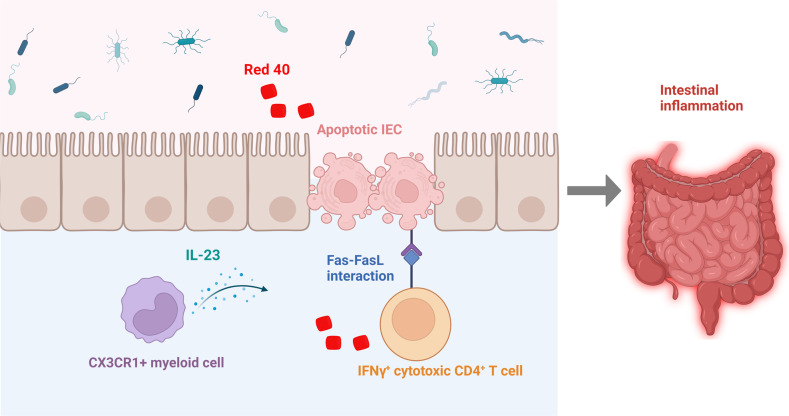
Food colorant Red 40 has been shown to induce colitis in the context of dysregulated IL-23. However, the mechanism involved is still not fully understood. A study by Chen et al. in this issue of Cellular Molecular Immunology [1] demonstrates that under dysregulated IL-23 production from myeloid cells, Red 40 induces IFNγ+ cytotoxic CD4+ T cells to promote colitis.
It is well established that the pathogenesis of inflammatory bowel diseases (IBD) involves dysregulated mucosal immune responses to gut microbiota in genetically susceptible individuals [2]. Over 200 risk loci for IBD have been identified by genome-wide association studies (GWAS), and some of these risk loci have been evaluated in various animal models of colitis [3]. However, the combination of all these genes/loci only accounts for a small proportion of disease heritability, suggesting that environmental factors also play a critical role in the pathogenesis of IBD. Accumulating evidence has revealed that various immune cell types coordinate their functions through cytokine-mediated intercellular communication to regulate the pathogenesis of IBD [4]. IL-23 is a heterodimer that is comprised of IL12p40 and IL23p19. It is primarily produced by innate myeloid cells and acts on Th17 cells, which are crucial in mediating the pathogenesis of IBD [5]. The coding variants in IL23R are associated with protection from IBD onset, and the IL12B locus, which encodes IL-12p40, is associated with IBD risk [6]. However, overexpression of IL-23 alone in myeloid cells does not cause colitis in animal models, indicating that other factors, including local environmental variables, coordinate disease development.
In addition to the gut microbiota, which profoundly affects intestinal homeostasis and IBD, certain dietary components have been shown to influence the pathogenesis of IBDs. The diet could regulate IBD incidence through various mechanisms, such as direct actions on host cells or indirect actions that alter the gut microbiota [7]. Artificial food colorants that are made from petroleum and approved by the US Food and Drug Administration (FDA) to enhance the color of processed foods are widely used in the food and pharmaceutical industries. The most popular food colorants are Red 40 (also known as Allura Red AC), Yellow 5, and Yellow 6. These three colorants make up 90% of all the food dyes used in the US and are found in many foods, beverages, and medicines [8]. Although they are reported to have no adverse cytotoxic effect on the host, whether and how they affect human diseases, including IBD, is still unclear. An elegant study by Chen et al. in this issue of Cellular Molecular Immunology [1] demonstrated that under high IL-23 production from myeloid cells, Red 40 induced IFNγ+ cytotoxic CD4+ T cells to cause colitis. In previous studies, the same group developed a strain of mice by crossing Rosa26-lox-STOP-lox-IL23 mice with CX3CR1CreER mice to conditionally express IL-23 in CX3CR1-positive myeloid cells (R23FR mice) [9, 10]. The R23FR mice developed colitis when treated with tamoxifen (TAM) to turn on IL-23 production, and exposed to repeated cycles of custom diet 2019 (TD. 160647, Envigo), which contains Red 40, but not to the standard diet used in their animal facility (Labdiet 5053). Interestingly, the mice entered remission after the 2019 diet was discontinued, and the subsequent treatment of the R23FR mice in remission with the 2019 diet caused colitis flares even without the overproduction of IL-23. Red 40 alone did not induce colitis in the control mice, but it did trigger severe colitis in IL-23-overexpressing R23FR mice. These findings are similar to those of the 2019 diet, which were mediated by CD4+ T cells. CD4+ T cells from colitic R23FR mice in remission transfer colitis to Rag1−/− mice treated with Red 40 [9, 10].
Among CD4+ T cells, the gut microbiota-reactive IFNγ-producing Th1 cells and IL-17-producing Th17 cells are central to the pathogenesis of certain types of IBD [11]. IL-12 promotes Th1-cell development, whereas IL-23 enhances Th17-cell development. However, it is unclear what types of CD4+ T cells mediate the pathogenesis of colitis in Red 40-treated R23FR mice. In the current study [1], Chen et al. investigated the mechanisms of how Red 40 induced colitis in IL-23-overexpressing R23FR mice mediated by CD4+ T cells. Using single-cell RNA sequencing, they identified 9 clusters of CD4+ T cells in colitic Rag1−/− mice that received CD4+ T cells from colitic R23FR mice. Among the 3 predominant clusters, Cluster 1 significantly overexpressed Ifng and genes associated with cytotoxic function, including Gzma, Gzmb, Nkg7, and others. These findings suggest that cells in Cluster 1 have higher cytotoxic potential. Indeed, CD4+ T cells in the intestine of Red 40-fed Rag1−/− recipient mice (R23FR→Rag mice) induced apoptosis of intestinal epithelial cells (IECs), as depletion of T cells suppressed apoptosis. To directly demonstrate whether colitic CD4+ T cells are cytotoxic to IECs, they cocultured CD4+ T cells isolated from adoptively transferred Rag1−/− mice that were treated with or without Red 40 with primary IECs or colonic enteroids. Intestinal CD4+ T cells from Rag1−/− recipient mice treated with Red 40, but not water, were cytotoxic to IECs. IECs are the primary cell type that is in direct contact with stimuli from the luminal microbiota and diets. They are also critical players in microbe-host interactions, contributing to the maintenance of intestinal homeostasis through various defense mechanisms and barrier functions [12]. Once IEC barrier function is impaired, the gut microbiota invades and causes inflammation in the gut and beyond. This suggests that CD4+ T-cell cytotoxicity against IEC is involved in the pathogenesis of colitis in Red 40-treated R23FR mice. Chen et al. then performed transwell experiments to determine whether such CD4+ T-cell cytotoxicity requires cell–cell contact or the production of soluble factors. The cytotoxicity was abolished when CD4+ T cells and IECs were separated, indicating that cell–cell contact is required for CD4+ T-cell cytotoxicity. Certain TNF family members, including FasL and TRAIL, and perforin/granzyme-mediated cytotoxic pathways have been implicated in the killing of target cells via cell–cell contact. By using various antibodies to block specific pathways, Chen et al. showed that CD4+ T-cell cytotoxicity on IECs involved triggering caspase activity in IECs. This activity was mediated by FasL but not TNFα, IFNγ, TRAIL, or perforin.
Although IFNγ is not directly involved in CD4+ T-cell cytotoxicity, it is required for CD4+ CTL generation and contributes to the development of colitis in R23FR mice and R23FR→Rag mice. When treated with TAM and Red 40, Ifng−/− R23FR mice developed much less severe colitis than Ifng+/+ R23FR mice. Furthermore, blockade of IFNγ with anti-IFNγ antibody decreased cleaved Caspase-3+ Pankeratin+ apoptotic epithelial cells in the intestine of Red 40-treated adoptively transferred Rag1−/− mice compared to control antibody-treated Rag1−/− recipient mice. Therefore, Chen et al. identified an essential role of IFNγ+ CD4+ CTLs in the pathogenesis of colitis induced by Red 40 in the presence of dysregulated IL-23 expression (Fig. 1).
Fig. 1.
Red 40 induces colitis. Red 40 induces colitis in the context of dysregulated IL-23 expression through the induction of IFNγ+ CD4+ cytotoxic T cells, which cause IEC apoptosis
The study by Chen et al. thus provides novel insights into the mechanism of Red 40-induced colitis in the context of dysregulated IL-23 production and how food colorants regulate intestinal inflammation. As with all elegant studies, it also raises many questions. The most intriguing question will be how Red 40 moderates CD4+ T-cell differentiation into IFNγ+ T cells in the context of IL-23. Does Red 40 directly cause host cells to promote the expression of IFNγ in T cells or through moderating gut microbiota, which in turn promotes the expression of IFNγ in T cells? IL-23 has been shown to promote Th17-cell development and function, leading to the pathogenesis of IBD. Thus, it is also unclear how IL-23 drives T cells to express IFNγ in mice treated with Red 40. As this study focuses on Red 40, it will be important to investigate whether other food colorants function similarly in regulating intestinal homeostasis in various contexts. Exploring such questions will provide novel insights into the understanding of the dietary regulation of intestinal homeostasis and novel mechanisms that drive the pathogenesis of IBD, thus offering novel therapeutic targets for the treatment of IBD.
Acknowledgements
This work was supported by NIH grants DK112436, DK125011, AI150210, and DK124132. Figure 1 was created with BioRender.com.
Competing interests
The authors declare no competing interests.
References
- 1.Chen L, He Z, Reis BS, Gelles JD, Chipuk JE, Ting AT, et al. IFNγ+ cytotoxic CD4+ T lymphocytes are involved in the pathogenesis of colitis induced by IL-23 and food colorant Red 40. Cell Mol Immunol. 2022;19:777–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elson CO, Cong Y. Host-microbiota interactions in inflammatory bowel disease. Gut Microbes. 2012;3:332–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang H, Fang M, Jostins L, Umicevic Mirkov M, Boucher G, Anderson CA, et al. Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature. 2017;547:173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamias G, Cominelli F. Cytokines and intestinal inflammation. Curr Opin Gastroenterol. 2016;32:437–42. [DOI] [PubMed] [Google Scholar]
- 5.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. [DOI] [PubMed] [Google Scholar]
- 6.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee D, Albenberg L, Compher C, Baldassano R, Piccoli D, Lewis JD, et al. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology. 2015;148:1087–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vojdani A, Vojdani C. Immune reactivity to food coloring. Alter Ther Health Med. 2015;21:52–62. [PubMed] [Google Scholar]
- 9.Chen L, He Z, Iuga AC, Martins Filho SN, Faith JJ, Clemente JC, et al. Diet modifies colonic microbiota and CD4(+) T-cell repertoire to induce flares of colitis in mice with myeloid-cell expression of interleukin 23. Gastroenterology. 2018;155:1177–1191. e1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Z, Chen L, Catalan-Dibene J, Bongers G, Faith JJ, Suebsuwong C, et al. Food colorants metabolized by commensal bacteria promote colitis in mice with dysregulated expression of interleukin-23. Cell Metab. 2021;33:1358–1371. e1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Globig AM, Hennecke N, Martin B, Seidl M, Ruf G, Hasselblatt P, et al. Comprehensive intestinal T helper cell profiling reveals specific accumulation of IFN-gamma+IL-17+coproducing CD4+ T cells in active inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:2321–9. [DOI] [PubMed] [Google Scholar]
- 12.Allaire JM, Crowley SM, Law HT, Chang SY, Ko HJ, Vallance BA. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 2018;39:677–96. [DOI] [PubMed] [Google Scholar]