5%) | 24 (45.3%) | 43 (41.0%) | |
| Female | 33 (63.5%) | 29 (54.7%) | 62 (59.0%) | |
| Race | ||||
| White | 46 (88.5%) | 47 (88.7%) | 93 (88.6%) | chi-square p = 0.9720 |
| Black | 2 (3.8%) | 2 (3.8%) | 4 (3.8%) | p = 0.9516 |
| Multiple | 3 (5.8%) | 1 (1.9%) | 4 (3.8%) | p = 0.2988 |
| Asian | 0 (0%) | 1 (1.9%) | 1 (1.0%) | p = 0.3293 |
| Other | 0 (0%) | 1 (1.9%) | 1 (1.0%) | p = 0.3293 |
| Did not answer | 1 (1.9%) | 1 (1.9%) | 2 (1.9%) | p = 0.9891 |
| Ethnicity | ||||
| Hispanic/Latino | 1 (1.9%) | 1 (1.9%) | 2 (1.9%) | p = 0.9891 |
| Not Hispanic/Latino | 45 (86.5%) | 43 (81.1%) | 88 (83.8%) | p = 0.4521 |
| Not specified | 6 (11.5%) | 9 (17.0%) | 15 (14.3%) | p = 0.9808 |
| BMI | t test p = 0.0668 | |||
| Mean (SD) | 31.4 (7.32) | 29.1 (4.68) | 30.2 (6.20) | |
| Median [Min, Max] | 30.0 [21.6, 58.9] | 28.5 [19.8, 42.7] | 29.3 [19.8, 58.9] | |
| Missing | 1 (1.9%) | 1 (1.9%) | 2 (1.9%) | |
| High-risk comorbidity | chi-square p = 0.6251 | |||
| Yes | 17 (32.7%) | 15 (28.3%) | 32 (30.5%) | |
| No | 35 (67.3%) | 38 (71.7%) | 73 (69.5%) | |
| Chronic Lung Disease | 9 (17.3%) | 10 (18.9%) | 19 (18.1%) | p = 0.7647 |
| Diabetes Mellitus | 5 (9.6%) | 5 (9.4%) | 10 (9.5%) | p = 1.0000 |
| Cardiovascular Disease | 3 (5.7%) | 3 (5.7%) | 6 (5.7%) | p = 0.9808 |
| Renal Disease | 0 | 0 | 0 | |
| Liver Disease | 0 | 0 | 0 | |
| Immunocompromised | 0 | 0 | 0 | |
| Smoking | ||||
| Current Smoker | 2 (3.8%) | 1 (1.8) | 3 (2.8%) | p = 0.5122 |
| Former Smoker | 12 (23.1%) | 8 (15.1%) | 20 (19.1%) | p = 0.0981 |
| Medications of Interest | ||||
| ACE inhibitor / ACE receptor blocker use | 10 (19.2%) | 5 (9.4%) | 15 (14.3%) | p = 0.1515 |
| Vitamin D use | 2 (3.8%) | 6 (11.3%) | 8 (7.6%) | p = 0.1489 |
| Oral Steroid use | 2 (3.8%) | 2 (3.8%) | 4 (3.8%) | p = 0.9845 |
| Inhaled Steroid use | 4 (7.6%) | 0 (0%) | 4 (4.8%) | p = 0.0395 |
SD = standard deviation; ACE = Angiotensin-converting enzyme.
p-values not adjusted for multiple comparisons.
There was no indication of systematic biases in randomization: 4/122 = 3% of hypothesis tests comparing baseline symptoms between randomized groups were statistically significant at the 5% level without adjustment for multiplicity, and none were statistically significant following a Bonferroni correction. (Supplemental Tables S-1, S-2a, and S-3a).
Compliance
Most participants completed the course of treatment. At the exit interview, 43 of 50 (86.0%) persons in the placebo group and 41 of 50 (82.0%) in the resveratrol group reported having completed at least 7 days of their respective treatments (Fisher’s exact test p-value = 0.786).
Primary endpoint—hospitalization within 21 days
One patient (2%) in the RV group and 3 (6%) in the placebo group (Table 2) were hospitalized within 21 days of symptom onset (risk ratio (RR) = 0.33; 95% confidence interval (CI) = 0.04–3.10; Risk difference = − 4.0%; 95% CI (− 11.6%–3.6%); Fisher’s exact test p value = 0.617; see Table 3). Tipping point analysis of missing outcome data indicate that no possible combination of outcomes among the five patients whose data are missing would have yielded a p value below 0.05. Supplemental Table S-4 shows outcomes stratified by patient characteristics. The differences in rates between study groups are not significant in any subgroup.
Table 2.
Characteristics of individual hospitalized participants.
| Placebo Patient 1 | Placebo Patient 2 | Placebo Patient 3 | Resveratrol Patient | |
|---|---|---|---|---|
| Reasons for hospitalization | Pneumonia, Pulmonary Embolism, Hypoxemia | Pneumonia, Hypoxemia, Not eating | Pneumonia, Hypoxemia, Transaminitis | Pneumonia, hypoxemia |
| Length of hospitalization (days) | 3 | 2 | 4 | 1 |
| Days after symptom onset when treatment started | 3 | 4 | 5 | 3 |
| Days after symptom onset when hospitalized | 12 | 11 | 14 | 10 |
| Days after treatment started when hospitalized | 9 | 7 | 9 | 7 |
| Baseline characteristics | ||||
| Age | 66 | 46 | 51 | 63 |
| Sex | Female | Female | Male | Male |
| Race | White | White | undisclosed | White |
| Hispanic/Latino | No | No | No | No |
| Not specified | ||||
| BMI | 30 | 37 | 35 | 43 |
| High-risk comorbidity | ||||
| Chronic Lung Disease | No | No | No | Yes |
| Diabetes Mellitus | No | No | No | No |
| Cardiovascular Disease | No | No | No | No |
| Current Smoker | No | No | No | No |
| Former Smoker | No | Yes | No | No |
| Medications of Interest | ||||
| ACE inhibitor/ACE receptor blocker use | No | Yes | Yes | Yes |
| Vitamin D | No | No | No | No |
| Oral Steroid Use | No | No | No | No |
| Inhaled Steroid Use | No | No | No | No |
This trial was conducted prior to the availability of COVID-19 vaccines.
Table 3.
Primary and secondary outcomes, as observed, by study group.
| Placebo | Resveratrol | Risk Ratio Risk Difference |
95% CI | p value | |
|---|---|---|---|---|---|
| N (%) | N (%) | ||||
| Primary outcome | |||||
| Hospitalization | 3 (6.0) | 1 (2.0) |
0.33 − 4.0% |
0.04–3.10 − 11.6–3.6% |
0.617 |
| Secondary outcomes | |||||
| Death | 0 (0) | 0 (0) | NA | NA | 1 |
| Invasive ventilation | 0 (0) | 0 (0) | NA | NA | 1 |
| ICU admission | 0 (0) | 0 (0) | NA | NA | 1 |
| ER visits for COVID | 7 (14.0) | 4 (8.0) |
0.57 − 6.0% |
0.18–1.83 − 18.2–6.2% |
0.525 |
| Pneumonia | 8 (16.0) | 4 (8.0) |
0.50 − 8.0% |
0.16–1.55 − 20.6–4.6% |
0.357 |
| Pulmonary embolism | 1 (2.0) | 1 (2.0) |
1.00 0% |
0.06–15.55 − 5.5–5.5% |
1 |
All outcomes evaluated over the 21 days that followed patient randomization to study group.
Outcomes observed for N = 50 patients per group.
NA = not applicable; CI = confidence interval; ICU = intensive care unit; ER = emergency room.
p-value from Fisher's exact test. p-values not adjusted for multiple comparisons.
Secondary endpoints
Among secondary endpoints, there were fewer events in the RV group than the placebo group for incidence of pneumonia and for emergency room visits due to COVID-19 (Table 3). Neither difference was statistically significant. There was one pulmonary embolism in each group, so those incidence rates were equal across study groups. There were no events and therefore no differences between study groups, for death, invasive ventilation, or ICU admission. If outcomes had been observed for the five patients who withdrew from the study, no secondary endpoint could have had a statistically significant difference between study groups, even if the five outcomes had been as favorable as possible for RV.
Notable events
One patient in the placebo group was diagnosed with pancreatitis that was attributed to COVID-19 by the patient's emergency department physician.
Adverse events
There were no serious adverse events reported. There were no significant differences in the proportion of patients from each study group reporting symptoms in a daily diary (Supplemental Table S-2b). When asked to think back over the previous seven days, the placebo group reported more severe dry mouth and more frequent general pain than the RV group, and the latter reported more frequent diarrhea (87.2% vs. 61.3%; p = 0.040) and more frequent nausea (23.1% vs. 5.7%; p = 0.050) than patients in the control group (Supplemental Table S-3b). No adjustments were made for multiple comparisons and only four p-values were statistically significant out of 110 symptom comparisons for study days 2–21.
Discussion
Resveratrol is an extensively studied plant phytoalexin that has demonstrated potential beneficial biologic effects in multiple human clinical trials. With respect to COVID-19, multiple publications have suggested its use in humans as a potential treatment. This has been supported by prior research describing resveratrol’s poly-mechanistic properties; computerized molecular docking analysis demonstrating resveratrol potential to interfere with coronavirus; as well as multiple in vitro studies demonstrating efficacy against MERS-CoV and SARS-CoV-2.
It should be noted that the much of the resveratrol literature is concerned about poor bioavailability and discounts possible effects of resveratrol metabolites such as the more intravascularly abundant resveratrol-glucuronides61,62. This dismissal of resveratrol’s metabolites is despite the fact that other drugs have demonstrated increased potency in their metabolized forms (i.e., morphine-6-glucuronide is known to be more potent than morphine)63. Molecular docking analysis suggest that resveratrol-glucuronide may be more potent against coronavirus since there is a higher binding affinity between resveratrol-glucuronides and coronavirus structures6.
The pandemic has had a significant impact on biomedical research including the development of this protocol. In the spring and summer of 2020, when this protocol was developed, many healthcare facilities were limiting access64. Elective procedures were cancelled, and many medical practices were transitioning to online appointments, partly due to limited availability of personal protective equipment (PPE). This project was limited to remote access to participants, in part, to reduce the risk to healthcare providers and eliminate the use of PPE, at a time when vaccines were not available. There was no physical contact with the participants which resulted in some missed opportunities to collect additional supporting data such as laboratory specimens or provide direct supervision. Data was collected solely by patient input dosing logs and online surveys which did result in inconsistent compliance with data collection.
The resveratrol treatment group (n = 50) was observed to have 1 hospitalization, 4 ER visits, and 4 pneumonias compared to the placebo group (n = 50) that had 3 hospitalization, 7 ER visits, and 8 pneumonias. The two-sided 95% confidence intervals for risk ratio are (0.04–3.10) for hospitalization, (0.18–1.83) for ER visits, and (0.16–1.55) for pneumonias indicating a wide range of plausible true treatment effects. The degree of evidence against the null hypothesis that the treatments are interchangeable is p = 0.617, p = 0.525, and p = 0.357 respectively (Table 3). The favorable risk ratios could be due to chance, but there are biological reasons to believe that RV would be effective and so the protective effect may be quite real, but not significant due to small sample size and low incidence of adverse outcomes. This study was limited to participants 45 and older based upon the early morbidity and mortality data published by the CDC in March of 2020 describing a hospitalization rate of > 25%55. However, we now know that the risk of admission is much lower than had been anticipated in the very early days of the pandemic65. Analysis of data for patients > 40 years old who tested positive for SARS-CoV-2 published by the state of Ohio for our local county between September 1, 2020 and December 31, 2020 resulted in a hospitalization rate of about 4%, which is consistent with our overall sample66. Recent data also suggests that vitamin D deficiency might be independent risk factor for hospitalization67. Our empiric use of early vitamin D in both groups might have played a role in our low rate of adverse events.
It is notable that in influenza, shorter time between the onset of symptoms and the start of antiviral treatment results in improved outcomes such that the CDC primarily recommends starting treatments within 48-h68. The median time from symptom onset to delivery of treatment packet was 5 days (Table 4). The magnitude of effect of resveratrol in COVID-19 might be greater if treatment could be started sooner, but due to delays in presentation, test results, and delivery, a 48-h treatment window was not feasible for this study.
Table 4.
Time (days) from symptom onset to delivery of treatment packets (start of treatment).
| Placebo (N = 52) | Resveratrol (N = 53) | Overall (N = 105) | |
|---|---|---|---|
| Average | 4.4 | 4.9 | 4.6 |
| Median | 4 | 5 | 5 |
| 75th percentile | 5.5 | 6 | 6 |
It is also notable that all the hospitalized patients had a BMI consistent with obesity (Table 2). Obese patients may require a larger dose than provided in this study given the increased volume of distribution.
There were no serious adverse events attributed to resveratrol in this study, and given resveratrol’s long safety history, the data presented here would support a larger clinical trial to determine efficacy, ideally starting treatment shortly after the onset of symptoms.
Dry mouth (p = 0.046), nausea (p = 0.05), and diarrhea (p = 0.04) was reported in higher frequency in the RV group. This is certainly consistent with known gastrointestinal side effects of resveratrol. However, these p-values for secondary measures are not adjusted for multiple comparisons.
Resveratrol treated patients had a lower incidence of overall pain (p = 0.04). This is consistent with prior preclinical literature demonstrating RV to have analgesic properties as a cyclooxygenase inhibitor (COX I & COX II)69. This would also support that orally administered resveratrol is able to achieve systemic effects despite concerns for limited bioavailability.
This study was a proof-of-concept to primarily determine the safety of using resveratrol in the setting of COVID-19. FDA guidance was to limit this study to no more than 200 participants with a planned interim safety analysis after the first 100 patients were enrolled. Enrollment in the study was slow initially but did rapidly increase in December as Ohio was starting its third COVID-19 wave. Enrollment was paused after the 100th patient so that an interim analysis could be performed. After completion of data collection and an interim analysis by an independent Data Safety Monitoring Board, Ohio’s third COVID-19 wave was ending. While the DSMB did approve continuation of the study, a feasibility analysis of daily case rate in the Mount Carmel Health System, and considering the prior rate of enrollment, it was estimated that it would take at least another 6–8 months to enroll another 100 patients (not knowing there were more COVID-19 waves yet to come). The enrollment rate would further be impacted by the availability of vaccinations and competing/exclusionary treatments (such a monoclonal antibodies). Furthermore, a statistical futility analysis also suggested that 100 more patients would be inadequate to determine efficacy, therefore the study was discontinued after the first 100 patients.
Additional limitations include limited geographic area, limited racial diversity, and a disproportionate number of heath care providers as subjects in the trial.
While 100 percent of the participants were contacted to determine their primary outcome measures, compliance with PRO-CTCAE was limited, resulting an incomplete picture of adverse events. A better funded clinical trial with larger research staff might be more effective at achieving patient compliance through more active patient contact (such as routine phone calls throughout the study).
Conclusions
This proof-of-concept study, along with the wealth of other resveratrol pre-clinical research, supports further investigation resveratrol as a potential treatment of COVID-19 and possibly other viral respiratory infections (including influenza, Respiratory Syncytial Virus, and Human Rhinovirus)70. If the magnitude of the effect of this small study was representative of a larger trial, the number needed to treat to prevent ER visits or hospitalization would compare favorably against currently available (i.e., monoclonal antibody therapy) outpatient treatments.
Given the scale of the health and economic impacts of COVID-19, any treatment that can reduce hospitalizations could have a significant impact in this pandemic. RV is generally recognized as safe and has been shown to have positive health benefits in human trials. Prior research in human trials related to lung disease, in vitro studies of RV of coronavirus, and animal studies of RV in other viral infections support investigating RV as a treatment for coronavirus disease. Given that RV is readily available and could be cheaply scaled up through the fermentation of yeast, it is potentially a scalable solution to treat COVID-19.
Supplementary Information
Acknowledgements
First and foremost, I (MRM) would like to thank my family who supported the use of our personal savings to fund this project and supported me volunteering countless hours to complete this project during my time off work. To my wife, Beth McCreary, and daughter, Marina McCreary, thanks for volunteering your time as editors of the protocol. Thanks to my daughter, Makayla McCreary, who turned complicated concepts into the art of Fig. 1. I would also like to thank Lynn Schaffer of Mount Carmel Health System’s Department of Research, who guided my evolution from physician to principal investigator. Thanks to my fellow Emergency Physician, Ashley Patel, who volunteered to help with recruitment and independently assessed patient safety. Thanks to Vita-Age Nutrition who agreed to manufacture the resveratrol and placebo at their cost to assist in finding a treatment for COVID-19. Their generosity made this project financially feasible. Thanks to the patient volunteers who provided their time and effort entering their data online while they simultaneously endured illness to help find a treatment for patients with COVID-19.
Author contributions
M.M.: conceptualization, methodology, software, writing original draft, supervision. P.S.: statistical analysis, writing original statistical protocol. D.R.: statistical analysis, writing – review and editing. All authors reviewed the manuscript.
Funding
The use of REDCap and the work of Patrick Schnell was supported by the Ohio State University Center for Clinical and Translational Science, CTSA Grant UL1TR002733. Remaining aspects of the research funded by the first author.
Data availability
Deidentified individual data that supports the results will be shared by written request to the communicating author; provided the requesting investigator has approval from an Institutional Review Board (IRB), Independent Ethics Committee (IEC), or Research Ethics Board (REB), as applicable, and executes a data use/sharing agreement with Mount Carmel Health System.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-13920-9.
References
- 1.Tomé-Carneiro J, et al. Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 2013;19:6064–6093. doi: 10.2174/13816128113199990407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abba Y, Hassim H, Hamzah H, Noordin MM. Antiviral activity of resveratrol against human and animal viruses. Adv. Virol. 2015;2015:1. doi: 10.1155/2015/184241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M, et al. De novo production of resveratrol from glucose or ethanol by engineered Saccharomyces cerevisiae. Metab. Eng. 2015;32:1–11. doi: 10.1016/j.ymben.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Wahedi HM, Ahmad S, Abbasi SW. Stilbene-based natural compounds as promising drug candidates against COVID-19. J. Biomol. Struct. Dyn. 2020;39:1–10. doi: 10.1080/07391102.2020.1762743. [DOI] [PubMed] [Google Scholar]
- 5.Pandey P, et al. Targeting SARS-CoV-2 spike protein of COVID-19 with naturally occurring phytochemicals: an in silico study for drug development. Null. 2020;39:1–11. doi: 10.1080/07391102.2020.1796811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranjbar, A., Jamshidi, M. & Torabi, S. Molecular modelling of the antiviral action of Resveratrol derivatives against the activity of two novel SARS CoV-2 and 2019-nCoV receptors. Eur. Rev. Med. Pharmacol. Sci. 7834–7844 (2020). [DOI] [PubMed]
- 7.Mishra, A., Pathak, Y. & Tripathi, V. Natural compounds as potential inhibitors of novel coronavirus (COVID-19) main protease: An in silico study. Res. Square. Preprint (2020) 10.21203/rs.3.rs-22839/v2.
- 8.Shin D, Mukherjee R, Grewe D, et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020;587:657–622. doi: 10.1038/s41586-020-2601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rathnayake, A., Zheng, J., Kim, Y., & et al. 3C-like protease inhibitors block coronavirus replication in vitro and improve survival in MERS-CoV-infected mice. Sci. Transl. Med.12, (2020). [DOI] [PMC free article] [PubMed]
- 10.El-Aziz, N. M. A., Shehata, M. G., Awad, O. M. E. & El-Sohaimy, S. A. Inhibition of COVID-19 RNA-dependent RNA polymerase by natural bioactive compounds: Molecular docking analysis. Res. Square. Preprint (2020). 10.21203/rs.3.rs-25850/v1.
- 11.Maity B, Bora M, Sur D. An effect of combination of resveratrol with vitamin D3 on modulation of proinflammatory cytokines in diabetic nephropathy induces rat. Orient. Pharm. Exp. Med. 2018;18:127–138. doi: 10.1007/s13596-018-0311-4. [DOI] [Google Scholar]
- 12.Estrov Z, Shishodia S, Faderl S, et al. Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-kappaB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood. 2003;102:987–995. doi: 10.1182/blood-2002-11-3550. [DOI] [PubMed] [Google Scholar]
- 13.Zhu J, Yong W, Wu X. Anti-inflammatory effect of resveratrol on TNF-alpha-induced MCP-1 expression in adipocytes. Biochem. Biophys. Res. Commun. 2008;369:471–477. doi: 10.1016/j.bbrc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 14.Sun J, et al. Suppression of TLR4 activation by resveratrol is associated with STAT3 and Akt inhibition in oxidized low-density lipoprotein-activated platelets. Eur. J. Pharmacol. 2018;836:1–10. doi: 10.1016/j.ejphar.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Shigematsu S, et al. Resveratrol, a red wine constituent polyphenol, prevents superoxide-dependent inflammatory responses induced by ischemia/reperfusion, platelet-activating factor, or oxidants. Free Radical Biol. Med. 2003;34:810–817. doi: 10.1016/S0891-5849(02)01430-2. [DOI] [PubMed] [Google Scholar]
- 16.Xia N, Förstermann U, Li H. Resveratrol and endothelial nitric oxide. Molecules. 2014;19:16102–16121. doi: 10.3390/molecules191016102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Åkerström S, et al. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79:1966–1969. doi: 10.1128/JVI.79.3.1966-1969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Åkerström S, Gunalan V, Keng CT, Tan Y-J, Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: Viral RNA production and palmitoylation of the S protein are affected. Virology. 2009;395:1–9. doi: 10.1016/j.virol.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren X, et al. Resveratrol inhibits NF-kB signaling through suppression of p65 and IkappaB kinase activities. Pharmazie. 2013;68:689. [PubMed] [Google Scholar]
- 20.Xuzhu G, Komai-Koma M, Leung B, et al. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann. Rheum. Dis. 2021;71:129–135. doi: 10.1136/ard.2011.149831. [DOI] [PubMed] [Google Scholar]
- 21.Polonikov A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infectious Diseases. 2020;6:1558–1562. doi: 10.1021/acsinfecdis.0c00288. [DOI] [PubMed] [Google Scholar]
- 22.Kode A, et al. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 23.Horowitz RI, Freeman PR, Bruzzese J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: A report of 2 cases. Respir. Med. Case. Rep. 2020;30:101063. doi: 10.1016/j.rmcr.2020.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuba K, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta P, et al. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholls J, Peiris M. Good ACE, bad ACE do battle in lung injury, SARS. Nat. Med. 2005;11:821–822. doi: 10.1038/nm0805-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu H, et al. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci. Rep. 2016;6:19840–19840. doi: 10.1038/srep19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan T, Xiao R, Lin G. Angiotensin-converting enzyme 2 in severe acute respiratory syndrome coronavirus and SARS-CoV-2: A double-edged sword? FASEB J. 2020;34:6017–6026. doi: 10.1096/fj.202000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moran CS, et al. Resveratrol inhibits growth of experimental abdominal aortic aneurysm associated with upregulation of angiotensin-converting enzyme 2. Arterioscler. Thromb. Vasc. Biol. 2017;37:2195–2203. doi: 10.1161/ATVBAHA.117.310129. [DOI] [PubMed] [Google Scholar]
- 30.Imai Y, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horne JR, Vohl M-C. Biological plausibility for interactions between dietary fat, resveratrol, ACE2, and SARS-CoV illness severity. Am. J. Physiol. Endocrinol. Metab. 2020;318:E830–E833. doi: 10.1152/ajpendo.00150.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magrone T, Magrone M, Jirillo E. Focus on receptors for coronaviruses with special reference to angiotensin-converting enzyme 2 as a potential drug target - a perspective. Endocr. Metab. Immune Disord. Drug Targets. 2020;20:1–11. doi: 10.2174/187153032001191213111933. [DOI] [PubMed] [Google Scholar]
- 33.Lin S-C, et al. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017;17:144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olejnik J, Hume AJ, Mühlberger E. Toll-like receptor 4 in acute viral infection: Too much of a good thing. PLoS Pathog. 2018;14:e1007390. doi: 10.1371/journal.ppat.1007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conti, P. et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by COVID-19: anti-inflammatory strategies. J. Biol. Regulat. Homeostatic Agents34, (2020). [DOI] [PubMed]
- 36.Patterson, B. K. et al. Disruption of the CCL5/RANTES-CCR5 Pathway Restores Immune Homeostasis and Reduces Plasma Viral Load in Critical COVID-19. 10.1101/2020.05.02.20084673 (2020).
- 37.Wang W, et al. Up-regulation of IL-6 and TNF-α induced by SARS-coronavirus spike protein in murine macrophages via NF-κB pathway. Virus Res. 2007;128:1–8. doi: 10.1016/j.virusres.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu X, Lei X, Dong W. Resveratrol as a potential therapeutic drug for respiratory system diseases. Drug Des. Dev. Ther. 2017;11:3591–3598. doi: 10.2147/DDDT.S148868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Culpitt SV, et al. Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax. 2003;58:942–946. doi: 10.1136/thorax.58.11.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeDiego ML, et al. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014;88:913–924. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alghetaa H, et al. Resveratrol protects mice against SEB-induced acute lung injury and mortality by miR-193a modulation that targets TGF-β signalling. J. Cell Mol. Med. 2018;22:2644–2655. doi: 10.1111/jcmm.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang L, et al. Resveratrol ameliorates LPS-induced acute lung injury via NLRP3 inflammasome modulation. Biomed. Pharmacother. 2016;84:130–138. doi: 10.1016/j.biopha.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 43.Chen H, Bai C, Wang X. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Rev. Respir. Med. 2010;4:773–783. doi: 10.1586/ers.10.71. [DOI] [PubMed] [Google Scholar]
- 44.Shah, A. Novel coronavirus-induced NLRP3 inflammasome activation: A potential drug target in the treatment of COVID-19. Front. Immunol.11, (2020). [DOI] [PMC free article] [PubMed]
- 45.Wang J-Z, Zhang R-Y, Bai J. An anti-oxidative therapy for ameliorating cardiac injuries of critically ill COVID-19-infected patients. Int. J. Cardiol. 2020;312:137–138. doi: 10.1016/j.ijcard.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao X, et al. Antiviral effect of resveratrol in piglets infected with virulent pseudorabies virus. Viruses. 2018;10:457. doi: 10.3390/v10090457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fagone P, et al. Transcriptional landscape of SARS-CoV-2 infection dismantles pathogenic pathways activated by the virus, proposes unique sex-specific differences and predicts tailored therapeutic strategies. Autoimmun. Rev. 2020;19:102571–102571. doi: 10.1016/j.autrev.2020.102571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palamara AT, et al. Inhibition of influenza a virus replication by resveratrol. J. Infect. Dis. 2005;191:1719–1729. doi: 10.1086/429694. [DOI] [PubMed] [Google Scholar]
- 49.Zang N, et al. Resveratrol-mediated gamma interferon reduction prevents airway inflammation and airway hyperresponsiveness in respiratory syncytial virus-infected immunocompromised mice. J. Virol. 2011;85:13061–13068. doi: 10.1128/JVI.05869-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grant W, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aslan MT, Aslan İÖ, Özdemir Ö. Is vitamin D one of the key elements in COVID-19 days? J Nutr Health Aging. 2020;24:1038–1039. doi: 10.1007/s12603-020-1517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ilahi M, Armas LAG, Heaney RP. Pharmacokinetics of a single, large dose of cholecalciferol. Am. J. Clin. Nutr. 2008;87:688–691. doi: 10.1093/ajcn/87.3.688. [DOI] [PubMed] [Google Scholar]
- 53.Harris PA, et al. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Obeid JS, et al. Procurement of shared data instruments for research electronic data capture (REDCap) J. Biomed. Inform. 2013;46:259–265. doi: 10.1016/j.jbi.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Cancer Institute Division of Cancer Control & Population Services. Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). https://healthcaredelivery.cancer.gov/pro-ctcae/.
- 56.CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. Morbidity Mortality Week. Rep.69, 343 (2020). [DOI] [PMC free article] [PubMed]
- 57.Saldanha J, et al. Effects of resveratrol supplementation in nrf2 and NF-κB expressions in nondialyzed chronic kidney disease patients: A randomized, double-blind, placebo-controlled, crossover clinical trial. J. Ren. Nutr. 2016;26:401–406. doi: 10.1053/j.jrn.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 58.R Core Team. R: A language and environment for statistical computing. (R Foundation for Statistical Computing, 2020).
- 59.StataCorp. Stata Statistical Software: Release 17. (2021).
- 60.White I, Joseph R, Best N. A causal modelling framework for reference-based imputation and tipping point analysis in clinical trials with quantitative outcome. J. Biopharm. Stat. 2020;30:334–350. doi: 10.1080/10543406.2019.1684308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walle T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- 62.Sergides C, Chirilă M, Silvestro L, Pitta D, Pittas A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016;11:164–170. doi: 10.3892/etm.2015.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christrup L. Morphine metabolites. Acta Anaesthesiol Scand. 1997;41:116–122. doi: 10.1111/j.1399-6576.1997.tb04625.x. [DOI] [PubMed] [Google Scholar]
- 64.Lauer, M. Continued Impact of COVID-19 on Biomedical Research. https://nexus.od.nih.gov/all/2020/11/04/continued-impact-of-covid-19-on-biomedical-research/ (2020).
- 65.The Economist. Pandemic mortality - See how age and illnesses change the risk of dying from covid-19. https://www.economist.com/graphic-detail/covid-pandemic-mortality-risk-estimator (2021).
- 66.Ohio Department of Health. COVID-19 Dashboard. https://coronavirus.ohio.gov/wps/portal/gov/covid-19/dashboards/overview (2021).
- 67.Merzon E, et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: An Israeli population-based study. FEBS J. 2020;287:3693–3702. doi: 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Centers for Disease Control and Prevention. Influenza Antiviral Medications: Clinician Summary. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm (2021).
- 69.Bertelli A, et al. Analgesic resveratrol? Antioxid. Redox Signal. 2008;10:403–404. doi: 10.1089/ars.2007.1926. [DOI] [PubMed] [Google Scholar]
- 70.Rossi GA, Sacco O, Capizzi A, Mastromarino P. Can resveratrol-inhaled formulations be considered potential adjunct treatments for COVID-19? Front. Immunol. 2021;12:1591. doi: 10.3389/fimmu.2021.670955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified individual data that supports the results will be shared by written request to the communicating author; provided the requesting investigator has approval from an Institutional Review Board (IRB), Independent Ethics Committee (IEC), or Research Ethics Board (REB), as applicable, and executes a data use/sharing agreement with Mount Carmel Health System.