Abstract
Background:
The risk of high-grade prostate cancer, given a family history of cancer, has been described in the general population, but not among men selected for prostate biopsy in an international cohort.
Objective:
To estimate the risk of high-grade prostate cancer on biopsy based on a family history of cancer.
Design, setting, and participants:
This is a multicenter study of men undergoing prostate biopsy from 2006 to 2019, including 12 sites in North America and Europe. All sites recorded first-degree prostate cancer family histories; four included more detailed data on the number of affected relatives, second-degree relatives with prostate cancer, and breast cancer family history.
Outcomes measurements and statistical analysis:
Multivariable logistic regressions evaluated odds of high-grade (Gleason grade group ≥2) prostate cancer. Separate models were fit for family history definitions, including first- and second-degree prostate cancer and breast cancer family histories.
Results and limitations:
A first-degree prostate cancer family history was available for 15 799 men, with a more detailed family history for 4617 (median age 65 yr, both cohorts). Adjusted odds of high-grade prostate cancer were 1.77 times greater (95% confidence interval [CI] 1.57–2.00, p < 0.001, risk ratio [RR] = 1.40) with first-degree prostate cancer, 1.38 (95% CI 1.07–1.77, p = 0.011, RR = 1.22) for second-degree prostate cancer, and 1.30 (95% CI 1.01–1.67, p = 0.040, RR = 1.18) for first-degree breast cancer family histories. Interaction terms revealed that the effect of a family history did not differ based on prostate-specific antigen but differed based on age. This study is limited by missing data on race and prior negative biopsy.
Conclusions:
Men with indications for biopsy and a family history of prostate or breast cancer can be counseled that they have a moderately increased risk of high-grade prostate cancer, independent of other risk factors.
Patient summary:
In a large international series of men selected for prostate biopsy, finding a high-grade prostate cancer was more likely in men with a family history of prostate or breast cancer.
Keywords: Family history, Biopsy, Prostate cancer, Breast cancer, Diagnosis
1. Introduction
A family history of prostate cancer is a well-established risk factor for being diagnosed with prostate cancer, with a roughly two- to three-fold increase in risk among men with an affected first-degree relative [1,2]. A first-degree family history of breast cancer has also been recognized as a risk factor for prostate cancer, and women with a family history of prostate cancer similarly have an increased risk of postmenopausal breast cancer [2–6]. More recent reports have also identified this trend in pancreatic and ovarian cancers, supporting a shared genetic predisposition to developing hormone-sensitive cancers [7,8]. The Cancer Genome Atlas Research Network found a germline alteration in 4.6% of localized prostate cancers [9]. In metastatic prostate cancer, the frequency of such alterations is higher, reported to be between 11.8% and 16.2% [10,11]. These germline mutations are most commonly found in BRCA2, CHEK2, ATM, HOXB13, and BRCA1 [12]. Importantly, a family history encompasses these genetic factors as well as shared environmental factors.
Describing germline alterations is important for advancing our understanding of hereditary prostate cancer, while estimating population risks can help inform decision-making about screening. The clinical setting of a man presenting for biopsy is different because of the availability of clinical information such as prostate-specific antigen (PSA) level or digital rectal examination (DRE). Moreover, these men, by definition, have an indication for biopsy and thus are all at a high risk of having prostate cancer. We sought to provide a contemporary estimate of risk for high-grade disease for varying degrees of family history of prostate or breast cancer, independent of other clinical risk factors, within a large multi-institutional biopsy cohort, the Prostate Biopsy Collaborative Group (PBCG) [13]. This analysis can inform counseling on the increased risk associated with a positive family history in men with other features prompting a biopsy. Here, we describe the association between high-grade prostate cancer and each type of family history (first- or second-degree prostate cancer, or first-degree breast cancer) among patients in whom more than one family history type was present.
2. Patients and methods
2.1. Data source
We utilized prostate biopsy outcomes and prebiopsy risk factors from PBCG. This multi-institutional protocol describes the histologic results of patients who underwent prostate biopsy. Prospective data collection from 12 sites started in 2014, although some sites provided data on biopsies from 2006 to 2014 that had prospectively been collected for institutional databases [13]. Four sites retrospectively reviewed data on biopsies performed from 2006 to 2014. Each site obtained local institutional review board approval to participate. The consortium is composed of eight tertiary care referral centers (Cleveland Clinic, Martini-Clinic Prostate Cancer Center at Hamburg, Mayo Clinic, Memorial Sloan Kettering Cancer Center, IRCCS Hospital San Raffaele, Triemli Hospital, University of California San Francisco [UCSF], and University Hospital Zurich), two Veterans Affairs (VA) centers (Durham, NC and San Juan, PR, USA), and two other health systems that include tertiary care referral centers and associated community or VA urology providers (Sunnybrook [University of Toronto] and University of Texas Health Science Center at San Antonio [UT Health]). All sites collected data on the first-degree family history of prostate cancer, except for UCSF, or retrospectively added cases at the San Juan VA, which were accordingly excluded from this analysis (n = 1681). Prospectively collected data from four sites (Durham VA, San Juan VA, UT Health, and Zurich) included information on the number (and type) of first- and second-degree relatives with prostate cancer and relatives with breast cancer.
We defined high-grade prostate cancer as Gleason 3 + 4/grade group ≥2 disease. Positive cases missing Gleason score (n = 9) were excluded. Additional prebiopsy variables of interest were collected at each site, including age, PSA (ng/ml) level, DRE, and history of prior negative biopsy. We included both magnetic resonance imaging (MRI)-guided and standard ten- to 12-core biopsies. In patients who underwent more than one biopsy during the study period (n = 1129), only the first biopsy was considered. We excluded patients with a prior diagnosis of prostate cancer. Additional descriptions of the cohort have been published [13].
2.2. Statistical analyses
To examine the relationship between a first-degree family history of prostate cancer and the risk of high-grade disease, a series of logistic regression models were fit, adjusting for the natural logarithm of PSA (logPSA), age, race (white, African American, or other), DRE (abnormal vs normal), type of biopsy (MRI fusion or standard transrectal ultrasonography guided), and history of a prior negative biopsy (yes/no). While continuing to adjust for these variables, additional models were fit including an interaction term between logPSA and a first-degree history, an interaction with PSA categorized as high or low (cutoff 3 ng/ml), an interaction between age and a first-degree history, and the number of affected first-degree relatives (0, 1, or ≥2). Two additional models evaluated the association between Gleason ≥3 + 4 cancer and a second-degree family history: one including only a second-degree family history and another that also included a first-degree family history.
Our second series of logistic regression models tested the association between a first-degree family history of breast cancer and high-grade prostate cancer. Adjusting for the same clinical characteristics, we investigated the family history of breast cancer alone, including an interaction with logPSA and with a first-degree history of prostate cancer also in the model. We included interaction terms in the analysis to test whether a prostate or breast cancer family history has a larger or smaller effect depending on the PSA levels.
For models that included both a first- and second-degree history of prostate cancer, or a first-degree history of both prostate and breast cancer, we sought to determine whether increases in the risk of high-grade prostate cancer were independently explained by different types of family histories. To account for decreases in precision due to missing predictor variables, including race, DRE, and prior negative biopsy, we performed a sensitivity analysis using multiple imputation with chained equations, replicating the primary analyses. All analyses were conducted using R 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Within the PBCG, data were available on 20 323 patients who underwent prostate biopsy from January 2006 to February 2020, of whom 4524 (22%) were missing a family history of first-degree prostate cancer, resulting in data on 15 799 patients available for analysis. Among the four sites that recorded additional family history detail, data were available for 4619 (99.9%) patients. In the overall cohort, there were proportionally fewer African American patients than in the four sites with detailed family history available, but other characteristics appeared similar (Table 1). Patients were most likely to have a first-degree relative with prostate cancer (19%), followed by a second-degree relative (8.0%), and a first-degree relative with breast cancer (7.5%). Among those with a first-degree relative, a father with prostate cancer (59%) was more common than a brother (49%) or son (0.7%). There was low correlation between family history types: in patients with a first-degree relative with prostate cancer, 15% also had an affected second-degree relative, while 11% had a first-degree relative with breast cancer (Supplementary Table 3). PSA levels at biopsy in men without (vs with) a first-degree family history of prostate cancer were slightly higher (median difference = 0.4, 95% confidence interval [CI] 0.3–0.6, p < 0.001), but no differences were detected based on first-degree breast cancer (median difference = –0.3, 95% CI –0.7 to 0.04, p = 0.7). This was further evaluated as an interaction term in regression models.
Table 1 –
Characteristics of the Prostate Biopsy Collaborative Group cohort, and among the four sites (Zurich, UTHSCSA, San Juan VA, and Durham VA) with additional family history data availablea
| Characteristic | Overall (N = 20 323) | Detailed family history available (N = 4621) |
|---|---|---|
|
| ||
| Age at biopsy (yr) | 65 (60–70) | 65 (60–69) |
| Missing | 2 | 0 |
| Race | ||
| White | 11 505 (80) | 2605 (56) |
| Black | 2128 (15) | 1779 (39) |
| Other | 708 (4.9) | 229 (5.0) |
| Missing | 5982 | 8 |
| Prostate-specific antigen (ng/ml) | 6.4 (4.6, 9.8) | 6.0 (4.6, 8.7) |
| Abnormal digital rectal exam | 4474 (25) | 1507 (34) |
| Missing | 2482 | 217 |
| Prior negative biopsy | 2798 (21) | 924 (20) |
| Missing | 6933 | 0 |
| Biopsy Gleason grade group | ||
| Negative | 9552 (47) | 2089 (45) |
| 1 | 3682 (18) | 925 (20) |
| 2 | 3185 (16) | 770 (17) |
| 3 | 1491 (7.3) | 365 (7.9) |
| Gleason 7 unspecified | 159 (0.8) | 0 (0) |
| 4 | 924 (4.5) | 247 (5.3) |
| 5 | 1330 (6.5) | 225 (4.9) |
| MRI-guided biopsy | 1234 (6.1) | 318 (6.9) |
| First-degree prostate cancer family history | 2889 (18) | 888 (19) |
| Missing | 4524 | 2 |
| Type of first-degree prostate cancer family history | ||
| Father | – | 446 (50) |
| Brother | – | 352 (40) |
| Father and brother | – | 83 (9.4) |
| Son | – | 6 (0.7) |
| Missing | – | 3 |
| Number of first-degree prostate cancer relatives | ||
| 1 | – | 751 (85) |
| 2 | – | 108 (12) |
| 3 | – | 24 (2.7) |
| 4 | – | 4 (0.5) |
| Missing | – | 3 |
| Second-degree prostate cancer family history | – | 371 (8.0) |
| Missing | – | 6 |
| First-degree breast cancer family history | – | 348 (7.5) |
| Missing | – | 8 |
IQR = interquartile range; MRI = magnetic resonance imaging; UTHSCSA = University of Texas Health Science Center at San Antonio; VA = Veterans Affairs.
Additional data collected at these sites include the number of first- and second-degree relatives with a family history of prostate or breast cancer. Median (IQR) and n (%) are shown for continuous and categorical variables, respectively.
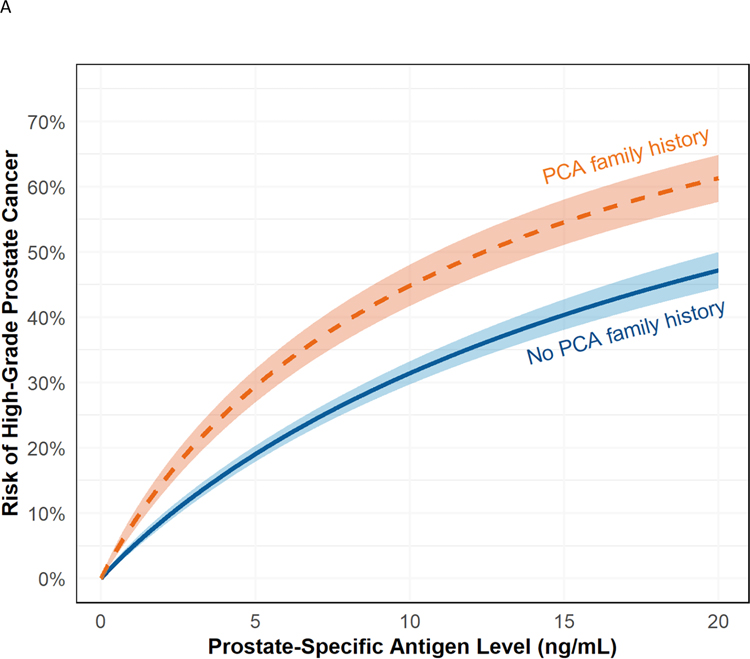
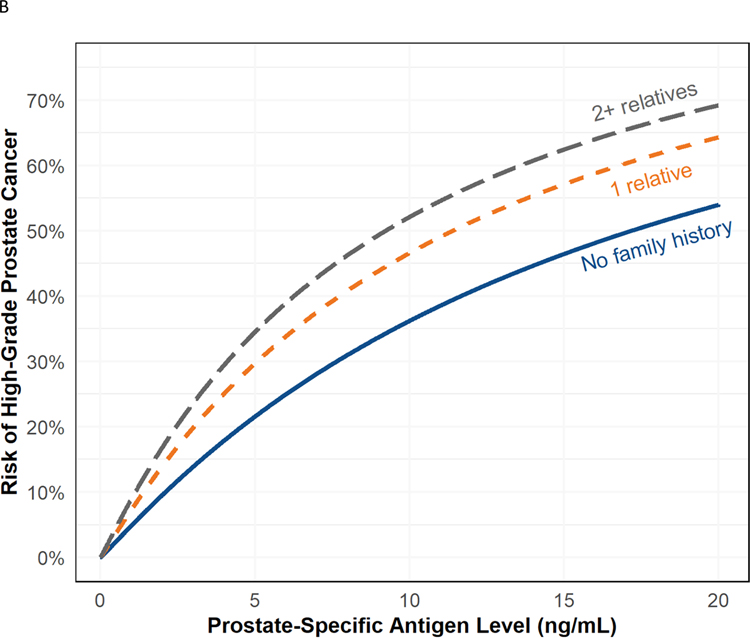
In multivariable logistic regression models, odds of high-grade prostate cancer were 1.77 times greater (95% CI 1.57–2.00, p < 0.001) for patients with a first-degree relative with prostate cancer (Table 2 and Fig. 1A). There was no evidence of heterogeneity across sites (p = 0.7; Supplementary Fig. 1). There was no statistically significant interaction between family history and PSA levels, whether dichotomized at ≥3 ng/ml (p = 0.3) or as a continuous variable (p = 0.4). There was an interaction between age and a first-degree prostate cancer family history, such that the family history was less strongly associated with high-grade prostate cancer with increasing age (p = 0.024; Supplementary Fig. 2). In the model evaluating the effect of multiple first-degree relatives with prostate cancer, the estimated odds of high-grade prostate cancer were higher with two or more (vs one) first-degree relative with prostate cancer (odds ratio [OR] 1.92 vs 1.54; Fig. 1B). In models exploring the effect of second-degree relatives with prostate cancer, a positive family history increased the risk (OR 1.38, 95% CI 1.07–1.77, p = 0.011). However, the risk associated with a second-degree history decreased to 1.27 (95% CI 0.98–1.63, p = 0.068) when a first-degree family history of prostate cancer was added to the model.
Table 2 –
Multivariable logistic regression model estimates for odds of high-grade prostate cancer, for various prostate cancer family history modelsa
| Family history characteristic | Odds ratio | 95% CI | p value |
|---|---|---|---|
|
| |||
| First-degree prostate cancer family history | 1.77 | 1.57–2.00 | <0.001 |
| First-degree prostate cancer family history | 1.52 | 1.03–2.22 | 0.031 |
| Log PSA × first-degree prostate cancer family history | 1.09 | 0.89–1.33 | 0.4 |
| First-degree prostate cancer family history | 1.27 | 0.78–2.03 | 0.3 |
| High PSA × first-degree prostate cancer family history | 1.31 | 0.81–2.18 | 0.3 |
| Number of first-degree prostate cancer relatives | |||
| 1 | 1.54 | 1.28–1.84 | <0.001 |
| 2+ | 1.92 | 1.28–2.84 | 0.001 |
| Second-degree prostate cancer family history | 1.38 | 1.07–1.77 | 0.011 |
| First-degree prostate cancer family history | 1.55 | 1.30–1.84 | <0.001 |
| Second-degree prostate cancer family history | 1.27 | 0.98–1.63 | 0.068 |
CI = confidence interval; MRI = magnetic resonance imaging; PSA = prostate-specific antigen.
All models are adjusted for age, race, digital rectal examination (normal or abnormal), MRI-guided technique, presence of a prior negative biopsy, and the natural log of PSA.
Fig. 1 –
(A) Risk of high-grade prostate cancer based on PSA for patients with (orange) and without (blue) a first-degree family history of prostate cancer with 95% confidence intervals. Note that there is no significant interaction between PSA and family history, so the effect of family history is consistent across PSA levels. (B) Probability of high-grade prostate cancer based on the number of first-degree relatives with prostate cancer. The solid line indicates no family history (blue), and the dashed lines indicate one (orange), and two or more (gray) affected relatives. Note that there is no significant interaction between PSA and family history, so the effect of family history is consistent across PSA levels.
PCA = first-degree prostate cancer; PSA = prostate-specific antigen.
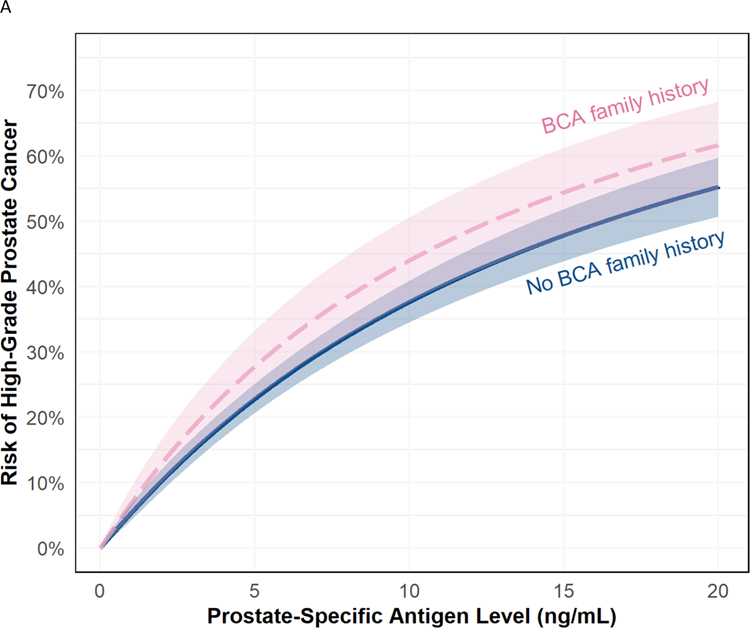
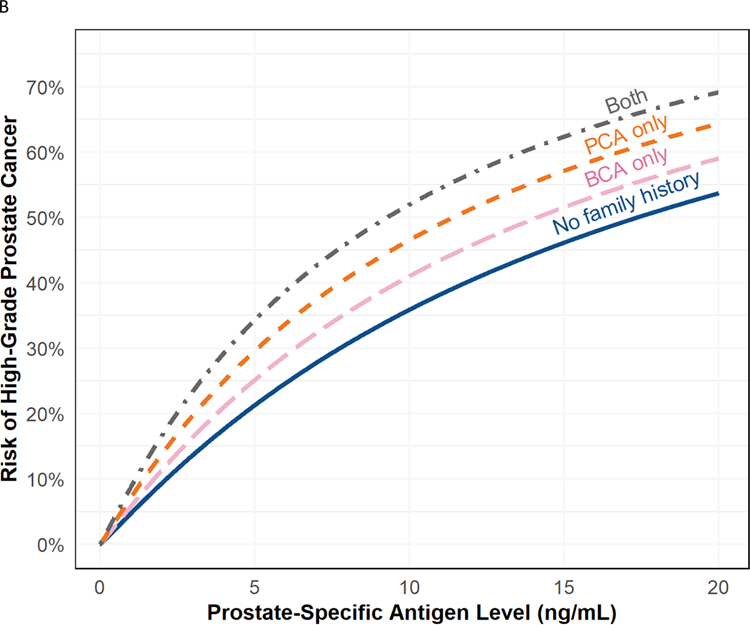
Having a first-degree relative with breast cancer was also associated with high-grade prostate cancer on biopsy (OR 1.30, 95% CI 1.01–1.67, p = 0.040; Table 3 and Fig. 2A). When also adjusting for a first-degree prostate cancer family history, the association between a breast cancer family history and high-grade prostate cancer was attenuated and no longer statistically significant (OR 1.24, 95% CI 0.96–1.60, p = 0.093; Fig. 2B). A model including an interaction term between a breast cancer family history and PSA revealed no evidence of effect modification (p > 0.9).
Table 3 –
Multivariable logistic regression model estimates for odds of high-grade prostate cancer for various first-degree breast cancer family history modelsa
| Family history characteristic | Odds ratio | 95% CI | p value |
|---|---|---|---|
|
| |||
| First-degree breast cancer family history | 1.30 | 1.01–1.67 | 0.040 |
| First-degree breast cancer family history | 1.34 | 0.55–3.07 | 0.5 |
| Log PSA × first-degree breast cancer family history | 0.98 | 0.65–1.55 | >0.9 |
| First-degree prostate cancer family history | 1.56 | 1.31–1.85 | <0.001 |
| First-degree breast cancer family history | 1.24 | 0.96–1.60 | 0.093 |
CI = confidence interval; MRI = magnetic resonance imaging; PSA = prostate-specific antigen.
All models are adjusted for age, race, digital rectal examination (normal or abnormal), MRI-guided technique, presence of a prior negative biopsy, and the natural log of PSA.
Fig. 2 –
(A) Risk of high-grade prostate cancer based on PSA for patients with (orange) and without (blue) a first-degree family history of breast cancer with 95% confidence intervals. Note that there is no significant interaction between PSA and family history, so the effect of family history is consistent across PSA levels. (B) Risk of high-grade prostate cancer based on PSA for patients with a family history of both first-degree prostate and breast cancer (gray), only a first-degree prostate cancer family history (orange), only a first-degree breast cancer family history (pink), or no family history or either cancer (blue). Note that there is no significant interaction between PSA and family history, so the effect of family history is consistent across PSA levels. Family history of first-degree prostate cancer and first-degree breast cancer was reported in 99 patients.
BCA = first-degree breast cancer; PCA = first-degree prostate cancer family history; PSA = prostate-specific antigen.
Sensitivity analyses using multiple imputation to account for missing predictor variables were largely similar, but with some notable differences (Supplementary Tables 1 and 2). The imputed models attenuated the increase in risk based on a first-degree family history alone (OR 1.55 [95% CI 1.39–1.74] compared with 1.77 [95% CI 1.57–2.00] in the primary model). Estimates comparing the imputed models for a second-degree history of prostate cancer with those for a first-degree history of breast cancer were very similar to the primary analysis. A separate sensitivity analysis using a definition of Gleason ≥4 + 3 (Grade group ≥3) yielded a lower OR for a first-degree prostate cancer family history (OR 1.38, 95% CI 1.18, 1.61; p < 0.001).
4. Discussion
We found that among men who meet the indications for a prostate biopsy, those with a first-degree relative with prostate cancer have an increased risk of grade group ≥2 disease compared with men who do not have a first-degree relative with prostate cancer, given equivalent PSA and other clinical risk factors. A positive family history of breast cancer, or a second-degree relative with prostate cancer, also increased the risk (roughly 1.2 times for each) of detecting high-grade cancer. Our models also showed an overlap in the risk associated with the family history of breast and prostate cancers in first-degree relatives, consistent with the shared genetic and environmental factors underlying the familial risk for these cancers. Finally, based on interaction terms added to the models, there was no evidence that the effect of a family history differed based on PSA level, but a first-degree prostate cancer family history was more strongly associated with high-grade cancer at younger ages.
Our study is unique in that it evaluated the adjusted risk of high-grade prostate cancer based on a family history of cancer in a heterogeneous, international cohort. This cohort allowed us to evaluate Gleason grade group ≥2 disease, a relevant outcome given the effort to decrease overtreatment of low-risk disease [14]. Thus, we provide a contemporary, generalizable estimate of risk for patient counseling, holding constant other factors that would prompt biopsy.
In our cohort of patients selected for biopsy, which therefore have a higher baseline risk, a lower observed effect for family history is expected. In a population-based cohort, the increased risk associated with a family history reflects the greater propensity to be screened as well as to detect cancer once screened. Population-based case-control studies in the late 1990s estimated 2.3–3.2 times greater odds (unadjusted) of prostate cancer in the presence of an affected first-degree relative, with similar findings reported in European cohorts [1,2,15]. Similarly, a 2015 study that linked Utah population-based genealogy data to the state’s cancer registry (n = 635 443) found a relative risk of 2.76 (95% CI 2.69–2.82) in men with one or more first-degree relatives and a relative risk of 1.51 (95% CI 1.47–1.56) in men with a second-degree relative with prostate cancer [16]. As expected, such population-based analyses are uniformly higher than the relative risk of 1.40 calculated from the adjusted OR of 1.77 (95% CI 1.57–2.00) that we report. In addition to differences in the cohorts analyzed (biopsy vs population based), our multivariable analyses account for other adverse prognostic factors in patients with a family history, also explaining the lower risk estimates. Owing to these differences, our results serve a separate purpose: counseling a patient on the independent risk accounted for due to their family history once they have already been screened and referred for consideration of prostate biopsy.
Moreover, we evaluated high-grade (as opposed to any) prostate cancer, a more clinically relevant endpoint for men undergoing biopsy. However, after refitting our models with any prostate cancer as the outcome, heterogeneity tests did not show that estimates differed significantly from the high-grade prostate cancer models (all p > 0.2).
The association between a breast cancer family history and prostate cancer detected in this study has also been identified in previous studies [3,7,17–19]. An analysis of the Health Professionals Follow-up Study, which included 37 002 men followed over a 16-yr period, estimated a hazard ratio (HR) of 1.21 (95% CI 1.10–1.34) for detecting prostate cancer in the presence of a breast cancer family history and a 34% increased risk of lethal prostate cancer [17]. Similar to our findings, the investigators estimated a greater increased risk with a family history of prostate cancer (age-adjusted HR 1.76, 95% CI 1.60–1.92) versus breast cancer (age-adjusted HR 1.26, 95% CI 1.14–1.39). Our analysis, including both family history types in the same model, suggested an overlap in the associated risk of prostate cancer. While germline mutations predisposing to both cancer types have been identified (BRCA1/2, CHEK2, ATM, and MLH1), it must be understood that a family history of cancer encompasses both inherited genes and shared environments among family members, and the interplay between the two [7,12,20]. Obesity, which results from both genetic and environmental factors, is thought to increase the risk of both aggressive prostate cancer and postmenopausal breast cancer [21,22]. Furthermore, when genetic factors predisposing to prostate cancer are present, they can be attenuated based on environmental factors [23]. A prior study found that family history was associated with prostate cancer in Europeans, but not in North Americans enrolled in the REDUCE study, highlighting the potential role of the environment [19]. Thus, family history remains an important clinical variable, even as understanding of the genetic basis of cancer continues to evolve.
This study is distinguished by its large cohort, reflecting patients treated in academic, VA, and community settings across North America and Europe. We were also uniquely able to control for risk factors beyond age (especially PSA), thus providing a relevant estimate for patients facing prostate biopsy. However, this study is not without limitations. Our study is affected by missing data, especially in the race (n = 5982 missing) and prior negative biopsy (n = 6933) covariates. After multiple imputation, most estimates were similar, except for first-degree family history, where the OR decreased from 1.77 to 1.55. Owing to population-based estimates of first-degree family member risk exceeding a relative risk of 2.0, we feel that our primary analysis estimate of 1.77 is likely closer to the true association in men undergoing biopsy. Patients with missing family history data were similar in clinical characteristics, except for very few patients with missing family history data having had MRI-guided biopsy and more having a prior negative biopsy (32% vs 21%; Supplementary Table 5). Finally, there were differences in the nuances of data collection at different sites, and an ascertainment bias is possible. For instance, if retrospectively reviewed cases where family history was truly negative were recorded as missing, it would bias our reporting toward lower risk estimates. That said, a sensitivity analysis showed similar findings when including only prospective cases (first-degree prostate cancer family history model OR 1.74, 95% CI 1.51, 2.00; p < 0.001).
5. Conclusions
In summary, we found that a first- or second-degree family history of prostate cancer and a first-degree family history of breast cancer are associated with Gleason ≥3 + 4 disease in men selected for biopsy. While these three types of family history are each associated with an increased risk while controlling for other clinical factors, our models indicate an overlap between the underlying genetic predispositions and environmental factors. This increased risk does not appear to vary by PSA level. In younger patients, a first-degree prostate cancer family history had a stronger association, which may be reflective of underlying genetic inheritance or that older men are biopsied only in the presence of other strong risk factors. These data support the wider inclusion of family histories of cancer in nomograms, patient counseling, and clinical decision-making. Further research is required to better understand the genetic and shared environmental factors that increase the risk of both breast and prostate cancer.
Supplementary Material
In a large, international cohort, men with indications for prostate biopsy have an increased risk of high-grade prostate cancer in the presence of a family history of prostate cancer, second-degree prostate cancer, and first-degree breast cancer, controlling for other risk factors. This risk did not vary based on prostate-specific antigen level, and having additional affected relatives conferred an increased risk of high-grade prostate cancer on biopsy.
Acknowledgments:
The contents of this publication do not represent the views of the VA Caribbean Healthcare System, the Department of Veterans Affairs, or the US Government. This material is based upon work supported by the Research and Development Service, Urology Section, Surgery Department and Department of Veterans Affairs, Caribbean Healthcare System San Juan, PR, USA.
Financial disclosures:
Andrew J. Vickers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Conflicts of interest: Andrew J. Vickers is a co-inventor of the 4K score test (OPKO Biotechnology, Inc.) and received stock options and rights to royalty income. Lourdes Guerrios-Rivera is a urology staff at VA Caribbean Healthcare System, San Juan, Puerto Rico. There are no other conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lesko SM, Rosenberg L, Shapiro S. Family history and prostate cancer risk. Am J Epidemiol 1996;144:1041–7. [DOI] [PubMed] [Google Scholar]
- [2].Cerhan JR, Parker AS, Putnam SD, et al. Family history and prostate cancer risk in a population-based cohort of Iowa men. Cancer Epidemiol Biomarkers Prev 1999;8:53–60. [PubMed] [Google Scholar]
- [3].Ren ZJ, Cao DH, Zhang Q, et al. First-degree family history of breast cancer is associated with prostate cancer risk: a systematic review and meta-analysis. BMC Cancer 2019;19:871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sellers TA, Potter JD, Rich SS, et al. Familial clustering of breast and prostate cancers and risk of postmenopausal breast cancer. J Natl Cancer Inst 1994;86:1860–5. [DOI] [PubMed] [Google Scholar]
- [5].Beebe-Dimmer JL, Yee C, Bock C, et al. Familial clustering of breast and prostate cancer and risk of postmenopausal breast cancer in the Women's Health Initiative Study. Cancer 2015;121:1265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Grill S, Fallah M, Leach RJ, et al. Incorporation of detailed family history from the Swedish Family Cancer Database into the PCPT risk calculator. J Urol 2015;193:460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Beebe-Dimmer JL, Kapron AL, Fraser AM, Smith KR, Cooney KA. Risk of prostate cancer associated with familial and hereditary cancer syndromes. J Clin Oncol 2020;38:1807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jacobs EJ, Chanock SJ, Fuchs CS, et al. Family history of cancer and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Int J Cancer 2010;127:1421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 2015;163:1011–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med 2016;375:443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Castro E, Romero-Laorden N, Del Pozo A, et al. PROREPAIR-B: a prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2019;37:490–503. [DOI] [PubMed] [Google Scholar]
- [12].Cheng HH, Sokolova AO, Schaeffer EM, Small EJ, Higano CS. Germline and somatic mutations in prostate cancer for the clinician. J Natl Compr Canc Netw 2019;17:515–21. [DOI] [PubMed] [Google Scholar]
- [13].Ankerst DP, Straubinger J, Selig K, et al. A contemporary prostate biopsy risk calculator based on multiple heterogeneous cohorts. Eur Urol 2018;74:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: risk stratification, shared decision making, and care options. J Urol 2018;199:683–90. [DOI] [PubMed] [Google Scholar]
- [15].Brandt A, Bermejo JL, Sundquist J, Hemminki K. Age-specific risk of incident prostate cancer and risk of death from prostate cancer defined by the number of affected family members. Eur Urol 2010;58:275–80. [DOI] [PubMed] [Google Scholar]
- [16].Albright F, Stephenson RA, Agarwal N, et al. Prostate cancer risk prediction based on complete prostate cancer family history. Prostate 2015;75:390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Barber L, Gerke T, Markt SC, et al. Family history of breast or prostate cancer and prostate cancer risk. Clin Cancer Res 2018;24:5910–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hemminki K, Chen B. Familial association of prostate cancer with other cancers in the Swedish Family-Cancer Database. Prostate 2005;65:188–94. [DOI] [PubMed] [Google Scholar]
- [19].Thomas JA 2nd, Gerber L, Moreira DM, et al. Prostate cancer risk in men with prostate and breast cancer family history: results from the REDUCE study (R1). J Intern Med 2012;272:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kicinski M, Vangronsveld J, Nawrot TS. An epidemiological reappraisal of the familial aggregation of prostate cancer: a meta-analysis. PLoS One 2011;6:e27130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol 2013;63:800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78. [DOI] [PubMed] [Google Scholar]
- [23].Loeb S, Peskoe SB, Joshu CE, et al. Do environmental factors modify the genetic risk of prostate cancer? Cancer Epidemiol Biomarkers Prev 2015;24:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.