IMPORTANCE:
The prevalence and causes of sepsis in patients hospitalized with COVID-19 are poorly characterized.
OBJECTIVES:
To investigate the prevalence, clinical characteristics, and outcomes of sepsis caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) versus other pathogens in patients hospitalized with COVID-19.
DESIGN, SETTING, AND PARTICIPANTS:
Cross-sectional, retrospective chart review of 200 randomly selected patients hospitalized with COVID-19 at four Massachusetts hospitals between March 2020 and March 2021.
MAIN OUTCOMES AND MEASURES:
The presence or absence of sepsis was determined per Sepsis-3 criteria (infection leading to an increase in Sequential Organ Failure Assessment score by ≥ 2 points above baseline). Sepsis episodes were assessed as caused by SARS-CoV-2, other pathogens, or both. Rates of organ dysfunction and in-hospital death were also assessed.
RESULTS:
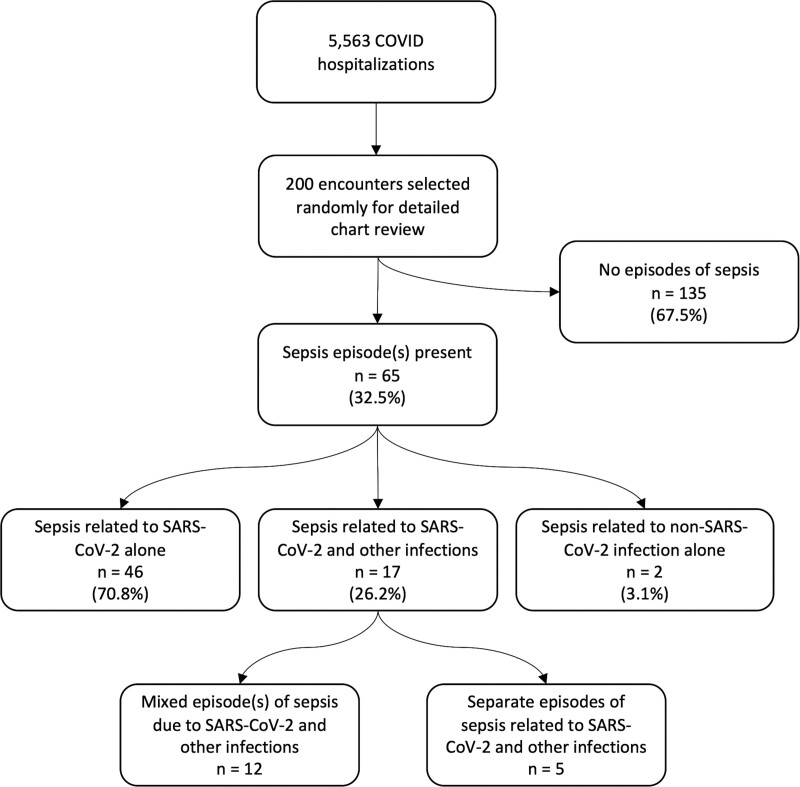
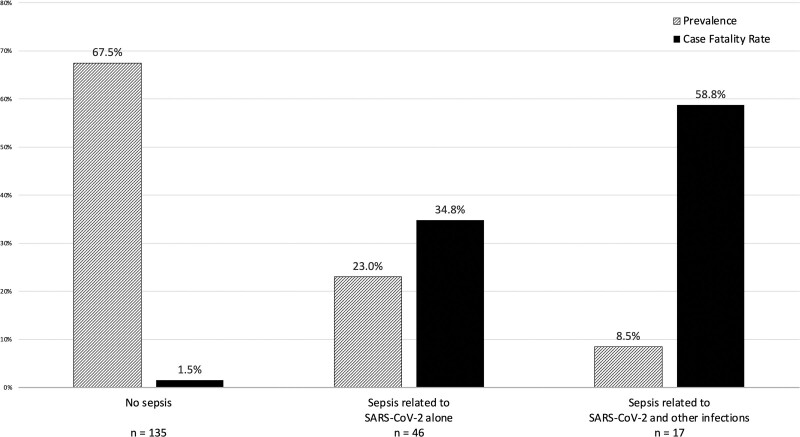
Sepsis was present in 65 of 200 COVID-19 hospitalizations (32.5%), of which 46 of 65 sepsis episodes (70.8%) were due to SARS-CoV-2 alone, 17 of 65 (26.2%) were due to both SARS-CoV-2 and non-SARS-CoV-2 infections, and two of 65 (3.1%) were due to bacterial infection alone. SARS-CoV-2–related organ dysfunction in patients with sepsis occurred a median of 1 day after admission (interquartile range, 0–2 d) and most often presented as respiratory (93.7%), neurologic (46.0%), and/or renal (39.7%) dysfunctions. In-hospital death occurred in 28 of 200 COVID-19 hospitalizations (14.0%), including two of 135 patients without sepsis (1.5%), 16 of 46 patients with sepsis (34.8%) due to SARS-CoV-2 alone, and 10 of 17 patients with sepsis (58.8%) due to both SARS-CoV-2 and bacterial pathogens.
CONCLUSIONS:
Sepsis occurred in one in three patients hospitalized with COVID-19 and was primarily caused by SARS-CoV-2 itself, although bacterial infection also contributed in a quarter of sepsis cases. Mortality in COVID-19 patients with sepsis was high, especially in patients with mixed SARS-CoV-2 and bacterial sepsis. These findings affirm SARS-CoV-2 as an important cause of sepsis and highlight the need to improve surveillance, recognition, prevention, and treatment of both viral and bacterial sepsis in hospitalized patients with COVID-19.
Keywords: COVID-19, organ dysfunction, sepsis, severe acute respiratory syndrome coronavirus 2, viral sepsis
BACKGROUND AND OBJECTIVES
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection most frequently manifests as respiratory dysfunction but can also impair other organs via direct and indirect mechanisms. Thus, SARS-CoV-2 is a cause of sepsis according to the Third International Consensus Definitions of Sepsis and Septic Shock (Sepsis-3), which defines sepsis as infection leading to an increase in Sequential Organ Failure Assessment (SOFA) score by greater than or equal to 2 points above baseline (1, 2). Sepsis tends to be underdiagnosed in COVID-19; however, rigorous data regarding its prevalence, clinical characteristics, and outcomes are lacking (3, 4). A recent meta-analysis estimated the prevalence of sepsis in patients hospitalized with COVID-19 using the pooled frequency of organ dysfunction and replacement from published COVID cohorts but was limited by heterogeneity in definitions, reliance on indirect proxies for sepsis, and inability to distinguish between sepsis caused by SARS-CoV-2 versus other pathogens (3). We therefore investigated the prevalence of sepsis caused by SARS-CoV-2 versus other pathogens in patients hospitalized with COVID-19 and compared their characteristics and outcomes to patients without sepsis using detailed medical record reviews.
METHODS
We performed a cross-sectional, retrospective study of a random sample of 200 adult patients hospitalized with COVID-19 at four acute care hospitals in Massachusetts between March 1, 2020, and March 1, 2021. COVID-19 hospitalizations were defined by positive SARS-CoV-2 polymerase chain reaction (PCR) tests from 3 days before admission through discharge or via institutional COVID-19 electronic medical record flags triggered by positive internal or external PCR tests. Encounters were excluded if the patient had a positive COVID-19 PCR or COVID-19 flag greater than 30 days prior to hospitalization or if the COVID-19 flag associated with the admission lasted less than 5 days, typically an indicator that the infection control team deemed infection to be resolved, remote, or a false positive result (5).
Of 5,563 COVID hospitalizations, 200 cases were randomly selected for medical record review including notes, laboratory and microbiology test results, vital signs, medication administration records, radiology reports and images, and pathology reports using a standardized data abstraction tool in the secure, web-based application Research Electronic Data Capture Version 11.1.26 (2022, Vanderbilt University, Nashville, TN). Charts were reviewed for reason for admission, presence of COVID-19 symptoms, date of symptom onset, first positive PCR date, presence of non-SARS-CoV-2 infections using previously described criteria for likelihood of infection (6), and presence or absence of sepsis. Receipt of anti-bacterial therapy was not considered sufficient evidence of bacterial infection in the absence of at least one other objective finding consistent with bacterial infection (e.g., positive clinical cultures or compatible imaging).
Sepsis was defined as an increase in SOFA score by greater than or equal to 2 points from baseline, which was definitely or probably related to SARS-CoV-2 or another infection as per Sepsis-3 criteria (2). Organ dysfunction was potentially attributable to non-SARS-CoV-2 pathogens only in cases where non-SARS-CoV-2 infection was deemed at least possibly present. Identification of a specific pathogen (e.g., a positive blood or respiratory culture) was not required for sepsis to be considered potentially due to a non-SARS-CoV-2 pathogen. A final determination was made of definite or probable, possible, or no SARS-CoV-2–related and non-SARS-CoV-2–related sepsis; for our primary analysis, sepsis was dichotomized as definite/probable/possible versus no sepsis. For encounters that met criteria for both SARS-CoV-2– and non-SARS-CoV-2–related sepsis, episodes were determined to be temporally separate or concurrent (e.g., mixed COVID-19 and bacterial pneumonia). See Supplement Table 1 (http://links.lww.com/CCX/A998) for criteria for infection and sepsis categories.
SOFA scores were manually calculated for the first 24 hours of each sepsis episode. While an increase in SOFA score greater than 2 points was required to identify sepsis episodes per Sepsis-3 criteria, all organ dysfunctions equivalent to greater than or equal to 1 SOFA point within the first 24 hours of the episode were abstracted and reported. Arterial oxygen saturation/Fio2 ratios were used to identify respiratory dysfunction when arterial blood gases were unavailable (7).
All 200 cases were reviewed by a Pulmonary and Critical Care Medicine physician (C.N.S.); challenging cases (n = 27) were flagged for joint review with two infectious disease physicians (M.K., C.R.) to achieve consensus and ensure a standardized approach for all other cases.
Descriptive statistics were used to determine the prevalence and in-hospital mortality of sepsis in patients hospitalized with COVID-19. Measures of association were not calculated; therefore, no statistical methods were used to control for confounding. Data analysis was done in Stata Version 17 (StataCorp, 2021, College Station, TX: StataCorp LLC). The study was approved by the Mass General Brigham Institutional Review Board (2020P001631).
RESULTS
Study Cohort
Among the 200 hospitalized patients with COVID-19, median age was 62 years (interquartile range [IQR], 47–78 yr), 90 (45.0%) were female, comorbidities were common, median length of stay was 5 days (IQR, 3–9 d), and 45 (22.5%) required ICU admission (Table 1). One-hundred seventy-two patients (86.0%) had symptomatic COVID infections and 171 (85.5%) were admitted for reasons related to COVID-19. The other 29 patients were admitted for unrelated medical conditions (n = 18), trauma (n = 6), and obstetric indications (n = 5); SARS-CoV-2 tests were positive at admission for 27 of 29 of these patients, while two of 29 first tested positive after admission.
TABLE 1.
Demographics and Clinical Characteristics of Patients Hospitalized With COVID-19, Stratified by Presence of Sepsis Related to Severe Acute Respiratory Syndrome Coronavirus 2 and Other Pathogens
| Category | No Sepsis | Sepsis Related to SARS-CoV-2 Alone | Sepsis Related to SARS-CoV-2 and Non-SARS-CoV-2 | Overalla |
|---|---|---|---|---|
| Overall, n (%) | 135 (67.5) | 46 (23) | 17 (8.5) | 200 (100) |
| Symptomatic COVID infection, n (%) | 107 (79.3) | 46 (100) | 17 (100) | 172 (86) |
| Non-SARS-CoV-2 infection, n (%) | 18 (13.3) | 18 (39.1) | 17 (100) | 55 (27.5) |
| Definite/probable | 10 (7.4) | 8 (17.4) | 8 (47.1) | 26 (13) |
| Possible | 8 (5.9) | 10 (21.7) | 9 (52.9) | 29 (14.5) |
| Age, median (IQR), yr | 59 (45–74) | 70.5 (56–82) | 66 (60–82) | 62 (47–78) |
| Sex, n (%) | ||||
| Women | 64 (47.4) | 17 (37.0) | 7 (41.2) | 90 (45) |
| Race, n (%) | ||||
| White | 70 (51.9) | 23 (50) | 4 (23.5) | 97 (48.5) |
| Black | 21 (15.6) | 5 (10.9) | 6 (35.3) | 33 (16.5) |
| Other | 43 (31.9) | 15 (32.6) | 6 (35.3) | 65 (32.5) |
| Missing | 1 (< 1) | 3 (6.5) | 1 (5.9) | 5 (2.5) |
| Comorbiditiesb, n (%) | ||||
| Cancer | 4 (3) | 3 (6.5) | 1 (5.9) | 8 (4) |
| Congestive heart failure | 12 (8.9) | 6 (13) | 5 (29.4) | 32 (16) |
| Chronic lung disease | 19 (14.1) | 13 (28.3) | 5 (29.4) | 44 (22) |
| Diabetes | 34 (25.2) | 21 (45.7) | 10 (58.8) | 66 (33) |
| Neurologic disease | 14 (10.4) | 2 (4.4) | 7 (41.2) | 23 (11.5) |
| Kidney disease | 23 (17) | 11 (23.9) | 7 (41.2) | 41 (20.5) |
| Elixhauser mortality score, median (IQR) | 4 (0–11) | 7 (0–19) | 13 (11–23) | 6 (0–13) |
| Hospital length of stay, median (IQR), d | 4 (3–6) | 11 (8–23) | 14 (9–24) | 5 (3–9) |
| Required ICU admission, n (%) | 5 (3.7) | 25 (54.4) | 13 (76.5) | 45 (22.5) |
| Any oxygen requirement, n (%) | 75 (55.6) | 45 (97.8) | 16 (94.1) | 137 (68.5) |
| Any MV, n (%) | 2 (1.5) | 20 (43.5) | 11 (64.7) | 33 (16.5) |
| Duration of MV, median (IQR), d | 1.5 (1–2) | 15.5 (5–23.5) | 16 (11–27) | 15 (5–25) |
| Discharge disposition, n (%) | ||||
| Home | 107 (79.3) | 14 (30.4) | 0 (0) | 122 (61) |
| Facility | 26 (19.3) | 16 (34.8) | 7 (41.2) | 50 (25) |
| Death | 2 (1.5) | 16 (34.8) | 10 (58.8) | 28 (14) |
IQR = interquartile range, MV = mechanical ventilation, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
aColumns do not sum to 200 because two patients meeting Sepsis-3 criteria due to non-SARS-CoV-2 infections alone were omitted from the table.
bComorbidities were derived using the Elixhauser index. “Cancer” includes solid tumor with and without metastases and lymphoma. “Diabetes” includes diabetes with and without complications.
Sepsis Prevalence and Etiologies
Sepsis was present in 65 of 200 (32.5%) of COVID-19 hospitalizations, of which 46 of 65 (70.7%) were related exclusively to SARS-CoV-2, 17 (26.2%) to SARS-CoV-2 with concurrent (n = 12) or subsequent (n = 5) bacterial infections, and 2 (3.1%) exclusively to bacterial infections. A flowchart summarizing the distribution of sepsis cases is shown in Figure 1 and representative cases from each category are described in Supplemental Table 2 (http://links.lww.com/CCX/A998).
Figure 1.
Flowchart summarizing distribution of sepsis cases in patients hospitalized with COVID-19. SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
SARS-CoV-2–Related Sepsis
Organ dysfunction (≥ 1 SOFA point increase above baseline within the first 24 hr of a sepsis episode) in SARS-CoV-2–related sepsis was most often respiratory (59/63, 93.7%), neurologic (29/63, 46%), renal (25/63, 39.7%), coagulopathy (21/63, 33.3%), and cardiovascular (19/63, 30.2%). SARS-CoV-2–related organ dysfunction occurred a median of 1 day after admission (range, 0–24; IQR, 0–2). Sixty-one of 63 patients (96.8%) with SARS-CoV-2–related sepsis were admitted for COVID-19; the other two were admitted for unrelated conditions and subsequently tested positive.
Non-SARS-CoV-2 Infections and Sepsis
Of the 65 COVID-19 hospitalizations with sepsis, 19 of 65 (29.2%) were potentially related to non-SARS-CoV-2 infections. Most (17/19) had both SARS-CoV-2 and non-SARS-CoV-2–related sepsis, but two of 19 cases were due to bacterial infections alone. Of the 17 patients with sepsis related to both COVID and non-COVID infections, most (12/17, 70.6%) had a single, mixed sepsis episode rather than separate episodes (5/17, 29.4%). The most common sites of non-SARS-CoV-2 infections contributing to sepsis were bacterial pneumonia (n = 8), gastrointestinal/intra-abdominal infection (n = 6), bloodstream infection (n = 5), and urinary tract infection (n = 5) (nonexclusive categories).
In addition to the 19 patients with sepsis potentially attributable in whole or in part to non-SARS-CoV-2 infections, there were an additional 36 patients with definite or possible non-SARS-CoV-2 infections that did not lead to sepsis. All told then, 55 of 200 patients (27.5%) hospitalized with SARS-CoV-2 had non-SARS-CoV-2 infections as well: all were proven or suspected bacterial infections, 36 of 55 infections (65.4%) were present at admission, and 19 of 55 (34.6%) only manifested after admission. The most common non-SARS-CoV-2 infection sites were pulmonary (n = 22), urinary (n = 17), gastrointestinal/intra-abdominal (n = 10), and bloodstream (n = 9). Twenty-five of 55 patients with non-SARS-CoV-2 infections had at least one organism identified, most commonly Escherichia coli (n = 8), Clostridium difficile (n = 4), Staphylococcus aureus (n = 3), Enterococcus faecalis (n = 3), and Klebsiella pneumoniae (n = 3).
Outcomes
In-hospital death occurred in 28 of 200 COVID-19 patients (14.0%). Mortality rates were 26 of 65 (40.0%) for those with sepsis versus two of 135 (1.5%) for those without sepsis. Among those with sepsis due to SARS-CoV-2 alone, in-hospital death occurred in 16 of 46 (34.8%), whereas 10 of 17 patients (58.8%) with both SARS-CoV-2 and bacterial sepsis died (Fig. 2). Neither of the two patients with bacterial sepsis alone died.
Figure 2.
Prevalence and case fatality rates for sepsis related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and other infections among patients hospitalized with COVID-19.
DISCUSSION
We found that sepsis occurred in one in three patients hospitalized with COVID-19. Almost all sepsis episodes were related to SARS-CoV-2 infection itself, although over one quarter were also definitely or possibly related to bacterial infections; only a small fraction (3%) had sepsis solely from bacterial infections. In-hospital mortality rates were very high for COVID-19 patients with sepsis, especially those with bacterial superinfections, and very low for those without sepsis.
Our findings are in line with a meta-analysis of global COVID-19 cohorts, which reported a sepsis prevalence of 33.3% in non-ICU patients (3). Our study is smaller but provides more granularity by distinguishing sepsis related to SARS-CoV-2 versus other pathogens on the basis of detailed medical record reviews and by quantifying organ dysfunction using standardized SOFA scores. Both studies highlight sepsis as a major complication of SARS-CoV-2 in hospitalized patients.
In-hospital mortality rates for SARS-CoV-2–related sepsis exceeded 40%, far higher than mortality rates in pre-pandemic Sepsis-3 cohorts, which generally ranged between 10% and 16% (8, 9). This may reflect the pathogenicity of the virus, its predilection to cause respiratory failure (which is associated with worse prognoses than most other organ dysfunctions), lack of effective therapeutics for COVID-19 during the early months of the pandemic, staffing and resource constraints during surges, or a combination of these. The results highlight the ongoing need for scalable and effective treatments for severe COVID-19 infections as well as vaccinations and other prevention measures.
The prevalence of confirmed or possible bacterial coinfections in hospitalized COVID patients in our cohort was 27.5%, which exceeds prior estimates of less than 10% (10). However, our analysis included suspected, culture-negative, and incidental infections; the prevalence of culture-proven bacterial infection was 12.5%. Mortality in patients who experienced sepsis from both SARS-CoV-2 and bacterial infections was extremely high (59%); this may reflect more severe disease due to concurrent infections and/or greater underlying severity-of-illness in patients prone to develop serious nosocomial infections.
Nearly all patients who developed SARS-CoV-2–related sepsis experienced respiratory dysfunction; many also experienced CNS, renal, coagulation, and cardiovascular dysfunction. This underscores the breadth of organs potentially affected with severe COVID-19 (11). However, our reported rates of organ dysfunction almost certainly represent underestimates of overall organ dysfunction prevalence as we only abstracted SOFA scores for the first 24 hours of the first sepsis episode resulting from each pathogen category, a limitation related to the resource intensiveness of case reviews.
Our study has additional limitations. First, the majority of medical record reviews were conducted by one physician and the post hoc determination of the presence of infection(s) and the cause of organ dysfunction can be challenging. However, there is no guarantee that using additional reviewers would increase accuracy given that diagnosing both infection and sepsis is often highly subjective (12–16). We attempted to mitigate this by using structured reviews with clear criteria adapted from our group’s prior work and discussing challenging cases among a group of at least three physicians to achieve consensus and ensure a standardized classification approach (6–8). Second, prior work in the pre-COVID era has demonstrated that a substantial fraction of patients with sepsis are culture-negative, that most culture-negative sepsis cases are caused by respiratory infections, and that most respiratory infections do not have a causative organism identified even with intensive testing for both bacterial and viral pathogens (17–19). Importantly, our classification scheme included possible or probable bacterial infections without microbiologic confirmation, but it is nonetheless possible that our analysis underestimated the frequency of non-SARS-CoV-2–related sepsis or potentially misattributed some sepsis cases to COVID-19. Third, our study was performed using data from early in the pandemic. Treatments and hospital conditions have evolved significantly since then, vaccination is increasingly widespread, and new SARS-CoV-2 variants vary in their severity; these changes could significantly affect our findings. Fourth, our study was conducted in a single healthcare system and may not be widely generalizable; larger studies with greater geographic, hospital-level, and patient-level diversity are needed.
Finally, some have questioned whether or not labeling SARS-CoV-2–associated organ dysfunction as “sepsis” adds value over simply reporting the rates and types of organ dysfunction in patients with “severe COVID-19,” and indeed whether this label risks harm by promoting inappropriate administration of broad-spectrum antibiotics and other sepsis bundle elements (20). We believe, however, that labeling severe SARS-CoV-2 cases as sepsis is conceptually consistent with all prior consensus definitions of sepsis that are agnostic to the specific pathogen type triggering the maladaptive host immune response (2, 21, 22). Furthermore, labeling SARS-CoV-2–associated organ dysfunction as sepsis may help convey the seriousness of a patient’s condition and high risk of death if left untreated, while also allowing for more accurate measurement of the true burden of sepsis and its underlying etiologies. Last, applying the sepsis label to patients with severe COVID-19 may actually represent an opportunity to break from the overly simplistic view of sepsis as a monolithic entity and ultimately facilitate more nuanced and individualized treatment approaches (4, 23). We advocate, however, that clinicians using the term sepsis append the likely cause and clinical syndrome (i.e., “SARS-CoV-2 sepsis with respiratory failure and acute kidney injury”) in recognition that different kinds of sepsis necessitate different management strategies and differ in their natural history and prognoses.
CONCLUSIONS
Sepsis was present in one in three patients hospitalized with COVID-19, primarily due to SARS-CoV-2 itself, although concurrent bacterial infections contributed to sepsis in more than a quarter of cases. Mortality in COVID-19 patients with sepsis was high, especially in those with mixed SARS-CoV-2 and bacterial infections, while mortality in COVID-19 patients without sepsis was very low. These findings affirm SARS-CoV-2 as an important cause of sepsis and highlight the need to improve surveillance, recognition, prevention, and treatment of both viral and bacterial sepsis in hospitalized patients with COVID-19.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by grant from the Centers for Disease Control and Prevention (U54CK000484) and National Institutes of Health (F32 Award 1F32GM143862-01).
Drs. Klompas and Rhee report royalties from UpToDate. Dr. Rhee also reports consulting fees from Pfizer and Cytovale for work unrelated to this topic. The remaining authors have disclosed that they do not have any potential conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control and Prevention or the National Institutes of Health.
REFERENCES
- 1.Li H, Liu L, Zhang D, et al. : SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet 2020; 395:1517–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karakike E, Giamarellos-Bourboulis EJ, Kyprianou M, et al. : Coronavirus disease 2019 as cause of viral sepsis: A systematic review and meta-analysis. Crit Care Med 2021; 49:2042–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shappell CN, Klompas M, Rhee C: Does severe acute respiratory syndrome coronavirus 2 cause sepsis? Crit Care Med 2020; 48:1707–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee C, Baker MA, Kanjilal S, et al. : Prospective clinical assessments of hospitalized patients with positive SARS-CoV-2 PCR tests for necessity of isolation. Open Forum Infect Dis 2021; 8:ofab194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shappell CN, Klompas M, Ochoa A, et al. : Likelihood of bacterial infection in patients treated with broad-spectrum IV antibiotics in the emergency department. Crit Care Med 2021; 49:e1144–e50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee C, Jones TM, Hamad Y, et al. ; Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program: Prevalence, underlying causes, and preventability of sepsis-associated mortality in US acute care hospitals. JAMA Netw Open 2019; 2:e187571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee C, Dantes R, Epstein L, et al. : Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA 2017; 318:1241–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seymour CW, Liu VX, Iwashyna TJ, et al. : Assessment of clinical criteria for sepsis: For the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:762–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langford BJ, So M, Raybardhan S, et al. : Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin Microbiol Infect 2020; 26:1622–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhoef PA, Kannan S, Sturgill JL, et al. : Severe acute respiratory syndrome-associated coronavirus 2 infection and organ dysfunction in the ICU: Opportunities for translational research. Crit Care Explor 2021; 3:e0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhee C, Kadri SS, Danner RL, et al. : Diagnosing sepsis is subjective and highly variable: A survey of intensivists using case vignettes. Crit Care 2016; 20:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerlin MP, Trick WE, Anderson DJ, et al. : Interrater reliability of surveillance for ventilator-associated events and pneumonia. Infect Control Hosp Epidemiol 2017; 38:172–178 [DOI] [PubMed] [Google Scholar]
- 14.Stevens JP, Kachniarz B, Wright SB, et al. : When policy gets it right: Variability in U.S. hospitals’ diagnosis of ventilator-associated pneumonia*. Crit Care Med 2014; 42:497–503 [DOI] [PubMed] [Google Scholar]
- 15.Klompas M: Interobserver variability in ventilator-associated pneumonia surveillance. Am J Infect Control 2010; 38:237–239 [DOI] [PubMed] [Google Scholar]
- 16.Lopansri BK, Miller Iii RR, Burke JP, et al. : Physician agreement on the diagnosis of sepsis in the intensive care unit: Estimation of concordance and analysis of underlying factors in a multicenter cohort. J Intensive Care 2019; 7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peake SL, Delaney A, Bailey M, et al. ; ARISE Investigators; ANZICS Clinical Trials Group: Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–506 [DOI] [PubMed] [Google Scholar]
- 18.Phua J, Ngerng W, See K, et al. : Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care 2013; 17:R202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain S, Self WH, Wunderink RG, et al. : Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oxman DA: Less lumping and more splitting: Why we should not call COVID sepsis. Crit Care Med 2021; 49:e656–e657 [DOI] [PubMed] [Google Scholar]
- 21.Bone RC, Balk RA, Cerra FB, et al. : Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–1655 [DOI] [PubMed] [Google Scholar]
- 22.Levy MM, Fink MP, Marshall JC, et al. : 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 2003; 31:1250–1256 [DOI] [PubMed] [Google Scholar]
- 23.Shappell CN, Klompas M, Rhee C: Quantifying the burden of viral sepsis during the coronavirus disease 2019 pandemic and beyond. Crit Care Med 2021; 49:2140–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.