Abstract
Rationale & Objective:
Recent reassessment of the use of race in estimated glomerular filtration rate (eGFR) in adults has instigated questions about the role of race in eGFR expressions for children. Little research has examined the associations of self-reported race with measured GFR (mGFR) adjusting for serum creatinine or cystatin C in children and young adults with chronic kidney disease (CKD). This study examined these associations and evaluated the performance of the previously published Under 25 (U25) eGFR equations in a large cohort of children and young adults with CKD.
Study Design:
Observational cohort study.
Setting & Participants:
Participants in the Chronic Kidney Disease in Children (CKiD) study including 191 Black and 674 non-Black participants contributing 474 and 1896 annual person-visits, respectively.
Exposures:
Self- or parental-reported race (Black, non-Black). Adjustment for serum creatinine and or cystatin C; body size; and socioeconomic status.
Outcome:
Measured iohexol clearance-based GFR.
Analytical Approach:
Linear regression with generalized estimating equations, stratified by age (<6, 6–12, 12–18 and ≥18 years) incorporating serum creatinine or serum cystatin C. Contrasting performance in different self-reported racial groups of the U25 eGFR equations.
Results:
Self-reported Black race was significantly associated with 12.8% higher mGFR among children in regression models including serum creatinine. Self-reported Black race was significantly associated with 3.5% lower mGFR after adjustment for cystatin C overall, but was not significant for those over 12 years. Results were similar after adjustment for body size and socioeconomic factors. The average of creatinine- and cystatin C-based U25 equations was unbiased by self-reported race groups.
Limitations:
Small number of children <6 years; estimated lean body mass.
Conclusions:
Differences in the creatinine-mGFR relationship by self-reported race were observed in children and young adults with CKD and were consistent with findings in adults. Smaller and opposite differences were observed for the cystatin C-mGFR relationship, especially in the younger age group. We recommend inclusion of children for future investigations of biomarkers to estimate GFR. Importantly, for GFR estimation among those under 25 years of age, the average of the new U25 creatinine and cystatin equations without race coefficients yields unbiased estimates of mGFR.
Plain language summary
Increased discussion about use of race in estimation of glomerular filtration rate (GFR) in adults motivated this investigation in children using self/parental-reported race and measured GFR data from the Chronic Kidney Disease in Children (CKiD) study. Self-reported Black race was systematically associated with slightly higher GFR, after adjustment for serum creatinine in children older than 6 and young adults. We observed a smaller and opposite difference when adjusting for cystatin C. The Under 25 (U25) eGFR equations, which are race independent, yielded significant but small bias in the SCr-only equation for self-reported Black race, but when averaged with the U25 CysC-equation, was unbiased. Our results were consistent with adult studies, and we recommend inclusion of children for future investigations of biomarkers to estimate GFR.
INTRODUCTION
Glomerular filtration rate (GFR) is considered the best measure of kidney health. Equations to estimate GFR (eGFR) are commonly used clinically and in research to quantify kidney function. In adults, eGFR equations are based on endogenous biomarkers that are filtered by the kidney, specifically serum creatinine (SCr)1,2 and cystatin C (CysC), as well as demographic characteristics, including age, sex and, in the case of SCr-based equations, race (as a binary term for Black and non-Black race). SCr is an endogenous compound and is a metabolite of creatine, which is generally found in muscle, is filtered by the kidney, but is also known to have non-GFR determinants and therefore has limitations as a direct marker of GFR2–4. The CKD-EPI equation based on CysC includes terms for age and sex, but not one for race because it was not a significant predictor of GFR (CysC was hypothesized to be independent of muscle mass)5. In contrast, the combined CKD-EPI equation included both SCr and CysC with a coefficient for Black race4,5. The inclusion and implications of a Black (or African-American) race coefficient in equations for adults has recently generated a great deal of attention, particularly in the United States.
New research and editorials have discussed the potential impact of removing race, a sociopolitical, and not a biological construct, from GFR estimating equations, in terms of the diagnosis of chronic kidney disease (CKD), access to organ availability, and medication dosing decisions4–10. In addition, adult equations were known to have heterogeneous classifications of race (self-reported or investigator assigned), which was a limitation.4 Most recently, the presidents of the National Kidney Foundation and the American Society of Nephrology issued a statement that race modifiers should not be included in estimates of GFR11 and a Task Force convened by the two organizations has issued its final report in 202112.
Pediatric eGFR equations from the Chronic Kidney Disease in Children (CKiD) cohort do not have a coefficient for Black race13,14, including the recent equations designed for populations under the age of 25 (“U25 equations”)15. The lack of race coefficients for earlier equations was informed by a lack of predictive value of self-reported race (likely due to smaller sample sizes)13,14, and recognizing the need for equations free of a race coefficient for the U25 equations15. However, the relationship between GFR, SCr, CysC, and self-reported race in children and young adults with kidney diseases has not been comprehensively characterized. CKiD data provides the opportunity to investigate these biomarkers in a pediatric and young adult population with CKD, using centrally measured GFR (mGFR), SCr, and CysC, in the context of self- and parental-reported race, to examine putative differences in these endogenous biomarkers across ages and levels of kidney function, by self-reported race.
We explored the relationships between SCr and mGFR, and CysC and mGFR, with adjustment for different metrics of body size, including estimated lean body mass (eLBM) because muscle mass is hypothesized to explain, at least in part, the relationship between SCr and GFR16. In addition, we adjusted for potential confounding socioeconomic indicators which have been associated with disease progression in children17,18. We investigated how the mGFR and biomarker relationships were modified by participants self-reporting mixed and not mixed Black race, and then evaluated the performance of the new U25 estimated GFR (eGFR) equations by self-reported race. Lastly, we discuss the implications of these pediatric results for GFR estimation more broadly.
METHODS
Study population
The CKiD study is a longitudinal multicenter cohort of 1095 children with a diagnosis of kidney disease and eGFR < 90/ml/min|1.73m2 at entry initiated in 2005 across 56 clinical sites in the United States and Canada. Annual in-person visit data consisted of markers of kidney function, including serum biomarkers, growth, cardiovascular health, and self- or parental-reported general health. Plasma disappearance of iohexol GFR was measured (mGFR) at the first and second annual study visit and every other year thereafter through 2018, at which point, the procedure became optional. A complete description of the study design has been previously published19. All participants and families provided informed consent/assent and study protocols were approved by the Institutional Review Boards of each site.
Self- or parental-reported race
Race was reported by the participant or their accompanying parent/guardian, if the participant was too young. The question was “Which of the following describe the race of (name of child)?” with the following response options: “White”, “Black/African American”, “American Indian/Alaskan Native”, “Asian”, “Native Hawaiian/Pacific Islander” and “Other”. Participants were instructed to circle “Yes”, “No”, or “Don’t Know” for each of those choices. For the primary analysis, to be consistent with adult GFR estimating equation categorization, Black race was defined as Black/African American with mixed race including Black/African American (i.e., checking another race in addition to Black). As a secondary analysis, race was categorized as “non-Black”, “Black with mixed race”, or “Black without mixed race”.
Kidney function measurement and serum creatinine and cystatin C
mGFR was measured in ml/min|1.73m2, by plasma disappearance of iohexol using validated protocols20–23. Briefly, mGFR was calculated based on a 2-compartment model with four plasma samples collected after iohexol injection; and a 1-compartment model, with either three or two plasma samples collected, and a universal correction equation22,24. mGFR was standardized to body surface area (BSA) using the Haycock formula25.
Serum samples were collected at the time of GFR measurement. Kidney function biomarkers (SCr and CysC) were measured at the CKiD Central Biochemistry Laboratory (University of Rochester) and used methods calibrated to International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) reference materials (mg/dL for SCr26, and mg/L for CysC27).
Markers of body size
Since SCr is suggested to be dependent in part on muscle mass, we compared the relationship between SCr and mGFR, by race, adjusting for a panel of markers of body size, overall and stratified by age (<6, ≥6 to <12, ≥12 to <18, ≥18 years). Markers of body size included sex, age, Tanner stage, height (meters), body surface area (m2), eLBM (kg) without and with a coefficient for Black race. There was little to no variability for Tanner stage among children <6 (100% Stage 1) and adults ≥18 years (87% Stage 5) and were not included for these groups. Lean body mass was not directly measured but eLBM was a proxy to adjust for a governing factor of creatinine28. These eLBM equations were sex-specific and based on height, weight, BMI z-score and age, without and with a coefficient for race (Black/non-Black).
Socioeconomic indicators
Parental/guardian report of annual household income at baseline was classified as <$36,000; $36,000 to $75,000; and, >$75,000. Maternal education was defined as college or more, or less than any college, as a binary variable. These variables were considered potential confounders of the exposure (self-reported race) to outcome (mGFR) relationships.
Statistical analyses
Since racial differences in SCr-derived eGFR equations were observed in adult studies, all analyses in this study were presented overall and stratified by age categories. To describe the relationship of SCr with mGFR, scatterplots displayed mGFR on SCr (both were log-transformed). To describe CysC, the same approach was used with CysC on the x-axis. Nonparametric lowess splines summarized the relationship by race with ellipses depicting the region in which 90% of the data distribute under bivariate normal assumptions29,24.
Linear regression models of mGFR on SCr (both in the natural log scale) with an independent variable for Black race, and body size metrics as additional independent variables. Each body size variable was included separately (none, sex, age as a continuous variable, height, BSA, eLBM without and with ancestry). Multivariable models were constructed with adjustment for a) age and sex (i.e., similar to CKD-EPI equation); b) age, sex and height (similar to CKiD equations); c) age, sex, height, and eLBM without ancestry; and d) age, sex, height, household income category, and maternal college education. A total of eleven models, including unadjusted, were fit and compared. We also tested the statistical interaction between self-reported Black race and age categories for each model. We note that in the multivariable model with height and eLBM, eLBM is derived from height as well as other markers of body size.
Generalized estimating equations (GEE) with an independent working correlation structure provided valid standard errors and corresponding 95% confidence intervals for repeated measurements within individuals. The same models were fit with log(CysC) as an independent variable instead of log(SCr). Sensitivity analyses restricted to one randomly selected observation per individual investigated robustness of inference in the absence of repeated measures. Lastly, we included self-reported race as a three-category variable (“non-Black”, “Black with mixed race”, and “Black without mixed race”) in the regression models above and report the percent differences in mGFR with non-Black being the reference after adjusting for age, sex, height, income category and maternal education, and SCr and CysC in separate respective models.
To assess performance of the U25 equations stratified by self-reported race, we reanalyzed the testing dataset which was independent from model development.15 We compared the U25 equations based on SCr, CysC, and the average of the two, to mGFR. Agreement metrics included bias (with 95% confidence intervals), proportions within 30% and within 10% of mGFR, and root mean square error (RMSE).
Statistical significance was assessed at p<0.05. All analyses and graphics were conducted and produced in SAS 9.4 and R 4.0.0.
RESULTS
A total of 190 self-reported Black participants contributed 473 person-visits, and 675 self-reported non-Black participants contributed1897 person-visits. Table 1 demonstrates that both groups had similar age distributions (median age= 9 years), and a similar proportion of males (66.2% and 61.3% among Black and non-Black person-visits, respectively). Among those who self-reported as Black race, 22.6% self-reported mixed race. A total of 85.0% of non-Black participants self-reported White race, and 5.6% reported non-Black mixed race. Black participants were more likely to report household income < $36,000 (65.5% vs. 33.3%), and a maternal education less than college (77.0% vs. 64.6%). Body size and Tanner stage were similar but average eLBM was greater among Black participants. Median SCr was the same between Black and non-Black participants (1.2 mg/dL), but CysC was lower (1.51 vs. 1.73 mg/dL, respectively). Median mGFR was higher among Black participants than non-Black participants (52.9 vs. 46.2 ml/min|1.73m2).
Table 1.
Demographic, clinical and longitudinal data characteristics of person-visits, stratified by self-reported race. Median [interquartile range] or n (%).
| Overall population n=2370 person-visits from 865 participants |
Self-reported Black race n=473 person-visits from 190 participants |
Self-reported Non-Black race n=1897 person-visits from 675 participants |
|
|---|---|---|---|
|
| |||
| Time-fixed variables | |||
| Male | 1476 (62.3%) | 1163 (61.3%) | 313 (66.2%) |
| Self-reported race | |||
| Black, no mixed race | 366 (15.4%) | 366 (77.4%) | NA |
| Black, with mixed race | 107 (4.5%) | 107 (22.6%) | NA |
| White | 1613 (68.1%) | NA | 1613 (85%) |
| Non-Black with mixed race | 106 (4.5%) | NA | 106 (5.6%) |
| Other race | 78 (3.3%) | NA | 78 (4.1%) |
| Asian | 51 (2.2%) | NA | 51 (2.7%) |
| American Indian | 38 (1.6%) | NA | 38 (2.0%) |
| Native Hawaiian | 4 (0.2%) | NA | 4 (0.2%) |
| Glomerular diagnosis | 613 (25.9%) | 170 (35.9%) | 443 (23.4%) |
| Time varying-variables | |||
| Demographic | |||
| Age, years | 13.1 [9.3, 16.2] | 13.3 [9.7, 16.2] | 13.0 [9.3, 16.2] |
| Age < 6y | 231 (9.7%) | 46 (9.7%) | 185 (9.8%) |
| Age 6 to <12 | 775 (32.7%) | 144 (30.4%) | 631 (33.3%) |
| Age 12 to <18 | 1066 (45%) | 222 (46.9%) | 844 (44.5%) |
| Age ≥ 18y | 298 (12.6%) | 61 (12.9%) | 237 (12.5%) |
| Body size characteristics | |||
| Tanner 1 | 970 (43.9%) | 175 (40%) | 795 (44.9%) |
| Tanner 2 | 209 (9.5%) | 36 (8.2%) | 173 (9.8%) |
| Tanner 3 | 192 (8.7%) | 33 (7.5%) | 159 (9%) |
| Tanner 4 | 347 (15.7%) | 76 (17.4%) | 271 (15.3%) |
| Tanner 5 | 492 (22.3%) | 118 (26.9%) | 374 (21.1%) |
| Height, cm | 151 [130, 166] | 153 [132, 166] | 150 [130, 166] |
| Weight, kg | 46.4 [28.6, 63] | 50.5 [29.8, 70.5] | 45.2 [28.5, 61.3] |
| Body mass index, kg/m2 | 19.6 [16.8, 23.9] | 20.6 [16.9, 26.5] | 19.3 [16.7, 23.3] |
| Body surface area, m2 | 1.40 [1.02, 1.71] | 1.48 [1.04, 1.83] | 1.38 [1.02, 1.68] |
| Estimated LBM without Black coefficient, kg | 29.3 [19.8, 41.3] | 32.1 [19.9, 45.2] | 29.1 [19.8, 40.4] |
| Estimated LBM with Black coefficient, kg | 29.2 [19.7, 41.0] | 32.5 [20.1, 45.6] | 28.8 [19.6, 40.0] |
| Socioeconomic | |||
| Income < $36,000 | 939 (39.7%) | 310 (65.5%) | 629 (33.3%) |
| Income $36,000 to $75,000 | 704 (29.8%) | 111 (23.5%) | 593 (31.4%) |
| Income > $75,000 | 720 (30.5%) | 52 (11.0%) | 668 (35.3%) |
| Maternal education less than college | 1584 (67.0) | 364 (77.0%) | 1220 (64.6%) |
| Kidney disease and function | |||
| Serum creatinine, mg/dL | 1.2 [0.9, 1.8] | 1.2 [0.9, 1.8] | 1.2 [0.9, 1.8] |
| Serum cystatin c, mg/dL | 1.7 [1.3, 2.4] | 1.5 [1.1, 2.0] | 1.7 [1.4, 2.5] |
| Iohexol mGFR, ml/min|1.73m2 | 47.3 [34.0, 63.8] | 52.9 [37.6, 72.7] | 46.2 [33.2, 61.8] |
| Longitudinal data per participant | |||
| 1 to 2 observations | 391 (45.2%) | 108 (56.8%) | 283 (41.9%) |
| 3 to 4 observations | 374 (43.2%) | 68 (35.8%) | 306 (45.3%) |
| ≥ 5 observations | 100 (11.6%) | 14 (7.4%) | 86 (12.7%) |
Abbreviations: Years (y), Not applicable (NA), kilograms (kg), meters (m), glomerular filtration rate (GFR); measured GFR (mGFR)
Table S1 presents characteristics stratified by four self-reported race groups. Missing data was minimal and fully described in Table S2. Tanner stage data was missing for approximately 5% among those ages 6 to 12, and 7% among those 12 to 18 years.
Racial differences in measured GFR and serum creatinine
Figure 1 presents the age-stratified relationship between SCr on the x-axis and directly mGFR on the y-axis, both in the log scale. Nonparametric splines summarize these relationships and 90% region ellipses illustrate variability. These panels show a systematic difference such that mGFR levels for a given SCr value were higher among Black compared to non-Black person-visits, but these differences were relatively small relative to the variance (depicted by the ellipses). For person-visits with mGFR <30 ml/min|1.73m2, this difference diminished, although there were few person-visits contributing data among those ≥18 years of age.
Figure 1.
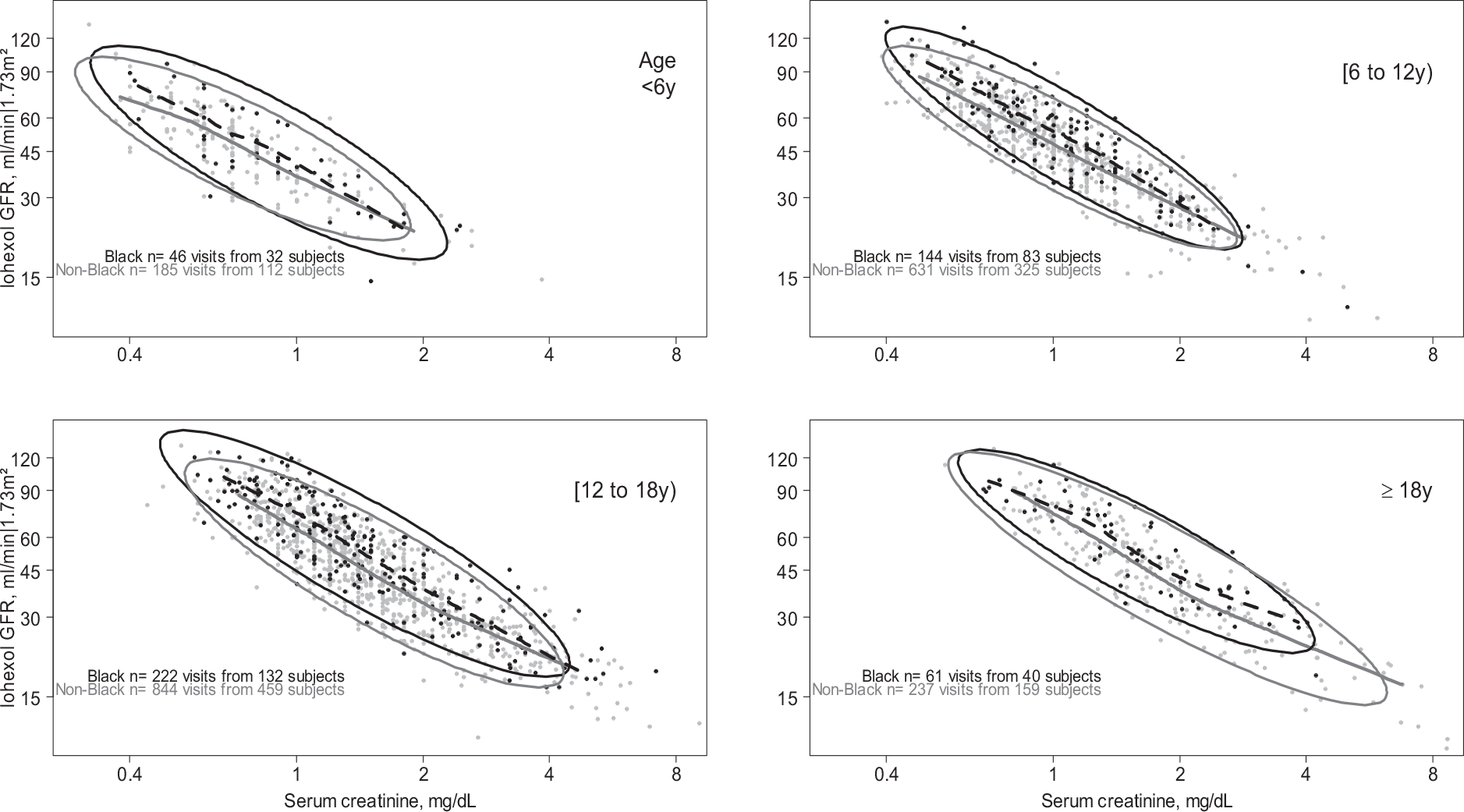
The relationship between serum creatinine (x-axis) and directly measured iohexol GFR (y-axis) by race (Black/non-Black), stratified by age groups. Data points represent person-visits with nonparametric Lowess splines over the middle 95% of the serum creatinine data (dashed are Black race, and continuous are non-Black race). Ellipses represent the region encompassing 90% of the data, stratified by race. Both serum creatinine and iohexol GFR are plotted on the log scale.
Table 2 presents results from regression models: in unadjusted models without age stratification, mGFR was 12.8% higher among Black participants, adjusting for SCr (95%CI: +7.8%, +18.1%). When separate models were fit by age groups older than 6, this difference ranged from +10.0% to +13.4%, and differences were significant for all age groups older than 6 years old. Among children contributing person-visits less than 6 years old, this difference was similar but not statistically significant (+7.4%, 95%CI: −1.0%, +16.5%). In models adjusting for markers of body size, the relationships were inferentially identical These differences persisted when adjusting for sex and age (similar to the CKD-EPI equation); sex, age, and height; sex, age, height and eLBM; and sex, age, height, household income and maternal education: the differences ranged from +4.9% to +6.2% for those<6, and from +9.6 to +12.8% among those ≥6 years of age. Differences by self-reported race were not significant across age categories.
Table 2.
Overall and age-stratified estimated percent difference in measured GFR between self-reported Black and non-Black person-visits (p-v) with adjustment for serum creatinine (in the log scale) and markers of body size, based on linear regression models.
| Model | Overall n= 474 Black p-v n= 1896 non-Black p-v |
Age < 6y n= 46 Black p-v n= 185 non-Black p-v |
≥6 to <12 year n= 144 Black p-v n= 631 non-Black p-v |
≥12 to <18 y n= 223 Black p-v n= 843 non-Black p-v |
≥18 y n= 61 Black p-v n= 237 non-Black p-v |
P-value for differences across ages |
|---|---|---|---|---|---|---|
|
| ||||||
| Unadjusted | +12.8% | +7.4% | +10.6% | +13.4% | +10.0% | 0.7 |
| (+7.8%, +18.1%) | (−1.0%, +16.5%) | (+5.8%, +15.7%) | (+8.7%, +18.2%) | (+1.8%, +18.9%) | ||
| Sex | +12.3% | +6.4% | +10.1% | +12.4% | +9.8% | 0.7 |
| (+7.3%, +17.5%) | (−1.7%, +15.2%) | (+5.5%, +14.9%) | (+8%, +16.8%) | (+3.8%, +16.1%) | ||
| Age | +11.7% | +6.0% | +10.7% | +13.8% | +10.0% | 0.5 |
| (+8.4%, +15.2%) | (−0.4%, +12.7%) | (+6.2%, +15.4%) | (+9.5%, +18.4%) | (+1.8%, +18.9%) | ||
| Tanner stage | NA | NA | +10.1% | +10.2% | NA | 0.9 |
| (+5.1%, +15.3%) | (+5.9%, +14.6%) | |||||
| Height | +10.9% | +6.4% | +10.1% | +11.9% | +11.1% | 0.6 |
| (+7.9%, +14%) | (+0.1%, +13.1%) | (+5.7%, +14.6%) | (+8.1%, +15.8%) | (+4.0%, +18.7%) | ||
| Body surface area | +8.8% | +6.2% | +10.3% | +9.9% | +6.4% | 0.7 |
| (+5.5%, +12.1%) | (+0.1%, +12.7%) | (+5.7%, +15.0%) | (+5.7%, +14.4%) | (−1.9%, +15.5%) | ||
| eLBM with ancestry | +8.5% | +5.2% | +9.4% | +8.5% | NA | 0.8 |
| (+5.5%, +11.6%) | (−0.9%, +11.7%) | (5.0%, 13.9%) | (+4.5%, +12.6%) | |||
| eLBM without ancestry | +9.6% | +6.5% | +10.1% | +9.6% | NA | 0.8 |
| (+6.5%, +12.7%) | (+0.4%, +12.9%) | (+5.7%, +14.7%) | (+5.6%, +13.8%) | |||
| Sex, age | +11.0% | +4.9% | +10.1% | +12.8% | +9.8% | 0.4 |
| (+8.0%, +14%) | (−1.1%, +11.3%) | (+5.9%, +14.6%) | (+8.8%, +16.9%) | (+3.8%, +16.1%) | ||
| Sex, age, height | +10.5% | +5.7% | +9.6% | +11.9% | +10.5% | 0.5 |
| (+7.7%, +13.3%) | (−0.4%, +12.1%) | (+5.4%, +13.9%) | (+8.2%, +15.6%) | (+4.4%, +16.8%) | ||
| Sex, age, height, eLBM without ancestry | +10.7% | +6.2% | +9.8% | +12.2% | NA | 0.4 |
| (+7.8%, +13.7%) | (+0.1%, +12.6%) | (+5.7%, +14.2%) | (+8.4%, +16%) | |||
| Sex, age, height, family income and maternal education | +11.3% | 5.7% | +9.9% | +12.7% | +12.7% | 0.5 |
| (+8.4%, +14.3%) | (−0.7%, 12.5%) | (+5.5%, +14.5%) | (+8.8%, +16.7%) | (+6.1%, +19.6%) | ||
GFR, as the dependent variable, was log transformed and results are expressed as percent difference (95% confidence interval).
Estimated lean body mass (eLBM) was calculated for children < 18 years.
Racial differences in measured GFR and serum cystatin c
Figure 2 presents the relationships between CysC and mGFR. In contrast to the SCr graph, minimal differences were observed between Black and non-Black person-visits. Among the youngest age group (<6 years old), Black person-visits had systematic, but slightly lower, mGFR level, adjusting for CysC, and these differences diminished in older age groups.
Figure 2.
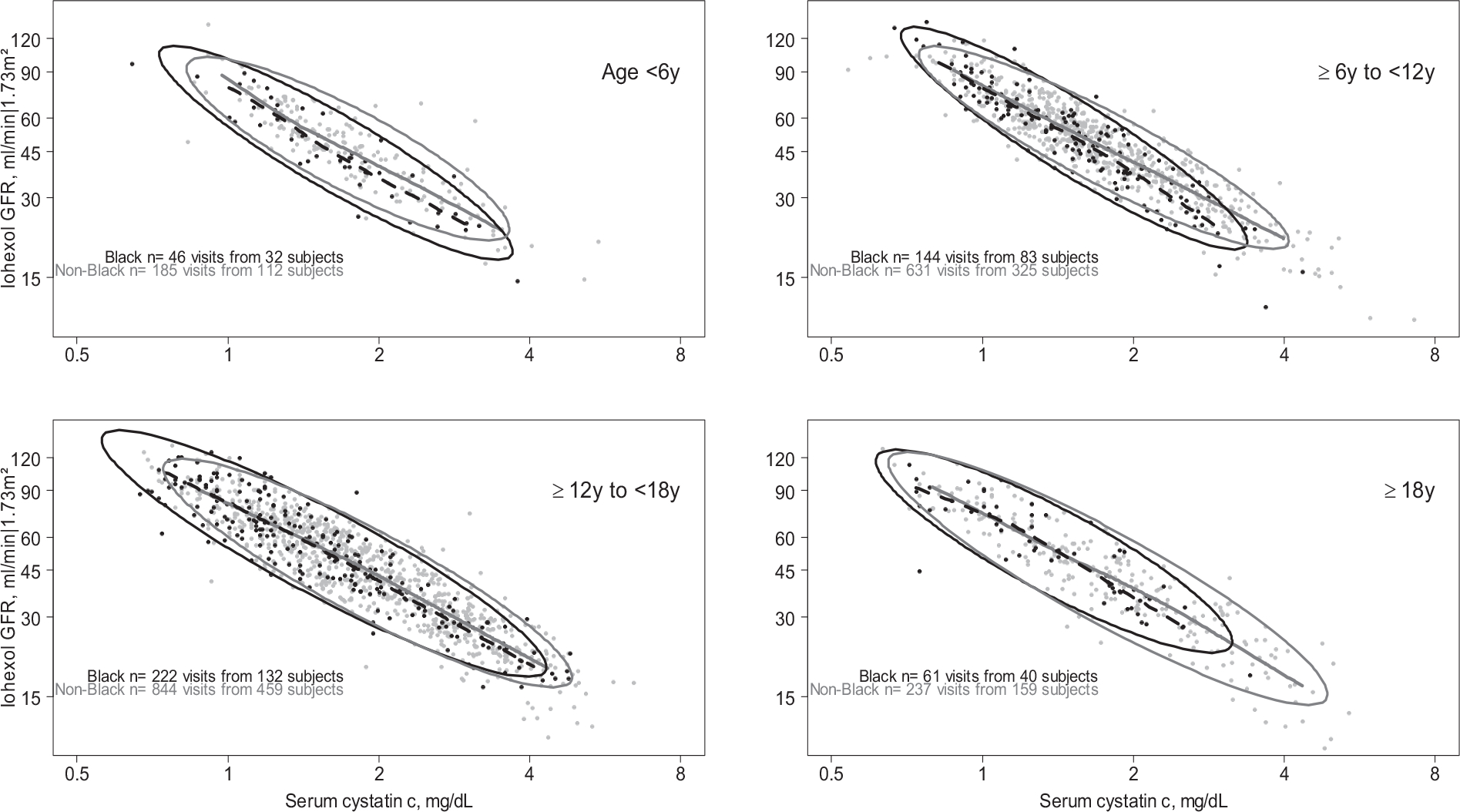
The relationship between serum cystatin c (x-axis) and directly measured iohexol GFR (y-axis) by race (Black/non-Black), stratified by age in 5 year bins. Data points represent person-visits with nonparametric Lowess splines over the middle 95% of the serum cystatin c data (dashed are Black race, and continuous are non-Black race). Ellipses represent the region encompassing 90% of the data, stratified by race. Both serum cystatin c and iohexol GFR are plotted on the log scale.
Table 3 presents the estimated racial differences in GFR after accounting for CysC. Across all ages in unadjusted models, Black race was associated with a 3.5% lower mGFR (95%CI: −5.7%, −1.4%). There was no interaction across ages, but differences were attenuated as age increased: for those aged 6 to 12, 12 to 18 and >18 years, the differences were −5.0% (95%CI: −8.3%, −1.5%), −2.5% (95%CI: −5.6%, +0.6%), and −0.9% (95%CI: −6.4%, +5.0%), respectively. These relationships were essentially the same in models that included additional adjustment for other markers of body size, and indicators of socioeconomic status. Among those under the age of 6, self-reported Black race was consistently associated with lower mGFR ranging from −7.5% to −9.3%. For those older than 18 years, non-significant differences ranged from −0.3% and −3.3%. In multivariable models, the associations were similar across ages, but there were no interactions by age group.
Table 3.
Overall and age-stratified estimated percent difference in measured GFR between self-reported Black and non-Black person-visits (p-v) with adjustment for serum cystatin c (in the log scale) and markers of body size, based on linear regression models.
| Model | Overall n= 474 Black p-v n= 1896 non-Black p-v |
Age < 6y n= 46 Black p-v n= 185 non-Black p-v |
≥6 to <12 year n= 144 Black p-v n= 631 non-Black p-v |
≥12 to <18 y n= 223 Black p-v n= 843 non-Black p-v |
≥18 y n= 61 Black p-v n= 237 non-Black p-v |
P-value for differences across ages |
|---|---|---|---|---|---|---|
|
| ||||||
| Unadjusted | −3.5% | −8.6% | −5.0% | −2.5% | −0.9% | 0.2 |
| (−5.7%, −1.4%) | (−14.5%, −2.1%) | (−8.3%, −1.5%) | (−5.6%, +0.6%) | (−6.4%, +5.0%) | ||
| Sex | −4.1% | −9.2% | −5.1% | −3.6% | −0.8% | 0.1 |
| (−6.1%, −2.0%) | (−15.1%, −3.0%) | (−8.5%, −1.6%) | (−6.5%, −0.7%) | (−6.0%, +4.7%) | ||
| Age | −3.5% | −8.6% | −5% | −2.8% | −0.8% | 0.3 |
| (−5.7%, −1.3%) | (−14.5%, −2.3%) | (−8.3%, −1.5%) | (−5.9%, +0.3%) | (−6.4%, +5.2%) | ||
| Tanner stage | NA | NA | −5.1% | −3.0% | NA | 0.2 |
| (−8.6%, −1.5%) | (−6.1%, +0.1%) | |||||
| Height | −3.7% | −8.6% | −5.1% | −2.6% | −0.3% | 0.2 |
| (−5.8%, −1.5%) | (−14.5%, −2.2%) | (−8.4%, −1.6%) | (−5.6%, +0.4%) | (−5.9%, +5.7%) | ||
| Body surface area | −3.7% | −8.6% | −4.9% | −3.0% | −3.3% | 0.3 |
| (−5.8%, −1.5%) | (−14.6%, −2.3%) | (−8.3%, −1.4%) | (−6.0%, +0.1%) | (−8.5%, +2.2%) | ||
| eLBM with ancestry | −4.2% | −7.7% | −5.2% | −3.6% | NA | 0.4 |
| (−6.4%, −2.0%) | (−13.8%, −1.3%) | (−8.5%, −1.7%) | (−6.5%, −0.5%) | |||
| eLBM without ancestry | −4.1% | −7.5% | −4.9% | −3.2% | NA | 0.4 |
| (−6.3%, −1.9%) | (−13.6%, −1.1%) | (−8.3%, −1.5%) | (−6.2%, −0.2%) | |||
| Sex, age | −4.1% | −9.3% | −5.2% | −4.0% | −0.7% | 0.2 |
| (−6.1%, −1.9%) | (−15%, −3.2%) | (−8.5%, −1.7%) | (−6.8%, −1.0%) | (−5.9%, +4.8%) | ||
| Sex, age, height | −4.2% | −9.3% | −5.3% | −4.2% | −0.5% | 0.1 |
| (−6.2%, −2.1%) | (−14.9%, −3.3%) | (−8.6%, −1.8%) | (−7.0%, −1.2%) | (−5.8%, +5.1%) | ||
| Sex, age, height, eLBM without ancestry | −4.8% | −8.3% | −5.1% | −4.4% | NA | 0.5 |
| (−7.0%, −2.6%) | (−14.1%, −2.1%) | (−8.4%, −1.6%) | (−7.3%, −1.3%) | |||
| Sex, age, height, family income and maternal education | −4.0% | −10.3% | −6.1% | −3.4% | 0.6% | 0.2 |
| (−6.2%, −1.7%) | (−16.4%, −3.7%) | (−9.7%, −2.3%) | (−6.5%, −0.3%) | (−4.9%, 6.4%) | ||
GFR, as the dependent variable, was log transformed and results are expressed as percent difference (95% confidence interval).
Estimated lean body mass (eLBM) was calculated for children < 18 years.
A sensitivity analysis restricted to one randomly selected visit per individual was assessed for models presented in Tables 2 and 3, and showed robust inferences in the setting of independence observations (Tables S3 and S4).
Race as three categories: non-Black, Black with mixed race, Black with non-mixed race
Figure 3 presents the results from using self-reported race as three categories with inclusion of SCr and CysC in separate models (Panel A and B, respectively), with additional adjustment for age, sex and height. Overall, Panel A demonstrates that Black person-visits, regardless of mixed or no mixed race had about 7% to 12% significantly higher mGFR compared with non-Black person-visits (as the reference group) after adjusting for SCr, sex, age, height, household income and maternal education. There were no significant differences between Black with mixed race and Black with no mixed race person-visits.
Figure 3.
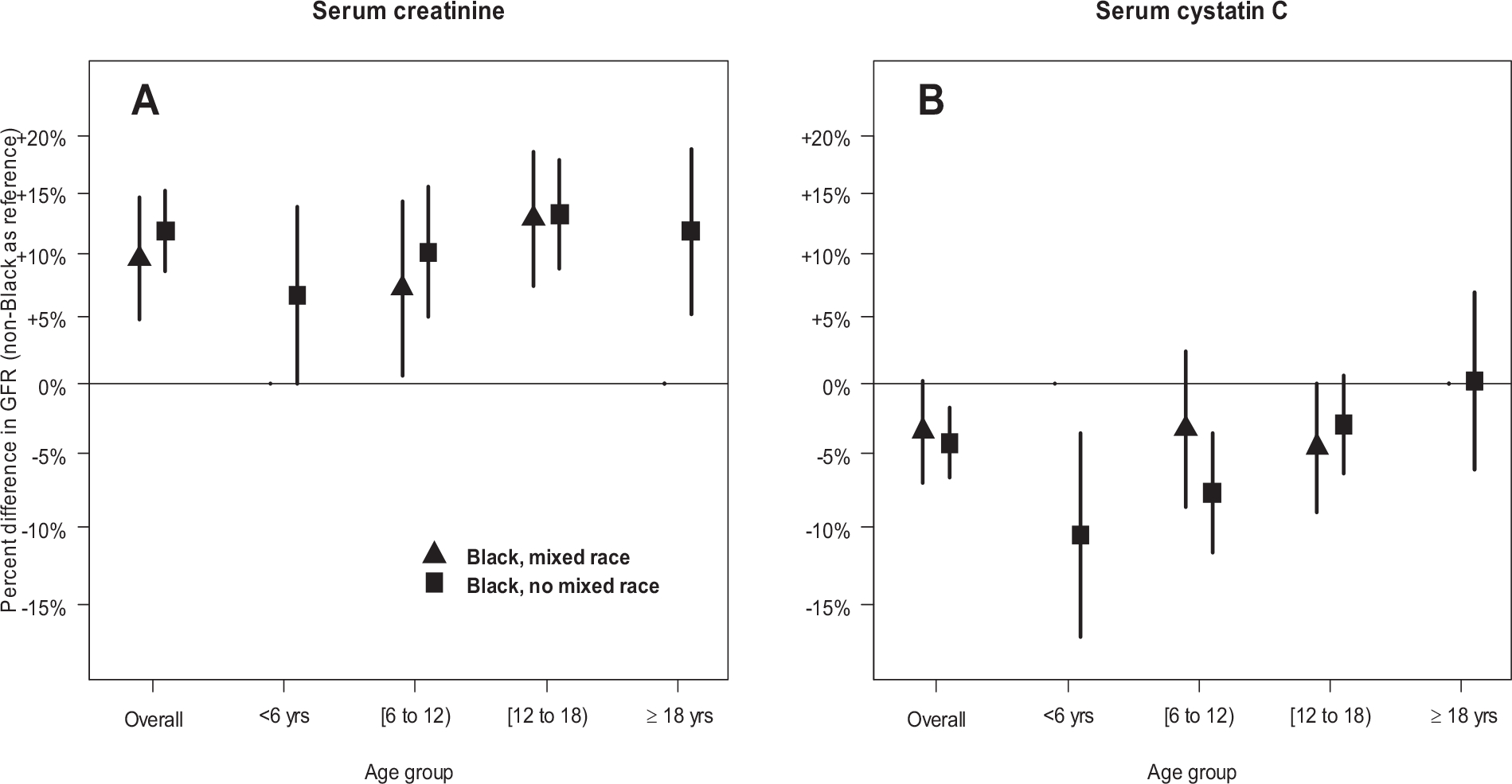
Percent differences in GFR for Black with mixed race, and Black with no mixed race, relative to non-Black participants when conditioning on serum creatinine (Panel A), and serum cystatin C (Panel B), overall and stratified by age category. Estimates additionally adjust for age (on the continuous scale), sex, height, family income category, and college or more maternal education. Due to sparse data, differences for Black with mixed race were not estimated for age < 6 years (n= 6 person-visits) and ≥ 18 years (n= 14 person-visits).
Panel B shows no significant within-Black race differences in CysC models, although those who self-reported Black with no mixed race had significantly lower mGFR than non-Black person-visits in a fully adjusted model. In general, there were no statistically significant patterns by self-reported Black race (with and without mixed race), although the differences between binary self-reported Black and non-Black person-visits were consistent and significant.
Under 25 (U25) estimating equation performance by self-reported race
Table 4 presents an internal validation assessment of agreement between the U25 eGFR equations and measured GFR. Among Black participants, SCr-based eGFR significantly underestimated mGFR (−3.37 ml.min|1.73m2, 95%CI: −6.07, −0.67) and this was not observed for the non-Black groups. The CysC-based U25 eGFR had no significant bias across self-reported racial groups. The average of the SCr and CysC equations yielded unbiased estimates, but also had the highest proportions within 30% and 10% of mGFR, and with the lowest RMSE.
Table 4.
Agreement analysis describing bias, accuracy (proportion within 30% and 10% of mGFR) and root mean square error (RMSE) comparing three U25 estimated GFR equations (serum creatinine-based, cystatin C-based, and the average of the serum creatinine-based and cystatin c-based equations) to directly measured iohexol GFR across three self-reported racial groups.
| Black n= 131 observations from 53 participants |
White n= 610 observations from 213 participants |
Other n= 102 observations from 38 individuals |
|
|---|---|---|---|
| Bias eGFR - mGFR, ml/min|1.73m2 (95%CI) | |||
| U25 SCr-based eGFR | −3.37 (−6.07, −0.67) | 0.98 (−0.09, 2.06) | 0.94 (−1.89, 3.76) |
| U25 CysC-based eGFR | 0.76 (−1.19, 2.72) | −0.92 (−1.89, 0.05) | −1.98 (−4.66, 0.70) |
| Average of U25 SCr- and CysC-based eGFR | −1.41 (−3.52, 0.69) | 0.04 (−0.80, 0.89) | −0.60 (−2.72, 1.51) |
| % of eGFR within 30% of mGFR | |||
| U25 SCr-based eGFR | 86.3% | 86.1% | 89.2% |
| U25 CysC-based eGFR | 81.7% | 87.7% | 85.3% |
| Average of U25 SCr- and CysC-based eGFR | 89.3% | 91.3% | 91.2% |
| % of eGFR within 10% of mGFR | |||
| U25 SCr-based eGFR | 35.1% | 37.0% | 40.2% |
| U25 CysC-based eGFR | 35.1% | 42.0% | 35.3% |
| Average of U25 SCr- and CysC-based eGFR | 42.0% | 46.2% | 39.2% |
| Test RMSE, ml/min|1.73m2 | |||
| U25 SCr-based eGFR | 12.04 | 9.41 | 9.70 |
| U25 CysC-based eGFR | 10.86 | 10.30 | 11.39 |
| Average of U25 SCr- and CysC-based eGFR | 10.01 | 8.24 | 8.23 |
The agreement analysis used data from the validation set of the equation development among those with available serum creatinine and cystatin C from Pierce et al., Kidney International, 202015, which was independent from equation development comprising 843 observations from 304 participants.
DISCUSSION
Our findings in this pediatric population with kidney diseases provide insight into self-reported racial differences in the relationships between GFR, SCr and CysC. Specifically, these data show that self-reported Black race was associated with a higher mGFR after adjusting for SCr, markers of body size, and socioeconomic indicators. This was a systematic phenomenon (observed in Figure 1), but there was substantial variability. These differences ranged between 7% and 12% higher for participants reporting Black race, and were generally consistent with adult equations. The CKD-EPI equation yields eGFR that is 15.9% higher among Black individuals after adjusting for Scr, age, and sex; in the MDRD equation, it was 21.2% higher. The confidence intervals of our estimates for those >6 years contained both of these values, although estimates were attenuated in our younger population.
We also noted that differences by self-reported race were not statistically significant among the children <6 years old. This may be due to smaller sample size (n= 46 person-visits) and we note that there was no statistical heterogeneity across ages. It was somewhat surprising that self-reported race differences were observed in those aged 6 to 12, comprising mostly pre-pubescent children, even after Tanner stage adjustment. Since muscle mass is considered an extrarenal modifier of creatinine excretion4,16, pre-pubertal muscle mass was expected to be more homogeneous. We hypothesized that differences would be minimal in this younger group, but this was not borne out in the data. Investigating factors influencing SCr that are not directly related to muscle mass across the full age spectrum will help understand these differences.
The finding that self-reported race differences between CysC and GFR were attenuated relative to SCr was less surprising, particularly for young adults. There were no significant differences among young adults (approximately −3.3% to −0.3%) which was also consistent with adult-based CysC equations free of a race coefficient. However, there was an underestimation bias among younger age groups. While the differences were not as extreme for those between the ages of 6 to 18 compared to SCr, Black children <6 years demonstrated 7.5% to 9.3% lower GFR when adjusting for CysC and markers of body size. This discrepancy between racial differences in CysC models compared to SCr models was noteworthy and indicates that more data are needed in this younger age group, since our sample size was relatively small.
We aimed to contribute to an ongoing discussion of how best to estimate GFR by investigating how these biomarkers relate to kidney function in in pediatric and young adult populations with mild to severe CKD. We demonstrated that the new U25 equations using SCr and CysC had excellent agreement across self-reported race groups. These equations do not include a term for Black race15 consistent with recent recommendations. While there was an underestimation of the U25 SCr-based eGFR for those who self-reported Black race, the average of SCr- and CysC-based U25 equations yielded even lower and non-significant bias. An online calculator U25 eGFR is readily accessible for clinical use (https://ckid-gfrcalculator.shinyapps.io/eGFR/).
This study has several strengths. The study population of children and young adults with CKD represents a wide spectrum of age and kidney function. In addition, measured GFR and biomarker data used in this analysis were obtained by standardized protocols and centralized measurement. We do note a limitation that the participant- or parent-reported question on race may have been interpreted differently by respondents, particularly when invited to report multiple races. However, this question was applied equally to all participants, was substantially stronger than investigator-assigned race, and despite potential heterogeneous interpretations, similar differences in mGFR were observed as reported by CKD-EPI. This was somewhat surprising since a limitation of the CKD-EPI equation development was that the variable for race was not well-defined or collected in a standardized way5. We recognize the potential for reporting heterogeneity, especially for mixed race and grouping all participants reporting non-Black race together, which was why we investigated mixed race as a potential modifier. We lacked data on genetic ancestry for consideration along with self-reported race. Genetic African ancestry, for example, has been previously associated with higher SCr levels30.
Another limitation was a lack of directly measured muscle mass, which governs creatinine production and serum levels. We attempted to address this by including eLBM based on a validated equation28. These equations did not strongly relate to racial differences in the SCr-mGFR relationship in this analysis. It was also interesting to note that the two proposed equations for eLBM were constructed with and without Black race coefficients. This was external evidence beyond the nephrology literature that racial differences in lean body mass may be present in children (and described in adults by Levey et al.4). Other measurements of muscle mass would be useful to fully understand potential non-renal influences on these biomarker relationships. Creatinine metabolism and diet as non-renal determinants of SCr may be important since adjustment for eLBM did not affect the SCr-mGFR relationship. Developing and testing theories for the biological or social mechanism for observed differences in children and adults remains important.
Another limitation was that we only adjusted for two socioeconomic variables (household income and maternal education) which we considered potential confounders. We lacked data on other factors, such as dietary patterns, which could have contributed to observed racial differences, especially variables that directly affect muscle mass or protein intake. The underlying reasons for these difference remain unclear, but further investigations must include pediatric and young adult populations which exhibit similar phenomena. Although our data covered the full range of kidney function from normal to severely diminished, all participants had a diagnosis of CKD and our results may not generalize to children free of kidney diseases. Ideally, using the same protocols with more representation among younger children (<6 years) and inclusion of young people with no kidney disease would improve inferences.
There is no dispute that serum biomarkers offer useful, but imperfect, estimates of GFR compared to direct measures like plasma clearance of iohexol. This analysis, along with several recent papers, demonstrate potential limitations in the use of SCr for GFR estimation. In this population, racial differences in GFR after accounting for CysC, while statistically significant, were of smaller magnitude than SCr. As barriers and costs for measuring CysC decrease, it is worth considering routine use of CysC for GFR estimation, particularly since taking the simple average of U25 SCr- and CysC-based equations yield unbiased estimates in this population. If a SCr-based U25 eGFR value is clinically questionable for a particular patient, CysC offers utility for confirmation and also for calculating the average of U25 eGFR values. Our results demonstrate that, in addition to adults, children should be included in future investigations of these biomarker relationships with GFR.
Supplementary Material
Acknowledgements:
The authors acknowledge Dr. Rick Kaskel for critical input in the manuscript preparation, and Dr. Alvaro Muñoz and Mr. Christopher Pierce for their contributions to the evaluation of differences in U25 eGFR equations.
Support:
The CKiD Study (https://statepi.jhsph.edu/ckid) is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (grants U01 DK066143, U01 DK066174, U24 DK082194, U24 DK066116). Data in this manuscript were collected by CKiD with clinical coordinating centers at Children’s Mercy Hospital and the University of Missouri - Kansas City (Principal Investigator [PI], Warady) and Children’s Hospital of Philadelphia (PI, Furth), Central Biochemistry Laboratory (PI, Schwartz) at the University of Rochester Medical Center, and data coordinating center (PIs, Muñoz and Ng) at the Johns Hopkins Bloomberg School of Public Health. The funders did not have any role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Article Information
CKiD Study Investigators: A list of the CKiD Study Investigators can be found in Table S5 and at https://statepi.jhsph.edu/ckid/site-investigators/.
Peer Review: Received April 29, 2021. Evaluated by 2 external peer reviewers, with direct editorial input from a Statistics/Methods Editor and an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form October 24, 2021. The involvement of an Acting Editor-in-Chief was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodenbach KE, Fuhrman DY, Maier PS, Schwartz GJ. Renal Response to a Protein Load in Healthy Young Adults as Determined by Iohexol Infusion Clearance, Cimetidine-Inhibited Creatinine Clearance, and Cystatin C Estimated Glomerular Filtration Rate. J Ren Nutr. 2017;27(4):275–281. doi: 10.1053/j.jrn.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney Disease, Race, and GFR Estimation. Clin J Am Soc Nephrol. 2020;15(8):1203–1212. doi: 10.2215/CJN.12791019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eneanya ND, Yang W, Reese PP. Reconsidering the Consequences of Using Race to Estimate Kidney Function. JAMA. 2019;322(2):113–114. doi: 10.1001/jama.2019.5774 [DOI] [PubMed] [Google Scholar]
- 7.Powe NR. Black Kidney Function Matters: Use or Misuse of Race? JAMA. 2020;324(8):737–738. doi: 10.1001/jama.2020.13378 [DOI] [PubMed] [Google Scholar]
- 8.Seegmiller JC, Eckfeldt JH. Racial Demographics in Glomerular Filtration Rate Estimating Equations. Clin Chem. Published online November 30, 2020. doi: 10.1093/clinchem/hvaa234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vyas DA, Eisenstein LG, Jones DS. Hidden in Plain Sight — Reconsidering the Use of Race Correction in Clinical Algorithms. New England Journal of Medicine. 2020;383(9):874–882. doi: 10.1056/NEJMms2004740 [DOI] [PubMed] [Google Scholar]
- 10.Diao JA, Wu GJ, Taylor HA, et al. Clinical Implications of Removing Race From Estimates of Kidney Function. JAMA. Published online December 2, 2020. doi: 10.1001/jama.2020.22124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Removing Race from Estimates of Kidney Function. National Kidney Foundation. Published March 9, 2021. Accessed March 12, 2021. https://www.kidney.org/news/removing-race-estimates-kidney-function
- 12.Delgado C, Baweja M, Crews DC, et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis. Published online September 22, 2021. doi: 10.1053/j.ajkd.2021.08.003 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82(4):445–453. doi: 10.1038/ki.2012.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierce CB, Muñoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. Published online December 7, 2020. doi: 10.1016/j.kint.2020.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu J, Johansen KL, Hsu C yuan, Kaysen GA, Chertow GM. Higher Serum Creatinine Concentrations in Black Patients with Chronic Kidney Disease: Beyond Nutritional Status and Body Composition. CJASN. 2008;3(4):992–997. doi: 10.2215/CJN.00090108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng DK, Moxey-Mims M, Warady BA, Furth SL, Muñoz A. Racial differences in renal replacement therapy initiation among children with a nonglomerular cause of chronic kidney disease. Ann Epidemiol. 2016;26(11):780–787.e1. doi: 10.1016/j.annepidem.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng DK, Antiporta DA, Matheson MB, Muñoz A. Nonparametric Assessment of Differences Between Competing Risk Hazard Ratios: Application to Racial Differences in Pediatric Chronic Kidney Disease Progression. Clin Epidemiol. 2020;12:83–93. doi: 10.2147/CLEP.S225763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furth SL, Cole SR, Moxey-Mims M, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1(5):1006–1015. doi: 10.2215/CJN.01941205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz GJ, Furth S, Cole SR, Warady B, Muñoz A. Glomerular filtration rate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney International. 2006;69(11):2070–2077. doi: 10.1038/sj.ki.5000385 [DOI] [PubMed] [Google Scholar]
- 21.Schwartz GJ, Abraham AG, Furth SL, Warady BA, Muñoz A. Optimizing iohexol plasma disappearance curves to measure the glomerular filtration rate in children with chronic kidney disease. Kidney Int. 2010;77(1):65–71. doi: 10.1038/ki.2009.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng DKS, Schwartz GJ, Jacobson LP, et al. Universal GFR determination based on two time points during plasma iohexol disappearance. Kidney Int. 2011;80(4):423–430. doi: 10.1038/ki.2011.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz GJ, Wang H, Erway B, et al. Multicenter Laboratory Comparison of Iohexol Measurement. The Journal of Applied Laboratory Medicine. 2018;2(5):711–724. doi: 10.1373/jalm.2017.024240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng DK, Levey AS, Shlipak MG, Muñoz A, Inker LA, Shafi T. Validation of a simple equation for glomerular filtration rate measurement based on plasma iohexol disappearance. Clin Kidney J. 2020;13(3):397–401. doi: 10.1093/ckj/sfz083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93(1):62–66. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz GJ, Kwong T, Erway B, et al. Validation of creatinine assays utilizing HPLC and IDMS traceable standards in sera of children. Pediatr Nephrol. 2009;24(1):113–119. doi: 10.1007/s00467-008-0957-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz GJ, Cox C, Seegmiller JC, et al. Recalibration of cystatin C using standardized material in Siemens nephelometers. Pediatr Nephrol. 2020;35(2):279–285. doi: 10.1007/s00467-019-04389-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster BJ, Platt RW, Zemel BS. Development and validation of a predictive equation for lean body mass in children and adolescents. Ann Hum Biol. 2012;39(3):171–182. doi: 10.3109/03014460.2012.681800 [DOI] [PubMed] [Google Scholar]
- 29.Ng DK, Schwartz GJ, Schneider MF, Furth SL, Warady BA. Combination of pediatric and adult formulas yield valid glomerular filtration rate estimates in young adults with a history of pediatric chronic kidney disease. Kidney Int. 2018;94(1):170–177. doi: 10.1016/j.kint.2018.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Udler MS, Nadkarni GN, Belbin G, et al. Effect of Genetic African Ancestry on eGFR and Kidney Disease. J Am Soc Nephrol. 2015;26(7):1682–1692. doi: 10.1681/ASN.2014050474 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


