OBJECTIVES:
In critically ill patients with neurologic disease, pupil examination abnormalities can signify evolving intracranial pathology. Analgesic and sedative medications (analgosedatives) target pupillary pathways, but it remains unknown how analgosedatives alter pupil findings in the clinical care setting. We assessed dexmedetomidine and other analgosedative associations with pupil reactivity and size in a heterogeneous cohort of critically ill patients with acute intracranial pathology.
DESIGN:
Retrospective cohort study.
SETTING:
Two neurologic ICUs between 2016 and 2018.
PATIENTS:
Critically ill adult patients with pupil measurements within 60 minutes of analgosedative administration. Patients with a history of intrinsic retinal pathology, extracranial injury, inaccessible brain imaging, or no Glasgow Coma Scale (GCS) data were excluded.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
We used mixed-effects linear regression accounting for intrapatient correlations and adjusting for sex, age, GCS score, radiographic mass effect, medication confounders, and ambient light. We tested the association between an initiation or increased IV infusion of dexmedetomidine and pupil reactivity (Neurologic Pupil Index [NPi]) and resting pupil size (mm) obtained using NeurOptics NPi—200 (NeurOptics, Irvine, CA) pupillometer. Of our 221 patients with 9,897 pupil observations (median age, 60 [interquartile range, 50–68]; 59% male), 37 patients (166 pupil observations) were exposed to dexmedetomidine. Dexmedetomidine was associated with higher average NPi (β = 0.18 per 1 unit increase in rank-normalized NPi ± 0.04; p < 0.001) and smaller pupil size (β = –0.25 ± 0.05; p < 0.001). Exploratory analyses revealed that acetaminophen was associated with higher average NPi (β = 0.04 ± 0.02; p = 0.02) and that most IV infusion analgosedatives including propofol, fentanyl, and midazolam were associated with smaller pupil size.
CONCLUSIONS:
Dexmedetomidine is associated with higher pupil reactivity (high NPi) and smaller pupil size in a cohort of critically ill patients with neurologic injury. Familiarity with expected pupil changes following analgosedative administration is important for accurate interpretation of pupil examination findings, facilitating optimal management of patients with acute intracranial pathology.
Keywords: analgesics, dexmedetomidine, neurocritical care, pupil reactivity, pupillometry, sedatives
Assessment of pupil reactivity and size is a cornerstone of the neurologic examination in critically ill patients. Pupil abnormalities can signify life-threatening acute intracranial pathology (1). In particular, pupil reactivity to light is a noninvasive indicator of midbrain integrity (2–5). In addition to conditions affecting primary pathways extending from the retina to the Edinger-Westphal Nucleus (EWN) and back through the oculomotor nerve, pupil reactivity and size can be influenced by networks of sympathetic and parasympathetic modulators (2, 3). Analgosedatives target these autonomic networks and can potentially modify pupil characteristics during the neurologic assessment (3).
There is conflicting evidence regarding the effect of IV infusion analgosedatives on pupil reactivity and size (6–10). Case series suggest that α2 agonists, such as dexmedetomidine and clonidine, may cause a paradoxical increase in reactivity (11–14). However, these studies are largely limited to anesthetized or healthy subjects and small sample sizes (10–20 participants). With the increased adoption of quantitative pupillometry, a noninvasive and reliable method of capturing objective data on pupil reactivity to light, there is a new opportunity and urgent need to study the relationship between analgosedatives and pupil characteristics in the neurologic ICU (neuro-ICU) (15–17).
There is accumulating evidence that Neurologic Pupil Index (NPi), a variable that is automatically computed from the quantitative pupillometer reflecting pupil reactivity (18), is predictive of neurologic outcome in critically ill patients (19, 20). To accurately interpret NPi in patients with acute intracranial pathology, the impact of analgosedatives on quantitative pupil characteristics must be understood.
Our primary objective was to assess dexmedetomidine’s effect on pupil reactivity and size in a heterogenous sample of critically ill patients with acute intracranial pathology. Our secondary objective was to explore how other IV infusions, oral per os (PO), and one-time IV analgosedatives affected pupil reactivity and size. We hypothesized that dexmedetomidine would be associated with increased pupil reactivity and unchanged pupil size. We reasoned that clonidine, an α2 agonist-like dexmedetomidine, would also associate with increased pupil reactivity and that other IV infusion analgosedatives would result in no change to pupil reactivity and smaller pupil size. We expected that other analgosedatives would not be associated with changes in pupil reactivity. A better understanding of analgosedative and pupil characteristic relations is imperative to the accurate interpretation of clinical pupillometry data and management of critically ill patients with acute intracranial pathology.
MATERIALS AND METHODS
Population and Study Design
We conducted a two-center retrospective study of critically ill patients from the Brigham and Women’s and Massachusetts General Hospital neuro-ICUs between 2016 and 2018. We used convenience sampling to collect data on patients with at least three complete (bilateral) quantitative pupil measurements and available medication data. We excluded patients with a history of retinal surgery, optic neuritis, traumatic pupil injury, glaucoma, cataracts, extracranial injuries, inaccessible brain imaging, or no Glasgow Coma Scale (GCS, a 12-point ordinal scale measuring eye opening, verbal, and motor responses to stimuli) (21) data (Fig. 1). Further information on this cohort is published previously (1). Our article was prepared following the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines (22).
Figure 1.
Inclusion and exclusion flowchart. BWH = Brigham and Women’s Hospital, GCS = Glasgow Coma Score, MGH = Massachusetts General Hospital, N = patients.
Data Collection
We collected demographic, diagnostic, procedural (intracranial pressure monitors, external ventricular drains, and craniectomies), radiologic (CT and MRI), GCS, medication, and mortality at discharge data from the electronic medical record (Epic Systems, Verona, WI). Trained team members (H.S., C.J.O.) identified radiologic mass effect (defined as at least local displacement caused by an intracranial lesion), midline shift, and uncal herniation. Trained nursing staff collected quantitative pupillometry data using the NeurOptics NPi—200 (NeurOptics, Irvine, CA) pupillometer every 2 hours as part of standard care. The NeurOptics NPi—200 automatically calculates the NPi, a composite score based on resting and constricted pupil size, percent change, constriction and dilation velocity, and latency that ranges from 0 to 5, in which greater than 3 is considered normal (18, 23). We used Research Electronic Data Capture, an electronic data capture tool, to store electronic medical record and radiographic feature data (24). The Boston Medical Center and Massachusetts General Brigham Institutional Review Boards (H-37699, 2016P002718) approved this study and waived the need for informed consent because quantitative pupillometry is part of routine care in participating neuro-ICUs.
Variables
Our primary exposure was the initiation or increased dose of dexmedetomidine up to 60 minutes prior to pupil measurement, dichotomously recorded. At our institutions, dexmedetomidine is typically started at 0.2 µg/kg/hr for patients with agitation, a need for sedation, or concerns regarding ventilator toleration and titrated for patient comfort. Secondary exposures included the initiation or increased dose of other analgosedatives commonly administered in the neuro-ICU, including continuous opioid or gamma aminobutyric acid-ergic infusions, immediate-release one-time sedative, and IV or PO analgesic agents. We set 60 minutes as the upper limit based on pharmacokinetic, pharmacodynamic profiles, and expected peak effect time. For cases in which two or more pupil observations existed in the 60-minute time window following dexmedetomidine exposure, we assigned the pupil measurement closest in time to the dexmedetomidine exposure as affected and considered the remaining pupil observations unaffected. The full list of analgosedatives is shown in Supplementary Table 1 (http://links.lww.com/CCX/A987).
Primary outcomes were average NPi and average resting pupil size between the left and right eyes. We averaged left and right eye measurements to mitigate biases related to unilateral pupillary changes that may be due to etiologies such as compressive mass effect. For our multivariable models, hypothesized confounders included sex, age (25), arousal via the GCS score (1), radiographic mass effect (mass effect and midline shift can affect pupils) (26), potential medication confounders from preliminary data (acetaminophen), and ambient light (captured indirectly by determining whether a pupil measurement occurred at nighttime between 7:00 pm and 7:00 am) (27). We had no missing demographic data at the patient level. Pupil observations not paired with GCS within 60 minutes (22%, 2,180 pupil observations) were imputed using the last-known GCS because it is standard practice to not record GCS if unchanged.
Analysis
We reported baseline characteristics of patients with at least one exposure to dexmedetomidine and those naïve to dexmedetomidine during their neuro-ICU stay. We used chi-square or Student t test for categorical and continuous variables, respectively.
We transformed average NPi and average resting pupil size using rank normalization to satisfy the normality assumption required for regressions (Supplementary Fig 1, http://links.lww.com/CCX/A987) (28). Univariate mixed-effects linear regression was used to test association between average NPi and resting pupil size and dexmedetomidine adjusting for intrapatient correlation using a random effects term (29). We then constructed two multivariable models to test the association between average NPi and resting pupil size with dexmedetomidine adjusting for sex, age, GCS score, radiographic mass effect, acetaminophen, a nighttime indicator as a proxy for ambient light, and intrapatient correlations. To account for our two primary hypotheses (that average NPi is positively associated and that resting pupil size is negatively associated with dexmedetomidine), we set our significance threshold at α = 0.025 using Bonferroni correction (30). In the subgroup who received at least one dose of dexmedetomidine, we tested association between average NPi and resting pupil size, treating dexmedetomidine as both a dichotomous variable (increase dose vs not) and as a continuous variable (absolute dose) using similar mixed-effects linear regressions.
We also explored associations between other analgosedatives and average NPi and resting pupil size using univariate mixed-effects models. To further elaborate on our observed relation between acetaminophen and average NPi, we also constructed an exploratory multivariable model adjusting for the potential confounders listed above.
We used RStudio Version 1.3.959 (RStudio Team (2020). RStudio: Integrated Development for R. RStudio, PBC, Boston, MA; http://www.rstudio.com/) for statistical analyses.
RESULTS
Our final analysis included 221 patients with 9,897 pupil observations. From an initial cohort of 319 patients, we excluded 98 patients who had less than three complete pupil observations, no available medication data, a history of retinal surgery, optic neuritis, traumatic pupil injury, glaucoma, cataracts, extracranial injury, inaccessible brain imaging, or no GCS data (Fig. 1). The median age was 60 years (interquartile range [IQR], 50–68 yr). Fifty-nine percent (N = 131) were male, and 69% (N = 153) were White. The most common diagnoses included spontaneous intraparenchymal hemorrhage (22%), ischemic stroke (22%), brain tumor (16%), and traumatic intraparenchymal hemorrhage (13%) (Table 1).
TABLE 1.
Baseline Characterization of Dexmedetomidine Group
| Variable | Total (N = 221) | Dexmedetomidine (+) (N = 37) | Dexmedetomidine (−) (N = 184) |
|---|---|---|---|
| Demographics | |||
| Median age (interquartile range), yr | 60 (50–68) | 55 (44–60) | 61 (51.75–68) |
| Male, n (%) | 131 (59) | 23 (62) | 108 (59) |
| Race, n (%) | |||
| White | 153 (69) | 22 (59) | 131 (71) |
| Black | 16 (7) | 3 (8) | 13 (7) |
| Asian | 9 (4) | 4 (11) | 5 (3) |
| Othera | 43 (19) | 8 (22) | 35 (19) |
| Diagnosis | |||
| Spontaneous IPH, n (%) | 49 (22) | 4 (11) | 45 (24) |
| Stroke, n (%) | 49 (22) | 5 (14) | 44 (24) |
| Brain tumor, n (%) | 35 (16) | 4 (11) | 31 (17) |
| Traumatic IPH, n (%) | 28 (13) | 11 (30) | 17 (9) |
| Aneurysmal subarachnoid hemorrhage, n (%) | 22 (10) | 6 (16) | 16 (9) |
| Seizure, n (%) | 13 (6) | 5 (14) | 8 (4) |
| Otherb, n (%) | 25 (11) | 2 (5) | 23 (12) |
| Markers of disease severity | |||
| Mass effect, n (%) | 162 (73) | 26 (70) | 136 (74) |
| Midline shift, n (%) | 112 (51) | 18 (49) | 94 (51) |
| Uncal herniation, n (%) | 72 (33) | 9 (24) | 63 (34) |
| Intracranial pressure monitor, n (%) | 78 (35) | 18 (49) | 60 (33) |
| External ventricular drain, n (%) | 24 (11) | 7 (19) | 17 (9) |
| Craniectomy, n (%) | 36 (16) | 8 (22) | 28 (15) |
| Mechanical ventilation, n (%) | 172 (78) | 37 (100) | 135 (73) |
| Death at discharge, n (%) | 74 (33) | 4 (11) | 70 (38) |
IPH = intraparenchymal hemorrhage, N = patients
aOther races: Native American (N = 1), and unspecified (N = 42).
bOther diagnoses: cerebral sinus venous thrombosis (N = 4), nonaneurysmal subarachnoid hemorrhage (N = 1), epidural hemorrhage (N = 1), subdural hemorrhage (N = 6), isolated interventricular hemorrhage (N = 1), isolated hydrocephalus (N = 2), infection (N = 5), moyamoya disease (N = 1), MCA aneurysm (N = 1), anti-NMDA encephalitis (N = 1), autoimmune encephalitis (N = 1), and posterior reversible encephalopathy syndrome (N = 1).
(+) dexmedetomidine represents an initiation or increase in dose, whereas (−) dexmedetomidine represents absent, static, or decrease in dose.
Thirty-seven patients (17%) had at least one pupil measurement within 60 minutes following initiation or increased infusion of dexmedetomidine. Patients in the dexmedetomidine group were younger (55 yr [IQR, 44–60 yr] versus 61 yr [IQR, 51.75–68 yr]). A greater percentage of patients in the dexmedetomidine group were diagnosed with traumatic intraparenchymal hemorrhage (30% vs 9%), required intracranial pressure monitoring (49% vs 33%), and required mechanical ventilation (100% vs 73%). The dexmedetomidine group was less likely to be deceased by discharge (11% vs 38%) and more likely to have received another analgesic (95% vs 69%) or another sedative (57% vs 15%) at some point during their ICU stay (Table 1, and Supplementary Table 2, http://links.lww.com/CCX/A987). The median time between dexmedetomidine administration and pupil measurement was 17 minutes (IQR, 9–30 min).
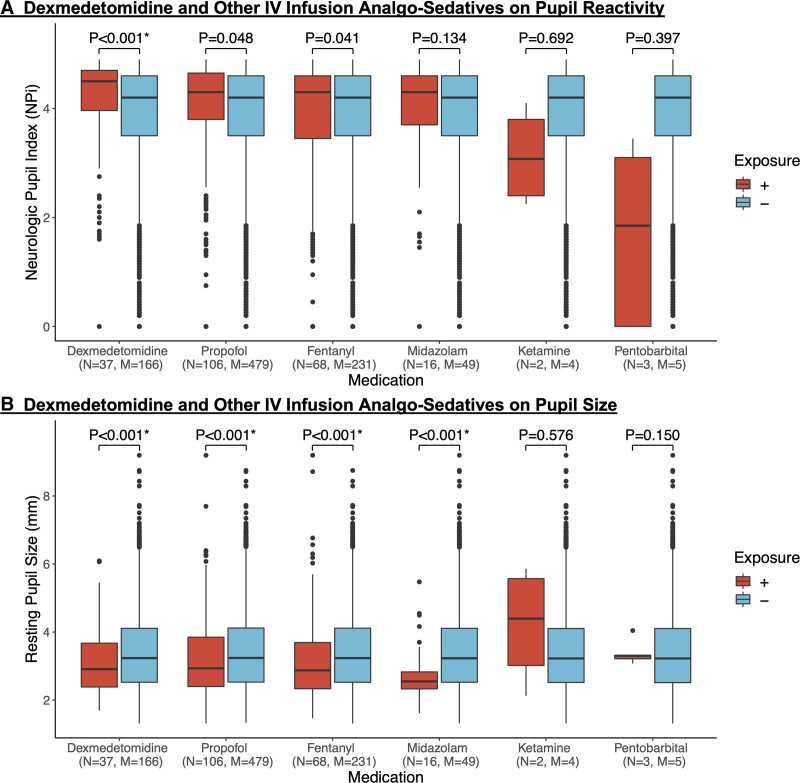
Regarding our primary hypotheses, we observed that dexmedetomidine was associated with higher average NPi (4.14 ± 0.87 vs 3.77 ± 1.22; p < 0.001) and smaller average resting pupil size (3.13 ± 0.96 vs 3.41 ± 1.13; p < 0.001) (Fig. 2, and Supplementary Table 3, http://links.lww.com/CCX/A987). These relationships remained significant in our multivariable model adjusting for sex, age, GCS, radiographic mass effect, acetaminophen, and nighttime indicator (NPi [β = 0.18 normalized unit increase when dexmedetomidine is present ± 0.04; p < 0.001] and pupil size [β = –0.25 ± 0.05; p < 0.001]) (Table 2). In the subset of patients with at least one exposure to dexmedetomidine, the direction and significance of these relations persisted (NPi [β = 0.18 ± 0.04; p < 0.001] and pupil size [β = –0.24 ± 0.06; p < 0.001]) (Supplementary Fig. 2, http://links.lww.com/CCX/A987). As a continuous variable, dexmedetomidine dose did not have a significant association with pupil reactivity and size (Supplementary Table 4, http://links.lww.com/CCX/A987).
Figure 2.
Grouped boxplots of IV infusion analgosedatives on average Neurologic Pupil Index (NPi) and average resting pupil size. Dexmedetomidine and other IV infusion analgosedatives on pupil reactivity and size. Grouped boxplots showing the effect of dexmedetomidine and other IV infusion analgosedatives on (A) average Neurologic Pupil Index (NPi) and (B) average resting pupil size between both eyes. Displayed are boxes outlining the median, 25th, and 75th quartiles. Sample sizes presented are the total number of patients and pupil observations associated with the medication. (+) exposure represents an initiation or increase in medication dose, whereas (−) exposure represents absent, static, or decrease in medication dose. p values were calculated using univariate mixed-effects linear regression. Our model was constructed as follows: average NPi (or average resting pupil size) ~ Primary medication exposure (dichotomous) + 1|patient indicator + error. α = 0.025* due to Bonferroni correction. M = pupil observations, N = patients.
TABLE 2.
Multivariable Model of Analgosedatives on Pupil Reactivity and Size
| Dexmedetomidine Model,a (N = 37, M = 166) | Neurologic Pupil Index β | p | Resting Pupil Size β | p |
|---|---|---|---|---|
| Dexmedetomidine | 0.18 ± 0.04 | < 0.001 | –0.25 ± 0.05 | < 0.001 |
| Male | 0.27 ± 0.12 | 0.03 | –0.22 ± 0.11 | 0.05 |
| Age, yr | 0.01 ± 0.004 | < 0.001 | -0.02 ± 0.004 | <0.001 |
| Glasgow Coma Score | 0.04 ± 0.003 | <0.001 | 0.07 ± 0.004 | < 0.001 |
| Mass effect | –0.19 ± 0.13 | 0.14 | 0.05 ± 0.13 | 0.71 |
| Acetaminophen | 0.04 ± 0.02 | 0.02 | 0.01 ± 0.02 | 0.54 |
| Nighttime | 0.15 ± 0.01 | < 0.001 | 0.11 ± 0.01 | < 0.001 |
| Acetaminophen Model (N = 139, M = 984) | Neurologic Pupil Index β | p | Resting Pupil Size β | p |
| Acetaminophen | 0.04 ± 0.02 | 0.02 | 0.01 ± 0.02 | 0.55 |
| Male | 0.26 ± 0.12 | 0.03 | –0.22 ± 0.11 | 0.05 |
| Age, yr | 0.01 ± 0.004 | < 0.001 | –0.02 ± 0.004 | < 0.001 |
| Glasgow Coma Score | 0.04 ± 0.003 | < 0.001 | 0.06 ± 0.004 | < 0.001 |
| Mass effect | –0.20 ± 0.13 | 0.14 | 0.05 ± 0.13 | 0.71 |
| Nighttime | 0.15 ± 0.01 | < 0.001 | 0.10 ± 0.01 | < 0.001 |
M = pupil observations, N = patients.
aPrimary hypothesis.
β coefficients represent the change in rank-normalized units of average Neurologic Pupil Index or resting pupil size in the presence of a dichotomous variable (dexmedetomidine, male, mass effect, acetaminophen, and nighttime indicator) or an increase in a continuous or ordinal scale (age and Glasgow Coma Score).
Sample sizes presented show the total number of patients and pupil observations associated with an initiation or an increase in medication dose less than 60 min prior to pupil examination.
p values were calculated using multivariable mixed-effects linear regression. Our model was constructed as follows: average Neurologic Pupil Index (or average resting pupil size) ~ primary medication exposure (dichotomous) + sex + age + Glasgow Coma Scale + mass effect + acetaminophen + night-indicator + 1 patient indicator + error.
No other IV infusion analgosedative was significantly associated with average NPi (Fig. 2). Among primary sedatives, clobazam (3.16 ± 1.42 vs 3.78 ± 1.21; p = 0.007) was associated with lower average NPi, whereas lorazepam (3.86 ± 1.21 vs 3.78 ± 1.22; p = 0.005) was associated with higher average NPi (Supplementary Table 3, http://links.lww.com/CCX/A987).
Among primary analgesics, acetaminophen significantly associated with higher average NPi (4.02 ± 0.94 vs 3.75 ± 1.24; p = 0.004) (Supplementary Table 3, http://links.lww.com/CCX/A987). To further explore this unexpected association, we conducted an exploratory multivariable analysis adjusting for sex, age, GCS, radiographic mass effect, and nighttime indicator. In our post hoc analysis, acetaminophen remained associated with higher average NPi (β = 0.04 ± 0.02; p = 0.02) (Table 2).
We observed smaller average resting pupil sizes following initiation or increased doses of IV infusion analgosedatives including propofol (3.19 ± 1.04 vs 3.41 ± 1.13; p < 0.001), fentanyl (3.15 ± 1.15 vs 3.41 ± 1.12; p < 0.001), and midazolam (2.71 ± 0.74 vs 3.41 ± 1.13; p < 0.001) (Fig. 2, and Supplementary Table 3, http://links.lww.com/CCX/A987).
Conversely, intermittent sedatives including clonidine (3.99 ± 1.15 vs 3.40 ± 1.13; p < 0.001), lorazepam (3.62 ± 1.18 vs 3.40 ± 1.12; p = 0.001), and clobazam (4.30 ± 1.22 vs 3.40 ± 1.12; p < 0.001) were associated with larger average resting pupil size. Among primary analgesics, meperidine (2.98 ± 0.91 vs 3.41 ± 1.13; p = 0.007) was associated with smaller average resting pupil size (Supplementary Table 3, http://links.lww.com/CCX/A987).
DISCUSSION
Changes in pupil reactivity and size can instigate management decisions and diagnostic workup. In patients monitored for evolving intracranial injuries, familiarity with pupil changes following analgosedative administration can be crucial for accurate pupil interpretation. Although prior studies have found associations, some contradictory, between different analgosedatives and pupil reactivity and size, they were primarily done in small cohorts of healthy or anesthetized individuals awaiting surgery. In our cohort of 221 critical care patients with intracranial injury, we found that dexmedetomidine was associated with higher pupil reactivity (β = 0.18 ± 0.04; p < 0.001) and smaller resting pupil size (β = –0.25 ± 0.05; p < 0.001) after adjusting for confounders (Table 2).
Pupil reactivity and size depend on a complex balance of neuronal pathways primarily involving the optic and oculomotor nerves (31). The locus coeruleus, carrying input from the amygdala, sleep and arousal networks, and nociceptive pathways, modulates pupil reactivity and size via noradrenergic projections terminating on the EWN, the parasympathetic nucleus of the oculomotor nerve (5, 11, 32, 33). Due to the abundance of receptors lining principal afferent/efferent and locus coeruleus-mediated modulatory pathways, exogenous agents can affect pupil reactivity and size in opposing directions (31).
Despite anecdotal assumptions that most analgosedatives decrease both pupil reactivity and size (7–10, 34), our work is consistent with Larson and Talke (12) finding that dexmedetomidine is associated with increased pupil reactivity. One hypothesized mechanism is that dexmedetomidine, an α2 agonist, activates autoreceptors in the locus coeruleus and disinhibits the EWN-mediated pupillary constriction (31, 35). Larson and Talke (12) proposed the possibility of an unknown accessory pathway because the eight patients the authors studied presumably already had depressed locus coeruleus activity from anesthesia (36). Our finding that dexmedetomidine associated with decrease in resting pupil size supports a previous case series that found decreased pupil size in patients administered dexmedetomidine prior to undergoing cataract surgery (37).
We did not find a significant association between clonidine (another α2 agonist) and NPi (Supplementary Table 3, http://links.lww.com/CCX/A987), counter to published work in both healthy and anesthetized humans (13, 14). One possible explanation is that dexmedetomidine is approximately 8–10 times more selective for the α2-adrenergic receptor, with α2:α1 receptor affinity 1,620:1 versus 200:1, respectively (38, 39). The increased selectivity produces more potent sedative-hypnotic effects through interactions with α2-adrenergic receptors in the locus coeruleus. Additionally, dexmedetomidine acts as a full agonist, as opposed to clonidine, a partial agonist at α2-adrenergic receptors. As such, the dose of clonidine may not have been sufficient to elicit a significant difference in pupil reactivity. The greater α1-adrenergic receptor agonist activity with clonidine may explain the larger resting pupil size compared with dexmedetomidine through increased contraction of the radial eye muscle (39). Finally, clonidine is often administered in the ICU for autonomic storming, which could modulate pupil reactivity and size. We did not have temporal information related to blood pressure or pulse to rigorously examine this association.
We found that the association between other IV infusion analgosedatives and NPi did not meet our threshold for significance (Supplementary Table 3, http://links.lww.com/CCX/A987). This opposes studies reporting fentanyl, propofol, and ketamine led to lower NPi in humans (9, 10, 34), and pentobarbital depressed the pupillary light reflex in cats (36). We acknowledge that the lack of robust associations may have been due to small sample size in the case of pentobarbital (N = 3) and ketamine (N = 2). However, the number of patients who received propofol (N = 106) and fentanyl (N = 68) was higher, and an absence in reactivity change was also supported by the work by Shirozu et al (10) and Kim et al (19). Smaller pupil size following propofol, fentanyl, and midazolam administration supports published findings in both healthy and anesthetized patients (Supplementary Table 3, http://links.lww.com/CCX/A987) (7–9).
Among primary analgesic or sedatives, acetaminophen and lorazepam were associated with higher average NPi, whereas clobazam was associated with lower average NPi. Acetaminophen associated with higher NPi after adjusting for confounders (β = 0.04 ± 0.02; p = 0.02). However, aside from acetaminophen (N = 139, M = 984), each of these medications had less than 20 patients and 60 pupil measurements.
Although the pharmacokinetic mechanisms of acetaminophen are not fully elucidated, its analgesic and antipyretic properties have been well documented (40, 41). Modulation of emotional factors, cognitive load, and pain by acetaminophen can influence pupil reactivity and size (5). It is also possible that secondary mechanisms of acetaminophen may affect pupillary response in ways that are yet to be determined, however, as other analgesics in our study did not have significant associations (42). Acetaminophen is widely used in the inpatient setting, and findings should be confirmed in prospective settings.
Our results should be interpreted with the following limitations. By averaging our primary outcomes, we are not able to draw conclusions on how medications affect pupil reactivity unilaterally, especially in the settings of ipsilateral or contralateral mass effect. We attempted to limit the effects of confounders that may asymmetrically impair pupil reactivity by excluding patients with a history of retinal surgery, optic neuritis, traumatic pupil injury, glaucoma, and cataracts. Due to the lack of prior studies, we conducted multiple tests of association, increasing the potential for false-positive findings. We attempted to mitigate this by selecting two primary hypotheses and an appropriate statistical correction. The remainder of our analyses are hypothesis-generating for more definitive studies. Sample size limited studying variation by the heterogeneous diagnoses present in the neuro-ICU and comparing findings with patients without intracranial pathology. We performed subgroup analyses of average NPi and average resting pupil size after dexmedetomidine administration stratified by diagnosis and found that the association between dexmedetomidine and average NPi remained significant in patients with brain tumors and parenchymal subarachnoid hemorrhage (Supplementary Fig. 3, http://links.lww.com/CCX/A987).
Because our study was observational and retrospective, we cannot exclude residual confounding or establish causal relations. Our 60-minute study window was selected to account for the heterogeneity of medication therapies and formulations, as time to onset and peak effects vary considerably due to medication-specific pharmacokinetics as well as route of administration. Dexmedetomidine, our primary medication exposure, has a rapid redistribution half-life of approximately 6 minutes, and its peak effect is expected to be observed within 30 minutes (43). Although more rapid time to peak effect may be seen after IV bolus, IV bolus therapy is rarely used in our institutions’ clinical practice. Our time window, however, is limited by the potential for multiple changes in pupil reactivity and size over the 60 minutes and should be decreased in the future as determined by medication-specific properties. To address bias from ambient light, we included a dichotomous night-indicator based on the assumption that ambient light levels are lower between 7 pm and 7 am in the ICU. Our variable, however, does not capture absolute lumens and account for staff and patient manipulation of room lighting. We did not have mechanical ventilator data at the pupil level and were not able to control for the potentially lower GCS scores during ventilation, which could spuriously lower GCS in our multivariable models. We adjusted our primary multivariable model only for acetaminophen because we observed an unexpected yet substantial association with the same directional association as dexmedetomidine, whereas we assumed that effects from other potentially influencing medications would bias us toward the null. We did not have sufficient sample sizes to further explore pupil changes following lorazepam and clobazam administration, which commonly occur in the setting of seizures and meperidine, which is indicated for shivering during targeted temperature management. Cognitive load pain could have also influenced pupil reactivity and size but was not accounted for in this study (5).
Despite limitations, this is the first study to our knowledge to test the effect of analgosedatives on quantitative pupillometry in a heterogenous cohort of critically ill patients with acute intracranial pathology, using over 9,000 pupil observations across two neuro-ICUs. For our primary investigation, we adjusted for patient- and pupil-level covariates including demographics and arousal state. Finally, our use of quantitative pupillometry and statistical methodology enables future validation and generalization beyond our institution.
CONCLUSIONS
Dexmedetomidine administration was associated with higher pupil reactivity and smaller resting pupil size in critically ill patients with acute intracranial pathology after adjusting for confounders. We observed smaller resting pupil size but no significant difference in NPi among other IV infusion analgosedatives. Of our tested PO and one-time IV analgesics, acetaminophen was associated with higher pupil reactivity after adjusting for confounders. Understanding expected pupil effects after commonly used medications is important for accurate interpretation of clinical pupil findings. Our foundational study provides the groundwork for larger prospective investigations, improves our interpretation of the pupil examination, and potentially facilitates more accurate evaluation, prognostication, and management of patients with neurologic disease in the neuro-ICU.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Mr. Chan contributed to statistical analysis, interpretation of results, article drafting and revisions, figure and table creation, and submission. Mr. Prescott contributed to study design, data collection, statistical analysis, interpretation of results, and article drafting. Dr. Barra contributed to data collection, interpretation of results, and article revisions. Dr. Chung contributed critical revisions to the article. Ms. Kim contributed critical revisions to the article. Dr. Saglam contributed to data collection and imaging review. Ms. Hutch contributed critical revisions to the article. Dr. Shin contributed critical revisions to the article. Dr. Zafar contributed critical revisions to the article. Dr. Smirnakis contributed critical revisions to the article. Dr. Benjamin contributed critical revisions to the article. Dr. Dupuis contributed to statistical analysis plan and review, and critical revisions to the article. Dr. Greer contributed to study design, interpretation of results, and critical revisions to the article. Dr. Ong contributed to study design, data collection, statistical analysis, interpretation of results, article drafting, table creation, and critical revisions.
Mr. Chan received support from 2T35AG038027-11. Dr. Zafar receives support from K23NS114201. Dr. Benjamin is supported in, part by, 2R01HL092577; 1R01 HL141434 01A1; 2U54HL120163; 1R01AG066010; 1R01AG066914; and American Heart Association, AHA_18SFRN34110082. Dr. Smirnakis is supported by R01EY024019 and VA Merit award I01RX002981. Dr. Greer is supported by R01NS102574. Dr. Ong receives support from NIH/NINDS K23NS116033. The authors whose names are listed immediately above certify that they have no affiliations with or involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in this article. The remaining authors have disclosed that they do not have any potential conflicts of interest.
This work was performed at Massachusetts General Hospital, Brigham and Women's Hospital and Boston Medical Center.
REFERENCES
- 1.Prescott BR, Saglam H, Duskin JA, et al. : Anisocoria and poor pupil reactivity by quantitative pupillometry in patients with intracranial pathology. Crit Care Med 2021; 50:e143–e153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belliveau AP, Somani AN, Dossani RH: Pupillary light reflex. In: StatPearls. Treasure Island, FL, StatPearls Publishing, 2021 [PubMed] [Google Scholar]
- 3.Hall CA, Chilcott RP: Eyeing up the future of the pupillary light reflex in neurodiagnostics. Diagnostics (Basel) 2018; 8:E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spector RH: The pupils. In: Clinical Methods: The History, Physical, and Laboratory Examinations. Walker HK, Hall WD, Hurst JW. (Eds). Boston, MA, Butterworths, 1990 [PubMed] [Google Scholar]
- 5.Larson MD, Behrends M: Portable infrared pupillometry: A review. Anesth Analg 2015; 120:1242–1253 [DOI] [PubMed] [Google Scholar]
- 6.Behrends M, Larson MD, Neice AE, et al. : Suppression of pupillary unrest by general anesthesia and propofol sedation. J Clin Monit Comput 2019; 33:317–323 [DOI] [PubMed] [Google Scholar]
- 7.Bokoch MP, Behrends M, Neice A, et al. : Fentanyl, an agonist at the mu opioid receptor, depresses pupillary unrest. Auton Neurosci 2015; 189:68–74 [DOI] [PubMed] [Google Scholar]
- 8.Sabourdin N, Meniolle F, Chemam S, et al. : Effect of different concentrations of propofol used as a sole anesthetic on pupillary diameter: A randomized trial. Anesth Analg 2020; 131:510–517 [DOI] [PubMed] [Google Scholar]
- 9.Hoshi T: [Influence of propofol and remifentanil on pupillary light reflex assessed by a hand-held point-and-shoot pupillometer]. Masui 2017; 66:174–176 [PubMed] [Google Scholar]
- 10.Shirozu K, Setoguchi H, Tokuda K, et al. : The effects of anesthetic agents on pupillary function during general anesthesia using the automated infrared quantitative pupillometer. J Clin Monit Comput 2017; 31:291–296 [DOI] [PubMed] [Google Scholar]
- 11.Samuels ER, Szabadi E: Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function part II: Physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol 2008; 6:254–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larson MD, Talke PO: Effect of dexmedetomidine, an alpha2-adrenoceptor agonist, on human pupillary reflexes during general anaesthesia. Br J Clin Pharmacol 2001; 51:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bitsios P, Szabadi E, Bradshaw CM: The effects of clonidine on the fear-inhibited light reflex. J Psychopharmacol 1998; 12:137–145 [DOI] [PubMed] [Google Scholar]
- 14.Clifford JM, Day MD, Orwin JM: Reversal of clonidine induced miosis by the alpha 2-adrenoreceptor antagonist RX 781094. Br J Clin Pharmacol 1982; 14:99–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson DM, Stutzman S, Saju C, et al. : Interrater reliability of pupillary assessments. Neurocrit Care 2016; 24:251–257 [DOI] [PubMed] [Google Scholar]
- 16.Ong CJ: Quantitative pupillometry. Chest 2020; 157:1049–1050 [DOI] [PubMed] [Google Scholar]
- 17.Phillips SS, Mueller CM, Nogueira RG, et al. : A systematic review assessing the current state of automated pupillometry in the NeuroICU. Neurocrit Care 2019; 31:142–161 [DOI] [PubMed] [Google Scholar]
- 18.Chen JW, Gombart ZJ, Rogers S, et al. : Pupillary reactivity as an early indicator of increased intracranial pressure: The introduction of the Neurological Pupil index. Surg Neurol Int 2011; 2:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TJ, Park SH, Jeong HB, et al. : Neurological pupil index as an indicator of neurological worsening in large hemispheric strokes. Neurocrit Care 2020; 33:575–581 [DOI] [PubMed] [Google Scholar]
- 20.Riker RR, Sawyer ME, Fischman VG, et al. : Neurological pupil index and pupillary light reflex by pupillometry predict outcome early after cardiac arrest. Neurocrit Care 2020; 32:152–161 [DOI] [PubMed] [Google Scholar]
- 21.Teasdale G, Jennett B: Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2:81–84 [DOI] [PubMed] [Google Scholar]
- 22.von Elm E, Altman DG, Egger M, et al. : Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007; 335:806–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson DM, Stutzman SE, Atem F, et al. : Establishing normative data for pupillometer assessment in neuroscience intensive care: The “END-PANIC” registry. J Neurosci Nurs 2017; 49:251–254 [DOI] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson AB, Yellott JI: A unified formula for light-adapted pupil size. J Vis 2012; 12:12. [DOI] [PubMed] [Google Scholar]
- 26.Osman M, Stutzman SE, Atem F, et al. : Correlation of objective pupillometry to midline shift in acute stroke patients. J Stroke Cerebrovasc Dis 2019; 28:1902–1910 [DOI] [PubMed] [Google Scholar]
- 27.Ong C, Hutch M, Smirnakis S: The effect of ambient light conditions on quantitative pupillometry. Neurocrit Care 2019; 30:316–321 [DOI] [PubMed] [Google Scholar]
- 28.Qiu X, Wu H, Hu R: The impact of quantile and rank normalization procedures on the testing power of gene differential expression analysis. BMC Bioinformatics 2013; 14:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Detry MA, Ma Y: Analyzing repeated measurements using mixed models. JAMA 2016; 315:407–408 [DOI] [PubMed] [Google Scholar]
- 30.Armstrong RA: When to use the Bonferroni correction. Ophthalmic Physiol Opt 2014; 34:502–508 [DOI] [PubMed] [Google Scholar]
- 31.Szabadi E: Functional organization of the sympathetic pathways controlling the pupil: Light-inhibited and light-stimulated pathways. Front Neurol 2018; 9:1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joshi S, Li Y, Kalwani RM, et al. : Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 2016; 89:221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa VD, Rudebeck PH: More than meets the eye: The relationship between pupil size and locus coeruleus activity. Neuron 2016; 89:8–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vide S, Costa CM, Gambus PL, et al. : Effects of ketamine on pupillary reflex dilation: A case report. A A Pract 2018; 10:39–41 [DOI] [PubMed] [Google Scholar]
- 35.Gertler R, Brown HC, Mitchell DH, et al. : Dexmedetomidine: A novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001; 14:13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharpe LG: Separate neural mechanisms mediate sufentanil-induced pupillary responses in the cat. J Pharmacol Exp Ther 1991; 256:845–849 [PubMed] [Google Scholar]
- 37.Dersu I: Dexmedetomidine associated with intraoperative floppy iris syndrome in ophthalmic surgery. J Clin Diagn Res 2020; 14:NL01. [Google Scholar]
- 38.Srivastava U, Sarkar ME, Kumar A, et al. : Comparison of clonidine and dexmedetomidine for short-term sedation of intensive care unit patients. Indian J Crit Care Med 2014; 18:431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giovannitti JA, Jr, Thoms SM, Crawford JJ: Alpha-2 adrenergic receptor agonists: A review of current clinical applications. Anesth Prog 2015; 62:31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohashi N, Kohno T: Analgesic effect of acetaminophen: A review of known and novel mechanisms of action. Front Pharmacol 2020; 11:580289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koch-Weser J: Drug therapy. Acetaminophen. N Engl J Med 1976; 295:1297–1300 [DOI] [PubMed] [Google Scholar]
- 42.Przybyła GW, Szychowski KA, Gmiński J: Paracetamol – an old drug with new mechanisms of action. Clin Exp Pharmacol Physiol 2021; 48:3–19 [DOI] [PubMed] [Google Scholar]
- 43.Iirola T, Aantaa R, Laitio R, et al. : Pharmacokinetics of prolonged infusion of high-dose dexmedetomidine in critically ill patients. Crit Care 2011; 15:R257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.