Abstract
Study Objectives:
To assess the incidence and risk factors of chronic opioid use after obstructive sleep apnea surgery.
Methods:
Using IBM MarketScan research database, adults (>18 years) who underwent a variety of sleep surgery procedures between 2007 and 2015 were identified. Individuals with 1 year of insurance coverage before and after the surgical procedure were included. Additional anesthesia event(s) in the year following the procedure of interest and those who filled an opioid prescription within the year prior to surgery (not naive) were excluded. Outcomes included rates of persistent opioid use (additional opioid prescriptions filled 90–180 days postoperatively), prolonged use (additional opioid prescriptions filled 181–365 days postoperatively), and inappropriate use (> 100 morphine milligram equivalents). Evaluated variables include demographics, surgical procedures, and comorbidities.
Results:
A total of 10,766 surgical procedures met the inclusion criteria. There was a trend of increased rates of perioperative opioid prescription. After multivariable logistic regression analysis, perioperative opioid prescription and smoking were independent risk factors for inappropriate opioid use (odds ratio [OR] = 31.51, P < .001; OR = 1.41, P = .016, respectively). Opioid prescription and hypertension were independent risk factors for persistent opioid use (OR = 37.8, P < .001, OR = 1.38, P = .008). Perioperative opioid prescription, previous opioid dependence diagnosis, smoking, and male sex were associated with continuous prolonged opioid use (OR = 73.1, 8.13, 1.95, and 1.55, respectively; P < .001, P = .020, P = .024, and P = .032, respectively).
Conclusions:
While efforts by different societies are being implemented to control the opioid crisis, we found that perioperative opioid prescription for airway surgery targeting obstructive sleep apnea is an independent risk factor for persistent, prolonged, and inappropriate opioid use.
Citation:
Abdelwahab M, Marques S, Howard J, et al. Incidence and risk factors of chronic opioid use after sleep apnea surgery. J Clin Sleep Med. 2022;18(7):1805–1813.
Keywords: obstructive sleep apnea, sleep surgery, opioid prescription, chronic opioid use, uvulopalatopharyngoplasty, maxillomandibular advancement
BRIEF SUMMARY
Current Knowledge/Study Rationale: Otolaryngologists recently showed a significant variability in opioid prescribing patterns, “with a tendency toward excess prescription.” In an effort to control the opioid crisis in the United States, we aimed to evaluate opioid prescription patterns specific to surgeries addressing obstructive sleep apnea and define possible risk factors for chronic opioid use.
Study Impact: In those who are opioid naive, perioperative opioid prescriptions were a significant predictor for persistent, prolonged, and inappropriate opioid use. This is a call to action for increased investigation and use of multimodal pain-control strategies and opioid de-escalation protocols, particularly in patients with obstructive sleep apnea. Skeletal surgery was associated with significantly less perioperative opioid prescription compared with soft tissue surgery.
INTRODUCTION
Over 2 million Americans have opioid use disorder, and tens of thousands die of opioid-related causes annually.1,2 Opioid prescribing patterns in the United States were heavily influenced by the pharmaceutical industry in the 1990s, which downplayed the addictive nature of these drugs according to the Department of Health and Human Services.3 Additionally, a 2001 report released by the Joint Commission declared pain as “the 5th vital sign” and chastised physicians for undertreating pain.4 While the assertions made in the 1990s and in the Joint Commission report have since been debunked,5 the subsequent overprescription by physicians has become the primary source of misused opiates in the United States.6
For a significant number of patients, surgery is the first exposure to such medications.7 Surgeons are the second-highest prescriber of opioids, responsible for nearly 40% of all prescriptions, trailing only behind pain specialists.6 Effective postoperative pain management is critical for patient satisfaction, recovery, and health outcomes.8 This is especially true in the context of many otolaryngologic procedures involving the upper aerodigestive tract, as inadequately managed pain can be a limiting factor for oral intake and managing oropharyngeal secretions; however, developing appropriate postoperative pain regimens can be challenging. A recent study evaluating US otolaryngologists characterized significant variability in opioid prescribing patterns, “with a tendency toward excess prescription.”9
Currently, there are limited data on opioid prescription patterns specific to surgeries addressing obstructive sleep apnea (OSA). These include uvulopalatopharyngoplasty (UPPP), additional nasal and tongue base procedures, as well as skeletal procedures such as maxillomandibular advancement and maxillary expansion.10–12 Patients with OSA often possess other risk factors that may be associated with a higher likelihood of chronic opioid use in the postoperative period.13 This study characterizes the pattern of opioid prescription among patients undergoing various sleep apnea surgeries and evaluates the risk of chronic use among opioid-naive patients with OSA. This is especially critical in patients with OSA as opioids can reduce the respiratory drive and therefore worsen the apneic events.14 We also aimed to assess potential variables and risk factors that may contribute to persistent and prolonged use postoperatively.
METHODS
Study design
This is a retrospective cohort study among adults (≥ 18 years old) with OSA who underwent procedures for OSA, utilizing the IBM Marketscan Research and Medicare Database, from January 1, 2007, to December 31, 2015. Data collection and analysis were carried out at Stanford University School of Medicine. An institutional review from the School of Medicine was not required since the study involved only deidentified, commercially available data.
Study population
Patients undergoing procedures of palatopharyngoplasty (International Classification of Diseases, Ninth Revision [ICD-9], code 421.45) and/or tonsillectomy (ICD-9 code 428.26) and skeletal procedures based on current procedural terminology (CPT) codes encountered and with an OSA primary and/or a component diagnosis (based on the ICD-9 code) were included (Table S1 (967.3KB, pdf) in the supplemental material). The soft tissue surgery group was further subdivided into single and multilevel surgery procedures according to the additional procedures performed on the same day (Table S1 (967.3KB, pdf) ). We did not include nasal surgery alone, given the limited evidence supporting its role as a sole treatment option for OSA. Patients who underwent other upper airway procedures that did not include tonsillectomy or UPPP or skeletal procedures, or those not having continuous insurance coverage for the year prior to or after surgery, were excluded.
Information regarding prescriptions for analgesics was obtained and classified based on therapeutic classes; 58 and 59 were considered nonopioid analgesics, while 60 and 62 were considered opioid analgesics. Perioperative opioid use in each group was initially based on prescriptions filled between 14 days preoperatively and 7 postoperatively. Opioid prescription fills were recorded for the year after surgery for all patients, and the total morphine milligram equivalents (MME) were calculated. Patients with additional surgeries in the year following their procedure were excluded by eliminating those with anesthetic events over that year. Only patients with insurance coverage for 1 year prior and after the procedure were included. Opioid-naive patients were defined as those without an opioid prescription in the interval of 12 months to 14 days prior to surgery.
The primary outcome was persistent opioid use, identified as patients receiving postoperative opioid prescriptions between 3 and 6 months (91 days–180 days). Secondary outcomes included prolonged opioid use, identified as opioid prescriptions after 6 months (> 180 days) to 1 year, and inappropriate opioid use, identified as receiving over 100 MME per day. Prolonged opioid use was divided into continuous and noncontinuous based on opioid use before 6 months postoperatively. These definitions were guided by the International Association for the Study of Pain.15 We also evaluated the rate of 30-day postoperative complications, including hemorrhage, intubation, tracheostomy, and hospital readmission rates.
Supplementary demographics and comorbidities were evaluated, including age, sex, tobacco use 1 year before surgery, diagnosis of autoimmune or immunodeficiency disorders, hypertension, diabetes mellitus and psychiatric disorders (schizophrenia, depression, other mood disorders), and opioid and substance dependence (Table S1 (967.3KB, pdf) ). Comorbidities were identified using the van Walraven modification of the Elixhauser index, which condenses medical comorbidities into a numerical score that is closely associated with mortality in acute settings.16,17
Data source
The IBM MarketScan Research and Medicare Databases capture patient-specific clinical utilization, expenditures, and enrollment across outpatient and inpatient services, medication prescriptions, and carve-out services. The data are assembled from a selection of large health plans, employers, government, and public companies representing over 273 million lives and have been used extensively for peer-reviewed studies for over 30 years. This entails patients with active health care insurance, and thus is a limitation.18
Statistical analysis
Univariate descriptive statistics were calculated for demographic variables and comorbidities. A logistic regression model was estimated to examine factors associated with different opioid misuse and rates of perioperative opioid prescription. Relevant characteristics for inclusion in the final model for each complication were identified using a stepwise multivariate logistic regression (P < .05 for inclusion) to adjust for confounders. A multinominal logistic regression was used to evaluate the P value and odds ratio (OR) of different outcomes and complication rates between patients receiving opioids and those who did not among surgery groups. Analysis of variance (ANOVA) was used to calculate the difference in perioperative opioid prescription between different procedures. A Tukey test was used to compare the 3 groups. Calculated P values were 2-tailed, with significance identified as P < .05. Statistical analysis was performed using R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).19
RESULTS
Demographics
Of 133,757,228 patients’ claims, 5,798,528 (4.34%) of patients had a diagnosis of OSA in the analyzed period of January 2007 to December of 2015, where 11,367 surgical procedures met inclusion criteria. After exclusion of patients under 18 years of age, a final cohort of 10,766 procedures among 10,522 patients met the inclusion criteria (Figure S1 (967.3KB, pdf) in the supplemental material), where 482 (4.5%) patients had two or more surgeries. The mean age (standard deviation) was 43 (11.3) years old following a normal distribution, with a 75.3% male predominance (Table 1). Opioid outcomes/terms based on time intervals are shown in Table S2 (967.3KB, pdf) in the supplemental material.
Table 1.
Demographic data according to perioperative opioid prescription.
| Filled Perioperative Opioid Prescription | Did Not Fill Perioperative Opioid Prescription | Total | |
|---|---|---|---|
| Surgery events | 6,735 (100%) | 4,031 (100%) | 10,766 (100%) |
| Male sex | 5,188 (77%) | 2,914 (72%) | 8,102 (75%) |
| Age, mean, y | 42.2 | 44.4 | 43.0 |
| Age, standard deviation, y | 11.4 | 11.8 | 11.6 |
| Age group | |||
| 18–34 years | 1,694 (25%) | 802 (20%) | 2,496 (23%) |
| 35–44 years | 2,216 (33%) | 1,172 (29%) | 3,388 (31%) |
| 45–54 years | 1,818 (27%) | 1,244 (31%) | 3,062 (28%) |
| 55–64 years | 887 (13%) | 692 (17%) | 1,579 (15%) |
| ≥ 65 years | 120 (2%) | 121 (3%) | 241 (2%) |
| Risk factors | |||
| Smoking | 436 (6%) | 361 (9%) | 797 (7%) |
| Hypertension | 2,543 (38%) | 1,880 (47%) | 4,423 (41%) |
| Diabetes | 690 (10%) | 636 (16%) | 1,326 (12%) |
| Autoimmune | 546 (8%) | 539 (13%) | 1,085 (10%) |
| Immune deficiency | 184 (3%) | 139 (3%) | 323 (3%) |
| Depression | 447 (7%) | 271 (7%) | 718 (7%) |
| Mood disorder | 88 (1%) | 49 (1%) | 137 (1%) |
| Schizophrenia | 14 (0%) | 9 (0%) | 23 (0%) |
| Opioid dependence | 13 (0%) | 10 (0%) | 23 (0%) |
| Substance dependence | 18 (0%) | 9 (0%) | 27 (0%) |
| Surgery type | 6,735 (100%) | 4,031 (100%) | 10,766 (100%) |
| UPPP alone | 3,037 (45%) | 1,788 (44%) | 4,825 (45%) |
| UPPP multilevel | 3,572 (53%) | 1,957 (49%) | 5,529 (51%) |
| UPPP + GGA | 50 (1%) | 19 (0%) | 69 (1%) |
| UPPP + nose | 3,110 (46%) | 1,666 (41%) | 4,776 (44%) |
| UPPP + nose + GGA | 29 (0%) | 12 (0%) | 41 (0%) |
| UPPP + nose + tongue | 220 (3%) | 135 (3%) | 355 (3%) |
| UPPP + tongue | 162 (2%) | 121 (3%) | 283 (3%) |
| UPPP + tongue + GGA | 1 (0%) | 4 (0%) | 5 (0%) |
| Skeleton surgery | 126 (2%) | 286 (7%) | 412 (4%) |
Data are presented as n (%) unless otherwise indicated. GGA = genioglossus advancement, UPPP = uvulopalatopharyngoplasty.
Demographic data based on the perioperative prescriptions are shown in Table 1. The most common comorbid conditions were hypertension (41.1%), diabetes (12.3%), autoimmune diseases (10.1%), and mood disorders (7.9%). The most common surgical procedure was multilevel surgical procedures, with UPPP accounting for 51.4% of all procedures performed, followed by UPPP alone (44.8%) and skeletal surgery (3.8%). A total of 7,030 patients (65.3%) were prescribed an analgesic perioperatively, of which 95.8% were opioids. In the years analyzed, there was a trend toward increased perioperative opioid prescriptions, overall prescriptions in 3–6 and 6–12 months after surgery, and prolonged use (continuous and noncontinuous). Opioid prescription in the 3- to 6-month interval after sleep surgery was reported in 4.1%, while prescription in the 6- to 12-month interval was 8.2%. Postoperative inappropriate opioid use was found in 6.8%. Persistent opioid use was reported in 2.8%, continuous prolonged use was reported in 1.1%, while noncontinuous prolonged opioid use was in reported in 6.4%. There was an increased association with a diagnosis of mood disorders (including depression) and psychosis (namely, schizophrenia) among patients undergoing surgery. There was a trend of increasing rates of UPPP alone (41.8–52%) and skeletal surgery (3.2–4.8%) across the years (Table S3 (967.3KB, pdf) in the supplemental material).
Table 2 shows the multivariate model for variables associated with perioperative opioid prescriptions by surgeons. Male sex and older age tend to receive fewer opioids (OR = 0.85 and 0.99, P < .001). Patients undergoing multilevel and skeletal surgery compared with UPPP alone (OR = 0.40 and 0.54, P < .001) and those with a smoking history, hypertension, diabetes, and autoimmune diseases (OR = 0.64–0.79, P < .001) were less likely to receive an opioid prescription.
Table 2.
Multivariate regression model of factors related to getting a perioperative opioid prescription.
| Opioid Prescription | Estimate | Std. Error | z Value | P (> |z|) | 95% CI | Interpretation | ||
|---|---|---|---|---|---|---|---|---|
| Min | OR | Max | ||||||
| (Intercept) | 0.7731 | 0.0899 | 8.6020 | <.001 | Odds Ratio | |||
| Multilevel surgery | −0.9107 | 0.0798 | −11.4080 | <.001 | 0.3714 | 0.4022 | 0.4357 | Protection factor |
| Skeletal surgery | −0.6072 | 0.0516 | −11.7640 | <.001 | 0.5174 | 0.5449 | 0.5737 | Protection factor |
| Age | −0.0093 | 0.0019 | −4.8920 | <.001 | 0.9888 | 0.9907 | 0.9926 | Protection factor |
| Male | −0.1535 | 0.0476 | −3.2260 | < .001 | 0.8179 | 0.8577 | 0.8995 | Protection factor |
| Smoking | −0.3964 | 0.0756 | −5.2450 | <.001 | 0.6238 | 0.6727 | 0.7255 | Protection factor |
| Hypertension | −0.2353 | 0.0444 | −5.3030 | <.001 | 0.7561 | 0.7904 | 0.8262 | Protection factor |
| Diabetes | −0.3186 | 0.0626 | −5.0860 | <.001 | 0.6830 | 0.7271 | 0.7742 | Protection factor |
| Autoimmune disease | −0.4560 | 0.0667 | −6.8410 | <.001 | 0.5929 | 0.6338 | 0.6775 | Protection factor |
| Immune deficiency | −0.0932 | 0.1180 | −0.7900 | .4295 | 0.8096 | 0.9110 | 1.0251 | No contribution |
| Depression | 0.0735 | 0.0837 | 0.8780 | .3801 | 0.9898 | 1.0763 | 1.1702 | No contribution |
| Other mood disorders | 0.1317 | 0.1867 | 0.7050 | .4806 | 0.9465 | 1.1408 | 1.3749 | No contribution |
| Schizophrenia | −0.1078 | 0.4390 | −0.2460 | .8059 | 0.5788 | 0.8978 | 1.3926 | No contribution |
| Opioid dependence | −0.0296 | 0.4490 | −0.0660 | .9475 | 0.6197 | 0.9709 | 1.5211 | No contribution |
| Substance dependence | 0.2537 | 0.4238 | 0.5990 | .5494 | 0.8436 | 1.2888 | 1.9691 | No contribution |
CI = confidence interval, Max = maximum, Min = minimum, OR = odds ratio, Std. = standard.
Outcomes
The percentage of perioperative opioid use went from 59.5% in 2008 to 78.6% in 2015. The impact of opioid-related sequelae is shown in Table 2, Table 3, and Table 4. Table 3 and Table 4 show that perioperative opioid prescription was significantly associated with opioid prescription refills in the 3- to 6- and 6- to 12-month intervals postoperatively (P < .001), as well as with inappropriate opioid prescription (P < .001). Persistent opioid use was associated with perioperative opioid prescription in UPPP and skeletal surgery (P < .001; OR = 27.04 and 9.63, respectively). Continuous prolonged opioid use was significantly associated with perioperative UPPP opioid prescription (P < .001, OR = 28.09). Among patients with continuous prolonged opioid use who had multilevel or skeletal surgery, all received a perioperative opioid prescription. Noncontinuous prolonged opioid use was significantly associated with perioperative opioid prescription in UPPP, multilevel, and skeletal surgery (P < .001 for all; OR = 48.52, 74.36, and 38.51, respectively).
Table 3.
Opioid prescription among different multilevel sleep surgeries according to time interval.
| UPPP Alone | UPPP Multilevel | Skeleton Surgery | All Surgeries | |
|---|---|---|---|---|
| Perioperative prescription | 3,037 | 3,572 | 126 | 6,735 |
| Opioid prescription 3 to 6 months | ||||
| Did not fill perioperative opioid prescription | 9 | 5 | 6 | 20 |
| Filled perioperative opioid prescription | 134 | 159 | 8 | 301 |
| P value | <0.001 | <0.001 | .03 | <0.001 |
| OR | 9.12 | 18.19 | 3.16 | 9.38 |
| (x; y) where x ≤ OR ≤ y | (4.63; 17.96) | (7.45; 44.38) | (1.07; 9.32) | (5.96; 14.78) |
| Opioid prescription 6 to 12 months | ||||
| Did not fill perioperative opioid prescription | 37 | 27 | 17 | 81 |
| Filled perioperative opioid prescription | 352 | 429 | 21 | 802 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 |
| OR | 6.20 | 9.76 | 3.16 | 6.59 |
| (x; y) where x ≤ OR ≤ y | (4.4; 8.75) | (6.59; 14.45) | (1.61; 6.23) | (5.23; 8.31) |
OR = odds ratio, UPPP = uvulopalatopharyngoplasty.
Table 4.
Opioid prescription among different multilevel sleep surgeries according to chronic opioid use.
| UPPP Alone | UPPP Multilevel | Skeleton Surgery | All Surgeries | |
|---|---|---|---|---|
| Perioperative prescription | 3,037 | 3,572 | 126 | 6,735 |
| Noncontinuous prolonged opioid use | ||||
| No perioperative opioid prescription | 4 | 3 | 1 | 8 |
| Filled perioperative opioid prescription | 298 | 366 | 15 | 679 |
| P value | .00 | .00 | .00 | .00 |
| OR | 48.52 | 74.36 | 38.51 | 56.38 |
| (x; y) where x ≤ OR ≤ y | (18.06; 130.38) | (23.84; 231.95) | (5.03; 295.05) | (28.05; 113.33) |
| Inappropriate opioid use | ||||
| No perioperative opioid prescription | 9 | 4 | 2 | 15 |
| Filled perioperative opioid prescription | 316 | 397 | 9 | 722 |
| P value | .00 | .00 | .00 | .00 |
| OR | 22.96 | 61.05 | 10.92 | 32.15 |
| (x; y) where x ≤ OR ≤ y | (11.8; 44.65) | (22.76; 163.73) | (2.32; 51.32) | (19.25; 53.69) |
| Continuous prolonged opioid use | ||||
| No perioperative opioid prescription | 1 | 0 | 0 | 1 |
| Filled perioperative opioid prescription | 47 | 62 | 5 | 114 |
| P value | .00 | .00 | .00 | .00 |
| OR | 28.09 | — | — | 69.39 |
| (x; y) where x ≤ OR ≤ y | (3.87; 203.78) | — | — | (9.69; 497.05) |
| Continuous persistent opioid use | ||||
| No perioperative opioid prescription | 3 | 0 | 2 | 5 |
| Filled perioperative opioid prescription | 132 | 156 | 8 | 296 |
| P value | .00 | .00 | .00 | .00 |
| OR | 27.04 | — | 9.63 | 37.01 |
| (x; y) where x ≤ OR ≤ y | (8.6; 85.04) | — | (2.01; 46.01) | (15.28; 89.67) |
The denominator was the number of surgeries of the same level that had or did not have opioids, respectively. OR = odds ratio, UPPP = uvulopalatopharyngoplasty.
Table 5 shows that receiving perioperative opioid prescriptions was associated with reduced rates of postoperative hemorrhage in patients with UPPP (3.8% vs 4.3%) and multilevel surgery (2.5% vs 3.2%) (P < .001, OR = 0.64, and P = .01, OR = 0.69). Perioperative opioid prescriptions were not associated with other complications, such as hospital readmission, tracheostomy, or intubation.
Table 5.
Opioid prescription among different multilevel sleep surgeries according to chronic opioid use.
| UPPP Alone | UPPP Multilevel | Skeleton Surgery | All Surgeries | |
|---|---|---|---|---|
| Perioperative prescription | 3,037 | 3,572 | 126 | 6,735 |
| Bleeding | ||||
| No perioperative opioid prescription | 116 | 90 | 5 | 211 |
| Filled perioperative opioid prescription | 130 | 115 | 0 | 245 |
| P value | .00 | .01 | .40 | .00 |
| OR | 0.64 | 0.69 | — | 0.68 |
| (x; y) where x ≤ OR ≤ y | (0.5; 0.83) | (0.52; 0.91) | — | (0.57; 0.83) |
| Hospital readmission | ||||
| No perioperative opioid prescription | 63 | 113 | 14 | 190 |
| Filled perioperative opioid prescription | 99 | 222 | 11 | 332 |
| P value | .62 | .51 | .13 | .61 |
| OR | 0.92 | 1.08 | 1.86 | 1.05 |
| (x; y) where x ≤ OR ≤ y | (0.67; 1.27) | (0.86; 1.37) | (0.82; 4.22) | (0.87; 1.26) |
| Tracheostomy | ||||
| No perioperative opioid prescription | 2 | 3 | 5 | 0 |
| Filled perioperative opioid prescription | 1 | 0 | 1 | 0 |
| P value | — | .26 | .52 | .02 |
| OR | 1.00 | 0.27 | — | 0.12 |
| (x; y) where x ≤ OR ≤ y | (0.92; 1.09) | (0.02; 3.02) | — | (0.01; 1.02) |
| Intubation | ||||
| No perioperative opioid prescription | 0 | 0 | 1 | 1 |
| Filled perioperative opioid prescription | 1 | 1 | 0 | 2 |
| P value | .64 | .66 | .71 | .88 |
| OR | — | — | — | 1.20 |
| (x; y) where x ≤ OR ≤ y | — | — | — | (0.11; 13.21) |
The denominator was the number of surgeries of the same level that had or did not have opioids, respectively. OR = odds ratio, UPPP = uvulopalatopharyngoplasty.
Table 1 shows the patterns of perioperative opioid prescriptions with different procedures. Patients with UPPP alone had a 63% rate of perioperative opioid prescription compared with UPPP with multilevel procedures (65%, P = .184). Skeletal surgery, on the other hand, was associated with a 31% rate of perioperative opioid prescription, which was significantly less than the other 2 categories (63% and 65%, respectively) using an ANOVA followed by a Tukey test (P < .001).
The univariate regression model evaluating potential risk factors, demographic details, various surgeries, and associated outcomes is summarized in Table S4 (967.3KB, pdf) in the supplemental material. A multivariate regression model was used to identify risk factors for complications and different opioid outcomes, as shown in Table S5 (967.3KB, pdf) in the supplemental material. Opioid prescription and smoking were significantly associated with inappropriate opioid use (OR = 31.51, P < .001, and OR = 1.41, P = .016, respectively). Depression was also associated with inappropriate opioid use, yet did not reach statistical significance (OR = 1.29, P = .078). Opioid prescription and hypertension were associated with persistent opioid use (OR = 37.8, P < .001, OR = 1.38, P = .008). Opioid prescription and previous opioid dependence diagnosis, smoking, and male sex were associated with continuous prolonged opioid use (OR = 73.1, 8.13, 1.95, and 1.55, respectively; P < .001, P = .020, P = .024, and P = .032, respectively). Perioperative opioid use and male sex were associated with noncontinuous prolonged opioid use (OR = 57.81 and 1.31, respectively; P < .001 and P = .029, respectively). Opioid use 3–6 months after surgery was significantly associated with perioperative opioid use and hypertension (OR = 9.68 and 1.39; P < .001 and .034). Opioid use 6–12 months after surgery was significantly associated with perioperative opioid use, opioid dependence, autoimmune disease, diabetes, male sex, and older age (OR = 7.16, 3.28, 1.27, 1.43, 1.35, and 1.01; P < .001, P = .028, P = .046, P < .001, P < .001, and P = .009, respectively).
Perioperative opioid prescription, male sex, increasing age, and multilevel surgery were associated with fewer bleeding events postoperatively (OR = 0.66, 0.55, 0.95, and 0.38; P < .001, < .001, .001, and .0028). Multilevel surgery was associated with more readmissions (OR = 1.52, P = .009), while skeletal surgery was associated with fewer readmissions (OR = 0.77, P = .012). Smoking, hypertension, and autoimmune diseases were associated with more readmissions (OR = 1.49, 1.55, and 1.76; P < .001, 0.006, and .008). Opioid dependence was significantly associated with increased reintubation rates (OR = 7.48, P = .006).
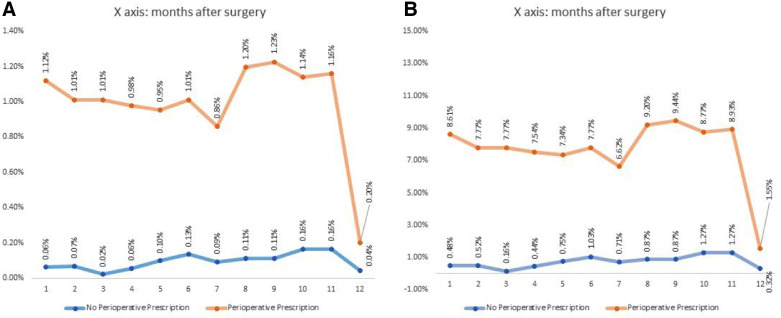
In Figure 1 we evaluated the percentage of opioid prescriptions after the first 30 days in this cohort. Patients who had perioperative opioid prescriptions were associated with more postoperative opioid prescriptions across the whole year when compared with those who did not. Table S3 (967.3KB, pdf) shows the changes in the rates of various surgical procedures over the span of 9 years.
Figure 1. Percentage of opioid prescriptions after the first 30 days in this cohort.
(A) The denominator is total opioid prescription. (B) The denominator is opioid prescription from month 1 through month 12 postoperatively. NDCNUM = National Drug Code Number, OSA = obstructive sleep apnea, UPPP = uvulopalatopharyngoplasty.
DISCUSSION
Opioid overdoses and deaths have been on the rise over the past several decades, which could be at least in part due to increased prescriptions.20 The literature on use of postoperative opioids suggests that patients often use less than half of the opioids prescribed after large thoracic or gynecologic surgeries.21 As surgeons are responsible for over one-third of all opioid prescriptions in the United States,6 it is of the upmost importance that we carefully examine these practice patterns with the goal of reducing excessive or unnecessary prescriptions. Considering this risk, the Centers for Disease Control and Prevention recommends that clinicians provide patients with the lowest effective dose for the shortest expected duration of severe pain.22
In this analysis, after evaluating the pattern of opioid prescriptions among opioid-naive patients undergoing OSA procedures, we found that 62.6% had opioid prescriptions in the identified perioperative period. The prevalence of OSA did not exceed 5%, highlighting that OSA remains underdiagnosed in the general population.23 Perioperative opioid prescriptions were significantly associated with increased persistent, prolonged (continuous and noncontinuous), and inappropriate opioid use. Similarly, perioperative opioid prescription was also a strong predictor of persistent and prolonged opioid use after different plastic and reconstructive procedures.24 These findings are also consistent with other studies, where increased risk of opioid use after 1 year has been associated with each additional day of opioid medication supplied, starting after the third day in opioid-naive patients.25
While there is a paucity of data, existing otolaryngology literature suggests that pain medications may be overprescribed. Two studies suggest that Acetaminophen alone is sufficient in treating pain following endoscopic sinus surgery.26,27 Taliercio et al28 reported that only 1 in 5 of patients require opioids following direct laryngoscopy. Patel et al29 found that only about 1 in 3 of opioids prescribed for postoperative rhinoplasty patients were used. A recent cross-sectional survey conducted by Schwartz et al9 found large variations in the opioid prescribing patterns for the same surgeries, based on individual surgeon preferences.
To our knowledge, this study is the first to evaluate the pattern of opioid prescriptions following OSA surgery, including UPPP, multilevel soft tissue, and facial skeletal procedures, based on a large population-based database, and rates of postoperative opioid use up to 1 year following these procedures. It also raises awareness with regard to the increasing trend in opioid prescriptions over the years that correlates with the overall increased use of opioids, echoing reports of opioid consumption in the United States and Europe.30
Some clinical implications that can be postulated include identifying individuals who are prone to chronic opioid misuse or inappropriate opioid doses. A large retrospective analysis performed on the administrative health claims data of over 640,000 opioid-naive surgical patients found that male sex, age greater than 50 years, and preoperative history of substance use disorder, alcohol use disorder, depression, benzodiazepine use, or antidepressant use were associated with chronic opioid use. Chronic opioid use in their study was defined as having filled 10 or more prescriptions, or more than 120 days’ supply, within the first year after surgery, excluding the first 90 postoperative days. These results may suggest that patients with OSA could be at higher risk for chronic opioid use than average surgical patients, as rates of substance use disorders and mood disorders are generally higher among patients with OSA.31–33
Overall, our analysis found increasing rates of mood disorders and psychosis among patients undergoing sleep surgery. Tobacco smoking was associated with inappropriate and prolonged opioid use. A former diagnosis of opioid dependence and male sex were also considered risk factors, and interestingly, hypertension was associated with persistent use. Similarly, this was noted as a risk factor for long-term use after orthopedic procedures.34
Our results show that UPPP is the most commonly performed procedure for OSA, alone or in combination. This is known to be a painful procedure, especially in the early postoperative period, with reported dysphagia due to pain in up to 60% and an average dysphagia rate of 31%.35 Therefore, pain management in this cohort of patients is critical and may require supplementary care.
Additional concerns about postoperative respiratory suppression are well known. Opiates/opioids bind to various receptors—namely, δ, κ, µ, and the nociception/orphanin receptors. These receptors not only control pain and stress but also affect respiration. Ligands that stimulate κ and µ receptors suppress the respiratory center and can result in reduction in both respiratory rate and tidal volume.36 Subsequently, opioids can reduce the chemo-responsiveness to respiratory stimulants as hypercapnia and hypoxia, resulting in alterations of ventilatory drive, and may impair the high loop gain in patients with OSA. The loop gain can be affected by opioid misuse resulting in failed restoration of normal arterial blood gas tension after an apneic event.37 Opioids also reduce the muscle tone, resulting in more airway floppiness.38 Although opioids/opiates did not show a reduction in apnea-hypopnea index or oxygen desaturation index, there are reports that demonstrate reduced lowest oxygen saturation and increased central apneic events.39 In children with OSA using codeine postoperatively, there were reports of post-tonsillectomy deaths attributed to genotypic and metabolic variations.40,41 Of note, there is a well-established link between opioids and all types of sleep-disordered breathing, with a dose-dependent relationship.42 Over two-thirds of patients on opioids are believed to have sleep-disordered breathing, with a predilection toward moderate-to-severe sleep-disordered breathing.43–45
Limitations of this study include the nature of database research and accuracy of the data coded. Another limitation is the definition of opioid-naive patients and perioperative opioids as patients may have opioid prescription after 7 days postoperatively. The lack of information regarding opioid diversion, other opioid resources, the uncertainty of prescription completion, sleep studies, body mass index, as well as having data only until 2015 are other limitations. Additionally, these results are only adopted from the United States. This study accounts for patients enrolled in employment-based insurance and therefore does not capture the whole population. While unlikely, patients who had additional procedures that were not part of the medical records can be a hypothetical reason for persistent or prolonged opioid use. Although one may argue that sleep surgery procedures are painful, particularly UPPP which comprises most of the procedures, this should not be an explanation for the use of opioids more than 1 month postoperatively.
Sleep surgeons should contemplate counseling patients in the perioperative visits regarding the possible risk of persistent and/or prolonged use, which reached up to 8% in this study, and contemplate the use of nonopioid analgesics (acetaminophen and ibuprofen) with different regimens to avoid opioid-related risks in the OSA population.46–48
CONCLUSIONS
Among patients undergoing surgery for OSA, there is a tendency of increased opioid prescriptions by sleep surgeons over the years. In those who are opioid naive, perioperative opioid prescriptions were a significant predictor for persistent, prolonged, and inappropriate opioid use. Among OSA procedures, skeletal surgery was associated with significantly less perioperative opioid prescription compared with soft tissue surgery. This is a call to action for increased investigation and use of multimodal pain-control strategies and opioid de-escalation protocols, particularly in patients with OSA who have an already compromised airway architecture.
DISCLOSURE STATEMENT
The authors report no conflicts of interest.
ABBREVIATIONS
- GGA
genioglossus advancement
- OR
odds ratio
- OSA
obstructive sleep apnea
- UPPP
uvulopalatopharyngoplasty
REFERENCES
- 1. Hedegaard H , Warner M , Miniño AM . Drug overdose deaths in the United States, 1999–2016 . NCHS Data Brief. 2017. : 1 – 8 . [PubMed] [Google Scholar]
- 2. Substance Abuse and Mental Health Services Administration . Key Substance Use and Mental Health Indicators in the United States: Results from the 2016 National Survey on Drug Use and Health. 2017. . https://www.samhsa.gov/data/report/key-substance-use-and-mental-health-indicators-united-states-results-2016-national-survey .
- 3. US Department of Health and Human Services . About the Epidemic. What is the U.S. Opioid Epidemic? Accessed May 24, 2021. .
- 4. Joing Commission on Accreditation of Healthcare Organizations . Joint Commission on Accreditation of Healthcare Organizations Pain Standards for. 2001. .
- 5. Mularski RA , White-Chu F , Overbay D , Miller L , Asch SM , Ganzini L . Measuring pain as the 5th vital sign does not improve quality of pain management . J Gen Intern Med. 2006. ; 21 ( 6 ): 607 – 612 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levy B , Paulozzi L , Mack KA , Jones CM . Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007-2012 . Am J Prev Med. 2015. ; 49 ( 3 ): 409 – 413 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waljee JF , Li L , Brummett CM , Englesbe MJ . Iatrogenic opioid dependence in the United States: are surgeons the gatekeepers? Ann Surg. 2017. ; 265 ( 4 ): 728 – 730 . [DOI] [PubMed] [Google Scholar]
- 8. American Society of Anesthesiologists Task Force on Acute Pain Management . Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management . Anesthesiology. 2012. ; 116 ( 2 ): 248 – 273 . [DOI] [PubMed] [Google Scholar]
- 9. Schwartz MA , Naples JG , Kuo CL , Falcone TE . Opioid prescribing patterns among otolaryngologists . Otolaryngol Head Neck Surg. 2018. ; 158 ( 5 ): 854 – 859 . [DOI] [PubMed] [Google Scholar]
- 10. Abdelwahab M , Poomkonsarn S , Ren X , Awad M , Capasso R , Riley R , et al . A Comprehensive Strategy for Improving Nasal Outcomes After Large Maxillomandibular Advancement for Obstructive Sleep Apnea . Facial Plast Surg Aesthet Med. 2021. ; 23 ( 6 ): 437 – 442 . [DOI] [PubMed] [Google Scholar]
- 11. Abdelwahab M , Yoon A , Okland T , Poomkonsarn S , Gouveia C , Liu SY-C . Impact of distraction osteogenesis maxillary expansion on the internal nasal valve in obstructive sleep apnea . Otolaryngol Head Neck Surg. 2019. ; 161 ( 2 ): 362 – 367 . [DOI] [PubMed] [Google Scholar]
- 12. Liu SY , Awad M , Riley R , Capasso R . The role of the revised stanford protocol in today’s precision medicine . Sleep Med Clin. 2019. ; 14 ( 1 ): 99 – 107 . [DOI] [PubMed] [Google Scholar]
- 13. Sun EC , Darnall BD , Baker LC , Mackey S . Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period . JAMA Intern Med. 2016. ; 176 ( 9 ): 1286 – 1293 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lyons OD , Bradley TD . Heart failure and sleep apnea . Can J Cardiol. 2015. ; 31 ( 7 ): 898 – 908 . [DOI] [PubMed] [Google Scholar]
- 15. Treede RD , Rief W , Barke A , et al . A classification of chronic pain for ICD-11 . Pain. 2015. ; 156 ( 6 ): 1003 – 1007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Walraven C , Austin PC , Jennings A , Quan H , Forster AJ . A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data . Med Care. 2009. ; 47 ( 6 ): 626 – 633 . [DOI] [PubMed] [Google Scholar]
- 17. Elixhauser A , Steiner C , Harris DR , Coffey RM . Comorbidity measures for use with administrative data . Med Care. 1998. ; 36 ( 1 ): 8 – 27 . [DOI] [PubMed] [Google Scholar]
- 18. IBM MarketScan Research Databases for Health Services Researchers . Published 2019. . https://www.ibm.com/downloads/cas/6KNYVVQ2 .
- 19. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. .
- 20. Centers for Disease Control and Prevention (CDC) . Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008 . MMWR Morb Mortal Wkly Rep. 2011. ; 60 ( 43 ): 1487 – 1492 . [PubMed] [Google Scholar]
- 21. Bartels K , Mayes LM , Dingmann C , Bullard KJ , Hopfer CJ , Binswanger IA . Opioid use and storage patterns by patients after hospital discharge following surgery . PLoS One. 2016. ; 11 ( 1 ): e0147972 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dowell D , Haegerich TM , Chou R . CDC guideline for prescribing opioids for chronic pain—United States, 2016 . MMWR Recomm Rep. 2016. ; 65 ( 1 ): 1 – 49 . [DOI] [PubMed] [Google Scholar]
- 23. Abdelwahab M , Marques S , Previdelli I , Capasso R . Perioperative Antibiotic Use in Sleep Surgery: Clinical Relevance . Otolaryngol Head Neck Surg. 2022. ; 166 ( 5 ): 993 – 1002 . [DOI] [PubMed] [Google Scholar]
- 24. Olds C , Spataro E , Li K , Kandathil C , Most SP . Assessment of persistent and prolonged postoperative opioid use among patients undergoing plastic and reconstructive surgery . JAMA Facial Plast Surg. 2019. ; 21 ( 4 ): 286 – 291 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shah A , Hayes CJ , Martin BC . Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006-2015 . MMWR Morb Mortal Wkly Rep. 2017. ; 66 ( 10 ): 265 – 269 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kemppainen TP , Tuomilehto H , Kokki H , Seppä J , Nuutinen J . Pain treatment and recovery after endoscopic sinus surgery . Laryngoscope. 2007. ; 117 ( 8 ): 1434 – 1438 . [DOI] [PubMed] [Google Scholar]
- 27. Kemppainen T , Kokki H , Tuomilehto H , Seppä J , Nuutinen J . Acetaminophen is highly effective in pain treatment after endoscopic sinus surgery . Laryngoscope. 2006. ; 116 ( 12 ): 2125 – 2128 . [DOI] [PubMed] [Google Scholar]
- 28. Taliercio S , Sanders B , Achlatis S , Fang Y , Branski R , Amin M . Factors associated with the use of postoperative analgesics in patients undergoing direct microlaryngoscopy . Ann Otol Rhinol Laryngol. 2017. ; 126 ( 5 ): 375 – 381 . [DOI] [PubMed] [Google Scholar]
- 29. Patel S , Sturm A , Bobian M , Svider PF , Zuliani G , Kridel R . Opioid use by patients after rhinoplasty . JAMA Facial Plast Surg. 2018. ; 20 ( 1 ): 24 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Volkow ND , McLellan TA , Cotto JH , Karithanom M , Weiss SR . Characteristics of opioid prescriptions in 2009 . JAMA. 2011. ; 305 ( 13 ): 1299 – 1301 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hobzova M , Prasko J , Vanek J , et al . Depression and obstructive sleep apnea . Neuroendocrinol Lett. 2017. ; 38 ( 5 ): 343 – 352 . [PubMed] [Google Scholar]
- 32. Gałecki P , Florkowski A , Zboralski K , Pietras T , Szemraj J , Talarowska M . [Psychiatric and psychological complications in obstructive sleep apnea syndrome] . Pneumonol Alergol Pol. 2011. ; 79 ( 1 ): 26 – 31 . [PubMed] [Google Scholar]
- 33. Gupta MA , Simpson FC . Obstructive sleep apnea and psychiatric disorders: a systematic review . J Clin Sleep Med. 2015. ; 11 ( 2 ): 165 – 175 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rao AG , Chan PH , Prentice HA , Paxton EW , Funahashi TT , Maletis GB . Risk factors for opioid use after anterior cruciate ligament reconstruction . Am J Sports Med. 2019. ; 47 ( 9 ): 2130 – 2137 . [DOI] [PubMed] [Google Scholar]
- 35. Franklin KA , Anttila H , Axelsson S , et al . Effects and side-effects of surgery for snoring and obstructive sleep apnea—a systematic review . Sleep. 2009. ; 32 ( 1 ): 27 – 36 . [PMC free article] [PubMed] [Google Scholar]
- 36. Stein C . Opioid receptors . Annu Rev Med. 2016. ; 67 : 433 – 451 . [DOI] [PubMed] [Google Scholar]
- 37. Eckert DJ , White DP , Jordan AS , Malhotra A , Wellman A . Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets . Am J Respir Crit Care Med. 2013. ; 188 ( 8 ): 996 – 1004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O’Keefe RJ , Domalik-Wawrzynski L , Guerrero JL , Rosow CE , Lowenstein E , Powell WJ Jr . Local and neurally mediated effects of sufentanil on canine skeletal muscle vascular resistance . J Pharmacol Exp Ther. 1987. ; 242 ( 2 ): 699 – 706 . [PubMed] [Google Scholar]
- 39. Mason M , Cates CJ , Smith I . Effects of opioid, hypnotic and sedating medications on sleep-disordered breathing in adults with obstructive sleep apnoea . Cochrane Database Syst Rev. 2015. ;(7): CD011090 . [DOI] [PubMed] [Google Scholar]
- 40. Ciszkowski C , Madadi P , Phillips MS , Lauwers AE , Koren G . Codeine, ultrarapid-metabolism genotype, and postoperative death . N Engl J Med. 2009. ; 361 ( 8 ): 827 – 828 . [DOI] [PubMed] [Google Scholar]
- 41. US Food and Drug Administration. Drug Safety Communications: Safety review update of codeine use in children; new Boxed Warning and Contraindication on use after tonsillectomy and/or adenoidectomy. Silver Spring, MD: US Food and Drug Administration; 2013.
- 42. Jungquist CR , Flannery M , Perlis ML , Grace JT . Relationship of chronic pain and opioid use with respiratory disturbance during sleep . Pain Manag Nurs. 2012. ; 13 ( 2 ): 70 – 79 . [DOI] [PubMed] [Google Scholar]
- 43. Webster LR , Choi Y , Desai H , Webster L , Grant BJ . Sleep-disordered breathing and chronic opioid therapy . Pain Med. 2008. ; 9 ( 4 ): 425 – 432 . [DOI] [PubMed] [Google Scholar]
- 44. Mogri M , Khan MIA , Grant BJB , Mador MJ . Central sleep apnea induced by acute ingestion of opioids . Chest. 2008. ; 133 ( 6 ): 1484 – 1488 . [DOI] [PubMed] [Google Scholar]
- 45. Correa D , Farney RJ , Chung F , Prasad A , Lam D , Wong J . Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations . Anesth Analg. 2015. ; 120 ( 6 ): 1273 – 1285 . [DOI] [PubMed] [Google Scholar]
- 46. Tan GX , Tunkel DE . Control of pain after tonsillectomy in children: a review . JAMA Otolaryngol Head Neck Surg. 2017. ; 143 ( 9 ): 937 – 942 . [DOI] [PubMed] [Google Scholar]
- 47. D’Souza JN , Schmidt RJ , Xie L , Adelman JP , Nardone HC . Postoperative nonsteroidal anti-inflammatory drugs and risk of bleeding in pediatric intracapsular tonsillectomy . Int J Pediatr Otorhinolaryngol. 2015. ; 79 ( 9 ): 1472 – 1476 . [DOI] [PubMed] [Google Scholar]
- 48. Liu C , Ulualp SO . Outcomes of an alternating ibuprofen and acetaminophen regimen for pain relief after tonsillectomy in children . Ann Otol Rhinol Laryngol. 2015. ; 124 ( 10 ): 777 – 781 . [DOI] [PubMed] [Google Scholar]