OBJECTIVES:
Catecholamines and vasopressin are commonly used in patients with post cardiovascular surgery vasoplegia (PCSV). Multimodal therapy, including methylene blue (MB), hydroxocobalamin, and angiotensin II (Ang II), may improve outcomes in patients who remain hypotensive despite catecholamine and vasopressin therapy. However, a standardized approach has not been established. We created a protocol at Emory Healthcare (Emory Protocol), which provides guidance on norepinephrine equivalent dose (NED) and the use of noncatecholamines in the setting of PCSV and sought to determine the clinical significance of adherence to the protocol.
DESIGN:
Retrospective study.
SETTING:
Multisite study at Emory University Hospital.
PATIENTS:
Patients receiving Ang II for PCSV in any cardiovascular ICU from 2018 to 2020.
INTERVENTIONS:
Patient encounters were scored on Emory Protocol compliance based on NED (1–5), use of vasopressin (1–2), use of MB (1–2), and documentation of high-output shock (1–4). A compliant score was less than 7, moderately compliant 7 to 8, and poorly compliant greater than 8. Demographics, clinical data, and outcomes were abstracted from the medical records.
MEASUREMENTS AND MAIN RESULTS:
Of the 78 consecutive patients receiving Ang II for PCSV, overall ICU mortality was 26.9%, with an average compliance score of 6.2. ICU mortality was 21.1% for compliant cases (n = 38), 29.7% for moderately compliant cases (n = 24), and 37.5% for poorly compliant cases (n = 16). In regression analysis, the cumulative compliance score to the Emory Protocol was predictive of ICU mortality (p = 0.027).
CONCLUSIONS:
Compliance with the Emory Protocol, emphasizing early initiation of the noncatecholamines vasopressin, MB, hydroxocobalamin, and Ang II at lower catecholamine doses in high-output shock, is associated with improved ICU mortality.
Keywords: angiotensin II, cardiovascular surgery, circulatory shock, protocolized therapy, vasoplegia
Patients undergoing cardiac surgery are at risk for postoperative hypotension despite adequate cardiac function and often require significant doses of vasopressors to support blood pressure following separation from cardiopulmonary bypass (CPB). Postoperative hypotension, also known as post cardiovascular surgery vasoplegia (PCSV), is characterized by low systemic vascular resistance with normal or enhanced cardiac output and associated with high mortality rates (1). Currently, the treatment of PCSV is similar to that of septic shock and includes administration of fluids and vasopressor support. Previous studies of PCSV have primarily examined these treatment modalities in this context.
More recently, the use of noncatecholamine vasopressors for PCSV has been emphasized (2). Patients undergoing cardiac surgery are often treated with renin-angiotensin-aldosterone system (RAAS) blockade. Further, the highest density of angiotensin-converting enzyme (ACE) is present on the pulmonary capillary endothelium, so the effect of CPB will further exacerbate RAAS dysfunction. Therefore, IV angiotensin II (Ang II) may be an ideal agent to treat PCSV. Endogenous Ang II is a peptide hormone naturally produced by the body that regulates blood pressure via vasoconstriction of both arteries and veins and sodium reabsorption, causing an increase mean arterial pressure (MAP) (3). The Angiotensin II for the Treatment of High-Output Shock 3 (ATHOS-3) study demonstrated that administration of Ang II supports low blood pressure and reduces the total amount of other vasopressors in patients with high-output shock (4). A post hoc analysis of ATHOS-3 identified 16 patients with PCSV and described a robust blood pressure effect in response to Ang II (5). Additional case reports describe the successful down-titration of catecholamines in the setting of Ang II for PCSV (6, 7).
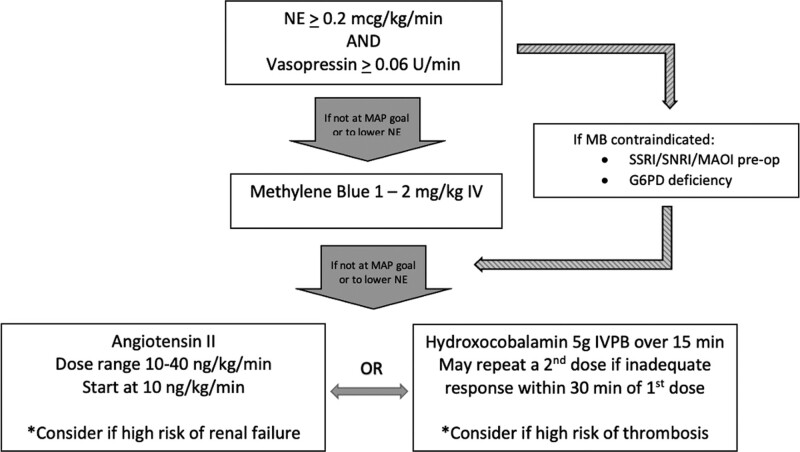
The practice of deploying multimodal therapy augments blood pressure by targeting different receptor pathways in order to achieve a desired effect while avoiding high doses of catecholamines, which have been shown to cause direct cardiotoxicity (8). Noncatecholamine therapies for PCSV include vasopressin and Ang II, as well as nonspecific nitric oxide scavengers methylene blue (MB) and hydroxocobalamin. Compared with the nitric oxide scavengers, Ang II is the only noncatecholamine that is safely titratable. This has served to increase interest in its use as a component of multimodal therapy for PCSV. Currently, however, there is no consensus on how to deploy multimodal therapy for PCSV. Following the commercial availability of Ang II, a standardized protocol was developed throughout cardiovascular ICUs (CVICUs) within the Emory Healthcare System (the Emory Protocol) (Fig. 1). PCSV is not an uncommon occurrence among Emory’s 2,900 cases annually, with approximately 10–15% of patients requiring vasopressor support postoperatively (of which one quarter receive Ang II). We sought to evaluate compliance to the Emory Protocol in a cohort of PCSV patients who ultimately received Ang II and determine whether such compliance translated into improved ICU survival.
Figure 1.
The Emory Protocol should be deployed in the proper clinical scenario, which requires temporal proximity (~48 hr) to cardiovascular surgery with cardiopulmonary bypass support. Patients should have features and documentation of high-output shock, including a cardiac index of greater than 2.3 or a mixed venous oxygen saturation greater than or equal to 70% in conjunction with a central venous pressure greater than or equal to 8 mm Hg. Shock should be supported with catecholamines at a norepinephrine equivalence (NE) dose of approximately 0.2 µg/kg/min as well as vasopressin at a dose of greater than or equal to 0.06 U/min before third-line therapy is considered. Methylene blue (MB) should be considered the primary third-line therapy unless contraindications exist. In the event of contraindications or failure of response to MB, angiotensin II (Ang II) or hydroxocobalamin may be considered, with the choice between the two informed by the clinical situation and likelihood of adverse events. G6PD = glucose-6-phosphate dehydrogenase, IVPB = IV piggyback/IV short-term infusion, MAOI = monoamine oxidase inhibitor, MAP = mean arterial pressure, SNRI = serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor.
MATERIALS AND METHODS
Patient Population
In this retrospective study, we enrolled 78 consecutive adult patients who received Ang II for PCSV and were admitted to any CVICU within the Emory Healthcare system (Atlanta, GA) between January 1, 2018, and December 31, 2020. Diagnostic parameters for PCSV included reduced MAP requiring greater than or equal to one vasopressor, evidence of high cardiac output, and a temporal relationship to cardiovascular surgery. This study was approved by the Emory University Institutional Review Board (IRB00113240).
Data Collection
Basic demographic and clinical information were collected through review of the electronic medical record and the Emory Healthcare cardiac surgery database, including age, sex, comorbid conditions, serum lactate at the time of initiation of Ang II, ICU length of stay, hospital length of stay, survival to ICU discharge, survival to hospital discharge, and 30-day mortality. Sequential Organ Failure Assessment (SOFA) scores were calculated and recorded for each patient within 24 hours preceding the initiation of Ang II. Doses of vasopressors at Ang II initiation were collected for each patient. Finally, operative data and the presence or absence of mechanical circulatory support were also collected.
Protocol for Post Cardiovascular Surgery Vasoplegia
Deployment of the Emory Protocol included publication, distribution, and education of the protocol to all potential hospital staff end users, as well as ongoing evaluation of compliance. Per the Emory Protocol, patients are required to have documentation of high-output shock along with initiation of catecholamines and vasopressin infusions. The MAP goal is ultimately determined by the provider. The concomitant norepinephrine equivalent catecholamine dose (NED), which includes the sum total of norepinephrine and epinephrine, is required to be greater than or equal to 0.2 mcg/kg/min in addition to a vasopressin dose greater than or equal to 0.06 U/min before a second noncatecholamine agent, MB, is added. Other catecholamines (phenylephrine and dopamine) are not included in calculation of NED due to infrequent usage. MB is bypassed if there is a contraindication to MB (selective serotonin reuptake inhibitor/serotonin-norepinephrine reuptake inhibitor/monoamine oxidase inhibitor use preoperatively or a documented glucose-6-phosphate dehydrogenase deficiency). If the MAP goal is not achieved with this regimen, a third noncatecholamine agent is added, which is dictated by the clinical scenario. Ang II (Giapreza, La Jolla Pharmaceutical Company, La Jolla, CA) is considered if there is a high risk of renal impairment, and hydroxocobalamin is considered if there is concern for thrombosis. A flowchart summarizing Emory Protocol is presented in Figure 1. Notably, and exclusive of the Emory Protocol, ACE inhibitors and angiotensin receptor blockers (ARBs) are held for 48 hours preoperatively for elective and semi-elective cases at Emory. For urgent or emergent cases, ACE inhibitors or ARBs are held at admission.
Inclusion Criteria and Protocol Compliance
All patients receiving Ang II for PCSV were included. We calculated Emory Protocol compliance by analyzing background NED, use and dose of vasopressin, the use of MB, and documentation of high-output shock at both the ordering time of Ang II as well as the time of initiation of Ang II. We then graded compliance based on a point system, with a lower score indicating higher compliance. NED compliance ranged from a score of 1 to 5 depending on the NED at initiation of Ang II. High-output shock compliance ranged from 1 to 4 based on acknowledgment and timely documentation of high-output shock, which was defined as a cardiac index of greater than 2.2 L/min/m2 or a combination of a mixed venous oxygen saturation of greater than or equal to 70% and a central venous pressure greater than or equal to 8 cm H2O. Vasopressin compliance was scored a 1 or a 2 based on the presence of and starting dose of vasopressin at the time of initiation of Ang II. MB compliance was also scored 1 to 2 based on the deployment of MB prior to the use of Ang II or the presence of a contraindication to MB. Supplemental Table 1 (http://links.lww.com/CCX/A985) shows details of compliance scoring. Patient encounters were scored for protocol compliance and further grouped as compliant (< 7), moderately compliant (7–8), or poorly compliant (> 8).
Statistical Analysis
The primary endpoint was ICU mortality based on protocol compliance with secondary endpoints including hospital length of stay, ICU length of stay, hospital mortality, and 30-day mortality. Differences among groups were analyzed using descriptive statistics with 95% CIs, through the Wilcoxon rank-sum test or analysis of variance for continuous or ordinal variables and chi-square or Fisher exact test for discrete variables. We performed binary logistic regression of ICU mortality, hospital mortality, and 30-day mortality (the dependent variable) using both discrete and continuous variables that included age, gender, SOFA score, serum lactate at time of initiation of Ang II, NED dose at the start of Ang II, the presence or absence of MB, hydroxocobalamin, or mechanical assist support, CPB time, and cumulative protocol compliance. Subsequent sensitivity analyses consisted of three binary logistic regression models of 1) variables that were unbalanced between groups (p ≤ 0.20), 2) variables likely to influence the primary outcome measure, and 3) a combination of variables that were statistically significant within the first two sensitivity analyses. A further sensitivity analysis consisted of binary regression against the components of the cumulative compliance score, including categorical variables of MB and vasopressin compliance and the continuous variables of NED dosing and high-output compliance. Data were analyzed using IBM SPSS Statistics, Version 27 (IBM Corporation, Armonk, NY).
RESULTS
Seventy-eight consecutive patients with PCSV were included in the analysis. The mean age was 59.6 (sd 12.4) and 61 (78.2%) patients were male. The mean SOFA score was 7.9 (sd 3.2). Average NED and vasopressin doses at time of initiation of Ang II were 0.31 µg/kg/min and 0.11 U/min, respectively. Twenty-one patients died (26.9%) while still in the ICU, while 23 died (29.5%) before hospital discharge. ICU and hospital LOS were 16.3 and 25.4 days. The mean Emory Protocol compliance score was 6.2 (sd 1.6). Cumulative compliance score was found to be correlated with ICU mortality (p = 0.03) (Table 1). Of the other continuous variables (age, SOFA score, vasopressor doses, CPB time, cross-clamp time, and serum lactate), only CPB time correlated with ICU mortality (p = 0.008). Thirty-eight patient (48.7%) encounters were graded as compliant, 24 (30.8%) as moderately compliant, and 16 (20.5%) as poorly compliant. Compliance grade did not correlate with ICU mortality (p = 0.44). Other categorical variables including gender, comorbidity status, the use of MB and the use of hydroxocobalamin, and the use of mechanical circulatory support did not correlate with mortality. Demographic and clinical data are presented in Table 2.
TABLE 1.
Binary Logistic Regression Models for ICU, Hospital, and 30-Day Mortality
| Variable | B (95% CI) | p |
|---|---|---|
| ICU mortality | ||
| Age | 0.006 (0.953–1.062) | 0.83 |
| Gender | –0.892 (0.096–1.743) | 0.23 |
| NE dose at start of Ang II | –0.669 (0.005–54.659) | 0.78 |
| SOFA score | 0.175 (0.972–1.459) | 0.09 |
| Serum lactate prior to Ang II | 0.090 (0.929–1.289) | 0.28 |
| Use of methylene blue | –0.111 (0.239–5.231) | 0.89 |
| Use of hydroxocobalamin | –0.564 (0.161–2.009) | 0.38 |
| Use of mechanical circulatory support | –0.620 (0.115–2.525) | 0.43 |
| Cardiopulmonary bypass time | 0.006 (0.998–1.015) | 0.15 |
| Cumulative compliance score | 0.434 (1.051–2.268) | 0.03 |
| Constant | –6.494 | 0.04 |
| Hospital mortality | ||
| Age | 0.018 (0.966–1.072) | 0.51 |
| Gender | –1.134 (0.079–1.317) | 0.12 |
| NE dose at start of Ang II | 0.014 (0.011–95.801) | 1.0 |
| SOFA score | 0.155 (0.962–1.419) | 0.12 |
| Serum lactate prior to Ang II | 0.090 (0.932–1.285) | 0.27 |
| Use of methylene blue | 0.216 (0.273–5.651) | 0.78 |
| Use of hydroxocobalamin | –0.645 (0.156–1.762) | 0.30 |
| Use of mechanical circulatory support | –0.936 (0.085–1.800) | 0.23 |
| Cardiopulmonary bypass time | 0.004 (0.996–1.013) | 0.30 |
| Cumulative compliance score | 0.464 (1.082–2.336) | 0.02 |
| Constant | –6.598 | 0.03 |
| 30-d mortality | ||
| Age | –0.008 (0.936–1.051) | 0.79 |
| Gender | 1.773 (1.217–28.510) | 0.03 |
| NE dose at start of Ang II | 1.977 (0.048–1,084.278) | 0.44 |
| SOFA score | –0.065 (0.742–1.183) | 0.58 |
| Serum lactate prior to Ang II | –0.195 (0.676–1.003) | 0.05 |
| Use of methylene blue | –0.257 (0.137–4.375) | 0.77 |
| Use of hydroxocobalamin | 0.841 (0.592–9.076) | 0.23 |
| Use of mechanical circulatory support | 0.593 (0.337–9.713) | 0.49 |
| Cardiopulmonary bypass time | –0.005 (0.986–1.005) | 0.34 |
| Cumulative compliance score | –0.560 (0.369–0.884) | 0.01 |
| Constant | 10.849 | 0.07 |
Ang II = angiotensin II, NE = norepinephrine equivalence, SOFA = Sequential Organ Failure Assessment.
Gender, use of methylene blue, use of hydroxocobalamin, and use of mechanical circulatory support were considered categorical variables, with all other variables treated as continuous.
TABLE 2.
Baseline Demographic and Clinical Data
| Demographic and Clinical Data | Died in ICU (n = 21) | Survived ICU (n = 57) | All Patients | p |
|---|---|---|---|---|
| Age, yr, mean (sd) | 57.0 (11.7) | 60.6 (12.6) | 59.6 (12.4) | 0.26 |
| Gender, n (%) | ||||
| Female | 6 (35.3) | 11 (64.7) | 17 (100.0) | 0.38 |
| Male | 15 (24.6) | 46 (75.4) | 61 (100.0) | |
| Sequential Organ Failure Assessment score, mean (sd) | 8.7 (3.7) | 7.6 (2.9) | 7.9 (3.2) | 0.17 |
| Comorbid conditions, n (%) | ||||
| Cerebrovascular accident | 6 (28.6) | 15 (71.4) | 21 (100.0) | 0.84 |
| Chronic lung disease | 5 (17.9) | 23 (82.1) | 28 (100.0) | 0.18 |
| Diabetes | 5 (22.7) | 17 (77.3) | 22 (100.0) | 0.60 |
| Hypertension | 19 (27.5) | 50 (72.5) | 69 (100.0) | 0.74 |
| Immunocompromise | 7 (36.8) | 12 (63.2) | 19 (100.0) | 0.26 |
| Liver disease | 2 (33.3) | 4 (66.7) | 6 (100.0) | 0.71 |
| Cumulative compliance score | 6.8 (1.8) | 6.0 (1.5) | 6.2 (1.6) | 0.04 |
| Compliance grade, n (%) | ||||
| Compliant | 8 (21.1) | 30 (78.9) | 38 (100.0) | 0.44 |
| Moderately compliant | 7 (29.2) | 17 (70.8) | 24 (100.0) | |
| Poorly compliant | 6 (37.5) | 10 (62.5) | 16 (100.0) | |
| Norepinephrine equivalence dose at start of Ang II, µg/kg/min (sd) | 0.33 (0.17) | 0.30 (0.13) | 0.31 (0.14) | 0.43 |
| Vasopressin dose at start of Ang II, U/min (sd) | 0.10 (0.07) | 0.12 (0.05) | 0.11 (0.05) | 0.24 |
| Ang II starting dose, ng/kg/min (sd) | 19.0 (17.7) | 16.4 (11.0) | 17.1 (13.1) | 0.43 |
| Methylene blue, n (%) | ||||
| Used | 4 (26.7) | 11 (73.3) | 15 (100.0) | 0.98 |
| Not used | 17 (27.9) | 46 (73.0) | 63 (100.0) | |
| Hydroxocobalamin, n (%) | ||||
| Used | 11 (34.4) | 21 (65.6) | 32 (100.0) | 0.22 |
| Not used | 10 (21.7) | 36 (78.3) | 46 (100.0) | |
| Mechanical circulatory support, n (%) | ||||
| Used | 16 (32.7) | 33 (67.3) | 49 (100.0) | 0.14 |
| Not used | 5 (17.2) | 24 (82.8) | 29 (100.0) | |
| Operative data | ||||
| Cardiopulmonary bypass time, min (sd) | 267.1 (70.7) | 203.9 (85.5) | 220.3 (86.1) | 0.004 |
| Cross-clamp time, min (sd) | 183.4 (68.1) | 166.2 (70.0) | 170.6 (69.4) | 0.36 |
| Serum lactate prior to Ang II, mg/dL (sd) | 8.9 (5.0) | 7.0 (3.6) | 7.5 (4.2) | 0.08 |
| ICU LOS, d (sd) | 16.5 (20.4) | 16.2 (11.3) | 16.3 (14.2) | 0.94 |
| Hospital LOS, d (sd) | 19.5 (22.3) | 27.5 (17.2) | 25.4 (18.9) | 0.10 |
Ang II = angiotensin II, LOS = length of stay.
χ2 and analysis of variance analyses reported with an alpha of 0.05.
In regression analysis, which included age, gender, NED dose, and serum lactate at the start of Ang II, SOFA score, the use of MB, hydroxocobalamin, or mechanical circulatory support, CPB time and cumulative compliance score, only the cumulative compliance score was able to predict either ICU (p = 0.03) or hospital (p = 0.02) mortality. In a similar model, gender (p = 0.03) and cumulative compliance score (p = 0.01) were predictive of 30-day mortality. The regression models are presented in Table 1.
As part of sensitivity analysis, we performed three binary logistic regression models of 1) variables that were unbalanced between groups (p ≤ 0.20), 2) variables likely to influence the primary outcome measure, and 3) a combination of variables that trended toward statistically significant (p ≤ 0.20) within the first two regressions. In all three models, cumulative compliance score remained statistically significant (Supplemental Tables 2–4, http://links.lww.com/CCX/A985). We further attempted to ascertain whether certain components of the Emory Protocol were more important than others by quantifying the effect size in a separate regression of the NED compliance score, the vasopressin compliance score, the MB compliance score, and the high-output documentation compliance score to ICU mortality. We treated the NED compliance (score 1–5) and cardiac output documentation compliance (score 1–4) as continuous variables and vasopressin compliance and MB compliance score (score 1 or 2) as categorical variables. Only high-output compliance score (p = 0.008) was noted to predict ICU mortality with statistical significance, while the vasopressin dose compliance score (p = 0.089) trended toward statistical significance. Neither the NED compliance score nor the MB compliance scores contributed in a statistically significant way (Supplemental Table 5, http://links.lww.com/CCX/A985).
DISCUSSION
In this retrospective study, we found improved ICU survival in PCSV patients receiving Ang II where adherence to the Emory Protocol was followed. This result may provide guidance on early initiation of noncatecholamines including vasopressin, MB, hydroxocobalamin, and Ang II at lower catecholamine doses in the setting of documented high-output shock. Furthermore, in regression analysis, we found that only the cumulative compliance score was associated with ICU mortality and hospital mortality, and only gender and cumulative compliance score were associated with 30-day mortality.
While other algorithms for PCSV have been proposed in the literature (9) and other studies have supported the use of noncatecholamine therapy in PCSV, this is the first algorithm of multimodal vasopressor support that has been linked to improved survival. Vasopressin was evaluated as part of the Vasoplegic Shock After Cardiac Surgery trial, which compared vasopressin against norepinephrine as first-line therapy (10). This trial reported a statistically significant composite endpoint benefit (which included mortality) but was mostly driven by lower rates of arrhythmia and kidney failure. MB has been evaluated as an adjunctive therapy for PCSV in multiple studies including Ozal et al (11) who showed a reduction in catecholamine support and reduced ICU and hospital LOS when prophylactic MB was given prior to surgery but did not report a mortality benefit.
Interestingly, our study found that background catecholamine dose did not contribute to the prediction of ICU mortality in both binary logistic regression analysis (Table 1) as well as in sensitivity analysis of known predictors of ICU mortality (Supplemental Table 3, http://links.lww.com/CCX/A985). Furthermore, in the regression analysis of individual elements of the Emory Protocol, background NED compliance was an insignificant contributor (Supplemental Table 5, http://links.lww.com/CCX/A985). These findings contradict previous evidence suggesting that higher catecholamine dose is associated with increased chance of mortality (12–14). We speculate that the relatively high compliance to the Emory Protocol resulted in relatively few patients in whom background NEDs exceeded dangerous thresholds. The majority (91.0%) of patients received NED less than or equal to 0.5 µg/kg/min, a cutoff frequently associated with higher mortality. An alternative explanation is that the addition of noncatecholamine therapy may exert a protective effect independent of the background catecholamine dose. While intuitively, this argument for multimodal therapy would make sense and has been argued (15), evidence of outcome improvement regardless of background NED when noncatecholamine therapy is added has been lacking. Finally, protocolized therapy and participation in well-defined bundled care have been associated with improved outcomes in sepsis and septic shock (16, 17). Improved outcomes may be independent of the intervention in question (18, 19). Conceivably, mere participation in the Emory Protocol appears to have provided a similar benefit, regardless of NED.
Our study has multiple strengths. The rationale for our study was based on a pragmatic and easily implementable approach to treatment of PCSV in a diverse set of postsurgical patients in the CVICU across multiple ICUs. Noncatecholamine therapy is already considered an essential element of the treatment of PCSV, as supported in the literature (10). As such, the Emory Protocol was easy to implement. While previous studies of multimodal therapy for PCSV have focused on background catecholamine dose reduction or other composite endpoints, this is the first study that sought to detect mortality as a primary endpoint. Finally, this study is the largest cohort of CVICU patients treated with a protocolized therapy for PCSV to date. The results of our analysis speak to the generalizability of this multimodal approach across a broad patient population.
Our study also has several limitations, including the retrospective nature of the analysis. Because of this, our data may not be capturing the true clinical scenario or urgency at the time inclusion in the dataset. For this reason, we used in our calculation of compliance either the time of ordering of Ang II or the initiation of Ang II, recognizing that the patient condition may be rapidly changing during this period. We chose the least noncompliant value among the two endpoints, as this was the most conservative approach given our endpoints (mortality based on noncompliance). Additionally, there were some imbalances in our data, including CPB time. However, our sensitivity analysis demonstrated that any imbalanced elements exhibited no predictive effect on the primary endpoint. Our collected data may not capture all relevant information driving Protocol compliance. Factors such as premorbid chronic kidney disease or deep vein thrombosis were not recorded and may influence the PCSV Protocol pathway for some patients. Third, though PCSV has been defined as occurring within temporal proximity (usually considered to be ~24 hr) to surgery, we used a 48-hour timeframe. All patients within this dataset continuously experienced shock subsequent to surgery prior to enrollment, and we felt that shock within this window was consistent with the diagnosis of PCSV. Fourth, patients were included for analysis only after they received Ang II, which is not a necessary component of the Emory Protocol but rather one optional element (as is the use of hydroxocobalamin). The inclusion of only patients in whom Ang II is used presupposes that consideration was given to the use of MB, which resides higher on the protocol algorithm. However, very poor compliance was found with MB administration. We maintain a separate registry of PCSV patients who received MB but not Ang II, which only totaled 30 patients from 2017 to 2020 (data not included). Given these circumstances, it is possible that our protocol may require adjustment to more accurately reflect emerging standard practice. Furthermore, we did not assign relative weight to the various components of the Emory Protocol, essentially rendering each compliance point equal. This may introduce a scoring bias, as some components of our protocol are binary (score 1 or 2), while others are continuous (score 1–4 or 1–5). Given this limitation, we found that NED and vasopressin compliance were fairly high (mean compliance score for NED compliance of 2 (for a scoring range of 1–5) and mean compliance score for vasopressin compliance of 1.06 (for a scoring range of 1–2). High output shock compliance mean score was 1.6 (for a scoring range of 1–4) and MB compliance mean score was 1.53 (for a scoring range of 1–2), suggesting these components contributed more to noncompliance. Finally, because our analysis includes only those patients who received Ang II, it does not address outcomes or protocol compliance when agents other than Ang II are used (e.g., just catecholamines and vasopressin). This may introduce a significant amount of sampling bias into our analysis. Nonetheless, because multimodal therapy is becoming increasingly common, our conclusions may still be applicable.
CONCLUSIONS
In conclusion, this retrospective study of patients with PCSV on multimodal therapy with catecholamines, vasopressin and Ang II ± MB and/or hydroxocobalamin demonstrates that compliance with the Emory Protocol, consisting of balanced vasopressor deployment in the setting of documented high-output shock, was associated with improved ICU mortality. A standardized algorithmic approach to the treatment of PCSV may be more impactful on patient survival than background catecholamine dose, which has heretofore been the goal of multimodal therapy. While prospective analyses and additional data are needed to validate these findings, the easy implementation of the Emory Protocol and improved outcomes associated with protocol adherence make the case for deployment of an organized, protocolized approach to the treatment of PCSV.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccxjournal).
Dr. Busse is a senior author.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Busse LW, Barker N, Petersen C: Vasoplegic syndrome following cardiothoracic surgery-review of pathophysiology and update of treatment options. Crit Care 2020; 24:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes TJ, Hockstein MA, Jabaley CS: Vasoplegia after cardiopulmonary bypass: A narrative review of pathophysiology and emerging targeted therapies. SAGE Open Med 2020; 8:2050312120935466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wakefield BJ, Busse LW, Khanna AK: Angiotensin II in vasodilatory shock. Crit Care Clin 2019; 35:229–245 [DOI] [PubMed] [Google Scholar]
- 4.Khanna A, English SW, Wang XS, et al. ; ATHOS-3 Investigators: Angiotensin II for the treatment of vasodilatory shock. N Engl J Med 2017; 377:419–430 [DOI] [PubMed] [Google Scholar]
- 5.Klijian A, Khanna AK, Reddy VS, et al. : Treatment with angiotensin II is associated with rapid blood pressure response and vasopressor sparing in patients with vasoplegia after cardiac surgery: A post-hoc analysis of Angiotensin II for the Treatment of High-Output Shock (ATHOS-3) study. J Cardiothorac Vasc Anesth 2021; 35:51–58 [DOI] [PubMed] [Google Scholar]
- 6.Evans A, McCurdy MT, Weiner M, et al. : Use of angiotensin II for post cardiopulmonary bypass vasoplegic syndrome. Ann Thorac Surg 2019; 108:e5–e7 [DOI] [PubMed] [Google Scholar]
- 7.Wieruszewski PM, Radosevich MA, Kashani KB, et al. : Synthetic human angiotensin II for postcardiopulmonary bypass vasoplegic shock. J Cardiothorac Vasc Anesth 2019; 33:3080–3084 [DOI] [PubMed] [Google Scholar]
- 8.Rona G: Catecholamine cardiotoxicity. J Mol Cell Cardiol 1985; 17:291–306 [DOI] [PubMed] [Google Scholar]
- 9.Ortoleva JP, Cobey FC: A systematic approach to the treatment of vasoplegia based on recent advances in pharmacotherapy. J Cardiothorac Vasc Anesth 2019; 33:1310–1314 [DOI] [PubMed] [Google Scholar]
- 10.Hajjar LA, Vincent JL, Barbosa Gomes Galas FR, et al. : Vasopressin versus norepinephrine in patients with vasoplegic shock after cardiac surgery: The VANCS randomized controlled trial. Anesthesiology 2017; 126:85–93 [DOI] [PubMed] [Google Scholar]
- 11.Ozal E, Kuralay E, Yildirim V, et al. : Preoperative methylene blue administration in patients at high risk for vasoplegic syndrome during cardiac surgery. Ann Thorac Surg 2005; 79:1615–1619 [DOI] [PubMed] [Google Scholar]
- 12.Brown SM, Lanspa MJ, Jones JP, et al. : Survival after shock requiring high-dose vasopressor therapy. Chest 2013; 143:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins CR, Gomersall CD, Leung P, et al. : Outcome of patients receiving high dose vasopressor therapy: A retrospective cohort study. Anaesth Intensive Care 2009; 37:286–289 [DOI] [PubMed] [Google Scholar]
- 14.Benbenishty J, Weissman C, Sprung CL, et al. : Characteristics of patients receiving vasopressors. Heart Lung 2011; 40:247–252 [DOI] [PubMed] [Google Scholar]
- 15.Chawla LS, Russell JA, Bagshaw SM, et al. : Angiotensin II for the Treatment of High-Output Shock 3 (ATHOS-3): Protocol for a phase III, double-blind, randomised controlled trial. Crit Care Resusc 2017; 19:43–49 [PubMed] [Google Scholar]
- 16.Angus DC, Barnato AE, Bell D, et al. : A systematic review and meta-analysis of early goal-directed therapy for septic shock: The ARISE, ProCESS and ProMISe Investigators. Intensive Care Med 2015; 41:1549–1560 [DOI] [PubMed] [Google Scholar]
- 17.Levy MM, Gesten FC, Phillips GS, et al. : Mortality changes associated with mandated public reporting for sepsis. The results of the New York State Initiative. Am J Respir Crit Care Med 2018; 198:1406–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peek GJ, Mugford M, Tiruvoipati R, et al. ; CESAR trial collaboration: Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009; 374:1351–1363 [DOI] [PubMed] [Google Scholar]
- 19.Zwischenberger JB, Lynch JE: Will CESAR answer the adult ECMO debate? Lancet 2009; 374:1307–1308 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.